Enhancing the efficiency of a dye sensitized solar cell due to the energy transfer between CdSe quantum dots and a designed squaraine dye†
Lioz
Etgar
*a,
Jinhyung
Park
b,
Claudia
Barolo
*b,
Vladimir
Lesnyak
*c,
Subhendu K.
Panda
c,
Pierluigi
Quagliotto
b,
Stephen G.
Hickey
c,
Md. K.
Nazeeruddin
a,
Alexander
Eychmüller
c,
Guido
Viscardi
b and
Michael
Grätzel
a
aLaboratoire de Photonique et Interfaces, Institut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), Station 6, CH-1015, Lausanne, Switzerland. E-mail: lioz.etgar@epfl.ch
bDipartimento di Chimica, NIS Centre of Excellence, Università di Torino, Via Pietro Giuria 7, I-10125, Torino, Italy. E-mail: claudia.barolo@unito.it
cPhysical Chemistry, TU Dresden, Bergstr. 66b, 01062, Dresden, Germany. E-mail: vladimir.lesnyak@chemie.tu-dresden.de
First published on 3rd February 2012
Abstract
The power conversion efficiency of a dye-sensitized solar cell with tailored squaraine dye enhanced by 47%, due to Förster resonance energy transfer from CdSe quantum dots to the squaraine dye. The incident photons to collection efficiency of electrons indicate panchromatic response from the visible to the near-infrared spectrum.
Introduction
Photovoltaic technology is one of the most promising alternative renewable energy sources, harvesting energy from the sun. Dye sensitized solar cells (DSSCs) are inexpensive and use abundant materials for large-scale solar energy conversion. In a typical DSSC the dye absorbs photons and goes to the excited state, generating electron and hole pairs. The electrons are injected into the TiO2 conduction band and diffuse to the front contact, simultaneously, the holes are scavenged by a redox couple. Currently DSSCs are exceeding power conversion of 12%,1 despite the fact that many dyes do not absorb strongly over 700 nm. Therefore, Förster resonance energy transfer (FRET) has already been demonstrated in a variety of optoelectronic applications and recently in DSSCs to harvest visible and near infrared (IR) absorption spectra.2–5 Usually, the traditional dyes used in DSSCs suffer from low molar extinction coefficients or limited absorption spectra regions.6 Using FRET to transfer energy from donor to acceptor inside the DSSCs paves a new way for enhancing the photovoltaic performance of DSSCs. This provides the possibility to employ a cascade combination of dyes, which have narrow absorption spectra.Squaraine dyes are well-known for their remarkable optical behavior because of strong absorption from charge transfer between an electron-deficient central squaric core and each side of the substituent in the red to near-infrared region,7 and they have been widely and successfully used also in DSSCs in recent years.8 A recent report on FRET based dye sensitized solar cells showed that the acceptor should be anchored to the TiO2. In addition it must have lower excitation energy than the donor, enabling fast and efficient electron transfer to TiO29 upon excitation of the energy relay dye.
Quantum dots (QDs) have high molar extinction coefficients and broad absorption spectra covering a wide part of the visible spectrum. Hence they can be good candidates as donors in FRET DSSCs in cases where IR dyes that absorb weakly in the visible region are used as acceptors.
On the other hand, QDs can be affected by the electrolyte, one possibility to avoid this was described by Zaban et al.,10–12 who created an amorphous thin TiO2 barrier to avoid direct contact with the electrolyte. Another option is to use a more “friendly” electrolyte, which will not quench the QDs.
Here, we apply the FRET concept to a hybrid QDs/dye-sensitized solar cell. The donors are TOP/TOPO-capped CdSe QDs, while the acceptor is a newly designed symmetric squaraine dye, called VG1-C10, possessing an additional carboxylic group13 as compared to standard squaraine dyes, and two C10-chains. This provides better dye stability and allows efficient energy transfer. The use of the cobalt complex (Co+2/Co+3) as an electrolyte in the cells permits direct contact between the QDs and the electrolyte. Moreover, there is no need to exchange the original ligands of the QDs prior to deposition, since the two C10 chains of the dye molecules and TOP/TOPO-capping of the QDs provide the optimum distance for FRET and make the preparation and the structure of the cell simple. As a result of the energy transfer, the cell power conversion efficiency was increased and its solar response was expanded from the visible to the near infrared spectral region.
Results and discussion
Different non-symmetric and symmetric squaraines were synthesized and characterized (see ESI†) in order to match their optical and hydrophobic properties with the selected QDs. Their interaction properties have been studied both in solution and on FTO glass, avoiding the titania substrate in order to eliminate the injection problem. Subsequently, the best couple (symmetrical long chain dyes VG1-C10 and CdSe TOP/TOPO capped QDs) have been used in the cell.The symmetric squaraine VG1-C10 was synthesized via a two step procedure using commercially available precursors (see Scheme 1). Symmetrical dyes have the advantage of simple synthesis and easy purification by crystallization.13 For the synthesis details and NMR data see the ESI.†
 | ||
| Scheme 1 Synthetic pathway for symmetric squaraine VG1-C10. | ||
The scheme of the energy transfer cell is presented in Fig. 1A. The TiO2 electrode was first dipped in the VG1-C10 dye solution overnight and then the CdSe QDs were spin coated on top of the dye-coated electrode. Finally the Co+2/Co+3 electrolyte was injected into the cell.
 | ||
| Fig. 1 (A) Schematic presentation of the cell structure; (B) energy level diagram of the components involved in the cell. Dashed arrows represent absorption, wavy arrows show energy transfer from the QDs excited state to the LUMO of the dye via dipole–dipole interaction; (C) the arrangement of the QDs and the VG1-C10 inside the cell. | ||
Fig. 1B shows the energy level diagram of the cell, which is a type I system. The energy level positions of the QDs and of the dye permit energy transfer between the QDs as donor and the dye as the acceptor. Moreover, the mismatch in the conduction band of the QDs and the LUMO of the dye does not allow for charge transfer from the dye to the QDs. As a rule, in the DSSC light is absorbed by the dye, an electron is excited from the HOMO to the LUMO and injected to the conduction band of TiO2. In the present hybrid cell, the QDs act as additional light harvesters absorbing the light and transferring the energy of their excited state to the LUMO of the dye via dipole–dipole interaction. Subsequently, as in standard DSSCs, the electron from the LUMO of the dye is injected to the conduction band of the TiO2 and the hole is transported to the counter electrode via the redox couple.
Fig. 2 shows FTIR spectra of the VG1-C10 as dye powder in KBr pellets and the VG1-C10 adsorbed on TiO2. The spectra reveal that the adsorption of VG1-C10 on TiO2 occurs via carboxy groups, showing two bands at 1609 cm−1 and 1353 cm−1,14 while the characteristic COOH band around 1730 cm−1–1680 cm−1 completely disappeared. Therefore, the VG1-C10 can be linked by both COOH groups to the titania surface, in a cis arrangement, probably directing the hydrocarbon chains far from the TiO2.
 | ||
| Fig. 2 FTIR spectra of VG1-C10 in KBr pellets (red), and VG1-C10 adsorbed on TiO2 electrodes (black). | ||
Relying on the FTIR measurements, Fig. 1C presents a possible explanation for the orientation of the QDs and the dye inside the cell, which permits FRET to occur. As discussed above, the VG1-C10 dye anchors the TiO2 by using its two carboxylic groups, as a result the C10H21-chains are free and away from the TiO2, which allows them to intertwine with the TOP/TOPO ligands of the QDs via efficient hydrophobic interactions. This kind of a connection results in an average distance between the donor and the acceptor, which lies within the Förster radius and allows an efficient energy transfer. This type of ligand interconnection was demonstrated by Vogel et al.15 showing FRET between QDs and lipids both containing long hydrocarbon chains.
Fig. 3 shows the absorption and emission spectra of the CdSe QDs and the VG1-C10 dye. For FRET it is essential that the emission spectrum of the donor (QDs) overlap with the absorption spectrum of the acceptor (dye).
 | ||
| Fig. 3 Absorption and emission spectra of the CdSe QDs and VG1-C10 dye. | ||
The Förster radius, R0, is the distance at which the probability for energy transfer is 50%. The Förster radius is given by:16
 | (1) |
 , ID is the emission profile spectrum of the donor and εA is the molar extinction coefficient of the acceptor. According to this formula, R0 in our system is 3.7 nm, which agrees well with our suggested orientation of the QDs and the dye. One of the main evidences for FRET is the shortening of the donor lifetime in the presence of the acceptor and the increase of the lifetime of the acceptor in the presence of the donor.
, ID is the emission profile spectrum of the donor and εA is the molar extinction coefficient of the acceptor. According to this formula, R0 in our system is 3.7 nm, which agrees well with our suggested orientation of the QDs and the dye. One of the main evidences for FRET is the shortening of the donor lifetime in the presence of the acceptor and the increase of the lifetime of the acceptor in the presence of the donor.
In order to show the energy transfer from the CdSe QDs to the VG1-C10 dye, mixed composite films of different QDs/VG1-C10 ratios were prepared. Absorption and photoluminescence (PL) spectra of the CdSe QDs and the VG1-C10 dye in solution are presented in Fig. 3. As is seen from the figure, the QDs sample chosen for the experiments as a potential energy donor meets two main requirements for investigation of energy relations in the system by means of optical spectroscopy: on the one hand, its emission spectrum overlaps with the absorption of the dye, on the other hand its PL maximum is quite distant from that of the dye, which allows for the separate detection of the time resolved emissions from the donor and from the acceptor, at 600 and 700 nm, respectively. Moreover, excitation sources for time resolved measurements (pulse laser diodes) allow for separate excitation of the QDs and the dye. As is seen from Fig. 3, neither the QDs absorb light at 670 nm (used for the dye emission measurements), nor does the dye absorb at 470 nm (used for the QDs PL excitation). Thus, emissions of the QDs and the dye do not interfere and consequently do not account for time resolved traces of each other.
As is clearly seen from Fig. 4A and B, an increase in the QDs content in the mixed films leads to strong enhancement of both emission lifetimes. Thus the more donors present in the system, the more efficient energy pumping of the acceptor is realized. FRET from the donors (QDs) is clearly shown by the appearance of a slow component in the acceptor PL lifetime.
 | ||
| Fig. 4 Time resolved PL spectra of the composite QDs/VG1C10 films with different ratios recorded at 600 nm (QDs emission) (A) and at 700 nm (VG1-C10 emission) (B); λex = 470 nm for all the samples except the pure VG1-C10 film excited at 635 nm (black curve in Fig. 4B). Note, QDs emission measured at 700 nm (ex. 470 nm) is very weak (not shown). The arrows indicate an increase in the QD/VG1 ratio. | ||
FRET efficiency can be calculated according to the following equation:17E = 1 − (τDA/τD), where τDA and τD are the lifetime of the donor in the presence of the acceptor and the lifetime of the donor alone, respectively. In our case, the PL lifetime was fitted by bi-exponential equation and the weighted average lifetime of each decay curve was calculated. The donor lifetime is 9.7 ± 0.1 ns and the lifetime of the donor in the presence of acceptor drops to 3 ± 0.1 ns. For the lifetime of the donor in the presence of the acceptor, we considered the QD/VG1-C10 ratio, which is the same as their ratio in the cell. The calculated FRET efficiency is E ≈ 69%.
Fig. 5 shows the incident photon to current efficiency (IPCE) of three different DSSCs: CdSe QDs donor cell, VG1-C10 acceptor cell and hybrid cell composed of the donor (QDs) and the acceptor (dye). The QDs cell (one layer of QDs deposited on the titania electrode) shows a very week response for the whole wavelength range. The dye cell exhibits an IPCE response between 500 nm to 750 nm, which matches well the absorption spectrum of the dye (see Fig. 3). As is seen from Fig. 5, the hybrid cell containing both the dye and the QDs layers has the best efficiency and demonstrates response over the whole visible range, reaching its maximum at 360 nm and 660 nm, corresponding to IPCE intensity of 36% and 30%, respectively. The coverage of the whole visible spectrum in the hybrid cell is evidence for the efficient FRET interaction between the constituents of the light-absorbing layer. Moreover, the cell fabrication method, consisting of dipping the TiO2 electrode in the dye solution with subsequent deposition of the QDs via spin coating, minimizes the possibility of co-sensitization. During the QDs deposition the whole TiO2 surface is already covered by the dye molecules, which prevents direct injection of electrons from the QDs to the TiO2 conduction band.
 | ||
| Fig. 5 Incident photon to current efficiency (IPCE) curves of the three cells. The CdSe cell (brown line); the VG1-C10 cell (green line) and the hybrid cell composed of the donor and acceptor (purple line). | ||
The photovoltaic performance (PV) of the acceptor cell composed of the VG1-C10 dye only and the donor-acceptor cell (composed of the dye and the QDs) is shown in Fig. 6 and Table 1. The acceptor cell exhibits a power conversion efficiency (PCE) of 0.79% under AM 1.5 G and short circuit current (Jsc), open circuit voltage (Voc), and fill factor (FF) of 2.73 mA cm−2, 542 mV, and 0.53, respectively. Upon incorporation of CdSe QDs into the device, the Jsc increases by 16% to 3.25 mA cm−2 with an increase in the PCE up to 1.48%. The Voc increased by 111 mV (17% increase) and the FF increased by 23%. The increase in the Voc is mainly due to an upward shift of the TiO2 conduction band. This can be explained in the difference of the dark current measurements shown in Fig. 7 (assuming the same recombination behaviour), and by the higher current density of the FRET cell, which indicates more electrons injected into the TiO2 conduction band. The 47% increase in the device efficiency is partially attributed to the increase in the Jsc due to the harvesting of visible photons in the 350–650 nm region resulting from energy transfer.
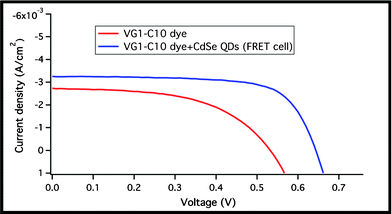 | ||
| Fig. 6 Photovoltaic response of the VG1-C10 cell and the VG1-C10 + CdSe QDs cell. | ||
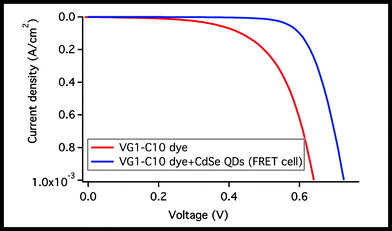 | ||
| Fig. 7 Dark current measurements of the VG1-C10 cell and the VG1-C10 + CdSe QDs cell (FRET cell). | ||
Conclusion
We demonstrated an enhancement of the light harvesting in dye sensitized solar cell due to Förster resonance energy transfer. The donors are CdSe QDs and the acceptor is a new designed symmetric squaraine dye with two carboxy groups and two long hydrocarbon chains. The use of the cobalt complex (Co+2/Co+3) as the electrolyte in the cells permits direct contact between the QDs and the electrolyte without affecting the QDs, which results in a simple structure FRET system employed in a hybrid QDs/dye-sensitized solar cell. PL lifetime measurements revealed FRET from the QDs to the dye. IPCE curves of the device exhibit a full coverage of the visible region. In addition, all the cell photovoltaic parameters were enhanced, proving efficient energy transfer within the QD–dye-sensitized solar cell.Acknowledgements
This research was funded by the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 227057, Project “INNOVASOL”. L. E. acknowledges the Marie Curie Actions-Intra-European Fellowships (FP7-PEOPLE-2009-IEF) under grant agreement n° 252228, project “Excitonic Solar Cell”. We gratefully acknowledge Christian Waurisch (TU Dresden) for providing the samples. J. P., C. B., P. Q. and V. G. thank Compagnia di San Paolo and Fondazione CRT for continuous equipment supply.References
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, Md. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629 CrossRef CAS.
- B. E. Hardin, E. T. Hoke, P. B. Armstrong, J. H. Yum, P. Comte, T. Torres, J. M. J. Frechet, M. K. Nazeeruddin, M. Grätzel and M. D. McGehee, Nat. Photonics, 2009, 3, 406 CrossRef CAS.
- K. Shankar, X. Feng and C. A. Grimes, ACS Nano, 2009, 3, 788 CrossRef CAS.
- J. H. Yum, B. E. Hardin, S. J. Moon, E. Baranoff, F. Nüesch, M. D. McGehee, M. Grätzel and M. K. Nazeeruddin, Angew. Chem., Ger. Edit., 2009, 48, 9277 CrossRef CAS.
- K. Driscoll, J. Fang, N. Humphry-Baker, T. Torres, W. T. S. Huck, H. J. Snaith and R. H. Friend, Nano Lett., 2010, 10, 4981 CrossRef CAS.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and A. H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS.
- (a) S. Yagi, Y. Hyodo, M. Hirose, H. Nakazumi, Y. Sakurai and A. Ajayaghosh, Org. Lett., 2007, 9, 1999 CrossRef CAS; (b) W. Y. Yan, A. L. Sloat, S. Yagi, H. Nakazumi and C. L. Colyer, Electrophoresis, 2006, 27, 1347 CrossRef CAS; (c) M. Matsui, S. Tanaka, K. Funabiki and T. Kitaguchi, Bull. Chem. Soc. Jpn., 2006, 79, 170 CrossRef CAS; (d) N. Fu, J. M. Baumes, E. Arunkumar, B.C. Noll and B.D. Smith, J. Org. Chem., 2009, 74, 6462 CrossRef CAS.
- (a) J.-H. Yum, P. Walter, S. Huber, D. Rentsch, T. Geiger, F. Nesch, F. De Angelis, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2007, 129, 10320 CrossRef CAS; (b) T. Geiger, S. Kuster, J.-H. Yum, S.-J. Moon, Md. K. Nazeeruddin, M. Grätzel and F. Nüesch, Adv. Funct. Mater., 2009, 19, 2720 CrossRef CAS; (c) S. Paek, H. Choi, C. Kim, N. Cho, S. So, K. Song, M. K. Nazeeruddin and J. Ko, Chem. Commun., 2011, 47, 2874–2876 RSC; (d) Y. Shi, R. B. M. Hill, J.-H. Yum, A. Dualeh, S. Barlow, M. Grätzel, S. R. Marder and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2011, 50, 6619–6621 CrossRef CAS.
- (a) K. Shankar, X. Feng and C.A. Grimes, ACS Nano, 2009, 3, 788 CrossRef CAS; (b) J. I. Basham, G. K. Mor and C. A. Grimes, ACS Nano, 2010, 4, 1253 CrossRef CAS; (c) G. K. Mor, J. Basham, M. Paulose, S. Kim, O. K. Varghese, A. Vaish, S. Yoriya and C. A. Grimes, Nano Lett., 2010, 10, 2387 CrossRef CAS.
- S. Buhbut, S. Itzhakov, E. Tauber, M. Shalom, I. Hod, T. Geiger, Y. Garini, D. Oron and A. Zaban, ACS Nano, 2010, 4, 1293–1298 CrossRef CAS.
- S. Buhbut, S. Itzhakov, D. Oron and A. Zaban, J. Phys. Chem. Lett., 2011, 2, 1917 CrossRef CAS.
- S. Itzhakov, S. Buhbut, E. Tauber, T. Geiger, A. Zaban and D. Oron, Adv. Energy Mater., 2011, 1, 626 CrossRef CAS.
- J. Park, C. Barolo, F. Sauvage, N. Barbero, C. Benzi, P. Quagliotto, S. Coluccia, D. Di Censo, M. Grätzel, M. K. Nazeeruddin and G. Viscardi, Chem. Commun., 2012, 48, 2782–2784 RSC.
- (a) K. Y. Law, F. C. Bailey and L. J. Bluett, Can. J. Chem., 1986, 64, 1607 CrossRef CAS; (b) E. Terpetschnig, H. Szmacinski, A. Ozinskas and J. R. Lakowicz, Anal. Biochem., 1994, 217, 197 CrossRef CAS.
- I. Geissbuehler, R. Hovius, K. L. Martinez, M. Adrian, K. R. Thampi and H. Vogel, Angew. Chem., Int. Ed., 2005, 44, 1388–1392 CrossRef CAS.
- T. Förster, Transfer Mechanisms of Electronic Excitation. Discuss., Faraday Soc., 1959, 7 Search PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Plenum Press, New York and London, 3rd edn, 1986, pp. 496 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental data includes synthesis of the squaraine dyes and the QDs. See DOI: 10.1039/c2ra20192e |
| This journal is © The Royal Society of Chemistry 2012 |
