Nathalie Erdeljac, Gerald Kehr, Marie Ahlqvist, Laurent Knerr and Ryan Gilmour
Chem. Commun., 2018,54, 12002-12005
DOI:
10.1039/C8CC05643A,
Communication
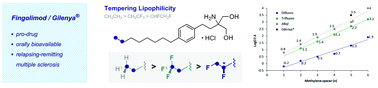
The direct, catalytic vicinal difluorination of terminal alkenes via an I(I)/I(III) manifold was exploited to install a chiral, hybrid bioisostere of the CF3 and Et groups (BITE) in Gilenya®; the first orally available drug for the clinical management of Multiple Sclerosis (MS). This subtle fluorination pattern allows lipophilicity (log D) to be tempered compared to the corresponding CF3 and Et derivatives (CH2CH3 > CH2CF3 > CHFCH2F).