Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Suppressing O-type stacking and cation migration with Mg and Si doping in P2-type Fe–Mn layered oxides for sodium-ion cathodes†
Arup
Chakraborty
 a,
Robert A.
House
a,
Robert A.
House
 ab and
M. Saiful
Islam
ab and
M. Saiful
Islam
 *ab
*ab
aDepartment of Materials, University of Oxford, Parks Road, Oxford, OX1 3PH, UK. E-mail: Saiful.islam@materials.ox.ac.uk
bThe Faraday Institution, Harwell Campus, Didcot, OX11 0RA, UK
First published on 29th April 2025
Abstract
Layered oxide cathodes for Na-ion batteries containing Mn or Fe are of considerable interest for sustainable energy storage applications largely due to cost issues, relative abundance and high capacities. However, these layered cathodes such as NaxFe0.5Mn0.5O2 exhibit a structural phase transformation (from P2 to O2) at high charge states accompanied by irreversible cation migration into the Na layers and formation of trapped O2. Here we investigate whether doping into NaxFe0.5Mn0.5O2 can effectively suppress this phase transformation and cation migration, focusing on the atomic-scale effects of Mg2+ and Si4+ substitution, using ab initio simulation techniques. These dopants are contrasting species in terms of bonding character from divalent ionic (Mg) to tetravalent covalent (Si). Our study indicates that Mg-doping delayed Fe migration into the Na-layers in line with the experiment. In contrast, Si-doping stabilised the P2 phase over the entire charging range and suppresses Fe migration with no O–O dimer formation, suggesting that the Si-doped system should be a promising Na-ion cathode material.
1. Introduction
Na-ion batteries are attracting considerable attention for energy storage applications largely due to the cost advantages and relative abundance of sodium compared to lithium.1–6 From the several types of Na-ion cathode materials, layered oxide cathodes are of particular interest as they provide high gravimetric capacities.7–11The Fe–Mn-based material, NaxFe0.5Mn0.5O2, is a promising cathode because of the inexpensive elements and high discharge capacity of ∼200 mA h g−1.12–16 However, this material suffers from a structural phase transition at high voltage (≥4.1 V) linked with the participation of oxide ions in the redox process and capacity fading with cycling. Previous work12–14,16 investigated the structural properties of NaxFe0.5Mn0.5O2 including the P2–O2 phase transition at a high charge state accompanied by Fe migration to the O-type Na-ion layers and oxygen redox; the notations P2 and O2 are two different structures in which Na-ions occupy trigonal prismatic (P) and octahedral (O) sites with oxygen stacking of AB⋯BA and AB⋯CB, respectively.17 Cation substitution of some of the transition metal ions (for example, with Mg2+ or Ni2+) has been explored to improve capacity retention in NaxFe0.5Mn0.5O2.18–22 Recently, efforts have been made to stabilize the P2 phase through a combined approach of Na2SiO3 coating and Si4+ doping.23 Studies on related layered oxide cathodes have explored the effects of cation substitution, including Mg2+, Cu2+, Ti4+, and Sb5+ dopants.20,24–36
It is clear that a key objective is to stabilise the P2 structure of NaxFe0.5Mn0.5O2 throughout the entire charging process. Prismatic sites in the P-type Na layer are not favourable for transition metal ions to migrate into, so preserving the P2 structure should allow higher capacity and better capacity retention. However, there are limited studies on NaxFe0.5Mn0.5O2 that have addressed the P2/O2 phase stability range, and the mechanistic features of O-redox and cation migration at the atomic level, which we investigate here using advanced ab initio simulation techniques. The atomic-scale effects of partial Mg2+ and Si4+ substitution on the structural and redox behaviour of NaxFe0.5Mn0.5O2 are examined; these dopants in the transition metal layers were selected as they are contrasting species in terms of bonding character from divalent ionic (Mg) to tetravalent covalent (Si).
The results indicate that the P2 phase remains stable versus O2 up to a high charge state of x = 0.16 for Mg-doped Na0.67Mg0.11Fe0.22Mn0.67O2 and up to the top of charge (x = 0.0) for Si-doped Na0.67Si0.11Fe0.22Mn0.67O2. At the top of charge, there is cation migration to the Na-layers in both the parent oxide NaxFe0.5Mn0.5O2 and Mg-doped system, but Fe migration and O–O dimerization are not found in Si-doped Na0.67Si0.11Fe0.22Mn0.67O2.
2. Results and discussion
2.1. Structural stability of P2/O2 phases at various charge states
The starting point for our phase stability analysis was modelling the P2 structure of Na0.67Fe0.5Mn0.5O2 and Na0.67Mg0.11Fe0.22Mn0.67O2; comparison of the lattice parameters with the available experimental data16 showed good agreement to within 1.1% (Table 1). One of our main aims was to compare our simulation results with experimental measurements. We therefore used the same composition as in a previous study of Boivin et al.16 on the Mg-doped material in which Mg0.11 was considered. Numerous studies on Mg-doped NaxMO2 (M = Mg, Ni, Mn etc.) materials have shown that Mg typically occupies the transition metal layer.37,38 In addition, we have not found any experimental studies on Mg substitution at Na sites in Fe–Mn-based layered oxides that we focused on. Therefore, for direct comparison and consistency, we proceeded with the same concentration for the Si-doped material to allow direct comparison between Mg and Si.There are no experimental reports of the lattice parameters for the Si-doped material Na0.67Si0.11Fe0.22Mn0.67O2; we note that Na0.67Si0.11Fe0.22Mn0.67O2 likely prefers the P2 structure, according to a new concept of cationic potential from Zhao et al.39 (discussed in detail in ESI Note 1†). Nevertheless, Si doping in layered oxide cathodes for Na-ion and Li-ion batteries has been successfully explored.23,40,41 Therefore, while synthesizing Si-doped layered oxide materials may be possible, it remains challenging. In addition, the formation of all three materials was confirmed from analysis of the phase diagram42 (Fig. S1 and ESI Note 2†).
| Material | Method | a (Å) | c (Å) | α (°) | β (°) | γ (°) |
|---|---|---|---|---|---|---|
| Na0.67Fe0.5Mn0.5O2 | Calc. | 2.94 | 11.12 | 89.61 | 90.47 | 120.84 |
| Expt. | 2.94 | 11.20 | 90.00 | 90.00 | 120.00 | |
| Δ (%) | 0.00 | 0.70 | 0.43 | 0.52 | 0.70 | |
| Na0.67Mg0.11Fe0.22Mn0.67O2 | Calc. | 2.91 | 11.06 | 90.25 | 90.06 | 119.33 |
| Expt. | 2.93 | 11.18 | 90.00 | 90.00 | 120.00 | |
| Δ (%) | 0.68 | 1.07 | 0.27 | 0.00 | 0.55 | |
| Na0.67Si0.11Fe0.22Mn0.67O2 | Calc. | 2.92 | 11.04 | 89.98 | 89.54 | 119.85 |
As noted, a key objective is to stabilize the P2 structure throughout the entire charging process to allow higher capacity and better capacity retention. We therefore compared the thermodynamic stability of the primary P2 and O2 phases of NaxFe0.5Mn0.5O2, NaxMg0.11Fe0.22Mn0.67O2, and NaxSi0.11Fe0.22Mn0.67O2 (Fig. 1). We calculated the difference in energies, ΔE = E(O2) − E(P2), for four charge states (x = 0.67, 0.33, 0.16, 0.00) as illustrated in Fig. 1c. Here, E(P2) and E(O2) represent the lowest energies of the P2 and O2 structures, respectively, which were taken separately from analysis of more than hundred configurations and the calculated convex hulls (Fig. S2 in ESI†). This approach represents a possible design principle for new P2-type cathode materials in which high capacities can be achieved.
In Fig. 1c, the calculated ΔE between the P2 and O2 phases of undoped and doped NaxFe0.5Mn0.5O2 at different charge states indicate three main features. First, the P2 phase is stable only in the pristine state (x = 0.67) in agreement with experimental observation,16 whereas the O2 structures are stable at other charge states, namely x = 0.33, 0.16, and 0.0. It should be noted that we validated these results for P2/O2 stability of the parent oxide NaxFe0.5Mn0.5O2 with a range of different density functional theory (DFT) functionals and found the same trend in accord with experiment (Fig. S3 in ESI†). Second, our findings suggest that Mg-doping into NaxFe0.5Mn0.5O2 extends the P2 phase stability from pristine to x = 0.16. Third, Si-doping maintains the P2 structure throughout the entire charging process, from pristine to the top of charge (x = 0.67 to 0.0). This result suggests that Si doping would allow a wider charging region, resulting in a higher capacity. Regarding high-valent dopants, recent studies have found Sb5+ substitution in a Mn-rich layered oxide cathode suppresses the P2–O2 phase transformation in the high voltage condition.34 In the following analysis, we explore the underlying reasons for these stability trends.
2.2. Redox activity and oxidation states on charging
To probe redox activities on charging, we examined the oxidation states of the ions in NaxFe0.5Mn0.5O2, NaxMg0.11Fe0.22Mn0.67O2, and NaxSi0.11Fe0.22Mn0.67O2 (using local magnetic moments) as a function of Na content (x) from the pristine material to high charge states (≥0.33), which are shown in Fig. 2. In the pristine state (x = 0.67), the Mn-ions in all three materials are found in two oxidation states, Mn3+ and Mn4+, and the Fe-ions are consistently in the Fe3+ state. Upon charging to x = 0.33, all Mn3+ ions in NaxFe0.5Mn0.5O2 and NaxMg0.11Fe0.22Mn0.67O2 oxidised to Mn4+, while approximately 33% of the Fe3+ ions oxidise to Fe4+. Further charging from x = 0.33 to x = 0.0 leads to oxidation of the remaining Fe3+ to Fe4+. These findings on Mn and Fe oxidation states at different charge states align with previous experimental results. XANES studies show that the Mn K-edge shifts to higher energy during initial charging states, followed by the Fe K-edge shift at high charge states in both the undoped parent oxide and Mg-doped material.16In contrast, for NaxSi0.11Fe0.22Mn0.67O2, the oxidation of Mn3+ continues up to x = 0.16, followed by oxidation of Fe3+. This extended Mn oxidation at and beyond x = 0.33 is due to the presence of high valent Si, which reduces the average Mn oxidation state in the pristine system.
In addition, the simulations indicate O-redox in which localized magnetic moments of 0.1–0.3μB exist for certain O ions (O(2−n)−), which may be attributed to undercoordinated O-ions. From Fig. 2, there is an enhancement of O-redox at high charge states due to Mg doping. In contrast, there is a general decreasing trend of the number of O(2−n)− ions in both NaxFe0.5Mn0.5O2 and NaxSi0.11Fe0.22Mn0.67O2.
We also analysed the density of states at various charge states to examine localised states of O ions and to provide clues to the O-redox mechanisms in all three materials (shown in Fig. S4†). The density of states for NaxFe0.5Mn0.5O2 revealed localised O-p states near the Fermi energy at high charge states which become more pronounced at the top of charge, suggesting that O-ions might participate in the redox process. We found a similar pattern with greater intensity of the localized O-p states near the Fermi energy for NaxMg0.11Fe0.22Mn0.67O2. In contrast, only weakly localized O-p states appear at the top of charge for NaxSi0.11Fe0.22Mn0.67O2, which suggests that Si-doping delays or inhibits O-redox participation at high charge states.
2.3. Cation migration and O-dimerization
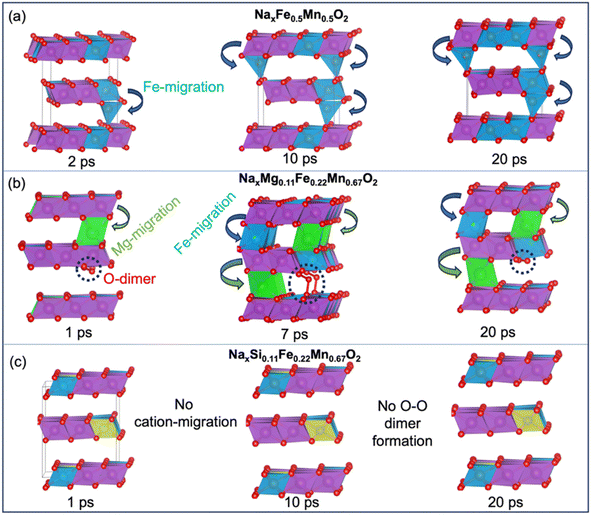
The nature of oxidised oxygen species and possible formation of O-dimers along with cation migration are critical to understanding the behaviour of these materials at high charge states. To capture kinetic behaviour, we performed ab initio molecular dynamics (AIMD) simulations at the top of charge (x = 0.0) for the O2 structures of NaxFe0.5Mn0.5O2, NaxMg0.11Fe0.22Mn0.67O2, and NaxSi0.11Fe0.22Mn0.67O2. Here, we considered the lowest energy structures from the previous structural calculations, which typically follow a ribbon-like pattern within the transition metal layers (Fig. S5 in ESI†).First, considering the parent oxide NaxFe0.5Mn0.5O2 (x = 0.0), the simulations indicate initial Fe ion migration to the Na-layers (Fig. 3a). The mean square displacement (MSD) analysis (Fig. 4) also suggests out-of-plane Fe migration into tetrahedral interstices within the O2-type stacked Na-layers. Boivin et al. similarly observed a reduction in the intensity of Fe ions in the transition metal layers in their Fe K-edge EXAFS spectra.16 Our results also indicate no out-of-plane Mn migration for all three compositions (Fig. 4b). The magnetic moments of the Fe-ions indicate that the migrated Fe ions have an oxidation state of Fe3+ which tends to prefer tetrahedral environments. Towards the end of the simulation run, the migrated Fe ions ultimately transitioned from tetrahedral to octahedral coordination (Fig. S6†). We confirmed that these kinetically accessible structures are thermodynamically stable by finding a decrease in total energy (Fig. S7a†) (which used a higher level DFT functional (r2SCAN)).
For the Mg-doped system, NaxMg0.11Fe0.22Mn0.67O2, the evolution of the structure along the AIMD trajectory (Fig. 3b) shows that Mg-ions start migrating in the early stages, followed by Fe migration into the Na layers, as shown in the MSD plots (Fig. 4). O-dimers start to form early on and persist throughout the rest of the simulation, with O–O bond distances ranged from 1.30 to 1.48 Å. It is found that the configurations involving Mg and Fe migration followed by O-dimer formation are thermodynamically stable (Fig. S7b†). Such Mg migration and O-redox were also observed from EXAFS and resonance inelastic X-ray spectroscopy (RIXS) measurements, respectively.16
The evolution of structures and MSDs for the Si-doped material NaxSi0.11Fe0.22Mn0.67O2 indicate that, in contrast to the Mg-doped system, there is no out-of-plane migration of either Fe, Mn or Si and no O-dimer formation (Fig. 3c and 4). This result may be related to the strong covalent Si–O bonds, which inhibit the formation of orphan O ions. Consequently, this reduces the likelihood of O-dimer formation or out-of-plane cation migration. Therefore, Si-doping is predicted to be beneficial in stabilizing the P2 structure at high charge states, suggesting that NaxSi0.11Fe0.22Mn0.67O2 would be a promising P2 cathode material. It is worth noting that in addition to the studies of the low energy ribbon structures, we investigated the effects of honeycomb superstructures (Fig. S8–S10†) and found very similar results on cation migration (detailed in ESI Note 5†).
To further analyse metal–oxygen bond strength (degree of covalency), we calculated the integrated crystal orbital bond index (ICOBI) and the integrated crystal orbital Hamiltonian population (ICOHP) of the metal–O bonds. The Si–O bonds are more robust than Mg–O bonds (Fig. S11†), which indicates that the Si–O bonds are covalent, while Mg–O bond are more ionic in nature. Consequently, these covalent bonds help to stabilise the P2 structure and inhibit cation migration in the Si-doped materials. A recent study showed a similar observation, where covalent Sb–O bonds stabilise the P2 structure in Na2/3Li1/4Sb1/12Mn2/3O2.43
Key objectives of our work are to provide design insights and atomistic understanding of Mg and Si doping in P2-type Fe–Mn layered oxides. Such insights are often difficult to obtain by experiment alone. Boivin et al. previously synthesized undoped and Mg-doped materials.16 Preparing Si-doped materials presents experimental challenges using conventional synthesis routes, although prior research has successfully explored Si doping in layered oxide cathodes for both Na-ion and Li-ion batteries.23,40,41
3. Conclusions
Our atomic-scale investigation suggests that the Si-doped material, NaxSi0.11Fe0.22Mn0.67O2, maintains the P2 structure even at the top of charge, which is an important goal for high capacity Na-ion cathodes. The parent oxide P2-type NaxFe0.5Mn0.5O2 transforms to an O2 structure at x = 0.33 in agreement with experimental observation, and Mg-doping extends the P2 phase stability to x = 0.16. Our kinetic study of NaxFe0.5Mn0.5O2 revealed Fe migration into tetrahedral interstices within the O-type stacked Na-layers, which is consistent with EXAFS experiments. In NaxMg0.11Fe0.22Mn0.67O2, O-dimers form when Mg ions migrate to the Na layers. In contrast, in NaxSi0.11Fe0.22Mn0.67O2, we find no migration of Fe, Mn or Si and no O–O dimer formation, indicating that the stronger Si–O covalency can enhance structural stability by strengthening O-binding in the transition metal layers. We recognise possible synthesis challenges for Si substitution, but this study suggests that the Si-doped cathode material should be a promising candidate for Na-ion batteries. Overall, these findings contribute to the broader effort of designing robust P2-type cathode materials.4. Methods
The DFT calculations were performed using the plane-wave-based Vienna Ab initio Simulation Package (VASP) in conjunction with the projector augmented wave (PAW) potential.44–46 We employed the generalized gradient approximation (GGA), Perdew–Burke–Ernzerhof (PBE) functional with Hubbard-U and Grimme's dispersion D3 correction.47 The effective Hubbard-U for Fe and Mn are 4 eV and 5 eV, respectively.48,49 We have also considered the higher-level meta-GGA functional, r2SCAN, to confirm our findings.50 An energy cut-off of 550 eV and a k-mesh of 3 × 3 × 2 were used for all static calculations. The AIMD simulations were also performed with VASP using the PBE + U + D3 functional. To speed up our AIMD simulations, we considered an energy cut-off of 400 eV and a single Γ-centered k-point.51 We noted that a larger supercell (4 × 3 × 1) and an enhanced k-mesh (2 × 2 × 2) also provides similar results (Fig. S12†). Before running the AIMD simulations, we fully optimized the geometries. We initially stabilised the system using NVE ensemble calculations and then selected NVT ensembles employing Nose–Hoover thermostat set at temperatures of T = 500 K and 900 K.Data availability
We have provided data in the main manuscript and the ESI.† Further any data is available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. C. acknowledges the Royal Society Newton International Fellowship grant (NIF\R1\211700) for funding. A. C. and M. S. I. thank the Faraday Institution CATMAT project (EP/S003053/1, FIRG016) for the Michael High-Performance Computing (HPC) facility (FIRG030). This work made use of the UK's National Supercomputer, ARCHER2, through the HEC Materials Chemistry Consortium (EP/R029431). R. A. H. acknowledges the Royal Academy of Engineering for support through the Research Fellowship scheme. We thank Daniel Kwok from Oxford Materials for fruitful discussions.References
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636–11682 CrossRef CAS.
- R. Usiskin, Y. Lu, J. Popovic, M. Law, P. Balaya, Y.-S. Hu and J. Maier, Nat. Rev. Mater., 2021, 6, 1020–1035 CrossRef CAS.
- N. Tapia-Ruiz, A. R. Armstrong, H. Alptekin, M. A. Amores, H. Au, J. Barker, R. Boston, W. R. Brant, J. M. Brittain, Y. Chen, M. Chhowalla, Y.-S. Choi, S. I. R. Costa, M. Crespo Ribadeneyra, S. A. Cussen, E. J. Cussen, W. I. F. David, A. V. Desai, S. A. M. Dickson, E. I. Eweka, J. D. Forero-Saboya, C. P. Grey, J. M. Griffin, P. Gross, X. Hua, J. T. S. Irvine, P. Johansson, M. O. Jones, M. Karlsmo, E. Kendrick, E. Kim, O. V. Kolosov, Z. Li, S. F. L. Mertens, R. Mogensen, L. Monconduit, R. E. Morris, A. J. Naylor, S. Nikman, C. A. O'Keefe, D. M. C. Ould, R. G. Palgrave, P. Poizot, A. Ponrouch, S. Renault, E. M. Reynolds, A. Rudola, R. Sayers, D. O. Scanlon, S. Sen, V. R. Seymour, B. Silván, M. T. Sougrati, L. Stievano, G. S. Stone, C. I. Thomas, M.-M. Titirici, J. Tong, T. J. Wood, D. S. Wright and R. Younesi, J. Phys.: Energy, 2021, 3, 031503 CAS.
- A. Rudola, A. J. R. Rennie, R. Heap, S. S. Meysami, A. Lowbridge, F. Mazzali, R. Sayers, C. J. Wright and J. Barker, J. Mater. Chem. A, 2021, 9, 8279–8302 RSC.
- D. Kundu, E. Talaie, V. Duffort and L. F. Nazar, Angew. Chem., Int. Ed., 2015, 54, 3431–3448 CrossRef CAS PubMed.
- E. Goikolea, V. Palomares, S. Wang, I. R. de Larramendi, X. Guo, G. Wang and T. Rojo, Adv. Energy Mater., 2020, 10, 2002055 CrossRef CAS.
- W. Zuo, A. Innocenti, M. Zarrabeitia, D. Bresser, Y. Yang and S. Passerini, Acc. Chem. Res., 2023, 56, 284–296 CrossRef CAS PubMed.
- C. Zhao, Z. Yao, Q. Wang, H. Li, J. Wang, M. Liu, S. Ganapathy, Y. Lu, J. Cabana, B. Li, X. Bai, A. Aspuru-Guzik, M. Wagemaker, L. Chen and Y.-S. Hu, J. Am. Chem. Soc., 2020, 142, 5742–5750 CrossRef CAS.
- J. Wang, Y.-F. Zhu, Y. Su, J.-X. Guo, S. Chen, H.-K. Liu, S.-X. Dou, S.-L. Chou and Y. Xiao, Chem. Soc. Rev., 2024, 53, 4230–4301 RSC.
- M. H. Han, E. Gonzalo, G. Singh and T. Rojo, Energy Environ. Sci., 2015, 8, 81–102 RSC.
- Z. Chen, Y. Deng, J. Kong, W. Fu, C. Liu, T. Jin and L. Jiao, Adv. Mater., 2024, 36, 2402008 CrossRef CAS.
- N. Yabuuchi, M. Kajiyama, J. Iwatate, H. Nishikawa, S. Hitomi, R. Okuyama, R. Usui, Y. Yamada and S. Komaba, Nat. Mater., 2012, 11, 512–517 CrossRef CAS PubMed.
- E. Talaie, V. Duffort, H. L. Smith, B. Fultz and L. F. Nazar, Energy Environ. Sci., 2015, 8, 2512–2523 RSC.
- B. Mortemard de Boisse, D. Carlier, M. Guignard, L. Bourgeois and C. Delmas, Inorg. Chem., 2014, 53, 11197–11205 CrossRef CAS PubMed.
- M. Kim, H. Kim, M. Cho and D. Kim, J. Mater. Chem. A, 2021, 9, 15179–15187 RSC.
- E. Boivin, R. A. House, J.-J. Marie and P. G. Bruce, Adv. Energy Mater., 2022, 12, 2200702 CrossRef CAS.
- C. Delmas, C. Fouassier and P. Hagenmuller, Physica B+C, 1980, 99, 81–85 CrossRef CAS.
- S. Xu, J. Wu, E. Hu, Q. Li, J. Zhang, Y. Wang, E. Stavitski, L. Jiang, X. Rong, X. Yu, W. Yang, X.-Q. Yang, L. Chen and Y.-S. Hu, J. Mater. Chem. A, 2018, 6, 20795–20803 RSC.
- J. W. Somerville, A. Sobkowiak, N. Tapia-Ruiz, J. Billaud, J. G. Lozano, R. A. House, L. C. Gallington, T. Ericsson, L. Häggström, M. R. Roberts, U. Maitra and P. G. Bruce, Energy Environ. Sci., 2019, 12, 2223–2232 RSC.
- J. W. Somerville, R. A. House, N. Tapia-Ruiz, A. Sobkowiak, S. Ramos, A. V. Chadwick, M. R. Roberts, U. Maitra and P. G. Bruce, J. Mater. Chem. A, 2018, 6, 5271–5275 RSC.
- Q. Shi, R. Qi, X. Feng, J. Wang, Y. Li, Z. Yao, X. Wang, Q. Li, X. Lu, J. Zhang and Y. Zhao, Nat. Commun., 2022, 13, 3205 CrossRef PubMed.
- F. Fu, X. Liu, X. Fu, H. Chen, L. Huang, J. Fan, J. Le, Q. Wang, W. Yang, Y. Ren, K. Amine, S.-G. Sun and G.-L. Xu, Nat. Commun., 2022, 13, 2826 CrossRef CAS PubMed.
- J. Jiao, K. Wu, R. Dang, N. Li, X. Deng, X. Liu, Z. Hu and X. Xiao, Electrochim. Acta, 2021, 384, 138362 CrossRef CAS.
- X. Zhang, W. Zuo, S. Liu, C. Zhao, Q. Li, Y. Gao, X. Liu, D. Xiao, I. Hwang, Y. Ren, C.-J. Sun, Z. Chen, B. Wang, Y. Feng, W. Yang, G.-L. Xu, K. Amine and H. Yu, Adv. Mater., 2024, 36, 2310659 CrossRef CAS PubMed.
- K. Zhang, Z. Xu, G. Li, R.-J. Luo, C. Ma, Y. Wang, Y.-N. Zhou and Y. Xia, Adv. Energy Mater., 2023, 13, 2302793 CrossRef CAS.
- G. Zhang, J. Li, Y. Fan, Y. Liu, P. Zhang, X. Shi, J. Ma, R. Zhang and Y. Huang, Energy Storage Mater., 2022, 51, 559–567 CrossRef.
- L. Yang, X. Li, J. Liu, S. Xiong, X. Ma, P. Liu, J. Bai, W. Xu, Y. Tang, Y.-Y. Hu, M. Liu and H. Chen, J. Am. Chem. Soc., 2019, 141, 6680–6689 CrossRef CAS PubMed.
- X. Wang, Q. Zhang, C. Zhao, H. Li, B. Zhang, G. Zeng, Y. Tang, Z. Huang, I. Hwang, H. Zhang, S. Zhou, Y. Qiu, Y. Xiao, J. Cabana, C.-J. Sun, K. Amine, Y. Sun, Q. Wang, G.-L. Xu, L. Gu, Y. Qiao and S.-G. Sun, Nat. Energy, 2024, 9, 184–196 CrossRef CAS.
- J.-k. Park, G.-g. Park, H. H. Kwak, S.-T. Hong and J.-w. Lee, ACS Omega, 2018, 3, 361–368 CrossRef CAS.
- Y. Niu, Z. Hu, B. Zhang, D. Xiao, H. Mao, L. Zhou, F. Ding, Y. Liu, Y. Yang, J. Xu, W. Yin, N. Zhang, Z. Li, X. Yu, H. Hu, Y. Lu, X. Rong, J. Li and Y.-S. Hu, Adv. Energy Mater., 2023, 13, 2300746 CrossRef CAS.
- Y. Niu, Z. Hu, H. Mao, L. Zhou, L. Wang, X. Lou, B. Zhang, D. Xiao, Y. Yang, F. Ding, X. Rong, J. Xu, W. Yin, N. Zhang, Z. Li, Y. Lu, B. Hu, J. Lu, J. Li and Y.-S. Hu, Energy Environ. Sci., 2024, 17, 7958–7968 RSC.
- L. Mu, S. Xu, Y. Li, Y.-S. Hu, H. Li, L. Chen and X. Huang, Adv. Mater., 2015, 27, 6928–6933 CrossRef CAS PubMed.
- Y. Liu, D. Wang, H. Li, P. Li, Y. Sun, Y. Liu, Y. Liu, B. Zhong, Z. Wu and X. Guo, J. Mater. Chem. A, 2022, 10, 3869–3888 RSC.
- S. Jamil, F. Mudasar, T. Yuan, M. Fasehullah, G. Ali, K. H. Chae, O. Voznyy, Y. Zhan and M. Xu, ACS Appl. Mater. Interfaces, 2024, 16, 14669–14679 CrossRef CAS PubMed.
- H. Hu, C.-W. Kao, C. Cheng, X. Xia, Y. Shen, X. Zhou, G. Liu, L. Wang, P. Zeng, J. Mao, T.-S. Chan and L. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 30332–30341 CrossRef CAS.
- Y.-J. Guo, P.-F. Wang, Y.-B. Niu, X.-D. Zhang, Q. Li, X. Yu, M. Fan, W.-P. Chen, Y. Yu, X. Liu, Q. Meng, S. Xin, Y.-X. Yin and Y.-G. Guo, Nat. Commun., 2021, 12, 5267 CrossRef CAS PubMed.
- G. Singh, N. Tapia-Ruiz, J. M. Lopez del Amo, U. Maitra, J. W. Somerville, A. R. Armstrong, J. Martinez de Ilarduya, T. Rojo and P. G. Bruce, Chem. Mater., 2016, 28, 5087–5094 CrossRef.
- Y. Li, K. A. Mazzio, N. Yaqoob, Y. Sun, A. I. Freytag, D. Wong, C. Schulz, V. Baran, A. S. J. Mendez, G. Schuck, M. Zając, P. Kaghazchi and P. Adelhelm, Adv. Mater., 2024, 36, 2309842 CrossRef CAS PubMed.
- C. Zhao, Q. Wang, Z. Yao, J. Wang, B. Sánchez-Lengeling, F. Ding, X. Qi, Y. Lu, X. Bai, B. Li, H. Li, A. Aspuru-Guzik, X. Huang, C. Delmas, M. Wagemaker, L. Chen and Y.-S. Hu, Science, 2020, 370, 708–711 CrossRef CAS.
- D.-H. Kim, J.-H. Song, C.-H. Jung, D. Eum, B. Kim, S.-H. Hong and K. Kang, Adv. Energy Mater., 2022, 12, 2200136 CrossRef CAS.
- D. Yuan, H. Zhai, Y. Shen, L. Wang, A. Chen and Y. Cheng, J. Energy Storage, 2024, 84, 110944 CrossRef.
- J. Buckeridge, D. O. Scanlon, A. Walsh and C. R. A. Catlow, Comput. Phys. Commun., 2014, 185, 330–338 CrossRef CAS.
- X. Cai, Z. Shadike, N. Wang, X.-L. Li, Y. Wang, Q. Zheng, Y. Zhang, W. Lin, L. Li, L. Chen, S. Shen, E. Hu, Y.-N. Zhou and J. Zhang, J. Am. Chem. Soc., 2025, 147, 5860–5870 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- D. A. Tompsett, S. C. Parker and M. S. Islam, J. Am. Chem. Soc., 2014, 136(4), 1418–1426 CrossRef CAS.
- S. Singh, A. Chakraborty, A. Neveu, P. K. Jha, V. Pralong, M. Fichtner, M. S. Islam and P. Barpanda, Chem. Mater., 2024, 36(16), 8088–8097 CAS.
- J. W. Furness, A. D. Kaplan, J. Ning, J. P. Perdew and J. Sun, J. Phys. Chem. Lett., 2020, 11(19), 8208–8215 CrossRef CAS PubMed.
- K. McColl, R. A. House, G. J. Rees, A. G. Squires, S. W. Coles, P. G. Bruce, B. J. Morgan and M. S. Islam, Nat. Commun., 2022, 13, 5275 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ta00804b |
| This journal is © The Royal Society of Chemistry 2025 |