Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Single-carbon-atom transfer to para-quinone methides from TMSCF2Br†
Ruikang Sun‡
a,
Pei Zhang‡a,
Yong Yana,
Jie Zhua,
Qirui Chena,
Chi Yangb,
Aijun Lin a,
Xuanyi Lia,
Shang Gao*a and
Hequan Yao
a,
Xuanyi Lia,
Shang Gao*a and
Hequan Yao *a
*a
aState Key Laboratory of Natural Medicines (SKLNM), Department of Medicinal Chemistry, School of Pharmacy, China Pharmaceutical University, Nanjing, 210009, P. R. China. E-mail: gaoshang1990@cpu.edu.cn; hyao@cpu.edu.cn
bAnhui Provincial Joint Key Laboratory for Innovative Drug Research and Industry Integration, School of Chemistry and Materials Engineering, Fuyang Normal University, Fuyang 236037, P. R. China
First published on 4th June 2025
Abstract
Single-carbon-atom transfer reactions offer a powerful strategy for constructing complex molecular architectures by sequential assembly of substituents around the atomic carbon core. However, the limited availability of atomic carbon sources has significantly hindered progress in this field. Herein, we demonstrate a single-carbon atom transfer reaction utilizing commercially available TMSCF2Br as an atomic carbon equivalent. Through a cascade of 1,6-addition and TBAF-catalyzed intramolecular cyclization with para-quinone methides (p-QMs), gem-difluorinated spiro[2.5]octa-4,7-dien-6-ones were efficiently formed. These spirocyclic intermediates exhibit remarkable electrophilicity, enabling stereoselective capture of diverse nucleophiles to access fluorinated alkenes with excellent stereocontrol. The resulting fluoroalkenes serve as versatile platforms for constructing tetrasubstituted alkenes via nucleophilic vinylic substitution (SNV), achieving excellent stereoselectivities. In the presence of a 1,3-bisnucleophile, for example a C2-substituted acetoacetate ester, cyclic 2-methylene-2,3-dihydrofuran was generated via a sequential SNV reaction with excellent stereoselectivities. Moreover, a computational study and a control experiment provide insight into the mechanism of the reaction.
Introduction
Molecular skeleton editing has emerged as a transformative strategy for diversifying molecular complexity in synthetic chemistry.1 While advancements in late-stage functionalization have enabled precise modifications of (hetero)aromatic backbones,2 the incorporation of a single carbon atom into molecular frameworks with simultaneous formation of four bonds provides a fascinating platform to enhance molecular complexity beyond aromatic compounds (Scheme 1(1)).3–10 Wherein, single C(sp)-atom transfer reactions have been well-developed to construct alkynes and allenes, including several textbook reactions.4 In contrast, single C(sp2)- and C(sp3)-atom transfer reactions are still largely underdeveloped due to the limited types of atomic carbon sources. With the development of novel atomic carbon sources from the Tobisu,5 Hansmann,6 Glorius7 and Suero8 groups, especially the carbene precursors, significant breakthrough has been made over the past few years (Scheme 1(2)). For example, using N-heterocyclic carbene (NHC) and diazosulfur ylide (Ph2S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N2) reagents as single C(sp3)-atomic sources, γ-lactams and highly strained carbon spiro-centers were constructed by the Tobisu and Hansmann groups, respectively (Scheme 1(3)).4,5 Shevlin and colleagues made seminal contributions to the development of C(sp2)-atom transfer reactions with arc-discharge-generated carbon atoms and tert-butylbenzene systems.9 However, the synthetic utility of this method remains limited due to its remarkably low efficiency (Scheme 1(4a)). In 2010, Baceiredo and Kato reported another example with mixed P,S-bis(ylide) as a carbon atom source, allowing the creation of vinyl phenyl sulfide in quantitative yield via sequential elimination of phosphine oxide and Ph2S (Scheme 1(4b)).10 Very recently, Glorius's group developed a new reagent, chloro-diazoacetyl diarylmethanone oxime (Cl-DADO), enabling access to C3-functionalized quinolines via stepwise Rh-catalyzed carbyne insertion/functionalization of the oxime ester (Scheme 1(4c)).7 Despite these advances, further exploration of novel single-carbon-atom transfer reaction remains highly desirable yet challenging.
N2) reagents as single C(sp3)-atomic sources, γ-lactams and highly strained carbon spiro-centers were constructed by the Tobisu and Hansmann groups, respectively (Scheme 1(3)).4,5 Shevlin and colleagues made seminal contributions to the development of C(sp2)-atom transfer reactions with arc-discharge-generated carbon atoms and tert-butylbenzene systems.9 However, the synthetic utility of this method remains limited due to its remarkably low efficiency (Scheme 1(4a)). In 2010, Baceiredo and Kato reported another example with mixed P,S-bis(ylide) as a carbon atom source, allowing the creation of vinyl phenyl sulfide in quantitative yield via sequential elimination of phosphine oxide and Ph2S (Scheme 1(4b)).10 Very recently, Glorius's group developed a new reagent, chloro-diazoacetyl diarylmethanone oxime (Cl-DADO), enabling access to C3-functionalized quinolines via stepwise Rh-catalyzed carbyne insertion/functionalization of the oxime ester (Scheme 1(4c)).7 Despite these advances, further exploration of novel single-carbon-atom transfer reaction remains highly desirable yet challenging.
Commercially available TMSCF2Br presents an attractive yet underexploited reagent, offering triple functionality as a TMSCF2 radical donor, a difluorocarbene precursor and an atomic carbon equivalent (Scheme 1(5)).11 While the efficacy of the first two variants has been empirically validated,12 its implementation potential in single-carbon-atom transfer reactions remains elusive.13 Concurrently, para-quinone methides (p-QMs) have gained prominence as versatile synthons due to their inherent aromaticity-driven reactivity, particularly in 1,6-addition cascades.14 We envisioned that merging the p-QMs' reactivity with TMSCF2Br's latent single atomic carbon-donating capability would provide unprecedented opportunities for advancing single-carbon-atom transfer reactions. Several challenges are apparent for this transformation: (1) functionalization of TMSCF2Br in a sequentially controllable manner is essential to generate single atomic carbon doped products in synthetically useful yields (Scheme 1(6)). (2) The regioselectivity of the ring-opening of 1,1-difluoro-spiro[2.5]octa-4,7-dien-6-one 3 is not clear for different nucleophiles (Scheme 1(7)). (3) It is challenging to construct the highly congested tetra-substituted olefins 5, 6 and 8. (4) The control of the stereoselectivities for the formation of fully substituted olefin 6 and the chemoselectivities for the intramolecular SNV reaction of intermediate 8 to generate 9 is another challenge.15 With our continuing interest in the construction of stereodefined alkenes and the chemistry of p-QMs,16 we herein report a skeleton editing of p-QMs via a single-carbon atom transfer reaction, enabling the stereoselective construction of sterically hindered tetra-substituted fluoroalkenes and 2-methylene-2,3-dihydrofurans. The fluoroalkenes could serve as a platform to undergo diversity-oriented synthesis, giving sterically hindered tetra-substituted alkenes via a formal SNV reaction with good retention of the olefinic configuration. The exo-alkene motif of 2-methylene-2,3-dihydrofurans could undergo a series of late-stage transformations to enrich the structural diversity of the products. Of note is that the TMSCF2Br works as an atomic sp2-hybridized carbon.
Results and discussion
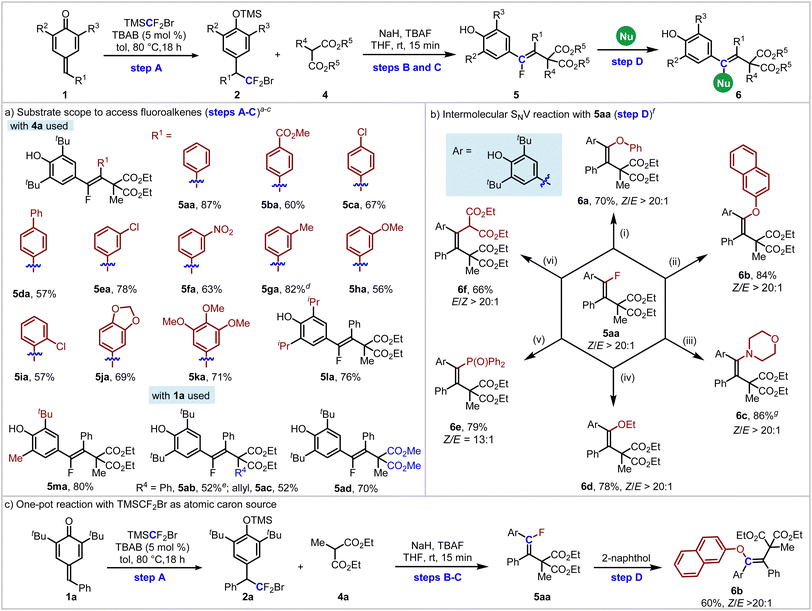
To probe our hypothesis, we initiated our studies with para-quinone methide 1a and diethyl 2-methylmalonate 4a as the model substrates to construct fluoroalkene 5aa. Substrate 1a was first treated with TBAB in the presence of TMSCF2Br in toluene at 80 °C for 18 h,17 followed by the addition of TBAF, 4a and tBuOK in THF. The tetra-substituted fluoroalkene 5aa was isolated in 42% yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z-selectivity (Table 1, entry 1). Screening of bases indicated that the base had a great impact on the yield (entries 2–5). While the yield of 5aa could be further promoted to 75% in the presence of NaH, only trace product was detected when PhCO2Na was used; no product was detected using K2CO3 or KHCO3. Subsequent solvent optimization (entries 6–10) failed to improve outcomes. The yield of 5aa could be further improved to 87% after reducing the loading of TBAF to 0.4 equivalent (entry 11). Further decreasing the equivalent of TBAF did not improve the yield of 5aa (entry 12).
1 Z-selectivity (Table 1, entry 1). Screening of bases indicated that the base had a great impact on the yield (entries 2–5). While the yield of 5aa could be further promoted to 75% in the presence of NaH, only trace product was detected when PhCO2Na was used; no product was detected using K2CO3 or KHCO3. Subsequent solvent optimization (entries 6–10) failed to improve outcomes. The yield of 5aa could be further improved to 87% after reducing the loading of TBAF to 0.4 equivalent (entry 11). Further decreasing the equivalent of TBAF did not improve the yield of 5aa (entry 12).
| Entry | Base | Solvent | Yield (%) | Z/E |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol, 1.0 equiv.), TMSCF2Br (2.0 equiv.), TBAB (5 mol%) and toluene (1.0 mL) in a sealed tube, 80 °C, 18 h, then 4a (1.0 mmol, 2.0 equiv.), TBAF (1.1 equiv.) and base (1.1 equiv.) in solvent (2.0 mL) was added. The reaction mixture was stirred at rt for 15 minutes.b Isolated yields.c The Z/E ratio was determined by 1H NMR.d 0.4 equiv. of TBAF was used.e 0.2 equiv. of TBAF was used. | ||||
| 1 | tBuOK | THF | 42 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | K2CO3 | THF | ND | — |
| 3 | KHCO3 | THF | ND | — |
| 4 | PhCO2Na | THF | Trace | — |
| 5 | NaH | THF | 75 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | NaH | MTBE | Trace | — |
| 7 | NaH | DMF | 38 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | NaH | MeCN | 36 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | NaH | Toluene | 33 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | NaH | DCM | ND | — |
| 11d | NaH | THF | 87 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12e | NaH | THF | 68 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
With the optimized conditions for the steps A-C established, we next evaluated the substrate scope of p-QMs and substituted malonates. As shown in Table 2a, p-QMs containing various electron-withdrawing or electron-donating groups at the para-, meta- or ortho-position of the phenyl ring are suitable substrates for this transformation, producing 5ba–5ia in 51–82% yields. Substrates bearing multiple substituents on the phenyl ring were also amenable to this reaction, and products 5ja and 5ka were isolated in 69% and 71% yields, respectively. When the tert-butyl groups were replaced with isopropyl groups, 5la was obtained in 76% yield. When unsymmetrical para-quinone methide 1m was used, the corresponding product 5ma was isolated in 80% yield. Diethyl 2-methylmalonate containing a phenyl or allyl group at the C2-position is also a suitable substrates for this transformation, delivering 5ab and 5ac in 52% yields. When dimethyl 2-methylmalonate was used, the product 5ad could be obtained in 70% yield. All transformations proceeded with exceptional stereocontrol (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z-selectivity), underscoring the robustness of this platform for constructing congested alkenes. To complete the C(sp2)-atomic transfer reaction, 5aa was further subjected to intermolecular functionalization with various nucleophiles, aiming to enrich the complexity and diversity of tetra-substituted alkenes (Table 2b, step D). Generally, in the presence of tBuOK, various O-, N-, P-, and C-nucleophiles could participate in intermolecular nucleophilic vinylic substitution (SNV) reactions18 under mild conditions, giving 6a–6e in 70–86% yields with 13–20:1 Z-selectivity and 6f in 66% yield with > 20
1 Z-selectivity), underscoring the robustness of this platform for constructing congested alkenes. To complete the C(sp2)-atomic transfer reaction, 5aa was further subjected to intermolecular functionalization with various nucleophiles, aiming to enrich the complexity and diversity of tetra-substituted alkenes (Table 2b, step D). Generally, in the presence of tBuOK, various O-, N-, P-, and C-nucleophiles could participate in intermolecular nucleophilic vinylic substitution (SNV) reactions18 under mild conditions, giving 6a–6e in 70–86% yields with 13–20:1 Z-selectivity and 6f in 66% yield with > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E-selectivity. In addition, as a proof of concept, this reaction was run in one pot, and the corresponding product 6b was isolated in 60% yield with > 20
1 E-selectivity. In addition, as a proof of concept, this reaction was run in one pot, and the corresponding product 6b was isolated in 60% yield with > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z-selectivity (Table 2c).
1 Z-selectivity (Table 2c).
| a 1 (0.5 mmol, 1.0 equiv.), TMSCF2Br (1.0 mmol, 2.0 equiv.), TBAB (5 mol%) and toluene (1.0 mL) in a sealed tube, 80 °C, 18 h, and then 4 (1.0 mmol, 2.0 equiv.), TBAF (0.4 equiv.) and NaH (3.0 equiv.) were added. The reaction mixture was stirred at rt for 15 minutes.b Isolated yields.c The Z/E ratio was determined by 1H NMR.d The reaction time for steps B and C was further prolonged to 30 min.e NaH (5.0 equiv.) was used.f Nucleophiles (0.4 mmol, 2.0 equiv.), 5aa (0.2 mmol, 1.0 equiv.) and tBu (4.0 equiv.) in DCM were stirred at rt to 60 °C for 12 h.g K3PO4 (0.4 mmol, 2.0 equiv.) and DMF were used. |
|---|
 |
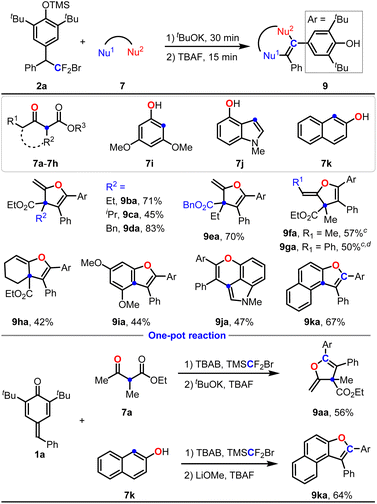
Encouraged by the above-mentioned single-carbon-atom transfer to p-QMs from TMSCF2Br with two different nucleophiles via stepwise functionalization, we next turned our attention to bisnucleophiles to examine the ability of this single-carbon-atom transfer reaction to construct cyclic compounds. First, ethyl 2-methyl-3-oxobutanoate 7a was tested. It's noteworthy that 7a contains three potential nucleophilic sites, and the chemoselectivity for the intramolecular SNV reaction of 8aa is another challenge. Under our previous standard conditions, the intramolecular O-substituted SNV product 9aa was successfully obtained in 17% yield, accompanied by the formation of 8aa in 47% yield, while the intramolecular C-substituted SNV product 9aa′ was not detected (Scheme 2(1)). At this stage, initiating condition screening with compound 2a as the precursor presented an enhanced efficiency profile for subsequent development. To our delight, the yield of 8aa could be further improved to 79% with tBuOK as the base (Scheme 2(2), see the ESI† for details of the condition screening).
Dihydrofuran skeletons and derivatives are important skeletons widely found in natural products and bioactive molecules (Scheme 2(3)).19 For example, Tunicamycin V is an N-glycosylation inhibitor, and has been used as a pharmacological inducer of endoplasmic reticulum (ER) stress.19f Acortatarins A and B are two spirocyclic alkaloids that significantly inhibit reactive oxygen species production in high-glucose-stimulated mesangial cells.19g In this context, we further explored the substrate scope of p-QMs for the construction of 2-methylene-2,3-dihydrofurans via single-carbon-atom transfer reactions. As shown in Table 3, substrates 2 containing various electron-donating groups and electron-withdrawing groups at the ortho-, meta- and para-positions are all compatible with this reaction, delivering products 9ab–9am in 46–82% yields. When the R1 group was a heterocycle such as indole and thiophene, the corresponding products 9an and 9ao were obtained in moderate yields. Cyclopropyl (R1) substituted p-QM could also deliver 9ap in 42% yield accompanied by some unknown byproducts detected. Other alkyl groups such as cyclohexyl, phenylethyl could not give the corresponding products (not shown in Table 3). The unsymmetric p-QM derived adduct 2q generated 9aq smoothly in 64% yield. When the reaction was run on a 4 mmol scale, the product 9aa could still be isolated in 75% yield (1.34 g) without loss of efficiency.
Next, the scope of substituted acetoacetic esters was examined. As shown in Table 4, acetoacetic esters containing various alkyl groups at the C2-position were all compatible with this reaction, giving products 9ba–9da in 45–83% yields. Notably, the generation of some uncertain byproducts led to a moderate yield for product 9ca while the conversion of 2a was complete. Switching the R3 group from an ethyl group to a benzyl group could still deliver product 9ea in 70% yield. Intriguingly, introducing an alkyl or aryl group at the α-position of the ketone (R1 = Me or Ph) could also afford the cyclic products 9fa and 9ga in moderate yields with excellent Z-selectivities. Ethyl 2-oxocyclohexane-1-carboxylate 7h could also deliver the corresponding product 7ha in 42% yield. Next, electron-rich phenolic substrates 7i–7k were tested, generating 9ia–9ka in 44–67% yields. Again, a one pot reaction was performed to probe the efficacy of this single-carbon-atom transfer reaction, giving 9aa in 56% yield and 9ka in 64% yield.
To demonstrate the synthetic utility of this single-carbon-atom transfer reaction, a series of transformations of the products were conducted. As shown in Scheme 3, Pd/C-catalyzed hydrogenation of 2-methylene-2,3-dihydrofuran 9aa proceeded smoothly, and the product 10 containing four contiguous chiral centres was isolated in 99% yield with > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. The relative configuration of 10 was determined by X-ray analysis. In the presence of N-iodosuccinimide (NIS), the terminal alkene 9aa was iodinated to afford 11 in 66% yield with > 20
1 dr. The relative configuration of 10 was determined by X-ray analysis. In the presence of N-iodosuccinimide (NIS), the terminal alkene 9aa was iodinated to afford 11 in 66% yield with > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z-selectivity. The vinyl iodide motif of compound 11 provided a handle for further functionalization, enriching the structural diversity of 2-methylene-2,3-dihydrofurans. For example, vinyl iodide could participate to a series of palladium-catalyzed stereoretentive cross-coupling reactions, including Heck, Sonogashira and Suzuki reaction, generating products 12–14 in 73–76% yields. The tert-butyl groups of 9ka were removed via AlCl3-catalyzed retro-Friedel–Crafts alkylation, yielding product 15 in 77% yield.
1 Z-selectivity. The vinyl iodide motif of compound 11 provided a handle for further functionalization, enriching the structural diversity of 2-methylene-2,3-dihydrofurans. For example, vinyl iodide could participate to a series of palladium-catalyzed stereoretentive cross-coupling reactions, including Heck, Sonogashira and Suzuki reaction, generating products 12–14 in 73–76% yields. The tert-butyl groups of 9ka were removed via AlCl3-catalyzed retro-Friedel–Crafts alkylation, yielding product 15 in 77% yield.
To gain insight into the reaction mechanism, a control experiment was conducted. As shown in Scheme 4(a), vinyl fluoride 8aa could be converted to 9aa smoothly in 96% yield in the presence of KOtBu, while no cyclic product 17 could be detected when compound 16 was used. Collectively, these results suggest that 8aa serves as the intermediate for the cyclic process and the hydroxyl group is essential for the intramolecular SNV reaction. Combined with previous studies,16g,17 a plausible mechanism for the formation of 2-methylene-2,3-dihydrofuran 9aa was proposed as shown in Scheme 4(b). In the presence of TBAF, 2a could be transformed to spiro INT-I via sequential desilylation/cyclization. Then the enolate II generated from 7a and basic KOtBu underwent regioselective nucleophilic substitution with INT-I to give INT-III. Dearomatization of INT-III via the elimination of fluoride and base promoted enolization generated INT-IV, which then underwent intramolecular 1,6-addition to yield INT-V. Phenolic anion or enol ether assisted elimination of the fluoride anion from INT-V would give INT-VI-1(2), and then proton transfer (P.T.) would produce the product 9aa. To probe the origin of the regioselectivity for the ring-opening step of INT-I, a computational study was conducted. As shown in Scheme 4(c), the computational study of INT-I showed that the C2–C3 bond possesses the smallest bond order in the cyclopropane unit.20 In other words, the C2–C3 bond is the weakest bond in the cyclopropane unit.
Conclusions
In summary, we have developed a single-carbon atom transfer reaction of the alkene motifs of p-QMs. In the presence of a nucleophile such as malonic acid ester, tetra-substituted fluoroalkenes were constructed with excellent stereoselectivities. The fluoroalkenes could further react with another nucleophile via formal SNV reaction to generate highly congested tetra-substituted olefins with good to excellent stereoselectivities. When 1,3-bisnucleophiles were used, cyclic products such as 2-methylene-2,3-dihydrofurans were obtained with excellent chemo- and stereo-selectivities. Sequentially controllable functionalization of TMSCF2Br enables it to act as an atomic carbon equivalent. More importantly, this reaction features with mild conditions, a broad substrate scope and the ability to enable diversity-directed synthesis. Further applications of TMSCF2Br as an atomic carbon source are currently ongoing in our laboratory.Data availability
The data supporting this article have been included as part of the ESI,† including detailed experimental procedures and characterization data for new compounds. Crystallographic data have been deposited with the CCDC with deposition numbers 2356255 (10).Author contributions
H. Y. and S. G. directed the project and revised the manuscript. R. S. and P. Z. performed the experiments, and analysed and interpreted the results; they contributed equally to this work. Y. Y., J. Z., Q. C. and C. Y. were involved in the preparation of the substrates. X. L. performed the computational studies. C. Y. and A. L. revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge generous financial support from the National Natural Science Foundation of China (NSFC22071267), the Project Program of the State Key Laboratory of Natural Medicines (SKLNMZZ202211), the Fundamental Research Funds for the Central Universities (2632024ZD09) and Natural Science Foundation of Fuyang Normal University (FYKFKT24004) and the Innovation and Entrepreneurship (Shuangchuang) Program of Jiangsu Province (2024).Notes and references
- For selected reviews, see: (a) P. Xu and A. Studer, Acc. Chem. Res., 2025, 58, 647 CrossRef CAS; (b) M. Peplow, Nature, 2023, 618, 21 CrossRef CAS PubMed; (c) Z. Liu, X. Zhang, P. Sivaguru and X. Bi, Acc. Chem. Res., 2025, 58, 130 CrossRef CAS PubMed; (d) E.-Q. Li, C. W. Lindsley, J. Chang and B. Yu, J. Med. Chem., 2024, 67, 13509 Search PubMed; (e) Y.-A. Xu, S.-H. Xiang, J.-T. Che, Y.-B. Wang and B. Tan, Chin. J. Chem., 2024, 42, 2656 Search PubMed; (f) Z. Liu, P. Sivaguru, Y. Ning, Y. Wu and X. Bi, Chem.–Eur. J., 2023, 29, e202301227 Search PubMed; (g) Z. Cheng, Z. Hu and N. Jiao, Acc. Chem. Res., 2025, 58, 1003 Search PubMed.
- For selected examples, see: (a) Z. Cheng, K. Huang, C. Wang, L. Chen, X. Li, Z. Hu, X. Shan, P.-F. Cao, H. Sun, W. Chen, C. Li, Z. Zhang, H. Tan, X. Jiang, G. Zhang, Z. Zhang, M. Lin, L. Wang, A. Zheng, C. Xia, T. Wang, S. Song, X. Shu and N. Jiao, Science, 2025, 387, 1083 CrossRef CAS; (b) B. Ghosh, P. Kafle, R. Mukherjee, R. Welles, D. Herndon, K. M. Nicholas, Y. Shao and I. Sharma, Science, 2025, 387, 102 CrossRef CAS PubMed; (c) X. Zhang, Q. Song, S. Liu, P. Sivaguru, Z. Liu, Y. Yang, Y. Ning, E. A. Anderson, G. de Ruiter and X. Bi, Nat. Chem., 2025, 17, 215 Search PubMed; (d) S. Liu, Y. Yang, Q. Song, Z. Liu, P. Sivaguru, Y. Zhang, G. de Ruiter, E. A. Anderson and X. Bi, Nat. Commun., 2024, 15, 9998 CrossRef CAS; (e) S. Liu, Y. Yang, Q. Song, Z. Liu, Y. Lu, Z. Wang, P. Sivaguru and X. Bi, Nat. Chem., 2024, 16, 988 CrossRef CAS; (f) Y. Yang, Q. Song, P. Sivaguru, Z. Liu, D. Shi, T. Tian, G. de Ruiter and X. Bi, Angew. Chem., Int. Ed., 2024, 63, e202401359 CrossRef CAS; (g) Z. Zhang, Q. Li, Z. Cheng, N. Jiao and C. Zhang, Nat. Commun., 2024, 15, 6016 CrossRef CAS PubMed; (h) H. Wang, H. Shao, A. Das, S. Dutta, H. T. Chan, C. Daniliuc, K. N. Houck and F. Glorius, Science, 2023, 381, 75 Search PubMed; (i) T. J. Pearson, R. Shimazumi, J. L. Driscoll, B. D. Dherange, D.-I. Park and M. D. Levin, Science, 2023, 381, 1474 CrossRef CAS; (j) J. C. Reisenbauer, O. Green, A. Franchino, P. Finkelstein and B. Morandi, Science, 2022, 377, 1104 CrossRef CAS; (k) H. Lyu, I. Kevlishvili, X. Yu, P. Liu and G. Dong, Science, 2021, 372, 175 Search PubMed; (l) J. Woo, A. H. Christian, S. A. Burgess, Y. Jiang, U. F. Mansoor and M. D. Levin, Science, 2022, 376, 527 CrossRef CAS PubMed; (m) S. H. Kennedy, B. D. Dherange, K. J. Berger and M. D. Levin, Nature, 2021, 593, 223 CrossRef CAS.
- For selected reviews: (a) M. Fedoryński, Chem. Rev., 2003, 103, 1099 CrossRef PubMed; (b) D. Habrant, V. Rauhala and A. M. P. Koskinen, Chem. Soc. Rev., 2010, 39, 2007 Search PubMed; (c) H. Fujimoto and M. Tobisu, Acc. Chem. Res., 2025, 58, 1168 Search PubMed , for selected examples: ; (d) F. Zhou, T.-D. Tan and M. J. Koh, Angew. Chem., Int. Ed., 2025, 64, e202505033 CrossRef CAS; (e) C. Mackay and R. Wolfgang, Science, 1965, 148, 899 CrossRef CAS PubMed; (f) B. M. Armstrong, F. Zheng and P. B. Shevlin, J. Am. Chem. Soc., 1998, 120, 6007 Search PubMed; (g) P. B. Shevlin, J. Am. Chem. Soc., 1972, 94, 1379 CrossRef CAS; (h) S. Kammula and P. B. Shevlin, J. Am. Chem. Soc., 1974, 96, 7830 CrossRef CAS.
- For seminal and recent examples, see: (a) J. C. Gilbert and U. Weerasooriya, J. Org. Chem., 1979, 44, 4997 Search PubMed; (b) E. J. Corey and P. L. Fuchs, Tetrahedron Lett., 1972, 13, 3769 Search PubMed; (c) W. von E. Doering and P. M. LaFlamme, Tetrahedron, 1958, 2, 75 CrossRef; (d) G. Liu, Z. Shi, C. Guo, D. Gu and Z. Wang, Angew. Chem., Int. Ed., 2025, 64, e202418746 CrossRef CAS PubMed.
- (a) M. Kamitani, B. Nakayasu, H. Fujimoto, K. Yasui, T. Kodama and M. Tobisu, Science, 2023, 379, 484 CrossRef CAS; (b) H. Fujimoto, B. Nakayasu and M. Tobisu, J. Am. Chem. Soc., 2023, 145, 19518 CrossRef CAS PubMed; (c) H. Fujimoto, T. Nishioka, K. Imachi, S. Ogawa, R. Nishimura and M. Tobisu, J. Am. Chem. Soc., 2025, 147, 8138 Search PubMed.
- (a) T. Koike, J.-K. Yu and M. M. Hansmann, Science, 2024, 385, 305 Search PubMed; (b) Y. Kutin, T. Koike, M. Drosou, A. Schnegg, D. A. Pantazis, M. Kasanmascheff and M. M. Hansmann, Angew. Chem., Int. Ed., 2025, e202424166 CAS; (c) Q. Sun, J.-N. Belting, J. Hauda, D. Tymann, P. W. Antoni, R. Goddard and M. M. Hansmann, Science, 2025, 387, 885 CrossRef CAS PubMed.
- F.-P. Wu, J. L. Tyler, C. G. Daniliuc and F. Glorius, ACS Catal., 2024, 14, 13343 CrossRef CAS.
- A. Puggioli, L. Jiang, A. G. Herraiz, L. J. Nannini, K. de la Vega-Hernández, A. Rey-Blanco, A. Diéguez-Vázquez, S. Cañellas and M. G. Suero, J. Am. Chem. Soc., 2025, 147, 11309 CrossRef CAS PubMed.
- (a) B. M. Armstrong, F. Zheng and P. B. Shevlin, J. Am. Chem. Soc., 1998, 120, 6007 CrossRef CAS; (b) W. Pan and P. B. Shevlin, J. Am. Chem. Soc., 1996, 118, 10004 CrossRef CAS; (c) P. B. Shevlin and S. Kammula, J. Am. Chem. Soc., 1977, 99, 2627 Search PubMed.
- N. Dellus, T. Kato, X. Bagán, N. Saffon-Merceron, V. Branchadell and A. Baceiredo, Angew. Chem., Int. Ed., 2010, 49, 6798 CrossRef CAS PubMed.
- For selected reviews, see: (a) Q. Xie and J. Hu, Acc. Chem. Res., 2024, 57, 693 Search PubMed; (b) A. D. Dilman and V. V. Levin, Acc. Chem. Res., 2018, 51, 1272 Search PubMed; (c) Z. Tu, J. Yu and J. Zhou, Chin. J. Org. Chem., 2023, 43, 3491 CrossRef CAS . For seminal and selected examples, see: ; (d) A. K. Yudin, G. K. S. Prakash, D. Deffieux, M. Bradley, R. Bau and G. A. Olah, J. Am. Chem. Soc., 1997, 119, 1572 CrossRef CAS; (e) L. Li, F. Wang, C. Ni and J. Hu, Angew. Chem., Int. Ed., 2013, 52, 12390 Search PubMed; (f) Q. Xie, Z. Zhu, C. Ni and J. Hu, Org. Lett., 2019, 21, 9138 CrossRef CAS; (g) R. Zhang, Z. Zhang, Q. Zhou, L. Yu and J. Wang, Angew. Chem., Int. Ed., 2019, 58, 5744 CrossRef CAS; (h) X. Wang, S. Pan, Q. Luo, Q. Wang, C. Ni and J. Hu, J. Am. Chem. Soc., 2022, 144, 12202 CrossRef CAS PubMed; (i) A. Liu, C. Ni, Q. Xie and J. Hu, Angew. Chem., Int. Ed., 2023, 62, e202217088 Search PubMed.
- For selected examples, see: (a) A. Liu, X. Zhang, F. Zhao, C. Ni and J. Hu, J. Am. Chem. Soc., 2024, 146, 1806 Search PubMed; (b) H. Sheng, J. Su, X. Li and Q. Song, CCS Chem., 2022, 4, 3820 CrossRef CAS; (c) J. D. Ellefsen and S. J. Miller, J. Org. Chem., 2022, 87, 10250 CrossRef CAS PubMed; (d) V. I. Supranovich, V. V. Levin, M. I. Struchkova, A. A. Korlyukov and A. D. Dilman, Org. Lett., 2017, 19, 3215 Search PubMed.
- (a) A. Liu, C. Ni, Q. Xie and J. Hu, Angew. Chem., Int. Ed., 2022, 61, e202115467 Search PubMed; (b) D. Ge, Z. Jia and X. Chu, Green. Synth. Catal., 2022, 3, 303 CrossRef.
- For selected reviews, see: (a) H. Wang, S. Yang, Y. Zhang and F. Shi, Chin. J. Org. Chem., 2023, 43, 974 Search PubMed; (b) J.-Y. Wang, W.-J. Hao, S.-J. Tu and B. Jiang, Org. Chem. Front., 2020, 7, 1743 Search PubMed; (c) A. Parra and M. Tortosa, ChemCatChem, 2015, 7, 1524 CrossRef CAS , for selected seminal and recent examples, see: ; (d) W.-D. Chu, L.-F. Zhang, X. Bao, X.-H. Zhao, C. Zeng, J.-Y. Du, G.-B. Zhang, F.-X. Wang, X.-Y. Ma and C.-A. Fan, Angew. Chem., Int. Ed., 2013, 52, 9229 CrossRef CAS; (e) L. Caruana, F. Kniep, T. K. Johansen, P. H. Poulsen and K. A. Jørgensen, J. Am. Chem. Soc., 2014, 136, 15929 Search PubMed; (f) X. Tan, Z. Deng, Q. Wang, S. Chen, G. Zhu and J. Sun, Nat. Synth., 2023, 2, 275 CrossRef CAS; (g) S. Liu, K. L. Chan, Z. Lin and J. Sun, J. Am. Chem. Soc., 2023, 145, 12802 CrossRef CAS PubMed; (h) S. Deswal, A. Guin and A. T. Biju, Angew. Chem., Int. Ed., 2024, 63, e202408610 CrossRef CAS.
- For selected reviews, see: (a) A. B. Flynn and W. W. Ogilvie, Chem. Rev., 2007, 107, 4698 CrossRef CAS; (b) B. E. Maryanoff and A. B. Reitz, Chem. Rev., 1989, 89, 863 CrossRef CAS.
- (a) X. Li, L. Kong, S. Yin, H. Zhou, A. Lin, H. Yao and S. Gao, Adv. Sci., 2024, 11, 2309706 CrossRef CAS PubMed; (b) Q. Wu, Q. Zhang, S. Yin, A. Lin, S. Gao and H. Yao, Angew. Chem., Int. Ed., 2023, 62, e202305518 CrossRef CAS PubMed; (c) M. Xu, Q. Lu, B. Gong, W. Ti, A. Lin, H. Yao and S. Gao, Angew. Chem., Int. Ed., 2023, 62, e202311540 CrossRef CAS PubMed; (d) Q. Li, X. Fang, R. Pan, H. Yao and A. Lin, J. Am. Chem. Soc., 2022, 144, 11364 CrossRef CAS PubMed; (e) D. Zhang, M. Li, J. Li, A. Lin and H. Yao, Nat. Commun., 2021, 12, 6627 CrossRef CAS; (f) K. Gai, X. Fang, X. Li, J. Xu, X. Wu, A. Lin and H. Yao, Chem. Commun., 2015, 51, 15831 RSC; (g) J. Zhu, M. Xu, B. Gong, A. Lin and S. Gao, Org. Lett., 2023, 25, 3271 CrossRef CAS.
- X. Song, J. Chang, D. Zhu, J. Li, C. Xu, Q. Liu and M. Wang, Org. Lett., 2015, 17, 1712 CrossRef CAS PubMed.
- For selected reviews, see: (a) C. F. Bernasconi and Z. Rappoport, Acc. Chem. Res., 2009, 42, 993 CrossRef CAS PubMed; (b) S. Chiba, K. Ando and K. Narasaka, Synlett, 2009, 16, 2549 Search PubMed; (c) Z. Rappoport, Acc. Chem. Res., 1981, 14, 7 CrossRef CAS , for selected examples, see:; (d) M. Chen, C. D. Knox, M. C. Madhusudhanan, T. H. Tugwell, C. Liu, P. Liu and G. Dong, Nature, 2024, 631, 18855 Search PubMed; (e) Y. Zong, Q. Ma and G. C. Tsui, Org. Lett., 2021, 23, 6169 CrossRef CAS.
- (a) J. B. J. Pavey, A. J. Lawrence, I. A. O'Neil, S. Vortler and R. Cosstick, Org. Biomol. Chem., 2004, 2, 869 Search PubMed; (b) T. P. Prakash, C. R. Allerson, P. Dande, T. A. Vickers, N. Siuofi, R. Jarres, B. F. Baker, E. E. Swayze, R. H. Griffey and B. Bhat, J. Med. Chem., 2005, 48, 4247 Search PubMed; (c) L. J. Baptiste, S. Yemets, R. Legay and T. Lequeux, J. Org. Chem., 2006, 71, 2352 Search PubMed; (d) A. Lorente, J. Lamariano-Merketegi, F. Albericio and M. Álvarez, Chem. Rev., 2013, 113, 4567 Search PubMed; (e) R. B. Teponno, S. Kusari and M. Spiteller, Nat. Prod. Rep., 2016, 33, 1044 RSC; (f) K. Yamamoto, A. Katsuyama and S. Ichikawa, Bioorg. Med. Chem., 2019, 27, 1714 Search PubMed; (g) X.-G. Tong, L.-L. Zhou, Y.-H. Wang, C. Xia, Y. Wang, M. Liang, F.-F. Hou and Y.-X. Cheng, Org. Lett., 2010, 12, 1844 CrossRef CAS.
- (a) G. N. Lewis, J. Am. Chem. Soc., 1916, 38, 762 CrossRef CAS; (b) T. A. Manz, RSC Adv., 2017, 7, 45552 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: CCDC 2356255. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc03234b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |