Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bioanalytical tools: half a century of application for potable reuse
Frederic D. L.
Leusch
*a and
Shane A.
Snyder
bc
aSmart Water Research Centre, Australian Rivers Institute, School of Environment, Griffith University, Southport Qld 4222, Australia. E-mail: f.leusch@griffith.edu.au
bUniversity of Arizona, 1133 E. James E. Rogers Way, Harshbarger 108, Tucson, AZ 85721-0011, USA
cNational University of Singapore, NUS Environmental Research Institute (NERI), 5A Engineering Drive 1, T-Lab Building, #02-01, Singapore 117411
First published on 10th July 2015
Abstract
In vitro bioassays, more recently referred to as “bioanalytical tools” in an attempt to emphasize their analytical purpose rather than the uncertain relation to adverse health outcomes, are often thought of as novel tools by water stakeholders. They have, however, been used for over half a century in assessment of recycled water quality. Today, millions of chemicals and formulations are available for commercial use and most have a high propensity to enter sewage collection systems. However, traditional health risk assessment methods involving animal testing at high doses and extrapolation to environmental relevant levels are vastly overwhelmed in capacity by the innumerable chemicals and transformation products potentially present in waters. Beyond the sheer number of chemicals, the interactions of these chemicals as complex mixtures is largely unaddressed in traditional regulatory schemes. Moreover, non-human animal models are often misleading due to differences in metabolism and associated pharmacokinetics. Thus, water professionals continue to struggle with ever increasing numbers of chemicals detected at trace levels in water and the potential interactions of these chemicals during mixture exposures. Bioanalytical tools offer a path forward towards more comprehensive chemical evaluations of water, which can provide greater public confidence in the ability of potable reuse schemes to produce clean and safe drinking water.
Water impactGrowing urban populations and uncertain climatic conditions have in the past decades led to increasingly severe and widespread water shortages, which require integration of novel water sources, such as water reclaimed from wastewater. Alternative water sources can present new chemical challenges that conventional chemical analysis cannot overcome. Bioanalytical tools offer a path forward towards more comprehensive chemical evaluations of water, by detecting chemicals not by their structure but by their biological activity. This provides an improved capacity to detect non-target compounds and some measure of mixture interactions. This is important, as it can provide greater public confidence in the ability of potable reuse schemes to produce clean and safe drinking water. |
1 Introduction
Conventional drinking water can contain a variety of chemical contaminants commonly found in surface waters, such as pesticides, chemicals formed during water treatment, human pharmaceuticals and other xenobiotics.1,2 These chemicals have differing human health risk profiles, as some can be acutely toxic and result in immediate adverse health effects, while others pose chronic health risks and only produce adverse effects after prolonged continuous exposure. Other chemicals that occur may not be toxic to human health, even after a lifetime of exposure. Drinking water standards are generally set for specific chemicals that are likely to be found in water sourced from conventional sources such as surface water or groundwater.3 However, those same drinking water standards may not be appropriate for less traditional water sources such as water reclaimed from wastewater, which may contain very different chemical composition and/or concentrations that introduce new, potentially unaccounted risks.Municipal wastewater can contain a wide range of natural and synthetic chemicals, including personal care products, household chemicals, industrial products, natural and synthetic hormones and pharmaceuticals, and chemicals formed during wastewater treatment.4 Some health authorities have therefore produced guidance documents specifically for water intentionally sourced from wastewater, which consider the much larger universe of chemical contaminants potentially present.5,6 Many water recycling schemes have conducted extensive chemical monitoring studies on reclaimed water,7,8 and these rich datasets can be used to determine the likelihood and significance of exceedance of chemical guidelines.9,10 However, even extensive chemical monitoring can only detect a limited subset of the vast number of chemicals that are likely present and only those above a methodologically defined detection limit that is constantly evolving. There are, for example, more than 4000 pharmaceutical compounds,11 up to 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds in daily use,12 and up to 65
000 compounds in daily use,12 and up to 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 chemicals and formulations commercially available.13 Beyond the large number of chemicals produced, each one has the propensity to form transformation products both during treatment and in the environment.14 Not only would it be impossible to write a drinking water guideline document that would consider all of those compounds, it is also not feasible to detect each of them by conventional chemical analysis. This is where toxicity testing may be a crucial additional tool to ensure the chemical safety of recycled water.15
000 chemicals and formulations commercially available.13 Beyond the large number of chemicals produced, each one has the propensity to form transformation products both during treatment and in the environment.14 Not only would it be impossible to write a drinking water guideline document that would consider all of those compounds, it is also not feasible to detect each of them by conventional chemical analysis. This is where toxicity testing may be a crucial additional tool to ensure the chemical safety of recycled water.15
Toxicity testing involves collecting whole water samples and testing for a range of toxicological endpoints in biological systems. Toxicity testing with whole animals has been the cornerstone of toxicology for a long time, but ethical and financial drivers to reduce, refine and replace whole animal tests16 combined with recent advances in molecular toxicology17–20 have led to an intense interest in alternative techniques such as in vitro bioassays. In vitro toxicity tests are performed at the molecular or cellular level, usually using concentrated organic extracts from water.21 They can detect the triggering molecular or cellular toxic event that occurs at low environmentally relevant concentrations, often below detection limits of chemical analysis and whole animal toxicity testing.22 The main limitation of in vitro assays is that they lack some of the metabolism and transport (toxicokinetic) mechanisms that modulate toxicity in whole organisms. While in vitro bioassays were developed for screening purposes and there is still much debate about their ability to predict whole-organism effects,15 the gap in our understanding of the link between an in vitro response and an adverse outcome in whole organisms is getting narrower. The concept of Adverse Outcome Pathway (AOP)23 provides a solid framework to link a molecular or cellular event (as measured in vitro) to a whole organism effect,20 providing a promising basis for the future of toxicity testing.24–26
In an attempt to emphasize their analytical purpose rather than the uncertain relation to adverse health outcomes, in vitro bioassays applied to water quality testing are sometimes referred to as “bioanalytical tools”.21 Bioanalytical tools are well-suited to monitoring of water quality, as they are significantly faster and cheaper than whole animal toxicity testing, are amenable to high-throughput screening, and allow the generation of relatively rapid toxicology data without the need for ethically and financially expensive whole-animal experimentation.16 In recent years, there has been a move towards standardising the various in vitro techniques available, with the creation of the European Centre for the Validation of Alternative Methods (ECVAM) in 1991 and the US National Toxicology Program Interagency Centre for the Evaluation of Alternative Toxicological Methods (NICEATM) in 1998. These two programs, and similar efforts by Organisation for Economic Cooperation and Development (OECD), have published an ever-growing catalogue of defined operating protocols for the testing of chemicals.
Bioanalytical tools are increasingly applied to water quality assessment21,27 and, considering the predicaments of conventional chemical risk assessment with complex water sources such as treated sewage, it is only logical to apply bioanalytical techniques in the context of recycled water quality assessment. A few bioanalytical tools have in fact been applied since the 1960s to assessment of recycled water quality, but recent developments have greatly expanded the number and scope of in vitro tools available for (recycled) water quality testing.
2 Types of bioanalytical tools
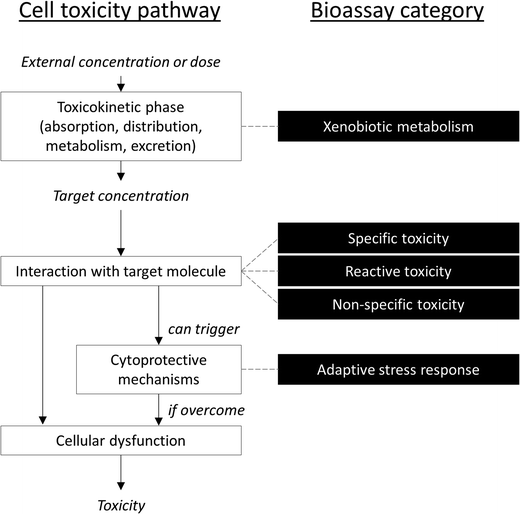
It is difficult to simplify the vast diversity of bioassays, which incorporate various (and oftentimes overlapping) modes of action, while remaining scientifically accurate. One compromise suggested by Escher and Leusch21 is to sort bioassays in five broad categories based on a simplified cellular toxicity pathway (Fig. 1): one is a measure of biological response in the toxicokinetic phase (xenobiotic metabolism), three are based on the type of interaction with the target molecule (non-specific, specific and reactive toxicity), and the fifth is a measure of cellular defense mechanisms (adaptive stress response).21 This section presents some of the assays that have been applied to recycled water quality assessment. Note that many more assays than those listed below may be suitable for water quality testing.13,27 In fact, many of the bioassays developed for drug discovery applications could potentially be used with environmental samples, although adaptation of the assay protocols are often necessary to make the assays compatible with water extracts, including validation and development of relevant quality control parameters (such as determination of accuracy, precision, robustness, selectivity, sensitivity, specificity and repeatability, identification of relevant reference compounds, and intra- and inter-assay comparisons). In a recent study,28 103 different bioassays were tested on a variety of water samples, including reclaimed water, and identified a few endpoints that have so far received little attention but appear to be highly relevant for water quality assessment.2.1 Non-specific toxicity
Non-specific toxicity assays measure cellular response due to non-specific interference with basic cellular functions, such as cell membranes, ATP production or intracellular homeostasis. This is often measured as cytotoxicity. In water quality assessment, bacterial toxicity assays such as the Microtox,29 ToxScreen30 and BLT-Screen31 are common and sensitive methods to detect non-specific toxicity. A variety of mammalian cell-based assays have also been applied to detect cytotoxicity in recycled water samples, using for example neutral red uptake in Caco2 (colon cancer), resazurin reduction in WIL2-NS (B lymphocyte) and HepG2/C3A (liver cancer),32 lactate dehydrogenase leakage in red blood cells33 and crystal violet uptake in CHO (Chinese Hamster Ovary) cells.34 Note that cytotoxicity is also usually concurrently measured (but rarely reported) in specific toxicity assays to ensure the validity of the assay results (i.e., exclude cytotoxic interference).In the context of potable reuse, applying a gastro-intestinal or liver human cell model would appear to be the most suitable. The bacterial assays are clearly more sensitive than the mammalian cytotoxicity assays,28 although it is more difficult to relate bacteria toxicity results to potential human health risks. On the other hand some of the human cell-based assays have been shown to correlate well with acute animal toxicity tests.35
2.2 Reactive toxicity
Reactive toxicity is caused by direct chemical reaction or covalent bonding between the xenobiotic and endogenous molecules such as DNA or proteins. Damage to DNA may lead to cancer in animals, either via mutagenicity (change in DNA sequence) or genotoxicity (damage to DNA structure). The widely used Ames test is a bacterial assay for mutagenicity, while the 6-thioguanine resistance assay measures mutagenicity in mammalian (V79 Chinese Hamster lung cancer) cells. Other commonly used assays for genotoxicity include bacteria assays such as the umu Chromotest (umuC assay)36 and mammalian cell-based assays like the Comet37 and micronucleus assays.32 Cell transformation assays have also been used to detect both genotoxic and non-genotoxic carcinogens (reviewed in ref. 15). Reactive toxicity assays are often run in combination with liver enzymes or microsomes (e.g., rat liver S9 fraction) because metabolism can play an important role in modulation of reactive toxicity (i.e., bioactivation).Toxicity to proteins has not received much attention in water quality assessment, but a recent study suggests a genetically modified bacteria assay (E. coli GSH±) may be suitable with highly treated waters.38
2.3 Specific toxicity
Specific toxicity assays measure interference with particular biological molecules (such as enzymes, nuclear receptors and other transcription factors) or pathways (such as photosynthesis) as a result of three-dimensional interactions between the xenobiotic and the target molecule. Endocrine activity, and in particular estrogenicity, is an example of a widely monitored specific toxicity endpoint.A wide variety of yeast and mammalian reporter gene assays (such as CALUX, GeneBLAzer, yeast estrogen and androgen screen) and cell proliferation assays (such as the E-SCREEN, A-SCREEN and T-SCREEN) have been applied to detect endocrine active compounds in water quality monitoring.39–43
The Imaging-PAM (I-PAM) assay has been widely applied to detect inhibition of photosynthesis in water samples44 and is not surprisingly highly sensitive to herbicides.45
Enzymatic activity, such as interference with the enzyme acetylcholinesterase (AChE), is generally measured with “naked” enzymes (i.e., not in cell-based assay), and can therefore be more sensitive to matrix interference in complex water samples.46 AChE inhibition has nevertheless been tested and detected in water samples, and is commonly associated with potent insecticides.32,36
2.4 Adaptive stress response
Adaptive stress response assays measure the cytoprotective defense mechanisms that cells can initiate to protect against chemically induced damage. These include, for example, production of proteins and enzymes to repair DNA damage or isolation of reactive oxygen species. Assays for adaptive stress response do not measure toxicity per se, but rather the cell's early response to potential toxic injury. Assays for adaptive stress response have only recently been applied in water quality assessment, and available assays appear sensitive and relevant to water samples, particularly oxidative stress and inflammation.282.5 Xenobiotic metabolism
Xenobiotic metabolism assays measure the induction of liver enzymes (such as cytochrome P450s) or stimulation of biological pathways involved in metabolising xenobiotics, such as aryl hydrocarbon receptor (AhR), peroxisome proliferator-activated receptor (PPAR), pregnane X receptor (PXR) or constitutive androstane receptor (CAR) responses. Xenobiotic metabolism assays likewise do not measure toxicity per se, but the cell's attempt to detoxify foreign chemicals. The AhR-CAFLUX is a reporter gene assay with rat liver cells commonly used to assess dioxins that has been frequently applied in water quality assessment.36 Induction of cytochrome P450 enzymes in metabolically active liver cells (such as the HepG2/C3A liver cancer cell line) has also been used to detect xenobiotics in water.322.6 Expression of bioassay data
For specific toxicity assays, results are often expressed as toxic equivalents, based on the assay's reference compound (positive control).47,48 For example, estrogenic activity is generally reported as Estradiol Equivalent (EEQ)49 or aryl hydrocarbon receptor-mediated dioxin activity is reported as TCDD equivalent (TCDDEQ).50 This is because the response in specific toxicity assays can often be explained by a relatively small subset of chemicals,39,41,45,51 while the response in the other assays classes discussed above (reactive and non-specific toxicity, adaptive stress and xenobiotic metabolism) can often be induced by a very large number of chemicals.51 Not only has this created difficulties in establishing the causative chemicals for a particular bioassay response, but it also means that it is uninformative to express the response as a toxic equivalent. The results of these assays are therefore often expressed as ECX_REF,48 which is the REF (Relative Enrichment Factor) required to produce a bioassay response of X%. This is a very useful measure to compare the toxicity of different water samples both within and among different studies, but can be difficult to explain to the uninitiated. It is therefore sometimes expressed as a Toxic Unit (TU), calculated as 1/ECX_REF.32 Nevertheless, some studies have reported results of non-specific and reactive toxicity assays as toxic equivalents, for example, Toxic Equivalent (TEQ) in the Microtox assay52 or 4-Nitroquinolone-1-Oxide Equivalent (4NQOEQ) in the umuC assay.483 Application of bioanalytical tools to recycled water quality assessment
Bioanalytical tools have been applied for several decades for validation and/or verification monitoring at a variety of water reclamation schemes (Table 1). It is important to note that the table focuses only on studies specifically investigating planned water reuse schemes. In a way, all studies that have applied bioanalytical tools to test the quality of treated wastewater (and there are many – see ref. 27) could be added here if we were to include unplanned water reuse.| Scheme/site name | Endpoints (assays) | Reference(s) |
|---|---|---|
| a Please refer to original citation for assay name abbreviations. Other abbreviations used here: “LDH” = Lactate Dehydrogenase; “AChE” = Acetylcholinesterase. | ||
| Dan Region Sewage Reclamation Project, Israel (1960-present) | Mutagenicity (Ames test) | 55 |
| Montebello Forebay Groundwater Recharge Project, California, USA (1962-present) | Mutagenicity (Ames test) | Reviewed in 15 |
| Carcinogenicity (mammalian cell transformation assay) | ||
| Orange County Water Factory 21 (1975–2004) and Groundwater Replenishment System (2004-present), California, USA | Mutagenicity (Ames test) | 15, 56 |
| Potomac Estuary Experimental Water Treatment Plant, Virgina, USA (1980–1982) | Mutagenicity (Ames test) | 15, 57 |
| Carcinogenicity (mammalian cell transformation assay) | ||
| Tampa Water Resource Recovery Project, Florida, USA (1987–1989) | Mutagenicity (Ames test) | Reviewed in 15 |
| Genotoxicity (sister chromatid exchange test) | ||
| San Diego Total Resources Recovery Project, California, USA (1981–1999) | Mutagenicity (Ames test) | 15, 61 |
| Genotoxicity (micronucleus test, 6-thioguanine resistance assay) | ||
| Carcinogenicity (mammalian cell transformation assay) | ||
| Tucson Reclaimed Water System, Arizona, USA (1989-present) | Mutagenicity (Ames test) | 102 |
| Windhoek Direct Potable Reuse Scheme, Namibia (1968-present), | Cytotoxicity to bacteria (bacterial growth test) | 33, 59, 60 |
| Cytotoxicity to human cells (LDH leakage assay with whole blood cells) | ||
| Mutagenicity (Ames test) | ||
| Neurotoxicity (AChE inhibition) | ||
| Immunotoxicity (cytokine production with whole blood cells) | ||
| Landsborough Water Reclamation Plant, Queensland, Australia | Cytotoxicity to bacteria (Microtox) | 66, 67 |
| Estrogenicity (E-SCREEN, ERBA) | ||
| Five water reclamation plants in the USA | Estrogenicity (E-SCREEN, YES) | 68 |
| Androgenicity (A-SCREEN, YAS) | ||
| Perth Groundwater Replenishment Scheme, Western Australia, Australia (2009-present) | Cytotoxicity to bacteria (Microtox) | 70 |
| Genotoxicity (umuC) | ||
| Estrogenicity (E-SCREEN) | ||
| Androgenicity (AR-CALUX) | ||
| Phytotoxicity (I-PAM) | ||
| Qld Western Corridor Recycled Water Scheme, Queensland, Australia (2009-present) | Cytotoxicity to bacteria (Microtox) | 36, 72 |
| Genotoxicity (umuC) | ||
| AhR induction (AhR-CAFLUX) | ||
| Estrogenicity (E-SCREEN) | ||
| Phytotoxicity (I-PAM) | ||
| Neurotoxicity (AChE inhibition) | ||
| South Caboolture Water Reclamation Plant, Queensland, Australia | Cytotoxicity to bacteria (Microtox) | 48, 67, 74, 75 |
| Estrogenicity (E-SCREEN) | ||
| AhR induction (AhR-CAFLUX) | ||
| Neurotoxicity (AChE inhibition) | ||
| Phytotoxicity (I-PAM) | ||
| Genotoxicity (umuC) | ||
| Gerringong Water Reclamation Plant, Victoria, Australia | Cytotoxicity to bacteria (Microtox) | 67 |
| Estrogenicity (E-SCREEN) | ||
| Unidentified water reclamation plant in Queensland, Australia | Cytotoxicity to bacteria (ToxScreen3) | 30 |
| Androgenicity (AR-CALUX) | ||
| Estrogenicity (ER-CALUX) | ||
| Genotoxicity (umuC) | ||
| Nine water reclamation plants in various Australian states | Cytotoxicity to human cells (Caco2 NRU, WIL2NS TOX, HepaTOX) | 32 |
| Mutagenicity (Ames test) | ||
| Genotoxicity (WIL2NS FCMN) | ||
| Endocrine activity (CALUX [ERα, AR, GR, PR and TRβ]) | ||
| Neurotoxicity (AChE inhibition) | ||
| Immunotoxicity (cytokine production with THP1 cells) | ||
| MFO induction (HepCYP1A2) | ||
| Two water reclamation plants in Australia | Cytotoxicity (AREc32 cell viability, Caco2 NRU, RTG2 MTT, DART lethality, SK-N-SH cytotoxicity, algae growth inhibition, Microtox, Photobacterium phosphoreum T3) | 28 |
| Phytotoxicity (I-PAM) | ||
| Endocrine activity (CALUX [ERα, AR, GR, PR and TRβ], GeneBLAzer [ER, AR, GR and PR], yeast screen [estrogen and androgen], E-SCREEN, hER yeast, medER yeast, HELN [ERα, ERβ, AR and TR], FACTORIAL [ERE-cis, ERα-trans, AR-trans, GR-trans, THRα1-trans and RORβ-trans], hERα-HeLa-9903, MCF7 [ERE and ARE], steroidogenesis, DART CYP19A1B aromatase, MDA-kb2 [AR and GR], switchgear-GR, T-SCREEN, P19/A15, hRAR yeast assay) | ||
| Neurotoxicity (AChE inhibition) | ||
| Immunotoxicity (THP1 cytokine production assay) | ||
| Mutagenicity (Ames [TA98, TA100 and TAmix]) | ||
| Genotoxicity (umuC, micronucleus assay) | ||
| Protein toxicity (E. coli GSH±) | ||
| Adaptive stress response (FACTORIAL [HSE-cis, HIF-1a-cis, NFκB-cis, Nrf2/ARE-cis and p53-cis], DART HSPB11 induction, switchgear-hypoxia, GeneBLAzer [NFκB and p53], CALUX [NFκB, Nrf2 and p53], Jurkat E6.1 IκB, AREc32, Nrf2-keap) | ||
| Xenobiotic metabolism (FACTORIAL [PXR-cis, PXR-trans, CAR-trans, PPARγ-cis, PPARγ-trans and AhR-cis], HG5LN PXR, CAR-yeast, CALUX [PPARα and PPARγ], MCF7-PPAR, PPARγ-GeneBLAzer, AhR-yeast, AhR-CAFLUX, H4IIEluc, MCF7-DRE, DART CYP1A induction) | ||
3.1 The early years: mutagenicity and genotoxicity (1960–1998)
Bioanalytical tools have been incorporated in assessment of water recycling schemes since the 1960s (reviewed in ref. 15). In the early decades, the focus was on detecting reactive toxicity, predominantly mutagenicity and genotoxicity. In particular, the Ames test for mutagenicity (developed for chemical risk assessment,53) was widely applied to recycled water in the 1970s and 80s. Unfortunately, bacterial cells have a high degree of inherent gene mutation, and some of the Ames tester strains have a relatively high rate of both false positives and false negatives, which has posed a challenge in evaluating some of the Ames test results with water samples.15,54A 1978 study applied the Ames assay to test the effect of ozonation on the mutagenicity of reclaimed water for groundwater recharge in Israel.55 The Dan Region Sewage Reclamation Project is a groundwater recharge scheme established in the 1960s that receives treated wastewater from eight wastewater treatment plants in Tel Aviv. After a basic mechanical and biological treatment step, the water is injected into the local aquifer and used mostly for agricultural purposes. The study showed no significant difference in mutagenicity between groundwater (reclaimed from wastewater) and distilled water, but ozonation of groundwater led to a 3–6× increase in mutagenicity. However, the specific mutagens could not be identified.
In the USA, the Ames test was applied in the late 1970s and early 80s to water samples collected from various treatment stages at Water Factory 21,56 a managed aquifer project in Orange County, CA (now the Groundwater Replenishment System). The results showed significant mutagenicity in the influent (i.e., treated wastewater) but a significant decrease (to non-detectable) after GAC treatment. Mutagenicity was, however, detected again after subsequent chlorination. Fractionation experiments suggested that the mutagenic activity was associated mostly with hydrophobic organic compounds, but the exact compounds responsible could not be identified.
The Ames test for mutagenicity was combined in the mid-1980s with a mammalian cell transformation assay (which provides a more comprehensive measure of carcinogenic potential but is more cumbersome and time-consuming to perform) to test recycled water produced in both the Montebello Forebay Groundwater Recharge Project and the Potomac Estuary Experimental Water Treatment Plant. The Montebello Forebay scheme is a managed aquifer recharge project in California, USA, in operation since 1962. The Potomac Estuary Experimental Water Treatment Plant was a US Army Corps of Engineers pilot project to provide highly treated water by blending Potomac estuary water with secondary effluent from a municipal WWTP in Washington DC treated by filtration, carbon adsorption and disinfection. In both studies, low-level mutagenic activity was detected in the Ames test with reclaimed water after chlorination, although interestingly the activity was lower than that in other water samples tested for comparison, including groundwater and local drinking water.15,57 More than half of the mutagenic activity in the reclaimed water samples appeared to be due to the chlorination process.15 The cell transformation assay also showed a small number of positive samples with both the reclaimed water and the local drinking water.57 The study concluded that the reclaimed water did not indicate any increase in potential chronic health effects compared to local drinking water, although a subsequent review commented that the limited number of toxicity tests was insufficient to clearly establish the safety of the water.15
Similar results were obtained in a study at the Stander Reclamation Plant in Pretoria, South Africa, a plant producing 4.5 ML d−1 of reclaimed water by coagulation, sand filtration, activated carbon absorption and chlorination. Carcinogenic activity, determined by cell transformation assay, was lower in reclaimed water than in local tap water.58
In the late 1980s, a study on a pilot plant in Florida also applied the Ames test for mutagenicity, but this time combining it with a sister chromatid exchange assay for genotoxicity (reviewed in ref. 15). The Water Resource Recovery Project in Tampa was a pilot plant to evaluate the acceptability of using reclaimed water to augment the city's water supply. The final treatment train included GAC and disinfection with ozone. No mutagenic or genotoxic activity was observed in any of the samples. This project provides an interesting early insight into some of the power of quick and rapid in vitro bioassay use during the early design stage. Three different treatment trains were initially trialled (GAC, RO and UF), but the project proponents settled on GAC based on better results with the Ames test. Likewise, ozonation was selected as disinfection agent instead of chlorine because the latter produced mutagenic activity in the final water. Extensive toxicity testing during validation, including chronic toxicity tests in whole animals, confirmed that the selected treatment train had no adverse effect on any of the endpoints monitored.15
Regular bioassay testing has also been carried out at the direct potable reuse plant in Windhoek, Namibia. The Goreangab Water Reclamation Plant has had several upgrades since the start of operations in 1968, with the current advanced water treatment train producing 21 ML d−1 with a treatment train consisting of high dose ozonation, activated carbon, and ultrafiltration (O3/BAC + GAC/UF) followed by chlorination. The monthly testing regime includes in vitro assays such as the Ames test and a bacterial growth inhibition assay.59 The source water (treated sewage) was on occasion mutagenic (up to 2.9× increase in number of revertants), however the reclaimed water never induced significant mutagenicity (all results <2× increase).60 Inhibition of bacterial growth was evident with both the source and product waters, with up to 34% inhibition of bacterial growth in reclaimed water. The authors attribute this inhibition to occasionally high iron, aluminium and manganese concentrations.60
Extending the bioassay battery yet further (but still focussing only on reactive toxicity), Olivieri et al.61 applied the Ames test for mutagenicity, the micronucleus test for genotoxicity, the 6-thioguanine resistance assay for mutagenicity in mammalian cells and mammalian cell transformation assay for carcinogenicity to reclaimed water from the Total Resources Recovery Project in San Diego, a pilot plant that included UV, RO and GAC to reclaim water for indirect potable reuse (reviewed in ref. 15). The results show weak mutagenic activity in both reclaimed and drinking water source waters, with lower activity in reclaimed water compared to the conventional alternative. The results with the mammalian cell transformation assay were not repeatable and were thus rejected, and the remaining two assays did not show any mutagenic or genotoxic activity in either water samples.15 The study, which also included additional chemical and microbiological tests, concluded that the health risks associated with the use of reclaimed water as a raw water supply were less or equal to the raw water sources used then.61
Several studies in the late 1980s and early 90s tried to identify mutagenic and genotoxic compounds in water (mostly drinking water, reviewed in ref. 62–64). Those studies confirmed that chlorination by-products were likely the cause of the reactive toxicity in water. Several highly mutagenic compounds were identified, such as MX,15 but even those compounds could not account for the total reactive toxicity in water samples, and the identity of the causative compound(s) is still unclear to this day. The results clearly emphasized that exposure to chlorination disinfection by-products in water should be minimized, although proper pathogen control should never be compromised.
3.2 A slow decade, but with increasing interest in endocrine activity (1998–2007)
After much initial enthusiasm in the promise of in vitro methods, very few studies applied bioanalytical tools in recycled water quality assessment in the decade that followed the NRC review,15 perhaps because of disappointment due to the limitations of the early testing. Growing concern about Endocrine Disrupting Compounds (EDCs) in the late 1990s, however, led to intensive development of reporter gene bioassays to detect hormonal activity in water, and particularly estrogenic activity.65A 2005 study measured estrogenic activity at the Landsborough Water Reclamation Plant in Australia66 using two bioassays: an estrogen receptor binding assay (ERBA) and the E-SCREEN. The treatment train consists of ozonation, biological activated carbon and UV treatment (O3/BAC/UV). Both assays detected high estrogenic activity in sewage influent, but the treatment train was very effective and no activity was detected in the final effluent: <0.75 ng L−1 and <0.03 ng L−1 EEQ in the ERBA and E-SCREEN, respectively. The same plant was investigated again in 2010 using the Microtox and the E-SCREEN assays.67 Low activity was detected in both bioassays, up to 0.94 mg L−1 Toxic Equivalents (TEQ) in the Microtox (51–60% lower than secondary treated wastewater) and up to 0.07 ng L−1 EEQ in the E-SCREEN (94–96% lower).
A 2006 study applied four bioassays for estrogenic and androgenic endocrine activity (E-SCREEN, A-SCREEN, yeast estrogen and yeast androgen screen) to test water from five unspecified water reclamation facilities in several US states.68 The results show that estrogenic and androgenic activity were detected in treated sewage (0.2–7.9 ng L−1 EEQ in the E-SCREEN and 1.6–9.1 ng L−1 testosterone equivalents, TTEQ, in the A-SCREEN), but that soil aquifer treatment and reverse osmosis were very effective at reducing the residual endocrine activity to below detection limits (<0.04 ng L−1 EEQ and <1 ng L−1 TTEQ). The results of the estrogenic bioassays were well correlated with chemical analysis of estrogen hormones, but androgenic activity was higher than predicted, indicating the likely presence of unknown androgenic compounds.
3.3 Renewed dynamism due to severe and widespread water scarcity (2007–2014)
Severe and widespread drought over much of Australia in the second half of the first decade of the 21st century prompted significant emergency investment into building, testing and monitoring water reclamation scheme for various potable and non-potable uses. Water industry and regulators faced with this unique situation recognized the unique ability of bioanalytical techniques to provide an additional level of assessment of recycled water quality, and intense research was focused towards developing comprehensive bioassay test batteries. This led to considerable efforts to apply bioanalytical tools to water quality assessment6,21 and to develop interpretive frameworks for bioanalytical results.29,69 The development and application of new bioassays have led to renewed recognition of the value of bioanalytical tools for water quality monitoring, and bioassay batteries used for testing of water quality have expanded in both application and complexity.27Two large scale projects in particular deployed batteries of bioanalytical tools to examine water quality: the Perth Groundwater Replenishment Scheme (GWRS) and the Queensland Western Corridor Recycled Water Scheme (WCRWS). The GWRS is a 75 ML d−1 scheme to reclaim water from urban wastewater by microfiltration and reverse osmosis (MF/RO) for aquifer recharge in Perth, Western Australia. A one-year study in 2008/09 combined chemical analysis with 5 in vitro bioassays (Microtox, umuC, I-PAM, E-SCREEN and AR-CALUX).70,71 The MF/RO treatment significantly reduced biological response in all assays, and only minimal basal toxicity was detected in the final effluent: up to 0.41 toxic units (TU) in the Microtox (56–>82% lower than secondary treated sewage), <0.04 genotoxic units (GTU) in the umuC+S9 and −S9, <0.03 μg L−1 diuron equivalents (DEQ) in the I-PAM, <1 ng L−1 EEQ in the E-SCREEN and <2.5 ng L−1 dihydrotestosterone equivalents (DHTEQ) in the AR-CALUX. Overall, the bioanalytical results confirmed the chemical results and showed MF/RO treatment was very effective at removing biologically active chemicals, with the reclaimed water of comparable quality to ultrapure laboratory grade water.70,71 These findings were again confirmed by a 2014 study at the same site, which showed a reduction in the bioassay response of 92% in the Microtox assay, 89% in the AREc32 oxidative stress assay, and >90% in both the I-PAM and umuC−S9 assays after treatment.51 The latter study combined bioassay analysis with comprehensive chemical analysis of almost 300 chemicals and showed that while both chemical and bioassay analysis showed the same extensive chemical removal by MF/RO, even the thorough screening of 300 chemicals could only account for 1–3% of the non-specific and reactive bioassay responses. This suggests that chemical and bioassay analysis methods only overlap to a small extent and that they are clearly complementary.
The WCRWS was designed to reclaim water from combined urban wastewater in Southeast Queensland by microfiltration, reverse osmosis and advanced oxidation (MF/RO/AO) and supplement a local drinking water dam, producing up to 250 ML d−1 (although it is currently not in operation due to wet climatic conditions). A variety of in vitro bioassays were applied to water produced from the WCRWS, including Microtox, AChE inhibition, I-PAM, E-SCREEN, AhR-CAFLUX, and umuC bioassays.36,72 Again, final effluent samples showed very low activity in all bioassays: up to 0.12 mg L−1 TEQ in the Microtox (87% decrease from secondary treated effluent), <0.06 μg L−1 parathion equivalent (PTEQ) in the AChE inhibition assay, up to 0.05 μg L−1 DEQ in the I-PAM (81% decrease), <0.01 ng L−1 EEQ in the E-SCREEN, up to 0.08 ng L−1 TCDDEQ in the AhR-CAFLUX (93% decrease), <0.05 μg L−1 4NQOEQ in the umuC−S9 and <0.8 μg L−1 BaPEQ in the umuC+S9. Interestingly, the same study also applied the same assays to a variety of other water samples from the urban water cycle, including surface, wastewater, drinking water and ultrapure laboratory blanks. The water produced by the WCRWS was better than current drinking water in all bioassay results, and almost identical to the ultrapure laboratory blank.36,72
A study funded by the National Water Commission of Australia investigated seven unidentified membrane water reclamation plants (5 RO and 2 UF) in several Australian states.32,73 A broad battery of 13 in vitro bioassays was applied: three assays for human cell cytotoxicity (Caco2-NRU, WIL2NS TOX and HepaTOX), two reactive toxicity assays (Ames and WIL2NS FCMN), six assays for specific toxicity (ERα-CALUX, AR-CALUX, GR-CALUX, PR-CALUX, TRβ-CALUX and acetylcholinesterase inhibition assay), one adaptive stress response (CPA in THP1 human monocyte cells) and one xenobiotic metabolism assay (HepCYP1A2). Biological activity was detectable in 10 out of 13 assays in the secondary treated effluent, and while UF/UV treatment had only minimal (if any) effect on the measured activity at the two UF plants, only 3 bioassays produced a response in the RO effluent: up to 0.87 ng L−1 EEQ and 4.4 μg L−1 Tamoxifen Equivalents (TMXEQ) in the ER-CALUX assay (66–>99% decrease from secondary treated sewage), up to 0.61 μg L−1 Dexamethasone Equivalents (DexaEQ) in the THP1-CPA (15–>98% decrease), and up to 0.09 TU in the WIL2NS TOX assay. The biological response in the final RO effluent was tentatively attributed to plasticizers from the RO membranes and disinfection by-products.32,73
Several studies between 2009 and 2012 have combined chemical analysis and a mix of bioassay methods including non-specific (Microtox, ToxScreen3), specific (E-SCREEN, ER-CALUX, AR-CALUX, AhR-CAFLUX, AChE inhibition, I-PAM) and reactive (umuC) toxicity assays to smaller non-RO water reclamation plants, including the South Caboolture Water Reclamation Plant (O3/BAC/O3),48,67,74,75 an unidentified Water Reclamation Plant for non-potable reuse in Queensland (MF/UF/UV),30 and the Gerringong Water Reclamation Plant (O3/BAC/MF/UV).67
Overall, these studies showed that alternative (non-RO) treatments can also be very effective at reducing the biological response, but the final effluent of the advanced water treatment plant often had detectable (albeit very low) activity in many of the assays: 0.57–0.72 mg L−1 TEQ in the Microtox (67–84% decrease from secondary treated effluent), <0.13 ng L−1 EEQ in the E-SCREEN and ER-CALUX (>99% decrease), up to 0.36 ng L−1 TCDDEQ in the AhR-CAFLUX (46–69% decrease), <0.01–0.04 GTUECIR1.5 in the umuC−S9 (83–>92% decrease), up to 0.03 GTUECIR1.5 in the umuC+S9, up to 1.2 μg L−1 PTEQ in the AChE inhibition (57–>90% decrease), and up to 0.05 μg L−1 DEQ in the I-PAM assay (50–>91% decrease). These results were comparable to those obtained using a mix of in vitro bioassays (including Microtox, algae inhibition assays, YES, YAS, I-PAM, AChE inhibition, AhR induction in the yeast dioxin screen, and the umuC assay) to investigate the efficacy of ozonation to reduce biological activity in Swiss and German wastewater treatment plant effluents.76,77
The increase in scope of bioanalytical batteries have of course been mirrored worldwide. At the Goreangab Water Reclamation Plant for example, four in vitro assays have recently been applied to test water quality, including an AChE inhibition assay (neurotoxicity), an LDH leakage assay with whole blood cells (cytotoxicity), and two cytokine production assays (IL-6 and IL-10) in whole blood cultures (immunotoxicity).33 The results show a reduction of biological response in the final effluent compared with the secondary treated sewage influent, up to 6% activity in the AChE inhibition assay (72–>95% decrease from secondary treated sewage), <1% cytotoxicity in the LDH leakage assay (>96% decrease), up to approximately 110 pg mL−1 IL6 in the first CPA (84–>99% decrease), and <1 pg mL−1 IL-10 in the second CPA (>99% decrease).
To evaluate the suitability of this ever expanding catalogue of bioanalytical tools to benchmark water quality and to assess efficacy of water treatment processes, a recent inter-laboratory study28 screened water samples from two Australian water reclamation plants (one RO/AO, the other ozonation and BAC) with a battery 103 different in vitro bioassays: 10 assays for cytoxicity (including Microtox and Caco2-NRU), 46 for specific toxicity (including the I-PAM and various assays for endocrine activity), 12 for reactive toxicity (including Ames and umuC tests), 16 for adaptive stress response and 19 for xenobiotic metabolism (including the AhR-CAFLUX). The study found that source water (treated sewage) produced a biological response in 53 and 60 out of 103 bioassays at each plant, but that advanced water treatment reduced the biological response in all bioassays, to below detection limit in most. The reclaimed water produced a low but detectable response in 5 and 13 of the 103 bioassays for the RO/AO and O3/BAC plants, respectively. The five assays responsive with RO/AO reclaimed water were two bacterial toxicity assays (Microtox and another bioluminescent bacteria assay), the Ames test, and two assays that detect induction of xenobiotic metabolism (specifically the AhR and CAR pathways). The reclaimed water from the O3/BAC plant produced a detectable response in those same assays as well as another Ames tests, the ER-CALUX assay, two assays for oxidative stress (a type of adaptive stress response) and four additional xenobiotic metabolism assays (for the AhR, CAR and PXR pathways). Overall, the study suggests that early indicators of cellular responses (adaptive stress response and xenobiotic metabolism), which are not measures of toxicity per se, may be useful measures of treatment efficacy, as they remain detectable even in highly treated waters that only trigger minimal response specific, non-specific and reactive toxicity assays. The results also confirm the capability of advanced water treatment to produce very high quality of water.
3.4 Bioanalytical tools today
With the exception of the Perth Groundwater Recharge Scheme, intense rain events have refilled most of Australia's dams and drinking water reservoirs on the East Coast and water recycling has currently dropped off the agenda, with water reclamation plants either running idle or being mothballed. But increasingly severe droughts and water shortages in the Western United States have brought water reclamation back into intense focus in California. Renewed interest in bioanalytical techniques, driven by the need for improved water quality assessment tools, has already led to several research publications.78,79 An expert panel convened by the State of California has suggested bioassay screening as a major key in the path forward for characterization of water quality from potable reuse schemes.6 Considering more than 35 million people rely on the Colorado River for water supplies, there are unique driving forces towards increased development of potable reuse. However, one of the greatest challenges in potable reuse implementation is public perception, which is largely influenced by fears surrounding unknown and uncharacterized organic chemical mixtures.80 Bioassay screening tools are quite likely to improve consumer confidence by providing a more comprehensive evaluation of chemical constituents and instilling a greater sense of security in that “unknowns” are being better addressed.3.5 Bioanalytical methods can provide a unique perspective for treatment validation
The studies listed above have allowed some conclusions to be drawn about treatment technologies. For example, the results show that reverse osmosis, which is an effective technique to remove organic contaminants, is likewise highly efficient at removing the biological response in in vitro assays. Some low residual activity is sometimes detected in membrane-based systems70,81 indicating that RO is an effective but not absolute barrier to biologically active compounds, as had been previously demonstrated for individual chemicals.82 Reverse osmosis generally achieves 99% salt rejection, not 100%, thus depending on the source water composition, one can reasonably assume that some degree of passage will also occur for bioactive substances through infrastructure imperfections like glue-line failures, o-rings, and others.83 Therefore, complete removal is not a reality for any process, regardless of the intensity of treatment, and is essentially limited to the detection limits of analytical or bioanalytical measures.Where ozonation and BAC were used, all of the tested final effluents produced only minimal biological response in the deployed bioanalytical tools. When biological activity was detected, it was always less than 10× above the assay quantification limit or activity in the ultrapure laboratory blank. This suggests that even in those cases where biological activity was detected in the final effluent, that activity is unlikely to be of significant health concern. Bioanalytical tools thus provide additional evidence that ozonation and BAC are effective technologies to produce high quality purified recycled water.
Microfiltration and ultrafiltration can be effective techniques to remove pathogens but they are not effective at removing trace organic contaminants82 or their associated biological response.32,73
There is a growing number of studies that have applied various in vitro bioassays in small-scale experiments to determine treatment efficacy,84–93 in particular because bioanalytical tools provide a measure of the total biological response. This can provide a considerable improvement over the commonly accepted method of conducting these tests, which only include chemical analysis of a select number of compounds. This standard type of analysis can show the removal of a specific chemical structure, but does not indicate whether potentially more toxic transformation products have formed during treatment. Applying standard chemical analysis for targeted compounds in combination with bioanalytical tools can overcome this limitation and provide a more comprehensive assessment of treatment efficacy. These bench-scale studies can provide a useful and comparatively cost-effective method to compare different treatment configuration allowing careful fine-tuning of the treatment train to minimize biological activity in the reclaimed water, as was done for example at the Tampa Water Resource Recovery Project (see above). A recent review of advanced oxidation processes in water and wastewater treatment strongly emphasized the need to combined chemical analysis with bioassay testing to detect toxic by-product formation from advanced oxidation processes.94In vitro methods effectively complement chemical analysis methods to provide a more comprehensive measure of treatment efficacy. For example, a recent study showed that while chemical analysis alone indicated that sand filtration was an effective method to polish wastewater from a Swedish wastewater treatment plant, bioassays clearly demonstrated that toxic compounds were present in sand filter effluent (even if the monitored compounds were not).88 This study highlights that conclusions from chemical analysis alone may incorrectly identify treatment options as suitable when they in fact produce toxic by-products.
4 Current limitations
There are of course limitations to bioanalytical tools. The limitations do not mean that bioanalytical methods should not be used in water quality assessment, but rather that care must be taken when relying on in vitro data. The main limitations currently recognized include the need to concentrate water samples, the use of cancer cells, and a lack of regulatory framework to interpret bioanalytical results.In vitro bioassays are almost always conducted with concentrated water samples, which have been extracted either by liquid-liquid extraction (LLE) or solid-phase extraction (SPE). This means that inorganic species, highly water soluble organics, and highly volatile organic compounds are generally not entirely isolated in current extraction/concentration processes. Moreover, as compared to modern targeted analyses where surrogate standards are added to correct analytical data for losses and inefficient extraction,95,96 surrogates are not considered viable for bioassays due to the high potential to interfere with the biological responses. Extraction is generally carried out for two reasons: 1) to concentrate the organic constituents in water samples, and 2) to focus bioassay responses on the world of organic chemicals and not inorganic substances. Thus substances like bromate and perchlorate that are relevant to water reuse projects would not likely be detected by existing bioassay procedures, and bioassays are intrinsically susceptible to variability in extraction efficiency and/or procedure. Therefore, it is important to ensure that a suitable extraction technique is used that retains as wide a spectrum of chemical compounds as possible.73,97 However, it is impossible to say that the every single organic constituent is adequately extracted and concentrated during these processes.
Most cell lines used for bioassays are cancerous cell line, which (as opposed to primary cells) easily proliferate under laboratory conditions. Cancer cells can exhibit morphological and genetic differences compared to normal/healthy cells, and these need to be taken into account when analysing bioassay results. This is not a particularly significant issue when bioassays are used as detection tools for biologically active contaminants, but can be very relevant when bioassays are as representative tissues in a human hazard context.
The issue that has plagued bioanalytical methods for a very long time is what to do with a positive (or negative) bioassay result. There are currently no bioanalytical guidelines in drinking or recycled water regulation (although it should be noted that some dioxin guidelines are based on bioanalytical toxic equivalency, and that bioassays for dioxin-like activity, such as the DR-CALUX, have been used to provide a sum-measurement of all dioxin-like compounds in water). There is currently significant scientific effort to develop bioassay-based “guidelines”, commonly called “effects-based trigger values” (EBT) to highlight that these are not meant to be enforceable standards but rather screening levels that would trigger further conventional chemical analysis to identify causative chemicals and, if deemed necessary, effective treatment options. Brand et al.98 proposed several EBT for endocrine activity, as measured by several CALUX assays. Escher et al.69 has recently proposed a generic framework to derive EBT values for receptor-mediated pathways. Tang et al.29 and Escher et al.99 proposed an approach to derive EBT for non-specific assays, such as the Microtox assay and the oxidative stress response. Other projects are currently underway, such as the DEMEAU project funded by the European Commission, that aim to provide guidance on EBT. All of these proposals are still very novel, and require some time to be fully evaluated and tested by regulators before they can be more widely used.
5 Future perspective
5.1 What role for bioanalytical tools in the future?
The studies presented in this review clearly show that bioanalytical tools have a valuable place in risk assessment of reclaimed water. This development is a consequence of the realisation that we cannot monitor every potential constituent in reclaimed water, and that a rational approach that takes into account the inherent limitations of different monitoring strategies is needed.22 A recent review by the US National Research Council remarks that while in vitro bioassays should not be used in isolation for the determination of human health risks, a battery of in vitro bioassays can provide a powerful approach to screening water samples,4 a suggestion echoed in the Australian Guidelines for Water Recycling.5One issue that is limiting greater uptake of bioanalytical methods is the lack of bioassay-based guidelines to compare bioanalytical results to. While there have been several proposals in this area, these still need to be evaluated by health regulators. However, it has long been recognized that at the very least bioanalytical tools can be used to compare alternative water supplies such as reclaimed water with current conventional drinking waters to give information on the toxic potential associated with different water supplies.15
It is important to keep in mind that adoption of bioanalytical tools for recycled water monitoring will most likely not lead to lower monitoring costs. The cost of testing samples in a thorough in vitro bioassay battery is equivalent to current chemical analysis costs. Bioanalytical tools do not replace chemical testing, but rather they present an important addition to our current monitoring strategies by providing a means to detect non-target chemicals and unexpected transformation products, and provide a sum measure of toxic chemicals acting via the same mode of action. However, recent developments in high-throughput testing are likely to lead to a reduction in the per sample cost of in vitro testing, and application of intelligent testing strategies combining a first screening (tier 1) stage with bioanalytical tools and suitable surrogate/indicator chemicals could lead to a reduction of total analytical costs associated with measurement of hundreds of chemicals.
5.2 What are the relevant endpoints to monitor in recycled water?
Based on the information currently available, the following endpoints appear particularly well-suited for recycled water quality assessment:(1) Assays for endocrine activity, in particular estrogenic and glucocorticoid activity. Reporter gene assays are exquisitely sensitive to hormonally active compounds, and provide a sensitive measure of potential endocrine disruption, which is of high public concern;
(2) While obviously not an issue specific to reclaimed water, it is important to continue to monitor disinfected water with assays for reactive toxicity such as mutagenicity and genotoxicity. Although the results from these assays have been and will continue to be difficult to fully comprehend without clearly identified causative chemicals, comparison with other water sources and drinking water provide an important context for the activity in reclaimed water. It is also important to understand the limitation of the current (mostly bacteria-based) assays for reactive toxicity in a human health perspective, and development of novel assays better able to detect human carcinogens should be encouraged;
(3) More difficult to connect to a health outcome at the moment (although future developments in molecular toxicology may fill in the gaps), adaptive response assays (particularly oxidate stress) and xenobiotic metabolism assays (particularly AhR and PXR pathways) appear highly sensitive to compound in both source and reclaimed waters.28 It is particularly important with these assays to compare the results with currently accepted water sources, as even highly treated water is likely to produce a biological response in those assays, which can respond to compounds that may not be toxic to whole organisms due to downstream defense and repair mechanisms.
(4) Finally, bacterial toxicity assays are more sensitive than cytotoxicity assays with human cells, although of course less relevant to human health assessment. Their sensitivity to a wide range of compounds29 may make them well suited as indicators of treatment, especially when applied online.
It should be noted that this list should not be seen as a comprehensive and final list, and future research may well identify other modes of toxic action that are relevant to drinking water.
5.3 A practical framework for application of bioanalytical tools in validation and/or verification of reclaimed water schemes
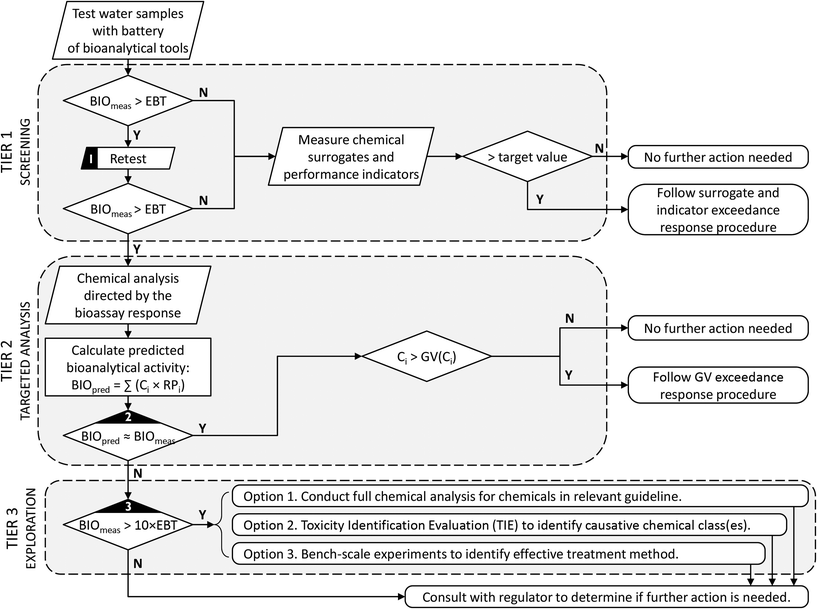
During treatment validation, bioanalytical testing can also be used to benchmark water samples (e.g., compare current drinking water sources with alternative water sources, or current drinking water with reclaimed water) and to determine the efficacy of different treatment technologies to remove bioactive compounds, including whether the process produced toxic transformation products. Several examples are presented earlier in this review.Perhaps the most difficult question faced by scheme operators is what to do when a water sample produces a positive bioanalytical result. The studies presented in this review clearly highlight that this is not an unexpected outcome. This does not imply that the water is unsafe, but rather is a consequence of the exquisite sensitivity of some of the in vitro assays that detect early molecular or cellular events of the adverse outcome pathway. Decades of experience, however, suggest that bioassays can be used to provide an improved monitoring programme with clear operational implications. As a starting point, it is crucially important to set specific targets, or trigger levels, prior to applying bioanalytical tools. Where possible, these trigger levels should be based on sound toxicological methods. If a trigger level is not available for a specific bioassay, the target could simply be the bioassay response with current drinking water, which would ensure that the reclaimed water is at least as good as current drinking water. Once the trigger level has been established, a simple step-wise decision framework can be followed, as described for example in Fig. 2.
5.4 Online monitoring: The next bioanalytical evolutionary stage?
As bioassays become recognized as useful indicators of treatment performance, a logical next stage of evolution of in vitro bioassays would be in the realm of online monitoring. Some bacterial and algal assays have already been adapted to online format,100 although issues of sensitivity in particular remain to be overcome.101 Once validated, these techniques would provide a real-time and sensitive tool to perform screening-level toxicity testing for routine monitoring.List of abbreviations
| AChE | Acetylcholinesterase |
| AOP | Adverse Outcome Pathway |
| A-SCREEN | a bioassay for androgenicity based on inhibition of proliferation of breast cancer cells |
| BAC | Biologically Activated Carbon |
| CAR | Constitutive Androstane Receptor |
| CHO | Chinese Hamster Ovary |
| CPA | Cytokine Production Assay |
| DEQ | Diuron Equivalent Concentration |
| DHTEQ | Dihydrotestosterone Equivalent Concentration |
| EBT | Effect Based Trigger value |
| ECVAM | European Centre for the Validation of Alternative Methods |
| EEQ | 17β-Estradiol Equivalent Concentration |
| ERBA | Estrogen Receptor Binding Assay |
| E-SCREEN | a bioassay for estrogenicity based on proliferation of breast cancer cells |
| FCMN | Flow Cytometry Micronucleus; GAC = Granular Activated Carbon |
| GSH | Glutathione |
| GTU | Genotoxic Unit |
| GWRS | Groundwater Replenishment Scheme |
| I-PAM | Imaging Pulse Amplitude Modulation, a method to measure photosynthesis inhibition |
| LDH | Lactate Dehydrogenase |
| LLE | Liquid-liquid Extraction |
| MF | Microfiltration |
| MFO | Multifunction Oxidase |
| MTT | a colorimetric assay for assessing cell metabolic activity |
| NICEATM | National Toxicology Program Interagency Centre for the Evaluation of Alternative Toxicological Methods (US) |
| NRC | National Research Council (US) |
| NRU | Neutral Red Uptake |
| OECD | Organisation for Economic Cooperation and Development |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| PTEQ | Parathion Equivalent Concentration |
| PXR | Pregnane X Receptor |
| REF | Relative Enrichment Factor |
| RO | Reverse Osmosis |
| SPE | Solid Phase Extraction |
| TCDDEQ | TCDD Equivalent Concentration |
| TEQ | Toxic Equivalents |
| TMXEQ | Tamoxifen Equivalent Concentration |
| T-SCREEN | a bioassay for thyroid activity based on proliferation of rat pituitary cells |
| TTEQ | Testosterone Equivalent Concentration |
| TU | Toxic Unit |
| UF | Ultrafiltration |
| UV | Ultraviolet |
| WCRWS | Western Corridor Recycled Water Scheme |
| WIL2NS | a human B lymphocyte cell line |
| WWTP | Wastewater Treatment Plant |
| YAS | Yeast Androgen Screen |
| YES | Yeast Estrogen Screen. |
Acknowledgements
This review was funded in part by the Australian Water Recycling Centre of Excellence. We thank Beate Escher and Keith Maruya for helpful discussions and comments on an earlier version of this manuscript, and Erik Prochazka for editorial comments.References
- M. Schriks, M. B. Heringa, M. M. E. van der Kooi, P. de Voogt and A. P. van Wezel, Water Res., 2010, 44, 461–476 CrossRef CAS PubMed
.
- M. J. Benotti, R. A. Trenholm, B. J. Vanderford, J. C. Holady, B. D. Stanford and S. A. Snyder, Environ. Sci. Technol., 2009, 43, 597–603 CrossRef CAS
.
- WHO, Guidelines for Drinking-water Quality, 4th edn, World Health Organisation, Geneva, Switzerland, 2011 Search PubMed
.
- National Research Council, Water Reuse. Potential for expanding the nation's water supply through reuse of municipal wastewater, National Academies Press, Washington, DC, USA, 2012 Search PubMed
.
- NWQMS, Australian guidelines for water recycling: managing health and environmental risks (phase 2). Augmentation of drinking water supplies, National Water Quality Management Strategy (NWQMS), Natural Resource Management Ministerial Council, Environment Protection and Heritage Council and National Health and Medical Research Council, Canberra, Australia, 2008 Search PubMed
.
-
P. Anderson, N. Denslow, J. E. Drewes, A. Oliveri, D. Schlenk and S. A. Snyder, Monitoring Strategies for Chemicals of Emerging Concern (CECs) in Recycled Water, State Water Resources Control Board, Sacramento, CA, USA, 2010 Search PubMed
.
- Department of Health, Premier's Collaborative Research Program (2005–2008): Characterizing Treated Wastewater For Drinking Purposes Following Reverse Osmosis Treatment, Department of Health, Western Australia, Perth, WA, Australia, 2009 Search PubMed
.
- WaterSecure, Water Quality Report, WaterSecure, Brisbane, Qld, Australia, 2010 Search PubMed
.
- C. Rodriguez, P. Van Buynder, R. Lugg, P. Blair, B. Devine, A. Cook and P. Weinstein, Int. J. Environ. Res. Public Health, 2009, 6, 1174–1203 CrossRef CAS PubMed
.
- J. E. Drewes, P. Anderson, N. Denslow, A. Olivieri, D. Schlenk, S. A. Snyder and K. A. Maruya, Water Sci. Technol., 2013, 67, 433–439 CrossRef CAS PubMed
.
- A. B. A. Boxall, M. A. Rudd, B. W. Brooks, D. J. Caldwell, K. Choi, S. Hickmann, E. Innes, K. Ostapyk, J. P. Staveley, T. Verslycke, G. T. Ankley, K. F. Beazley, S. E. Belanger, J. P. Berninger, P. Carriquiriborde, A. Coors, P. C. DeLeo, S. D. Dyer, J. F. Ericson, F. Gagne, J. P. Giesy, T. Gouin, L. Hallstrom, M. V. Karlsson, D. G. J. Larsson, J. M. Lazorchak, F. Mastrocco, A. McLaughlin, M. E. McMaster, R. D. Meyerhoff, R. Moore, J. L. Parrott, J. R. Snape, R. Murray-Smith, M. R. Servos, P. K. Sibley, J. O. Straub, N. D. Szabo, E. Topp, G. R. Tetreault, V. L. Trudeau and G. Van Der Kraak, Environ. Health Perspect., 2012, 120, 1221–1229 CrossRef PubMed
.
- R. P. Schwarzenbach, B. I. Escher, K. Fenner, T. B. Hofstetter, C. A. Johnson, U. von Gunten and B. Wehrli, Science, 2006, 313, 1072–1077 CrossRef CAS PubMed
.
- S. A. Snyder, J. – Am. Water Works Assoc., 2014, 106, 38–52 CrossRef CAS
.
- D. M. Cwiertny, S. A. Snyder, D. Schlenk and E. P. Kolodziej, Environ. Sci. Technol., 2014, 48, 11737–11745 CrossRef CAS PubMed
.
- National Research Council, Issues in potable reuse – The viability of augmenting drinking water supplies with reclaimed water, National Academy of Sciences, Washington DC, USA, 1998 Search PubMed
.
- J. Zurlo, D. Rudacille and A. M. Goldberg, Environ. Health Perspect., 1996, 104, 878–880 CrossRef CAS
.
-
U. A. Boelsterli, Mechanistic Toxicology: The molecular basis of how chemicals disrupt biological targets, 2nd edn, Informa Healthcare, New York, NY, USA, 2009 Search PubMed
.
- T. Hartung and M. McBride, ALTEX: Alternatives to Animal Experimentation, 2011, 28, 83–93 Search PubMed
.
- T. Seidle and M. L. Stephens, Toxicol. In Vitro, 2009, 23, 1576–1579 CrossRef CAS PubMed
.
- S. J. Shukla, R. Huang, C. P. Austin and M. Xia, Drug Discovery Today, 2010, 15, 997–1007 CrossRef CAS PubMed
.
-
B. Escher and F. Leusch, Bioanaytical tools in water quality assessment, With contributions by Chapman H and Poulsen A, IWA Publishing, London, UK, 2012 Search PubMed
.
- T. Asano and J. A. Cotruvo, Water Res., 2004, 38, 1941–1951 CrossRef CAS PubMed
.
- G. T. Ankley, R. S. Bennett, R. J. Erickson, D. J. Hoff, M. W. Hornung, R. D. Johnson, D. R. Mount, J. W. Nichols, C. L. Russom, P. K. Schmieder, J. A. Serrrano, J. E. Tietge and D. L. Villeneuve, Environ. Toxicol. Chem., 2010, 29, 730–741 CrossRef CAS PubMed
.
- F. S. Collins, G. M. Gray and J. R. Bucher, Science, 2008, 319, 906–907 CrossRef CAS PubMed
.
- T. Hartung, Eur. J. Pharm. Biopharm., 2011, 77, 338–349 CrossRef CAS PubMed
.
- National Research Council, Toxicity Testing in the 21st Century: A Vision and a Strategy, National Academies Press, Washington DC, USA, 2007 Search PubMed
.
-
A. Poulsen, H. Chapman, F. Leusch and B. Escher, Application of bioanalytical tools for water quality assessment, Urban Water Security Research Alliance, Brisbane, Qld, Australia, 2011 Search PubMed
.
- B. I. Escher, M. Allinson, R. Altenburger, P. A. Bain, P. Balaguer, W. Busch, J. Crago, N. D. Denslow, E. Dopp, K. Hilscherova, A. R. Humpage, A. Kumar, M. Grimaldi, B. S. Jayasinghe, B. Jarosova, A. Jia, S. Makarov, K. A. Maruya, A. Medvedev, A. C. Mehinto, J. E. Mendez, A. Poulsen, E. Prochazka, J. Richard, A. Schifferli, D. Schlenk, S. Scholz, F. Shiraishi, S. Snyder, G. Su, J. Y. M. Tang, B. V. D. Burg, S. C. V. D. Linden, I. Werner, S. D. Westerheide, C. K. C. Wong, M. Yang, B. H. Y. Yeung, X. Zhang and F. D. L. Leusch, Environ. Sci. Technol., 2014, 48, 1940–1956 CrossRef CAS PubMed
.
- J. Y. M. Tang, S. McCarty, E. Glenn, P. A. Neale, M. S. J. Warne and B. I. Escher, Water Res., 2013, 47, 3300–3314 CrossRef CAS PubMed
.
- K. Watson, G. Shaw, F. D. L. Leusch and N. L. Knight, Water Res., 2012, 46, 6069–6083 CrossRef CAS PubMed
.
- J. P. van de Merwe and F. D. L. Leusch, Environ. Sci.: Processes Impacts, 2015, 17, 947–955 CAS
.
- F. D. L. Leusch, S. J. Khan, S. Laingam, E. Prochazka, S. Froscio, T. Trinh, H. F. Chapman and A. Humpage, Water Res., 2014, 49, 300–315 CrossRef CAS PubMed
.
- A. K. Faul, E. Julies and E. J. Pool, Water SA, 2013, 39, 499–506 Search PubMed
.
- M. Plewa, E. Wagner and W. Mitch, Environ. Sci. Technol., 2011, 45, 4159–4165 CrossRef CAS PubMed
.
- R. Konsoula and F. A. Barile, Toxicol. In Vitro, 2005, 19, 675–684 CrossRef CAS PubMed
.
- M. Macova, S. Toze, L. Hodgers, J. F. Mueller, M. E. Bartkow and B. I. Escher, Water Res., 2011, 45, 4238–4247 CAS
.
- M. J. Plewa, E. D. Wagner, D. H. Metz, R. Kashinkunti, K. J. Jamriska and M. Meyer, Environ. Sci. Technol., 2012, 46, 7811–7817 CrossRef CAS PubMed
.
- J. Y. M. Tang, E. Glenn, H. Thoen and B. I. Escher, J. Environ. Monit., 2012, 14, 1073–1081 RSC
.
- S. A. Snyder, D. L. Villeneuve, E. M. Snyder and J. P. Giesy, Environ. Sci. Technol., 2001, 35, 3620–3625 CrossRef CAS
.
- Global Water Research Coalition, Bioanalytical tools to analyse hormonal activity in environmental waters, Global Water Research Coalition, London, UK, 2012 Search PubMed
.
- F. D. L. Leusch, C. de Jager, Y. Levi, R. Lim, L. Puijker, F. Sacher, L. A. Tremblay, V. S. Wilson and H. F. Chapman, Environ. Sci. Technol., 2010, 44, 3853–3860 CrossRef CAS PubMed
.
- A. M. Soto, J. M. Calabro, N. V. Prechtl, A. Y. Yau, E. F. Orlando, A. Daxenberger, A. S. Kolok, L. J. Guillette, B. le Bizec, I. G. Lange and C. Sonnenschein, Environ. Health Perspect., 2004, 112, 346–352 CrossRef CAS
.
- S. C. van der Linden, M. B. Heringa, H.-Y. Man, E. Sonneveld, L. M. Puijker, A. Brouwer and B. van der Burg, Environ. Sci. Technol., 2008, 42, 5814–5820 CrossRef CAS
.
- R. Muller, U. Schreiber, B. I. Escher, P. Quayle, S. M. Bengtson Nash and J. F. Mueller, Sci. Total Environ., 2008, 401, 51–59 CrossRef CAS PubMed
.
- J. Y. M. Tang and B. I. Escher, Environ. Toxicol. Chem., 2014, 33, 1427–1436 CrossRef CAS PubMed
.
- P. A. Neale and B. I. Escher, Environ. Toxicol. Chem., 2013, 32, 1526–1534 CAS
.
- M. Wagner, E. L. M. Vermeirssen, S. Buchinger, M. Behr, A. Magdeburg and J. Oehlmann, Environ. Toxicol. Chem., 2013, 32, 1906–1917 CrossRef CAS PubMed
.
- M. Macova, B. I. Escher, J. Reungoat, S. Carswell, K. L. Chue, J. Keller and J. F. Mueller, Water Res., 2010, 44, 477–492 CrossRef CAS PubMed
.
- W. Körner, V. Hanf, W. Schuller, C. Kempter, J. Metzger and H. Hagenmaier, Sci. Total Environ., 1999, 225, 33–48 CrossRef
.
- D. E. Tillitt, G. T. Ankley, D. A. Verbrugge, J. P. Giesy, J. P. Ludwig and T. J. Kubiak, Arch. Environ. Contam. Toxicol., 1991, 21, 91–101 CrossRef CAS
.
- J. Y. M. Tang, F. Busetti, J. W. A. Charrois and B. I. Escher, Water Res., 2014, 60, 289–299 CrossRef CAS PubMed
.
- B. I. Escher, N. Bramaz, J. F. Mueller, P. Quayle, S. Rutishauser and E. L. M. Vermeirssen, J. Environ. Monit., 2008, 10, 612–621 RSC
.
- B. N. Ames, F. D. Lee and W. E. Durston, Proc. Natl. Acad. Sci. U. S. A., 1973, 70, 782–786 CrossRef CAS
.
- A. Magdeburg, D. Stalter, M. Schlüsener, T. Ternes and J. Oehlmann, Water Res., 2014, 50, 35–47 CrossRef CAS PubMed
.
- N. Gruener, Bull. Environ. Contam. Toxicol., 1978, 20, 522–526 CrossRef CAS
.
-
P. L. McCarty, M. Reinhard, N. L. Goodman, J. W. Graydon, G. D. Hopkins, K. E. Mortelmans and D. G. Argo, Advanced treatment for wastewater reclamation at Water Factory 21, Department of Civil Engineering, Stanford University, CA, USA, 1982 Search PubMed
.
- National Research Council, The Potomac Estuary Experimental Water Treatment Plant. A review of the U.S. Army Corps of Engineers, Evaluation of the Operation, Maintenance, and Performance of the Experimental Estuary Water Treatment Plant, National Academy Press, Washington, DC, USA, 1984 Search PubMed
.
- R. Kfir and O. W. Prozesky, Water Res., 1982, 16, 1561–1568 CrossRef CAS
.
- G. I. Iiputa, K. Nikodemus and J. Menge, presented in part at the WISA 2008 Biennial Conference, Sun City, South Africa, 18–22 May 2008, 2008 Search PubMed.
- J. G. Menge and J. L. Slabbert, presented in part at the 9th International Symposium on Toxicity Assessment (ISTA), Pretoria, 26 Sep - 1 Oct 1999, 1999 Search PubMed.
- A. W. Olivieri, D. M. Eisenberg, R. C. Cooper, G. Tchobanoglous and P. Gagliardo, Water Sci. Technol., 1996, 33, 285–296 CrossRef CAS
.
- J. C. Loper, Mutat. Res., Genet. Toxicol., 1980, 76, 241–268 CrossRef CAS
.
- J. R. Meier, Mutat. Res., Genet. Toxicol., 1988, 196, 211–245 CrossRef CAS
.
- R. G. Stahl Jr, Ecotoxicol. Environ. Saf., 1991, 22, 94–125 CrossRef CAS
.
- S. A. Snyder, P. Westerhoff, Y. Yoon and D. L. Sedlak, Environ. Eng. Sci., 2003, 20, 449–469 CrossRef CAS
.
- F. D. L. Leusch, H. F. Chapman, W. Körner, S. R. Gooneratne and L. A. Tremblay, Environ. Sci. Technol., 2005, 39, 5781–5786 CrossRef CAS
.
-
J. Reungoat, B. I. Escher, M. Macova, M. J. Farré, F. X. Argaud, M. Rattier, W. Gernjak and J. Keller, Wastewater reclamation using ozonation combined with biological activated carbon filtration, Urban Water Security Research Alliance, Brisbane, Qld, Australia, 2012 Search PubMed
.
-
J. E. Drewes, J. D. C. Hemming, J. J. Schauer and W. C. Sonzogni, Removal of endocrine disrupting compounds in water reclamation processes, Water Environment Reseach Foundation and IWA Publishing, London, UK, 2006 Search PubMed
.
- B. I. Escher, P. A. Neale and F. D. L. Leusch, Water Res., 2015, 81, 137–148 CrossRef CAS PubMed
.
- F. D. L. Leusch, S. J. Khan, M. M. Gagnon, P. Quayle, T. Trinh, H. Coleman, C. Rawson, H. F. Chapman, P. Blair, H. Nice and T. Reitsema, Water Res., 2014, 50, 420–431 CrossRef CAS PubMed
.
-
T. Reitsema, H. E. Nice, F. D. L. Leusch, P. Quayle, H. F. Chapman, S. J. Khan, T. Trinh, H. Coleman, C. Rawson, M. M. Gagnon and P. Blair, Development of an 'ecotoxicity toolbox' to characterise water quality for recycling, Department of Water, Government of Western Australia, Perth, WA, Australia, 2010 Search PubMed
.
-
M. Macova, B. Escher, J. Mueller and S. Toze, Bioanalytical tools to evaluate micropollutants across the seven barriers of the indirect potable reuse scheme, Urban Water Security Research Alliance, Brisbane, Qld, Australia, 2010 Search PubMed
.
- National Water Commission, A national approach to health risk assessment, risk communication and management of chemical hazards from recycled water. Waterlines report No 48, National Water Commission (NWC), Canberra, Australia, 2011 Search PubMed
.
- J. Reungoat, B. I. Escher, M. Macova and J. Keller, Water Res., 2011, 45, 2751–2762 CrossRef CAS PubMed
.
- J. Reungoat, M. Macova, B. I. Escher, S. Carswell, J. F. Mueller and J. Keller, Water Res., 2010, 44, 625–637 CrossRef CAS PubMed
.
- B. I. Escher, N. Bramaz and C. Ort, J. Environ. Monit., 2009, 11, 1836–1846 RSC
.
- D. Stalter, A. Magdeburg, M. Wagner and J. Oehlmann, Water Res., 2011, 45, 1015–1024 CrossRef CAS PubMed
.
- A. Jia, B. I. Escher, F. D. L. Leusch, J. Y. M. Tang, E. Prochazka, B. Dong, E. M. Snyder and S. A. Snyder, Water Res., 2015, 80, 1–11 CrossRef CAS PubMed
.
- A. C. Mehinto, A. Jia, S. A. Snyder, B. S. Jayasinghe, N. D. Denslow, J. Crago, D. Schlenk, C. Menzie, S. D. Westerheide, F. D. L. Leusch and K. A. Maruya, Water Res., 2015, 83, 303–309 CrossRef CAS PubMed
.
- WateReuse Research Foundation, Downstream--Context, Understanding, Acceptance: Effect of Prior Knowledge of Unplanned Potable Reuse on the Acceptance of Planned Potable Reuse (WRF 09–01), WaterReuse Research Foundation, Alexandria, VA, USA, 2012 Search PubMed
.
- B. I. Escher, M. Lawrence, M. Macova, J. F. Mueller, Y. Poussade, C. Robillot, A. Roux and W. Gernjak, Environ. Sci. Technol., 2011, 45, 5387–5394 CrossRef CAS PubMed
.
-
S. A. Snyder, E. C. Wert, L. Hongxia, P. Westerhoff and Y. Yoon, Removal of EDCs and pharmaceuticals in drinking and reuse treatment processes, Awwa Research Foundation, USA, 2007 Search PubMed
.
- S. A. Snyder, S. Adham, A. M. Redding, F. S. Cannon, J. DeCarolis, J. Oppenheimer, E. C. Wert and Y. Yoon, Desalination, 2006, 202, 156–181 CrossRef PubMed
.
- N. Cao, T. Miao, K. Li, Y. Zhang and M. Yang, J. Environ. Sci., 2009, 21, 409–413 CrossRef CAS
.
- N. Cao, M. Yang, Y. Zhang, J. Hu, M. Ike, J. Hirotsuji, H. Matsui, D. Inoue and K. Sei, Sci. Total Environ., 2009, 407, 1588–1597 CrossRef CAS PubMed
.
- A. Kontana, C. A. Papadimitriou, P. Samaras, A. Zdragas and M. Yiangou, Water Sci. Technol., 2008, 57, 947–953 CrossRef CAS PubMed
.
- A. Kontana, C. A. Papadimitriou, P. Samaras, A. Zdragas and M. Yiangou, Water Sci. Technol., 2009, 60, 1497–1505 CrossRef CAS PubMed
.
- E. Lundstrom, M. Adolfsson-Erici, T. Alsberg, B. Bjorlenius, B. Eklund, M. Laven and M. Breitholtz, Ecotoxicol. Environ. Saf., 2010, 73, 1612–1619 CrossRef PubMed
.
- M. Petala, P. Samaras, A. Kungolos, A. Zouboulis, A. Papadopoulos and G. P. Sakellaropoulos, Chemosphere, 2006, 65, 1007–1018 CrossRef CAS PubMed
.
- M. Petala, P. Samaras, A. Zouboulis, A. Kungolos and G. Salkellaropoulos, Environ. Toxicol., 2006, 21, 417–424 CrossRef CAS PubMed
.
- J. Xu, C. Zhao, D. Wei and Y. Du, J. Environ. Sci., 2014, 26, 1961–1969 CrossRef PubMed
.
- X. Zhang and X. Zhao, Bull. Environ. Contam. Toxicol., 2013, 91, 499–502 CrossRef CAS PubMed
.
- X. Zhang, X. Zhao, M. Zhang and Q.-y. Wu, Desalination, 2011, 281, 185–189 CrossRef CAS PubMed
.
- L. Rizzo, Water Res., 2011, 45, 4311–4340 CrossRef CAS PubMed
.
- B. J. Vanderford and S. A. Snyder, Environ. Sci. Technol., 2006, 40, 7312–7320 CrossRef CAS
.
- T. Anumol, S. Merel, B. Clarke and S. Snyder, Chem. Cent. J., 2013, 7, 104 CrossRef PubMed
.
- WateReuse Research Foundation, Development of bio-analytical techniques to assess the potential human health impacts of recycled water, WateReuse Research Foundation, 2014 Search PubMed
.
- W. Brand, C. M. de Jongh, S. C. van der Linden, W. Mennes, L. M. Puijker, C. J. van Leeuwen, A. P. van Wezel, M. Schriks and M. B. Heringa, Environ. Int., 2013, 55, 109–118 CrossRef CAS PubMed
.
- B. I. Escher, C. van Daele, M. Dutt, J. Y. M. Tang and R. Altenburger, Environ. Sci. Technol., 2013, 47, 7002–7011 CAS
.
- M. V. Storey, B. van der Gaag and B. P. Burns, Water Res., 2011, 45, 741–747 CrossRef CAS PubMed
.
- M. Woutersen, S. Belkin, B. Brouwer, A. Wezel and M. Heringa, Anal. Bioanal. Chem., 2011, 400, 915–929 CrossRef CAS PubMed
.
- D. M. Quanrud, R. G. Arnold, K. E. Lansey, C. Begay, W. Ela and A. J. Gandolfi, J. Water Health, 2003, 1, 33–44 CAS
.
| This journal is © The Royal Society of Chemistry 2015 |