Hiroaki Itoh and Masayuki Inoue
Org. Biomol. Chem., 2019,17, 6519-6527
DOI:
10.1039/C9OB01130G,
Review Article
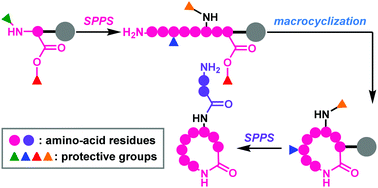
Fmoc-based solid-phase synthesis provides efficient access to both linear and macrocyclic peptides. To synthesize complex macrocyclic polyamides using Fmoc chemistry, multiple protective groups with orthogonal reactivities are generally employed because the free amines and carboxylic acids of specific residues must be selectively exposed prior to amide formation. This review focuses on four-dimensionally orthogonal protective group strategies for the full solid-phase synthesis of macrocyclic peptides with branched chains (polymyxin E2 and daptomycin) and a tricyclic natural peptide (lacticin 481).