Diksha Rai and Suvarn S. Kulkarni
Org. Biomol. Chem., 2020,18, 3216-3228
DOI:
10.1039/D0OB00297F,
Review Article
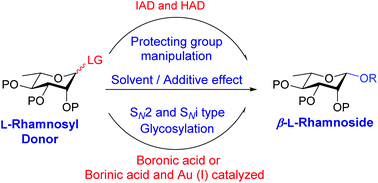
L-Rhamnose forms the key components of important antigenic oligo- and polysaccharides of a variety of pathogens. Obtaining 1,2-cis stereoselectivity in the glycosylation of L-rhamnoside is quite challenging due to the unavailability of neighboring group participation and disfavoring of the anomeric effect and stereoelectronic effect of the substituents on the C-2 axial position. Nevertheless, various methodologies have been developed exploiting diverse pathways for obtaining β-stereoselectivity in the glycosylation of L-rhamnose. This review describes the recent advances in β-L-rhamnosylation and its applications in the total synthesis of β-L-rhamnose-containing biologically important oligosaccharides.