Victoria Dimakos and Mark S. Taylor
Org. Biomol. Chem., 2021,19, 514-524
DOI:
10.1039/D0OB02009E,
Review Article
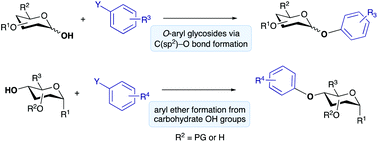
Methods for the O-arylation of hydroxyl and hemiacetal groups in carbohydrates via C(sp2)–O bond formation are discussed. Such methods provide an alternative disconnection to the traditional approach of nucleophilic substitution between a sugar-derived electrophile and a phenol or phenoxide nucleophile. They have led to new opportunities for stereoselectivity, site-selectivity and chemoselectivity in the preparation of O-aryl glycosides and carbohydrate-derived aryl ethers, compounds that are useful for a broad range of applications in medicinal chemistry, glycobiology and organic synthesis.