Open Access Article
Open Access ArticleA critical assessment of the photodegradation of pharmaceuticals in aquatic environments: defining our current understanding and identifying knowledge gaps†
Jonathan K.
Challis
a,
Mark L.
Hanson
b,
Ken J.
Friesen
c and
Charles S.
Wong
*acd
aDepartment of Chemistry, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada. E-mail: wong.charles.shiu@alum.mit.edu; Fax: +1-204-774-2401; Tel: +1-204-786-9335
bDepartment of Environment and Geography, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada
cDepartment of Chemistry, Richardson College for the Environment, The University of Winnipeg, Winnipeg, Manitoba R3B 2E9, Canada
dDepartment of Environmental Studies and Sciences, Richardson College for the Environment, The University of Winnipeg, Winnipeg, Manitoba R3B 2E9, Canada
First published on 19th March 2014
Abstract
This work presents a critical assessment of the state and quality of knowledge around the aquatic photochemistry of human- and veterinary-use pharmaceuticals from laboratory experiments and field observations. A standardized scoring rubric was used to assess relevant studies within four categories: experimental design, laboratory-based direct and indirect photolysis, and field/solar photolysis. Specific metrics for each category are defined to evaluate various aspects of experimental design (e.g., higher scores are given for more appropriate characterization of light source wavelength distribution). This weight of evidence-style approach allowed for identification of knowledge strengths and gaps covering three areas: first, the general extent of photochemical data for specific pharmaceuticals and classes; second, the overall quality of existing data (i.e., strong versus weak); and finally, trends in the photochemistry research around these specific compounds, e.g. the observation of specific and consistent oversights in experimental design. In general, those drugs that were most studied also had relatively good quality data. The four pharmaceuticals studied experimentally at least ten times in the literature had average total scores (lab and field combined) of ≥29, considered decent quality; carbamazepine (13 studies; average score of 31), diclofenac (12 studies; average score of 31), sulfamethoxazole (11 studies; average score of 34), and propranolol (11 studies; average score of 29). Major oversights and errors in data reporting and/or experimental design included: lack of measurement and reporting of incident light source intensity, lack of appropriate controls, use of organic co-solvents in irradiation solutions, and failure to consider solution pH. Consequently, a number of these experimental parameters were likely a cause of inconsistent measurements of direct photolysis rate constants and quantum yields, two photochemical properties that were highly variable in the literature. Overall, the assessment rubric provides an objective and scientifically-defensible set of metrics for assessing the quality of a study. A major recommendation is the development of a method guideline, based on this rubric, for conducting and reporting on photochemical studies that would produce consistent and reliable data for quantitative comparison across studies. Furthermore, an emphasis should be placed on conducting more dual-fate studies involving controlled photolysis experiments in natural sunlight, and whole system fate studies in either natural or artificial systems. This would provide accurate data describing the actual contribution of photolysis to the overall fate of pharmaceuticals in the environment.
Environmental impactHuman- and veterinary-use pharmaceuticals are present in surface waters globally. Understanding the fate of these contaminants is important for characterizing the occurrence in the environment and exposure to non-target organisms. Photolysis of pharmaceuticals is a major degradation process occurring in natural systems, and has been a topic of significant research. This critical review is an evaluation of the state and quality of knowledge regarding this topic using a weight of evidence-style approach. Specifically, the review aims to highlight inconsistencies in experimental design and reporting that contribute to unreliable data found throughout the literature. Major recommendations are made towards the goal of developing accepted testing guidelines for the study of photolytic fate of pharmaceuticals in aquatic environments. |
Introduction
Human- and veterinary-use pharmaceuticals are a diverse class of aquatic contaminants whose physiochemical properties, use patterns, and means of disposal, can result in significant quantities entering surface waters, making them ubiquitous in many aquatic environments.1,2 Once in surface waters, they can produce a broad range of responses in non-target organisms, including at environmentally relevant concentrations.3–5 Significant research into the environmental occurrence, fate, and effects of pharmaceuticals were in part motivated by a number of influential studies and reviews in the late 1990s.1,6–8 This current critique is an extension of these efforts.Improved understanding of pharmaceutical fate is needed to characterize better the risk these compounds pose to both ecosystems and human-health via aquatic exposure. Specifically, defining their environmental fate processes and removal mechanisms is vital, as these influence the magnitude and duration of exposure to a particular pharmaceutical, and hence risk. Considering the growing call and regulatory requirements for the ecological effects of pharmaceuticals to be included in formal risk assessments, it is paramount that high-quality fate data be generated to define and rank accurately the risks these compounds might pose. In turn, characterizing strengths and weaknesses of available data on fate processes for pharmaceuticals, specifically photolysis here, will allow researchers and regulators to direct their resources appropriately to address knowledge gaps. Therefore, we used a weight-of-evidence-style approach to assess the quality of existing data on the photolysis of pharmaceuticals in aquatic environments.
Photolysis is a major mechanism of removal from the aquatic environment for many pharmaceuticals.9,10 Individual compounds can undergo photolysis to varying degrees, depending on their chemical structure. The presence of aromatic rings and conjugated π systems, as well as various functional groups and heteroatoms, facilitate the direct absorption of solar radiation.9 Such structures result in strong absorption in the UV-C wavelength range, with tailing absorption into the UV-B and in some cases UV-A ranges. The potential spectral overlap with natural sunlight (λ > 290 nm11suggests that these pharmaceuticals may degrade at least partially by direct photolysis. As well, pharmaceuticals can also react with photosensitizing species (i.e., indirect photolysis) such as photolytically excited natural organic matter (NOM), nitrate, carbonate, or iron present in the water column.9
The extent of direct photolysis is commonly determined experimentally by obtaining a direct photolysis rate constant under a given irradiation source (sunlight or artificial light). This has been done throughout the literature for a wide range of pharmaceuticals and is relatively simple experimentally.12–19 Furthermore, direct photolysis can be predicted to a large extent by two factors: the rate of light absorption, dependent on the molar absorption properties of a chemical and light intensity in the UV-B and UV-A ranges; and the quantum yield, a measure of how efficiently a compound reacts upon absorption of a photon.20 While quantum yields serve as a much better predictor of direct photolytic fate than just simply rate constants and half-lives, fewer studies tend to measure them as their determination is more complex. Quantum yields are a characteristic property of a compound over a given wavelength range that can be compared across studies and used to predict real environmental fate,20 while a degradation rate constant is completely dependent on the specific light conditions used. In part, this review will touch on the predictive ability of quantum yields and discuss the experimental problems leading to inconsistent quantum yield determination for pharmaceuticals.
Indirect photolysis mechanisms often play a major role in the overall photolytic fate of pharmaceuticals, especially for those drugs that do not appreciably absorb light above 290 nm (e.g., ibuprofen19). Indirect mechanisms are increasingly complex and are much harder to predict, as chemicals can react via multiple pathways through interaction with naturally occurring photo-generated transient species. Many studies have detailed various mechanisms involving triplet excited dissolved organic matter (3DOM), singlet oxygen (1O2), hydroxyl radicals (˙OH), and others.13,21–27 Identifying specific species responsible for indirect photolysis involves detailed kinetic work that may be complicated in some cases by multiple mechanisms and competing effects. These types of issues make predictions regarding indirect photolysis difficult, and thus, pharmaceuticals generally need to be studied, for now, on a case-by-case, compound-by-compound basis. There are, however, methods that can be utilized to isolate possible reactive species and measure bimolecular second-order reaction rate constants for individual pharmaceuticals. These techniques, in some cases borrowed from the radiation chemistry literature, include pulsed radiolysis, spectroscopy, and competition kinetics.28,29
The general classes of pharmaceuticals (e.g., antibiotics, anti-psychotics, non-steroidal anti-inflammatory drugs or NSAIDs, etc.) are an extremely diverse collection of chemical compounds, which can be broken down further into different families of compounds on the basis of some structural similarity and pharmacological function. In many studies evaluated for this review, a subset of compounds from a family of drugs were examined to compare how the extent of photodegradation differs based on structural differences.13,21,23,30 For example, the family of sulfonamide antibiotics contain an identical backbone structure comprised of an aniline ring and a sulfonamide group, differing only in their R-heterocyclic functional groups. Boreen et al. studied a number of five-13 and six-21membered ring sulfonamides and observed differing photochemical reactivity amongst the very similarly structured compounds. Piram et al. also observed differing degrees of photolysis amongst a number of structurally related β-blocker drugs.30 These examples further illustrate the complexity of predicting the environmental photolysis of pharmaceuticals.
Two separate approaches were taken to evaluate and summarize the state of knowledge regarding the aquatic photochemistry of pharmaceuticals. The primary and overarching focus of this critical review employed a weight of evidence-style approach drawing from the work of Van Der Kraak et al.31 in order to assess the quality of data available in the literature as it relates to the photolytic fate of pharmaceuticals in aquatic systems. To this end, we developed a transparent scoring rubric to ascertain the overall quality of the studies under evaluation. Alternatively, a more traditional approach to a review paper was taken whereby relevant studies with similar/contrasting focus and/or findings were summarized and discussed. This took the form of both summary tables of data and discussions throughout the text. The objective of this specific approach, and specifically the use of summary tables, was to create an easily accessible summary of photolytic fate, and to readily identify conflicting conclusions regarding photolysis mechanisms for specific pharmaceuticals. While the two approaches are somewhat separate in practice, the discussion was tied back whenever possible to the weight of evidence-style approach in order to substantiate examples and draw concrete conclusions.
The weight-of-evidence approach is increasingly being employed in ecotoxicology, and is often applied to toxicological data to determine evidence for hazard in risk assessments, and to facilitate consistent and reliable data evaluation.32 Overall, there are a number of drivers that prompted this critical review into pharmaceutical photolysis. Firstly, many regulators (e.g., US-, EU-, Japanese-Environmental Protection Agencies, OECD – The Organisation for Economic Co-operation and Development) are working towards the establishment of testing programs and strategies to assess human and wildlife health risks associated with pharmaceuticals in the environment prior to their approval.33 As regulations become more stringent, they will require regulated and consistent testing protocols for their implementation. This is exemplified with the case of endocrine disrupting chemicals (EDCs, e.g., 17α-ethinylestradiol), for which attempts have been made at harmonizing testing strategies and decision-making criteria regarding their fate and effects.33
While post-production release of pharmaceuticals is not currently regulated globally, there is an emerging effort to do so in select jurisdictions. For example, in 1999 the European Commission adopted the “Community Strategy for Endocrine Disruptors” to develop strategies addressing research, communication, and policy, and provide short-, medium-, and long-term recommendations regarding the presence of EDCs in the environment.34 A recent report35 on the implementation of the “Community Strategy for Endocrine Disruptors” summarized a number of these short-, medium-, and long-term actions, including the establishment of a list of priority EDCs, monitoring programs, development of accepted criteria and testing strategies for identification and assessment of EDCs, and legislative actions involving the inclusion of EDCs in the European Union's REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) program (EC 1907/2006).36
Therefore, a critical assessment of the data around fate, in this case photolytic fate, of pharmaceuticals in the environment is needed, with a specific focus on testing criteria and guidelines that will facilitate consistent and reliable research in this area. Additionally, the last review on the photolysis of pharmaceuticals is now a decade old (2003),9 while a more recent (2011) review10 focused on the photodegradation products of pharmaceuticals. Significant research and progress has been made since 2003, with over 120 studies published on the photolysis of pharmaceuticals in the environment since that year – suggesting the need for a detailed critical review.
To this end, we developed a scoring rubric for available peer-reviewed literature based in part on OECD 31637 and US EPA OPPTS 835.221038 guidelines on direct photolysis. Both of these guidelines currently deal expressly with direct photolysis. The rubric developed here encompasses lab/field experimental design and direct/indirect photolysis criteria. Each study was weighted and scored based on how well it met the specific criteria outlined in the rubric. The scores then allow for simple, easily interpreted 2-dimensional plots (y-axis = laboratory studies, x-axis = field studies) to assist in identifying both significant knowledge strengths and gaps in our understanding of photolysis by pharmaceutical class, as well as the quality of the data generated to date. Relevant studies were highlighted, and their contributions to photochemical knowledge as well as their limitations are summarized using the rubric. The rubric approach ultimately helps to outline parameters to enable the drawing of comprehensive conclusions regarding the photolytic fate of pharmaceuticals. In various contexts, this critical review evaluated laboratory versus field photolysis investigations, direct and indirect photolysis, rate constants and quantum yields, photodegradation products, the quality of experimental design, and the reporting of data and information, all as they pertain to the aquatic photochemistry of pharmaceuticals.
Methods
Literature used in the review
A total of 120 papers were critically evaluated and scored, representing a large majority of the relevant literature available on this topic.12–19,21–26,30,39–143 Papers for inclusion were obtained via searches of the Web of Knowledge and SciFinder databases between June and September 1, 2013. The following search terms were used in various combinations: photolysis, photodegradation, pharmaceuticals, drugs, polar organic pollutants, environment(al), aquatic, surface waters, wetlands, experiment(s). In summary, studies included used either natural sunlight or artificial light emitting wavelengths greater than ≈290 nm (environmentally relevant) as an irradiation source (i.e., simulating natural sunlight). Studies done in non-aqueous solution or under non-environmentally relevant wavelengths (<290 nm, e.g., for advanced oxidation treatment) were not included in this review.Of these papers, 83% were published in the last ten years (2004–2013) and 42% in the last four (2010–2013), suggesting a significant increase in both the interest and knowledge regarding the photochemical fate of pharmaceuticals in aquatic environments. From this list of evaluated works and scores, the volume and quality of knowledge, respectively, for a given pharmaceutical drug can be ascertained by considering the number of times a given drug was studied and the overall scores those studies obtained. This type of information highlights data gaps regarding specific pharmaceuticals and/or the quality of data generated for a given compound. Generally speaking, there were seven major classes of pharmaceuticals that were among the most studied in terms of their photolytic fate in aquatic environments. These were (1) antibiotics (59/50 – # publications studying the respective compound class/# of different compounds studied in the class); (2) non-steroidal anti-inflammatory drugs or NSAIDs (22/7); (3) anti-psychotics (22/15); (4) β-blockers (19/10); (5) cholesterol-lowering drugs (12/6); (6) hormones (11/7); and, (7) analgesics (8/6). The studies included in this review represented a total of 116 different pharmaceutical compounds. Table S1A–G (ESI†) provides a list of all the studies evaluated and scored using the rubric, and the scores obtained by each study, organized alphabetically by pharmaceutical class. Individual scoring sheets for specific studies are available from the corresponding author upon request.
Weight-of-evidence-style scoring
The full scoring rubric is provided in Table S2.† The rubric is divided into four sections: (1) experimental design; as well as important parameters or metrics involved in laboratory-based study of (2) direct photolysis, (3) indirect photolysis, and (4) field/solar photolysis. Each scoring metric was chosen to encompass as much information as possible about the quality of the study that could be examined objectively and rapidly. Additionally, wherever possible, criteria were designed as ‘yes’ or ‘no’ questions in order to make the system as simple as possible. The last criterion in the rubric “Other processes considered as they relate to photolysis” was the one area that allowed some discretion in scoring. This criterion gave additional points for aspects of a study that either did not fit into any of the developed criteria or were not photolysis-specific, but related and important in some way to the overall fate of the drug.It is important to note that for the purposes of this work, the scoring rubric was designed specifically for environmentally relevant, experimental photolysis studies and thus the rubric criteria were chosen accordingly. It is important that the rubric be somewhat focused, otherwise evaluations simply become too time consuming and difficult to conduct. As a result, studies that did not fit well with our rubric criteria were generally not evaluated (e.g., photolysis modeling-type studies). However, this rubric is highly adaptable to fit different types of studies, and it is strongly encouraged that it be modified accordingly so that this technique can be used in other contexts and applications. While not explicitly scored, conducting studies either under or in the spirit of good laboratory practice (GLP) as well as open dissemination are highly encouraged.
A majority of the metrics making up the Experimental section of the rubric were adapted from the OECD Guidelines for the Testing of Chemicals no. 316: Phototransformation of Chemicals in Water – Direct Photolysis37 and the US EPA Fate, Transport, and Transformation Test Guideline OPPTS 835.2210: Direct Photolysis Rate in Water By Sunlight.38 Specific recommendations from the guidelines were either used as ‘yes’ (=1) or ‘no’ (=0) criteria (e.g., triplicate irradiations, dark control) or adapted to work as weighted criteria (e.g., wavelength distribution of light source, sample vessel, measured light flux) on a number scale, 0–4 (Table S2†). Other rubric criteria were designed from, and based on, experimental precedents set in the literature, with common trends across studies being incorporated into certain criteria. For example, detailed photo-product studies generally apply high-resolution mass spectrometry (and to a lesser extent, nuclear magnetic resonance), monitor formation/loss of photodegradation products, and propose mechanisms/pathways for the breakdown of parent pharmaceuticals to photo-products. Thus, these aspects of a study represented top scores (3–4) for the photo-product criterion.
The direct and indirect photolysis sections focused on the quality and thoroughness of the kinetic data, and for the most part were derived from experimental aspects common throughout kinetic photolysis literature. The criterion ‘R2-value of first-order plots’ (i.e., the degree of linearity) evaluates the uncertainty in the kinetic data, giving an indication as to the appropriateness of fitting (pseudo) first-order kinetics to the data. Furthermore, reporting errors in rate constants and quantum yields is important to lend transparency to the study in terms of precision of the experimental technique and the spread in the data. Determination of rate constants and quantum yields, consideration of pH and pKa, measurement of photosensitizing species, and the absorbance spectra of both compound and matrix are important metrics addressed across a large number of published photolytic fate studies, and thus, are important to consider when evaluating the quality of a study. The above mentioned rubric sections apply to both laboratory and sunlight photolysis experiments. However, extra considerations are necessary to account for different experimental methods used in the field versus the laboratory, hence the field/solar photolysis criteria (Table S2 in ESI†). While we recognize that other definitions of appropriate criteria are possible, our rubric approach allowed for data evaluation in a quantitative and consistent manner, and can be easily modified as needed.
Briefly, a study would be described by the pharmaceutical compound(s) studied and a set of pre-defined keywords, chosen based on the type and nature of the experiments conducted (see Table S3† for two examples of evaluated studies). These seven keywords were: lab irradiations, sunlight irradiations, field experiment, direct photolysis, indirect photolysis, photo-products, and quenching/competition mechanism experiments. The rubric was then filled out, including an explanation for why each criterion was scored as it was. The scores from each rubric section were totalled, and a brief description of the general findings was given.
One of the purposes of this rubric was to assess and identify which experimental and kinetic aspects of a photolysis study were necessary to produce both environmentally relevant and reliable data (i.e., information that can be used to understand and/or predict photolytic fate processes of pharmaceuticals) and give a score accordingly. For example, wavelengths below ≈ 290 nm and/or the use of organic solvents in the irradiation solutions – which may quench photosensitizing/intermediate species, altering the photolysis mechanism – were considered fundamental flaws in terms of environmental relevance of a study. Consequently, a study with these fundamental flaws in the experimental design would receive a lower score. This rubric also served to highlight any significant insufficiency in the basic reporting of information. This was especially evident for specific experimental criteria including number of replicates, dark controls, organic solvent carrier, solution pH, and temperature. If specific criteria were not explicitly reported in a study, it was assumed that these were not met, thus resulting in lower scores. In general, higher scores should equate to stronger, higher quality data and thus, predict environmental fate more accurately. However, as many of the studies scored were conducted in the laboratory, comparison to environmental fate data was in many cases not possible given a lack of field photolysis data for many pharmaceuticals. Where possible, comparisons to this end are made and discussed in the relevant sections of this review.
Results
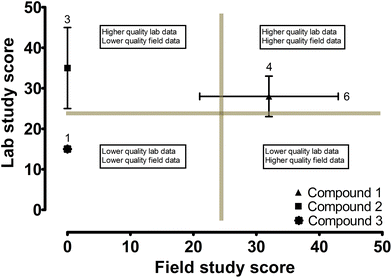
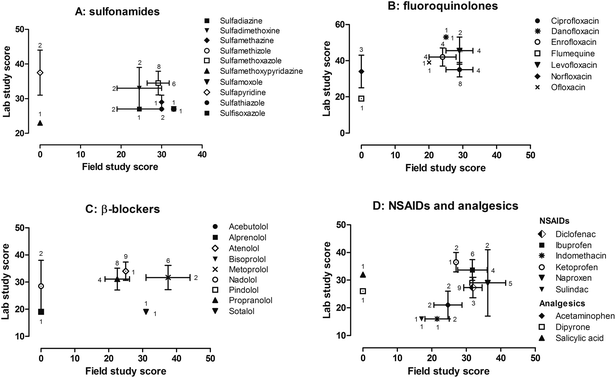
A hypothetical ‘maximum score’ was 73, based on the highest score in every criterion of the rubric. Studies were considered high quality if they obtained an overall score (lab and/or field) >40; mid-quality, 25–40; and low-quality, <25. While these rankings of high-, mid-, and low-quality studies, and the respective scoring range are somewhat arbitrary and specific to this rubric only, they are largely based on the average overall score for all laboratory and field studies (32), essentially following a normal distribution around the average. Scores ranged from 12–53, having a mode of 19 (obtained by 31 studies). To ensure the scoring exercise was consistent and reliable, 5% (six studies) of the total evaluated studies were chosen at random and re-scored. An average standard deviation of 1.1 (SD range = 0–2.1) was obtained between the initial and re-scored scores.An example score plot is shown in Fig. 1, with mock laboratory (y-axis) and field (x-axis) study scores plotted for three compounds, to orientate the reader as to how these score plots should be interpreted. The quality of data generated by a given study can be understood through the scores of given pharmaceuticals, based on where they fall on the plots. Generally speaking, compounds clustering closer to the upper right corner of these plots suggest high quality of laboratory and field data, while the lower left corner implies low quality data. The purpose of this exercise is to represent graphically the quality of laboratory and/or field data for specific pharmaceuticals within a family, via the rubric scores. The scoring of studies in this case is a surrogate for the quality of data, so a low score implies low quality data while a high score implies high quality data. A select set of our compiled results from our scoring exercise are depicted in Fig. 2, grouped by five major drug families: sulfonamides, fluoroquinolones, β-blockers, non-steroidal anti-inflammatory drugs (NSAIDs), and analgesics.
The photochemical fate of the β-blocker family has been studied extensively in the literature. Atenolol (open diamond; Fig. 2C) is a good example of how these plots can help identify data gaps for individual compounds. It has been studied nine times in the laboratory producing relatively strong data (average score >30, with a small standard error), but has only been studied under natural sunlight once, with a score of 25. Moreover, we note that acebutolol, alprenolol, bisoprolol, and pindolol – comprising close to half of the β-blockers that have been studied photochemically in the peer-reviewed literature – all overlap each other in the lower left hand corner of the plot (Fig. 2C) with a score of 19, suggesting relatively low quality of data for these compounds. Thus, conducting future investigations for atenolol under field conditions, and for the other β-blockers in general, are two recommendations from our scoring exercise.
The sulfonamide antibiotics are another family of drugs commonly studied in the literature. Sulfamethoxazole (open square, Fig. 2A) has been studied eight times in the lab and six times in the field, with an average score near or above 30 in both cases. The relatively small error bars for sulfamethoxazole indicate that all fourteen studies share similar scores (i.e., similar quality of data), which in this case should be considered a strong data set overall. Alternatively, for sulfadiazine (closed small square, Fig. 2A) and sulfadimethoxine (down-facing small triangle, Fig. 2A) we note large horizontal error bars (N = 2 field studies), indicating that one study scored significantly greater than the other for these two compounds. In such a case, a potential application of this scoring technique could be to differentiate the reliability of data from the scores of two (or more) respective studies. Furthermore, when there are discrepancies between data sets, this approach can aid in determining which data should be used and applied, e.g., within risk assessments,32 development of regulatory guidelines, and/or environmental fate modeling exercises. Of course, aside from just comparing scores, the data itself must also be critically examined (e.g., assessing the consistency within the science of photochemistry) to make a final decision as to the validity of one study over another.
There are other gaps that can be identified by using the rubric, besides lack of knowledge around specific compounds or classes of pharmaceuticals. For example, it reveals aspects often missed or overlooked in photochemical studies due to lack of reporting and/or oversights in experimental design, as noted previously by Fatta-Kassinos et al.10 and Hu et al.144 Some of the major and common oversights amongst the papers included:
• A lack of information regarding the irradiation source, wavelength range, light intensity/flux, and the radiometer/actinometer measurement technique – 44% of studies either did not report anything regarding light intensity measurement or reported a light intensity but not the measurement technique used (i.e., actinometer);
• Whether multiple irradiations were conducted – 63% of studies either did not report if replicate irradiations were done or reported single replicates;
• The use of dark controls – a number of studies made no mention of a control sample – 33% of studies did not report the use of dark controls;
• The presence of a solvent carrier in the aqueous irradiation solutions, which may quench photosensitizer species – 54% of studies either did not explicitly report the presence or absence of solvent in irradiation solutions or reported the use of solvents known to quench photosensitizing species (e.g., methanol for hydroxyl radicals);
• Consideration or reporting of pH in the irradiation solutions – 22% of studies did not report the pH of the irradiation solutions.
These criteria were considered fundamental to a strong photochemical investigation, yet they are frequently underreported in the literature, making interpretation and comparisons amongst studies difficult. For example, if a study simply fails to report the pH of the irradiation solution, comparison is difficult given that the protonation state can have a significant effect on the photochemical kinetics of many pharmaceuticals.13,15,21–23,65
Discussion: current progress and specific challenges
As noted, a significant amount of work has been done since the late 1990s to investigate the photochemical fate of pharmaceuticals in water, culminating in a large collection of data. Progress has been made in understanding and elucidating the photolytic mechanisms that limit the persistence of many pharmaceutical contaminants in aquatic environments. Herein, we summarize current knowledge and challenges regarding kinetics and mechanisms of photolysis, quantum yield determination, and photodegradation products, based on data and trends observed during the scoring exercise. Specifically, this discussion will focus on many of the rubric metrics that were frequently overlooked throughout the literature. The general direct and indirect photolytic processes dominating the fate of seven well-studied pharmaceuticals representing the six most-studied classes (ciprofloxacin – antibiotic; sulfamethoxazole – antibiotic; ibuprofen – NSAID; carbamazepine – anti-psychotic; atenolol – β-blocker; clofibric acid – cholesterol lowering agent; and 17α-ethinylestradiol – synthetic hormone) are summarized in Table 1. The following discussions will be focused on the experimental techniques used and inconsistencies in the resultant data produced.| Compound (CAS) | Study typea | Photolysis mechanism | Ref. |
|---|---|---|---|
| a Type of study indicates if the experiment was done in the laboratory = L (artificial light), in isolated systems under natural sunlight = S, or in the field = F (‘whole system’ fate study, e.g., using mesocosms or natural system). b Rapid degradation: t1/2 < 3 h. Quick degradation: t1/2 = 3–6 h. Moderate degradation: t1/2 = 6–12 h. Slow degradation: t1/2 > 12 h. | |||
| Ciprofloxacin (85721-33-1) | L | Direct photolysis identified as major mechanism – rapid degradation (t1/2 = 1.2 min). Slightly reduced degradation rates in synthetic waste water and river water | Babic et al.12 |
| L | Photolysis in water spiked with CaCO3 (direct versus indirect mechanisms not separated) was significant without fine particulate organic matter (FPOM) – rapid degradation (t1/2 = 2.9 h). FPOM reduced aqueous concentration significantly (sorption), slowing photolytic degradation | Belden et al.48 | |
| L | Direct photolysis identified as major mechanism – rapid degradation (t1/2 = 23 min). Also suggested that self-sensitized photo-oxidation via ˙OH and 1O2 is an important mechanism. Indirect photolysis species (humic acids, nitrate, iron) did not increase or decrease degradation rates compared to direct photolysis | Ge et al.23 | |
| L | Direct photolysis identified as major mechanism – rapid degradation (t1/2 = 13 min). Variable but small indirect effects. Faster and slower rates compared to direct photolysis depending on the concentrations of nitrate, cDOM, and carbonate | Lam et al.26 | |
| L | Only direct photolysis was considered, at 5 different pH values. Most reactive at pH 8.6. Rapid degradation (t1/2 ≈ 20 min). The extent of photolysis ranged, depending on pH | Torniainen et al.120 | |
| L | Only direct photolysis was considered, at 3 different pH values. Most reactive at pH 7. Rapid degradation (t1/2 ≈ 9 min). The extent of photolysis ranged, depending on pH | Vasconcelos et al.124 | |
| L | Only direct photolysis was considered at 11 pH values between 2–12. Most reactive at pH 8. Rapid degradation (t1/2 = 23 min). The extent of photolysis ranged, depending on pH | Wei et al.131 | |
| L, F | Photolysis in sterilized mesocosm water (direct versus indirect mechanism not separated) was significant in lab (t1/2 = 1.9 h) and field mesocosms (t1/2 = 1.1 h) (low levels of particulate organic carbon) – rapid degradation. Presence of amended DOC slowed degradation in lab. Lab mesocosm water spiked with POC reduced soluble ciprofloxacin at a rate more rapid then photolysis | Cardoza et al.53 | |
| S | Photolysis experiments only conducted in raw, unfiltered river water samples (no direct photolysis). Complete decomposition (≈0% remaining) of ciprofloxacin was observed after 20 minutes. Rapid degradation | Sturini et al.116 | |
| S | Direct and indirect photolysis. Slow degradation. Complete decomposition (≈0% remaining) took >150 days in pure water and ≈125 days in river water. Based on general consensus in the presented literature, these results appear to be an obvious outlier, thus omitted from the summary. The low score this study received (25) further supports the omission from the summary statement | Turiel et al.123 | |
| Summary | — | Consensus amongst all studies that direct photolysis is the major mechanisms responsible for photodegradation of ciprofloxacin. Photolysis rates in general are rapid for this compound. Degradation is most rapid at slightly basic pH | — |
| Recommendation | — | Data for this compound is generally consistent across studies. The two sunlight studies are of lower quality based on the rubric scores. Should be studied more under natural sunlight and in the field for comparisons to laboratory derived data | — |
| Sulfamethoxazole (723-46-6) | L | Only direct photolysis was assessed. Degradation was rapid. The neutral form at pH 3.2 was most reactive (t1/2 = 0.031 h) | Bonvin et al.49 |
| L | Direct and indirect photolysis were important processes. Direct photolysis degradation was quick (t1/2 ≈ 4 h, approximated from graph). In wetland water containing significant levels of DOC, nitrate, and carbonate, degradation increased (t1/2 ≈ 2 h, approximated from graph). ˙CO3− and 3DOM were important photosensitizing species | Jasper and Sedlak75 | |
| L | Direct photolysis identified as major mechanism. Rapid degradation (t1/2 = 1.5 h). Indirect experiments in synthetic field water (varying concentrations of DOM, nitrate, carbonate) resulted in slower degradation (t1/2 = 2.8–6 h, depending on concentration of photosensitizers). Indicated that ˙OH radicals mediated degradation however this effect was smaller than the light screening | Lam et al.84 | |
| L | Direct photolysis was identified as the major photolysis mechanism. Degradation was rapid (t1/2 = 12.6 min). Greatest reactivity observed at acidic pH. Indirect photolysis experiments in the presence of fulvic acids and suspended particles decreased degradation rates | Niu et al.100 | |
| L | Direct photolysis was found to be the major mechanism. Degradation was rapid (t1/2 ≈ 1 h, exact half life values were not reported). Indirect photolysis experiments in the presence of humic material or nitrate resulted in much slower degradation | Trovo et al.122 | |
| L, S | Direct and indirect photolysis was found to be important photolysis mechanisms. Direct photolysis was slow (t1/2 = 2.4 days in sunlight – calculated using measured quantum yield). In the presence of nitrate or humic acids photolysis half lives were 4.3 and 3.1 times faster compared to direct photolysis | Andreozzi et al.41 | |
| L, S | Direct photolysis was identified as the primary mechanism. Degradation was quick, and most rapid in its neutral state (t1/2 = 3.2 h in sunlight). Indirect photolysis may be important in some waters. 1O2 and ˙OH-radicals may only play a role in nitrate and humic rich waters where the concentration of these species would be high | Boreen et al.13 | |
| L, S | Only direct photolysis was assessed. Degradation was rapid under lab-light and sunlight, with half lives around 1 h (t1/2 values not given). Photolysis was pH dependent and most reactive in acidic solution | Moore et al.98 | |
| L, S | Direct photolysis was identified as primary mechanism in pure water (t1/2 = 1.7 h) and lake water (t1/2 = 1.6 h). In STP effluent indirect photolysis was significant (t1/2 = 1 h), attributed to ˙OH radicals and 3DOM. Also, deoxygenation led to more rapid direct photolysis indicating that direct photolysis proceeds through a triplet excited state | Ryan et al.111 | |
| S, F | Photolysis in natural river water (direct versus indirect mechanism not separated). No photodegradation observed, however samples were only irradiated for 6 h | Kunkel et al.80 | |
| S, F | Photolysis experiments done in natural mesocosm water (direct versus indirect mechanism not separated). No kinetic data given, but photolysis was concluded to be an important removal mechanism from mesocosms | Lam et al.85 | |
| Summary | — | Direct photolysis seems to be the primary mechanism for many studies. Indirect photolysis is variable between studies. Some report light screening effects while others observe small increases in degradation due to 1O2, ˙OH-radicals, or 3DOM. Indirect photolysis seems to be largely dependent on the type of water used in the experiments and the concentrations of photosensitizing species. In general photolysis of sulfamethoxazole is rapid to quick. The neutral form is most reactive | — |
| Recommendation | — | Data for this compound is consistent. Sulfamethoxazole is very well studied in the lab and field. No recommendations | — |
| Ibuprofen (15687-27-1) | L | Direct photolysis was of minor importance (t1/2 = 277 h). Rates increased significantly in the presence of fulvic acids (t1/2 = 36, 25, 9 h depending on type of fulvic acid). ˙OH radicals (terrestrial-DOM) and 3DOM (algal/bacterial-DOM) were important photosensitizing species | Jacobs et al.73 |
| L | Direct photolysis was of minor importance. Slow degradation (t1/2 = 205 h). In river water, degradation rate increased significantly (t1/2 = 15 h). Indirect photolysis identified as major mechanism – photosensitizing species not identified | Lin et al.18 | |
| L | No significant degradation was observed for direct photolysis experiments (t1/2 = 1437–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 931 h depending on light source). Indirect experiments in the presence of humic material significantly increased half lives (t1/2 = 556–921 h, depending on light source). Indirect photolysis was identified as the primary mechanism 931 h depending on light source). Indirect experiments in the presence of humic material significantly increased half lives (t1/2 = 556–921 h, depending on light source). Indirect photolysis was identified as the primary mechanism |
Peuravuori et al.104 | |
| L | Direct photolysis was of minor importance. Slow degradation (half life not reported, but from plots t1/2 ≫ 24 h). Indirect photolysis of some importance (rates not given), with ˙OH being the primary photosensitizing species | Vione et al.126 | |
| L, S | In lab experiments, direct photolysis was identified as the primary mechanism. Moderate degradation (t1/2 = 4 h). Degradation rates slowed slightly in seawater and river water. For sunlight experiments done in river water degradation was slow (t1/2 = 324 h) | Matamoros et al.96 | |
| L, S | No significant degradation was observed under natural sunlight, thus laboratory lamp (5× stronger than sunlight) was used. Direct photolysis showed moderate degradation (t1/2 = 11.6 h). Degradation rates increased significantly in river water (t1/2 = 2.6 h). Indirect photolysis was identified as the primary mechanism. ˙OH radicals played a role in degradation, likely along with other reactive oxygen species | Packer et al.19 | |
| S | Only direct photolysis was considered. Slow degradation (t1/2 = 9900 h in May to 600 h in August) | Yamamoto et al.139 | |
| S, F | Direct photolysis identified as major mechanism. Slow degradation (t1/2 = 6.3 days). Experiments in river water did not increase or decrease degradation rates compared to direct photolysis. In situ field rates (natural river system) were slightly faster (t1/2 = 4.6 days) | Fono et al.68 | |
| S, F | Photolysis in natural river water (direct versus indirect mechanism not separated). No photodegradation observed, however samples were only irradiated for 6 h | Kunkel et al.80 | |
| Summary | — | A majority of the studies observe indirect photolysis to play a major role in photodegradation of ibuprofen. There are some studies that found direct photolysis to be important. ˙OH radicals seem to be the main photosensitizer responsible for this degradation. In general, photolysis of ibuprofen is slow | — |
| Recommendation | — | With few exceptions, data for this compound is consistent. Ibuprofen has been studied multiple times in the lab and field. No recommendations | — |
| Carbamazepine (298-46-4) | L | Only direct photolysis experiments conducted. Photolysis was for the most part slow but varied significantly with pH and dissolved oxygen (t1/2 = 0.5–95 h). Reactivity was greatest at acidic pH and low dissolved oxygen levels | Calisto et al.14 |
| L | Photolysis experiments were done in natural river water (direct versus indirect mechanism not separated). Degradation was slow (t1/2 ≈ 5 days, from zero-order plots). Kinetic data not reported | Calza et al.52 | |
| L | Direct and indirect photolysis was important. Indirect photolysis experiments in artificial river water (humic acids, iron, nitrate, Cl−) showed slightly decreased degradation compared to the direct photolysis rates. Iron and Cl− in solution caused degradation rates to increase significantly. Half lives were not reported | Chiron et al.59 | |
| L | Direct photolysis was of minor importance. Degradation in pure water was slow (t1/2 = 19 h). Indirect photolysis experiments with NOM enhanced photolysis of carbamazepine significantly (t1/2 = 6.8 h) | Doll et al.64 | |
| L | Direct photolysis processes were insignificant. Indirect photolysis in wetland water resulted in moderate degradation (t1/2 ≈ 8.5 h, estimated from plot). 3DOM was found to contribute slightly to removal, but degradation was primarily through reaction with ˙OH radicals at pH 8.5. At pH 10.5 degradation rate decreased by 70% because of low reactivity with ˙CO3− radicals | Jasper and Sedlak75 | |
| L | Direct photolysis was of minor importance. Degradation in pure water was slow (t1/2 = 115 h). Indirect photolysis increased degradation rates significantly (t1/2 = 6–55 h, depending on the concentrations of DOM, nitrate, and carbonate). ˙OH radicals suggested as the species likely responsible for degradation | Lam et al.84 | |
| L | Photolysis overall was very slow (t1/2 ranging from 33 days in pure water to 21 days in humic rich water) | Peuravuori et al.104 | |
| L, S | Direct and indirect photolysis rates were slow. Direct photolysis was slow (t1/2 ≈ 100 days in sunlight – calculated using measured quantum yield). In the presence of nitrate, photolysis was 2.3 times faster compared to direct photolysis. Humic acids acted as a light screen, slowing degradation down four-fold | Andreozzi et al.42 | |
| L, S | Direct and indirect photolysis contributed to degradation. Direct photolysis was slow (t1/2 ≈ 38.5 h; lab-light). Indirect photolysis experiments in seawater and two different natural river waters significantly increased degradation rates (t1/2 ≈ 14.4, 12.8, 8.25 h, respectively; lab-light). In natural sunlight degradation in one of the river waters was slow (t1/2 ≈ 67.4 h) | Matamoros et al.96 | |
| S | Direct photolysis degradation was slow (t1/2 = 121.6 h). Indirect photolysis experiments increased degradation significantly (t1/2 = 69, 24.5, 11.2 h – half lives decreasing with increasing concentrations of nitrate). Humic acid acted as a light screen, slowing degradation down significantly compared to direct photolysis (t1/2 = 233.7 h). In natural river water the half life was increased further to 907 sunlight hours | Andreozzi et al.41 | |
| S | Only direct photolysis was considered. Slow degradation (t1/2 = 2100 h in May to 84 h in August) | Yamamoto et al.139 | |
| S, F | Photolysis experiments were done in natural river water (direct versus indirect mechanism not separated). No photodegradation observed, however samples were only irradiated for 6 h | Kunkel et al.80 | |
| S, F | Photolysis experiments done in natural mesocosm water (direct versus indirect mechanism not separated). Degradation was slow (t1/2 ≈ 10 days). Photolysis was concluded to be an important removal mechanism from mesocosms | Lam et al.85 | |
| Summary | — | Overall, photodegradation is slow. Carbamazepine seems to be relatively persistent towards direct photolysis. Indirect photolysis in most cases increased degradation rates. Humic material reported to enhance or slow degradation, varying from study to study. Likely to do with type and concentration of humic material. ˙OH radicals seem to the primary photosensitizer responsible for degrading carbamazepine | — |
| Recommendation | — | Data for this compound is consistent. Carbamazepine is very well studied in the lab and field. No recommendations | — |
| Atenolol (29122-68-7) | L | Only direct photolysis was considered. Degradation was slow at pH 7.4 (t1/2 = 45.2 h) to moderate at pH 4 (t1/2 = 6.87 h) | Andrisano et al.43 |
| L | Direct photolysis was not a significant degradation process. Indirect photolysis in the presence of fulvic acids was moderate in air saturated solutions (t1/2 = 10.8 h) to rapid in nitrogen purged solutions (t1/2 = 5.9 min). Degradation increased with increasing pH = 6–10. Presence of metal cations slowed degradation. 3DOM was identified as the species responsible for the indirect photolysis | Chen et al.22 | |
| L | Direct photolysis processes were insignificant. Indirect photolysis in wetland water resulted in moderate degradation (t1/2 ≈ 9 h, estimated from plot). Degradation was primarily through reaction with ˙OH radicals at pH 8.5, accounting for 80% of removal. At pH 10.5 degradation rate was mostly unchanged because of high reactivity with ˙CO3− radicals | Jasper and Sedlak75 | |
| L | Only indirect photolysis was tested. Degradation in the presence of nitrate was moderate (t1/2 = 11.4 h) and increased to rapid at higher nitrate concentrations (t1/2 = 1.6 h). Reactivity was slightly greater at pH 4.8 versus pH 10.4. Humic substances slowed degradation acting as a light filter. Nitrate induced ˙OH radicals were identified as the primary degradation mechanism | Ji et al.76 | |
| L | Only direct photolysis experiments were conducted. Direct photolysis was slow (t1/2 = 350 h) | Liu et al.92 | |
| L | Direct photolysis was of minor importance. Degradation was slow (t1/2 = 670 h). In three different types of natural river water degradation was slow but increased significantly compared to direct photolysis (t1/2 = 35–127 h). A small portion of this degradation was attributed to biodegradation | Liu et al.91 | |
| L | Direct photolysis in pure water and indirect photolysis in sewage effluent were not observed. Stable towards photolysis | Piram et al.30 | |
| L | Direct and indirect photolysis experiments. Direct photolysis was slow (t1/2 = 8.2 days) and accounted for ≈7% of total degradation. Presence of NOM significantly increased degradation. Primary reactive species was 3DOM accounting for 85% of degradation. Hydroxyl radicals accounted for ≈7% | Wang et al.129 | |
| L | Direct photolysis experiments did not result in any degradation. The presence of DOM caused photodegradation. Rates increased with concentration of DOM. Half lives were at least >20 h (estimated from plots) and depended on the type of DOM. When nitrate was present, DOM slowed down degradation (t1/2 = 6.8–8.9 h, depending on DOM type) compared to nitrate itself (t1/2 = 4 h). In solutions of iron and DOM, degradation was enhanced significantly compared to DOM alone. ˙OH radicals identified as the main reactive species in the photolysis process | Zeng et al.140 | |
| S | Only direct photolysis was considered. Degradation was slow (t1/2 = 730 h in May to 77 h in August) | Yamamoto et al.139 | |
| Summary | — | Direct photolysis of atenolol is of minor importance. Indirect photolysis is the major mechanism. Nitrate seems to be an important species mediating ˙OH radical reactions – a photosensitizing species responsible for atenolol degradation. 3DOM is the other major photosensitizing species responsible for atenolol photodegradation. The importance of ˙OH versus3DOM is not obvious but likely depends on the type of NOM, steady state concentrations, and water chemistry (e.g., nitrate, pH). Overall, photolysis of atenolol is slow | — |
| Recommendation | — | Data for this compound is consistent and quite extensive in the laboratory. Atenolol has only been studied once under natural sunlight with very limited data from the single sunlight study. More photolysis experiments should be conducted under sunlight and in the field | — |
| Clofibric acid (882-09-7) | L | Direct photolysis was slow (t1/2 > 70 h, from plot). Half lives not reported. Indirect photolysis data not given | Doll et al.64 |
| L | Direct photolysis was slow (t1/2 = 19.3 h). Degradation was increased most significantly in synthetic natural water containing high DOM and low nitrate and carbonate (t1/2 = 9.5 h). At other various concentrations of DOM, nitrate, and carbonate, degradation was only enhanced slightly. ˙OH radicals were suggested as the primary photosensitizing species | Lam et al.26 | |
| L, S | Direct and indirect photolysis rates were slow. Direct photolysis was slow (t1/2 ≈ 100 days in sunlight – calculated using measured quantum yield). In the presence of nitrate or humic acids, photolysis was 1.3 or 2.1 times faster, respectively, compared to direct photolysis | Andreozzi et al.41 | |
| L, S | Direct photolysis was slow in sunlight (t1/2 = 144 h). Indirect photolysis in natural river water increased degradation significantly (t1/2 = 50 h). Approximated that ˙OH radicals accounted for ≈20% of the increased degradation | Packer et al.19 | |
| S, F | Direct photolysis in pure water did not occur. In natural river water indirect photolysis was slow (t1/2 = 2.4 days) | Radke et al.107 | |
| Summary | — | Clofibric acid is relatively persistent towards photodegradation. Direct photolysis in some cases is reported to not occur at all for this compound, and at most plays a minor role in degradation. In all cases indirect photolysis significantly increased degradation of clofibric acid, indicating that this is the major photolysis mechanism degrading this compound. ˙OH radicals appear to be largely responsible | — |
| Recommendation | — | Data for clofibric acid is consistent. Clofibric acid has been studied in the lab and field. No recommendations | — |
| 17α-Ethinylestradiol (57-63-6) | L | Different light intensities were tested. These results are for high intensity UV-B treatment. Direct photolysis was slow (t1/2 = 18 h). Photolysis in two natural river water samples was reduced (t1/2 = 23–46 h). The river sample with higher DOC concentration resulted in reduced degradation suggesting light screening | Atkinson et al.45 |
| L | Direct photolysis in pure water was slow (t1/2 = 28.4 h). In natural river water degradation rates increased significantly (t1/2 = 2.3 h) | Lin et al.18 | |
| L | Direct photolysis was slow. 10% degraded after 4 h. No other kinetic data given. Rate increased slightly as pH increased. The presence of iron increased degradation rates (half lives not reported) | Liu et al.93 | |
| L | Only direct photolysis was considered. Degradation was quick (t1/2 ≈ 5 h, estimated from plot). Half lives not reported | Mazellier et al.97 | |
| L | Direct photolysis was moderate (t1/2 = 7.4 h). Indirect photolysis increased degradation slightly (t1/2 = 5.2 h) in the presence of fulvic acids | Whidbey et al.136 | |
| L, S | Under lab-light photodegradation was rapid and relatively constant across all matrices. Half lives in pure water (direct photolysis), seawater and two natural river waters (indirect photolysis) ranged from 0.95–1.13 h, indicating that direct photolysis was the primary mechanism. Under sunlight in river water degradation was slow (t1/2 = 106 h) | Matamoros et al.96 | |
| S, F | Photolysis in natural lake water was considered (direct versus indirect mechanism not separated). Under sunlight in the lake water a half life of 23 hours was observed | Zuo et al.143 | |
| Summary | — | Photodegradation of 17α-ethinylestradiol is somewhat variable in the literature. Overall photolytic degradation rates ranged from rapid to slow. Most commonly they fell in the moderate range. Indirect mechanisms seem to depend on the type of water and concentration of DOM, since studies report both enhancement and reduction in degradation for indirect photolysis experiments | — |
| Recommendation | — | Data for 17α-ethinylestradiol is variable. More systematic and thorough experiments should be conducted to determine more reliably the extent of direct photolysis and how indirect photolysis mechanisms vary with the presence of natural water constituents | — |
Direct photolysis quantum yields
Direct photolysis experiments are generally done regardless of the study focus, since they are relatively straightforward to perform. At minimum, they are often run alongside other photolysis experiments for the sake of comparison. The basic approach to direct photolysis experiments is to obtain a (pseudo) first-order decay curve of the target compound(s) in pure laboratory water, from which a direct photolysis rate constant and half-life can be determined. The difficulty with a rate constant is that it is directly related to the intensity and wavelength distribution of the light source. A greater intensity light source will produce a larger direct photolysis rate constant, and thus a shorter half-life.61,102 If these experiments are done under natural sunlight, a sufficient approximation can generally be obtained regarding the contribution of direct photolysis towards limiting the persistence of that compound in a real system. Because most sunlight photolysis experiments are done in small isolated vessels, it is generally assumed only to approximate near-surface processes, as light attenuation becomes significant with increasing depth.145A majority of photolysis experiments are conducted only in the laboratory, largely for logistical convenience. Of the studies evaluated here, 70% involved only laboratory-based photolysis investigations. Laboratory light sources range from filtered monochromatic emissions (e.g., filtered mercury lamp – 313 nm) to narrow wavelength range polychromatic emissions (e.g., filtered mercury lamp – 290–400 nm) to polychromatic emissions simulating natural sunlight (e.g. filtered xenon arc lamp – 290–800 nm). The appropriately filtered Xe arc lamp matches sunlight relatively closely in the 290–800 nm range and is the most common sunlight simulator used in the literature. The specific wavelength distribution of the light source and any respective light filter chosen for an environmental photolysis experiment is of utmost importance and cannot be stressed enough. The environmental photolysis-relevant wavelength range of sunlight reaching Earth's surface is very often reported as 290–800 nm, both throughout the literature and in many fundamental texts on photochemistry.11,145 However, for practical purposes (i.e., simulating natural sunlight for environmental photolysis experiments) the wavelength cut-off should be closer to 300 nm, given that at 290 nm the sunlight irradiance at the Earth's surface is sufficiently small that it is often omitted from tabulated sunlight irradiance tables.11,145
The American Society for Testing and Materials (ASTM) defines a standard terrestrial solar spectral irradiance distribution at an absolute air mass of 1.5 (AM1.5 – corresponding to a zenith angle of 48.19°).146 Between 290–300 nm the ASTM document G173-03 reports spectral solar irradiance values of 5.15 × 10−10 W m−2 nm−1 at 290.0 nm, 3.22 × 10−6 W m−2 nm−1 at 295.0 nm, and 5.00 × 10−4 W m−2 nm−1 at 300.0 nm.146 Thus, simply defining 290 nm as the lower limit wavelength cut-off may not be entirely accurate, and in some instances could have profound consequences on the resulting photolysis data. The wavelength cut-off becomes especially important for light sources that have their light intensity maxima at/near 290–300 nm (e.g., some Hg-vapour lamps) and when the target compound only absorbs light up to 290–300 nm, and is thus potentially very sensitive to the exact lower limit wavelength cut-off. Significant caution must be taken when employing light sources that do not match closely the intensity distribution of natural sunlight (specifically near the lower wavelength cut-off range of 290–300 nm), as photolysis rates can be significantly overestimated. This is a major contributing reason as to why laboratory derived photodegradation rate constants (and half lives) generally bear little meaning to environmental photodegradation rates, and highlights the importance for the determination and use of quantum yields, as will be discussed further on in this section.
Related to this issue is the use of Pyrex (borosilicate) glass filters as a 290 nm lower limit wavelength cut-off, a very common practice throughout the literature. Given that the exact wavelength cut-off of Pyrex glass is dependent on the glass thickness,147 inconsistencies can arise with the improper use and reporting of Pyrex glass as a wavelength filter. At a standard Pyrex glass thickness (50 mL tubes ≈ 2 mm thick glass, measured in our laboratory), some amount of light is transmitted down to approximately 280 nm,147 suggesting that depending on the light source used, photolysis rates could be significantly overestimated as a result of using a Pyrex glass wavelength filter. It should be noted that laboratory solar simulators that replicate the AM1.5 solar spectrum distribution and intensity (e.g., Luzchem solar simulator) are optimal for laboratory applications, and should provide the most reliable data in terms of replicating environmental photolytic fate.
The careful consideration and reporting of light source and filter specifics is an important issue that should be more appropriately addressed in future photolysis experiments. Specifically, caution must be taken when using light sources that do not match that of natural sunlight (intensity and distribution) and Pyrex light filters.
As stated, actual environmental behaviour can be approximated through use of an artificial light source with a wavelength and intensity distribution that matches closely to that of natural sunlight, but still discrepancies are unavoidable. Matamoros et al.96 studied the photolysis of carbamazepine, ibuprofen, ketoprofen, and 17α-ethinylestradiol using a sunlight simulator (Atlas SUNTEST CPS© apparatus equipped with a filtered Xe lamp) and under natural sunlight. While the irradiance of the artificial light source (300–800 nm, 507.5 W m−2) and natural sunlight (day average of 270 W m−2) were in general agreement, rate constants were between 5 and 111 times faster under simulated versus natural sunlight, depending on the compound.96 While these large discrepancies can likely be attributed to the diurnal cycle (day–night changes in light intensity and wavelength distribution) of natural sunlight, this is a good example of how direct photolysis rate constants determined in laboratories often may not reflect behaviour under natural sunlight. Unfortunately, because this study did not measure quantum yields, a more quantitative, direct comparison between the two light sources is impossible. Caution must be taken when using laboratory-determined direct photolysis rate constants to predict environmental fate, even when the artificial light source appropriately simulates natural sunlight.
Direct photolysis quantum yields measure the efficiency with which a compound breaks down upon absorption of light. This property should be independent of the light source over a single electronic transition (absorption band) of a compound,20,148 and is thus, much more useful for predictive and comparative purposes. However, quantum yields are less frequently determined in the literature. Consequently, many pharmaceuticals have no quantum yield data. Furthermore, reported quantum yields for a given compound can be highly variable (Table 2). This lack of quantum yield data and the variability therein of existing determinations are closely linked, and are likely a result of the more involved nature of determining this property.
| Drug | Irradiation informationb | Matrix/pH | Φ | Comments | Ref. (score) |
|---|---|---|---|---|---|
| a SMX = sulfamethoxazole; CBZ = carbamzaepine; EE2 = 17α-ethinylestradiol; E2 = 17β-estradiol; CLO = clofibric acid; DIC = diclofenac; IBU = ibuprofen; NAP = naproxen; PRO = propranolol; HP = high pressure; MP = medium pressure; LP = low pressure. b Irradiation information includes type of light source, wavelength range of light source, filters, light intensity, actinometer used. Any parameters missing in the table are parameters that were not reported in that specific study. c Suntest CPS Photosimulator equipped with Xe lamp as the UV radiation source and glass filters restricting the transmission of wavelengths below 290 nm. d Sun 2000 solar simulator-1000 W Xe lamp-AM1.5 filter. e Solarbox 1500 equipped with a 1500 W arc xenon lamp and special outdoor UV filters that restrict the transmission of light with wavelengths below 290 nm. | |||||
| SMX | Filtered Xe lamp;c 765 W m−2; PNA/PYR | Pure water/pH not reported | 0.02 | — | Lam et al.84 (38) |
| HP Hg lamp; ≈6 × 10−7 Ein s−1 (305, 313, 366 nm); H2O2, valerophenone, radiometer | Bi-distilled water/5.5 | 0.0043 | No report of filtering HP Hg emissions <290 nm | Andreozzi et al.41 (20) | |
| Natural sunlight (45°N; summer); PNA/PYR | DI water/2.6; 5.3; 10.8 | 0; 0.50; 0.09 | Φ for fully-protonated, singly-protonated, de-protonated species, respectively | Boreen et al.13 (45) | |
| Filtered Xe lamp;d 76 W m−2 (265–430 nm); PNA | Nanopure water/3.2; 8.4 | 0.96; 0.074 | — | Bonvin et al.49 (41) | |
| Pyrex filtered MP Hg lamp; 25 W m−2; radiometer; ferrioxalate | Bi-distilled water/3; 9 | 0.47; 0.084 | — | Moore et al.98 (22) | |
| CBZ | Pyrex filtered MP Hg lamp; 3.1 × 10−5 (313–578 nm) | Milli-Q water/not reported | 1.5 × 10−4 | — | Chiron et al.59 (29) |
| Filtered Xe lamp;c 765 W m−2; PNA/PYR | Pure water/pH not reported | 1.3 × 10−4 | — | Lam et al.84 (38) | |
| Natural sunlight (34°N; summer); PNAP/PYR | Not reported | 2.7 × 10−5; 6 × 10−6 | Two quantum yield values measured in August and May | Yamamoto et al.139 (25) | |
| Natural sunlight (40°N; summer); PNA/PYR | Bi-distilled water/5.5 | 4.8 × 10−5 | — | Andreozzi et al.41 (20) | |
| Filtered Xe lamp;e 55 W m−2; radiometer | Nanopure water/2.9; 4; 5.8; 9 | 6.4 × 10−5; 2.9 × 10−6; 1.1 × 10−5; 2 × 10−5 | — | Calisto et al.14 (36) | |
| EE2 | Filtered Xe lamp;c 765 W m−2; radiometer; PNA(P)/PYR | Milli-Q water/5.5 | 0.0048 | — | Lin et al.18 (40) |
| Fluorescent lamp (λ > 285–350 nm); phenol | Milli-Q water/5.5 | 0.08 | — | Mazellier et al.97 (19) | |
| E2 | Filtered Xe lamp;c 765 W m−2; radiometer; PNA(P)/PYR | Milli-Q water/5.5 | 0.0048 | — | Lin et al.18 (40) |
| Fluorescent lamp (λ > 285–350 nm); phenol | Milli-Q water/5.5 | 0.07 | — | Mazellier et al.97 (19) | |
| CLO | Natural sunlight (40°N; summer); PNA/PYR | Bi-distilled water/5.5 | 5.5 × 10−3 | — | Andreozzi et al.41 (20) |
| Natural sunlight (45°N; summer); PNA(P)/PYR | Milli-Q water/7 | 0.002 | — | Packer et al.19 (44) | |
| DIC | Natural sunlight (47°N; fall) | Not reported | 0.13 | Quantum yield estimated from GCSOLAR | Buser et al.50 (29) |
| HP Hg lamp; ≈6 × 10−7 Ein s−1 (305, 313, 366 nm); H2O2, valerophenone, radiometer | Bi-distilled water/5.5 | 0.031 | No report of filtering HP-Hg emissions <290 nm | Andreozzi et al.41 (20) | |
| Natural sunlight (40°N; summer); PNA/PYR | Bi-distilled water/5.5 | 0.038 | — | Andreozzi et al.41 (20) | |
| Natural sunlight (45°N; summer); PNA(P)/PYR | Milli-Q water/7 | 0.094 | — | Packer et al.19 (44) | |
| Natural sunlight (47°N; summer) | DI water/not reported | 0.018 | — | Poiger et al.105 (32) | |
| Natural sunlight (47°N; summer) | DI water-N2 sparged/not reported | 0.019 | — | Poiger et al.105 (32) | |
| Fluorescent tube (≈290–400 nm) | Lab water/8.5 | 0.22 | — | Eriksson et al.66 (20) | |
Sunlight simulator (315–800 nm); 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 μW cm−2; radiometer 910 μW cm−2; radiometer |
Pure water/6.4 | 9.3 × 10−5 | Type of lamp not reported | Peuravuori102 (31) | |
| LP Hg lamp (280–400 nm); 1871 μW cm−2; radiometer | Pure water/6.4 | 0.097 | Wavelengths <290 nm not filtered | Peuravuori102 (31) | |
| IBU | Pyrex filtered UV-vis lamp; 2 × 10−5 Ein L−1 s−1 (300–500 nm); ferrioxalate | Not reported/2; 8 | 1.01; 0.33 | — | Vione et al.126 (26) |
| Natural sunlight (34°N; summer); PNAP/PYR | Not reported | 3 × 10−5; 4.2 × 10−4 | Two quantum yield values measured in August and May | Yamamoto et al.139 (25) | |
| NAP | Filtered Xe lamp;c 765 W m−2; radiometer; PNA(P)/PYR | Milli-Q water/5.5 | 0.026 | — | Lin et al.18 (40) |
| Natural sunlight (45°N; summer); PNA(P)/PYR | Milli-Q water/7 | 0.036 | — | Packer et al.19 (44) | |
| PRO | Filtered Xe lamp;c 765 W m−2; radiometer; PNA(P)/PYR | Milli-Q water/5.5 | 0.0052 | — | Lin et al.18 (40) |
| Natural sunlight (34°N; summer); PNAP/PYR | Not reported | 0.017; 0.019 | Two quantum yield values measured in August and May | Yamamoto et al.139 (25) | |
| Natural sunlight (40°N; summer); PNA/PYR | Bi-distilled water/5.5 | 0.0022 | — | Andreozzi et al.41 (20) | |
| Pyrex filtered 150 W Xe short arc lamp | Water spiked with humic and fulvic acids/8 | 1.2 × 10−5; 6.6 × 10−5 | Quantum yields determined in the presence of humic and fulvic acids, respectively | Chen et al.16 (37) | |
| UV-254 germicidal lamp: 1920 μW cm−2 (254 nm); radiometer | DI water/3.9 | 0.07 | Not environmental/sunlight relevant | Dantas et al.61 (17) | |
Quantum yields are dependent upon multiple chemical properties and light characteristics, parameters often requiring experimental determination, which can be a source of uncertainty in a calculated quantum yield. The exact calculation required depends on the type of light source. Polychromatic light sources are often used in environmental photolysis studies to determine quantum yields, thus eqn (1) can be used over a given wavelength range, so long as the pharmaceutical(s) and chemical actinometer are irradiated under identical conditions, generally done simultaneously:145
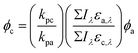 | (1) |
Alternatively, eqn (2) is analogous to eqn (1), but applies to a monochromatic light source:
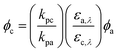 | (2) |
At single wavelengths, the Iλ terms cancel when chemical and actinometer are irradiated under identical light conditions. Other equations can be used depending on specific experimental conditions.145 The quantum yield of the target chemical (c), φc, is related to the direct photodegradation (p) rate constant (kp) of chemical and actinometer (a), the quantum yield of the actinometer (φa), and the product of the wavelength-specific incident light intensity (Iλ – generally in units of photons per area per time) and molar absorption coefficient (ελ) of actinometer and chemical, summed over the wavelength range of overlap (eqn (1)).
Generally speaking, the large majority of studies use polychromatic light sources to simulate natural sunlight more closely. Thus, eqn (1) is relevant and demonstrates the higher degree of difficulty required to determine direct photolysis quantum yields versus rate constants. The difficulty comes with accurately measuring the incident light intensity (Iλ), most commonly done using a chemical actinometer, a compound with a known quantum yield (φa). p-Nitroanisole/pyridine (PNA/PYR) and p-nitroacetophenone/pyridine (PNAP/PYR) are the two most common actinometers used for environmental photolysis studies as they represent a tunable system (i.e., the quantum yield can be systematically adjusted experimentally) that allows the matching of actinometer half life to that of the chemical.149 Of the studies that reported a measurement of light intensity (67% of all studies scored), 45% used the PNA/PYR or PNAP/PYR actinometer to do so. Other systems include the ferrioxalate actinometer150 and a radiometer.
We note that for a number of the drugs in Table 2, quantum yields were determined using both artificial and natural light. While theoretically they should be the same, there were large discrepancies in some cases. Sulfamethoxazole quantum yields have been repeatedly determined under natural sunlight, Xe light, and Hg light (Table 2), with general agreement amongst the studies. However, the value of Andreozzi et al.41 is approximately two orders of magnitude smaller (Table 2). One explanation for this discrepancy may be related to the three different actinometer systems used by Andreozzi et al. to characterize the incident light intensity, likely complicating the calculations and possibly introducing compounding errors in combining the three measurements.
Other discrepancies in Table 2 also point to characterization of light intensity as a potential factor leading to major uncertainties in quantum yield determination. Peuravuori102 found that the quantum yield for diclofenac determined using a full spectrum sunlight simulator was three orders of magnitude smaller than with the low pressure mercury lamp (280–400 nm),102 pointing to the incident light measurement as a major source of uncertainty. However, other aspects of this study raise concern regarding the validity of the experimental data. Peuravuori used a relatively high concentration of acetone (5.5 × 10−3 M) in the irradiation solutions; close to three-orders of magnitude greater than the test compound, diclofenac (1.3 × 10−6 M). The exact effects of using elevated concentrations of co-solvent are largely unknown and therefore unpredictable, putting into question the resulting data. This study is one of the few examples that obtains an average score (31), based largely on other criteria in the study, while producing weak data that is considered inconsistent with the literature.
Although this rubric exercise does a good job at identifying strong and weak aspects of a study, the resulting score should always be accompanied by a thorough analysis of the data. This is an important point also noted by Hu et al.144 in their discussion regarding the ambiguity surrounding optical properties (many relating to photolysis) reported in the field of oceanography. This rubric exercise focuses mainly on the common aspects of all photochemical studies, which, in large part makes this scoring technique feasible. However, original research always contains unique features that cannot necessarily be generalized through the use of pre-developed criteria, and simply require in depth analysis and expertise from the reader, as demonstrated here.
Carbamazepine is another compound for which quantum yields under a number of different light conditions have been reported. Again, there is general agreement between studies (Table 2), within an order of magnitude. An interesting result is the two different values (August, 2.7 × 10−5 and May, 6 × 10−6) reported by Yamamoto et al.,139 which were determined in August and May under natural sunlight (34°N). While sunlight intensity will be significantly different at these two times, the actinometer should account for these differences. With the rest of the experimental conditions being identical between these two dates,139 the most likely source of this discrepancy is the difficulty in accurately and consistently measuring the sunlight intensity during these two experiments. A confounding factor making this information difficult to interpret is the fact that Yamamoto et al. does not report the type of water used or the pH of the irradiation solutions. The discrepancy in the two quantum yield measurements could therefore also be the result of differences in solution pH between experiments. This explanation is consistent with our observation of a similarly-sized difference (an order of magnitude) between the two quantum yield values Calisto et al.14 observed as the pH of the solution changed (Table 2). While this remains speculative, it does raise another important issue that is often overlooked when determining quantum yields under artificial or natural light: pH dependence.
Many pharmaceuticals have pKa values in the environmentally relevant pH range of 4–9, meaning that they will often exist as a mixture of their protonated/deprotonated species. Speciation can change the electronic environment of the molecule, and in most cases alter the absorption spectra, subsequently altering the quantum yield. This effect has been observed for many pharmaceuticals.13,15,21–23,65 A quantum yield determined at a pH near a compound's pKa will be highly sensitive to pH changes, and more appropriately should be referred to as an ‘apparent quantum yield’. This greatly reduces the applicability and predictive property of the quantum yield considering it only applies to a system at that same pH. Alternatively, if quantum yields are determined as individual species (i.e., ≥2 pH units above or below the pKa) these values can then be used for cases when the compound is present as a mixture of protonation states. The fraction of each species can be determined using the pH of the system and the appropriate pKa of the compound, as demonstrated by Challis et al.15 for the sulfonamide antibiotic sulfapyridine.
Using the rubric scores, attempts were made to correlate kinetic data for individual pharmaceuticals with the total scores obtained for that compound. This exercise used quantum yields from Table 2 (photolysis decay rate constants are not comparable across studies, given the dependence on light source intensity) to assess whether or not a positive linear correlation existed between higher scores and the reliability/accuracy of the measured quantum yield. The quantum yield values and respective scores obtained by each study were plotted for sulfamethoxazole and diclofenac (data not shown). No strong correlation was observed, with R2-values < 0.21. This result was not unexpected given the small sample sizes and many of the factors discussed above. Specifically, too much variation existed between studies in terms of experimental design, e.g., differences between pH, actinometer, and light intensity, likely drowning out any potential existing relationship.
This discussion of direct photolysis rate constants and quantum yields has highlighted some important challenges regarding their determination, interpretation, and use. Rate constants determined in the laboratory are relatively easy to measure, but have little capability to predict actual photolytic fate in the environment. An important recommendation going forward would be that direct photolysis experiments be conducted under natural sunlight, providing a stronger environmental relevancy to the rate constant in terms of the conclusions that can be drawn regarding environmental fate overall.
Alternatively, while quantum yields are difficult to measure, they are a characteristic property of a chemical, carrying much greater predictive ability. The examples discussed above suggest the way in which incident light intensity is measured can have a significant effect on quantum yield results. Careful consideration should be taken when approaching the experimental characterization of a light source. Future research should be aimed at the specific causes of quantum yield discrepancies throughout the literature, and the factors leading to incorrect determinations of quantum yields. Furthermore, closer attention should be paid to the experimental design regarding determination of quantum yields, with close consideration of pH and use of co-solvent as factors that can significantly affect measurements.
A specific focus going forward should be the determination of a quantum yield over a wavelength range (spanning a single electronic transition) versus a monochromatic light source. Part of the issue may result from the use of a chemical actinometer that absorbs light at wavelengths significantly different from that of the target compound. If the wavelength overlap of actinometer and chemical with the light source are drastically different, the actinometer and chemical are absorbing different photons. The light intensity determined by the actinometer will not necessarily apply to the chemical, thus confounding the calculations and introducing significant error. Moreover, quantum yield measurements over a large wavelength distribution may have further implications if two electronic transitions occur in this range. It is well documented, most notably by Kahsa,148 that a quantum yield should be constant over a single transition. However, over large wavelength ranges, a tailing absorption band defined by a unique quantum yield, overlapping slightly with the absorption band of interest (i.e., >290 nm), may contribute to the overall measurement, and thus, resulting uncertainty. This idea should also be considered when examining quantum yields measured over single versus multiple wavelengths.
Indirect photolysis
Compared to direct photolysis, indirect mechanisms can be much more complex, making their characterization difficult experimentally. Additionally, it is also difficult to develop standardized methods to test indirect photolysis, since there are many confounding factors that may alter photolysis mechanisms and pathways, which are often unpredictable, and therefore challenging to capture in a standard testing guideline. One kinetic parameter defining indirect photolysis is bimolecular second-order rate constants between compound and photosensitizer. Considering the reactivity between two species (in this case pharmaceutical and reactive species) is constant; this parameter is theoretically comparable across studies, in a similar way to direct photolysis quantum yields. Almost exclusively, these second-order rate constants are measured for hydroxyl radicals and/or singlet oxygen species, and to a lesser extent carbonate radicals and hydrated electrons. Of the studies evaluated in this review, a total of fourteen (12% of all studies) measured second-order rate constants for reaction with photosensitizing species, with only a select number of compounds having more than one measurement (for example, sulfamethoxazole13,84 and atorvastatin84,108). There is, however, a body of literature aimed strictly at accurate measurement of these bimolecular rate constants, often utilizing pulsed radiolysis, that do not fit well with our developed rubric, and thus were not scored here.28,151–159 A complete list of relevant literature regarding this work is not presented. Regardless, the existing literature currently still does not present a large enough dataset – multiple measurements on a wide array of pharmaceuticals – to permit direct comparisons. For these reasons, it is difficult to discuss indirect photolysis in the context of the rubric scores, as was done for direct photolysis with quantum yields (Table 2). This section will thus focus less on comparing specific results and scores among studies and more on a critical evaluation of both the general mechanisms observed in the literature, and the different approaches that are taken to study indirect photolysis mechanisms. Table 1 does, however, give a summary of the general indirect (and direct) photolysis mechanisms observed across studies for seven well-studied pharmaceuticals, in a way comparing indirect photolysis results throughout the literature.The term ‘indirect photolysis’, when used in the literature, generally refers to the ‘total’ photolysis of a compound in an aqueous system containing photosensitizing species. Since indirect mechanisms cannot be determined separate from direct mechanisms experimentally, total photolysis is, in fact, being measured. Direct and indirect rate constants determined under the same conditions are additive, and thus can be separated out:15
| ktotal = kdirect + kindirect | (3) |
The matrix used for indirect photolysis investigations can take on any number of forms. It may be laboratory water spiked with naturally occurring constituents (i.e., synthetic ‘natural’ water) or natural water sampled from a water body (e.g., surface water, wastewater, experimental mesocosm water). Synthetic natural water is often used in controlled experiments with the goal of determining specific indirect photolysis mechanisms (i.e., which photosensitizing species are involved). Alternatively, sampled natural water, while sometimes more difficult to work with, more closely resembles a natural system, though it might be highly specific and lack broad applicability.
Many pharmaceuticals undergo both direct and indirect photolysis to varying degrees, which at a superficial level can be predicted to some extent based on the compound's light absorption properties. The NSAID ibuprofen is a compound with no spectral overlap with sunlight (λ > 290 nm), and thus, cannot directly absorb light energy. Photo-excited species are necessary for ibuprofen to photodegrade.19 Compounds such as propranolol (β-blocker) and naproxen (NSAID) undergo direct photolysis, but also exhibit increased degradation in natural waters (t1/2 = 1.1 min and 1.4 h, respectively) compared to direct photolysis alone (t1/2 = 4.4 h and 1.9 h, respectively).18 Alternatively, some compounds that exhibit rapid direct photolysis, such as ketoprofen (NSAID), show slower removal in natural waters (half-life of 2.5 min in pure water versus 4.1 h in natural water).18 Amongst pharmaceutical classes, and even within drug families, both the extent and mechanism of indirect photolysis can vary significantly, making predictions difficult and experimental work necessary on a compound-to-compound basis.
The natural water species studied most in the literature and deemed important to the indirect photolysis of aquatic organic contaminants include dissolved nitrates, carbonates, iron, and dissolved organic matter (DOM).16,160–168 These species can promote the production, and in some cases the inhibition or scavenging, of many different photosensitizers that may mediate contaminant breakdown. These include the formation of hydroxyl radicals (˙OH), singlet oxygen (1O2), carbonate radicals (˙CO3−), triplet excited state DOM (3DOM), hydrated electrons, and many other reactive oxygen species. The importance of each of these towards contaminant removal depends largely on what species (e.g., nitrates, carbonates, iron, DOM) are present, at what levels, and the nature of the contaminant present.
There have been some attempts at correlating species reactivity to specific functional groups and moieties. While these approaches have potential, many pharmaceutical compounds contain multiple functional groups. This structural diversity makes the application of these correlations less meaningful and predictive. Larson and Zepp169 investigated the reaction pathway of carbonate radicals (˙CO3−) with aromatic amine compounds, and concluded that electron-rich systems react rapidly. Huang and Mabury163 studied this reaction pathway with a few simple electron-rich sulfur-containing pesticides, and stated that ˙CO3− was of intermediate importance in indirect photolysis of these compounds. While both studies show strong evidence for this specific selective mechanism, generalizing the mechanism to a family of drugs containing multiple reactive moieties, for example, becomes increasingly difficult. Attempts at correlating structural composition of a pharmaceutical family to reactivity of specific species, using quantitative structure–activity relationships, may warrant further research. However, the complex nature of these compounds and the mechanisms involved require caution.
Predictions aside, much progress has been made experimentally on the indirect photolysis of pharmaceuticals in the last ten years. Of the studies scored in this review, 96 (80% of all studies) have investigated the indirect photolysis of pharmaceuticals. Only seven of these studies were published prior to 2003, the time of the last review on this topic.9 Generally speaking, studies investigating indirect photolysis will isolate the importance of specific species by amending irradiation solutions with known amounts of individual water constituents (e.g., standardized fulvic/humic acids, nitrate, carbonate, etc.) to assess the importance of each species. Quenching experiments often follow to determine the specific photosensitizer responsible and in some cases, second-order reaction rates are determined. Table 1 summarizes some of the main photolysis processes and mechanisms (indirect and direct) involved in the degradation of a select set of well-studied pharmaceuticals.
A majority of studies test the influence of individual and/or multiple species in a single matrix, at a single concentration, i.e., test a single type of water, natural or artificial. Lam et al.26 took a different approach by using a systematic multivariate technique to assess the effect of different species at varying concentrations on the indirect photolysis of pharmaceuticals and pesticides. The laboratory-based test system (“PhotoFate”) studied three natural water constituents that are known radical producers and scavengers: nitrate, bicarbonate, and DOM. Each species had a low, medium, and high concentration and were mixed with each other to make 16 different synthetic natural water test solutions – essentially a factorial approach. This approach is unique in that it simulates differing natural water systems to demonstrate that indirect photolysis can vary significantly depending on the identity and concentration of water constituents present.26 Only two pharmaceuticals were studied, and ciprofloxacin showed rapid direct photolysis, making indirect mechanisms less important. Clofibric acid showed increased removal with increasing DOM but an inconsistent trend with varying nitrate concentration. This type of approach may warrant further study on a larger and more representative group of pharmaceuticals. Since publication in 2003, Lam et al.26 have been cited over 120 times. Four studies23,170–172 adopted similar experimental designs to investigate indirect photolysis, with only Ge et al.23 investigating pharmaceuticals; the others were pesticide studies. One downside to this approach is the large number of samples and analyses required, likely a large reason this type of technique has not been more widely adopted.
Generally speaking, the most important oxidant is the ˙OH radical, an indiscriminate, fast reacting oxidant that works by either hydrogen abstraction or addition to a double bond.160 Second-order reaction rate constants with ˙OH range from 108 M−1 s−1 up to the diffusion controlled limit of 1010 M−1 s−1 for compounds containing aromatic rings and/or abstractable hydrogen atoms.160 Singlet oxygen tends to be a much more selective oxidant with slower reaction rates, generally varying from 105 to 108 M−1 s−1.173 Despite these slower reaction rates, 1O2 does play a role in the degradation of certain drugs.21,87 Ge et al.23 studied the photolysis of eight fluoroquinolone antibiotics and used quenching experiments to assess the roles of ˙OH and 1O2 in the overall photolysis of these drugs. Isopropanol and sodium azide – two very commonly used quenchers – were used to quench ˙OH and 1O2, respectively. Many of the freshwater constituents (DOM, nitrate, Fe(III)) inhibited degradation by acting as an inner filter. However, they did observe the interesting phenomenon of self-sensitized photo-oxidation via ˙OH and 1O2 in the direct photolysis experiments. A marked decrease in drug removal upon addition of the quenchers to pure water solutions was reported, indicating that the fluoroquinolones themselves were mediating the formation of ˙OH and 1O2, that subsequently degraded them.23
Edhlund et al.65 studied the direct and indirect photolysis of three nitrofuran antibiotics, and determined their bimolecular second-order reaction rate constants with ˙OH and 1O2 using competition kinetics. For the determination of ˙OH-rate constants, Fenton's reagent was used to generate hydroxyl radicals, with acetophenone as a reference compound. Steady-state photolysis was used to obtain a rate constant with singlet oxygen, using Rose Bengal as a sensitizer and furfural as the reference compound. Second-order reaction rate constants for the three nitrofuran antibiotics ranged between 109 M−1 s−1 with hydroxyl radicals and 105 to 106 M−1 s−1 with singlet oxygen.65 These rate constants were then used to estimate photolysis half-lives based on these two specific mechanisms (˙OH and 1O2), assuming environmental steady-state concentrations of the oxidants. This is a good example of how second-order rate constants for photosensitizing species can be applied to help predict environmental fate, either through a simple exercise as above, or as input parameters facilitating more accurate fate assessment in modelling programs.
As alluded earlier, more experimental data regarding second-order rate constants of pharmaceuticals is required to allow direct comparisons of measured rates to assess the consistency of the data (as was done for quantum yields), and facilitate more detailed predictions and modelling exercises. To this end, determining second-order rate constants for a wider range of pharmaceutical compounds should be considered a research focus going forward. Furthermore, it might be of interest to adapt the scoring rubric developed here to assess the quality of data regarding measurement and reporting of bimolecular reaction rate constants for pharmaceuticals with photosensitizing species.
DOM represents a complex and dynamic species that can have a broad range of effects on a chemical contaminant, including photosensitization,21,25 light screening,23,84 scavenging,174–176 and oxidative inhibition.27,177 There are many examples illustrating the complex nature and role of DOM in photolytic processes. Boreen et al.21 found the enhanced degradation of six-membered ring sulfonamides to be attributed mostly to interaction with 3DOM. However, not all DOM is created equally. More recent work has shown that the origins of the DOM – autochthonous (aquatic) or allochthonous (terrestrial) – can have significant effects on possible photo-mechanisms. Guerard et al.25 demonstrated with sulfadimethoxine and triclocarban that aquatic DOM will primarily mediate degradation through 3DOM intermediates, while terrestrial DOM is more reactive in promoting degradation by reactive oxygen species. Similar results were observed by Ryan et al.111 for sulfamethoxazole and trimethoprim. Moreover, further studies have shown that DOM is capable of inhibiting the excited triplet-induced oxidation of many aromatic aquatic contaminants, specifically those containing aniline functionalities (e.g., sulfonamides).27,177,178 This inhibition has been attributed to anti-oxidant moieties (i.e. phenolic groups) present in DOM, with allochthonous DOM expected to inhibit triplet-induced oxidation more efficiently than less aromatic DOM (autochthonous).178 This work was corroborated by Jacobs et al.73 by examining the direct and indirect photolysis of ibuprofen. The dynamic behaviour of DOM as a photosensitizer can be attributed to its complex and variable makeup, which is also the reason it is so difficult to characterize. As more research reveals the structure and function of different types of DOM,151,179 its roles as a photosensitizer will become more apparent.
Photodegradation products
The recent (2011) review by Fatta-Kassinos et al.10 provided a detailed treatise regarding the transformation products of pharmaceuticals in surface waters and wastewaters during photolysis and advanced oxidation processes. For this reason, an exhaustive search was not conducted for studies focused purely on the identification of photodegradation products, and likely some studies were left out. Furthermore, these types of studies often present little to no photochemical data and related experimental parameters, and thus, do not fit well with our designed rubric. This may also explain the lower scores obtained by these types of studies, despite the fact that high-quality data pertaining to photo-product identification is often obtained (for example, Aguera et al.39).With this, only a brief discussion will follow highlighting some important findings and trends that emerged from the scoring exercise conducted. One important and positive trend is that an increasing number of studies characterize, to varying degrees of detail, photodegradation products as part of a photolysis study. It is becoming standard in photolysis studies, almost certainly due to the advancement of analytical technologies, specifically mass spectrometry. Of all the studies scored and evaluated in this critical review, 65% (78 of 120) investigated photodegradation products in some capacity. A number of studies used high-resolution mass spectrometry to identify upwards of 20–30 photodegradation products.23,39,51,70,122 Furthermore, a small subset of these studies included toxicity assays with the photo-products, either in a single study with the kinetic data,23,115,122,180 or as completely separate photo-product toxicity studies.181,182 Nearly all such studies are laboratory-based, as controllable systems simplify the analysis of complex and varied photo-products, which are produced at relatively low concentrations that cannot be readily quantified given lack of authentic standards, and may be difficult to isolate from other matrix components. The development of relevant toxicity tests and screening for persistent photodegradation products for testing in toxicity bioassays should be a major research focus going forward.
Whole system fate studies
Up to this point in this review, literature referred to as field or sunlight studies have, for the most part, involved highly controlled experiments in natural sunlight, generally done in small (<1L), isolated sample vessels. A large majority of sunlight photolysis studies are done in this manner, as such experiments lend to more mechanistic photolysis experiments and thus to a more focused discussion on photolytic fate specifically. However, what these study designs gain through controlled setups, they lose in their ability to simulate real aquatic environments, and thus, true environmental fate. The following section of this critical review will examine ‘whole system’ (e.g., large outdoor microcosms/mesocosms or natural water bodies) fate studies existing in the literature for pharmaceuticals. The importance of these types of investigations will be highlighted and discussed, with specific focus on how they relate to laboratory studies.Logistically, ‘whole system’ fate studies are more involved, requiring more time and resources, and generally take one of two approaches to the experimental design: manipulation of full scale mesocosms53,85,183 or observations of a carefully chosen natural system.50,184–186 Mesocosms are designed to simulate a functional aquatic ecosystem with trophic level interactions (e.g., microorganisms, invertebrates, macrophytes) and multiple environmental compartments (e.g., sediment and water). Alternatively, in situ fate studies are generally done on impacted surface waters where elevated contaminant concentrations are observed. Rivers and/or lakes with single, well defined inputs and outputs50 are generally chosen as study sites. In most cases, a mass balance approach is taken to account for the fate processes responsible for contaminant removal/loss.50
Comparisons between isolated photolysis studies and whole system fate investigations is often a difficult task considering that many of the metrics used to evaluate laboratory photolytic fate are inherently different, and often less specific for field studies. For example, generally speaking, a quantum yield cannot be determined in a large outdoor mesocosm fate study. Similarly, a (pseudo) first-order rate constant determined in a mesocosm study will generally be a dissipation rate constant, accounting for multiple loss processes. It is difficult to separate out individual mechanisms. For example, recent work has examined the efficacy of wetland systems for the removal of pharmaceuticals from impacted waters at both laboratory-187,188 and mesocosm-scale,189–191 and in natural constructed wetland systems.192–198 These studies generally focus on overall removal efficiency. While such characterization is valuable, only qualitative mention is typically made of specific attenuation processes responsible for this removal – in particular, the importance of photodegradation for pharmaceuticals. Photolysis rates are generally not reported, nor are predictions of such rates based on light fluxes, known quantum yields, and measured or estimated steady-state concentrations of photosensitizing radical species for indirect photolysis. Rate constants for other dissipation processes, such as non-photolytic abiotic transformation, biotransformation, and sorption, are also generally not reported.
A small collection of studies have used various approaches to further delineate specific fate processes responsible for removal of pharmaceuticals in full scale systems. Staying with constructed wetland studies, Cardinal et al.199 investigated the efficacy of constructed wetlands for the removal of pharmaceuticals using planted and unplanted outdoor mesocosms. In addition to measuring the overall removal rates, the authors used a basic modelling approach to further elucidate specific loss processes occurring for individual pharmaceuticals. Specifically, the extent of sorption to natural particulate organic carbon (POC) in the water column and sediments, and direct and indirect photolysis, were estimated and compared to the observed overall loss rates. Plant uptake of pharmaceuticals was a minor process compared to photodegradation and sorption. Carbamazepine, for example, was found to be removed predominantly by photolysis (mostly indirect; kindirect,estimated = 0.076 per day), and to a lesser extent, sorption and sedimentation. The estimated half-life of carbamazepine based on these processes (7.6 days) agreed reasonably well with the observed half-life over the 28 days mesocosm experiment (9.1 days).199
Cardoza et al.53 conducted experiments both in lab and field to assess the fate of the antibiotic ciprofloxacin in aquatic systems. All experiments used the full-scale mesocosm water as a matrix. The authors observed rapid photodegradation of ciprofloxacin, with half-lives of 1.9–46 h in lab (depending on artificial light source) to approximately 1 h in the full-scale mesocosms under natural sunlight (3400–3900 μE m−2 s−1 at start of experiment). Further laboratory experiments amended dark treatments with POC and observed a significant reduction in the dissolved fraction of ciprofloxacin after only 15 min (in acidic conditions) – an observed removal rate far greater than the lab-observed photolysis. Although the mesocosm water contained very low levels of POC, allowing photolysis to dominate ciprofloxacin removal, the authors noted that both adsorption and photolysis are important fate processes for this compound, with the dominant mechanisms being dictated largely by POC level, light intensity, and pH of the system.53 Lin et al.184 used a similar approach for studying the fate of three pharmaceuticals (gemfibrozil, ibuprofen, and naproxen) along a 12 km stretch of river. Utilizing model simulations (GCSOLAR) and laboratory experiments, the authors concluded that the primary removal mechanisms for these three drugs were photodegradation (naproxen) and biotransformation (gemfibrozil and ibuprofen). Tixier et al.200 used field measurements and modeling to predict the environmental fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in the effluents of three wastewater treatment plants, two rivers, and in the water column of Lake Greifensee (Switzerland) over a three month period. These publications represent high quality studies that consider multiple fate processes in laboratory and full-scale systems (constructed and natural). These studies bridged the data obtained through the lab and field experiments – an important research focus going forward, as an aspect where strictly laboratory-based studies often fall short.
A very recent study made significant progress in isolating the role of specific photolysis mechanisms in the removal of pharmaceuticals from open-water cells in unit process treatment wetlands.75 Jasper et al.75 measured direct and indirect photolysis rates in pure and wetland water respectively, for atenolol, propranolol, sulfamethoxazole, and carbamazepine. In addition, steady-state radical concentrations of ˙OH and ˙CO3− in the wetland water and quantum yields for formation of ˙OH from DOM and nitrate were measured. Bimolecular second-order reaction rate constants with ˙CO3− were also measured. Other parameters, such as singlet oxygen concentrations and second order reaction rate constants of the pharmaceuticals with reactive oxygen species, were either predicted using quantitative structure–activity relationships (QSARs) (1O2) or borrowed from the literature (˙OH). These parameters were used to design a photolysis model to predict these processes in the open-water cells. Jasper et al.75 were able to draw conclusions as to the treatment efficiency of these open-water cell systems based on the photolysis predicted by the model. For example, this system would provide year-round treatment of photo-labile propranolol, while substantial removal of the more stable compound sulfamethoxazole would only occur in the spring and summer months. Tixier et al.118 used a similar approach to understand the total fate of triclosan in a model system (Lake Greifensee). Like Jasper et al., they used extensive experimental data (e.g., rate constants, quantum yields, pH consideration) and field measurements to predict the photo-transformation in Lake Greifensee.118
These studies take a unique approach to understanding photolysis, and more generally, fate of pharmaceuticals in aquatic systems. Most commonly, studies begin with isolated laboratory photolysis experiments that are often limited to the exact experimental conditions used and may lend little meaning to actual environmental fate, as illustrated previously. Jasper et al.,75 however, start at a full-scale environment, and use it as a model to design more isolated and directed experiments based in part on the observations from the natural system. This approach helps produce more realistic and applicable fate data.
With some exceptions, the studies cited in this section of the review were not assessed using the scoring rubric, as it is not, in its current state, designed to evaluate ‘whole system’ fate studies. That having been said, in addition to acting as an evaluation technique of and guideline for photolysis fate studies, the rubric developed here should also be treated as a template to be adapted for different applications and research streams.
Conclusions, future research needs, and recommendations
This critical assessment of the literature regarding photochemical fate of pharmaceuticals in aquatic environments has highlighted a number of key issues warranting consideration by researchers forwarding the field, and identified areas for potential future research focus:• Add to, and improve on, the study of important pharmaceuticals that had either low quality data or lacked laboratory or field data, or both, based on the scoring exercise (Fig. 2). For future photolytic fate investigations, some of these compounds should be considered for study in order to fill these existing knowledge gaps (e.g., β-blockers acebutolol, alprenolol, bisoprolol, nadolol, and pindolol; Fig. 2C).
• Make the execution and reporting of experimental methods more consistent, which will be central to producing more transparent data. The rubric developed here can aid in this process. The ability to have expansive supplementary information sections for many journals will assist as well.
• Shift the focus to the determination of photolysis rate constants under natural sunlight as opposed to in the laboratory, which would allow for more direct and reliable correlations to environmental fate.
• More detailed reporting of the light source and filter should be addressed in future photolysis experiments. Caution must be taken when using light sources and filters (e.g., Pyrex glass) that do not result in a light output closely matching natural sunlight in both intensity and distribution. A systematic study assessing the effects of light sources and filters on resulting kinetic data, specifically in the sensitive 290–300 nm range, may be of interest.
• Elucidate the root causes of inconsistent and variable quantum yield measurements in the lab and field. Research should focus on the use of actinometers for the determination of incident light intensity, and specifically how the choice of actinometer will affect light measurements, and ultimately quantum yield calculations. Also, the use of poly- versus mono-chromatic light for quantum yield measurements should be considered in regards to the assumption of a single electronic transition in a given wavelength range. Additionally, closer consideration to such factors as pH and the use of co-solvents should be given.
• An emphasis should be placed on measuring second-order rate constants towards photosensitizing species for a larger number of pharmaceuticals. A larger database of second-order rate constants will facilitate more accurate modeling of photochemical fate that can include both direct and indirect processes.
• Investigate specific indirect photolysis pathways and make correlations between pharmaceutical structure and species reactivity. While challenging, this interesting path will ultimately lead to mechanism predictions based on the structure of the drug.
• Consider performing simple toxicity assays simultaneously (e.g., Microtox), alongside the photo-product identification of pharmaceuticals to efficiently identify potentially toxic degradates.
• Greater focus on dual level fate studies – combining full-scale outdoor fate experiments, using mesocosms or natural systems, with more directed laboratory/sunlight experiments and modeling exercises. This will further our understanding of how photochemical fate fits with other fate processes and give a better sense of actual environmental fate.
The large amount of existing data regarding environmental photolysis of pharmaceuticals is absolutely necessary to understand the fundamental fate of these contaminants. However, at a point, a change in research focus is necessary to advance and develop any field of study. Thus, a concerted effort should be made towards the application of existing (and future) data, acquired in lab and to some extent in the field, to our knowledge of true environmental fate of these chemicals. This may involve studies done under natural sunlight, whole system fate studies, and modeling exercises, with a specific focus on bridging data from all three, allowing for more concrete conclusions to be drawn regarding environmental fate. Ultimately, this will allow for better regulatory and policy decisions regarding this issue. To this end, harmonizing the experimental methodologies of photolytic fate studies would be central to producing more consistent and comparable data. The scoring rubric developed here could assist in this goal by developing a methodological framework for use in future studies. This aim of developing consistent methods and standardized tests is by no means a novel idea and has been done throughout the scientific community across various research streams. This approach, while not specifically applied to pharmaceuticals at present, has been used for chemicals which are more heavily regulated (e.g., pesticides) and thus require more consistent evaluation, testing, and reporting36 – an idea that will likely be required of certain pharmaceuticals in the near future.34,35
It is important to note that the topics discussed in this critical evaluation by no means diminish the significance of the cited works. Simply, these observations highlight the need for better communication amongst the scientific community to develop more consistent and accepted testing methods. Though photochemical testing guidelines do exist37,38 they are often under-utilized by much of the scientific community.10 A renewed push towards this goal, beginning here, might be the needed reminder to help re-focus this field of research in order to produce high quality and relevant data.
Acknowledgements
We thank Julie Anderson for helpful technical assistance. Funding for this work was provided by the Canada Research Chairs Program and Canada's Natural Sciences and Engineering Research Program (NSERC). J.K.C. was supported in part by a University of Manitoba Graduate Fellowship and by a NSERC Canada Graduate Scholarship.References
- C. G. Daughton and T. A. Ternes, Environ. Health Perspect., 1999, 107, 907–938 CrossRef CAS PubMed.
- S. K. Khetan and T. J. Collins, Chem. Rev., 2007, 107, 2319–2364 CrossRef CAS PubMed.
- T. Brodin, J. Fick, M. Jonsson and J. Klaminder, Science, 2013, 339, 814–815 CrossRef CAS PubMed.
- K. Fent, A. A. Weston and D. Caminada, Aquat. Toxicol., 2006, 76, 122–159 CrossRef CAS PubMed.
- K. A. Kidd, P. J. Blanchfield, K. H. Mills, V. P. Palace, R. E. Evans, J. M. Lazorchak and R. W. Flick, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8897–8901 CrossRef CAS PubMed.
- B. Halling-Sørensen, S. N. Nielsen, P. F. Lanzky, F. Ingerslev, H. C. H. Lützhoft and S. E. Jørgensen, Chemosphere, 1998, 36, 357–393 CrossRef.
- R. Hirsch, T. Ternes, K. Haberer and K. L. Kratz, Sci. Total Environ., 1999, 225, 109–118 CrossRef CAS PubMed.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202–1211 CrossRef CAS PubMed.
- A. L. Boreen, W. A. Arnold and K. McNeill, Aquat. Sci., 2003, 65, 320–341 CrossRef CAS.
- D. Fatta-Kassinos, M. I. Vasquez and K. Kuemmerer, Chemosphere, 2011, 85, 693–709 CrossRef CAS PubMed.
- B. J. Finlayson-Pitts and J. N. Pitts Jr, Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications, Academic Press, San Diego, 1999 Search PubMed.
- S. Babić, M. Periša and I. Škoric, Chemosphere, 2013, 91, 1635–1642 CrossRef PubMed.
- A. L. Boreen, W. A. Arnold and K. McNeill, Environ. Sci. Technol., 2004, 38, 3933–3940 CrossRef CAS PubMed.
- V. Calisto, M. R. M. Domingues, G. L. Erny and V. I. Esteves, Water Res., 2011, 45, 1095–1104 CrossRef CAS PubMed.
- J. K. Challis, J. C. Carlson, K. J. Friesen, M. L. Hanson and C. S. Wong, J. Photochem. Photobiol., A, 2013, 262, 14–21 CrossRef CAS.
- Y. Chen, C. Hu, X. Hu and J. Qu, Environ. Sci. Technol., 2009, 43, 2760–2765 CrossRef CAS PubMed.
- D. E. Latch, J. L. Packer, B. L. Stender, J. VanOverbeke, W. A. Arnold and K. McNeill, Environ. Toxicol. Chem., 2005, 24, 517–525 CrossRef CAS PubMed.
- A. Y.-C. Lin and M. Reinhard, Environ. Toxicol. Chem., 2005, 24, 1303–1309 CrossRef CAS PubMed.
- J. L. Packer, J. J. Werner, D. E. Latch, K. McNeill and W. A. Arnold, Aquat. Sci., 2003, 65, 342–351 CrossRef CAS.
- R. G. Zepp, Environ. Sci. Technol., 1978, 12, 327–329 CrossRef CAS.
- A. L. Boreen, W. A. Arnold and K. McNeill, Environ. Sci. Technol., 2005, 39, 3630–3638 CrossRef CAS PubMed.
- Y. Chen, H. Li, Z. Wang, H. Li, T. Tao and Y. Zuo, Water Res., 2012, 46, 2965–2972 CrossRef CAS PubMed.
- L. Ge, J. Chen, X. Wei, S. Zhang, X. Qiao, X. Cai and Q. Xie, Environ. Sci. Technol., 2010, 44, 2400–2405 CrossRef CAS PubMed.
- J. J. Guerard and Y.-P. Chin, J. Agric. Food Chem., 2012, 60, 9801–9806 CrossRef CAS PubMed.
- J. J. Guerard, P. L. Miller, T. D. Trouts and Y.-P. Chin, Aquat. Sci., 2009, 71, 160–169 CrossRef CAS.
- M. W. Lam, K. Tantuco and S. A. Mabury, Environ. Sci. Technol., 2003, 37, 899–907 CrossRef CAS PubMed.
- J. Wenk, U. von Gunten and S. Canonica, Environ. Sci. Technol., 2011, 45, 1334–1340 CrossRef CAS PubMed.
- W. Song, W. Chen, W. J. Cooper, J. Greaves and G. E. Miller, J. Phys. Chem. A, 2008, 112, 7411–7417 CrossRef CAS PubMed.
- S. Ayatollahi, D. Kalnina, W. Song, M. Turks and W. J. Cooper, Radiat. Phys. Chem., 2013, 92, 93–98 CrossRef CAS.
- A. Piram, A. Salvador, C. Verne, B. Herbreteau and R. Faure, Chemosphere, 2008, 73, 1265–1271 CrossRef CAS PubMed.
- G. J. Van Der Kraak, A. J. Hosmer, M. L. Hanson, W. Kloas and K. R. Solomon, Crit. Rev. Toxicol., 2014 Search PubMed , submitted.
- H.-J. Klimisch, M. Andreae and U. Tillmann, Regul. Toxicol. Pharmacol., 1997, 25, 1–5 CrossRef CAS PubMed.
- M. Hecker and H. Hollert, Environ. Sci. Eur., 2011, 23, 15 CrossRef.
- Communication from the Commission to the Council and the European Parliament-Community Strategy for Endocrine Disrupters. COM (1999) 706 final, 1999.
- SEC(2011) 1001: 4th Report on the implementation of the “Community Strategy for Endocrine Disrupters” a range of substances suspected of interfering with the hormone systems of humans and wildlife (COM (1999) 706), European Commission, Brussels, 2011.
- Regulation (EC) no 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, 2006.
- OECD Guidelines for the Testing of Chemicals no. 316: Phototransformation of Chemicals in Water – Direct Photolysis, 2008.
- U.S. EPA Direct photolysis rate in water by sunlight – Fate, transport, and transformation test guidelines, OPPTS 835.2210, 1998.
- A. Agüera, L. A. P. Estrada, I. Ferrer, E. M. Thurman, S. Malato and A. R. Fernández-Alba, J. Mass Spectrom., 2005, 40, 908–915 CrossRef PubMed.
- R. Andersin and S. Tammilehto, Int. J. Pharm., 1995, 123, 229–235 CrossRef CAS.
- R. Andreozzi, M. Raffaelle and P. Nicklas, Chemosphere, 2003, 50, 1319–1330 CrossRef CAS PubMed.
- R. Andreozzi, R. Marotta, G. Pinto and A. Pollio, Water Res., 2002, 36, 2869–2877 CrossRef CAS PubMed.
- V. Andrisano, R. Gotti, A. Leoni and V. Cavrini, J. Pharm. Biomed. Anal., 1999, 21, 851–857 CrossRef CAS PubMed.
- T. Araki, Y. Kawai, I. Ohta and H. Kitaoka, Chem. Pharm. Bull., 2002, 50, 229–234 CrossRef CAS PubMed.
- S. K. Atkinson, V. L. Marlatt, L. E. Kimpe, D. R. S. Lean, V. L. Trudeau and J. M. Blais, Arch. Environ. Contam. Toxicol., 2011, 60, 1–7 CrossRef CAS PubMed.
- P. Bartels and W. von Tuempling, Jr, Sci. Total Environ., 2007, 374, 143–155 CrossRef CAS PubMed.
- P. Bartels and W. von Tuempling, Jr, Sci. Total Environ., 2008, 405, 215–225 CrossRef CAS PubMed.
- J. B. Belden, J. D. Maul and M. J. Lydy, Chemosphere, 2007, 66, 1390–1395 CrossRef CAS PubMed.
- F. Bonvin, J. Omlin, R. Rutler, W. B. Schweizer, P. J. Alaimo, T. J. Strathmann, K. McNeill and T. Kohn, Environ. Sci. Technol., 2013, 47, 6746–6755 CrossRef CAS PubMed.
- H.-R. Buser, T. Poiger and M. D. Müller, Environ. Sci. Technol., 1998, 32, 3449–3456 CrossRef CAS.
- V. Calisto, M. R. M. Domingues and V. I. Esteves, Water Res., 2011b, 45, 6097–6106 Search PubMed.
- P. Calza, C. Medana, E. Padovano, V. Giancotti and C. Minero, Environ. Sci. Pollut. Res., 2013, 20, 2262–2270 CrossRef CAS PubMed.
- L. A. Cardoza, C. W. Knapp, C. K. Larive, J. B. Belden, M. Lydy and D. W. Graham, Water, Air, Soil Pollut., 2005, 161, 383–398 CrossRef CAS.
- E. Caupos, P. Mazellier and J.-P. Croue, Water Res., 2011, 45, 3341–3350 CrossRef CAS PubMed.
- M. Cermola, M. DellaGreca, M. R. Iesce, L. Previtera, M. Rubino, F. Temussi and M. Brigante, Environ. Chem. Lett., 2005, 3, 43–47 CrossRef CAS.
- Y. Chen, C. Hu, J. Qu and M. Yang, J. Photochem. Photobiol., A, 2008, 197, 81–87 CrossRef CAS.
- Y. Chen, Q. Liang, D. Zhou, Z. Wang, T. Tao and Y. Zuo, J. Hazard. Mater., 2013, 252–253, 220–226 CrossRef CAS PubMed.
- Z. Chen, G. Cao and Q. Song, Environ. Chem. Lett., 2010, 8, 33–37 CrossRef CAS.
- S. Chiron, C. Minero and D. Vione, Environ. Sci. Technol., 2006, 40, 5977–5983 CrossRef CAS PubMed.
- R. R. Chowdhury, P. A. Charpentier and M. B. Ray, J. Photochem. Photobiol., A, 2011, 219, 67–75 CrossRef CAS.
- R. F. Dantas, O. Rossiter, A. K. Ribeiro Teixeira, A. S. M. Simoes and V. L. da Silva, Chem. Eng. J., 2010, 158, 143–147 CrossRef CAS.
- S.-L. Ding, X.-K. Wang, W.-Q. Jiang, X. Meng, R.-S. Zhao, C. Wang and X. Wang, Environ. Sci. Pollut. Res., 2013, 20, 3195–3201 CrossRef CAS PubMed.
- L. G. Dodson, R. A. Vogt, J. Marks, C. Reichardt and C. E. Crespo-Hernández, Chemosphere, 2011, 83, 1513–1523 CrossRef CAS PubMed.
- T. E. Doll and F. H. Frimmel, Chemosphere, 2003, 52, 1757–1769 CrossRef CAS PubMed.
- B. L. Edhlund, W. A. Arnold and K. McNeill, Environ. Sci. Technol., 2006, 40, 5422–5427 CrossRef CAS PubMed.
- J. Eriksson, J. Svanfelt and L. Kronberg, Photochem. Photobiol., 2010, 86, 528–532 CrossRef CAS PubMed.
- E. Fasani, M. Rampi and A. Albini, J. Chem. Soc., Perkin Trans. 2, 1999, 1901–1907 RSC.
- L. J. Fono, E. P. Kolodziej and D. L. Sedlak, Environ. Sci. Technol., 2006, 40, 7257–7262 CrossRef CAS PubMed.
- Gangwang, G. Liu, H. Liu, N. Zhang and Y. Wang, Sci. Total Environ., 2012, 435, 573–577 CrossRef PubMed.
- M. J. Garcia-Galán, M. S. Díaz-Cruz and D. Barceló, Water Res., 2012, 46, 711–722 CrossRef PubMed.
- M. J. Gomez, C. Sirtori, M. Mezcua, A. R. Fernandez-Alba and A. Aguera, Water Res., 2008, 42, 2698–2706 CrossRef CAS PubMed.
- C. Goncalves, S. Perez, V. Osorio, M. Petrovic, M. F. Alpendurada and D. Barcelo, Environ. Sci. Technol., 2011, 45, 4307–4314 CrossRef CAS PubMed.
- L. E. Jacobs, R. L. Fimmen, Y.-P. Chin, H. E. Mash and L. K. Weavers, Water Res., 2011, 45, 4449–4458 CrossRef CAS PubMed.
- L. E. Jacobs, L. K. Weavers, E. F. Houtz and Y.-P. Chin, Chemosphere, 2012, 86, 124–129 CrossRef CAS PubMed.
- J. T. Jasper and D. L. Sedlak, Environ. Sci. Technol., 2013, 47, 10781–10790 CrossRef CAS PubMed.
- Y. Ji, C. Zeng, C. Ferronato, J.-M. Chovelon and X. Yang, Chemosphere, 2012, 88, 644–649 CrossRef CAS PubMed.
- S. Jiao, S. Zheng, D. Yin, L. Wang and L. Chen, Chemosphere, 2008, 73, 377–382 CrossRef CAS PubMed.
- K. Kawabata, K. Sugihara, S. Sanoh, S. Kitamura and S. Ohta, J. Toxicol. Sci., 2013, 38, 215–223 CrossRef CAS PubMed.
- N. D. H. Khaleel, W. M. M. Mahmoud, G. M. Hadad, R. A. Abdel-Salam and K. Kuemmerer, J. Hazard. Mater., 2013, 244–245, 654–661 CrossRef CAS PubMed.
- U. Kunkel and M. Radke, Water Res., 2012, 46, 5551–5565 CrossRef CAS PubMed.
- J.-W. Kwon and K. L. Armbrust, Environ. Toxicol. Chem., 2004, 23, 1394–1399 CrossRef CAS PubMed.
- J.-W. Kwon and K. L. Armbrust, Environ. Toxicol. Chem., 2005, 24, 1618–1623 CrossRef CAS PubMed.
- J.-W. Kwon and K. L. Armbrust, Environ. Toxicol. Chem., 2006, 25, 2561–2568 CrossRef CAS PubMed.
- M. W. Lam and S. A. Mabury, Aquat. Sci., 2005, 67, 177–188 CrossRef CAS.
- M. W. Lam, C. J. Young, R. A. Brain, D. J. Johnson, M. A. Hanson, C. J. Wilson, S. M. Richards, K. R. Solomon and S. A. Mabury, Environ. Toxicol. Chem., 2004, 23, 1431–1440 CrossRef CAS PubMed.
- M. W. Lam, C. J. Young and S. A. Mabury, Environ. Sci. Technol., 2005, 39, 513–522 CrossRef CAS PubMed.
- D. E. Latch, B. L. Stender, J. L. Packer, W. A. Arnold and K. McNeill, Environ. Sci. Technol., 2003, 37, 3342–3350 CrossRef CAS PubMed.
- D. M. Leech, M. T. Snyder and R. G. Wetzel, Sci. Total Environ., 2009, 407, 2087–2092 CrossRef CAS PubMed.
- A. Y.-C. Lin, X.-H. Wang and W.-N. Lee, Environ. Sci. Technol., 2013, 47, 4104–4112 CrossRef CAS PubMed.
- A. Lindström, I. J. Buerge, T. Poiger, P.-A. Bergqvist, M. D. Müller and H.-R. Buser, Environ. Sci. Technol., 2002, 36, 2322–2329 CrossRef.
- Q.-T. Liu, R. I. Cumming and A. D. Sharpe, Photochem. Photobiol. Sci., 2009, 8, 768–777 CAS.
- Q.-T. Liu and H. E. Williams, Environ. Sci. Technol., 2007, 41, 803–810 CrossRef CAS PubMed.
- X. L. Liu, F. Wu and N. S. Deng, Environ. Pollut., 2003, 126, 393–398 CrossRef CAS PubMed.
- B. T. Lunestad, O. B. Samuelsen, S. Fjelde and A. Ervik, Aquaculture, 1995, 134, 217–225 CrossRef CAS.
- X. Luo, Z. Zheng, J. Greaves, W. J. Cooper and W. Song, Water Res., 2012, 46, 1327–1336 CrossRef CAS PubMed.
- V. Matamoros, A. Duhec, J. Albaigés and J. M. Bayona, Water, Air, Soil Pollut., 2009, 196, 161–168 CrossRef CAS.
- P. Mazellier, L. Méité and J. De Laat, Chemosphere, 2008, 73, 1216–1223 CrossRef CAS PubMed.
- D. E. Moore and W. Zhou, Photochem. Photobiol., 1994, 59, 497–502 CrossRef CAS PubMed.
- T. Morimura, T. Ohno, H. Matsukura and Y. Nobuhara, Chem. Pharm. Bull., 1995, 43, 1000–1004 CrossRef CAS PubMed.
- J. Niu, L. Zhang, Y. Li, J. Zhao, S. Lv and K. Xiao, J. Environ. Sci., 2013, 25, 1098–1106 CrossRef CAS.
- H. Oka, Y. Ikai, N. Kawamura, M. Yamada, K. Harada, S. Ito and M. Suzuki, J. Agric. Food Chem., 1989, 37, 226–231 CrossRef CAS.
- J. Peuravuori, Int. J. Environ. Anal. Chem., 2012, 92, 1470–1492 CrossRef CAS.
- J. Peuravuori, Environ. Sci. Pollut. Res., 2012, 19, 2259–2270 CrossRef PubMed.
- J. Peuravuori and K. Pihlaja, Anal. Bioanal. Chem., 2009, 394, 1621–1636 CrossRef CAS PubMed.
- T. Poiger, H.-R. Buser and M. D. Müller, Environ. Toxicol. Chem., 2001, 20, 256–263 CrossRef CAS PubMed.
- D. Prabhakaran, P. Sukul, M. Lamshoeft, M. A. Maheswari, S. Zuehlke and M. Spiteller, Chemosphere, 2009, 77, 739–746 CrossRef CAS PubMed.
- M. Radke, H. Ulrich, C. Wurm and U. Kunkel, Environ. Sci. Technol., 2010, 44, 2968–2974 CrossRef CAS PubMed.
- B. Razavi, S. Ben Abdelmelek, W. Song, K. E. O'Shea and W. J. Cooper, Water Res., 2011, 45, 625–631 CrossRef CAS PubMed.
- P. F. Robinson, Q.-T. Liu, A. M. Riddle and R. Murray-Smith, Chemosphere, 2007, 66, 757–766 CrossRef CAS PubMed.
- P. C. Rüa-Gömez and W. Puettmann, Chemosphere, 2013, 90, 1952–1959 CrossRef PubMed.
- C. C. Ryan, D. T. Tan and W. A. Arnold, Water Res., 2011, 45, 1280–1286 CrossRef CAS PubMed.
- L. Sanchez-Prado, M. Llompart, M. Lores, C. García-Jares, J. M. Bayona and R. Cela, Chemosphere, 2006, 65, 1338–1347 CrossRef CAS PubMed.
- H. Santoke, W. Song, W. J. Cooper and B. M. Peake, J. Hazard. Mater., 2012, 217–218, 382–390 CrossRef CAS PubMed.
- P. Schmitt-Kopplin, J. Burhenne, D. Freitag, M. Spiteller and A. Kettrup, J. Chromatogr. A, 1999, 837, 253–265 CrossRef CAS.
- C. Sirtori, A. Agueera, W. Gernjak and S. Malato, Water Res., 2010, 44, 2735–2744 CrossRef CAS PubMed.
- M. Sturini, A. Speltini, F. Maraschi, L. Pretali, A. Profumo, E. Fasani, A. Albini, R. Migliavacca and E. Nucleo, Water Res., 2012, 46, 5575–5582 CrossRef CAS PubMed.
- M. Sturini, A. Speltini, F. Maraschi, A. Profumo, L. Pretali, E. Fasani and A. Albini, Environ. Sci. Technol., 2010, 44, 4564–4569 CrossRef CAS PubMed.
- C. Tixier, H. P. Singer, S. Canonica and S. R. Müller, Environ. Sci. Technol., 2002, 36, 3482–3489 CrossRef CAS PubMed.
- L. Tong, P. Eichhorn, S. Pérez, Y. Wang and D. Barceló, Chemosphere, 2011, 83, 340–348 CrossRef CAS PubMed.
- K. Torniainen, S. Tammilehto and V. Ulvi, Int. J. Pharm., 1996, 132, 53–61 CrossRef CAS.
- C. Trautwein and K. Kuemmerer, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2012, 889–890, 24–38 CrossRef CAS PubMed.
- A. G. Trovó, R. F. P. Nogueira, A. Agueera, C. Sirtori and A. R. Fernández-Alba, Chemosphere, 2009b, 77, 1292–1298 Search PubMed.
- E. Turiel, G. Bordin and A. R. Rodriguez, J. Environ. Monit., 2005, 7, 189–195 RSC.
- T. G. Vasconcelos, D. M. Henriques, A. König, A. F. Martins and K. Kuemmerer, Chemosphere, 2009, 76, 487–493 CrossRef CAS PubMed.
- D. Vione, J. Feitosa-Felizzola, C. Minero and S. Chiron, Water Res., 2009, 43, 1959–1967 CrossRef CAS PubMed.
- D. Vione, P. R. Maddigapu, E. De Laurentiis, M. Minella, M. Pazzi, V. Maurino, C. Minero, S. Kouras and C. Richard, Water Res., 2011, 45, 6725–6736 CrossRef CAS PubMed.
- K. H. Wammer, A. R. Korte, R. A. Lundeen, J. E. Sundberg, K. McNeill and W. A. Arnold, Water Res., 2013, 47, 439–448 CrossRef CAS PubMed.
- K. H. Wammer, M. T. Slattery, A. M. Stemig and J. L. Ditty, Chemosphere, 2011, 85, 1505–1510 CrossRef CAS PubMed.
- L. Wang, H. Xu, W. J. Cooper and W. Song, Sci. Total Environ., 2012, 426, 289–295 CrossRef CAS PubMed.
- X.-H. Wang and A. Y.-C. Lin, Environ. Sci. Technol., 2012, 46, 12417–12426 CrossRef CAS PubMed.
- X. Wei, J. Chen, Q. Xie, S. Zhang, L. Ge and X. Qiao, Environ. Sci. Technol., 2013, 47, 4284–4290 CrossRef CAS PubMed.
- J. J. Werner, W. A. Arnold and K. McNeill, Environ. Sci. Technol., 2006, 40, 7236–7241 CrossRef CAS PubMed.
- J. J. Werner, M. Chintapalli, R. A. Lundeen, K. H. Wammer, W. A. Arnold and K. McNeill, J. Agric. Food Chem., 2007, 55, 7062–7068 CrossRef CAS PubMed.
- J. J. Werner, K. McNeill and W. A. Arnold, Chemosphere, 2005, 58, 1339–1346 CrossRef CAS PubMed.
- C. E. West and S. J. Rowland, Environ. Sci. Technol., 2012, 46, 4749–4756 CrossRef CAS PubMed.
- C. M. Whidbey, K. E. Daumit, T.-H. Nguyen, D. D. Ashworth, J. C. C. Davis and D. E. Latch, Water Res., 2012, 46, 5287–5296 CrossRef CAS PubMed.
- B. Wu, T. Zhang, J. Li, Y. Ye and H. Chen, Water Sci. Technol., 2012, 66, 735–740 CrossRef CAS PubMed.
- H. Xu, W. J. Cooper, J. Jung and W. Song, Water Res., 2011, 45, 632–638 CrossRef CAS PubMed.
- H. Yamamoto, Y. Nakamura, S. Moriguchi, Y. Nakamura, Y. Honda, I. Tamura, Y. Hirata, A. Hayashi and J. Sekizawa, Water Res., 2009, 43, 351–362 CrossRef CAS PubMed.
- C. Zeng, Y. Ji, L. Zhou, Y. Zhang and X. Yang, J. Hazard. Mater., 2012, 239–240, 340–347 CrossRef CAS PubMed.
- N. Zhang, G. Liu, H. Liu, Y. Wang, Z. He and G. Wang, J. Hazard. Mater., 2011, 192, 411–418 CAS.
- Q. Zhao, L. Feng, X. Cheng, C. Chen and L. Zhang, Water Sci. Technol., 2013, 67, 1605–1611 CrossRef CAS PubMed.
- Y. Zuo, K. Zhang and S. Zhou, Environ. Sci.: Processes Impacts, 2013, 15, 1529–1535 CAS.
- C. Hu, F. E. Muller-Karger and R. G. Zepp, Limnol. Oceanogr., 2002, 47, 1261–1267 Search PubMed.
- A. Leifer, The kinetics of environmental aquatic photochemistry: theory and practice, American Chemical Society, Washington, DC, United States of America, 1988 Search PubMed.
- ASTM International – Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface, G173–03, 2012.
- A. M. Winer, G. M. Breuer, W. P. L. Carter, K. R. Darnall and J. N. Pitts Jr., Atmos. Environ., 1979, 13, 989–998 CrossRef CAS.
- M. Kasha, Discuss. Faraday Soc., 1950, 14–19 RSC.
- D. Dulin and T. Mill, Environ. Sci. Technol., 1982, 16, 815–820 CrossRef CAS PubMed.
- C. G. Hatchard and C. A. Parker, Proc. R. Soc. London, Ser. A, 1956, 235, 518–536 CrossRef CAS.
- S. Ayatollahi, D. Kalnina, W. Song, B. A. Cottrell, M. Gonsior and W. J. Cooper, Water Sci. Technol., 2012, 66, 1941–1949 CrossRef CAS PubMed.
- J. Jeong, J. Jung, W. J. Cooper and W. Song, Water Res., 2010, 44, 4391–4398 CrossRef CAS PubMed.
- J. Jeong, W. Song, W. J. Cooper, J. Jung and J. Greaves, Chemosphere, 2010, 78, 533–540 CrossRef CAS PubMed.
- S. P. Mezyk, T. J. Neubauer, W. J. Cooper and J. R. Peller, J. Phys. Chem. A, 2007, 111, 9019–9024 CrossRef CAS PubMed.
- B. Razavi, W. Song, W. J. Cooper, J. Greaves and J. Jeong, J. Phys. Chem. A, 2009, 113, 1287–1294 CrossRef CAS PubMed.
- B. Razavi, W. Song, H. Santoke and W. J. Cooper, Radiat. Phys. Chem., 2011, 80, 453–461 CrossRef CAS.
- H. Santoke, W. Song, W. J. Cooper, J. Greaves and G. E. Miller, J. Phys. Chem. A, 2009, 113, 7846–7851 CrossRef CAS PubMed.
- W. Song, W. J. Cooper, S. P. Mezyk, J. Greaves and B. M. Peake, Environ. Sci. Technol., 2008, 42, 1256–1261 CrossRef CAS PubMed.
- H. Yu, E. Nie, J. Xu, S. Yan, W. J. Cooper and W. Song, Water Res., 2013, 47, 1909–1918 CrossRef CAS PubMed.
- G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513–886 CrossRef CAS.
- D. Vione, D. Bagnus, V. Maurino and C. Minero, Environ. Chem. Lett., 2010, 8, 193–198 CrossRef CAS.
- D. Vione, V. Maurino, C. Minero, M. E. Carlotti, S. Chiron and S. Barbati, Comptes Rendus Chimie, 2009, 12, 865–871 CrossRef CAS.
- J. Huang and S. A. Mabury, Chemosphere, 2000, 41, 1775–1782 CrossRef CAS PubMed.
- L. E. Jacobs, L. K. Weavers and Y.-P. Chin, Environ. Toxicol. Chem., 2008, 27, 1643–1648 CrossRef CAS PubMed.
- P. L. Miller and Y. P. Chin, Environ. Sci. Technol., 2005, 39, 4454–4462 CrossRef CAS PubMed.
- A. G. Trovo, R. F. P. Nogueira, A. Aguera, A. R. Fernandez-Alba, C. Sirtori and S. Malato, Water Res., 2009a, 43, 3922–3931 Search PubMed.
- D. Vione, G. Falletti, V. Maurino, C. Minero, E. Pelizzetti, M. Malandrino, R. Ajassa, R. I. Olariu and C. Arsene, Environ. Sci. Technol., 2006, 40, 3775–3781 CrossRef CAS PubMed.
- E. M. White, P. P. Vaughan and R. G. Zepp, Aquat. Sci., 2003, 65, 402–414 CrossRef CAS.
- R. A. Larson and R. G. Zepp, Environ. Toxicol. Chem., 1988, 7, 265–274 CrossRef CAS.
- J. M. Fisher, J. G. Reese, P. J. Pellechia, P. L. Moeller and J. L. Ferry, Environ. Sci. Technol., 2006, 40, 2200–2205 CrossRef CAS PubMed.
- D. F. Wallace, L. H. Hand and R. G. Oliver, Environ. Toxicol. Chem., 2010, 29, 575–581 CrossRef CAS PubMed.
- S. S. Walse, S. L. Morgan, L. Kong and J. L. Ferry, Environ. Sci. Technol., 2004, 38, 3908–3915 CrossRef CAS PubMed.
- F. Wilkinson, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1995, 24, 663–1021 CrossRef CAS.
- P. L. Brezonik and J. Fulkerson-Brekken, Environ. Sci. Technol., 1998, 32, 3004–3010 CrossRef CAS.
- J. V. Goldstone, M. J. Pullin, S. Bertilsson and B. M. Voelker, Environ. Sci. Technol., 2002, 36, 364–372 CrossRef CAS PubMed.
- P. Westerhoff, S. P. Mezyk, W. J. Cooper and D. Minakata, Environ. Sci. Technol., 2007, 41, 4640–4646 CrossRef CAS PubMed.
- S. Canonica and H.-U. Laubscher, Photochem. Photobiol. Sci., 2008, 7, 547–551 CAS.
- J. Wenk and S. Canonica, Environ. Sci. Technol., 2012, 46, 5455–5462 CrossRef CAS PubMed.
- A. Nebbioso and A. Piccolo, Anal. Bioanal. Chem., 2013, 405, 109–124 CrossRef CAS PubMed.
- W. Baran, J. Sochacka and W. Wardas, Chemosphere, 2006, 65, 1295–1299 CrossRef CAS PubMed.
- M. Isidori, A. Nardelli, A. Parrella, L. Pascarella and L. Previtera, Chemosphere, 2006, 63, 785–793 CrossRef CAS PubMed.
- M. Schmitt-Jansen, P. Bartels, N. Adler and R. Altenburger, Anal. Bioanal. Chem., 2007, 387, 1389–1396 CrossRef CAS PubMed.
- H. Sanderson, B. Laird, L. Pope, R. Brain, C. Wilson, D. Johnson, G. Bryning, A. S. Peregrine, A. Boxall and K. Solomon, Aquat. Toxicol., 2007, 85, 229–240 CrossRef CAS PubMed.
- A. Y.-C. Lin, M. H. Plumlee and M. Reinhard, Environ. Toxicol. Chem., 2006, 25, 1458–1464 CrossRef CAS PubMed.
- D. Sabaliunas, S. F. Webb, A. Hauk, M. Jacob and W. S. Eckhoff, Water Res., 2003, 37, 3145–3154 CrossRef CAS PubMed.
- H. Singer, S. Müller, C. Tixier and L. Pillonel, Environ. Sci. Technol., 2002, 36, 4998–5004 CrossRef CAS PubMed.
- A. V. Dordio, C. Duarte, M. Barreiros, A. J. P. Carvalho, A. P. Pinto and C. T. da Costa, Bioresour. Technol., 2009, 100, 1156–1161 CrossRef CAS PubMed.
- V. Matamoros, L. X. Nguyen, C. A. Arias, V. Salvadó and H. Brix, Chemosphere, 2012, 88, 1257–1264 CrossRef CAS PubMed.
- D. Q. Zhang, R. M. Gersberg, T. Hua, J. Zhu, M. K. Goyal, W. J. Ng and S. K. Tan, Environ. Pollut., 2013, 181, 98–106 CrossRef CAS PubMed.
- D. Q. Zhang, T. Hua, R. M. Gersberg, J. Zhu, W. J. Ng and S. K. Tan, Chemosphere, 2013, 91, 14–21 CrossRef CAS PubMed.
- D. Q. Zhang, T. Hua, R. M. Gersberg, J. Zhu, W. J. Ng and S. K. Tan, Ecol. Eng., 2012, 49, 59–64 CrossRef.
- J. C. Anderson, J. C. Carlson, J. E. Low, J. K. Challis, C. S. Wong, C. W. Knapp and M. L. Hanson, Chem. Cent. J., 2013, 7, 54 CrossRef PubMed.
- M. Breitholtz, M. Näslund, D. Stråe, H. Borg, R. Grabic and J. Fick, Ecotoxicol. Environ. Saf., 2012, 78, 63–71 CrossRef CAS PubMed.
- J. L. Conkle, J. R. White and C. D. Metcalfe, Chemosphere, 2008, 73, 1741–1748 CrossRef CAS PubMed.
- M. Hijosa-Valsero, V. Matamoros, J. Martín-Villacorta, E. Bécares and J. M. Bayona, Water Res., 2010, 44, 1429–1439 CrossRef CAS PubMed.
- V. Matamoros, C. A. Arias, L. X. Nguyen, V. Salvadó and H. Brix, Chemosphere, 2012, 88, 1083–1089 CrossRef CAS PubMed.
- V. Matamoros, J. García and J. M. Bayona, Water Res., 2008, 42, 653–660 CrossRef CAS PubMed.
- V. Matamoros and V. Salvado, Chemosphere, 2012, 86, 111–117 CrossRef CAS PubMed.
- P. Cardinal, J. C. Anderson, J. C. Carlson, J. E. Low, J. K. Challis, S. A. Beattie, C. N. Bartel, A. D. Elliott, O. F. Montero, S. Lokesh, A. Favreau, T. Kozlova, C. W. Knapp, M. L. Hanson and C. S. Wong, Sci. Total Environ., 2014 DOI:10.1016/j.scitotenv.2014.02.095.
- C. Tixier, H. P. Singer, S. Oellers and S. R. Müller, Environ. Sci. Technol., 2003, 37, 1061–1068 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3em00615h |
| This journal is © The Royal Society of Chemistry 2014 |