A pyridyl carboxamide molecule selectively stabilizes DNA G-quadruplex and regulates duplex–quadruplex competition†
Liang
Xu
a,
Weixin
Wu
a,
Jie
Ding
a,
Shuo
Feng
a,
Xiwen
Xing
a,
Minggang
Deng
a and
Xiang
Zhou
*ab
aCollege of Chemistry and Molecular Sciences, Key Laboratory of Biomedical Polymers of Ministry of Education, State Key Laboratory of Virology, Wuhan University, Hubei, Wuhan, 430072, P. R. of China. E-mail: xzhou@whu.edu.cn; Fax: 86-27-68756663
bState Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Shanghai, China
First published on 30th November 2011
Abstract
G-Quadruplexes formed by G-rich DNA are of broad interest due to their involvement in telomere function, gene transcription and recombination. Small ligands that interact strongly with G-quadruplexes have been considered to further influence telomeric function and gene transcription. Because most G-rich sequences are trapped in duplex structures in gene promoters, ligands that can stabilize G-quadruplexes in the presence of their complimentary strands would likely have strong effects on gene transcription. Here, we report a novel simple small molecule (pyridyl carboxamide), consisting of three pyridine rings and four amide bonds. Comparing with some reported G-quadruplex ligands, this molecule not only selectively stabilizes G-quadruplexes rather than duplexes, but also maintains a G-quadruplex structure even if the G-rich region was trapped in long double-stranded DNA (dsDNA). It is widely believed that the dissociation of duplexes is involved in gene transcription and that the formation of the G-quadruplex influences some oncogene expression. Py-Am exhibited strong G-quadruplex-forming ability within a long dsDNA sequence, suggesting it would have potent effects on the G-quadruplex-forming sequences involved in gene transcription.
Introduction
Growing evidence suggests that G-quadruplexes formed by guanine-rich DNA sequences may play an important biological rolein vivo.1,2 G-rich DNA sequences have been identified extensively in telomeric and some non-telomeric genomic DNA sequences and form G-quadruplex structures under pseudo-physiological conditions.3–8G-quadruplexes are involved in numerous cellular processes, including telomere function,2,4,5transcription6–8 and recombination,9,10 and have been the subject of increasing attention due to their potential as a therapeutic target for cancer.2,11–13 Besides human telomeric DNA, G-quadruplexes are also prevalent in non-telomeric portions of the genome,6–8 particularly in gene promoters, where quadruplex formation must be accordant with duplex dissociation. However, the ability of duplex DNA to form quadruplexes regardless of its complementary sequence in vivo is still unclear. Understanding the mechanism of the duplex–quadruplex competition is fundamental for regulating gene transcriptionin vivo and designing drugs for anticancer therapies.14–16Small molecules that interact strongly with G-quadruplexes to further influence telomeric function and gene transcription have been widely reported.14–21 Because most G-rich sequences are trapped in duplex structures in gene promoters, ligands that can stabilize G-quadruplexes in despite of their complementary strands would likely have strong effects on gene transcription. However, most reported quadruplex ligands induce the formation of a G-quadruplex from a single-stranded sequence and stabilize it;17–21 few ligands have been observed to stabilize G-quadruplexes in the presence of their complementary strands. To our own knowledge, several pathways were reported for regulating DNA duplex–quadruplex competition up to now.22–26 It was reported recently that low pH would benefit the duplex-to-quadruplex equilibrium of the DNA sequence of the c-kit transcription initiation site.23Lead ions were found to have ability to drive the duplex to form a G-quadruplex, but this duplex was just partially matched with a G-rich sequence, as fully matched duplex would not be influenced by lead ions.24 Besides, PNAs were also utilized to bind to the complementary C-rich strand in order to stabilize the G-quadruplex in the long dsDNA in the BCL2 promoter region.25 Recently, the abilities of several small ligands were evaluated for duplex–quadruplex competition, but the G-rich region of the selected duplex DNA was just partially matched with the other single strand.26 Actually, a small molecule that could selectively stabilize G-quadruplex regardless of its complementary strand within a long well matched duplex by its own ability was still void but significant.
Here, we reported a simple small molecule composed of pyridine and amide (Py-Am, Scheme 1). Pyridine and amide groups had been utilized in some previous reported G-quadruplex ligands.27–28 But unlike other generally reported G-quadruplex ligands, this molecule has none condensed aromatics. Besides G-quadruplex stabilization, we found that this pyridyl carboxamide molecule exhibited some other novel properties, that it could regulate duplex–quadruplex competition. This molecule could selectively maintain and stabilize G-quadruplex regardless of its complementary strand even if the G-rich region was trapped in long double-stranded DNA, implying its potential effects for the G-quadruplex forming sequences involved in gene transcription.
 | ||
| Scheme 1 Structures of Py-Am, TMPyP4 and 307A. | ||
Materials and methods
Materials
Human telemeric DNA, G4A (AG3(T2AG3)3) and 21G (G3(T2AG3)3), and its complementary oligonucleotide 21G-c (C3(TA2C3)3), c-kit (GGGCGGGCGCGAGGGAGGGG) and its complementary oligonucleotide, Pu27 (TGGGGAGGGTGGGGAGGGTGGGGAAGG) and its complementary oligonucleotide, and other non-labeled DNA were all purchased from the Invitrogen company. FAM-21G (5′-FAM-GGGTTAGGGTTAGGGTTAGGG-3′), F21T (5′-FAM-GGGTTAGGGTTAGGGTTAGGG-TMR-3′), FAM-c-kit (5′-FAM- GGGCGGGCGCGAGGGAGGGG-3′), F-c-kit-T (5′-FAM- GGGCGGGCGCGAGGGAGGGG-TMR-3′) FAM-Pu27 (5′-FAM-TGGGGAGGGTGGGGAGGGTGGGGAAGG-3′), the c-myc long DNA with FAM labeled FAM-c-myc (5′-FAM-CCCGGGAGGGGCGCTTATGGGGAGGGTGGGGAGGGTGGGGAAGGTGGGGAGGAGACTCA-3′) and its complementary sequence with TAMRA labeled TAMRA-c-myc-c (5′-TAMRA-TGAGTCTCCTCCCCACCTTCCCCACCCTCCCCACCCTCCCCATAAGCGCCCCTCCCGGG -3′) and the c-myc long DNA-mu (5′-FAM-CCCGGGAGGGGCGCTTATGGCGAGGCTGGCGAGGCTGGCGAAGGTGGGGAGGAGACTCA-3′), where FAM is carboxyfluorescein, and both TMR and TAMRA mean tetramethyl-6-carboxyrhodamine, were all purchased from TaKaRa Clontech. TE buffer: Tris–HCl 10 mM, EDTA 1 mM, pH = 7.4, Li-phosphate buffer: LiOH reacted with H3PO4 (pH = 7.0). TMPyP4 was purchased from Aldrich. 307A was synthesized according to the reported method.27,29Preparation of Py-Am
The target molecule Py-Am was prepared from the initial agents, 2,6-diamminopyridine and 2,6-dicarboxylpyridine. Synthetic details can be found in the ESI.†Py-Am were stored in DMSO solution with a concentration of 10 mM before measurements. All the other concentrations of Py-Am were diluted from this stock solution.Circular dichroism experiments
Circular dichroism (CD) experiments were carried out with a Chariscan circular dichroism photomultiplier (Applied Photophsics Limited. UK) equipped with a Quantum Nothwest TC125 temperature controller. All the CD spectra were measured from 220 nm to 320 nm in a 0.1 cm or 1 cm path-length cuvette with a scanning speed of 200 nm min−1, the 3 nm bandwidth and a 2 s response time. Thermal denaturation was performed as described below. After reaching the designed temperature, the sample would maintain this temperature for 2 min in order to totally achieve thermoequilibrium before each scan.Fluorescence experiments
The fluorescence spectra were collected with a Perkin Elmer LS55 Fluorescence spectrometer. Samples were excited at 480 nm for FAM, and emission spectra were collected from 500–650 nm.FRET melting assay
A FRET melting assay is performed with a real-time PCR machine (Rotor-Gene 2000, Corbett, Australia), using a total reaction volume of 20 μL, with 0.5 μM of labelled oligonucleotide. Each sample was boiled at 95 °C for 5 min first and then cooled down to room temperature to form G-quadruplex structures. After a first equilibration step at 35 °C during 5 min, a stepwise increase of 1 °C every minute for 61 cycles to reach 95 °C was performed and the fluorescence measurements of FAM were made after each “cycle” with excitation. Final analysis of the data was carried out using Excel and GraphPad software. Emission of FAM was normalized between 0 and 1, and Tm was defined as the temperature for which the normalized emission is 0.5.SPR experiments
The measurements were carried out by BIAcore 3000 optical biosensor system (BIAcore AB, Uppsala, Sweden) using a CM5 sensor chip covered streptavidin by an Amine Coupling Kit. Experimental procedures were described in the reported papers.30Native gel electrophoresis
1 μM FAM-labeled and 1.5 μM unlabelled complementary sequence were mixed together to form G-rich duplex in the solution containing 10 mM Tris-HCl (pH = 7.4), 100 mM KCl and 1 mM EDTA. Compounds were then added into the mixture to achieve indicated concentrations. The total volume of each sample was 10 μL. Each sample was treated with heat denaturation and renaturation before loaded onto gel. Native gel electrophoresis was run on 20% or 12% polyacrylamide gel containing and 100 mM KCl at 4 °C, 5 V cm−1 in 1 ×TBE buffer containing 100 mM KCl. Gels with FAM-labeled oligonucleotides were photographed under irradiation of UV light (Vilber Lourmat, Bio-Print, VL).S1 nuclease experiment
In the S1 nuclease experiment, 0.5 μM TAMRA-c-myc-c and 0.75 μM unlabelled c-myc were mixed together in a solution containing 10 mM Tris–HCl (pH = 7.4), 50 mM KCl with indicated concentrations of compounds. The total volume of each sample was 10 μL. Each sample was treated with heat denaturation and renaturation before addition of 50U S1 nuclease. The mixture was maintained at 37 °C for 0.5 h before inactivation of enzyme at 70 °C for 10 min in the presence of EDTA, and then analyzed by 20% denaturing PAGE. The gel was photographed with a Pharos FX Molecular imager.Dimethyl sulfate (DMS) footprinting
20 picomoles of 5′-FAM-labeled c-myc or c-myc-mu long DNA was prepared in 10 mM Tris–HCl pH 7.5, 100 mM KCl, with 22 picomoles of its complementary oligonucleotide in the absence of Py–Am or presence of Py–Am (2 nanomoles). Samples treated with heat at 95 °C for 5 min, and then slowly cooled down to r.t. Dimethyl sulfate was then added to a final concentration of 2%, and the reaction mix was incubated at room temperature for 10 min at which point the reaction was stopped with addition of stop buffer (2 M β-mercaptoethanol, 300 mM sodium acetate, 250 μg mL−1 sheared salmon sperm DNA). Three volumes of 100% ethanol were added immediately, and the DNA was precipitated at −20 °C overnight. The modified DNA was treated with 10% (v/v) piperidine in water and then heated at 90 °C for 30 min, followed by ethanol precipitation. The precipitated DNA was dissolved in 50% (v/v) deionized formamide in water, denatured at 95 °C for 5 min and resolved on a denaturing 20% polyacrylamide gel.Results and discussion
Binding ability of Py-Am with G-quadruplex DNA
This pyridyl carboxamide consists of three pyridine rings and four amide bonds (Scheme 1), prepared according to the method described in ESI.† Like other DNA G-quadruplex ligands, CD inducing and fluorescence resonance energy transfer (FRET) experiments indicated that the human telomeric DNA formed a G-quadruplex structure in the absence of cations (ESI,† Fig. S1). More importantly, Py-Am significantly stabilized the G-quadruplex structures (Fig. 1b and 1c). Three G-rich sequences, i.e., the G-rich portions of the c-kit and c-myc oncogene promoters (Pu27 [5′-TG4AG3TG4AG3TG4AAGG-3′] and c-kit [5′-G3CG3CGCGAG3AG4-3′]) and the human telomeric sequence [5′-AG3(T2AG3)3-3′], were selected for FRET and CD melting experiments. In the presence of Py-Am, these G-quadruplexes could not be completely unfolded at temperatures below 95 °C in 5 mM K+ solution (Fig. 1a), demonstrating extremely high thermodynamic stability. The differences in the melting temperatures (ΔTm) of these quadruplexes before and after treatment with Py-Am were significant (Fig. 1a and 1b) and comparable to other reported quadruplex ligands.17–21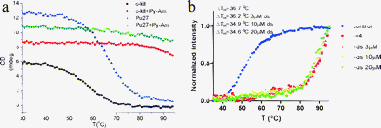 | ||
| Fig. 1 Binding abilities of Py-Am with G-quadruplex DNA. (a) CD melting curves of Pu27 and c-kit (both 10 μM in 10 mM Tris–HCl buffer (pH = 7.4), 5 mM KCl) in the absence and presence of 40 μM Py-Am. (b) FRET melting experiments of human telomeric DNA carried out with F21T (5 mM Li–phosphate buffer (pH 7.0), 10 mM KCl) with Py-Am in the absence and presence of ds20. r means the ratio of Py-Am and DNA. | ||
Because Py-Am efficiently stabilizes G-quadruplexes, we next evaluated the selectivity of Py-Am for quadruplex and duplex structures. FRET melting experiments were performed in the presence of various amounts of ds20 (20 bp duplex) as a duplex-DNA competitor to explore the selectivity between quadruplexes and duplexes (Fig. 1b). F21T (5′-FAM-G3[T2AG3]3-TAMRA-3′) was used as the G-rich sequence. Py-Am appears to be highly selective because F21T was highly stabilized even in the presence of 50 μm ds20. Because no platform was observed at the high temperature, the normalized Tm values were likely to be lower than the actual values. The binding constants for Py-Am binding to G-quadruplex DNA were determined by SPR (Fig. S2† and Table 1). The dissociation constants of Py-Am and the G-quadruplexes were all in the nanomolar range and much smaller than for the duplex structure, indicating the presence of strong interactions between Py-Am and the G-quadruplexes and high selectivity between the G-quadruplexes and duplexes.
Regulation of duplex–quadruplex competition in short dsDNA
With the exception of the G-rich single-stranded DNA of the telomere overhang sequence, most G-rich sequences are involved in duplexes, including the duplex region of telomere DNA and some non-telomeric DNA. As a good G-quadruplex binding ligand, Py-Am was further considered to figure out that whether it would stabilize G-quadruplexes in the presence of their complementary strands as most G-rich sequences are involved in duplexes. We mainly investigated G-rich duplexes from oncogene promoters (c-myc and c-kit), and human telomere duplex was also explored in this study. We firstly explored whether Py-Am could interact with the short G-rich duplexes to obtain G-quadruplexes. In order to investigate the equilibrium states of duplex–quadruplex competition in the presence and absence of Py-Am, all the samples for studies were treated with heat denaturation and renaturation.To demonstrate the formation of G-quadruplexes despite their complementary strands in the presence of Py-Am, native PAGE was performed to observe the G-quadruplexes on gels. Only duplex structures were observed on gels in the absence of Py-Am, while faster migrating bands were emerging when Py-Am was added into the system (Fig. 2). When the concentration of Py-Am reached 20 μM, the duplex bands disappeared almost completely. The new bands that migrated faster than the duplex bands were accordant with the band for the G-quadruplex except the human telomeric sequence shown in Fig. 2c, demonstrated the formation of the G-quadruplex. Interestingly for human telomeric DNA, new bands appeared that migrated faster than the duplex bands but slower than the G-quadruplex band without Py-Am and were just accordant with the band for the G-quadruplex in the presence of Py-Am (Fig. 2c). Most probably, Py-Am can interact with the human telomeric G-quadruplex to form a ligand–DNA complex.
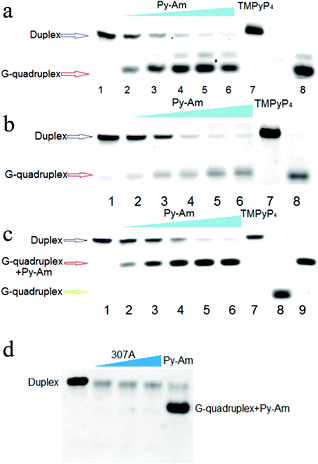 | ||
| Fig. 2 Gel analysis of duplex-quadruplex competition regulated by Py-Am. (a–c) shows the native PAGE analysis of the G-quadruplex formation. (a) is the FAM-c-kit's gel, (b) is the FAM-Pu27′s gel and (c) is the FAM-21G's gel. Each sample included 1 μM FAM-labeled DNA. Lane 1: duplexes without Py-Am; Lane 2–6: duplexes treated with 1, 2, 5, 10 and 20 μM Py-Am respectively; Lane 7: duplexes treated with 20 μM TMPyP4; Lane 8: free G-quadruplexes; Lane 9 in c: FAM-21G treated with 20 μM Py-Am. (d) Duplex–quadruplex competition in the presence of 307A. Lane 1, duplex FAM-21G in absence of 307A and Py-Am; Lane 2, 3 and 4, duplex FAM-21G with 5 μM, 10 μM and 20 μM 307A, respectively; Lane 5, duplex FAM-21G in the presence of Py-Am (10 μM). All the concentrations of FAM labeled 21G were 1 μM . | ||
In order to reveal the advantages of Py-Am, we furthermore selected a widely reported small molecule, TMPyP4 (Scheme 1) for comparison to evaluate whether other quadruplex ligands could also affect duplex–quadruplex competition. No new bands could be observed even with TMPyP4 (Fig. 2a–c, Lane 7), confirming the superiority of Py-Am. Besides, as TMPyP4 could tightly bind to the duplex as well, a structurally similar molecule, a 2,6-pyridin-dicarboxamide derivative27,29 (307A, Scheme 1), which has been reported to target the G-quadruplex, was also utilized for comparison. The results indicated that 307A lacked the ability to stabilize the G-quadruplex in the presence of its complementary strand shown in Fig. 2d. It was necessary to mention that the relative weaker bands with 307A in Fig. 2d were probably due to the fluorescent influence of 307A to FAM.
Besides, FRET experiments were performed to further confirm G-quadruplex formation. The FAM- and TAMRA-labeled c-kit sequence formed an excellent duplex structure with its complimentary sequence c-kit-c in K+ solution without Py-Am; no peak was observed around 580 nm in the fluorescence spectrum (Fig. S3a, ESI†). However, in the presence of Py-Am, we observed a remarkable increase of intensity at 580 nm, confirming the formation of the G-quadruplex. Similar results were observed with human telomeric duplex DNA (Fig. S3b, ESI†), further demonstrating the G-quadruplex formation in the presence of Py-Am.
Formation of G-qaudruplex during a long duplex system
Actually, Py-Am also regulates the formation of the G-quadruplex in a long duplex system. In genomic DNA, most quadruplex-forming sequences are trapped at internal positions in long double-stranded DNA, constrained at both ends by long DNA duplexes with complementary strands in close proximity to compete for duplex formation. Thus, investigation of G-quadruplex formation in a long duplex system is critical for understanding and regulating gene transcription. The c-myc gene sequence was chosen to explore the influence of Py-Am on G-quadruplex formation in long dsDNA (Fig. 3a). Similarly, in order to investigate the equilibrium states of duplex–quadruplex competition, all the samples were also treated with heat denaturation and renaturation. In the absence of Py-Am, the long dsDNA formed a well-matched duplex structure (Fig. 3b, Lane 1). When Py-Am was added to the long dsDNA system, slower migrating bands emerged along with the disappearance of duplex bands, implying that Py-Am promoted the formation of a much looser structure. Py-Am regulated nearly complete formation of the G-quadruplex within a long dsDNA when the concentration of Py-Am reached 50 μM, while TMPyP4 did not affect the duplex structure (see Fig. S4† for details). In order to eliminate the possibility that Py-Am interacted with the duplex to form a slower migrating Py-Am-duplex complex, a duplex (dsDNA-mu) with the same GC amount as the c-myc dsDNA but without a quadruplex-forming region was used for comparison. We clearly observed that Py-Am could not influence this duplex (Fig. 3b, Lane 9), confirming that the slower migration of the c-myc dsDNA was due to G-quadruplex formation stabilized by Py-Am. | ||
| Fig. 3 Formation of G-qaudruplex regulated by Py-Am during a long duplex system. (a) The selected long DNA sequence located at c-myc gene promoters and the mutant sequence. (b) Native PAGE analysis of G-quadruplex formation. Each sample included 1 μM FAM-labeled DNA. Lane 1: the long c-myc dsDNA only; Lane 2–6: the long c-myc dsDNA treated with 2, 5, 10, 20 and 50 μM Py-Am; Lane 7: the long c-myc dsDNA treated with 50 μM TMPyP4; Lane 8: dsDNA-mu only; Lane 9: dsDNA-mu treated with 50 μM Py-Am. (c) Sketch of S1 nuclease cleavage pathway. (d) S1 nuclease cleavage gel. Lane 1 was control with 0.5 μM labelled DNA; samples of Lane 2–6 were treated with 1, 2, 5, 10 and 20 μM Py-Am; samples of Lane 7 and 8 were treated with 20 μM TMPyP4 and 307A, respectively. (e) DMS footprinting experiment. The left lane was the sample of the long dsDNA without Py-Am; the right lane was the sample treated with Py-Am. | ||
Besides, S1 nuclease experiments were also performed to verify the relaxation of this long dsDNA regulated by Py-Am. In the absence of Py-Am, dsDNA was a well-matched duplex that could not be cleaved by S1 nuclease. However, in the presence of Py-Am, the duplex was loosened to form a G-quadruplex, resulting in the cleavage of the opposite strand by S1 nuclease (Fig. 3c). Thus, we could observe shorter oligonucleotides on gels, indicating G-quadruplex formation (Fig. 3d). While no phenomena could be observed in the control experiments using TMPyP4 and 307A as ligands (Fig. 3d, Lane 7 and 8).
To further demonstrate that the looser structure of the long c-myc dsDNA was the G-quadruplex, dimethyl sulfate (DMS) footprinting experiment was performed to identify the quadruplex formation. DMS modifies the N7 of guanosine and thus can directly probe G-quadruplex structure formation. Analysis of the footprinting pattern in Fig. 3e showed that the G-rich regions, which had been demonstrated to form G-quadruplex structure alone, were protected from DMS modification within the long dsDNA in the presence of Py-Am in K+ solution. However, the protection was lost in the absence of Py-Am. The DMS modification pattern abolished by Py-Am also suggests the presence of a G-quadruplex structure within the G-rich section. Besides, according to this footprinting pattern, we could identify that the red labeled G-rich part 3 in Fig. 3e was not involved in the G-quadruplex formation. Thus, we could figure out that the G-quadruplex regulated by Py-Am was composed of G-rich part 1, 2, 4, and 5 during this long dsDNA.
Another exception must be considered that whether the protection from DMS modification was due to the interaction of Py-Am with the duplex structure. In order to eliminate this possibility, the mutant dsDNA was also investigated with the treatment of DMS. The result that no protection was observed for this mutant, shown in Fig. S5 (ESI†), further demonstrated the formation of G-quadruplex in the long c-myc dsDNA.
Conclusions
In this work, we reported a novel example of organic compounds stabilizing DNA G-quadruplexes in the presence of their complementary strands even if the G-rich region was trapped in a long double-stranded DNA. Compared to other reported quadruplex ligands,17–21Py-Am strongly induced and stabilized the formation of G-quadruplex DNA. In order to reveal its unique properties, TMPyP4 and a much more structurally similar reported molecule (307A) were selected as control. We surprisingly found that neither TMPyP4 nor 307A could regulate duplex–quadruplex competition, but only Py-Am could realize this process. From the structural aspect, we proposed that the four carboxamide groups, which differ from 307A, might play important roles for G-quadruplex binding interaction.Relaxation of long double-stranded DNA is likely to be involved in gene transcription, and the formation of the G-quadruplex will influence some oncogene expression.6–8Py-Am could selectively stabilize G-quadruplex regardless of its complementary strand even if the G-rich region was trapped in long double-stranded DNA, which would be of large significance as the regulation of G-quadruplexes trapped in a long dsDNA may strongly influence gene transcription in living cells.
Acknowledgements
The authors thank the National Basic Research Program of China (973 Program) (2012CB720600), the National Science of Foundation of China (No. 90813031, 30973605, 20921062, 21072115), the National Grand Program on Key Infectious Disease (2012ZX10003-002), Program for Changjiang Scholars, Innovative Research Team in University (IRT1030), and the Fundamental Research Funds for the Central Universities.References
- A. M. Zahler, J. R. Williamson, T. R. Cech and D. M. Prescott, Nature, 1999, 350, 718–720 CrossRef.
- S. Neidle and G. Parkinson, Nat. Rev. Drug Discovery, 2002, 1, 383–393 CrossRef CAS.
- P. Luigi, O. T. John and J. B. Chaires, J. Am. Chem. Soc., 2008, 130, 16530–16532 CrossRef.
- W. I. Sundquist and A. Klug, Nature, 1989, 342, 825–829 CrossRef CAS.
- F. W. Smith and J. Feigon, Nature, 1992, 356, 164–168 CrossRef CAS.
- A. Siddiqui-Jain, C. L. Grand, D. J. Bearss and L. H. Hurley, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 11593–11598 CrossRef CAS.
- K. Guo, A. Pourpak, K. Beetz-Rogers, V. Gokhale, D. Sun and L. H. Hurley, J. Am. Chem. Soc., 2007, 129, 10220–10228 CrossRef CAS.
- S. Cogoi and L. E. Xodo, Nucleic Acids Res., 2006, 34, 2536–2549 CrossRef CAS.
- H. Sun, R. J. Bennett and N. Maizels, Nucleic Acids Res., 1999, 27, 1978–1984 CrossRef CAS.
- H. Sun, J. K. Karow, I. D. Hickson and N. Maizels, J. Biol. Chem., 1998, 273, 27587–27592 CrossRef CAS.
- S. Neidle and M. A. Read, Biopolymers, 2000, 56, 195–208 CrossRef CAS.
- J. L. Mergny and C. Helene, Nat. Med., 1998, 4, 1366–1367 CrossRef CAS.
- H. M. Wong, L. Payet and J. L. Huppert, Curr. Opin. Mol. Ther., 2009, 11, 146–155 CAS.
- M. Bejugam, S. Sewitz, P. S. Shirude, R. Rodriguez, R. Shahid and S. Balasubramanian, J. Am. Chem. Soc., 2007, 129, 12926–12927 CrossRef CAS.
- D. Sun, W. J. Liu, K. Guo, J. J. Rusche, S. Ebbinghaus, V. Gokhale and L. H. Hurley, Mol. Cancer Ther., 2008, 7, 880–889 CrossRef CAS.
- Y. Qin, E. M. Rezler, V. Gokhale, D. Sun and L. H. Hurley, Nucleic Acids Res., 2007, 35, 7698–7713 CrossRef CAS.
- A. Arola and R. Vilar, Curr. Top. Med. Chem., 2008, 8, 1405–1415 CrossRef CAS.
- K. Shin-ya, K. Wierzba, K. Matsuo, T. Ohtani, Y. Yamada, K. Furihata, Y. Hayakawa and H. Seto, J. Am. Chem. Soc., 2001, 123, 1262–1263 CrossRef CAS.
- J. E. Reed, A. A. Arnal, S. Neidle and R. Vilar, J. Am. Chem. Soc., 2006, 128, 5992–5993 CrossRef CAS.
- P. S. Shirude, E. R. Gillies, S. Ladame, F. Godde, K. Shin-Ya, I. Huc and S. Balasubramanian, J. Am. Chem. Soc., 2007, 129, 11890–11891 CrossRef CAS.
- R. Kieltyka, P. Englebienne, J. Fakhoury, C. Autexier, N. Moitessier and H. F. Sleiman, J. Am. Chem. Soc., 2008, 130, 10040–10041 CrossRef CAS.
- P. Alberti and J. L. Mergny, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 1569–1573 CrossRef CAS.
- P. Bucek, J. Jaumot, A. Aviñó, R. Eritja and R. Gargallo, Chem.–Eur. J., 2009, 15, 12663–12671 CrossRef CAS.
- T. Li, S. Dong and E. Wang, J. Am. Chem. Soc., 2010, 132, 13156–13157 CrossRef CAS.
- M. I. Onyshchenko, T. I. Gaynutdinov, E. A. Englund, D. H. Appella, R. D. Neumann and I. G. Panyutin, Nucleic Acids Res., 2009, 37, 7570–7580 CrossRef CAS.
- L. Lacroix, A. Séosse and J. L. Mergny, Nucleic Acids Res., 2011, 39, e21 CrossRef.
- G. Pennarun, C. Granotier, L. Gauthier, D. Gomez, F. Hoffschir, E. Mandine, J. Riou, J. L. Mergny, P. Mailliet and F. D. Boussin, Oncogene, 2005, 24, 2917–2928 CrossRef CAS.
- A. De Cian, E. Delemos, J. L. Mergny, M. P. Teulade-Fichou and D. Monchaud, J. Am. Chem. Soc., 2007, 129, 1856–1857 CrossRef CAS.
- T. Lemarteleur, D. Gomez, R. Paterski, E. Mandine, P. Mailliet and J.-F. Riou, Biochem. Biophys. Res. Commun., 2004, 323, 802–808 CrossRef CAS.
- Z. A. Waller, P. S. Shirude, R. Rodriguez and S. Balasubramanian, Chem. Commun., 2008, 1467–1469 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: Synthetic details, circular dichroism spectra, SPR sensorgrams, and gel electrophoresis. See DOI: 10.1039/c1ra00851j/ |
| This journal is © The Royal Society of Chemistry 2012 |
