High concentration DNA solubility in bio-ionic liquids with long-lasting chemical and structural stability at room temperature†
Mukesh Sharmaab,
Dibyendu Mondalab,
Nripat Singhab,
Nitin Trivediab,
Jitkumar Bhattab and
Kamalesh Prasad *ab
*ab
aMarine Biotechnology and Ecology Division, CSIR-Central Salt & Marine Chemicals Research Institute, G. B Marg, Bhavnagar-364002, Gujarat, India. E-mail: kamlesh@csmcri.org; drkamaleshp@gmail.com; Fax: +91-278-2567562; Tel: +91-278-2567760
bAcSIR-Central Salt & Marine Chemicals Research Institute, G. B Marg, Bhavnagar-364002, Gujarat, India
First published on 28th April 2015
Abstract
DNA (Salmon testes) was solubilized in two bio-based ionic liquids (bio-ILs) namely choline-pyruvate (Cho-Pyr) and choline-glycolate (Cho-Gly) up to 2.0 and 8.0 wt% respectively. The solubilized DNA was found to maintain its chemical and structural stability for up to one year when stored at room temperature (25 °C). Excessive H-bonding along with electrostatic interactions between the DNA and the ILs, as observed in the isothermal titration calorimetry (ITC) studies, was found to be the major reason behind the high concentration of DNA dissolution in Cho-Gly and its long term stability. Measurements carried out on a UV-Vis spectrophotometer, FT-IR, circular dichroism (CD) spectrometer, agarose-gel electrophoresis and PCR amplification of recovered DNA from the ILs solutions confirmed the chemical and structural integrity of DNA during dissolution.
Introduction
Ionic liquids (ILs) are molten salts at room temperature with unique characteristics such as high ion conductivity, electrochemical stability, low volatility, nonflammability and some of them have the ability to decrystallise or dissolve woody biomass and other oceanic polysaccharides.1–4 Application of ILs is increasing day by day and they find applications as dissolution and stabilizing media for bio-macromolecules such as protein or DNA and many more applications are being envisaged for these newly emerging solvents.5–9 The toxicity of a few ILs led to the development of ILs of bio origin, which enjoy properties such as biocompatibility, bio-degradability and non-toxicity along with sustainable resources making them exploitable for a number of applications.10–12 DNA, the carrier of biological information is evolving as a fascinating biomaterial and building block for new materials due to its unique properties such as molecular recognition, nanoscale structural distribution, biocompatibility etc.13–17 However, the major bottlenecks associated with the use of DNA for designing advanced functional materials are its poor solubility in a wide range of solvents and long term structural instability under ambient conditions and at different pH values. Apart from conventional aqueous buffer, DNA is reported to be soluble in non-aqueous organic solvents such as formamide, DMSO etc., however it loses its structural integrity in many such organic solvents.18,19 A significant improvement in the field of DNA dissolution and its long-lasting structural preservation (up to six months) was explored by us using bio-ILs (3.5 wt% DNA in choline-indole-3-acetate) and bio-deep eutectic solvents (5.5 wt% in the mixture of choline chloride and ethylene glycol),7,8 which turned to be advantageous towards preparation of a DNA based material having anti bacterial and magnetic properties.20This research is a result of our continuous efforts towards finding new bio-ILs structures for the improved dissolution of DNA with much longer structural and chemical stability upon storage at room temperature. This may add further wings to DNA research towards its wider application in material preparation. Herein, we have dissolved DNA in choline glycolate and choline pyruvate up to 8 and 2 wt% respectively and observed the stability of the delicate bio-molecule up to one year when stored at room temperature. Moreover, the chemistry behind the high concentration dissolution of DNA in the IL was explored. Furthermore the ILs were recycled and reused in the subsequent dissolution processes.
Experimental section
Materials
Deoxyribonucleic acid sodium salt extracted from Salmon testes (CAS no. 9007-49-2), analytical grade glycolic acid and pyruvic acid were purchased from TCI Chemicals, Tokyo, Japan. The ratio of the absorbance of the DNA stock solution at 260 nm to that at 280 nm was found to be 1.87, which suggested the absence of proteins as contamination.21 Due to this high purity it was used as received without any further processing. Choline hydrogen carbonate and tris (hydroxymethyl) aminomethtane HCl (Tris–HCl) was purchased from Sigma-Aldrich, USA. Deionized water (conductivity ≤ 12.95 μs cm−1) was used throughout the experiments. A stock solution of DNA was prepared by dissolving an appropriate amount of the solid DNA in Tris–HCl buffer solution and stored at 4 °C for more than 24 h with occasional gentle shaking to ensure homogeneity. DNA concentrations in solutions were determined by using an extinction coefficient of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1 at 260 nm on UV-Vis spectrophotometer and expressed in terms of base molarity.22
200 M−1 cm−1 at 260 nm on UV-Vis spectrophotometer and expressed in terms of base molarity.22
Synthesis of ionic liquids
Bio-ILs containing choline and glycolic acid/pyruvic acid were synthesized by metathesis reactions reported by Petkovic et al., (2010).23 In a typical reaction, solution of glycolic acid or pyruvic acid in methanol/water mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added drop-wise to choline hydrogen carbonate in equimolar ratio under ambient conditions until the formation of the ILs. Water was then removed under reduced pressure using a rotary evaporator (e.g. 80 °C, 2 h). The synthesized bio-ILs was finally washed with ethyl acetate and structures were confirmed by 1H NMR and electro spray-mass spectrometer (ESI-MS).
1) was added drop-wise to choline hydrogen carbonate in equimolar ratio under ambient conditions until the formation of the ILs. Water was then removed under reduced pressure using a rotary evaporator (e.g. 80 °C, 2 h). The synthesized bio-ILs was finally washed with ethyl acetate and structures were confirmed by 1H NMR and electro spray-mass spectrometer (ESI-MS).
Dissolution of DNA in bio-ionic liquids
The typical procedure for the dissolution of DNA in bio-ILs was identical to our previous reports.7,8 Briefly, 10 to 100 mg of DNA powders was added gradually into the vials containing 1 g of solvent (Cho-Gly & Cho-Pyr) at room temperature (25 °C) under nitrogen gas atmosphere with gentle stirring (1–10 h). Once the turbidity or the presence of particles were visualised by the cloud point method, the samples were equilibrated for at least 24 h to check the disappearance of the cloudiness and absence of insoluble particles were confirmed by observing the aliquots under a optical light microscope (100×). No solubility was considered when the addition of 0.1 wt% of solute showed turbidity and appearance of particles under microscope.Characterization
The UV-Vis absorption spectra of standard DNA at concentration of 2.3 × 10−5 M (20 μg mL−1) and regenerated DNA recovered from Cho-Gly and Cho-Pyr in Tris–HCl buffer (pH = 7.2) were recorded on a Varian CARY 500 UV-Vis-NIR spectrophotometer. Circular dichroism (CD) spectra were recorded on a Jasco model J-815 CD Spectrometer using measurement range at 230–330 nm and at optimized sample concentrations in Tris–HCl Buffer (pH = 7.2) at a scanning speed of 10 nm min−1 and band width of 1 nm. The spectra were acquired in a 1.0 cm path-length quartz cuvette at 25 °C. The 31P NMR spectra of standard DNA and recovered DNA from Cho-gly solution was recorded at 25 °C on a Bruker Avance 200 MHz spectrometer. The measurements were carried out with a DDO probe at a resonance frequency of 80.96 MHz. The phosphorous chemical shifts of DNA were externally referenced to 85% o-phosphoric acid. FT-IR of standard DNA and regenerated DNA from Cho-Gly and from Cho-Pyr were carried out on a Perkin-Elmer FT-IR machine (Spectrum GX, USA) using KBr disc (2 mg sample in 600 mg KBr). Presence of insoluble DNA particles in the solutions was monitored using ordinary light microscope with 100× magnification (Fine Vision Microscope, India). Karl-Fischer titration to estimate moisture content in the ILs were carried out on a Radiometer/TIM 880 instrument (UK) using 2 mL of the liquids. The shear viscosity of DNA solutions was measured on an Anton Paar, Physica MCR 301 rheometer USA, using parallel plate PP50/P-PTD200 geometry (50 mm diameter; 0.2 mm gap). Agarose gel electrophoresis was carried out with Salmon testes DNA (5 μL, 500 μg mL−1) and a DNA marker (lambda DNA Hind III digest) (4 μL). The 1.5% agarose gel was made in TAE buffer (tris acetate EDTA) and was run at a constant volt of 60 V apply initially for 15 min. and after 15 min 75 V was applied for 1 h. Isothermal Titration Calorimetry (ITC) of Cho-Gly with DNA in Tris–HCl buffer (pH = 7.2) solution was examined using a MicroCal ITC 200 micro calorimeter instrument and titration was done using an auto controlled Hamilton Micro syringe of 40 μL volume capacity. In a typical procedure for ITC measurements, DNA solution (600 μL of 5.0 × 10−5 mol L−1) was filled in sample cell and reference cell was filled with Tris–HCl buffer. Stock solution of Cho-Gly (0.1 mol L−1) was prepared in Tris–HCl buffer and was filled in Hamilton Micro syringe (40 μL). The titration was done by adding 2 μL of titrant (Cho-Gly) into the sample cell containing DNA solution with continuous stirring (500 rpm). The physical parameters like time of addition, rotation speed of syringe and duration between each addition were auto controlled by software. ITC plot for the titration of 600 μL of 5.0 × 10−5 mol L−1 DNA in Tris–HCl buffer with successive addition of 2 μL of 0.1 mol L−1 choline glycolate at 298.15 K was measured and was corrected by the subtraction of the corresponding heat changes derived from the titration of Cho-Gly into Tris–HCl buffer alone. The plot of enthalpy changes vs. concentration of titrant was used to describe the interaction between Cho-Gly and DNA.PCR of seaweed DNA after recovering from DES
The genomic DNA of the seaweed or marine macrophytic algae Ulva spp. was extracted following the method of Gupta et al., 2011.24 1.0 microgram of Ulva DNA was first dissolved separately in 100 μL of Cho-Gly or Cho-Pyr at room temperature followed by precipitation with ice cold 70% v/v aqueous ethanol and centrifugation. The DNA samples thus obtained were finally dried at room temperature. To evaluate the affect of solvents on DNA structure, PCR amplification of the rbcL gene of standard and regenerated Ulva genomic DNA was performed using the primers and PCR reaction profile described by Hayden et al., 2003.25Result & discussion
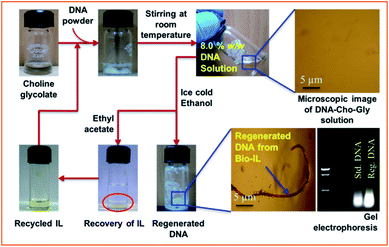
For the dissolution of DNA, two ionic liquids (ILs) were synthesized by a simple metathesis reaction as described in the experimental section using nontoxic and bio-based substrates (Fig. 1). The structure and purity of the ILs were confirmed by 1H NMR and ESI-MS measurements (ESI, Fig. S1–S6†). The physicochemical properties of the ILs are provided in Table S1† and found to be similar to those reported earlier.10 As photographically shown in Scheme 1, 2 to 10 wt% of DNA was added gradually separately in to 1 g of Cho-Gly or Cho-Pyr with gentle stirring at room temperature.8 wt% of the DNA was found to form transparent solution in Cho-Gly upon 6 h of stirring at room temperature but more than this concentration of DNA resulted formation of turbid solutions in the IL (ESI, Fig. S7†). However, maximum solubility of DNA in Cho-Pyr was found to be only 2 wt% upon 10 h of stirring at room temperature (ESI, Fig. S8†). The phase contrast microscopic images of DNA in the two ILs at different time interval and concentration shows absence of insoluble residues of DNA in Cho-Gly at 8 wt% and 2 wt% in Cho-Pyr, while above these concentrations presence of insoluble DNA was visible in both the ILs (ESI, Fig. S9†). It should be noted that the apparent viscosity of Cho-Pyr (204.7 mPa s) is about two times higher than Cho-Gly (93.7 mPa s) [Table S1†], the higher viscosity of the former results restricted mobility of cholinium ion in Cho-Pyr in comparison to Cho-Gly during dissolution. The lesser degree of freedom available for DNA in the former is perhaps the reason of low solubility of the molecule in the IL in comparison to the latter.8 The DNA was isolated from the DNA-IL solutions by adding ice cold ethanol and was characterised. The liquid stream containing mixture of IL and ethanol was phase separated by adding ethyl acetate (Scheme 1) and the respective ILs were recovered by decantation (>92% recovery). The purity was confirmed by 1H NMR (ESI, Fig. S10a and S10b†), where no additional peak due to contaminations were visible. The ILs were successfully recycled for 3 times and reused in the dissolution of DNA.
The FT-IR spectra of the standard DNA and regenerated DNA from Cho-Gly and Cho-Pyr were recorded and depicted in Fig. 2a and S11† respectively. In the DNA structure, the bands at 1073 and 1223 cm−1 are due to the symmetric and anti-symmetric stretching vibrations of the PO2− groups. Vibrational bands at 1713 cm−1 are due to in plane C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of amide I. Base pairing and base stacking of the molecule are observed between 1750–1550 cm−1.7,8 These characteristic bands were not disturbed in the DNA sample regenerated from Cho-Gly indicative of structural intactness of DNA during dissolution. The same was true for the regenerated DNA from Cho-Pyr (ESI, Fig. S11†). The FT-IR spectrum of DNA in Cho-Gly in solution state was also recorded to understand the interaction of IL with DNA (ESI, Fig. S12†). It was observed that, the symmetric and anti-symmetric stretching vibrations of the PO2− groups of DNA shifted from 1073 cm−1 to 1086 cm−1 and 1223 cm−1 to 1241 cm−1 respectively in the solution, which may be due to the electrostatic interaction between choline cation of the IL and phosphate group of DNA and facilitated the dissolution process. Fig. 2b shows the 31P NMR of std DNA and regenerated DNA from Cho-Gly. The strong peak at 0 ppm was ascribed to H3PO4 used as internal standard. The chemical shift of the phosphate group of the standard DNA and regenerated DNA were observed at −1.24 ppm and −1.26 ppm respectively. The similar chemical shift confirmed the structural and chemical stability of DNA during dissolution process. The possible electrostatic interaction between choline cation and phosphate group of the DNA was also confirmed using 31P NMR of DNA in Cho-Gly (ESI, Fig. S13†). The spectrum shows shifting of characteristic peak at −1.26 ppm to −1.36 indicating interaction of the choline cation with phosphate group of DNA.
O stretching of amide I. Base pairing and base stacking of the molecule are observed between 1750–1550 cm−1.7,8 These characteristic bands were not disturbed in the DNA sample regenerated from Cho-Gly indicative of structural intactness of DNA during dissolution. The same was true for the regenerated DNA from Cho-Pyr (ESI, Fig. S11†). The FT-IR spectrum of DNA in Cho-Gly in solution state was also recorded to understand the interaction of IL with DNA (ESI, Fig. S12†). It was observed that, the symmetric and anti-symmetric stretching vibrations of the PO2− groups of DNA shifted from 1073 cm−1 to 1086 cm−1 and 1223 cm−1 to 1241 cm−1 respectively in the solution, which may be due to the electrostatic interaction between choline cation of the IL and phosphate group of DNA and facilitated the dissolution process. Fig. 2b shows the 31P NMR of std DNA and regenerated DNA from Cho-Gly. The strong peak at 0 ppm was ascribed to H3PO4 used as internal standard. The chemical shift of the phosphate group of the standard DNA and regenerated DNA were observed at −1.24 ppm and −1.26 ppm respectively. The similar chemical shift confirmed the structural and chemical stability of DNA during dissolution process. The possible electrostatic interaction between choline cation and phosphate group of the DNA was also confirmed using 31P NMR of DNA in Cho-Gly (ESI, Fig. S13†). The spectrum shows shifting of characteristic peak at −1.26 ppm to −1.36 indicating interaction of the choline cation with phosphate group of DNA.
The UV-Vis spectra of DNA showed a single characteristic absorbance band at 260 nm and the purity of DNA depends on the absorbance ratio at 260 nm to 280 nm. The ratio was found to be 1.87 for pure DNA and 1.86 for regenerated from Cho-Gly (Fig. 2c). These comparable values confirmed that π stacking interaction between the base pair of the DNA was not affected during dissolution phenomenon. However, the absorbance ratio of DNA regenerated from Cho-Pyr was only 1.67 (ESI, Fig. S14†), which is lower than that of std DNA. The circular dichroism (CD) spectra of standard DNA and regenerated DNA from Cho-Gly were recorded and depicted in Fig. 2d. The CD spectra of DNA showed characteristic B-form of DNA with long wave positive band at 277 nm corresponding to π–π base packing and a shorter wave negative band at 246 nm corresponding to helicity. It should be noted that, the CD spectrum of DNA regenerated from Cho-Pyr (ESI, Fig. S15†) also showed similar bands indicating the conformation of DNA in Cho-Pyr was also intact.
To study the chemical and structural stability of the regenerated DNA in different environments, effect of temperature and pH on DNA conformation was also carried out (Fig. 3a and b). Fig. 3a shows the plot of absorbance ratio (A260/A280) at different temperature range from 25 °C to 70 °C for std DNA and regenerated DNA from Cho-Gly. The inset of Fig. 3a is the UV-Vis spectra of regenerated DNA at variable temperature. The absorbance ratio was 1.87 at 25 °C and 1.75 at 70 °C for std DNA and identical values were observed for the regenerated DNA, confirming the stability of the DNA at elevated temperatures, which is not affected during dissolution in Cho-Gly. Further to understand the change in the secondary structure of DNA due to pH, the CD spectra of regenerated DNA from Cho-Gly in varying pH (pH 2 to 10) were recorded in Tris–HCl buffer (Fig. 3b). B-form of the DNA was found to be intact at pH 3 to pH 10. However, at pH 2, the long wave positive band shifted from 277 nm to 279 nm and shorter wave band was shifted from 246 nm to 243 nm which may be due to the conformational change of the DNA. The study was extended to explore the long term stability of the DNA in Cho-Gly at room temperature. For this purpose DNA dissolved in Cho-Gly was stored at 25 °C for 12 months. After 12 months, UV-Vis spectrum of the regenerated DNA from the IL showed similar absorbance pattern that with std DNA and the absorbance ratio (A260/A280) was 1.82 (Fig. 3c). The UV-Vis absorbance data of DNA after 12 months stored in Cho-Gly was also supported by CD spectra (Fig. 3d). There were no conformational changes found which further confirmed the structural and chemical stability of DNA for long-lasting period in Cho-Gly. However the DNA stored in Tris–HCl buffer for the six month at room temperature was found to be degraded as observed previously.8 The DNA regenerated from Cho-Pyr also did not show any sign of degradation (UV-Vis and CD spectra of regenerated DNA are shown in ESI, Fig. S14 and S15†).
Isothermal titration calorimetry (ITC) was used to investigate the intermolecular interaction between DNA and ILs. Fig. 4 shows the ITC profile corresponding to differential enthalpy change of DNA solution against Cho-Gly concentrations, which was corrected by the subtraction of the corresponding heat changes derived from the titration of Cho-Gly into Tris–HCl buffer alone. At the first injection the observed ΔH was about +0.1 kJ mol−1 and it was increased up to +0.175 kJ mol−1 upon further addition of Cho-Gly. This indicates the binding of Cho-Gly to DNA is endothermic process. The small positive enthalpy change indicate there is some sort of electrostatic interaction26 which may arises due to interaction between choline and phosphate group of DNA as confirmed by FT-IR and 31P NMR (Fig. S12 and S13† respectively). These electrostatic interactions may be responsible for the enhanced solubility of DNA in Cho-Gly. As can be further seen from Fig. 4, the value of dH decreases with the extra addition of Cho-Gly and switches to exothermic enthalpy at a concentration of 11.0 mmol L−1. The negative enthalpies reveal that the system get stabilised exclusively by H-bonding.27 The extensive H-bonding interaction between the hydroxyl group of Cho-Gly and the DNA base pair may be accountable for long term stability of DNA at room temperature. On the other hand ITC profile of DNA solution titrated with Cho-Pyr showed dH value −0.28 kJ mol−1 at first injection and it increase up to −0.104 kJ mol−1 upon further addition of Cho-Pyr (ESI Fig. S16†). After that the enthalpy change was constant. This indicated the binding of Cho-Pyr to DNA was an endothermic process which was governed mainly by electrostatic interaction.28 Thus, only electrostatic interaction besides higher viscosity may be another reason for limited solubility of DNA in Cho-Pyr as compared to Cho-Gly.
In order to study the structural integrity of DNA during dissolution in the ILs, agarose-gel electrophoresis of recovered DNA from ILs were carried out and compared with standard DNA. A typical band was appeared for standard Salmon testes DNA in agarose gel electrophoresis as shown in ESI Fig. S17.† Similar typical bands for the DNA were also observed by other researchers.29 In the electrophoresis experiment, regenerated DNA from both of Cho-Gly and Cho-Pyr showed similar elution profile when compared that with standard DNA under identical conditions, this indicated that there was no degradation of DNA structure during the dissolution in the ILs. The PCR amplification of the standard and regenerated Ulva genomic DNA showed successful amplification of rbcL gene at around 750 bp indicating stability of the DNA sample during dissolution in the ILs (ESI, Fig. S18†).
Conclusion
In conclusion, DNA (Salmon testes) was solubilized in two different bio-based ionic liquids, namely choline-glycolate and choline-pyruvate. The macromolecule was found to soluble up to 8 wt% in the former, while only 2 wt% was soluble in the later. Multiple chemical investigations confirmed room temperature stability of DNA upon storage in the ionic liquids up to one year. The excessive hydrogen bonding and electrostatic interactions between ionic liquids and DNA was found to be responsible for the long term stability and high concentration solubility of the bio-macromolecule in the ionic liquids.Acknowledgements
CSIR-CSMCRI communication no. 022/2015. KP thanks CSIR, New Delhi for the grant of CSIR-Young Scientist Awardees Project and overall financial support. MS, NS and DM thanks UGC and CSIR for Research Fellowships and AcSIR for PhD registration. Analytical and centralized instrumentation facility division is acknowledged for providing instrumentation facility.References
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792 CrossRef PubMed.
- (a) H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519 RSC; (b) R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974 CrossRef CAS PubMed.
- (a) K. Prasad, M.-A. Murakami, Y. Kaneko, A. Takada, Y. Nakamura and J.-I. Kadokawa, Int. J. Biol. Macromol., 2009, 45, 221 CrossRef CAS PubMed; (b) W.-T. Wang, J. Zhu, X.-L. Wang, Y. Huang and Y.-Z. Wang, J. Macromol. Sci., Part B: Phys., 2010, 49, 528 CrossRef CAS PubMed.
- K. Prasad, Y. Kaneko and J. Kadokawa, Macromol. Biosci., 2009, 9, 376 CrossRef CAS PubMed.
- K. Fujita, D. R. MacFarlane and M. Forsyth, Chem. Commun., 2005, 4804 RSC.
- H. Zhao, J. Chem. Technol. Biotechnol., 2015, 90, 19–25 CrossRef CAS PubMed.
- C. Mukesh, D. Mondal, M. Sharma and K. Prasad, Chem. Commun., 2013, 49, 6849–6851 RSC.
- D. Mondal, M. Sharma, C. Mukesh, V. Gupta and K. Prasad, Chem. Commun., 2013, 49, 9606 RSC.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC.
- Y. Fukaya, Y. Iizuka, K. Sekikawa and H. Ohno, Green Chem., 2007, 9, 1155 RSC.
- K. M. S. Meera, R. M. Sankar, S. N. Jaisankar and A. B. Mandal, Colloids Surf., B, 2011, 86, 292 CrossRef CAS PubMed.
- S. Zhang, J. Sun, X. Zhang, J. Xin, Q. Miao and J. Wang, Chem. Soc. Rev., 2014, 43, 7838 RSC.
- T. H. LaBean and H. Li, Nano Today, 2007, 2, 26 CrossRef.
- P. W. K. Rothemund, Nature, 2006, 440, 297 CrossRef CAS PubMed.
- D. Schiffels, T. Liedl and D. K. Fygenson, ACS Nano, 2013, 7, 6700 CrossRef CAS PubMed.
- D. Yang, M. J. Campolongo, T. N. N. Tran, R. C. H. Ruiz, J. S. Kahn and D. Luo, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 648 CrossRef CAS PubMed.
- D. Liu, E. Cheng and Z. Yang, NPG Asia Mater., 2011, 3, 109 CrossRef.
- C. Sadhu, S. Dutta and K. P. Gopinathan, J. Biosci., 1984, 6, 817 CrossRef CAS.
- S. A. Markarian, A. M. Asatryan, K. R. Grigoryan and H. R. Sargsyan, Biopolymers, 2006, 82, 1 CrossRef CAS PubMed.
- D. Mondal, J. Bhatt, M. Sharma, S. Chatterjee and K. Prasad, Chem. Commun., 2014, 50, 3989 RSC.
- W. Saenger, Principles of Nuclei Structure, Springer-Verlag, New York, 1984 Search PubMed.
- M. E. Reichmann, S. A. Rice, C. A. Thomas and P. Doty, J. Am. Chem. Soc., 1954, 76, 3047–3053 CrossRef CAS.
- M. Petkovic, J. L. Ferguson, H. Q. N. Gunaratne, R. Ferreira, M. C. Leit˜ao, K. R. Seddon, L. P. Rebelo and C. S. Pereira, Green Chem., 2010, 12, 643–649 RSC.
- V. Gupta, R. S. Baghel, M. Kumar, P. Kumari, V. A. Mantri, C. R. K. Reddy and B. Jha, Aquaculture, 2011, 318, 389 CrossRef PubMed.
- H. S. Hayden, J. Blomster, C. A. Maggs, P. C. Silva, M. Stanhope and J. R. Waaland, Eur. J. Phycol., 2003, 38, 277 CrossRef PubMed.
- D. Matulis, I. Rouzina and V. A. Bloomfield, J. Mol. Biol., 2000, 296, 1053 CrossRef CAS PubMed.
- S. C. Bizley, A. C. Williams and V. V. Khutoryanskiy, Soft Matter, 2014, 10, 8254 RSC.
- I. Saha, M. Hossain and G. S. Kumar, Phys. Chem. Chem. Phys., 2010, 12, 12771 RSC.
- Z. Teng, J. Li, F. Yan, R. Zhao and W. Yang, J. Mater. Chem., 2009, 19, 1811 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03512k |
| This journal is © The Royal Society of Chemistry 2015 |