Wearable chem-biosensing devices: from basic research to commercial market
Bin
Yang
a,
Xingyu
Jiang
 *b,
Xueen
Fang
*b,
Xueen
Fang
 *a and
Jilie
Kong
*a
*a and
Jilie
Kong
*a
aDepartment of Chemistry and Institutes of Biomedical Sciences, Fudan University, Shanghai, 200433, P. R. China. E-mail: fxech@fudan.edu.cn; jlkong@fudan.edu.cn
bDepartment of Biomedical Engineering, Shenzhen Bay Laboratory, Southern University of Science and Technology, Shenzhen, 518055, P. R. China. E-mail: jiang@sustech.edu.cn
First published on 8th October 2021
Abstract
Wearable chem-biosensors have been garnering tremendous interest due to the significant potential in tailored healthcare diagnostics and therapeutics. With the development of the medical diagnostics revolution, wearable chem-biosensors as a rapidly emerging wave allow individuals to perform on-demand detection and obtain the required in-depth information. In contrast to commercial wearables, which tend to be miniaturized for measuring physical activities, the recent progressive wearable chem-biosensing device have mainly focused on non-invasive or minimally invasive monitoring biomarkers at the molecular level. Wearables is a multidisciplinary subject, and chem-biosensing is one of the most significant technologies. In this review, the currently basic academic research of wearable chem-biosensing devices and its commercial transformation were summarized and highlighted. Moreover, some representative wearable products on the market for individual health managements are presented. Strategies for the identification and sensing of biomarkers are discussed to further promote the development of wearable chem-biosensing devices. We also shared the limitations and breakthroughs of the next generation of chemo-biosensor wearables, from home use to clinical diagnosis.
1. Introduction
When we watch science fiction movies, we cannot help being surprised by the portrayal of invulnerable and invincible wearable devices, such as when Iron Man Tony Stark's exoskeleton is embedded with an integrated weapon system of flight, jet propulsion, communication and sensing; Wonder Woman wears a bracelet that can absorb energy and emit shock waves; or Star Trek member Spock uses a tricorder to assess environmental conditions. It seems to be fictitious scenes. However, recent research studies in materials science, electronics, biosensing technology, device designs, wireless communication, and power supply support the foundation for all types of wearables, which possess their distinct characterizations, such as non-invasiveness, conformal contact with skin, qualitative analysis ability, different modes of deployment from the epidermis and eyes to mouth guard and face mask. The tricorder in Star Trek is no longer a pipe dream. A realistic version of the tricorder has been designed and can monitor physiological indicators related to human health diseases. In 2017, the winner of the Qualcomm Tricorder XPrize competition went to Eugene Chan, who is the chief scientist in DNA Medicine Institute (DMI, USA). They developed a handheld device to conduct a serial of tests, including red blood cells or white blood cells counting. More and more wearable devices are being recognized and accepted all over the world. Wearable devices based on human-machine exchange technology are common in shopping malls. Users can achieve human-machine interactions through touch, sound, behavior, and eye movement. These wearable devices can realize intelligent entertainment activities.The term “wearable technology” was originated from the word “wearable device” at the Massachusetts Institute of Technology (MIT). Wearable was defined by the MIT media laboratory as follows: devices (e.g., clothes, bracelets, glasses), that are connected to a network database through computer multimedia and wireless communication without foreign body sensation and act as the user's personal intelligent assistant to handle the process the information. After decades of development, wearable technology has its own definition; that is, a portable device that directly adds any smart device on the wearers' body, clothes or accessories, and it enables processing data and send signals at the same time. Wearable technology is a new research field, including the intersection of physics, chemistry, biology, medicine, energy, electronics, materials, machinery, communication and other disciplines. It is the product of the rapid development of the Internet of Things (IoT) in the 21st century. With the mutual promotion and development of IoT, diversified wearable smart products have successfully evolved into a new commercial market with huge potential, occupying a place in the market and becoming a wave of industry and scientific research. This emerging wave should be attributed to wearable medical devices, as they allow users to operate self-management health monitoring instead of conventional laboratory. However, recent commercial wearable medical devices have focused on continuous glucose monitoring. Hence, wearable medical devices will remain preferable to health monitoring in the future, which might pave the way for personalized medical requirements.
Recently, there is an increasing number of comprehensive reviews in wearables topics in the field of wearables, covering multifunctional materials, point-of-care testing, chemical analysis, device designs, electrochemical sensors, sensing modalities, and power supply. These representative research groups include those of Joseph Wang, John A. Rogers, Zhenan Bao, Zhong Lin Wang, Wei Gao, and others.1–3e By contrast, this review highlights the chem-biosensing technology in a comprehensive manner, which combines fundamental research in biosensing and engineering with their technology achievement transformations in the commercial market, which have the potential of wide application in human healthcare. Interestingly, with the advent of personal medicine revolution, the recently reported wearable chem-biosensing devices have greatly improved in terms of modality, stability, sensitivity, and practicability. Thus, it is of great significance to highlight the latest development of wearable chem-biosensing devices to broaden the subject of biochemical sensing discipline. This review summarizes the latest progress of wearable chem-biosensing technology to 2021, aiming to provide a comprehensive and concise overview of the latest advances for peer researchers who want to engage in wearable chem-biosensing research. Meanwhile, we are delighted to provide practical suggestions and prospects of commercial transformation for those readers from different education levels and research areas, who want to get involved in the field of wearable biosensor market. Thus, in order to avoid some sophisticated jargons, this review might be a combination of popular and academic science for readers.
This review classified wearable chem-biosensors into four types according to the wearing manners, including epidermis wearable, mouth guard wearables, breath-based wearables, and ocular wearables. The origin, commercial products, core technologies, chem-biosensing strategies and prospects of wearable chemical biosensing devices are reviewed.
2. The history and development of wearable devices
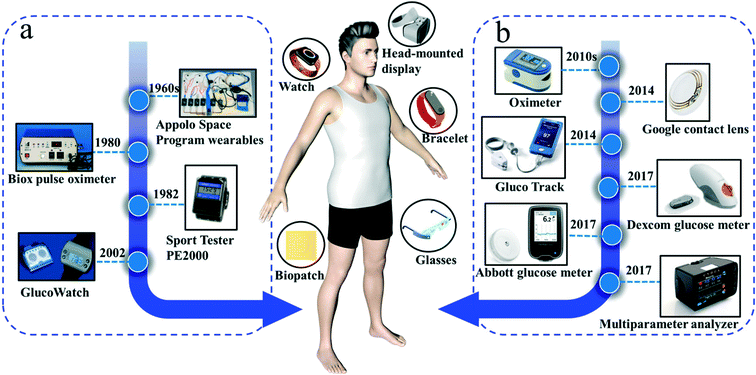
The origin and evolution of wearable devices can be seen in Fig. 1. The concept of wearable devices was first proposed by the MIT Media Lab in the 1960s. They proposed to incorporate emerging technologies, such as sensors, multimedia components, and wireless communications, into clothing or accessories to enrich and improve the quality of life. However, due to the limitations of science and technology at the time, some wearable prototypes were stagnant in the laboratory. Later, as shown in Fig. 1a, in the course of space exploration, the staff of the Apollo space program knew very well that astronauts in space flight would be exposed to extreme conditions. Therefore, it was necessary to continuously monitor the health of astronauts by means of three methods: (I) application of wearable electrocardiography (ECG); (II) detection of the cooling of air in the inlet and outlet by heating thermistors to monitor respiration; and (III) the use of a rectal probe to accurately measure body temperature.2 Finally, the relevant data were sent back to the earth. At that time, the MIT research team innovatively integrated sensing and communication technology into clothing accessories, which could not only monitor the health of users in real time, but also integrate art, fashion, and technology to improve the quality of life.3a In 1982, Polar launched the Sport Tester PE2000, which was a turning point. Additionally, in that year, Biox (Colorado, USA) first launched a commercial pulse oximeter. Within a few years, pulse oximetry has become a universal method for anesthesia.Commercial wearable products on the market, such as wearable blood glucose meters, undergo a long period of evolution before they can be meaningfully promoted. The predecessor of wearable blood glucose meters is a biosensor for glucose detection developed by Leland Clark and Ann Lyons of Cincinnati Children's Hospital in 1962. After a long period of research on non-invasive wearables, Cygnus launched the GlucoWatch product in 2002.
GlucoWatch has achieved remarkable results in glucose biosensing in a non-invasive way, reducing the pain of blood sampling for diabetic patients. The wearable device uses two gel-based pads attached to the surface of the human skin. With a direct current (DC) voltage, the patch utilizes the principle of reverse iontophoresis to non-invasively extract tissue fluid and glucose. The working principle of this watch-type wearable blood glucose meter device is using a current density of approximately 0.5 mA cm−2 to pass through the dense microchannels (such as sweat ducts and hair follicles) in the stratum corneum, which is at a rate of approximately 5–50 nL min−1 to extract interstitial fluid (ISF).3a Reverse iontophoresis leads to the electroosmotic flow of ISF in the paracellular pathway because the surface of the human skin is negatively charged, which promotes the electroosmotic flow of sodium ions. The device reversibly circulates the potential of the DC power supply on two gel-like pads every 10 minutes to maintain the pH of skin. Then, a commercial mature immobilized glucose oxidase electrode system is used to detect glucose in the ISF. Cygnus has obtained FDA approval for GlucoWatch for diabetes monitoring. This is a non-invasive wearable blood glucose meter that is an excellent achievement because if glucose changes are not monitored in a timely, accurate, and real-time manner, it may endanger the lives of diabetic patients. However, due to the repeated need to rely on the instrument, the traditional finger blood sampling method is required to calibrate the wearable device.
However, GlucoWatch also has some drawbacks. For one thing, sweating can lead to errors and biases in the recording of glucose concentration. On the other hand, a few hours after the reverse iontophoresis, abnormal stinging or skin damage can occur on the human skin surface. Therefore, GlucoWatch cannot be used as a popular product. This is one of the reasons why non-invasive wearable sensors have not yet existed in the commercial market as broad-spectrum products.
Wearable devices for personal health monitoring have been developed in recent years, as shown in Fig. 1b. Oximeters are one of the most common wearable items, and are commonly used for clinical or home use. An oximeter on the patient's finger provides real-time and non-invasive monitoring of SpO2 in blood, where hemoglobin shows different absorption intensities at the wavelength of 660 nm and 910 (controlled by two mini-LED), thus providing an individual blood oxygen signal. It was estimated that the number of older patients with diabetes would increase by nearly 4.5 times in 2050.4 Furthermore, the number of young adults with diabetes (type II) has been on the rise in recent years.5 Fortunately, wearable devices can shed light on glucose real-time monitoring. As can be seen from Fig. 1b, recent wearable products mainly focused on the market of non-invasive or minimally invasive glucose monitoring. In 2014, a breakthrough in the wearable commercial market came with Google contact lens for monitoring blood glucose in tears. This was good news for patients with diabetes (type I or type II) because the device can non-invasively monitor tear glucose to avoid the fingerstick bleeding. A glucose biosensor was integrated in the contact lens, and the real-time monitoring signal was transmitted wirelessly through an antenna. However, this project of the glucose contact lens has since been halted. Some reasons might be as follows: (I) the correlation between blood glucose and tears glucose is not so clear; (II) the fluid volume of the left and right tears is inconsistent, making sample extraction difficult; (III) the test results of tear samples are easily affected by external environmental factors, including temperature, humidity, eye pressure, and metabolization, leading to a fluctuation in glucose detection; (IV) a switch calibration is not sufficient for a long-term monitoring device. In 2014, Gluco Track (Cnoga Inc., Israel), a truly invasive wearable finger clip, was used for glucose detection. The detection principle was based on the optical reflection, where the absorption changes of different molecules reflected the concentration of glucose in the blood or ISF.
Later, in 2017, two competitive wearable products appeared on the market, namely Freestyle Libre (Abbott Inc.) and G5 continuous glucose monitoring (Dexcom Inc.), which were breaking wearables for ISF glucose monitoring. The detection principle of these two wearables was based on electrochemical glucose biosensor by a soft needle coated with glucose oxidase. The wearable glucose meter measures the ISF glucose concentration in a minimally invasive method, and the user does not need to correct the glucose concentration during the entire use. The waterproof device allows users to wear it for 24 hours without feeling any foreign sensation. The glucose concentration is recorded and scanned by a tiny needle, and data is transmitted to a portable device by a microcircuit board. The device is non-volatile, where all data collected remain even if the power is suddenly cut off. The wearers could obtain real-time ISF glucose concentration by a portal detector anytime and anywhere. Up to now, Freestyle Libre has received CE certification, U.S. Food and Drug Administration (FDA), and China Food and Drug Administration (CFDA), while Dexcom G5 continuous glucose monitoring has been approved by the FDA.
In Fig. 1b, Cnoga incorporation (Israel) launched a multiparameter health portable analyzer (Matrix Monitor) in 2017 for simultaneously monitoring the heart rate, blood pressure, blood oxygen, and pulse by a finger clip. It could be applied for the continuous self-management of the blood glucose level. Especially, this kind of minimally invasive device might be acceptable for pregnant women with diabetes. The glucose calibration should be conducted every one or two weeks by its supplied consumables. Interestingly, there is no time limitation in real-time monitoring. It is a truly non-invasive wearable device, merely weighing ∼100 g, and approved by CE certification, FDA, and CFDA successively.
Obviously, the present wearable devices are mainly dominated by watch-type, glass-type, bracelet-type, and biopatch sensing devices, such as FitBit, Apple watch, Google Glass, and Medtronic's SEEQ heart monitoring medical biopatch. It is worth noting that these wearable devices mainly perform simple electrochemical or optical detections for the epidermis, which have been popularly used over the past decades. These wearable devices tend to measure the physical indicators of users and cannot provide another important source of human health monitoring, namely, the monitoring of biomarkers in body fluids (including blood, sweat, ISF, saliva, tears, and urine).
The development of the combination of medical diagnosis and wearable medical devices has certain strategic significance. Medical diagnosis is a complex process, whose efficiency needs to be improved. Intelligent, portable, and miniaturized wearable medical devices are also used to extend the controllable useful information for diagnostic processes. Additionally, due to the rapid development of microfluidic technology in the fields of life analysis and medical testing, more and more lab-on-a-chips have been transforming into wearable system. It is well known that the microfluidic device with nano or micro channels can be used to study the kinetics of chemical reactions or physical systems, with high temporal resolution and decreased consumption of samples.
For users with different educational backgrounds and levels, a user-friendly wearable product should have the following characteristics: one-step operation, real-time detection, and comfortable wearing. Predictably, with the industrial technology revolution and the development of the IoT, current commercial wearable devices have risen rapidly in three categories: (I) smart wearable products, such as glasses and watches for human-machine exchange; (II) somatosensory interaction products, such as somatosensory games and eye-movement control; and (III) medical and health diagnostic products, such as wearable blood glucose meters. It can be seen from the market development in the field of wearable devices in recent years that wearables have become more intelligent, diversified, fashionable and popular.2
According to market research, Table 1 lists several wearable medical products. It is obvious that these products are mainly focused on minimally invasive or non-invasive glucose monitoring equipment, driven by the huge market of wearable medical sensors. Nevertheless, the listed commercial wearables still need to have massive verification, additional equipment for data collection, market approvals, and final marketing channels. At present, the most successful case of wearable medical equipment on the market is probably the blood glucose meter. Minimally invasive or non-invasive blood glucose meters have also been a hot spot in scientific research in recent years. As shown in Table 1, wearable medical devices, as the technical preface of mobile medical treatment, have the characteristics of fast updating, continuous technological progress, and diversity. Additionally, wearable medical devices have the following advantages: (I) dynamic monitoring provides a full range of medical diagnostic data; (II) it is beneficial for preventing sudden diseases and realizing early diagnoses and prognoses; (III) through real-time tracking to realize remote medical diagnosis, the medical staff can communicate with the patient more conveniently.
| Product and corporation | Analyte | Wearable mode | Detection method | Current status |
|---|---|---|---|---|
| Smart contact lens, Google and Novartis | Tear glucose | Contact lens | Electrochemistry | Updated in 2018 but currently suspended |
| GlucoWatch, Cygnus, Inc. | ISF glucose | Watch | Electrochemistry | FDA, but currently off the market |
| Symphony, Echo Therapeutics | ISF glucose | Watch | Electrochemistry | CE certification |
| Optical glucose meter, C8 Medisensors | Blood glucose | Waistband | Raman spectrum | CE certification |
| Gluco Track, Integrity Applications | Blood glucose | Clip | Ultrasound, electromagnetism, and heat | CE certification, FDA |
| Freestyle Libre, Abott | ISF glucose | Patch | Electrochemistry | CE certification, FDA, CFDA |
| Dexcom G6 CGM, Dexcom | ISF glucose | Patch | Electrochemistry | CE certification, FDA, CFDA |
| Eversense, Senseonics | ISF glucose | Subcutaneous implantation | Fluorescence spectrum | FDA |
| Diasensor 1000, Biocontrol Technology | Blood glucose | Clip | Near-infrared spectrum | CE certification |
| BioMKR, Prediktor Medical | Blood glucose | Waistband | Near-infrared spectrum | Clinical testing |
| GlucoWise, MediWise | Blood glucose | Clip | Radio frequency | Clinical testing |
| Glucontrol GC 300, Samsung Fine Chemicals | Blood glucose | Clip | Near-infrared spectrum | Unknown |
| Non-invasive glucose meter, NIMOS | ISF glucose | Patch | Electrochemistry | Unknown |
| Life guide system, Inverness Medical Technology | ISF glucose | Patch | Electrochemistry | Unknown |
| SugarTrac, LifeTrac System | ISF glucose | Earphone | Infrared spectrum | Unknown |
| Multianalyte Meter, Magnetic Diagnostics | ISF glucose | Patch | Magnetic resonance imaging | Development |
| NovioSense tear glucose sensor, NovioSense | Tear glucose | Implantation | Electrochemistry | Animal trial |
3. The core components of wearable devices
In this section, we will deeply discuss the core components of wearable devices during the design process. Wearable technology is an interdisciplinary subject that generally integrates materials, micromachining, fluid mechanics, biosensing technology, communication and other disciplines. Thus, we herein mainly categorized wearable core technologies into four parts: materials science, power supply, wireless signal communication, and chem-biosensing.3.1 Materials science
In this section, we will focus on materials that show flexibility, ductility, and biocompatibility in wearable skin biosensors for medical or diagnostic fields. With the rapid development of materials science, the wearable devices have the advantages of flexibility and multidimensional controllability. In essence, these materials can develop their own performance in response to changes in external conditions (such as temperature, pressure, light). For example, a piezoelectric material changes its own voltage when the external pressure changes; a thermal memory polymers changes shape at a certain temperature; and a photosensitive material responds to the intensity of light and exhibits structural property.The first consideration of flexible smart materials in wearable applications is their mechanical properties. Some professional terms, such as electronic tattoo, skin-like, epidermis, and electronic skin, refer to skin devices that have physical properties (thickness, mechanical properties, thermal mass) similar to human epidermis. Since the elastic deformation of the human epidermis can be as high as 15%, the elastic modulus of these materials ranges from 10 kPa to 200 kPa.6 An epidermal electronic device should be able to bend to fit the skin and adapt to the stress generated by the body natural movement. Fig. 2 shows the modulus of elasticity values for some materials. From the figure, we can find strategies for achieving highly flexible and stretchable devices on the skin.7a As shown in Fig. 2, there is a large mismatch between the mechanical properties of materials (silicon, gold, etc.) usually utilized for activated functionalized components and biological tissues (such as the brain, skin, and cartilage).
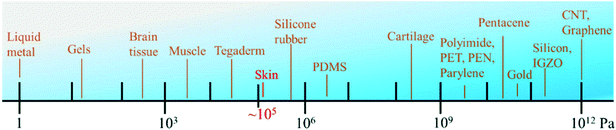 | ||
| Fig. 2 Comparison of the elastic modulus values of electronic materials, polymers and biological materials.7 | ||
One research hotspot is implantable devices. The elastic modulus of the supporting and encapsulating polymers is very close to the elastic modulus of biological tissue, which is beneficial to the wearable outer packaging and shaping. Chronic toxicity and usability should be taken into consideration for wearable medical biosensors. It was reported that carbon-based materials are deemed as an attractive application for wearables because of its low toxicity.3 Furthermore, it has been reported that some carbon-based materials are toxic to cells and tissues, which is related to its length, size, and aggregations.7b,c However, it is notable that there is no obvious evidence for the toxicity of carbon nanotubes, while it was reported that carbon nanotubes could facilitate cell growth.7c
To realize a flexible device with similar elastic modulus to the skin, a thin membrane was used to construct a highly flexible device. According to the prediction of the Euler–Bernoulli beam theory, the bending rigidity of the film, which can be defined as the ability of the material to resist bending, is positively correlated to the cubic function of the film thickness.8 According to the progress of thin-membrane processing and fabrication, electronic devices have been successfully formed by means of ultrathin polymers film, metals and semiconductor materials. For example, a single-crystal silicon nanofilm with a thickness of 100–200 nm can be transferred from an insulator silicon plate to an ultrathin polymer substrate.9 Since the bending stiffness is proportional to the cubic function of the thickness, the bending stiffness is reduced by several orders of magnitude. However, after the integration of the functional equation, the film is allowed to be bent to a smaller radius of curvature without breaking.9 Some recent work has also reported that massive organic or inorganic devices can be produced on ultrathin substrates with a large elastic modulus, so that the bending radius can be as small as tens of microns.10,11 Encapsulating the active layer of the embedding material on a zero-strain plane is another strategy to achieve mechanical stability.12 Materials with high fracture strengths, such as carbon nanotubes13 and graphene, are another effective strategy for further enhancing the mechanical strength of electronic devices.14,15
In addition, some materials show physical properties including electrical properties, optical properties, and acoustics, such as metal oxides, which may replace silicone as flexible materials in wearable devices.16–18 These materials can be deposited on a large scale with an electron mobility greater than 10 cm2 V−1 s−1 at room temperature. It is foreseeable that the current research and development of p-type oxide semiconductors may bring new opportunities for flexible optoelectronic wearable devices and digital circuits.
In addition to these semiconductor materials, dielectric materials used in wearable devices include more conventional silicon-based materials (SiO2, Si3N4) and inorganic high dielectrics (Al2O3, HfO2) materials. Ferroelectrics and piezoelectric materials are also used as coatings in the fields of flexible nonvolatile memory, pressure sensing and energy removal.19 Among the metal materials used in wearable devices, gold and platinum are widely used for flexible electrodes or solid electrodes that directly contact the skin due to their chemical inertness and low contact resistance. Copper is a preferred substrate material for antennas used for the interconnection of external communication equipment due to its low resistivity. In the field of wearable devices, there are also some composite conductive materials, such as composite conductors based on flexible polymers with dispersed conductive nanoparticles. This composite conductive material can conduct electricity and mechanical properties according to its specific application. Of course, there is also a widely reported method that may replace the conventional preparation of stretchable wires and antennas,20 in which liquid metal is embedded with a small elastic modulus through chemical bonds. This not only ensures the conformity of the wearable device but also realizes conduction and signal transmission.
As mentioned above, the epidermal deformation not only partially destroys the interface of the flexible substrate, but also detaches it from epidermis, which might give rise to an unstable recording of the electrical signal output. Wearable biosensors should be compatible with the epidermis or tissue; thus, a soft substrate is essential to narrow the mechanical gap between the device surface and biological tissues.21a To this aim, Xingyu Jiang's group utilized poly(vinyl pyrrolidone) for deposition onto metal liquid nanoparticles (eutectic gallium indium, EGaIn), which showed long-term stability in water (30 days) and ethanol (60 days).21b They also introduced stretchable and biocompatible electronics, where the metal liquid was modified with 11-mercaptoundecanoic acid and embedded into a polystyrene-block-polybutadiene-block-polystyrene substrate.21c Besides the good electrical performance (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S cm−1) and stretchability (800% of elongation), this composite metal liquid was enabled to produce on the large scale. To further achieve a balance between the device interface and epidermis, Xingyu Jiang's group proposed a flexible tattoo-based electronics device, where this multilayered skin-based wearable device integrated with 1 heater and 15 strain sensors.21d This conductive tattoo could be transferred to various soft substrates and have a close attachment without solvent. This liquid metal tattoo showed conformal property and high stretchability (800%).
000 S cm−1) and stretchability (800% of elongation), this composite metal liquid was enabled to produce on the large scale. To further achieve a balance between the device interface and epidermis, Xingyu Jiang's group proposed a flexible tattoo-based electronics device, where this multilayered skin-based wearable device integrated with 1 heater and 15 strain sensors.21d This conductive tattoo could be transferred to various soft substrates and have a close attachment without solvent. This liquid metal tattoo showed conformal property and high stretchability (800%).
Another method to address the deformation of wearable sensors lies in woven fabric substrates. Modali et al. proposed a wearable biosensor based on a woven substrate, which was highly robust for signal output under different deformation tests.21e Compared with the conventional screen-printed electrode, this electrochemical platform had inherent advantages of robustness for continuous non-invasively biosensing. In comparison to the usage of plastics and ceramics in commercial electrochemical sensors, Choudhary et al. utilized the biocompatible silk yarn as the substrate materials to fabricate the woven electrochemical biosensors for the determination of multiplexed targets.21f
3.2 Power supply
One of the milestones for wearable technology might be the sustainable power. In this section, we divided these flexible batteries into two categories, namely biofuel and non-biofuel cells. With the increasing application demands of miniaturized wearable platforms in sensing, data processing, signal communication and other tasks, the development of an efficient and sustainable power supply has become an urgent demand in this field. Wearable devices that can provide power are called active devices. For active wearable devices, the components that provide power should have the following characteristics: flexibility, stretchability, and high conformability and mechanical strain.Researchers have tried to expand the alternative methods for conventional solid-state batteries. For years, they have been exploring the use of advanced materials or nanomaterials to fabricate flexible batteries for wearable platforms. They have tested the applicability of different materials under different strain conditions. Researchers mainly solve this wearable energy problem through two complementary research fronts: non-biofuel cells and biofuel cells. Namely, biofuel cells refer to those utilizing selective enzyme to generate an electron between the anode and cathode; non-biofuel cells refer to conventional methods without a natural enzyme, such as solar cells and button-typed batteries. These two directions aim to develop electronic products with the advantages of flexibility, low-energy consumption, ergonomics, and high-density energy.
Compared with nickel–cadmium batteries, lithium batteries occupy most of the market. To achieve cooperation with miniaturized equipment, manufacturers often mold lithium batteries into a coin shape or a button shape. A lithium battery of this shape has the advantages of a high penetration rate, wide application range, low cost, and low environmental hazard.22a However, it also has some problems, such as low power storage, easy aging, and explosion.22a Although lithium batteries were once the most commonly used energy supply for wearable devices, researchers have gradually turned to new methods of obtaining energy in the environment. In a way, it is urgent that the power supply for wearables should not require frequent changes.
Therefore, here comes the energy-harvesting battery. The so-called energy harvesting refers to the process of collecting energy from the external environment, and converting the obtained energy into electrical energy through chemical reactions or physical changes. External environmental energy includes kinetic energy, solar energy, thermal energy, and piezoelectric energy.22b As the functional module of wearable devices, energy-harvesting batteries have the advantage of being self-powered without external power. This mode of energy supply with minimal loss improves energy efficiency. For example, collected solar energy directly enters the clothing worn, and the hand warmer directly contacts the human skin. However, this type of battery has some drawbacks, such as in the energy module of the wearable device. The energy conversion rate is not high enough, which limits the application of wearable devices in indoor scenes.
Graphene batteries have recently been discussed and developed by researchers in recent years, due to their high energy density and ability to store electricity, and are also expected to lead the next wave of battery technology revolution.23 Graphene is a planar two-dimensional nanomaterial composed of a single layer of carbon atoms. Graphene can be used as the negative electrode material of lithium batteries in a setup called graphene batteries, which uses the rapid shuttling process of lithium ions between the graphene surface and the electrode for energy conversion. Graphene batteries have a highly power storage capacity, but most are still in the development stage. In addition, thin-film batteries are made in a two-dimensional way. Their thinness provides certain flexibility and applicability in the wearable market. Although some thin-film batteries meet the standards for flexibility and thickness of wearable devices, they are still made of lithium-based chemicals, which are potentially dangerous and toxic to the environment.
Another type of battery used for energy supply to wearable devices should be a biofuel cell. As manifested, biofuel cells depend on biological components for their energy supply. These biological components are immobilized on the surface, such as glucose oxidase or lactase that can hydrolyze lactose into galactose and glucose, and produce the required energy by consuming spontaneous and continuous biofuels. At the same time, biofuel cells can be divided into microbial fuel cells and enzyme fuel cells.
The concept of microbial fuel cells was first proposed in the 1970s.24 Recently, microbial fuel cell research has been conducted in many disciplines of materials science, microbiology and biochemistry. Under common progress and mutual promotion, the actual model of microbial fuel cells can be truly applied.25 Microbial fuel cells oxidize a variety of fuels to give them long-term stability, such as the textile-based microbial fuel cell developed by Xie et al. as a strategy for wastewater treatment.26 However, microbial fuel cells also have some inherent limitations and shortcomings. For example, the active sites of enzymes are embedded in the cell membrane and cell wall of microorganisms, which hinders contact and reactions between fuel and enzymes and greatly reduce the active sites of enzymes, as well as the efficiency of the electron shuttle between the electrode and the electrode. One of the compound effects of this microbial fuel cell is a lower power density. Therefore, if microbial fuel cells are used as the power sources for wearable devices, cytotoxicity becomes another problem that urgently needs to be solved. These research results suggest that there may be an alternative biofuel cell energy supply that may be used as a battery for wearable devices.
Enzymatic biofuel cells have brought a new light to wearables. Researchers have been inspired by enzyme-modified electrodes. The enzyme-catalyzed model may be able to solve the low power density and biocompatibility issues. The surface of the enzyme-modified electrode has a sensitive membrane with a molecular structure that has a catalytic effect. This biosensitive membrane can promote rapid movement of electrons between the active site of the enzyme and the electrode surface. Based on this, researchers have developed a series of enzyme biofuel cells with high power densities.27 At the same time, based on the biocompatibility of the sensitive membrane construction method, some research has reported several implantable enzymatic devices with low cytotoxicity.28 Therefore, enzyme-based biofuel cells are expected to provide energy power generation for various wearables, and a large number of reported studies have promoted the development and practical application of enzyme-based biofuel cells.29a
An enzyme biofuel cell should include an enzyme-functionalized bioanode and biocathode. For the bioanode, the oxidation reaction occurs to generate electrons, relying on an immobilized enzyme such as glucose and lactate, which shows high selectivity to the substrate. In a biological cathode, the reaction typically involves the reduction of O2 to H2O in the presence of four electrons.
Hybrid enzyme biofuel cells are based on enzyme-functionalized biological anodes and metal-modified cathodes. The oxidation reaction that occurs on the surface of the biological anode generates electrons. This process is catalyzed by specific and selective biological enzymes on the substrate, such as glucose, lactic acid, pyruvate, ethanol, and cellulose.29b On the cathode, four electrons are obtained to reduce oxygen to H2O. The cathode reaction can be achieved by conventional noble metal catalysts based on reducing oxygen (such as Pt, Pd, Ru), laccase or bilirubin oxidase. Although noble metal-based cathodes can provide a suitable current density, they are susceptible to poisoning and low open-circuit potentials.29c On the other hand, enzyme-based biocathodes offer a higher open-circuit potential, and the high selectivity of enzymes makes the biocathode relatively less affected by the byproducts of the anode reaction. Some research groups in this field have obtained reaction substrates from fruits, beverages or biological tissues, which are used in the catalyzed reaction of corresponding biological enzymes and as enzyme-fueled cells to generate electricity.30–32 The rate of conversion of chemical energy into electrical energy by enzyme biofuel cells is slow. One of the main reasons behind this characteristic is that the rate of the oxygen reduction reaction is slow. Laccase and bilirubin oxidase have biological enzyme activity under weak acidic conditions, but their activity is inhibited when chloride ions and uric acid ions are present in the system. To solve these problems, Elouarzaki et al. constructed a double-enzyme effect biocathode, that is, a composite system of glucose oxidase, which completely reduced oxygen to H2O.33 In this system, by improving the biocompatible enzyme immobilization strategy, the catalytic performance of the biocathode was significantly improved. Thereby, the loss of enzymes on the electrode surface was reduced, and it ensured the diffusion of glucose and O2 and the local production of H2O2. Since two kinds of enzymes catalyzed the reaction simultaneously and independently, the biocathode had a higher open-circuit potential (0.60 ± 0.01 V) under simulated physiological conditions. Bandodkar et al. fabricated an electronic-skin-based self-power wearable device consisting of a series of anodes and cathodes. In these inkjet-printed electrode arrays, anode compartments were modified with lactate oxidase on a carbon nanotube-naphthoquinone substrate, and cathode compartments were modified with silver oxide on carbon nanotube substrate.34a This stretchable wearable biofuel cell had an open circuit voltage of 0.5 V with a power density of 1.2 mW cm−2 at 0.2 V. More recently, Joseph Wang's team fabricated a wearable biofuel cell based on lactate oxidase (anode compartment) and bilirubin oxidase (cathode compartment) using buckypaper consisting of carbon nanotubes.34b It showed a higher open circuit voltage of 0.74 V. It is believed that in the future, based on human sweat and tears, research on related biofuel cells will make a great contribution to the energy supply for wearable devices.
3.3 Wireless signal communication
Wireless communication is one of the key components to realize wearable devices through wearable antennas, which have the characteristics of lightness, flexibility, low cost and high conformability. With the advent of the fifth-generation (5G) network era, IoT will fully deploy 5G networks and wirelessly interconnect all network-related things, from home devices to daily consumer devices. The 5G network is a promising technology, which can not only meet the requirements of mobile terminals for exponentially increasing data rates, but can also be integrated with various services.35 According to Ericsson's predictions, it is estimated that by the end of 2021, approximately 28 billion smart devices will be connected worldwide using traditional technologies and new wireless RF formats.36The technology used for wearable device communication can be called wireless body local area networks. In recent years, certain advances have been achieved in this field, showing great potential in many medical and healthcare applications.37 This is also one of the reasons why various wearable sensors have become popular in recent years, including electrocardiograms and electroencephalograms. The workflow of wireless communication technology on wearable devices is as follows. First, the electrical signal from the sensor is amplified by the front-end amplifier and then converted into a digital signal by an analog-to-digital converter, and the digital signal is processed by the micro-control unit and sent by radio to the base station. Finally, the base station can collect various signals.37
According to the distance between the sensor and the signal receiver,38 wireless human body local area networks can be divided into four types: body area networks (BANs, distance < 2 m), personal area networks (PANs, 2 m < distance < 10 m), local area networks (LANs, 10 m < distance < 100 m), and wide area networks (WANs, distance > 100 m). A personal spatial biofeedback system is implemented in the BAN, in which the sensors and actuators attached to the epidermis use wireless communication. The function of the BAN roughly corresponds to the user of the biofeedback system, and the BAN can process the unit to collect data for postprocessing or real-time processing of concurrent feedback. The implementation of a closed-space biofeedback system with a guidance function can be easily realized in LAN or PAN. The sensors and actuators are wirelessly connected to the laptop via a wireless local area network (WLAN). The cloud application can realize an open-space personal biofeedback system. Due to the delay in communication with the cloud, it becomes difficult to transmit signals in real time.
However, radio frequency (RF) is the main bottleneck affecting the miniaturization of communication modules for wearable devices. Studies have shown that radio consumes most of the power in wireless wearable devices, which limits the size of wearable devices due to battery size or capacity.39,40 For example, the sweat monitoring wristband system reported by researchers uses a Bluetooth transceiver for data communication, but this Bluetooth communication method affects the power consumption of the system.41 Although the development and research of flexible batteries have received certain attention and reporting, their energy density and reliability are not as good as those of traditional lithium batteries.42–44 In addition, it is difficult to fully integrate RF on complementary metal oxide semiconductor (CMOS). This is because some components are passive, such as antennas, capacitors, and inductors. At the same time, there are certain difficulties in communication for those who are often in relatively low-frequency environments. In 2012, the IEEE802.15.6 standard defined wearable transceivers as being able to work under three protocols, namely, ultrawideband (UWB), narrowband (NB) and bulk channel communication (BCC).38 This benefited from the research over the past decade. The research and application of UWB and NB transceivers are relatively mature. In contrast, BCC is an emerging technology for wearable electronic products with huge potential.
3.4 Chem-biosensing technology
Chemical biosensor technology is one of the critical components of wearable devices.2 It measures external signals through circuits, and then converts it into recordable data in the required form according to a certain change rule. There are many types of sensors that can be roughly divided into different application scenarios: motion sensing (such as pressure sensors and acceleration sensors), chemical and biological sensing (such as pH sensors, gas sensors, blood glucose meters, and ECG sensors), and environmental sensing (humidity sensors and UV sensors). However, in the academic field of wearable medical devices, the most widely used type and what we mainly discuss here are chemical and biological sensing technologies, namely chem-biosensing.According to the International Union of Pure and Applied Chemistry (IUPAC), a chemical sensor is defined as a device that converts chemical information from the concentration of specific sample components relative to the analysis range of the total components into useful signals for analysis.45 The biosensor, as a branch of chemical sensors, usually consists of a recognition element (also called a receptor) and an actuator (also called transducer). The function of the identification element is to provide highly selective identification and induction of the target analyte in the presence of potentially interfering chemical substances, and the actuator is the key component that converts the related information into measurable analysis signals.46 In addition to the core technologies mentioned above, chem-biosensing technology is another major challenge for wearable medical devices. At the level of chem-biosensing technology, there are many factors that need to be considered, such as sensor calibration, sample processing, stability, sensitivity, detection limits, and multiple analysis. These issues will be discussed in depth in this section.
The first thing that wearable sensing technology has to face is the preprocessing and calibration of the device. Conventional sensors need to be calibrated once they are used and incubated with sample solutions, but such procedures are obviously not compatible with wearable devices. Secondly, many sensors have drift problems, which can cause large deviations in the calculation of analyte concentration.47 Therefore, such biosensors need to be pre-calibrated regularly. The developed calibration-free wearable sensors might tackle this issue.48 For wearable devices with strong operability, it is also challenging to pretreat the sample solution before testing. This is obviously beyond the reach of some sensors that can only detect analytes under a harsh environment. For instance, some gas sensors can only detect toxic gases at temperatures much higher than the ambient temperature.49,50 Therefore, in the case of portable and one-click wearable devices, the current chem-biosensing technology can hardly be used to directly detect analytes.
Chemical and biological sensors, especially wearable sensors, generally are faced with a major problem, namely, stability.2 Since chemical biosensors rely on biological recognition elements, the stability of these bioreceptors is the main basis for investigating the long-term storage and usage of these devices. Biological recognition elements (such as enzymes, DNA, antibodies and aptamers) are very sensitive to their chemical environment, such as the temperature, pH, ionic strength, humidity or pressure.51 Deviation from the optimal conditions will result in the denaturation of these biological receptors, thereby reducing their sensing capabilities. Unlike in a controllable analytical chemical laboratory, the stability of biometrics is particularly important for wearable devices that are often exposed to the harsh environment. Wearables should have the ability to adapt to constant changes in temperature, pH, ionic strength, humidity and pressure, caused by long-term indoor or outdoor activities. It is challenging to maintain a rapid and accurate response for a long time in such an uncertain circumstance. Therefore, the stability of wearable sensors should be considered in the development process.
In terms of the trace detection of low-abundance targets, wearable devices also need to have a certain sensitivity and low detection limits. For example, the glucose concentration in tears and skin ISF is in the micromolar range, which is 2 orders of magnitude lower than the glucose concentration in blood.2 Hence, the sensitivity of wearable sensor devices is critical. Trace detection is also particularly important for environmental and national defense applications. Some analytes require highly precision, selectivity and rapid detection at low concentrations, such as toxic pollutants, chemical weapons, explosives, and illegal drugs.52–55 At present, the detection of these samples is mainly concentrated on benchtop analyzers, but wearable versions of the same type can be developed. Continuous monitoring of analytes is an attractive aspect for wearable technology. Therefore, wearable sensors should be able to quickly detect analytes at dynamic concentration changes and have a quick response. This requires that the wearable sensor should have a reversible response and no residual byproducts, and provide accurate data under the premise of negligible hysteresis.
Bio-affinity capability is also an important parameter that needs to be investigated. Wearable devices on the market require the determination of bio-affinity. The so-called bio-affinity sensing capability refers to a sensing mechanism constructed by the active substrate on the interface, which provides affinity for bonding and recognizing the analyte through the specific affinity between biomolecules.56 The bio-affinity technology can fundamentally change the development of wearable forms, which has certain development potential in the field of the IoT for critical healthcare, safety and environmental monitoring. However, the realization of wearable biosensors based on bio-affinity is challenging. The reasons are as follows: first, when bio-affinity receptors are in the environment of wearable applications for a long time, they become very unstable and degenerate quickly, as is the case with antibodies, DNA, RNA, and aptamers; second, these bioreceptors rely on molecular-switch mechanisms to detect analytes, and even small changes in their 3D structure will greatly affect their ability to recognize analytes, which leads to a decrease in the sensing performance of the device.
It is indeed challenging for a wearable sensor to meet the two conditions of bio-affinity and continuous monitoring at the same time because the bioreceptor is firmly bonded to the analyte through chemical bonds, which is difficult to regenerate. One of the required conditions for wearable sensors to achieve continuous monitoring is rapid detection. For biological enzyme sensors, due to the catalytic kinetics, it is easy to meet this requirement. However, for sensors based on bio-affinity, the incubation time is usually longer (generally greater than 15 minutes), which is very challenging for the construction of continuous and fast wearable sensors based on bio-affinity.56 In addition, to obtain accurate and precise information, the sensor interface should be thoroughly cleaned after the sample incubation process in order to eliminate nonspecific binding of interfered substances. However, this kind of manual intervention is daunting for wearable sensor applications.
When the concentration of target analytes is as low as nM or aM, the wearable sensor is supposed to show a low detection limit. However, the low detection limit is inherently related to the aforementioned challenges. To achieve a lower detection limit, the binding affinity of the bioreceptor on the sensor surface to the target should be stable, which means that the regeneration of the biosensor surface would be very arduous. At the same time, low-detection limit sensing requires a thorough cleaning of the sensor surface to reduce nonspecific adsorption, which might be addressed by surface hydrophobic treatment or immuno-affinity mediated capture method for one-off or disposable wearable applications. At present, researchers mainly rely on the sandwich-type method to achieve low detection limits for biosensors.57,58 However, this method involves multiple procedures and cannot be easily implemented on a wearable platform.
Multiparameter monitoring is a current trend for chem-biosensing, which refers to the simultaneous monitoring of multiple parameters, such as chemical parameters and physiological indicators, to obtain comprehensive information about the wearer or the surroundings. For example, multiple physiological indicators of disease can allow determination of the health condition of the wearer. Similarly, for environmental and food safety applications, a series of chemical substances should be tested to comprehensively evaluate samples. This multifactor detection mode requires that the wearable device platform contains an array of single miniature chemical sensors, and the crosstalk between these multianalyte sensor arrays is negligible.59 By using a specific receiver, the spacing of the sensor array was controlled and the working point of each sensor interface was removed. Thus, the independence of each sensor in the multifactor measurement in this wearable sensor array can be guaranteed.60
4. Application and sensing mechanism of wearable devices
4.1 Epidermis-based wearable chem-biosensing devices
As an important organ of the human body, the epidermis covers most of the body. Therefore, among various types of wearable chem-biosensors, skin-wearable devices have garnered great attention. The epidermal biosensor can realize the real-time analysis of biomarkers in epidermal biological fluids, such as sweat and ISF, and has the ability to continuously monitor various physiological indicators. These devices rely on the sampling of sweat or ISF on the surface of the skin, and then further transmit and separate these biological fluids on the wearable biosensor. Such wearable skin devices usually rely on different transduction modes, such as optical, electrochemical and mechanical properties, to bind to biocatalytic and ion recognition receptors. A fully integrated wearable platform should be further combined with data processing and signal transmission. However, most state-of-the-art research studies have focused on electrochemistry and colorimetry.2 Significant advances have been made on various skin-wearing platforms, which can easily provide comfortable sampling of epidermal biological fluids to the wearer. The current reported skin-wearable devices (including watch-like, bandage, biopatch, and bracelet-like) can successfully achieve the following two functions: (I) directly transferring the sensor to the skin, such as electronic skin or a temporary tattoo, and (II) incorporating the sensor into a wristband or patch, where the sensor is directly embedded into the textile to ensure close contact with the skin, so that devices with mechanical stress can ensure chem-biosensing during body movement.For wearable sensors, sweat is a preferable biological fluid because sweat glands are distributed throughout the body, and human skin has a high density of sweat glands (100 glands per cm2).3 The physiological features provide a feasible sampling point and high specific surface area for wearable skin sensors. However, sweat is secreted to the outer surface of the skin for collection and detection, which occurs during active perspiration.3 The process of perspiration can also be stimulated by the outside, which is passive perspiration, including by heat conduction, body movement, pressure, electric field, etc. The sweat secreted by the human body contains small organic molecules (glucose, lactic acid, urea, ethanol, and cortisol), electrolytes (sodium, potassium, chloride, and ammonium), trace elements (zinc and copper) and biological macromolecules (nucleic acid, protein, microRNA, and inflammatory factors).61
Currently, in most reported studies, skin wearability is based on an epidermal biosensor system for measuring the concentrations of analytes in ISF or sweat. Among viable skin tissue cells, ISF is the main microenvironment component that directly offers molecules diffusing from the capillary endothelium. Depending on the microenvironment in ISF and its components, there is a reliable correlation between the concentration of analytes in ISF and the blood level. These analytes include inorganic electrolytes (sodium ions, phosphates, magnesium ions, potassium ions, and calcium ions), physiological metabolites (glucose, alcohol, lactic acid and cortisol) and proteins.62 Epidermis-wearable chem-biosensing equipment can be divided into two categories: the exercise type, which relies on the active perspiration amount and rate; and the inductive type, which can promote sweat or ISF by applying certain conditions to the skin through an external device that causes passive perspiration.
A new era of multitarget wearable biosensor platforms for sweat quantitative analysis has made significant progress on the basis of a fully integrated biological patch wearable sensor array. Huisheng Peng's group proposed a wearable multianalyte electrochemical sensing platform with flexible braids,68 which maintained real-time sensing performance under repeated motion and deformation (Fig. 3a). The platform forms a coaxial structure by coating the active material onto the carbon nanotube fibers and forms an electrode array on the braid. Each array uses the flexible Ag/AgCl as the reference electrode and the corresponding selective electrode as the working electrode, which simultaneously monitors glucose, Na+, K+, Ca2+ and pH values. In situ multiplexed target monitoring is important for the diagnosis and physiological monitoring of functions for wearable devices. The flexible wearable device can provide a sweat flow rate to calibrate the target analyte signal and improve the physiological correlation. The electrochemical wearable system is beneficial for monitoring health parameters during exercise, but relies on physical exertion to generate sweat and passively deliver target substances. Thus, it was limited in the storage of sweat sample for continuous monitoring. Recently, Huisheng Peng's group investigated a wearable textile display driven by an electric field based on ZnS phosphor.69 As shown in Fig. 3b, the flexible weaved textile display consisted of 5 × 105 electroluminescent units activated by an alternating electric field, which would be a promising tool for subjects with communication impairment.
 | ||
| Fig. 3 Exercise-based epidermis-wearable biosensor. (a) Fiber-based flexible wearable patch with working, reference, and counter electrodes woven into fibres.68 (b) Large-scaled electroluminescence wearable electronics based on woven-fiber materials.69 (c) All laser-engraved technology for constructing a wearable sensor for the determination of sweat uric acid and tyrosine, microfluidic channel of the device for sweat storage via laser engraving.70 (d) Body-worn wearable microfluidic system powered by a sweat-activated battery, Mg sheet as the anode, Ag/AgCl as the cathode, and a cellulose membrane as the separator.71 (e) A time-dependence epidermal wearable sensor based on a microfluidic valve system for the biofluid storage and biomarkers analysis.72 (f) Smart bandage-like wearable for real-time monitoring wound state and on-demand treatment,74 (i)–(iii) referring to the real-time monitoring of the wound temperature, lighting up a mini-LED to release antibiotics, and the wound treated by the released antibiotics, respectively. | ||
However, epidermis-wearable biosensors for the accurate measurement of physiologically relevant sweat concentrations face the following major challenges: (I) uncontrolled operating conditions, such as temperature and pH changes; (II) sample contamination of the skin surface due to different outer factors; (III) variable sampling rate; (IV) low sampling volumes. Although many reports have shown that the concentration of some analytes in sweat may be related to blood levels, the abovementioned limitations can also seriously impair the accuracy of the collected data. To solve these limitations, Wei Gao's group fabricated an all-laser-engraved epidermis wearable biosensor, enabling sweat sampling, low-abundance molecular biosensing and multiple signal communication.70 As shown in Fig. 3c, in regard to the biosensing function, the functionalized graphene is used to prepare stretchable array electrodes to enhance the electrochemical biosensing of uric acid/tyrosine detection during physical exercise. Multiple sensing can solve the immobilized enzyme activity changes caused by pH, temperature and humidity fluctuations so that measurement results can be continuously corrected and the operational accuracy can be improved.
However, sample contamination derived from the skin surface, as well as the external environment, is still a problem to be considered. In Fig. 3d, John A. Rogers' group proposed a sweat-powered wearable microfluidic device, where a microfluidic channel not only was used for storing sweat sample, but also self-discharged for battery.71 The self-powered wearable device, containing biosensing, wireless communication and power modules, was capable of continuously recording the change of the heart rate, chloride and pH. This wearable platform also consisted of a biocompatible sweat-active biofuel that conducted continuous epidermal physiological data recording in human trials.
One of the significant considerations for sweat sampling is the volume. Traditionally, adapted from a lateral flow strip, some colorimetric epidermis wearable devices utilized an absorption pad for driving the sweat sample, where the capillary force on the pad platform induced a sample for analyte detection. Since no power supply is needed, the colorimetrical wearable platform becomes convenient, small and easy to wear and use, but detection and data analysis require other readout devices, such as mobile phones with color analysis software functions. However, this sampling mode has some drawbacks: (I) unable to accommodate the variable sweat flow rate; (II) lack of diverse sweat management, such as flow direction, time-course compartmentalization, incubation; (III) sample contamination due to the large-area skin contact. Thus, Lin et al. fabricated an active wearable device based on a microfluidic valve system, which consisted of a network of the thermo-responsive poly(N-isopropylacrylamide) hydrogel (Fig. 3e).72 This hydrogel-valve-gated wearable system, comprising glucose and lactate sensors, could allow for sweat chrono-sampling-to-sensing to occur in a continuously manner.
Owing to the development of bioelectronics, another widespread application of active epidermis wearable devices is wound bandage. Bacterial infections might result in chronic wound healing, such as Pseudomonas aeruginosa.73a In order to identify bacteria with different subtypes, Edgar D. Goluch's group proposed a quorum sensing method for chronic diabetic wounds, which might offer an innovative research area for chronic wound dressings devices.73b The wearable bandage can monitor several wound parameters, including pH, uric acid, tyrosinase, and temperature, which can offer a feedback of the wound healing statue.73c Especially for those with chronic wounds, this can impair the healing process and carries a risk of amputation and death. To overcome this Pandora box of the wound statue, Pang et al. reported an integrated wearable bandage, not only enabling real-time monitoring wound healing process in the early stage, but also on-demand treatment by UV-controlled drug management and delivery.74Fig. 3f shows the smart bandage with a double-layer structure, of which the polydimethylsiloxane-based upper layer integrated a temperature sensor and UV light control, and a UV-responsive antibacterial hydrogel delivering gentamicin was embedded into the lower layer. According to real-time wound temperature sensing, the smart bandage could offer on-demand infection therapy via releasing gentamicin from the UV-controlled hydrogel. This integrated bandage is promising for timely wound treatments and chronic wound managements.
In vitro diagnostics (IVD) has certain advantages in the detection of early infections during global public health emergencies, such as SARS-CoV-2. However, an instrument-free IVD device in the clinical practice can not only enable rapid and highly sensitive detection, but also allow isolators to detect themselves. Thus, a bandage-like wearable sensor was constructed for fast and visual detection of nucleic acids. This wearable sensor based on recombinase polymerization reaction (RPA) was triggered by body heat (30–37 °C) for visual nucleic acid detection within 10 min.75 Due to the wide range of RPA reaction temperatures, this bandage sensor proved to be feasible by human trials under various circumstances. The smart bandage-like wearable device is expected to be used as a broad-spectrum detection method for pandemic diseases in resource-limited areas.
Early in 2001, the Cygnus corporation developed a sensing platform based on reverse iontophoresis called the GlucoWatch Biographer.76 Approved by the U.S. Food and Drug Administration (FDA), this device can non-invasively monitor glucose within 12 hours. However, GlucoWatch was withdrawn from the market in the early 2000s for the following reasons: (I) reverse iontophoresis might damage the skin during long-term operation, and (II) an invasive blood glucose meter was needed for GlucoWatch calibration. Thus, the commercialization of wearable biosensors requires a careful evaluation of accuracy and versatility to become popularized.
In regard to iontophoresis wearable patch, Joseph Wang's group has been engaging in comprehensive research studies in this filed. For the first time, they used iontophoresis electrodes and glucose biosensing electrodes, and developed a wearable platform based on reverse iontophoresis. This tattoo-like flexible device has a high degree of conformity with human skin.77 The integrated device solved the limitations of the GlucoWatch: (I) it reduced the interference in glucose detection by reducing the current applied from the outside and simultaneously minimized discomfort during the reverse iontophoresis process; (II) the flexible wearable platform utilized silk-screen printing tattoo technology, which was easy to fabricate and had a low cost; (III) due to the high degree of conformity, the device was highly fitted to human skin. The properties of the tattoo wearable sensor were evaluated by comparing the data obtained before and after a meal, and then simultaneously using commercial blood glucose test paper to compare the results. This study reported for the first time that a tattoo-based disposable wearable glucose biosensing platform could use reverse iontophoresis for ISF sampling, but it still lacked electronic integration functions and long-term operation verification for continuous monitoring applications.
Joseph Wang and colleagues further modified the reverse iontophoresis wearable device. They integrated the sweat extraction module and the sensor module into an epidermal wearable patch device. Pilocarpine was used in the sweat extraction module to induce sweat glands to secrete sweat, and then the sensor module detected sodium and chloride ions in cystic fibrosis, as well as the concentration of glucose in healthy individuals.78 The study evaluated the performance of the device by comparing the electrolyte data of healthy volunteers and cystic fibrosis testers. In addition, the ability of the sensor to detect glucose was evaluated by monitoring the glucose consumption of healthy volunteers. This method can non-invasively monitor target biomarkers in a fully integrated wearable platform with a sweat generating module. The sweat production can last for 60 minutes. Then, the wearer's sweat secretion rate may affect the real-time monitoring of the flexible device.
Although significant progress has been made in epidermal wearable flexible devices, most reports are limited to the analysis of a single target biological fluid. Joseph Wang's group further optimized the reverse-iontophoresis flexible device using a combined iontophoresis method to simultaneously sample and analyze two different epidermal biological fluids using the same wearable platform.79 As shown in Fig. 4a, pilocarpine was transported by iontophoresis to stimulate sweat glands to secrete sweat, while reverse iontophoresis was used to extract ISF so that the biomarkers in the two biological fluids could be analyzed simultaneously. The system used separate parts on a wearable tattoo platform to control and sample two biological fluids simultaneously on demand. The biosensing performance was confirmed by measuring sweat alcohol and ISF glucose levels in volunteers who consumed food and alcoholic beverages. The research report said that before the actual application of the epidermis wearable biosensing system, a detailed study of the correlations of the target substances in the blood level should be required.
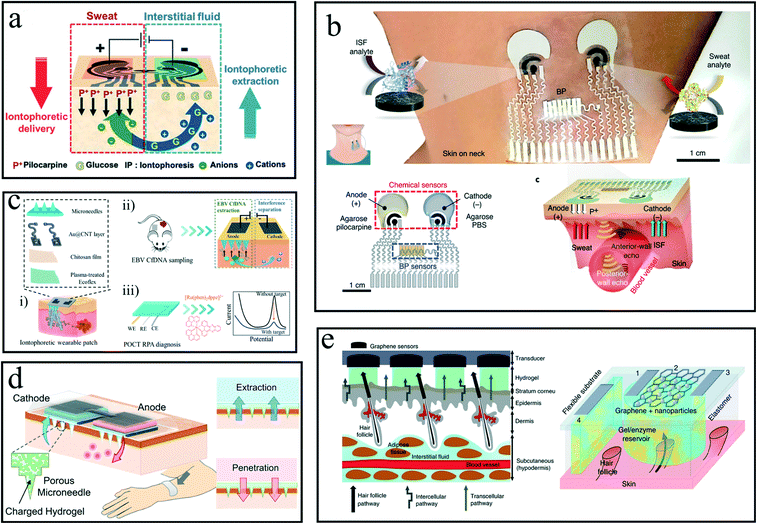 | ||
| Fig. 4 Induced epidermis wearable chem-biosensor. (a) The dual-function reverse iontophoresis wearable platform for the detection of ISF glucose and sweat alcohol, pilocarpine loaded on the anode to stimulate sweat secretion, and ISF glucose transferred to the cathode by a electroosmosis.79 (b) Multi-module epidermis iontophoresis wearable for physiological metrics and multiple biomarkers detection in a parallel manner.81 (c) An engineered wearable patch in combination of reverse iontophoresis and microneedles patch,86 (i)–(iii) referring to the construction of the wearable system, dual-extraction by the iontophoretic wearable patch on mouse, an off-line flexible biosensor for the detection of the extracted DNA, respectively. (d) A porous conductive MNs patch based on transdermal electroosmosis, the self-powered system consisting of a biofuel cell to offer energy for iontophoresis.87 (e) Wearable sensing of ISF glucose based on a graphene pixel array that specifically matched the epidermis pore in order to avoid sample contamination.89 | ||
As mentioned, in these wearable, multiple chemical sensor arrays were integrated into a single flexible patch. However, it could not meet the requirements for individual health daily management. Up-to-date, there is a growing tendency for multi-module wearable sensors based on the integration of physical and chemical sensors into a single patch for the monitoring of body heat, heart rate, glucose, and lactate.80 To this end, recently in 2021, Joseph Wang's group proposed a multimodule chem-biosensing wearable platform, which could real-time monitor the physical index (heart rate, blood pressure) and biomarkers (sweat lactate and alcohol; ISF glucose and caffeine).81 As shown in Fig. 4b, the physical index was monitored by ultrasonic transducers via stamp transfer technology, and biomarker levels were detected by chemical sensors arrays via screen-printing functional conductive materials. A reverse iontophoresis compartment was also used in this wearable platform for facilitating sweat secretion and separating the analyte of interest. Thus, these targeting biomarkers could be enriched around the corresponding anode or cathode, and then detected by chemical sensors arrays. This rational active epidermis-wearable design overcame the challenging milestones in the integration of different sensing modules, where the level of the physiological index and biofluid biomarkers could be real-time monitored in parallel without signal crosstalk.
However, this kind of epidermis wearable patch did not avail in capturing macro-biomolecules, such as proteins or nucleic acids existing in ISF or the dermis, because these methods could not transcutaneously transport these biomarkers of large molecular weights (>10 kDa).82 Notably, microneedles (MNs) can painlessly penetrate the epidermal or upper dermis layer, which have been reported to extract samples from ISF, including cells, protein, nucleic acid.83–86 Our group reported an epidermal wearable device in combination with reverse iontophoresis and MNs for the capture and sensing of cell-free DNA.86Fig. 4c shows that it consisted of three components, including a flexible modified Ecoflex film, spray-printed carbon nanotube layer, and hydrogel MNs. This wearable system can extract cell-free DNAs from ISF, attributed to a dual-extraction effect of reverse iontophoresis and MNs.
Another promising application of MNs is small molecule extractions and drug delivery. Kusama et al. recently proposed a self-powered wearable device based on conductive MNs and a biofuel cell.87 As shown in Fig. 4d, under reverse iontophoresis, ISF glucose was enriched around the conductive MNs patch. Meanwhile, a drug was released from MNs. This porous conductive MNs had high electroosmotic flow due to its high specific area. This MNs could improve the efficiency of ISF glucose sampling to achieve accurate sensing. Obviously, reverse iontophoresis has been applied widely for ISF enrichment to fabricate integrated wearable devices.88 However, due to individual differences, the extraction efficiency of reverse iontophoresis on the target is uncontrollable, and the collected ISF volume is inconsistent, which might ultimately affect the accuracy of measurement.
To address the challenge of consistency in analyte extraction by reverse ion transfer, researchers at the University of Bath developed a glucose monitoring patch based on path selection and graphene pixels.89 As shown in Fig. 4e, the monitoring platform uses a series of nanosized graphene to form an array of pixel points. The size of a single array is roughly the same as the size required to sample ISF from a single hair follicle, because ISF preferentially comes from follicles, with low resistance and high extraction reproducibility. Graphene pixel arrays perform multiple measurements on a single platform to improve accuracy. This extraction method may play a crucial role in the implementation of epidermal wearable biosensors. The graphene flexible device has successfully performed non-invasive blood glucose monitoring for more than 6 hours, while clinical trials and medical conversion will be carried out next.
Epidermis-wearable sensing devices have made increasing progress in sensing arrays, signal crosstalk, sweat and ISF collection, detection accuracy, and artificial self-healing materials, which can recover after chemical or physical damage. Among four types of wearable chem-biosensors, there is great commercialization potential for skin-wearing wearables, as some typical epidermis wearable prototypes have been emerging in the market (e.g., Abbott Freestyle Libre, Dexcom G5 continuous glucose monitoring). Unmistakably, the aforementioned research studies have confirmed that the sweat or ISF biomarkers have a close relationship with the blood level, which could offer feasibility for non-invasive or minimally invasive epidermis wearable devices. However, the future focus of epidermis-based wearable device research should be directed more toward clinical diagnostic capabilities, long-term use, detection of multiple targets in blood, and effective sampling before commercialization. Therefore, it is also necessary to explore the correlation of biomarkers between biological fluid and blood at first, and further improve the extraction efficiency. The multi-analysis sensing platform can also improve the reliability of monitoring. In addition, non-invasive or minimally invasive monitoring of various macromolecule biomarkers by epidermal wearable devices is anticipated.
4.2 Mouth-based wearable chem-biosensing devices
In recent years, the application of saliva as a biological fluid diagnostic in vitro has attracted widespread attention. Many biomarkers in saliva are directly transported from the blood through transcellular or paracellular pathways, reflecting the physiological state of saliva.90 The saliva containing highly protein components is an attractive candidate for detecting disease biomarkers for biomedical and health monitoring2 because saliva can be easily collected, and in vitro diagnosis can be performed through saliva test strips or portable device platforms. Mouth cavity-based wearability in a non-invasive manner may be an alternative for blood analysis.Saliva is mainly secreted by the parotid gland and composed of many complex components, such as microorganisms, inorganic ions, hormones, and proteins. Some saliva biomarkers have been used clinically, such as drugs, hormones, and antibodies, which provide meaningful information for disease diagnosis.91 However, there have been few efforts to develop oral wearable biosensors. This may be due to the high saliva protein content and low target biomarker concentrations. Mouth wearable devices have been reported to detect biomarkers in saliva non-invasively and dynamically, and such wearable platforms mainly rely on biosensors installed in the mouth cavity, such as mouth guards.
The first mouth wearable sensor was developed in 1960. The device replaces part of the user teeth with a sensor.2 The targets to be monitored include dental plaque and masticatory muscles. The mouth cavity sensor was integrated with a resonant coil, and could be used without a battery to detect salivary bacteria at the single-cell level in vitro. The main function of the mouth wearable biosensing conceptual model was to remotely monitor the development of bacterial membranes on the teeth, and it could be extended to the monitoring of other saliva biomarkers. Based on the early development of wearable braces, Joseph Wang's group further developed mouth wearable sensors to monitor the level of uric acid in saliva using a model of mouthguard braces and used them for clinical applications.92 The sensor integrates miniaturized electronic devices, including voltage regulators, microcontrollers and Bluetooth low-energy transceivers. Uric acid in the blood is an important biomarker of diseases, such as renal syndrome and hyperuricemia. The platform can perform non-invasive monitoring of sialic uric acid to replace blood uric acid. The wearable sensor has good sensitivity, selectivity, and stability. It can also quickly collect dynamic chemical data on mouth saliva biomarkers.
The components in the blood are actively transported or diffused to the salivary glands, so there is a certain correlation between the glucose in blood and saliva, which provides a non-invasive and convenient method for glucose sampling. For diabetic patients, changes in hormonal and nerve balances may affect the salivary glands. It also acts as a blood filter and can lead to an increase in the concentration of salivary glucose. Therefore, salivary glucose can provide another painless monitoring method for diabetic patients.93 Soni et al. found that the correlation between blood and saliva glucose concentrations in healthy subjects was R = 0.64, while the relationship in diabetic patients was closer, with R = 0.95.94
Recently, researchers have developed a wearable sensor based on an oral platform and successfully verified the feasibility of the device in volunteer experiments.95 As shown in Fig. 5a, the platform used a biocompatible hydrogel material to prepare dental stickers. Electronic dental stickers were attached to the surface of the teeth and could wirelessly monitor food intake. Through radio frequency wireless transmission of signals, the sensor measured multiple indicators of food in the oral cavity, including the alcohol content, salinity, sweetness, pH and temperature. However, applying this strategy to actual scenarios required a rigorous evaluation of the selectivity of the target analyte to ensure accuracy.
 | ||
| Fig. 5 Oral wearable chem-biosensor. (a) Hydrogel tooth bio-patch to detect multiple indicators of food intake.95 (b) Oral wearable system monitoring the sodium intake of hypertensive patients.96 (c) A fluorescence wearable mouthguard for the sensitive detection and accurate localization of volatile sulfur compounds for oral lesion spots,97 (i) and (ii) referring to before and after being worn on a volunteer with dental caries. (d) An artificial tongue based on a flexible substrate patch for the sensing of astringency perception.98 | ||
Real-time detection of sodium consumption and intake is an essential test for the control and management of hypertension. Researchers have developed a wearable device that replaced oral monitoring and detected oral sodium intake through remote wireless technology.96 The oral sensing platform used ultrathin retractable electronic devices and miniaturized sensors on the premise of ensuring user comfort. Tests conducted on this device in volunteers have proven the feasibility of monitoring sodium consumption (Fig. 5b). At the same time, this study evaluated the cytotoxicity of the device without the sensing layer. Therefore, future oral biosensors need to further rigorously evaluate biocompatibility in all aspects. It is also necessary to further measure sodium intake during food and beverage intake. In general, the currently developed oral wearable sensing platform requires other critical evaluations to ensure the safety and reliability of such systems. It is also necessary to pay attention to surface dirt and contamination caused by saliva components and food residues, ensuring the biosafety of these devices.
It is well known that disease of the oral cavity is usually caused by periodontitis and dental caries, and should be detected in an early stage. The above methods might detect its secretion of volatile sulfur compounds (VSC) qualitatively and quantitatively, but could not locate the lesion site. Li et al. developed a fluorescent mouthguard based on ZnO/PDMS nanomaterials. This oral wearable device could detect VSC in order to accurately locate lesion spots in the oral cavity.97 As shown in Fig. 5c, ZnO/PDMS on the mouthguard could be quenched by VSC in the oral cavity, thus it can be visualized by naked-eye at an excitation wavelength of 360 nm to achieve accurate dental lesion locations. It also had high selectivity and sensitivity of 9.43 ppm for the determination of VSC. Another development of an oral cavity wearable device is in artificial tongues.98 In Fig. 5d, Yeom et al. used a flexible patch to mimic an artificial tongue, where an ionic hydrogel was used to mimic the mechanism of astringency perception. When the flexible patch contacts with an astringent molecule, the hydrogel was transformed into a porous structure, producing highly ionic conductivity. In the determination of tannic acid, this oral wearable showed high sensitivity within ∼10 s response time, in the range of 0.0005–1 wt%.
Although these studies have shown that saliva can be used as a biological fluid for non-invasive diagnosis, there are still some problems before mass-production into the market. Since the concentrations of biomarkers in saliva are usually slightly lower than blood levels, oral wearable devices are required to be highly sensitive. Compared with other non-invasively collected biological fluids, saliva can be easily obtained without complicated procedures. However, complex components of saliva may contaminate samples to a certain extent and affect the detection accuracy. A selective protective coating can be used to remove macromolecules or impurities attached to the sensor surface. Before oral wearable flexible devices are widely used, it is necessary to strictly evaluate the relevance of the target at the oral and blood levels, as well as the biocompatibility, potential toxicity, sterilization and intraoral operational stability. The continuous discovery and research of saliva biomarkers will help expand the scope of saliva diagnosis.
4.3 Breath-based wearable chem-biosensing devices
The mixture of gas and vapor that the human body removes from the nasal cavity or oral cavity through respiration has great potential for non-invasive measurement of breath. The composition of breath is very complicated, which contains a mixture of N2, O2, CO2, and H2O (including up to 500 different compounds). These compounds include endogenous substances through physiological processes and external substances through food and air, including acetone, carbon monoxide, ammonia and nitric oxide.99These respiratory endogenous substances are very useful. For example, the respiratory carbon monoxide test has been used to diagnose neonatal jaundice; respiratory ammonia can be used to assess asthma and hemodialysis; the respiratory nitric oxide test is used to monitor the treatment process of asthma; and breathing acetone can replace blood sampling for monitoring diabetes.99 In addition to respiratory vapor components, the respiratory rate and condensate can be used for biochemical analysis. The respiratory frequency distribution chart is very important for patients with sleep apnea, asthma and chronic obstructive pulmonary disease.3,100 Respiratory condensate is a mixture of cold air containing liquid from the airway and lung tissue. The cytokines and reactive oxygen species in the condensate of the respiratory tract can be used for disease diagnosis and monitoring.101 Breath biosensing technology has been developed since the 1970s, the process of which required bulky equipment to collect respiratory waste. In the late 2000s, a portable breathing sensing device was developed.
The global pandemic outbreak of coronavirus 2019 (COVID-19) has called for an urgent need for respiration monitoring, which are also major factors in patients with COVID-19. Jesse Jokerst's group from University of California, San Diego investigated a mask-typed wearable integrated with a collateral flowing strip. In Fig. 6a, similar to convention pregnancy test strips, the principle of this wearable mask relied on the antibody immobilized on a test line, which specifically recognized the protein-cleaving molecules released by SARS-CoV-2. The wearable mask is a disposal device, where virus particles could be absorbed onto the paper-based strip. Then wearers could self-test the target in their breathing by means of squeezing out the preincubation reagents (e.g., colloidal nanomaterials).
 | ||
| Fig. 6 Breath-based wearable chem-biosensor. (a) A disposal wearable mask for SARS-CoV-2 visual detection: i) before and ii) after squeezing out the colloidal nanomaterials as a colorimetric indicator to represent viral loading. (b) A wearable humidity sensor based on graphene oxide non-woven fabric.102 (c) Modified porous graphene-based wearable sensor for respiration monitoring.103 (d) A wearable breath based on nanocrystalline graphite.104 (e) A fully integrated wearable mask sensor for breath pattern recognition.105 | ||
Respiration is another important physiological indicator to monitor individual health and activities during COVID-19. Typically, humidity is commonly used as an important parameter to evaluate respiration rate. Wang et al. developed a humidity sensor to fabricate a breath-based wearable device based on non-woven fiber modified with graphene oxide and bovine serum albumin.102 In Fig. 6b, due to its sensitivity and rapid response, the device was capable of differentiating nose or mouth breath during the monitoring fast or deep breath. It could also identify the wearers' spoken words. Meanwhile, Pang et al. proposed a wearable humidity sensor based on graphene network for respiration monitoring.103Fig. 6c shows the porous graphene network modified by graphene oxide, poly(3, 4-ethylenedioxythiophene)-poly(styrenesulfonate) and Ag colloids. The wearable sensor based on porous graphene showed excellent ability of monitoring various breathing rates, such as normal or deep respiration.
Another wearable humidity sensor utilized nanocrystalline graphite for real-time breath pattern analysis.104 In Fig. 6d, for constructing the wearable sensor, plasma-enhanced chemical vapor deposition was used to directly deposit graphite nanocrystalline onto the surface of the SiO2 substrate, resulting in rapid response, as well as mechanical stability. This wearable platform was expected to transform into a smart application by means of CMOS production technology. However, these mask-like sensors lacked relative accessories, so that the respiratory might be often overlooked. To this aim, Tipparaju et al. proposed a fully integrated mask-like wearable sensor for the precious monitoring of breath pattern in real-world conditions.105 It could offer comprehensive breath pattern information in combination with the disposable mask, housing compartment, circuit, venturi tube, battery, and thermistor (Fig. 6e). The breath-based wearable sensor could not only accurately detect the breath rate, but also identify the respiration pattern for individuals, especially for patients with asthma or chronic obstructive pulmonary disease.
As the epidemic of COVID-19 spreads, masks are considered to be a daily necessity. Before commercial translation, some issues need to be considered. Sensitivity should be taken into account at the first step. Convention visual analysis methods have not yet reached the detection limit of low-abundance SARS-CoV-2. Second, the biomarkers in the gas are greatly affected by humidity. To solve the humidity interference, increasing the temperature of the sensor for dehydration should be considered. Hygroscopic materials such as NiO can be used to separate water molecules from the target analyte. Then, compared with the real-world scenario, the oncoming wearable mask-like biosensor should be able to adapt to harsh environments, including variable temperature, humidity and airflow.
4.4 Ocular wearable chem-biosensing devices
Unlike blood, tears are a biological fluid that protects the eyes and has antifouling properties. These characteristics make tears an attractive diagnostic biological fluid for medical monitoring, and eye-wearable biosensing devices allow non-invasive sampling from tears rather than directly contacting with blood.106 Human tears are secreted by the lacrimal glands and act as a protective film covering the eyes, which is attributed to its antifouling properties. Tears contain low and high molecular weight compounds, such as proteins, peptides, lipids, metabolites and electrolytes. Tears are not as complicated as blood. The biomarkers in tears not only diffuse directly from the blood, but also provide an opportunity for the diagnosis, which shows a close correlation of blood-tear concentration.106 Especially for diabetic patients, it is important to manage blood glucose levels every day. Thus, this kind of ocular wearable device might be an alternative for daily glucose monitoring and self-management.However, there are some problems with tear samples used for in vitro diagnosis. The main problems are as follows: (I) small volume of samples;107 (II) easy evaporation during sample collection; (III) tear production differences among individuals;108 and (IV) the concentration of the collected samples.109 Different tear collection methods will affect the accuracy of tear-based in vitro diagnostic methods to a certain extent. The most commonly used tear collection method is glass capillaries or Schirmer test strips.108 The emotional tears and reflex tears produced in the process of human emotional changes or mechanical stimulation have different components from basic tears. Basic tears refer to protective tear films that cover the surface of the eyes. Therefore, there is an urgent need to develop a wearable sensing platform that does not cause eye irritation.
Wearing systems based on contact lenses are a solution for tear collection because they can be worn without irritating the eyes and directly contact basic tears.110 All necessary biosensing modules are integrated into the contact lens platform, including data processing and power supply, which is challenging in design. Due to the rapid development of flexible materials, new manufacturing methods have been provided for eye wearable devices, which not only minimize eye irritation, but also provide certain air permeability to avoid hypoxia and improve the accuracy of real-time monitoring.
According to an early research report, there is a good correlation between the concentration of tear glucose and blood glucose levels in animals and humans, including in patients with diabetes type I.111 Tears seem to be a good candidate for non-invasive detection in a wearable platform. A small contact lens electrochemical sensor developed by NovioSense was composed of multiple spiral electrodes modified with glucose hydrogel materials. The device placed under the conjunctiva can be in contact with tears continuously. The back of the eyelid can provide continuous tear glucose measurements without causing discomfort. In addition, it transmitted signals wirelessly. Verily is a part of Google, and has worked with this smart contact lens. However, this project was recently halted because there is a lack of glucose concentration correlation between tears and blood. Accordingly, a blog was posted in the Verily corporation, asserting “Our clinical work on the glucose-sensing lens demonstrated that there was insufficient consistency in our measurements to support the requirements of a medical device”.112 In addition, in John Smith's book, it was claimed that the non-invasive detection for glucose is the hunting of a deceitful turkey.112 Thus, before translation to commercial applications, the correlation of the glucose concentration between tears and blood needs further explorations.
Another application of the ocular-based wearable sensor is for electroretinogram recording, in combination with transparency and flexible substrate. A graphene-based contact lens was fabricated for full-cornea measurement of an electroretinogram from rabbit and monkey (Fig. 7a). This ocular wearable device was constructed from graphene grown on the surface of copper foil, and the grown graphene was transferred to a quartz mold.113 The device showed good signal amplitude in response to different full-field electroretinograms. Additionally, it showed that this device consisted of a multi-electrode array that could record well a resolved electroretinogram signal from rabbit. In regard to ocular health management, intraocular pressure is considered as a vital indicator for diagnosis and care. Kim et al. reported a flexible ocular wearable device, which could not only record intraocular pressure, but also monitor intraocular transplantation non-invasively for diabetes patients.114 In the device (Fig. 7b), there was a strain sensor inside the contact lens for monitoring comprehensive information caused by intraocular pressure. Finally, the continuous recording signal could be transmitted wirelessly via an antenna.
 | ||
| Fig. 7 Ocular wearable chem-biosensing device. (a) A soft graphene contact lens wearable for electroretinography.113 (b) Intraocular pressure wearable contact lens.114 (c) A soft ocular wearable for cortisol immunosensing.115 (d) A fully integrated ocular wearable for real-time glucose detection and therapy.116 (e) Contact lens based on microfluidics for multiplexed electrolyte analysis.117 (f) An ocular wearable for tears analytes semi-quantitative detection.118 | ||
Besides the monitoring of physiological and physical indexes, biomarker detection is also a growing tendency in ocular wearable devices. Thus, a biosensor combined with contact lens is thought to be an attractive platform for monitoring these tears biomarkers non-invasively. Ku et al. proposed a soft contact lens enabling the real-time detection of tear cortisol.115 As shown in Fig. 7c, this transparent ocular wearable sensor was composed of wireless antenna, capacitors, resistors, circuit, and cortisol sensor, which were protected by elastic layers from each other's crosstalk. For a chem-biosensing component of the device, a graphene-based field-effect transistor was utilized for tear cortisol biosensing with a detection limit of 10 pg mL−1. Similarly, this device was combined with an antenna and a near-field communication system for transmitting the recording signal to a portable mobile wirelessly.
Although there is widespread research on ocular wearable devices for target monitoring, a loop of real-time detection and drug-delivery system for health management is extremely urgent. Recently, to address this, Kim et al. developed a soft contact lens for simultaneous real-time glucose detection and drug-delivery treatment.116 In Fig. 7d, for real-time biosensing, this device was based on a three-electrode system, where a glucose sensor as working electrode was modified by glucose oxidase/hydrogel. As for the treatment component, the SU-8 reservoir array was fabricated for drug loading, and the anode/cathode module selectively controlled the on-demand drug releasing by an on/off voltage mode. This eye wearable device has verified the feasibility of the non-invasive detection and treatment of blood glucose in diabetic rabbit models.
Furthermore, a microfluidic channel can be used as a sample storage compartment for wearable contact lens. Yetisen et al. proposed a contact lens for multiplexed analysis, including Na+, K+, Ca2+, Mg2+, and Zn2+, which was visually detected by a portable handheld device.117 In Fig. 7e, the microchannel on the wearable lens was used to store low-volume tears, where the bio-receptor was previously immobilized on the detection zone, and then specifically bound with the target analyte. The quantitative results based on colorimetric chemistry was also obtained via a handheld readout device. Recently, Moreddu et al. fabricated this kind of contact lens prototype again to visually detect pH, glucose, protein and nitrite.118 As shown in Fig. 7f, tears could be divided into four compartments through a capillary of the microchannel, where the specific receptor was incubated. Consequently, the semi-quantitative results were obtained by different color indicators.
The approaching ocular wearable chem-biosensors could be developed for the determination of biomarkers and physiological indicators. As for biomarker detection (e.g., glucose, catecholamine, lysozyme), in order to avoid the inevitable failure case of the Google contact lens, the first consideration should be taken into the fact that the correlation of these biomarkers in tears and blood ought to be validated through a series of explorations. Second, there should be a deeper understanding of the relationship between these biomarkers and ocular diseases. At the same time, it should be noted that the tears sampling procedure should not destroy its components. As for the physiological indicators detection, intraocular pressure is a promising candidate for the approaching ocular wearable chem-biosensors, which is a direct indicator and closely related to glaucoma.116 Although the ocular biosensors for biomarkers detections has remained uncertain until now, contact lens biosensors for intraocular pressure testing have been rapidly developed and already commercialized. Glaucoma might provoke irreversible damage to the optic nerve, which is a common eye disease. Intraocular pressure is also a widely recognized primary indicator for glaucoma disease. To this aim, some recent follow-ups have been reported for intraocular pressure detection.119–121 Maeng et al. reported an embedded wearable ocular sensor for intraocular pressure, which could be visually achieved by color changes without external power, with a detection limit of 3.2 and 5.12 mmHg.120 As for the commercial market, there have been already some wearable products for intraocular pressure, such as Triggerfish Sensor (SENSIMED, Inc., Switzerland) and Eye Mate (Implandata Ophthalmic Products, Inc., Germany). As for the Triggerfish Sensor, a flexible disposable contact lens is embedded into a telemetry chip and strain gauge sensor for real-time continuous monitoring of the intraocular pressure.121
5. Outlook and challenges
These research reports demonstrate that wearable biosensing devices have great potential for real-world applications due to their reliability, monitoring capabilities and wearability. In the future, wearable biosensing devices might enable the non-invasive monitoring of various biomarkers, including proteins and nucleic acids, and ultimately achieve comprehensive medical diagnosis and performance evaluation. Despite recent advances in wearable biosensors, the latest technology in the field is still a proof-of-concept test for a few representative biomarkers, which can be an effort toward practical application. Wearable biosensing devices are faced with fundamental theoretical challenges, and overcoming these technical challenges is a key to realizing the widespread commercialization. At the same time, the acceptance of such non-invasive or minimally invasive biosensing equipment in the medical community still needs to be extensively verified through human testing, and a better understanding of the clinical relevance of sensor information is required. Only when these challenges have been solved will the era of wearable chem-biosensors truly arrive.5.1 Stability and accurate diagnosis
Before being accepted by the market, the stability and detection accuracy of wearable sensing devices need to be repeatedly and rigorously investigated. Surface contamination usually reduces the detection accuracy of wearable devices, posing a major challenge to continuous operation. In order to ensure stability during long-term manual operation, repeatable measurements can be performed over a longer period of time. At the same time, the surface anti-fouling and effective calibration mechanism should be strengthened. Bioaccumulation is caused by proteins, cells or macromolecules on the surface of the wearable biosensors via nonspecific binding. It prevents the target analyte from spreading to the surface of the wearable biosensor, resulting in a gradual decrease in the output signal, i.e., sensing drift. Compared with implantable or minimally invasive sensors, fouling on the surface of wearable biosensing devices is expected to be less serious, especially for the operating in sweat or tears for a short time. In contrast, there may be much dirt on the surface of the oral biosensing device because the protein content of saliva is much higher than that of non-invasive biological fluids. Therefore, wearable oral biosensors need to pay special attention to surface protective coatings to minimize the impact of oral fouling and eliminate interference from coexisting electroactive substances.Unlike traditional laboratories, wearable chem-biosensing devices exposed to the outdoors for a long time in an uncontrolled environment may face harsh and changeable conditions. Such severe conditions may affect the surface modification of biological components. The long-term operation and storage stability of wearable biological devices should consider the immobilization and surface chemistry principles of biological modifications. Accurate measurement by wearable devices also involves the surrounding environment, residues, and continuous signal drift. It is possible to solve such problems through appropriate microfluidic sampling systems and optimized sensitive membrane modification techniques.
5.2 Macromolecule biomarker real-time detection
To date, most of the reported wearable biosensing devices have focused on measuring small molecules, organics, and a small number of biomarkers. Future research may focus more on exploring new biosensor models, performing non-invasive or minimally invasive sampling of biological fluids, and targeting different macromolecular biomarkers.We have summarized three bottlenecks for this issue. The first challenge is feasibly detectible targets in the collected sample on wearables. The most common detection method on a wearable platform might be non-invasive or minimally invasive. However, not all of the macromolecule biomarkers are abundant to reliably exist in such a tiny sample volume. Taking the pipe dream of Theranos for example, there are fewer feasibly detectable analytes in small-volume blood than what they said. Currently, one of the most successful wearables is probably the customer-based wearable glucose meter. The reasons may be as follows: (I) quantities of research studies verified the good correlation between the ISF glucose and blood glucose; (II) the molecular structure of glucose is stable and simple; (III) glucose in ISF or blood is abundant enough to be detected in a small-volume sample. Following this train of thought, the oncoming wearable device for macromolecule biomarkers should be focused on the question: which macromolecule biomarkers is feasibly abundant in drops of samples.
The second challenge is how to conduct sample treatment on wearables. Conventionally, the whole blood is treated by sample centrifugation and cellular separation of components that contain DNA or RNA, but it seems to be ridiculous for a wearable device in a one-off act. That is to say, multi-step sample preparation overshadowed the concept of fully integrated wearable chem-biosensors.
The third challenge lies in exploring the composition of biological fluids in the human peripheral system and their correlation with blood levels. The real-time correlation between the analyte concentration in non-invasive biological fluid and the blood is of great research significance for the popularization of wearable devices. Systematic and in-depth analysis of the composition of different biological fluids is also an important way to discover new macromolecular biomarkers, such as metabolites, proteins and nucleic acids. In addition, evaluating the dynamic concentration of these macromolecular biomarkers under different circumstances can provide important information about circadian rhythms, disease development and health statuses. The currently reported non-invasive wearable platforms can be further expanded from a limited range of metabolites and electrolyte measurements to disease-related macromolecular biomarkers. In addition to the aforementioned ISF, sweat, tears and saliva, exploring new body fluids, such as urine, mucus and semen, may provide more opportunities for wearable biosensing devices.
At present, most wearable devices mainly focus on single-target measurements. Future work should continue to develop non-invasive monitoring methods of multiple macromolecular biomarkers. This not only allows a more extensive evaluation of the physiological state, but also calibrate the sensor signal to ensure the accuracy of the detection. In addition, the composite sensing detection strategy can improve the reliability of wearable flexible devices, which relies on the development of multimode wearable devices with chemical, electrophysiological and physical sensors. This multimode wearable device can promote more comprehensive monitoring of human physiology, and may expand the scope of applicable populations. Another concern of macromolecule biomarkers wearable devices is ethics. A customer-based wearable device might be a boon in emergency settings or resource-limited places, such as developing countries, military zones, or areas of pandemic disease outbreaks. However, unlike handheld glucose meters or oximeters, healthy users can run the risk of getting a false positive result when they choose to test for protein, DNA or RNA on a home wearable, sometimes causing panic.
5.3 Multimodalities
With the increase of precious medicine, single analyte monitoring might not be able to meet the requirement for self-monitoring compliance and healthcare quality for patients. More recently, it can be seen that there is a growing tendency for wearable devices to be more multi-functional, consisting of chemical and physical sensor arrays on a single patch, in order to offer a comprehensive information for individual health management. These multimodule wearable sensors integrated different health indexes into an in-depth single patch, involved in cardiovascular or diabetic biomarkers. However, some issues need to be addressed, such as signal cross-talk, miniaturization, long-term usage, and pre-calibration.5.4 Market regulation
According to a market report, the global wearable biosensing equipment market was valued at approximately 150 million in 2016, and is expected to reach $2.86 billion by 2025. Wearable biosensing equipment is expected to occupy a large share of the future market, especially for applications. Some multinational companies have developed point-of-care devices for quick testing, including Abbott (USA), Roche (Switzerland), Apple (USA) and Samsung (Korea). It is believed that these competitive companies would launch its corresponding wearable platforms in the near future. However, market oversight is a healthy thing for this industry to avoid the implosion of Theranos. Gunn at Genalyte pointed out that startups that can withstand repeated scrutiny would produce satisfactory products.122It is known to us that a successful translation of proof-of-concept demonstrations into commercial products faces various obstacles correlated with fundamental research. If there is no effective path to commercial market, wearables are hard to be realized. To date, most commercial products for wearable medical devices have focused on using electrochemical methods. Due to the inability of intimate epidermis interfaces, sensor stability, reagent contamination, as well as degradation over time, there is still a lack of wearable medical products for molecular monitoring in the commercial market. For most wearable medical devices, they should belong to the class III devices. It is also essential to obtain premarket approval from FDA or other types of regulatory approval in order to ensure the safety and effectiveness of the device. In this regard, long-term stability and safety are one of the most important facts that should be extensively studied before clinical applications. Besides, the reproducibility and functional reliability of the wearable medical devices require repeated and careful evaluation.
However, these constraints do not prevent the public from adopting wearable medical devices to simply monitor some physiological parameters, such as the pulse, heart rate, and blood glucose level. As for non-invasive blood glucose monitoring, with the rapid development of academic research, many people are still very optimistic about the market prospects of wearable biosensing devices.123 The transition of these wearable glucose biosensing equipment from basic theoretical research to commercial markets also faces some difficulties. Wearable glucose devices usually work continuously in some uncontrolled environments. These wearable devices should not only have a stable operation performance, but also should not require continuous recalibration for glucose concentration. Therefore, in the process of sensor preparation, it is necessary to ensure the stability of glucose oxidase and avoid sample contamination and residue, which is very important for the sensing ability and signal accuracy of the sensor. To date, there are few commercial wearable medical devices for continuous monitoring of sweat, tears, ISF or saliva. Future wearable medical devices might require the appropriate use of fluid sampling systems, such as microfluidics, to provide the effective and rapid transmission of biological fluids on the sensor and ensure reproducibility and accuracy. After solving all of the above obstacles, wearable biosensing technology can begin to realize the integration of industry and research.
Author contributions
Bin Yang conceived the research and prepared the manuscript. Bin Yang and Xueen Fang wrote the manuscript. Xingyu Jiang and Jilie Kong supervised the research. All of the authors have read and corrected the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We thank the National Natural Science Foundation of China (21974028), the Natural Science Foundation of Shanghai (19441903900), Scientific and Technological Innovation Action Plan (18142201000), the Shanghai Outstanding Academic Leaders program (19XD1433000), China National Postdoctoral Program for Innovative Talents (BX20200090), and Shanghai Post-doctoral Excellence Program (2020066).References
- J. Min, J. R. Sempionatto, H. Teymourian, J. Wang and W. Gao, Biosens. Bioelectron., 2021, 172, 112750 CrossRef CAS PubMed.
- J. Kim, A. S. Campbell, B. E. F. D. Ávila and J. Wang, Nat. Biotechnol., 2019, 37, 389–406 CrossRef CAS PubMed.
- (a) Y. Yang and W. Gao, Chem. Soc. Rev., 2019, 48, 1465–1491 RSC; (b) S. Shrivastava, T. Q. Trung and N. Lee, Chem. Soc. Rev., 2020, 49, 1812–1866 RSC; (c) G. Lee, H. Moon, H. Kim, G. H. Lee, W. Kwon, S. Yoo, D. Myung, S. H. Yun, Z. Bao and S. K. Hahn, Nat. Rev. Mater., 2020, 5, 149–165 CrossRef; (d) T. R. Ray, J. Choi, A. J. Bandodkar, S. Krishnan, P. Gutruf, L. Tian, R. Ghaffari and J. A. Rogers, Chem. Rev., 2019, 119, 5461–5533 CrossRef CAS PubMed; (e) Z. Liu, H. Li, B. Shi, Y. Fan, Z. L. Wang and Z. Li, Adv. Funct. Mater., 2019, 29, 1808820 CrossRef.
- C. Y. Wu, L. Terhorst, J. F. Karp, E. R. Skidmore and J. Rodakowski, Diabetes care, 2018, 41, 2072–2078 CrossRef PubMed.
- R. Consortium, Diabetes care, 2018, 41, 1707–1716 CrossRef PubMed.
- M. Amjadi, K. U. Kyung, I. Park and M. Sitti, Adv. Funct. Mater., 2016, 26, 1678–1698 CrossRef CAS.
- (a) S. Wagner and S. Bauer, MRS Bull., 2012, 37, 207–213 CrossRef; (b) Y. Liu, Y. Zhao, B. Sun and C. Chen, Acc. Chem. Res., 2013, 46, 702–713 CrossRef CAS PubMed; (c) J. Ren, Q. Xu, X. Chen, W. Li, K. Guo, Y. Zhao, Q. Wang, Z. Zhang, H. Peng and Y. G. Li, Adv. Mater., 2017, 29, 1702713 CrossRef PubMed.
- Ö. Civalek and Ç. Demir, Appl. Math. Model., 2011, 35, 2053–2067 CrossRef.
- Y. Lee, J. Park, A. Choe, S. Cho, J. Kim and H. Ko, Adv. Funct. Mater., 2020, 30, 1904523 CrossRef CAS.
- S. A. Hashemi, S. Ramakrishna and A. G. Aberle, Energy Environ. Sci., 2020, 13, 685–743 RSC.
- S. Huang, B. Zhang, Z. Shao, L. He, Q. Zhang, J. Jie and X. Zhang, Nano Lett., 2020, 20, 2478–2485 CrossRef CAS PubMed.
- S. Yuvaraja, A. Nawaz, Q. Liu, D. Dubal, S. G. Surya, K. N. Salama and P. Sonar, Chem. Soc. Rev., 2020, 49, 3423–3460 RSC.
- F. Yang, M. Wang, D. Zhang, J. Yang, M. Zheng and Y. Li, Chem. Rev., 2020, 120, 2693–2758 CrossRef CAS PubMed.
- F. Palumbo, C. Wen, S. Lombardo, S. Pazos, F. Aguirre, M. Eizenberg, F. Hui and M. Lanza, Adv. Funct. Mater., 2020, 30, 1900657 CrossRef CAS.
- Q. Zheng, J. Lee, X. Shen, X. Chen and J. K. Kim, Mater. Today, 2020, 36, 158–179 CrossRef CAS.
- S. K. Garlapati, M. Divya, B. Breitung, R. Kruk, H. Hahn and S. Dasgupta, Adv. Mater., 2018, 30, 1707600 CrossRef PubMed.
- H. Xu, A. Ren, J. Wu and Z. Wang, Adv. Funct. Mater., 2020, 30, 2000907 CrossRef CAS.
- S. Cheng, S. Han, Z. Cao, C. Xu, X. Fang and X. Wang, Small, 2020, 16, 1907461 CrossRef CAS PubMed.
- E. C. Ahn, H. S. P. Wong and E. Pop, Nat. Rev. Mater., 2018, 3, 18009 CrossRef CAS.
- J. Kim, A. Banks, H. Cheng, Z. Xie, S. Xu, K. Jang, J. W. Lee, Z. Liu, P. Gutruf, X. Huang, P. Wei, F. Liu, K. Li, M. Dalal, R. Ghaffari, X. Feng, Y. Huang, S. Gupta and U. Paik, Small, 2015, 11, 906–912 CrossRef CAS.
- (a) R. Dong, X. Liu, S. Cheng, L. Tang, M. Chen, L. Zhong, Z. Chen, S. Liu and X. Jiang, Adv. Healthcare Mater., 2021, 10, 2000641 CrossRef CAS PubMed; (b) Y. Liu, Q. Wang, S. Bi, W. Zhang, H. Zhou and X. Jiang, Nanoscale, 2020, 12, 13731–13741 RSC; (c) L. Mou, J. Qi, L. Tang, R. Dong, Y. Xia, Y. Gao and X. Jiang, Small, 2020, 16, 2005336 CrossRef CAS PubMed; (d) L. Tang, J. Shang and X. Jiang, Sci. Adv., 2021, 7, eabe3778 CrossRef CAS PubMed; (e) A. Modali, S. R. K. Vanjari and D. Dendukuri, Electroanalysis, 2016, 28, 1276–1282 CrossRef CAS; (f) T. Choudhary, G. P. Rajamanickam and D. Dendukuri, Lab Chip, 2015, 15, 2064–2072 RSC.
- (a) D. Liu, Z. Shadike, R. Lin, K. Qian, H. Li, K. Li, S. Wang, Q. Yu, M. Liu, S. Ganapathy, X. Qin, Q. H. Yang, M. Wagemaker, F. Kang, X. Q. Yang and B. Li, Adv. Mater., 2019, 31, 1806620 CrossRef PubMed; (b) Z. Wen, M. H. Yeh, H. Guo, J. Wang, Y. Zi, W. Xu, J. Deng, L. Zhu, X. Wang, C. Hu, L. Zhu, X. Sun and Z. L. Wang, Sci. Adv., 2016, 2, e1600097 CrossRef PubMed.
- S. Casaluci, M. Gemmi, V. Pellegrini, A. D. Carlo and F. Bonaccorso, Nanoscale, 2016, 8, 5368–5378 RSC.
- A. J. Bandodkar and J. Wang, Electroanalysis, 2016, 28, 1188–1200 CrossRef CAS.
- L. Su, W. Jia, C. Hou and Y. Lei, Biosens. Bioelectron., 2011, 26, 1788–1799 CrossRef CAS.
- X. Xie, M. Ye, L. Hu, N. Liu, J. R. Mcdonough, W. Chen, H. N. Alshareef, C. S. Criddle and Y. Cui, Energy Environ. Sci., 2012, 5, 5265–5270 RSC.
- N. Mano and A. D. Poulpiquet, Chem. Rev., 2017, 118, 2392–2468 CrossRef PubMed.
- X. Xiao, H. Xia, R. Wu, L. Bai, L. Yan, E. Magner, S. Cosnier, E. Lojou, Z. Zhu and A. Liu, Chem. Rev., 2019, 119, 9509–9558 CrossRef CAS PubMed.
- (a) C. Zhao, P. Gai, R. Song, Y. Chen, J. Zhang and J. Zhu, Chem. Soc. Rev., 2017, 46, 1545–1564 RSC; (b) S. Hao, X. Sun, H. Zhang, J. Zhai and S. Dong, J. Mater. Chem. B, 2020, 8, 3393–3407 RSC; (c) C. Zhan, T. Wu, J. Lu and K. Amine, Energy Environ. Sci., 2018, 11, 243–257 RSC.
- L. Halámková, J. Halámek, V. Bocharova, A. Szczupak, L. Alfonta and E. Katz, J. Am. Chem. Soc., 2012, 134, 5040–5043 CrossRef PubMed.
- M. Rasmussen, R. E. Ritzmann and I. Lee, J. Am. Chem. Soc., 2012, 134, 1458–1460 CrossRef CAS PubMed.
- B. Shi, Z. Li and Y. Fan, Adv. Mater., 2018, 30, 1801511 CrossRef PubMed.
- K. Elouarzaki, M. Bourourou, M. Holzinger, A. L. Goff, R. S. Marks and S. Consier, Energy Environ. Sci., 2015, 8, 2069–2074 RSC.
- (a) A. J. Bandodkar, J. M. You, N. H. Kim, Y. Gu, R. Kumar, A. M. V. Mohan, J. Kuiniawan, S. Imani, T. Nakagawa, B. Parish, M. Parthasarathy, P. P. Mercier, S. Xu and J. Wang, Energy Environ. Sci., 2017, 10, 1581–1589 RSC; (b) X. Chen, L. Yin, J. Lv, A. J. Gross, M. Le, N. G. Gutierrez, Y. Li, I. Jeerapan, F. Giroud, A. Berezovska, R. K. O'Reilly, S. Xu, S. Cosnier and J. Wang, Adv. Funct. Mater., 2019, 29, 1905785 CrossRef CAS.
- M. Agiwal, A. Roy and N. Saxena, IEEE Commun. Surv. Tutor., 2016, 18, 1617–1655 Search PubMed.
- K. N. Paracha, S. K. A. Rahim, P. J. Soh and M. Khalily, IEEE Access, 2019, 7, 56694–56712 Search PubMed.
- J. Mao, H. Yang, Y. Lian and B. Zhao, IEEE Trans. Biomed. Circuits Syst., 2017, 11, 1001–1012 Search PubMed.
- A. Kos, V. Milutinović and A. Umek, Future Gener. Comput. Syst., 2019, 92, 582–592 CrossRef.
- Y. Gao, S. J. Cheng and W. D. Toh, IEEE J. Solid-State Circuits, 2013, 48, 2717–2733 Search PubMed.
- Y. Shi, M. Choi and Z. Li, IEEE J. Solid-State Circuits, 2016, 51, 2570–2583 Search PubMed.
- W. Gao, S. Emaminejad, H. Y. Y. Nyein, S. Challa, K. Chen, A. Peck, H. M. Fahad, H. Ota, H. Shiraki, D. Kiriya and D. Lien, Nature, 2016, 529, 509–514 CrossRef CAS PubMed.
- A. M. Zamarayeva, A. E. Ostfeld, M. Wang, J. K. Duey, I. Deckman, B. P. Lechêne, G. Davies, D. A. Steingart and A. C. Arias, Sci. Adv., 2017, 3, e1602051 CrossRef PubMed.
- S. Huang, Y. Liu, Y. Zhao, Z. Ren and C. F. Guo, Adv. Funct. Mater., 2019, 29, 1805924 CrossRef.
- Z. Wu, Y. Wang, X. Liu, C. Lv, Y. Li, D. Wei and Z. Liu, Adv. Mater., 2019, 31, 1800716 CrossRef PubMed.
- A. Hulanicki, S. Glab and F. Ingman, Pure Appl. Chem., 1991, 63, 1247–1250 Search PubMed.
- D. Chen and Q. Pei, Chem. Rev., 2017, 117, 11239–11268 CrossRef CAS PubMed.
- X. U. Zou, X. V. Zhen, J. H. Cheong and P. Bühlmann, Anal. Chem., 2014, 86, 8687–8692 CrossRef CAS PubMed.
- R. Cisternas, H. Kahlert, F. Scholz and H. Wulff, Electrochem. Commun., 2015, 60, 17–20 CrossRef CAS.
- Z. Wen, J. Chen, M. H. Yeh, H. Guo, Z. Li, X. Fan, T. Zhang, L. Zhu and Z. L. Wang, Nano Energy, 2015, 16, 38–46 CrossRef CAS.
- J. Deng, J. Ma, L. Mei, Y. Tang, Y. Chen, T. Lv, Z. Xu and T. Wang, J. Mater. Chem. A, 2013, 1, 12400–12403 RSC.
- S. T. Gaylord, T. L. Dinh, E. R. Goldman and G. P. Anderson, Anal. Chem., 2015, 87, 6570–6577 CrossRef CAS PubMed.
- E. S. Cho, J. Kim, B. Tejerina, T. M. Hermans, H. Jiang, H. Nakanishi, M. Yu, A. Z. Patashinski, S. C. Glotzer, F. Stellacci and B. A. Grzybowski, Nat. Mater., 2012, 11, 978–985 CrossRef CAS PubMed.
- R. Lu, W. W. Li, B. Mizaikoff, A. Katzir, Y. Raichlin, G. Sheng and H. Yu, Nat. Protoc., 2016, 11, 377–386 CrossRef CAS PubMed.
- Y. Wang, A. La, Y. Ding, Y. Liu and Y. Lei, Adv. Funct. Mater., 2012, 22, 3547–3555 CrossRef CAS.
- A. S. Emrani, N. M. Danesh, M. Ramezani, S. M. Taghdisi and K. Abnous, Biosens. Bioelectron., 2016, 79, 288–293 CrossRef CAS PubMed.
- P. Jolly, V. Tamboli, R. L. Harniman, P. Estrela, C. J. Allender and J. L. Bowen, Biosens. Bioelectron., 2016, 75, 188–195 CrossRef CAS PubMed.
- S. Mariani, S. Scarano, J. Spadavecchia and M. Minunni, Biosens. Bioelectron., 2015, 74, 981–988 CrossRef CAS PubMed.
- J. Li, K. W. Chang, C. H. Wang, C. H. Yang, S. C. Shiesh and G. B. Lee, Biosens. Bioelectron., 2016, 79, 887–893 CrossRef CAS PubMed.
- S. Y. Kim, S. Park, H. W. Park, D. H. Park, Y. Jeong and D. H. Kim, Adv. Mater., 2015, 27, 4178–4185 CrossRef CAS PubMed.
- Y. Huang, F. Li, M. Qin, L. Jiang and Y. Song, Angew. Chem., Int. Ed., 2013, 52, 7296–7299 CrossRef CAS PubMed.
- Z. Sonner, E. Wilder, J. Heikenfeld, G. Kasting, F. Beyette, D. Swaile, F. Sherman, J. Joyce, J. Hagen, N. K. Loughnane and R. Naik, Biomicrofluidics, 2015, 9, 031301 CrossRef CAS PubMed.
- N. F. Andersen, B. M. Altura, B. T. Altura and O. S. Andersen, Clin. Chem., 1995, 41, 1522–1525 CrossRef.
- A. S. Campbell, J. Kim and J. Wang, Curr. Opin. Electrochem., 2018, 10, 126–135 CrossRef CAS PubMed.
- A. J. Bandodkar, W. Jia and J. Wang, Electroanalysis, 2015, 27, 562–572 CrossRef CAS.
- A. J. Bandodkar, D. Molinnus, O. Mirza, T. Guinovart, J. R. Windmiller, G. V. Ramírez, F. J. Andrade, M. J. Schöning and J. Wang, Biosens. Bioelectron., 2014, 54, 603–609 CrossRef CAS PubMed.
- J. Kim, W. R. D. Araujo, I. A. Samek, A. J. Bandodkar, W. Jia, B. Brunetti, T. R. L. C. Paixão and J. Wang, Electrochem. Commun., 2015, 51, 41–45 CrossRef CAS.
- W. Jia, A. J. Bandodkar, G. V. Ramírez, J. R. Windmiller, Z. Yang, J. Ramírez, G. Chan and J. Wang, Anal. Chem., 2013, 85, 6553–6560 CrossRef CAS PubMed.
- L. Wang, L. Wang, Y. Zhang, J. Pan, S. Li, X. Sun, B. Zhang and H. Peng, Adv. Funct. Mater., 2018, 28, 1804456 CrossRef.
- X. Shi, Y. Zuo, P. Zhai, J. Shen, Y. Yang, Z. Gao, M. Liao, J. Wu, J. Wang, X. Xu, Q. Tong, B. Zhang, B. Wang, X. Sun, L. Zhang, Q. Pei, D. Jin, P. Chen and H. Peng, Nature, 2021, 591, 240–245 CrossRef CAS PubMed.
- Y. Yang, Y. Song, X. Bo, J. Min, O. S. Pak, L. Zhu, M. Wang, J. Tu, A. Kogan, H. Zhang, T. K. Hsiai, Z. Li and W. Gao, Nat. Biotechnol., 2020, 38, 217–224 CrossRef CAS PubMed.
- A. J. Bandodkar, S. P. Lee, I. Huang, W. Li, S. Wang, C. J. Su, W. J. Jeang, T. Hang, S. Mehta, N. Nyberg, P. Gutruf, J. Choi, J. Koo, J. T. Reeder, R. Tseng, R. Ghaffari and J. A. Rogers, Nat. Electron., 2020, 3, 554–562 CrossRef CAS.
- H. Lin, J. Tan, J. Zhu, S. Lin, Y. Zhao, W. Yu, H. Hojaiji, B. Wang, S. Yang, X. Cheng, Z. Wang, E. Tang, C. Yeung and S. Emaminejad, Nat. Commun., 2020, 11, 4405 CrossRef CAS PubMed.
- (a) C. R. Santiveri, H. J. Sismaet, M. Kimani and E. D. Goluch, ChemistrySelect, 2018, 3, 2926–2930 CrossRef CAS; (b) P. J. Buch, Y. Chai and E. D. Goluch, Wound Repair Regen., 2021, 29, 106–116 CrossRef PubMed; (c) Y. Lei, W. Zhao, Y. Zhang, Q. Jiang, J. H. He, A. J. Baeumner, O. S. Wolfbeis, Z. L. Wang, K. N. Salama and H. N. Alshareef, Small, 2019, 15, 1901190 CrossRef PubMed.
- Q. Pang, D. Lou, S. Li, G. Wang, B. Qiao, S. Dong, L. Ma, C. Gao and Z. Wu, Adv. Sci., 2020, 7, 1902673 CrossRef CAS PubMed.
- B. Yang, J. Kong and X. Fang, Talanta, 2019, 204, 685–692 CrossRef CAS PubMed.
- M. J. Tierney, J. A. Tamada, R. O. Potts, L. Jovanovic, S. Garg and C. R. Team, Biosens. Bioelectron., 2001, 16, 621–629 CrossRef CAS PubMed.
- A. J. Bandodkar, W. Jia, C. Yardımcı, X. Wang, J. Ramirez and J. Wang, Anal. Chem., 2015, 87, 394–398 CrossRef CAS PubMed.
- S. Emaminejad, W. Gao, E. Wu, Z. A. Davies, H. Y. Y. Nyein, S. Challa, S. P. Ryan, H. M. Fahad, K. Chen, Z. Shahpar, S. Talebi, C. Milla, A. Javey and R. W. Davis, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 4625–4630 CrossRef CAS PubMed.
- J. Kim, J. R. Sempionatto, S. Imani, M. C. Hartel, A. Barfidokht, G. Tang, A. S. Campbell, P. P. Mercier and J. Wang, Adv. Sci., 2018, 5, 1800880 CrossRef PubMed.
- Y. J. Hong, H. Lee, J. Kim, M. Lee, H. J. Choi, T. Hyeon and D. H. Kim, Adv. Funct. Mater., 2018, 28, 1805754 CrossRef.
- J. R. Sempionatto, M. Lin, L. Yin, E. D. L. Paz, K. Pei, T. Sonsa, A. N. D. L. Silva, A. A. Khorshed, F. Zhang, N. Tostado, S. Xu and J. Wang, Nat. Biomed. Eng., 2021, 5, 737–748 CrossRef CAS PubMed.
- J. W. Coffey, S. C. Meliga, S. R. Corrie and M. A. F. Kendall, Biomaterials, 2016, 84, 130–143 CrossRef CAS PubMed.
- P. P. Samant, M. M. Niedzwiecki, N. Raviele, V. Tran, J. M. Lapaix, D. I. Walker, E. I. Felner, D. P. Jones, G. W. Miller and M. R. Prausnitz, Sci. Transl. Med., 2020, 12, eaaw0285 CrossRef CAS PubMed.
- A. Mandal, A. V. Boopathy, L. K. W. Lam, K. D. Moynihan, M. E. Welch, N. R. Bennett, M. E. Turvey, N. Thai, J. H. Van, J. C. Love, P. T. Hammond and D. J. Irvine, Sci. Transl. Med., 2018, 10, eaar2227 CrossRef PubMed.
- J. W. Coffey, S. R. Corrie and M. A. F. Kendall, Biomaterials, 2018, 170, 49–57 CrossRef CAS PubMed.
- B. Yang, X. Fang and J. Kong, Adv. Funct. Mater., 2020, 30, 2000591 CrossRef CAS.
- S. Kusama, K. Sato, Y. Matsui, N. Kimura, H. Abe, S. Yoshida and M. Nishizawa, Nat. Commun., 2021, 12, 658 CrossRef CAS PubMed.
- Y. Yao, J. Chen, Y. Guo, T. Lv, Z. Chen, N. Li, S. Cap, B. Chen and T. Chen, Biosens. Bioelectron., 2021, 179, 113078 CrossRef CAS PubMed.
- L. Lipani, B. G. R. Dupont, F. Doungmene, F. Marken, R. M. Tyrrell, R. H. Guy and A. Llie, Nat. Nanotechnol., 2018, 13, 504–511 CrossRef CAS PubMed.
- L. G. Carmona, A. Martín, J. R. Sempionatto, J. R. Moreto, M. C. Gonzalez, J. Wang and A. Escarpa, Anal. Chem., 2019, 91, 13883–13891 CrossRef PubMed.
- F. G. Bellagambi, T. Lomonaco, P. Salvo, F. Vivaldi, M. Hangouet, S. Ghimenti, D. Biagini, F. D. Francesco, R. Fuoco and A. Errachid, TrAC, Trends Anal. Chem., 2020, 124, 115781 CrossRef CAS.
- J. Kim, S. Imani, W. R. D. Araujo, J. Warchall, G. V. Ramírez, T. R. L. C. Paixão, P. P. Mercier and J. Wang, Biosens. Bioelectron., 2015, 74, 1061–1068 CrossRef CAS PubMed.
- W. Zhang, Y. Du and M. L. Wang, Sens. Bio-Sens. Res., 2015, 4, 23–29 CrossRef.
- A. Soni and S. K. Jha, Biosens. Bioelectron., 2015, 67, 763–768 CrossRef CAS PubMed.
- P. Tseng, B. Napier, L. Garbarini, D. L. Kaplan and F. G. Omenetto, Adv. Mater., 2018, 30, 1703257 CrossRef PubMed.
- Y. Lee, C. Howe, S. Mishra, D. L. Lee, M. Mahmood, M. Piper, Y. Kim, K. Tieu, H. Byun, J. P. Coffey, M. Shayan, Y. Chun, R. M. Costanzo and W. Yeo, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5377–5382 CrossRef CAS PubMed.
- X. Li, C. Luo, Q. Fu, C. Zhou, M. Ruelas, Y. Wang, J. He, Y. Wang, Y. S. Zhang and J. Zhou, Adv. Mater., 2020, 32, 2000060 CrossRef CAS PubMed.
- J. Yeom, A. Choe, S. Lim, Y. Lee, S. Na and H. Ko, Sci. Adv., 2020, 6, eaba5785 CrossRef CAS PubMed.
- T. H. Risby and S. F. Solga, Appl. Phys. B: Lasers Opt., 2006, 85, 421–426 CrossRef CAS.
- J. Hodgkinson and R. P. Tatam, Meas. Sci. Technol., 2012, 24, 012004 CrossRef.
- K. H. Kim, S. A. Jahan and E. Kabir, TrAC, Trends Anal. Chem., 2012, 33, 1–8 CrossRef CAS.
- Y. Wang, L. Zhang, Z. Zhang, P. Sun and H. Chen, Langmuir, 2020, 36, 9443–9448 CrossRef CAS PubMed.
- Y. Pang, J. Jian, T. Tu, Z. Yang, J. Ling, Y. Li, X. Wang, Y. Qian, H. Tian, Y. Yang and T. L. Ren, Biosens. Bioelectron., 2018, 116, 123–129 CrossRef CAS PubMed.
- T. Y. Ling, L. H. Wah, J. W. McBride, H. M. H. Chong and S. H. Pu, 2019 IEEE Inter. Confer. Sens. Nanotechnol., 2019, vol. 1, pp. 1–4 Search PubMed.
- V. V. Tipparaju, D. Wang, J. Yu, F. Chen, F. Tsow, E. Forzani, N. Tao and X. Xian, Biosens. Bioelectron., 2020, 169, 112590 CrossRef CAS PubMed.
- N. M. Farandos, A. K. Yetisen, M. J. Monteiro and C. R. Lowe, Adv. Healthcare Mater., 2015, 4, 792–810 CrossRef CAS PubMed.
- S. Mishima, A. Gasset, S. D. Klyce and J. L. Baum, Invest. Ophthalmol. Visual Sci., 1966, 5, 264–276 CAS.
- R. J. Fullard and L. G. Carney, Exp. Eye Res., 1984, 38, 15–26 CrossRef CAS PubMed.
- Q. Yan, B. Peng, G. Su, B. E. Cohan, T. C. Major and M. E. Meyerhoff, Anal. Chem., 2011, 83, 8341–8346 CrossRef CAS PubMed.
- M. B. Moshe, V. L. Alexeev, S. A. Asher and S. A. Asher, Anal. Chem., 2006, 78, 5149–5157 CrossRef PubMed.
- T. V. Zubareva and Z. M. Kiseleva, Int. J. Ophthalmol., 1977, 175, 339–344 CAS.
- J. L. Smith, Revised and Expanded, copyright, 2015 Search PubMed.
- R. Yin, Z. Xu, M. Mei, Z. Chen, K. Wang, Y. Liu, T. Tang, M. K. Priydarshi, X. Meng, S. Zhao, B. Deng, H. Peng, Z. Liu and X. Duan, Nat. Commun., 2018, 9, 2334 CrossRef PubMed.
- J. Kim, J. Kim, M. Ku, E. Cha, S. Ju, W. Y. Park, K. H. Kim, D. W. Kim, P. O. Berggren and J. U. Park, Nano Lett., 2019, 20, 1517–1525 CrossRef PubMed.
- M. Ku, J. Kim, J. E. Won, W. Kang, Y. G. Park, J. Park, J. H. Lee, J. Cheon, H. H. Lee and J. U. Park, Sci. Adv., 2020, 6, eabb2891 CrossRef CAS PubMed.
- S. K. Kim, J. Koo, G. H. Lee, C. Jeon, J. W. Mok, B. H. Mun, K. J. Lee, E. Kamrani, C. K. Joo, S. Shin, J. Y. Sim, D. Myung, S. H. Yun, Z. Bao and S. K. Hahn, Sci. Adv., 2020, 6, eaba3252 CrossRef PubMed.
- A. K. Yetisen and N. Jiang, Adv. Mater., 2019, 32, 1906762 CrossRef PubMed.
- R. Moreddu, J. S. Wolffsohn, D. Vigolo and A. K. Yetisen, Sens. Actuators, B, 2020, 317, 128183 CrossRef CAS.
- C. G. D. Moraes, K. Mansouri, J. M. Liebmann and R. Ritch, JAMA Ophthalmol., 2018, 136, 779–785 CrossRef PubMed.
- B. Maeng, H. Chang and J. Park, Lab Chip, 2020, 20, 1740–1750 RSC.
- Y. Zhang, Y. Chen, T. Man, D. Huang, X. Li, H. Zhu and Z. Li, Microsyst. Nanoeng., 2019, 5, 39 CrossRef PubMed.
- E. Waltz, Nat. Biotechnol., 2017, 35, 11–15 CrossRef CAS PubMed.
- J. Heikenfeld, A. Jajack, J. Rogers, P. Gutruf, L. Tian, T. Pan, R. Li, M. Khine, J. Kim, J. Wang and J. Kim, Lab Chip, 2018, 18, 217–248 RSC.
| This journal is © The Royal Society of Chemistry 2021 |