A photoconductive charge-transfer crystal with mixed-stacking donor–acceptor heterojunctions within the lattice†
Wei
Yu‡
a,
Xiao-Ye
Wang‡
b,
Jing
Li
a,
Zhi-Ting
Li
a,
Yu-Kun
Yan
a,
Wei
Wang
*a and
Jian
Pei
*b
aCenter for Synthetic Soft Materials, Key Laboratory of Functional Polymer Materials of the Ministry of Education, Institute of Polymer Chemistry, Nankai University, Tianjin 300071, China. E-mail: weiwang@nankai.edu.cn
bBeijing National Laboratory for Molecular Science (BNLMS), the Key Laboratory of Bioorganic Chemistry and Molecular Engineering, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: jianpei@pku.edu.cn
First published on 12th November 2012
Abstract
A pyrene derivative as the donor and a butyl-viologen as the acceptor were used to construct a novel charge-transfer cocrystal consisting of mixed-stacking structure and having switchable photoconductivity stemming from the donor–acceptor heterojunctions within the lattice.
Charge-transfer (CT) crystals are well-known solid-state organic functional materials which are composed of electron-rich molecules as donors (D) and electron-deficient molecules as acceptors (A). Since the discovery of extraordinary electrical conductivity in tetrathiafulvalene-tetracyanoquinodimethane (TTF-TCNQ) CT crystals,1 extensive research has been conducted on organic metals.2 Besides, additional intriguing properties (e.g. superconductivity,3 ferro-electricity,4 photonicity,5 and photovoltaic characteristics6) have also been discovered. Delightfully, recent studies demonstrated their great potential application in organic electronics.2 For instance, field-effect transistors with CT crystals as the active component7 or source–drain electrodes8 exhibited good performance.
The properties of CT crystals appear to be associated with their stacking arrangements of electron donors and acceptors.2 There are two major stacking modes: segregated- and mixed-stack. In segregated-stacking CT crystals, the donor and acceptor molecules form separate D- and A-stacks; while in mixed-stacking CT crystals, adjacent donor and acceptor molecules stack alternately in a face-to-face manner within the same stack. Segregated-stacking CT crystals are normally metallic conductors but most mixed-stacking CT crystals are unfortunately insulators,2,9 with only a few examples as semiconductors.10 It is encouraging, however, that the latest research provides a theoretical prediction of ambipolar charge transport characteristics for mixed-stack CT crystals,11 which were observed in a cocrystal recently.12
Up to now, more than two thousand organic CT crystals have been synthesized mainly based on TTF-TCNQ or acene-TCNQ and their derivatives.2 It is highly desirable to explore novel mixed-stacking CT systems with semiconducting properties, but this is quite difficult due to the phase separation problem. This challenge will be more formidable if the CT crystals are prepared from the familiar and simple compounds to avoid sophisticated molecular design and tedious synthesis. It is well-known that photoconductive materials are applicable to photodetectors,13 optical switches,14 and sensors.15 Up to now, only a few organic one-dimensional (1D) nanomaterials demonstrated photoconductive responses,16 but this attractive property has seldom been observed in 1D organic CT crystals with mixed-stacking structures.
In this communication, we report the preparation of a novel CT crystal with a mixed-stacking structure and the study of its photoconductive properties. To avoid tedious synthesis, commercially available 1,3,6,8-pyrenetetrasulfonic acid tetrasodium salt (PyTs) was chosen as the donor and 1,1′-bibutyl-4,4′-bipyridinium dibromide (BuV) as the acceptor (Fig. 1a), which was easily obtained by a simple synthetic route. Viologen is a kind of charge-accepting molecules with curious physicochemical properties,17 but was seldom used in the investigation of CT crystals. We also report the first observation of switchable photoconductivity in the PyTs–BuV CT cocrystal. This function stems from the donor–acceptor heterojunctions within the lattice of this cocrystal.
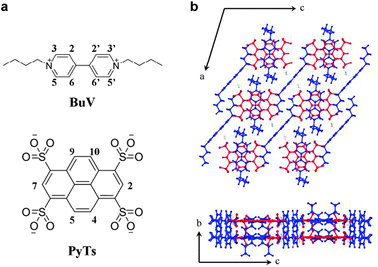 | ||
| Fig. 1 (a) Chemical structures of BuV (top) and PyTs (bottom); (b) molecular packing in the PyTs-BuV CT cocrystal on the ac plane (up) and bc plane (bottom). Red: PyTs. Blue: BuV. | ||
In aqueous solution, the fluorescence of PyTs was efficiently quenched by increasing the amount of BuV (Fig. 2a), an important evidence for the formation of CT complexes. The Stern–Volmer plot was not linear but an upward curve, especially at higher quencher BuV concentration. The details of the quenching mechanism could be explained by the sphere-of-action model,18 which revealed the strong multiple electrostatic interactions between PyTs and BuV assisted by elementary static quenching of stable stacking complexes. The formation of the CT complexes could also be confirmed by UV-vis spectra (Fig. 2b). In a concentrated solution (0.1 M), the CT complex formation resulted in a red shift compared to PyTs, while no significant shift was observed in dilute solutions.19 The CT interaction could be indicated by direct observation of an obvious colour change upon mixing the colourless aqueous solutions of PyTs and BuV (Fig. S3. ESI†). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture showed a deep yellow colour, which is a characteristic change for a CT complex.5b
1 mixture showed a deep yellow colour, which is a characteristic change for a CT complex.5b
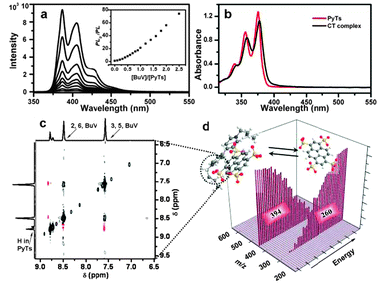 | ||
| Fig. 2 (a) Fluorescence spectra of PyTs with different contents of BuV. The inset is a Stern–Volmer plot; (b) UV-vis spectra of PyTs and CT crystals; (c) 2D NOESY NMR spectrum; (d) 2D tandem mass spectra. | ||
1H NMR features show upfield shifts of proton resonances in these two molecules in D2O, owing to the face-to-face stacking interactions between PyTs and BuV (Fig. S2, ESI†). The 2D NOESY NMR spectrum in Fig. 2c shows the detailed stacking formation. The protons of PyTs exhibit two signals from polar positions (2- and 7-positions) and equatorial positions (4-, 5-, 9-, and 10-positions). Stronger NOE cross-couplings were observed between protons of equatorial positions of PyTs and pyridyl rings of BuV, than the effects related to polar protons. This suggested a directed packing of the PyTs and BuV molecules in the solution, as the result of a minimized stacking free energy.
To investigate the details of PyTs and BuV recognition and assembly in the solution, tandem mass spectrometry (ESI-MS/MS) was performed. A 394 Da molecular ion peak, which is precisely tallied with the sum of the PyTs–BuV complex with two negative charges, was selected as the primary ion in the first stage. When the bombardment voltage in the second mass analyzer was enhanced in a stepwise manner, the peak intensity of the two-molecule complex ion was gradually weakened. Simultaneously, a new molecular ion peak at 260 Da, which just corresponds to the di-protonation of PyTs with two negative charges (M + 2H+)2−, emerged gradually (Fig. 2d). This bombardment voltage dependence represents a stacking–destacking process of the PyTs–BuV complex by strong intermolecular interactions.
Starting from dilute solutions of the PyTs–BuV complex, the supersaturated solution was slowly formed through the evaporation of H2O in the mixture of H2O–DMF. As a result, a plenty of needle-like orange crystals gradually separated out. If carefully cultivated in the supersaturated solution, the crystals would continuously grow to a size good for further studies. Structural analysis by single crystal X-ray diffraction shows that the crystal is a cocrystal of PyTs and BuV molecules with a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, just for the neutrality per unit cell. The PyTs–BuV cocrystal adopts a monoclinic crystal system. Along the b axis PyTs and BuV stacked alternately to form the mixed-stacking columns (Fig. 1b). The distance of neighbouring PyTs–BuV stacking molecules is 3.50 Å, which corresponds to the characteristic distance for π–π stacking. The notable hydrogen bond bridging two PyTs molecules assists the stability of the stacking columns. Moreover, another equivalent BuV molecules arranged beside the mixed-stacking columns, with the N atom near the sulphuric acid group of PyTs, referred to as a component of salts, by electrostatic interaction. The excess BuV molecules formed an isolated segregated-stacking column, which may play a pivotal role in exhibiting characteristic physical features of the CT cocrystals.
2, just for the neutrality per unit cell. The PyTs–BuV cocrystal adopts a monoclinic crystal system. Along the b axis PyTs and BuV stacked alternately to form the mixed-stacking columns (Fig. 1b). The distance of neighbouring PyTs–BuV stacking molecules is 3.50 Å, which corresponds to the characteristic distance for π–π stacking. The notable hydrogen bond bridging two PyTs molecules assists the stability of the stacking columns. Moreover, another equivalent BuV molecules arranged beside the mixed-stacking columns, with the N atom near the sulphuric acid group of PyTs, referred to as a component of salts, by electrostatic interaction. The excess BuV molecules formed an isolated segregated-stacking column, which may play a pivotal role in exhibiting characteristic physical features of the CT cocrystals.
A TEM image of the needle-like CT cocrystal is shown in Fig. 3a and the corresponding selected area electron diffraction (SAED) pattern is displayed in the inset of Fig. 3a. Based on the SAED pattern and monoclinic crystal structure, we can ascertain that the lattice plane of the mixed-stacking structure is just normal to the axis of the needle-like CT cocrystal, as schematically marked in Fig. 3a. To investigate the photoconductive properties of the CT cocrystal, a suspension of micro-sized CT cocrystals was spin-coated onto polystyrene-coated SiO2/Si substrates, and a polyethylene fibre as a shadow mask was mounted on the substrate. Finally, a 50 nm silver layer was deposited onto the crystals with an improved plastic-fibre-mask method.20 Photoconductivity measurements were carried out on the CT cocrystal-based devices in air (Fig. 3b).
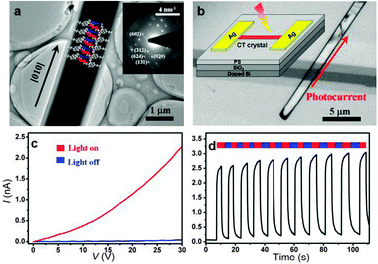 | ||
| Fig. 3 (a) TEM image of a CT cocrystal. Inset: SAED pattern of the cocrystal (lattice planes partly marked); (b) optical microscopy image of a CT cocrystal device plus a schematic of the device fabrication for the photocurrent measurements; (c) I–V curves of a photoconductive device under dark (blue line, I value near 0) and illumination (red line); (d) photoconductive characteristic at a bias of 30 V in response to turning on/off the illumination. | ||
The CT cocrystal-based devices exhibited a distinct photoconductive character as shown in Fig. 3c and d. The photocurrent exhibited a strong sublinear dependence on the voltage and reached ca. 2.3 nA at a bias of 30 V, under the illumination intensity of 100 mW mm−2. The photocurrent could be switched promptly several times with light on and off at a bias of 30 V, without any appreciable attenuation at an on/off ratio of about 102. The fast photoresponsiveness and high stability with the light turned on and off implied that the CT cocrystal with the molecular heterojunctions in the lattice is profitable for application in organic optoelectronic devices.
The previous studies have stated that the difference in the degree of charge transfer can lead to a charge rearrangement in CT crystals with different energies for CT transition.21 Among all CT pairs with various packing patterns, only a few cases showed a significant photoconductive property,21 while those with alternate stacking are rare but important. The observed efficient fluorescence quenching in aqueous solution illuminated a photoinduced electron transfer taking place between D and A molecules. So, the critical factor, which barricades the directed movement of the charge carriers, should be the recombination of the photogenerated charge carriers because the dissociation of the initially separated charges could be blocked.22 Although the strong donor–acceptor interaction leads to localization of the charges under dark, under light radiation, the specific structure of the D–A molecules in the cocrystal could at first result in a strong ionization of the mixed-stacking donor–acceptor molecules and then could prevent the unprofitable recombination process. The further transport of the dissociated charge carriers contributes to the photoconductive property of this mixed-stacking CT cocrystal.
In summary, we have successfully constructed a novel organic CT cocrystal with a mixed-stacking structure from an aqueous solution of a pyrene derivative (donor) and a viologen derivative (acceptor) and found a switchable photoconductive property of the cocrystal. The formation of the alternate stacking derives from the strong donor–acceptor interaction of the CT complex in solution. Unlike all segregated-stacking cases, this mixed-stacking structure presents a donor–acceptor heterojunction within the lattice. It is this unique stacking that promotes the efficient photogeneration and transport of the charge carriers, so this CT cocrystal exhibits the switchable photoconductive property. This desirable function will provide this novel mixed-stacking CT cocrystal with high potential in optoelectronic applications and evoke further theoretical investigations on this unique CT system and analogs.
The Nankai group greatly appreciates the kind help of Dr. X. L. Kong on the ESI-MS/MS.
Notes and references
- (a) J. Ferraris, D. O. Cowan, V. Walatka Jr and J. H. Perlstein, J. Am. Chem. Soc., 1973, 95, 948 CrossRef CAS; (b) L. B. Coleman, M. J. Cohen, D. J. Sandman, F. G. Yamagishi, A. F. Garito and A. J. Heeger, Solid State Commun., 1973, 12, 1125 CrossRef CAS.
- (a) T. Hasegawa and J. Takeya, Sci. Technol. Adv. Mater., 2009, 10, 024314 CrossRef; (b) T. Mori, J. Phys.: Condens. Matter, 2008, 20, 184010 CrossRef; (c) G. Saito and Y. Yoshida, Bull. Chem. Soc. Jpn., 2007, 80, 1 CrossRef CAS.
- M. Kurmoo, A. W. Graham, P. Day, S. J. Coles, M. B. Hursthouse, J. L. Caulfield, J. Singleton, F. L. Pratt and W. Hayes, J. Am. Chem. Soc., 1995, 117, 12209 CrossRef CAS.
- (a) S. Horiuchi, Y. Okimoto, R. Kumai and Y. Tokura, Science, 2003, 299, 229 CrossRef CAS; (b) S. Horiuchi and Y. Tokura, Nat. Mater., 2008, 7, 357 CrossRef CAS.
- (a) Q. Liao, H. Fu, C. Wang and J. Yao, Angew. Chem. Int. Ed., 2011, 50, 4942 CrossRef CAS; (b) J.-Y. Wang, J. Yan, L. Ding, Y. Ma and J. Pei, Adv. Funct. Mater., 2009, 19, 1746 CrossRef CAS.
- J. Tsutsumi, T. Yamada, H. Matsui, S. Haas and T. Hasegawa, Phys. Rev. Lett., 2010, 105, 226601 CrossRef.
- (a) T. Hasegawa, K. Mattenberger, J. Takeya and B. Batlogg, Phys. Rev. B: Condens. Matter, 2004, 69, 245115 CrossRef; (b) M. Sakai, H. Sakuma, Y. Ito, A. Saito, M. Nakamura and K. Kudo, Phys. Rev. B: Condens. Matter, 2007, 76, 045111 CrossRef.
- (a) Y. Takahashi, T. Hasegawa, Y. Abe, Y. Tokura, K. Nishimura and G. Saito, Appl. Phys. Lett., 2005, 86, 063504 CrossRef; (b) Y. Takahashi, T. Hasegawa, Y. Abe, Y. Tokura and G. Saito, Appl. Phys. Lett., 2006, 88, 073504 CrossRef.
- J. Zhang, H. Geng, T. S. Virk, Y. Zhao, J. Tan, C. Di, W. Xu, K. Singh, W. Hu, Z. Shuai, Y. Liu and D. Zhu, Adv. Mater., 2012, 24, 2603 CrossRef CAS.
- J. B. Torrance, J. E. Vazquez, J. J. Mayerle and V. Y. Lee, Phys. Rev. Lett., 1981, 46, 253 CrossRef CAS.
- G. Saito, S. Pac and O. O. Drozdova, Synth. Met., 2001, 120, 667 CrossRef CAS.
- L. Zhu, Y. Yi, Y. Li, E.-G. Kim, V. Coropceanu and J. Brédas, J. Am. Chem. Soc., 2012, 134, 2340 CrossRef CAS.
- (a) J. Wang, M. S. Gudiksen, X. Duan, Y. Cui and C. M. Lieber, Science, 2001, 293, 1455 CrossRef CAS; (b) X. Gong, M. Tong, Y. Xia, W. Cai, J. S. Moon, Y. Cao, G. Yu, C.-L. Shieh, B. Nilsson and A. J. Heeger, Science, 2009, 325, 1665 CrossRef CAS; (c) H.-F. Zhu, T. Li, Y.-J. Zhang, H.-L. Dong, J.-S. Song, H.-P. Zhao, Z.-M. Wei, W. Xu, W.-P. Hu and Z.-S. Bo, Adv. Mater., 2010, 22, 1645 CrossRef CAS.
- Y.-Q. Zhang, P.-L. Chen, L. Jiang, W.-P. Hu and M.-H. Liu, J. Am. Chem. Soc., 2009, 131, 2756 CrossRef CAS.
- D. Wang, C. Hao, W. Zheng, Q. Peng, T. Wang, Z. Liao, D. Yu and Y. Li, Adv. Mater., 2008, 20, 2628 CrossRef CAS.
- (a) Y. Yamamoto, T. Fukushima, Y. Suna, N. Ishii, A. Saeki, S. Seki, S. Tagawa, M. Taniguchi, T. Kawai and T. Aida, Science, 2006, 314, 1761 CrossRef CAS; (b) Q. Tang, L. Li, Y. Song, Y. Liu, H. Li, W. Xu, Y. Liu, W. Hu and D. Zhu, Adv. Mater., 2007, 19, 2624 CrossRef CAS; (c) L. Jiang, Y. Fu, H. Li and W. Hu, J. Am. Chem. Soc., 2008, 130, 3937 CrossRef CAS; (d) X. Zhang, J. Jie, W. Zhang, C. Zhang, L. Luo, Z. He, X. Zhang, W. Zhang, C. Lee and S. Lee, Adv. Mater., 2008, 20, 2427 CrossRef CAS; (e) Y. Zhou, L. Wang, J. Wang, J. Pei and Y. Cao, Adv. Mater., 2008, 20, 3745 CrossRef CAS; (f) H. Dong, H. Zhu, Q. Meng, X. Gong and W. Hu, Chem. Soc. Rev., 2012, 41, 1754 RSC.
- P. M. S. Monk, The Viologens: Physicochemical Properties, Synthesis, and Applications of the Salts of 4,4′-bipyridine, 1998, Wiley-VCH Verlag GmbH & Co., KGaA, Weinheim Search PubMed.
- J. Wang, D. Wang, E. K. Miller, D. Moses, G. C. Bazan and A. J. Heeger, Macromolecules, 2000, 33, 5153–5158 CrossRef CAS.
- H. Urayama, G. Saito, T. Inabe, T. Mori and Y. Maruyama, Synth. Met., 1987, 19, 469 CrossRef CAS.
- Y. Zhou, T. Lei, L. Wang, J. Pei, Y. Cao and J. Wang, Adv. Mater., 2010, 22, 148 Search PubMed.
- (a) Y. Che, X. Yang, G. Liu, C. Yu, H. Ji, J. Zuo, J. Zhao and L. Zang, J. Am. Chem. Soc., 2010, 132, 5743 CrossRef CAS; (b) K. E. Martin, Z. Wang, T. Busani, R. M. Garcia, Z. Chen, Y. Jiang, Y. Song, J. L. Jacobsen, T. T. Vu, N. E. Schore, B. S. Swartzentruber, C. J. Medforth and J. A. Shelnutt, J. Am. Chem. Soc., 2010, 132, 8194 CrossRef CAS; (c) Y. Che, H. Huang, M. Xu, C. Zhang, B. R. Bunes, X. Yang and L. Zang, J. Am. Chem. Soc., 2011, 133, 1087 CrossRef CAS.
- (a) O. Nicolet and E. Vauthey, J. Phys. Chem. A, 2002, 106, 5553 CrossRef CAS; (b) R. J. Dillon and C. J. Bardeen, J. Phys. Chem. A, 2012, 116, 5145 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic details, general methods, device fabrication, photoconductive measurements, 1H NMR, UV-Vis, ESI-mass, CV, POM, SEM, XPS, SAED, TG data or images. CCDC 906628. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2cc37655e |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry 2013 |
