DOI:
10.1039/D4RA07430K
(Paper)
RSC Adv., 2024,
14, 40198-40221
Exploring the role of Chrysanthemum coronarium leaves distillation waste as a green inhibitor for carbon steel in acidic environment: an integrated study†
Received
16th October 2024
, Accepted 28th November 2024
First published on 23rd December 2024
Abstract
In this study, the assessment of the Chrysanthemum coronarium leaves' co-product resulting from the hydrodistillation process was conducted to evaluate its anticorrosive potential for carbon steel in the hydrochloric acid medium. Phytochemical analysis of this biomass revealed its abundance in terms of polyphenols and flavonoids; hence the determination of total polyphenol content recorded a value of 75.4 mg GAE per g extract. This was corroborated by FTIR spectroscopy, which revealed the presence of various functional groups, thereby providing positive indications regarding the anticorrosive properties of this plant material. Electrochemical impedance spectroscopy and Tafel extrapolation analysis of polarization curves indicated that the extract from Chrysanthemum coronarium leaves reduced the corrosion rate of steel in 1 M HCl medium, reaching 78% in corrosion inhibition efficiency while following an adsorption process governed by the Langmuir isotherm. Furthermore, temperature effect investigations at a range between 293–313 K on the corrosion rate of carbon steel in the acidic medium in the presence and absence of CCLE revealed that the latter undergoes chemisorption-type adsorption on the active metal surface, thereby minimizing its degradation rate at elevated temperatures. The synergistic effect between the Chrysanthemum coronarium leaf extract and potassium iodide was examined using both electrochemical techniques, thus reflecting the cooperative abilities of the two compounds in inhibiting carbon steel corrosion. Additionally, scanning electron microscopy images of the surface state confirmed these findings, thereby providing significant insight into the anticorrosive properties of this plant material in corrosive environments. Similarly, a theoretical study using DFT and MD for the major compounds of CCLE confirmed the obtained results, concluding that the plant material derived from the hydrodistillation process of Chrysanthemum coronarium leaves exhibits remarkable corrosion inhibition capacity for carbon steel in acidic environments.
1 Introduction
Ensuring the seamless and dependable functioning of equipment and products under severe corrosive conditions is fundamental for sustaining efficient technological processes across various industries, both domestically and globally. This challenge has garnered escalating significance, driven by the ever-evolving demands of modern industrial operations. As highlighted by Global Industry Analysts, Inc. (2021), the trajectory of the market demand for products that inhibit corrosion is marked by a robust upward trend, poised for significant expansion. Projections indicate an impressive compounded annual growth rate of the corrosion monitoring market size at a CAGR of 8.5% during the forecast period 2021–2026, with anticipated market valuation soaring to a substantial $3.1 billion by 2026.1 This growth underscores the critical role that corrosion protection plays in safeguarding assets, enhancing operational reliability, and sustaining industrial productivity in the face of corrosive challenges. Across various industries, a plethora of corrosion regulations have been implemented to address the pervasive concern of metal deterioration for both existing and prospective assets.2 Amid these diverse approaches, the exploitation of organic corrosion inhibitors appears as a significant approach for corrosion suppression in harsh conditions. To date, corrosion inhibitors have been systematically categorized into three primary classifications: cathodic, anodic, and mixed-type inhibitors. Cathodic inhibitors primarily reduce the cathodic reaction, such as hydrogen evolution or oxygen reduction, by forming a protective barrier or interfering with the reduction process. Anodic inhibitors, on the other hand, suppress the anodic metal dissolution reaction by forming a passivating film over the metal surface. Mixed-type inhibitors simultaneously impact both anodic and cathodic processes, providing balanced protection by minimizing both metal dissolution and reduction reactions.3 Leveraging their inherent chemical attributes, characterized by the presence of lone non-bonding electron pairs facilitated by nitrogen and oxygen heteroatoms, alongside their propensity to engage with metal surfaces via vacant d-orbitals, organic corrosion inhibitors stand as robust tools for mitigating the deleterious effects of harsh environments. Consequently, their application leads to a discernible reduction in the corrosion rates of metals.4 Nonetheless, notwithstanding their significant inhibitory effect on metal degradation, the overwhelming majority of these inhibitors are comprised of synthesized chemical compounds, posing potential harm to the ecosystem, entailing high expenses, and possibly exerting adverse effects on human health. In response to the pressing environmental concerns associated with traditional corrosion inhibitors, there has been a burgeoning interest in the development of eco-friendly alternatives. Among these alternatives, organic inhibitors derived from natural sources have emerged as promising solutions. A diverse array of environmentally friendly inhibitor categories, including biopolymers,5 ionic liquids,6 amino acids,7 and plant extracts, have garnered significant attention from researchers and practitioners alike. The exploit of plant extracts, in particular, has attracted considerable interest due to several compelling reasons.8 Plant extracts offer a more accessible and cost-effective solution, in which the extraction procedures of these natural compounds typically require minimal processing and can be achieved using simple techniques, without the need for sophisticated facilities or solvents.9 Moreover, plant extracts contain a rich array of phytochemicals, including heteroatoms, which confer notable anticorrosion properties. These natural compounds possess inherent electron-donating capabilities, enabling them to form protective layers on metal surfaces and inhibit corrosion processes effectively. Additionally, their biodegradable nature and low environmental impact make them highly attractive for use in various industrial applications.10–16 However, it is imperative to recognize that certain plant extracts may exhibit modest and erratic corrosion inhibition properties. Consequently, the synergistic pairing of these extracts presents an intriguing avenue for exploration. Particularly noteworthy is the interaction between halide ions such as chloride (Cl−), bromide (Br−), or iodide (I−), and the phytochemical constituents of the plant extract.17 This interaction has the potential to substantially augment the corrosion inhibition effect of the electrolyte, all while minimizing the need for substantial quantities of the extract.18 In our investigation, we sought to employ potassium iodide as a synergistic catalyst due to its high synergistic potential when compared to other halide compounds. This advantage arises mostly from its capacity to swiftly generate a dipole moment owing to its greater molecular size.19 Within the frame of this work, we opted to investigate the corrosion resistance capability of Chrysanthemum coronarium leaves through electrochemical assessments. This genus of the Asteraceae botanical family serves multiple purposes, primarily for the food and pharmaceutical sectors.20 In formulating the methodological framework of this research endeavor, we were particularly intrigued by the prospect of exploiting the liquid waste generated from the hydrodistillation processes commonly employed in numerous cosmetic industries. This byproduct, often deemed undesirable in such industries, harbors a vast reservoir of organic molecules, which we deemed potentially viable as corrosion inhibitors, aligning with our overarching goal of developing cost-effective and environmentally friendly corrosion inhibitors. To elucidate the composition of the green inhibitor, we conducted a comprehensive array of analyses including phytochemical screening, determination of total polyphenol and flavonoid content, and Fourier Transform Infrared (FT-IR) spectroscopy on the aqueous extract of Chrysanthemum coronarium leaves. Electrochemical methods of measurement including potentiodynamic polarization as well as electrochemical impedance spectroscopy were applied to assess the inhibitory efficiency towards the carbon steel in a 1 M HCl solution. Carbon steel is widely used in various industries due to its mechanical strength, availability, and cost-effectiveness. However, it is highly susceptible to corrosion in acidic environments, particularly during processes such as acid cleaning, descaling, and acidizing of oil wells. Hydrochloric acid (HCl) is commonly employed in these operations, making it a relevant medium for corrosion studies.21 The choice of 1 M HCl solution for this study provides a controlled and aggressive environment to evaluate the performance of corrosion inhibitors, mimicking industrial conditions where carbon steel is at significant risk. This allows for an accurate assessment of the inhibitor's efficiency and practical applicability Table 1. Furthermore, we investigated the influence of temperature under identical conditions to ascertain the mechanistic inhibition regime occurring at the metal–solution interface. Furthermore, the synergistic effectiveness of potassium iodide when combined with Chrysanthemum coronarium leaves extract (CCLE) in 1 M HCl was assessed, alongside an examination of the surface morphology of carbon steel in the presence and absence of CCLE in the hydrochloric medium. Quantum chemical analyses were also conducted on the primary constituents of the leaf extract identified from existing literature,22–26 as depicted in Fig. 1.
Table 1 A contrast of some corrosion inhibitors derived from natural sources according to the literature
| Plant name |
Substrate |
Corrosive medium |
Findings |
Ref. |
| Methods |
IE (%) |
| Peanut shells |
Carbon steel |
HCl |
1 M |
PDP/EIS |
81% |
27 |
| Passiflora edulis sims peel |
Mild steel |
Simulated circulating cooling water |
— |
PDP/EIS |
85% |
28 |
| Ammophila arenaria |
Mild steel |
HCl |
1 M |
WL/PDP/EIS |
84.32% |
29 |
| Thymus vulgaris |
304 steel |
HCl |
1 M |
PDP/EIS |
62.15% |
30 |
| Camellia chrysantha |
Q235 steel |
HCl |
1 M |
WL/PDP/EIS |
65.13% |
31 |
| Chrysanthemum coronarium leafs |
Carbon steel |
HCl |
1 M |
PDP/EIS |
78% |
This study |
2 Experimental instruments and methods
2.1 Samples preparation and phytochemical investigations
The leaves of Chrysanthemum coronarium sourced from a field in Casablanca, Morocco underwent careful harvesting and immediate washing to ensure cleanliness. A hydrodistillation process using a Clevenger-Apparatus efficiently extracted desired compounds from the leaves. The resulting solid substrate underwent filtration and evaporation before further analysis. Dilutions of Chrysanthemum coronarium leaves extract were prepared by adding it to a 1 M HCl solution derived from a stock solution. Various concentrations ranging from 0.1 to 2 mg mL−1 were thus obtained. Following a prior study's protocols, phytochemical evaluation, total phenolics, and flavonoid contents analysis (TPC and TFC) were performed on the extract.17 These analyses unveiled insights into its chemical composition. FT-IR spectroscopy through an IRAffinity-1S spectrophotometer revealed the extract's primary functional groups and aided in understanding its molecular structure.
2.2 Metal characteristics
The investigation involved the analysis of a carbon steel working electrode with the composition containing C values between 0.350 and 0.390, Si at 0.400, Mn ranging from 0.50 to 0.80, S within 0.015–0.035, and a total of Cr + Ni + Mo at 0.63. Before each assessment, the active area underwent meticulous polishing using silicon carbide sheets across 180, 600, and 1200 grades and was subsequently cleaned with distilled water and degreased using acetone.
2.3 Electrochemical measurements
The corrosion behavior of the carbon steel was investigated in this study using electrochemical techniques, in which a PGZ 301 potentiostat–galvanostat controlled by Voltamaster 4 software was operated for the measurements. A typical three-electrode cell configuration was employed, with a rotating carbon steel disk electrode as the working electrode having a surface area of 0.283 cm2, a platinum rod as the counter electrode, and a saturated Ag/AgCl electrode as the reference. Electrochemical impedance spectroscopy (EIS) assessments were carried out under the natural electrical condition of the steel-solution interface, covering frequencies from 100 kHz to 10 mHz with a voltage magnitude of 10 mV and ten data points per logarithmic decade. Additionally, potentiodynamic polarization (PDP) curves were generated using the same setup, with a scanning rate of 0.5 mV s−1 and a potential range of ±200 mV relative to the Open Circuit Potential. To ensure reliability, each experiment was repeated thrice for result averaging and error minimization.
2.4 SEM-EDX analysis
The effect of CCLE on the surface of steel was studied by examining the surface morphology of the steel through the SEM-EDX technique at 293 K using QUATTRO S-FEG-Thermofisher scientific equipment. After immersing the samples in 1 M HCl for 15 hours, they were cleaned thoroughly with deionized water and dried before each SEM-EDX examination.
2.5 DFT details
The inhibitors under study were assessed for potential protonation sites using the Marvin Sketch program.32 The results indicated that neutral forms predominated at pH = 0.0 (1 M). As a result, the decision was made to examine the anticorrosive impact of the neutral forms of the constituents from Chrysanthemum coronarium leaf extract, as illustrated in Fig. 1. The structures of the examined components were constructed and visualized using Gauss View 6.1 software,33 with all computations conducted using the Gaussian09 suite of programs.34 The geometries of the neutral forms of the phytochemicals were optimized using the B3LYP method35–37 in conjunction with the 6-31+g(d,p) basis set. B3LYP has been demonstrated to yield consistent geometries for various systems.17,38–40 All calculations were performed in an aqueous solution using the Polarized Continuum Model of Solvation (PCM).41 The optimized geometries were confirmed as energy minima on the potential energy surface (with no imaginary frequencies) through frequency calculations at the optimized geometries using the same level of theory. Additionally, single-point calculations utilizing the Natural Bond Orbital (NBO) analysis code42 were conducted to determine the Natural Population Analysis (NPA) and the Hirshfeld charges for systems with N, N + 1, and N − 1 electrons at the neutral systems' optimized geometries.
 |
| | Fig. 1 Chrysanthemum coronarium leaves extract major components subjected to investigations. | |
The HOMO (EinhHOMO) and LUMO (EinhLUMO) energies were used to calculate the energy gap as their difference (ΔE = EinhLUMO − EinhHOMO), as well as to calculate the metal → inhibitor (ΔE1 = EinhLUMO − EFeHOMO) and inhibitor → metal (ΔE2 = EFeLUMO − EinhHOMO) interactions. It's recognized that adding or removing an electron affects not only the HOMO or LUMO orbitals but all of them. Therefore, using the ionization potential and electron affinity as the negative eigenvalues of HOMO and LUMO respectively, as proposed by the Kohn–Sham orbital theorem, does not produce satisfactory results.17,43,44 Instead, the vertical ionization potential (Iv = E0+ − E0) and vertical electron affinity (Av = E0− − E0) were employed to compute global quantum chemical reactivity descriptors. Here, E0 represents the total energy of the optimized structure for the system with N electrons, while E0− and E0+ pertain to the energies of the systems with N − 1 and N + 1 electrons at the optimal structure of the neutral species with N electrons.
Eqn (1)–(7) were used to calculate the following quantum chemical descriptors.45 Using the following equations:38,46–52
| |
 | (1) |
| |
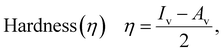 | (2) |
| |
 | (3) |
| |
 | (4) |
| |
 | (5) |
Electron-donating power (ω−), electron accepting power (ω+), net electrophilicity (Δω±)
| |
 | (6) |
The charge transfer model for donation and back-donation (ΔEb-d)
| |
 | (7) |
2.6 Local chemical reactivity (LCR)
Fukui Functions (FFs) are essential local reactivity parameters that help explain how electrons in a molecule respond to minor perturbations.49,53,54 These functions are linked to the electron density and provide local reactivity descriptors by approximating the sites responsible for nucleophilic f+(r), and electrophilic f−(r) attacks centers over the molecules54–56 as well as the dual Fukui functions f2(r).
2.7 Molecular dynamic simulations details
Calculations of the adsorption energies were carried out using DFT by Vienna Ab initio Simulation Package (VASP).55 Generalized gradient-correlated approximation (GGA)/exchange–correlation functionals Perdew–Burke–Ernzerhof (PBE) were employed with a plane-wave cut-off of 400 eV. An iterative self-consistent of 10−4 was employed to optimize geometry. The Fe (110) surface was selected for this investigation because, of all the Fe surfaces, it has the lowest energy and the densest structure.56,57 Fe (110) surface consists of three layers and its supercells are 4 × 5. Monkhorst–Pack 4 × 4 × 1 (ref. 58) and Fermi level smearing of 0.1 eV were selected to find the electronic structures. van der Waals (VDW) forces between Fe atoms and inhibitors have been considered by DFT-D3 model.59 The calculations were carried out in an aqueous solution using the Poisson–Boltzmann implicit solvation model in VASP-sol.60 Applying eqn (8), the energy of the adsorption systems has determined:| | |
Eads = E(inh@Fe)110 − (E(inh) + E(Fe110))
| (8) |
where E(inh@Fe)110 is the total energy of the adsorbed inhibitor on Fe, EFe is the total energy of the Fe clean surface, E(inh) is the total energy of the isolated optimized inhibitor. As well, the charge density differences were calculated using the following equation:| | |
Δρ(r) = Δρ(inh@Fe)110(r) − Δρ(Fe110)(r) − Δρ(inh)(r)
| (9) |
3 Experimental results
3.1 Total phenolic, flavonoid content, and phytochemical screening
Polyphenols, coumarin, flavonoids, and tannins derivatives, are natural substances produced by plants, playing crucial roles in shielding them from herbivores and diseases. These substances often form the basis of the healing properties of plants, exhibiting multiple properties such as anti-inflammatory, antioxidant, and anticancer properties. The examination of the Chrysanthemum coronarium leaf extract revealed a high concentration of these secondary metabolites, as demonstrated by phytochemical screening, as well as total polyphenol (TPC) and total flavonoid (TFC) analyses (Table 2). Notably, the extract displayed increased levels of polyphenols, tannins, flavonoids, and coumarin derivatives, all renowned for their bioactive qualities.61 The lower concentrations of terpenoids and quinone derivatives in the extract could be attributed to the solvent's affinity for phenolic compounds during the extraction process. These results suggest that the aqueous Chrysanthemum coronarium leaf extract shows potential as a corrosion inhibitor under acidic conditions. These active secondary metabolites are likely responsible for the extract's corrosion inhibition properties, warranting further research and development as a corrosion inhibitor.
Table 2 Potential chemical classes, TPC, and TFC of CCLE extracta
| |
Polyphenols |
Flavonoids |
Coumarin |
Terpenoids |
Tannins |
Quinones |
TPC (mg GAE per g extract) |
TFC (mg QE per g extract) |
| ++: higher presence, +: moderate presence, −: negative. |
| CCLE |
++ |
+ |
+ |
− |
+ |
− |
75.4 ± 0.6 |
13.6 ± 0.3 |
3.2 IR analysis
The study employed Fourier Transform Infrared (FTIR) spectroscopy to examine the chemical functional groups present in plant substances derived from the leaf waste of Chrysanthemum coronarium. He infrared spectra (Fig. 2) displayed distinct absorption peaks, each representing a specific molecular functional group. For example, the peaks at 3442 cm−1 and 2939 cm−1 indicated the presence of O–H stretching bonds typical of phenolic structures and C–H stretching vibrations characteristic of alkanes, respectively.62 Additionally, the peak at 1631 cm−1 suggested C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations.63–66 The peak at 1413 cm−1 was linked to the skeletal vibration of C–O–O bonds, while the peaks at 1269 cm−1 and 1107 cm−1 were attributed to C–O bonds in esters and alcohol derivatives.67 The FTIR analysis confirmed the presence of phenolic compounds in the aqueous extract of Chrysanthemum coronarium leaves, as indicated by the phytochemical analysis. These results further substantiate the presence of complex functional groups in the Chrysanthemum coronarium leaf extract, highlighting the potential therapeutic properties of its compounds.
O stretching vibrations.63–66 The peak at 1413 cm−1 was linked to the skeletal vibration of C–O–O bonds, while the peaks at 1269 cm−1 and 1107 cm−1 were attributed to C–O bonds in esters and alcohol derivatives.67 The FTIR analysis confirmed the presence of phenolic compounds in the aqueous extract of Chrysanthemum coronarium leaves, as indicated by the phytochemical analysis. These results further substantiate the presence of complex functional groups in the Chrysanthemum coronarium leaf extract, highlighting the potential therapeutic properties of its compounds.
 |
| | Fig. 2 FTIR spectra of the Chrysanthemum coronarium leaf extract through infrared spectroscopy. | |
3.3 Electrochemical experiments
The open circuit potential (OCP) plot for carbon steel in 1 M HCl illustrates the temporal variation of potential in the presence of varying concentrations of the CCLE. A noticeable positive shift in the OCP values at higher extract concentrations indicates the development of a protective layer on the metal surface. This shift suggests a reduction in the anodic dissolution of the metal or a suppression of cathodic reactions, thereby enhancing the corrosion resistance of the material. Furthermore, the stabilization of OCP values over time reflects the progressive formation of a stable inhibitor film, which effectively reduces the interaction between the aggressive chloride ions in the acid solution and the steel surface. Conversely, at lower extract concentrations, the OCP exhibits less pronounced positive shifts, signifying a weaker protective effect. This behavior can be attributed to insufficient coverage of the steel surface by the extract molecules, leading to a less effective barrier against corrosion processes. The concentration-dependent trend observed in the OCP profiles highlights the role of the extract concentrations in enhancing inhibition efficiency, with higher concentrations providing more robust protection (Fig. 3).
 |
| | Fig. 3 Open circuit potential (OCP) plots of the carbon steel in the presence of CCLE in 1 M HCl. | |
The meticulous recording of potentiodynamic polarization curves within a 1 M HCl solution, meticulously executed under controlled conditions of temperature (298 K), unveiled intriguing insights into the corrosion behavior of the system under study. The experimental setup encompassed a comprehensive exploration of corrosion dynamics under varying scenarios: in the presence and absence of the CCLE, administered at different concentrations. This systematic investigation aimed to discern the efficacy of the leaf extract as a potential corrosion inhibitor. Fig. 4, a visual rendition of the polarization curves post-extrapolation, serves as a graphical testament to the complex interplay between the corrosive environment and the inhibitive effects exerted by the Chrysanthemum coronarium leaf extract. Meanwhile, the tabulated data encapsulated in Table 3 provides a quantitative snapshot of the extracted parameters, offering a meticulous breakdown of the corrosion characteristics observed across the experimental spectrum. Analyzing the data reveals a stark contrast between corrosion behaviors in the absence and presence of the leaf extract. Notably, in the absence of the inhibitor, the corrosion current density (icorr) registers a significant value of 0.73 mA cm−2, alongside a corrosion potential (Ecorr) of −421.37 mV per AgCl, indicative of the aggressive nature of the corrosive medium. However, upon the introduction of varying concentrations of Chrysanthemum coronarium leaf extract, a palpable reduction in both cathodic and anodic current densities emerges, underscoring the inhibitive potential of the extract. This inhibitive effect is particularly pronounced even at low concentrations of the extract, as evidenced by the substantial decrease in corrosion current density, reaching a noteworthy low of 0.16 mA cm−2 at 2 g L−1, thereby yielding an impressive 78% corrosion inhibition efficiency. Moreover, the observed stability in corrosion potential values across multiple measurements, albeit with subtle deviations towards positive values, hints at the nuanced interplay between the inhibitor and the electrochemical processes governing corrosion kinetics.68 Of significant interest is the consistency observed in the slopes of both cathodic and anodic Tafel lines (βc), indicating a parallel increase unaffected by the presence of Chrysanthemum coronarium leaf extract. This suggests that the inhibitive action of the extract does not perturb the underlying electrochemical mechanisms transpiring across the anodic and cathodic regions of the C38 steel surface.69 In essence, this comprehensive analysis underscores the potential of Chrysanthemum coronarium leaf extract as an effective corrosion inhibitor in 1 M HCl environment. The extract's ability to mitigate corrosion, even at minimal concentrations, highlights its promising utility in industrial applications aimed at safeguarding metallic structures against corrosive degradation.70
Table 3 Tafel characteristics of carbon steel at various concentrations of the CCLE inhibitor
| |
Concentration (g L−1) |
Ecorr vs. AgCl (mV) |
icorr (mA cm−2) |
−βc (mV dec−1) |
βa (mV dec−1) |
θ |
I.EPDP (%) |
| CCLE |
0 |
−421.37 ± 0.57 |
0.73 ± 0.05 |
119.33 ± 1.20 |
81.10 ± 1.61 |
— |
— |
| 0.1 |
−401.8 ± 3.8 |
0.38 ± 0.01 |
133.10 ± 3.89 |
101.00 ± 8.35 |
0.493 |
48 |
| 0.25 |
−414.3 ± 3.6 |
0.27 ± 0.01 |
107.27 ± 3.46 |
103.20 ± 5.31 |
0.630 |
63 |
| 0.5 |
−396.2 ± 3.2 |
0.23 ± 0.01 |
132.77 ± 4.29 |
87.63 ± 4.71 |
0.685 |
68.5 |
| 1 |
−389.7 ± 2.2 |
0.18 ± 0.01 |
120.73 ± 8.21 |
90.43 ± 7.07 |
0.753 |
75.3 |
| 2 |
−397.2 ± 3.2 |
0.16 ± 0.01 |
139.40 ± 8.80 |
83.6 ± 1.60 |
0.780 |
78 |
 |
| | Fig. 4 Tafel plots of the working electrode in the presence of CCLE. | |
Fig. 5 provides a comprehensive visual representation of Nyquist and Bode diagrams, meticulously generated at the corrosion potential (Ecorr), offering valuable insights into the corrosion behavior of metallic substrates in the presence of varying concentrations of Chrysanthemum coronarium leaf extract (CCLE) at a temperature of 293 K. This analytical approach allows for a nuanced exploration of the electrochemical processes occurring at the metal–solution interface under different inhibitive conditions. The examination of the Nyquist diagrams reveals a consistent capacitive loop for both inhibited and uninhibited metallic samples, positioned beneath the real impedance axis (Z′). This characteristic positioning underscores the predominant role of charge transfer processes in governing the electrochemical corrosion mechanism at play.13 Such a configuration suggests that the inhibitive action of the Chrysanthemum coronarium leaf extract primarily operates through the alteration of charge transfer dynamics, thereby mitigating the corrosion propensity of the metallic substrate. Furthermore, the diameter of the capacitive loop exhibits a notable augmentation with increasing concentrations of the leaf extract within the corrosive medium. This observed trend is corroborated by the data presented in Table 4, which highlights a corresponding rise in polarization resistance, reaching a value of 98.87 Ω cm2 at a concentration of 2 g L−1 of the Chrysanthemum coronarium leaf extract. This trend underscores the direct correlation between inhibitor concentration and the impedance of the metallic substrate, implying a more robust inhibitive effect at elevated inhibitor concentrations. Moreover, despite variations in inhibitor concentration, the general morphology of the Bode module curves remains largely invariant. This consistency suggests that the addition of the green inhibitor does not induce significant alterations in the fundamental electrochemical corrosion mechanism governing carbon steel behavior in hydrochloric acid.71,72 The presence of a singular peak in all Bode phase illustrations further supports this assertion, indicating the presence of a singular time constant characterizing the corrosion process. Additionally, the observed increase in the phase angle peak with escalating inhibitor concentrations signifies an enhancement in capacitive behavior at the metal–solution interface. This enhanced capacitive behavior is indicative of greater adsorption of phytochemical compounds from the Chrysanthemum coronarium leaf extract onto the metal surface. Consequently, this phenomenon leads to a pronounced attenuation of the electrochemical corrosion process, particularly at higher inhibitor concentrations.
 |
| | Fig. 5 Nyquist and Bode graphs of the C38 steel in a 1 M HCl solution with different amounts of CCLE introduced. | |
Table 4 EIS parameters of the carbon steel at various concentrations of the CCLE inhibitor
| |
Conc. (g L−1) |
Rs (Ω cm2) |
Rp (Ω cm2) |
CPE |
C (μF cm−2) |
χ2 |
I.EEIS (%) |
| Q (μΩ−1 sn cm−2) |
n |
| CCLE |
0 |
0.81 ± 0.01 |
25.47 ± 0.12 |
424 ± 0.36 |
0.83 ± 0.01 |
168 |
0.002 |
— |
| |
0.1 |
0.92 ± 0.17 |
45.01 ± 2.54 |
335 ± 0.1 |
0.79 ± 0.01 |
109.8 |
0.001 |
46 |
| |
0.25 |
1.04 ± 0.44 |
61.89 ± 1.40 |
263 ± 0.08 |
0.79 ± 0.01 |
88 |
0.001 |
59.5 |
| |
0.5 |
0.69 ± 0.03 |
73.02 ± 1.39 |
228 ± 0.06 |
0.79 ± 0.01 |
76.7 |
0.001 |
66.2 |
| |
1 |
0.78 ± 0.19 |
88.51 ± 1.60 |
193 ± 0.05 |
0.78 ± 0.01 |
61.2 |
0.001 |
73.5 |
| |
2 |
0.73 ± 0.01 |
98.87 ± 6.62 |
180 ± 0.05 |
0.78 ± 0.01 |
57.7 |
0.001 |
74.2 |
3.4 Temperature effect
Temperature plays a pivotal role in influencing the corrosion mechanisms of metals in harsh environments, affecting the kinetics of corrosion. To assess the corrosion inhibition effectiveness of the Chrysanthemum coronarium leaves extract, experiments were conducted across a temperature range from 293 K to 313 K. The optimal concentration of CCLE, set at 2 g L−1, was employed under acidic conditions, using Tafel extrapolation methods. The resulting data, depicted in Fig. 6, illustrate the Tafel diagrams of carbon steel, with and without the inclusion of the green extract, at different temperatures. Remarkably, regardless of the presence of CCLE, the plots display similar shapes, showing an increase in current densities as temperature rises. However, the rate of increase in the inhibited medium appears to be more gradual compared to the uninhibited environment. Table 5 provides a summary of the kinetic parameters obtained from the Tafel extrapolation of the carbon steel electrode. Analysis reveals that increasing temperature leads to higher icorr values in both inhibited and uninhibited media, resulting in progressively reduced inhibitory efficiency. These observations suggest that elevated temperature promotes the adsorption of CCLE molecules from the steel surface, exposing it to corrosive agents and accelerating the corrosion rate.73 To gain deeper insights into the inhibitory process occurring at the surface interface, investigations into the activation thermodynamics parameters were conducted following established Arrhenius laws:74| |
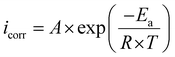 | (10) |
| |
 | (11) |
Activation energy denoted as Ea along with the Arrhenius pre-exponential factor, represented by A, and constants like the universal gas constant (R) and absolute temperature (T), are fundamental parameters in understanding reaction kinetics. In addition, Avogadro's number (N) and the Planck constant (h) play crucial roles in theoretical frameworks. Activation entropy (ΔSa) and enthalpy (ΔHa) further illuminate the energetics of reactions. Arrhenius plots, as illustrated in Fig. 7, provide graphical insights into both inhibited and uninhibited media. These plots offer a theoretical lens to understand the adsorption coordination process between CCLE constituents and specimen active sites. Results tabulated in Table 6 reveal that activation energy values, derived from ln(icorr) vs. 1000/T plots, increase upon CCLE introduction, suggesting adsorption occurs at active centers with lower energies, while metal dissolution takes place at those with higher energies. This underscores a chemical coordination between inhibitor molecules and the carbon steel surface.75 Moreover, activation enthalpy and entropy parameters, computed from ln(icorr/T) vs. 1000/T plots (Table 6), shed light on the endothermic nature of steel dissolution, indicated by positive ΔHa values, whether in the presence or absence of CCLE, contributing to its sluggish rate.76 Notably, the rise in activation entropy in the presence of CCLE molecules signifies a more orderly prohibitive layer has been developed associated with the formation of the steel-CCLE complex.77
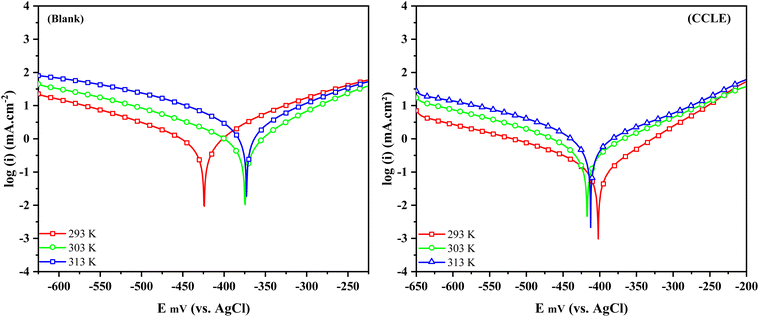 |
| | Fig. 6 Potentiodynamic polarization (PDP) curves recorded under various temperature conditions, both in the presence and absence of CCLE, within a 1 M HCl solution. | |
Table 5 Tafel parameters of the carbon steel at various temperatures with the incorporation of the CCLE inhibitor
| Medium |
Temperature (K) |
Ecorr vs. AgCl (mV) |
icorr (mA cm2) |
βa (mV dec−1) |
−βc (mV dec−1) |
I.EPDP% |
| Blank |
293 |
−421.37 ± 0.57 |
0.73 ± 0.05 |
81.10 ± 1.61 |
119.33 ± 1.20 |
— |
| 303 |
−373.6 ± 1.64 |
1.09 ± 0.06 |
88.77 ± 2.79 |
122.33 ± 1.5 |
— |
| 313 |
−370.53 ± 1.5 |
2.17 ± 0.09 |
99.8 ± 2.55 |
113.13 ± 2.52 |
— |
| |
293 |
−397.2 ± 3.2 |
0.16 ± 0.01 |
83.60 ± 1.60 |
139.40 ± 8.80 |
78 |
| CCLE |
303 |
−412.6 ± 8.9 |
0.22 ± 0.04 |
80.20 ± 5.09 |
124.63 ± 3.20 |
79.2 |
| |
313 |
−412.9 ± 6.7 |
0.33 ± 0.03 |
109.47 ± 7.49 |
118.33 ± 6.12 |
84.5 |
 |
| | Fig. 7 Arrhenius plots and ln(icorr/T) versus 1000/T graphs created for carbon steel (CS) immersed in 1 M HCl, both with and without the addition of CCLE. | |
Table 6 Entropy, enthalpy, and activation energies with and without the incorporation of CCLE
| |
Ea (kJ mol−1) |
ΔHa (kJ mol−1) |
ΔSa (J mol−1 K−1) |
| Blank |
41.86 |
39.17 |
−114.1 |
| CCLE |
33.35 |
30.68 |
−188.5 |
3.5 Synergetic effect
Synergistic studies were conducted to enhance the inhibitory efficiency of Chrysanthemum coronarium leaf extract (CCLE) on carbon steel in 1 M HCl medium using potassium iodide (KI/6 10−3 M) at both minimal and maximal concentrations of the extract. Potentiodynamic polarization and electrochemical impedance spectroscopy were employed at 293 K to assess the effectiveness of this combination. Graphical parameters and polarization resistance data obtained from Tafel extrapolation during potentiodynamic polarization evaluations, both in the presence and absence of the leaf extract in the solution, are presented in Fig. 8 and Table 7. The curves reveal no significant alteration in the shape of the cathodic and anodic branches in the presence of CCLE with KI. However, a notable decrease in the βa and βc slope values is observed in the presence of CCLE with KI, indicating that the introduction of both compounds mitigates the corrosion process, both in the anodic region for metal dissolution and in the cathodic region for hydrogen evolution. Additionally, icorr values also decrease for both concentrations when the extract and halide are present, indicating strengthened inhibitory effects, reaching 87.7% in an acidic medium with 2 g L−1 of CCLE, demonstrating significant synergistic interaction between the halide ions and Chrysanthemum coronarium leaf extract compounds. Fig. 9 and Table 8 illustrate the collected EIS analysis data. In the presence of CCLE with KI, the capacitive loop's semicircle significantly increases compared to CCLE alone in the acidic medium, with no significant modification in shape. The polarization resistances recorded in Table 8 attest to this improvement. When the leaves extract concentration associated with halide ions is lowest, Rp increases from 45.01 to 98.87 Ω cm2 due to increased compound adsorption on the steel surface, resulting in a significant decrease in capacitance. Similar results were obtained for the maximum concentration of CCLE associated with the halide ion, where inhibitory efficiency appears significantly increased in the presence of both compounds. These electrochemical results are confirmed by these observations, indicating that the combination of KI and CCLE significantly reduces the corrosive effect of the acidic environment. The synergy observed between the simultaneous presence of corrosion inhibitors and iodide ions can be explained either by a competitive adsorption mechanism between these substances, where each opposes the other for adsorption sites on the surface, leading to their simultaneous coating, or by a cooperative mechanism where one compound chemisorbs to an active center while the second undergoes physisorption,78 facilitating their combined action, as follows:| |
 | (12) |
where S, η, η′, η′′ are the synergistic parameter, the inhibitory efficiency in the presence of KI, CCLE, and CCLE combined with the halide catalyst, respectively. According to Table 9, the calculated synergistic parameter exceeds unity for both extract concentrations, highlighting that the incorporation of Chrysanthemum coronarium leaf extract and halide ions markedly diminishes the corrosion rate of carbon steel in a hydrochloric medium. This reduction is attributed to the formation of a predominant protective layer at the interface, facilitated by the synergistic interaction between the two compounds.
 |
| | Fig. 8 Tafel curves depicting the performance of carbon steel in the presence and absence of both potassium iodide (KI) and CCLE. | |
Table 7 The corrosion rate features of the carbon steel influenced by the presence and absence of the CCLE inhibitor and potassium iodide (KI)
| Concentration |
Ecorr vs. AgCl (mV) |
icorr (mA cm2) |
βa (mV dec−1) |
−βc (mV dec−1) |
I.EPDP (%) |
| Blank |
−421.37 ± 0.57 |
0.73 ± 0.05 |
81.10 ± 1.6 |
119.33 ± 1.2 |
— |
| Blank + 6 × 10−3 M KI |
−360.23 ± 2.01 |
0.44 ± 0.02 |
70.77 ± 2.4 |
124.93 ± 4.2 |
40 |
| 0.1 g per L CCLE |
−401.8 ± 3.8 |
0.38 ± 0.01 |
101.00 ± 8.35 |
133.10 ± 3.89 |
47.9 |
| 0.1 g per L CCLE + 6 × 10−3 M KI |
−392.3 ± 4.2 |
0.16 ± 0.01 |
80.47 ± 4.35 |
89.53 ± 6.43 |
78 |
| 2 g per L CCLE |
−397.2 ± 3.2 |
0.16 ± 0.01 |
83.60 ± 1.60 |
139.40 ± 8.80 |
78 |
| 2 g per L CCLE + 6.10−3M KI |
−391.8 ± 6.8 |
0.09 ± 0.01 |
73.53 ± 4.32 |
−94.53 ± 4.51 |
87.7 |
 |
| | Fig. 9 Nyquist plots illustrating the behavior of carbon steel when subjected to 1 M HCl with the addition of both potassium iodide (KI) and CCLE. | |
Table 8 EIS assessments conducted for the working electrode immersed in a 1 M HCl at 293 K, with both potassium iodide (KI) and CCLE simultaneously present
| Concentration |
Rs (Ω cm2) |
Rp (Ω cm2) |
CPE |
C (μF cm−2) |
χ2 |
I.EEIS (%) |
| Q (μΩ−1 sn cm−2) |
n |
| Blank |
0.81 ± 0.01 |
25.47 ± 0.12 |
424 ± 0.36 |
0.83 ± 0.01 |
168 |
0.002 |
— |
| Blank + 6 × 10−3 M KI |
0.76 ± 0.01 |
44.3 ± 2.35 |
344 ± 0.15 |
0.81 ± 0.01 |
129 |
0.004 |
42.5 |
| 0.1 g per L CCLE |
0.92 ± 0.17 |
45.01 ± 2.54 |
335 ± 0.1 |
0.79 ± 0.01 |
109.8 |
0.001 |
46 |
| 0.1 g per L CCLE + 6.10−3M KI |
0.83 ± 0.07 |
116.0 ± 1.34 |
150 ± 0.01 |
0.82 ± 0.01 |
61.6 |
0.001 |
78 |
| 2 g per L CCLE |
0.73 ± 0.01 |
98.87 ± 6.62 |
18 ± 0.05 |
0.78 ± 0.01 |
57.7 |
0.001 |
74.2 |
| 2 g per L CCLE + 6 × 10−3 M KI |
0.81 ± 0.08 |
149.8 ± 3.95 |
141 ± 0.02 |
0.79 ± 0.01 |
50.5 |
0.001 |
83 |
Table 9 Synergistic parameter of CCLE and KI in a 1 M hydrochloric acid solution through EIS and PDP analysis
| Concentration |
PDP |
EIS |
| I.EPDP (%) |
SPDP |
I.EEIS (%) |
SEIS |
| 6 × 10−3 M KI |
40 |
1.12 |
42.5 |
1.13 |
| 0.1 g per L CCLE |
47.9 |
|
46 |
|
| 0.1 g per L CCLE + 6 × 10−3 M KI |
78 |
|
78 |
|
| 6 × 10−3 M KI |
40 |
|
42.5 |
|
| 2 g per L CCLE |
78 |
1.35 |
74.2 |
1.4 |
| 2 g per L CCLE + 6 × 10−3 M KI |
87.7 |
|
83 |
|
3.6 Adsorption isotherm
Exploring adsorption isotherms remains a valuable method for deepening our understanding of the chemical interactions between corrosion inhibitors and metal surfaces. Typically, organic molecules hinder corrosion by attaching to the metal's active surface, displacing water molecules initially present on that surface. This process forms a protective layer that restricts corrosive agents' access to the metal surface. To investigate the interaction between a corrosion inhibitor (CCLE) and the metal surface in an HCl environment, various isotherms such as Langmuir, Frumkin, and Temkin were exploited to analyze the adsorption dynamics at the interface as follows:79,80| |
 | (13) |
| | |
Temkin: exp(−2aθ) = KCinh
| (14) |
| |
 | (15) |
| |
 | (16) |
Within this framework, the symbol K denotes the equilibrium adsorption constant, which reflects the affinity of the corrosion inhibitor for the metal surface. The parameter θ signifies the fractional surface coverage, indicating the proportion of the metal surface occupied by the inhibitor molecules. Additionally, the symbol a represents the constant of molecular interaction, portraying the strength of the interactions between the inhibitor molecules and the metal surface. Analysis of the adsorption isotherms suggests that the introduction of CCLE prompts the expulsion of water atoms from the metal surface's active locations. Consequently, a suppressive monolayer form on the metallic surface aligns with the Langmuir isotherm model. This model assumes that a fixed number of active sites exist on the adsorbent's surface, each capable of accommodating only one adsorbate molecule.81 As a result, adsorption occurs in a manner that forms a monolayer of inhibitor molecules without lateral interactions between them. However, the Langmuir model's simplicity encounters challenges when applied to complex systems like plant extracts, which contain a multitude of compounds with diverse chemical structures. The intricate composition of CCLE makes it arduous to designate a specific inhibitory action for the extract. This complexity hinders the straightforward determination of the thermodynamic properties of the extract's compounds. Nevertheless, employing an isotherm model facilitates the exploration of the thermodynamic properties by evaluating the equilibrium constant Kads. This constant characterizes the equilibrium between the adsorbed and unadsorbed inhibitor molecules on the metal surface. Despite its utility, the phytochemical diversity of the CCLE presents a significant challenge in accurately determining its molecular weight. Consequently, this complexity complicates the precise determination of the solution's molarity, affecting the interpretation of experimental results and the formulation of effective corrosion inhibition strategies (Fig. 10).17,73,82,83
 |
| | Fig. 10 Isotherm model graphs depicting the interaction of CCLE with carbon steel in a 1 M HCl solution at 293 K. | |
3.7 SEM-EDX study
For further exploration of the influence of phytoconstituents derived from the Chrysanthemum coronarium leaves extract on the surface of carbon steel in an acidic environment, a 15 hours thorough surface investigation was carried out using the SEM-EDX method at 293 K. The state of the steel surface under various testing settings is clearly shown by the SEM pictures, as shown in Fig. 11. When as opposed to the unexposed metal surface, it is clear that the steel surface shows considerable damage in the absence of CCLE, which is shown by the presence of pits and fractures. This damage is a result of the continuous metallic breakdown process in the acidic medium. Nevertheless, a notable inhibition of the corrosion process is seen after adding CCLE to the mixture. There is a discernible decrease in the frequency of local corrosion pits, and the surface seems to be insulated. The EDX data, which is compiled in Table 10, supports this conclusion even more. The examination of the data indicates that there are significant oxygen atoms on the steel surface in the inhibited medium. These atoms are often linked to the production of ferrous oxide as a consequence of the corrosion process. It's interesting to note that the fraction of oxygen atoms connected to the corrosion by-product decreases when CCLE is added to the medium (Fig. 12). This implies that the leaf substances found in Chrysanthemum coronarium plants have the capacity to mitigate the pace at which carbon steel dissolves in a hydrochloric solution. This outcome is likely a result of the cohesive interaction between these compounds and the active centers residing on the C38 active surface. This interaction facilitates the formation of a protective layer, effectively hindering the corrosive action of the environment on the metal surface.
 |
| | Fig. 11 SEM images depicting carbon steel are presented as follows: (a) in its original, untreated state, (b) after immersion in 1 M HCl, and (c) after exposure to 1 M HCl containing 2 g L−1 of CCLE. | |
Table 10 Atom percentage recorded in the EDX spectrum with the incorporation of CCLE
| Conditions |
Atom percentage |
| Iron |
Carbon |
Oxygen |
Total |
| 1 M HCl |
86.95 |
6.9 |
6.14 |
100 |
| 1 M HCl + CCLE |
91.32 |
6.57 |
2.11 |
100 |
 |
| | Fig. 12 EDX analysis results for carbon steel are provided for (a) its original state, (b) after immersion in 1 M HCl, and (c) after exposure to 1 M HCl with the addition of 2 g L−1 of CCLE. | |
3.8 Analysis of DFT results
Continuing our investigation into the constituents of Chrysanthemum coronarium stems and flower extracts17,82 this study focuses on the DFT analysis of the components found in the leaf extract. Illustrated in Fig. 1, these components include: L1 – chlorogenic acid, L2 – 4,5-dicaffeoylquinic acid, L3 – 3,4-dicaffeoylquinic acid, L4 – 3,5-dicaffeoylquinic acid, L5 – 4-succinyl-3,5-dicaffeoylquinic acid, and L6 – rutin. Notably, L1 and L6 are also present in the stem and flower extracts, thus their results are included solely for comparative purposes with the other components of the leaf extract. Comprehensive discussions regarding their HOMOs, LUMOs, and ESP maps were previously provided.17,82 Moreover, due to their isomeric nature, the electronic properties of L2, L3, and L4 are nearly identical.
3.8.1 Analysis of HOMOs, LUMOs, ESP maps. Fig. 13 illustrates the optimized structures, HOMOs, LUMOs, and electrostatic potential maps (ESP) of the Chrysanthemum coronarium stem and flower extracts (L1–L6). Notably, for L2, L3, and L4, the HOMO is predominantly localized over one of the dicaffeoylic acid units, whereas their LUMOs are spread across both units of the dicaffeoylic acid. Similarly, for the L5 inhibitor, the findings indicate that the HOMO is also concentrated over one of the dicaffeoylic acid units, while the LUMO is dispersed over the other dicaffeoylic acid unit. These results shed light on the electron donation tendency of one dicaffeoylic acid unit to the metal surface, while the other exhibits a susceptibility to accept electrons from the metal surface.
 |
| | Fig. 13 From left to right: optimized molecular structures, HOMOs, LUMOs, and electrostatic potential (ESP) maps for the components of the Chrysanthemum coronarium leaves extract as obtained using the B3LYP/6-31+G(d,p) level of theory in aqueous solution. | |
Plotting of the ESP maps can give additional insight into the electron-rich and electron-poor regions. The strong red color (negative potential) covers the carbonyl oxygen atom region of the dicaffeoylic acid units, while the strong blue color (positive potential) covers the carbonyl carbon atom. In addition, the faint red color (weak negative potential) covers the aromatic ring of the dicaffeoylic acid unit. The red regions represent the nucleophilic regions, which are electron-rich and are subjected to electrophilic attacks. On the contrary, the blue region corresponds to the electrophilic region, which are electron-poor centers and they are the favorable sites for nucleophilic attacks. Again, these results are also confirmed by plotting the total densities at the HOMOs and LUMOs as shown in Fig. SD1 of the ESI.† The red and blue trajectories correspond to the electron-rich and electron-poor regions, which are responsible for electrophilic and nucleophilic attacks, respectively.
3.8.2 Analysis of global chemical reactivity descriptors. The energies of HOMO and LUMO of the inhibitor molecules (EinhHOMO and EinhLUMO) are quantum chemical descriptors, which are usually related to the electron donating and accepting tendency of the inhibitor molecules. Thus, inhibitors having high values of EinhHOMO incline to donate electrons to the unoccupied 3d-orbitals of the metal acceptors, while the lower EinhLUMO value, the higher ability to accept electrons from the occupied 3d-orbital of the metal. On the other hand, when the metaland inhibitor become close to each other, this permits the electrons to flow from the inhibitor to the metal or vice versa. Table 11 displays the values of EinhHOMO, EinhLUMO, ΔE1 and ΔE2. To simplify the representation of the results, the graphical representation of the ΔE, ΔE1, and ΔE2 are shown in Fig. 14.
Table 11 Energy gaps (ΔE), metal → inhibitor (ΔE1) and inhibitor → metal (ΔE2) interactions of for the components of the Chrysanthemum coronarium leaves extract as obtained using the B3LYP/6-311+G(d,p) level of theory in aqueous solution
| |
EinhHOMO |
EinhLUMO |
ΔE |
ΔE1 |
ΔE2 |
| L1 |
−6.129 |
−2.177 |
3.951 |
5.835 |
5.925 |
| L2 |
−6.096 |
−2.157 |
3.940 |
5.725 |
5.978 |
| L3 |
−6.102 |
−2.173 |
3.929 |
5.701 |
5.951 |
| L4 |
−6.099 |
−2.164 |
3.936 |
5.746 |
5.945 |
| L5 |
−6.102 |
−2.327 |
3.775 |
5.746 |
5.945 |
| L6 |
−6.076 |
−2.068 |
4.009 |
5.739 |
5.948 |
 |
| | Fig. 14 Energy gaps, ΔE, metal → inhibitor (ΔE1) and inhibitor → metal (ΔE2) interactions for the components of the Chrysanthemum coronarium leaves extract as obtained using the B3LYP/6-311+G(d,p) level of theory in aqueous solution. | |
According to the EinhHOMO values in Table 12 and Fig. 14, the following order of electron-donating ability from the inhibitor to the metal surface is accepted as follows: L6 > L2 > L4 > L3 > L5 > L1. Whereas, according to EinhLUMO, order of accepting electron ability power from the metal is given as follows: L5 > L1 > L3 > L4 > L2 > L6.
Table 12 Quantum chemical reactivity descriptors for all components of the Chrysanthemum coronarium leaves extract as obtained using the B3LYP/6-311+G(d,p) level of theory in aqueous solution. Softness and nucleophilicity are in the eV−1 unit, dipole moment in Debye, ΔN110 is unitless, and the other descriptors are in the eV unit
| |
L1 |
L2 |
L3 |
L4 |
L5 |
L6 |
| E0 |
−35311.751 |
−50884.298 |
−50884.293 |
−50884.298 |
−61239.972 |
−61243.003 |
| E0− |
−35314.071 |
−50886.607 |
−50886.635 |
−50886.610 |
−61242.693 |
−61245.210 |
| E0+ |
−35305.711 |
−50878.308 |
−50878.306 |
−50878.292 |
−61234.530 |
−61237.041 |
| Iv |
6.040 |
5.990 |
5.987 |
6.006 |
5.442 |
5.961 |
| Av |
2.321 |
2.309 |
2.342 |
2.312 |
2.721 |
2.208 |
| μ |
−4.180 |
−4.150 |
−4.165 |
−4.159 |
−4.082 |
−4.085 |
| χ |
4.180 |
4.150 |
4.165 |
4.159 |
4.082 |
4.085 |
| η |
1.860 |
1.840 |
1.822 |
1.847 |
1.361 |
1.877 |
| σ |
0.538 |
0.543 |
0.549 |
0.542 |
0.735 |
0.533 |
| ω |
4.698 |
4.678 |
4.759 |
4.684 |
6.123 |
4.444 |
| ε |
0.213 |
0.214 |
0.210 |
0.214 |
0.163 |
0.225 |
| ω− |
7.021 |
6.983 |
7.069 |
6.994 |
8.333 |
6.721 |
| ω+ |
2.841 |
2.833 |
2.905 |
2.835 |
4.252 |
2.637 |
| Δω± |
9.861 |
9.817 |
9.974 |
9.829 |
12.585 |
9.358 |
| ΔEb-d |
0.172 |
0.182 |
0.180 |
0.179 |
0.271 |
0.196 |
| ΔN110 |
−0.465 |
−0.460 |
−0.456 |
−0.462 |
−0.340 |
−0.469 |
The energy gap is an important quantum global descriptor, which measures the chemical reactivity and kinetic stability of the molecule. The lower the energy gap, the higher the chemical reactivity and the lower the chemical kinetic stability. Results in Table 11 and shown in Fig. 14 declare the following chemical reactivity order as follows: L5 > L3 > L4 > L2 > L1 > L6. Therefore, among the investigated inhibitors, L5 has the highest corrosion inhibition efficiency, while L6 has the lowest corrosion inhibition efficiency.
The electron flow ability from metal to inhibitor or vice versa can be explained by calculating ΔE1 and ΔE2 descriptors. The results are also tabulated in Table 11 and graphically shown in Fig. 14. Our results reveal that for all investigated inhibitors, the ΔE1 < ΔE2, signifying that the metal acts as Lewis base and the inhibitors act as Lewis acids. Therefore, the ability to electron from form the occupied 3d-orbitals of the metal to the lying unoccupied orbitals of the inhibitors is energetically favored compared to the reverse process. Among all the investigated inhibitors, the ability to electron flow from metal to inhibitor (ΔE1) is ordered as follows: L5 > L1 > L3 > L4 > L2 > L6, while the tendency to electron flow from inhibitor to metal (ΔE2) is ordered as follows: L6 > L2 > L4 > L3 > L5 > L1.
The vertical ionization potential and the vertical electron affinity have been used to calculate the most relevant global chemical reactivity descriptors for all components of the Chrysanthemum coronarium leaves extract as obtained using the B3LYP/6-311+G(d,p) level of theory in aqueous solution and results are summarized in Table 12.
It was reported that the inhibitors with low electronegativity value have a good corrosion inhibition performance. Based on this assumption and monitoring our results in Table 11, the expected corrosion inhibition efficiency is as follows: L5 > L6 > L2 > L3 ≈ L4 > L1. Considering the change in electronegativity of Fe metal (χFe = 7.0 eV) and the inhibitor as ΔχχFe – χinh, the following order is expected for increasing of Δχ as follows: L5 (2.918 eV) > L6 (2.915 eV) > L2 (2.850 eV) > L3 (2.841 eV) ≈ L4 (2.841 eV) > L1 (2.820 eV). Consistent with Sanderson's electronegativity equalization principle,84 L5 and L6 with the lowest χ and highest Δχ values and hence high reactivity are expected to have the highest corrosion inhibition efficacy.
It was reported by Obi-Egbedi et al.85 that a hard molecule has a larger energy gap, while a soft molecule has a small energy gap. According to our results of hardness and softness, as shown in Table 12, the corrosion inhibition efficiency of the investigated inhibitor is ordered as follows: L5 > L3 > L2 > L4 > L1 > L6. Therefore, L5 and L3 inhibitors are the most active components for the protection of the metal surface against corrosion.
The tendency of chemical species to accept electrons can be measured by calculating the electrophilicity index (ω). It was reported that as the ω increases, the tendency behavior of the molecule to accept electrons increases. Thus, a good electrophile is characterized by higher values of μ and ω, while, on the contrary, a good nucleophile is known for its low values of μ and ω. Asindicated in Table 12, the corrosion inhibition efficiency ranking of the different components is given as follows: L5 > L3 > L1 > L4 > L2 > L6.
The set of eqn (4) has been used to calculate the values of the electron-accepting tendency (ω−), electron-donating behavior (ω+), and net electrophilicity (Δω±) for all components of the Chrysanthemum coronarium leaves extract as obtained using the B3LYP/6-311+G(d,p) level of theory in aqueous solution, and the results are also summarized in Table 12. The results obtained revealed that the order of electron-donating power follows the order L5 > L3 > L1 > L4 > L2 > L6, and the same order is also achieved for the electron-accepting power and the net electrophilicity. Therefore, these results indicate that L5 and L3 inhibitors have the highest possibility to accept and donate electrons simultaneously. Whereas, L6 is characterized by the lowest ω−, ω+, and Δω±.
Another quantum chemical descriptor is the ΔEb-d, which describes the transfer of charge from a molecule, followed by its return to the molecule. González Gómez et al.86 proposed that the ΔEb-d value is decreased and becomes energetically favored with the increase in the harness value. In addition, as ΔEb-d becomes less negative, the corrosion inhibition efficiency of the molecule increases. As indicated from the results in Table 11, the corrosion inhibition efficacy of the investigated components can be arranged as follows: L5 > L3 > L2 > L4 > L1 > L6.
As the metal and inhibitor become close to each other, electrons can flow from an inhibitor with lower χ to a metal with higher χ, until equilibrium is reached (χin = χFe). Therefore, at this point, the fraction of electrons transferred, ΔNmax, is given by eqn (17).
| |
 | (17) |
Here,
χFe = 7.0 eV,
87 and the global hardness (
ηFe = 0) for iron, considering
I =
A for metallic bulk atoms.
88 Recent studies reported that using
χFe = 7.0 eV is not correct, and instead, the work function of Fe metal (
ΦFe = 4.82 eV for Fe
110 plane) is used.
89| |
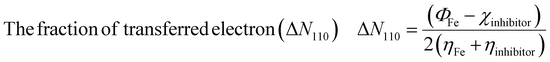 | (18) |
Eqn (10) provides a more accurate measure of ΔN110 then using χFe = 7 eV because it is considered without accounting for electron–electron interactions but for the free electron gas Fermi energy of iron only.89,90 The results obtained for ΔN110 are also summarized in Table 12. It was outlined previously that the positive value (ΔN110) indicates that the inhibitors act as electron donors (Lewis base)91,92 Inspection of Table 12 indicates that ΔN110 values for species are positive and <3.6 values,92 illustrating that all of the inhibitors have the ability to donate electrons to the unoccupied 3d-orbital of the Fe metal. Additionally, our results show that L5 and L6 molecules have the greatest ΔN110 values of 0.271 and 0.196, respectively followed by L2, L3, and L4 with ΔN110 values of 0.182, 0.180 and 0.179, respectively, while L5 has the smallest ΔN110 value of 0.172. As can be seen, L2–L4 inhibitors have almost the same ΔN110 values, which can be attributed to their similar structures as isomers.
3.8.3 Analysis of local reactivity indices. FFs, which were originally introduced by Kenichi Fukui, are a concept53 that considers the effects of structural differences on the reactivity of the different active centers in the molecule. Fukui's theory explains the behavior of electrons in a molecule when exposed to small perturbations.53,84 Fukui functions are valuable tools for pinpointing specific regions or atoms within a molecule that exhibit nucleophilic or electrophilic characteristics, indicating their roles in donating or accepting electrons.38,51,84 Fig. 15 shows the 3D iso-surfaces of the Fukui functions for the most active electrophilic (f−(r)), electrophilic (f+(r)) and dual (f2(r) attacks centers of the investigated inhibitor molecule.
 |
| | Fig. 15 3D-isosurfaces (isovalue = 0.005 a.u.) of the electrophilic, nucleophilic, and dual attacks for the components of the Chrysanthemum coronarium leaves extract as obtained using the B3LYP/6-311+G(d,p) level of theory in aqueous solution. | |
To measure the Fukui functions, Hirshfeld charges were employed to derive the condensed Fukui descriptors for both nucleophilic (fk+) and electrophilic (fk−) interactions, along with the dual condensed Fukui descriptor (fk2) based on conceptual density functional theory (CDFT)93,94 as implemented in the Multiwfn code95 using the following equations:84
| | |
fk+ = qkN − qkN+1, fA− = qkN−1 − qkN, and fk2 = fk+ − fk−
| (19) |
Here,
qNA,
qN+1A and
qN−1A are the Hirshfeld charges over the
k atom for systems with
N,
N + 1, and
N − 1 electrons, respectively. The strong electrophile character at an atom
k is measured by the maximum value of the
fk+ descriptor indicates a strong electrophilic character, while the strong nucleophilic character at atom
k is identified by the maximum value of
fk− descriptor.
96 Morell
et al.97 introduced the most accurate descriptor, known as the condensed dual Fukui descriptor (
fk2), which extends the understanding of the
fk+ and
fk− indices and objects to describe concurrently the nucleophilic and electrophilic potencies at an atomic site. Morell
et al.97 reported that if
fA2 > 0, an atom's tendency to undergo nucleophilic attack designates it as a favored location for donating electrons.
Contrary, fA2 < 0 indicates the atom's ability to undergo electrophilic attack, making it a preferred site for accepting electrons.97,98 Fig. 16 graphically presents the most representative results of the condensed Fukui indices for nucleophilic (fA+), electrophilic (fA−), and (fA2) dual interactions of the investigated leaves compounds. The most representative results of the condensed Fukui nucleophilic (fA+), electrophilic (fA−) and dual (fA2) indices of the investigated phytochemical compounds are graphically shown in Fig. 16. In order to follow up on the number of active centers, Fig. SD2† shows the optimized structures of the probed inhibitors with the number of atoms. The complete results, including the Hirshfeld charges for the N, N + 1, and N − 1 electron systems, as well as the condensed Fukui indices for nucleophilic (fA+), electrophilic (fA−), and dual interactions (fA2) of the examined inhibitor molecules, are summarized in Tables SD1–SD6 of the ESI.† In this study, we are dealing only with the results of fA2. For L1 inhibitor, the most active centers for electrophilic attacks are correspondingly, O9, C20, and O8, while the centers that are active for nucleophilic attacks are C19 and C17, respectively, see Fig. 16a and Table SD1†. For L2 inhibitor, as indicated in Fig. 16b and Table SD2,† the centers O11, O9, and C26 show the maximum propensity for electrophilic attacks, respectively, while the most favorable centers for nucleophilic attacks are, correspondingly, C25 and C21. For the L3 inhibitor, the results shown in Fig. 16c and listed in Table SD3† declare that the centers O12 and O10 have the highest tendency to undergo electrophilic attacks, while the C24 and C25, C20 and C21 atoms show the maximum capability to undergo nucleophilic attacks. For the L4 inhibitor, our results show that the centers O11 and O9 undergo electrophilic attacks, while the C25 and C24 can undergo nucleophilic attacks, see Fig. 16d and Table SD4.† The results listed in Table SD5† and shown in Fig. 16e for the L5 inhibitor indicate that O72 and O9 have the maximum ability to undergo electrophilic attacks, while C47 and C45 show the maximum tendency for nucleophilic attacks. Finally, for the L6 inhibitor, the atoms C31, O12, and C30 are the most favorable centers for nucleophilic attacks, while the atoms O16, C33, and C29 are the most favorable centers for nucleophilic attacks, see Fig. 16f and Table SD6.†
 |
| | Fig. 16 Condensed Fukui functions for nucleophilic, electrophilic, and dual attacks (a)–(f) for the components of the Chrysanthemum coronarium leaves extract as obtained using the B3LYP/6-311+G(d,p) level of theory in aqueous solution. | |
The above results indicate that the most favorable sites for electrophilic attacks, which are able to donate electrons to the unoccupied 3d-orbitals of Fe metal are the oxygen atoms and some carbon atoms, while the most favorable sites for nucleophilic attacks, which are responsible for accepting electrons from the occupied 3d-orbitals of the Fe-metal are the carbon atoms of the carbonyl groups.
3.9 Adsorption inhibitors on Fe (110)
To illustrate how the inhibitors interact with Fe (110) surface by PBE-D3, Fig. 17 depicts adsorption systems with their adsorption energies. Each inhibitor studied contains –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –OH functional groups that are chemically bonded to the surface. According to the calculations, the process of forming bonds between Fe and inhibitors is exothermic. As well, all inhibitors exhibited negative adsorption energies between −108 and −275 kJ mol−1.
O and –OH functional groups that are chemically bonded to the surface. According to the calculations, the process of forming bonds between Fe and inhibitors is exothermic. As well, all inhibitors exhibited negative adsorption energies between −108 and −275 kJ mol−1.
 |
| | Fig. 17 The side view of optimized structures for the investigated inhibitors on the Fe (110) surface at the aqueous solution with their adsorption energies (Eads in kJ mol−1) and covalent bond lengths between Fe and C and O atoms (dFe–C, dFe–O in Å) in black font. The dashed blue and grey lines indicated non-classical hydrogen bonds (dFe⋯H) and hydrogen bonding (dO⋯H), respectively. Code colure: Fe in light brown, O in red, C in dark brown, and H in white. | |
Among all investigated systems, Rutin@Fe is the one that is strongly interaction with surface with adsorption energy of −274.50 kJ mol−1. Due to multiple bonds with Fe surface. It is observed that adsorption energies have been increasing as L6 (−274.5 kJ mol−1) > L3 (−188.5 kJ mol−1) > L4 (−186.9 kJ mol−1) ≈ L2 (−186.9 kJ mol−1) > L1 (−140.0 kJ mol−1) > L5 (−115.3 kJ mol−1). Both 34dca and 35dca are binding with Fe surface by two –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the hydrogen of hydroxyl groups is transferred to bond with Fe atoms. Those Fe–H bonds have bond lengths between 1.57 to 1.79 Å. On the other hand, the other systems Chlorogenic acid@Fe, Rutin@Fe, and 4s35dca@Fe formed one or two non-classical Fe–H bonds. Their bond lengths range between 2.98 to 1.61 Å, which is different from the standard (1.50 Å) about 1.48 to 0.11 Å. Several studies have demonstrated nonclassical interactions between the H atoms in inhibitors and the transition metal center.99,100
O and the hydrogen of hydroxyl groups is transferred to bond with Fe atoms. Those Fe–H bonds have bond lengths between 1.57 to 1.79 Å. On the other hand, the other systems Chlorogenic acid@Fe, Rutin@Fe, and 4s35dca@Fe formed one or two non-classical Fe–H bonds. Their bond lengths range between 2.98 to 1.61 Å, which is different from the standard (1.50 Å) about 1.48 to 0.11 Å. Several studies have demonstrated nonclassical interactions between the H atoms in inhibitors and the transition metal center.99,100
The interactions between the investigated inhibitor and with Fe surface can be further characterized using the charge density difference (CDD) tool, see Fig. 18. By using DFT calculations, this tool illustrates the redistribution of charge caused by chemical/physical bonds. Their idea is based on the change between the system of interest and its reference. All inhibitors cause an electron density deficit between the carbonyl groups and Fe and iron atoms, indicating that an O–Fe chemical bond is formed.
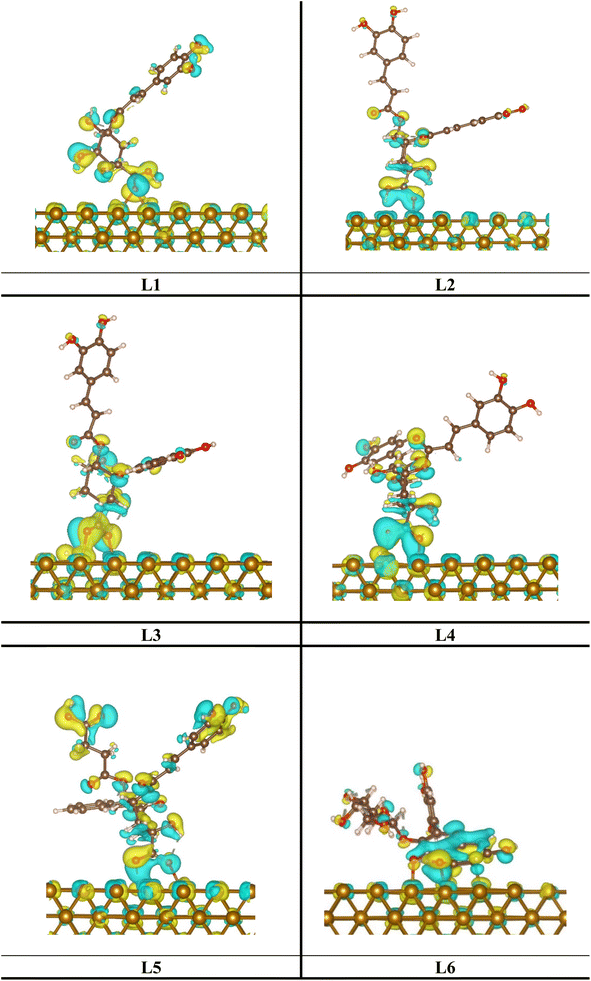 |
| | Fig. 18 CDD for the investigated inhibitors on the Fe surface at the aqueous solution. Note: the electron excess and deficit regions are represented with blue and yellow colors, respectively, and isosurfaces of 0.04 e bohr−3. | |
3.10 Suggested corrosion mechanism
When presented in an acidic environment, botanical-derived organic compounds, such as those found in the examined extract, provide a substantially more sustainable and enduring substitute to conventional corrosion inhibitors.27,101 The compounds analyzed in this study, which are chlorogenic acid, rutin, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, and 4-succinyl-3,5-dicaffeoylquinic acid, exhibits structural features that facilitate chemisorption onto the steel surface, a key mechanism in corrosion inhibition. When exposed to a corrosive electrolyte, an electric charge accumulates at the interface, resulting in a positive charge on the metal surface.102,103 Chemisorption occurs primarily through the interaction of the π–electrons of aromatic rings and the vacant d-orbitals of metallic iron, as well as through the donation of unshared electrons from oxygen atoms present in the phenolic, carboxylic, and glycosidic moieties of these compounds.104 Fig. 19 illustrates these processes, showcasing the interplay of π–electron interactions, oxygen electron donation, and the formation of a cohesive adsorbed layer that enhances the corrosion resistance of the metal. The aromatic systems within chlorogenic acid and dicaffeoylquinic acids serve as electron-rich regions, with their delocalized π–electrons forming coordinate bonds with the positively charged steel surface. This interaction creates a stable adsorbed layer that minimizes the exposure of the steel to corrosive ions. The oxygen atoms in the hydroxyl (–OH) groups and carboxylic (–COOH) functionalities also play a significant role, with their lone pairs forming strong coordination bonds with the metal surface. For instance, the carboxylic groups in dicaffeoylquinic acids can deprotonate in acidic media, enhancing their electron-donating ability and facilitating direct bonding with the metallic substrate. Similarly, the multiple hydroxyl groups in rutin, combined with its conjugated aromatic rings, provide numerous active sites for chemisorption, resulting in a dense protective barrier. The bulky structure of 4-succinyl-3,5-dicaffeoylquinic acid, containing both caffeoyl and succinyl groups, further contributes to a compact and uniform organic layer that hinders the penetration of aggressive ions such as chloride.105 This synergistic interaction, involving π–electrons, unshared oxygen electrons, and the surface d-orbitals, highlights the ability of the extract's compounds to form a robust chemisorbed layer, effectively reducing the corrosion rate of carbon steel in acidic conditions.
 |
| | Fig. 19 The adsorption regime of the CCLE phytoconstituents towards the carbon steel active sites: (a) chlorogenic acid, (b) rutin, (c) 3,4 dicaffeoylquinic acid, (d) 3,5 dicaffeoylquinic acid, (e) 4,5 dicaffeoylquinic acid, (f) 4-succinyl-3,5-dicaffeoylquinic acid. | |
4 Conclusion
In conclusion, this study delved into the intricate mechanisms underlying the corrosion inhibition properties of Chrysanthemum coronarium leaf extract (CCLE) on carbon steel in acidic environments. Through a comprehensive investigation involving phytochemical analysis, spectroscopic techniques, electrochemical studies, and theoretical modeling, significant insights have been gained. The phytochemical analysis revealed the richness of CCLE in polyphenols and flavonoids, suggesting its potential as a corrosion inhibitor. This was corroborated by spectroscopic surface studies, which identified the formation of an organic-based protective layer on the steel surface through adsorption of the phytoconstituents from the Chrysanthemum coronarium leaves extract. Furthermore, electrochemical studies demonstrated that CCLE effectively reduces the corrosion rate of carbon steel in acidic media. This inhibition mechanism involves multiple interactive processes, including chemisorption processes, leading to the formation of a uniform adsorbed layer that hinders the ingress of corrosive ions onto the metal surface. Theoretical modeling using Density Functional Theory (DFT) and Molecular Dynamics (MD) simulations provided further insights into the molecular-level interactions between CCLE compounds and the steel surface, in which, the DFT method using B3LYP/6-31+G(d,p) in aqueous solution has been used to study the anticorrosive performance of the of the components of the Chrysanthemum coronarium leaves extract. Analysis of the global chemical reactivity descriptors reveals that the 4-succinyl-3,5-dicaffeoylquinic acid (L5) component has the maximum corrosion inhibition efficiency, while rutin (L6) has the least contribution in the protection of the Fe-metal against corrosion. The analysis of local reactivity indices (Fukui indices) concludes that the oxygen atoms are able to interact with the Fe-metal by electron donation process, while the carbonyl carbon atoms have the ability to interact with the Fe-metal by electron acceptance process. Molecular dynamic (MD) simulation has been performed to calculate the adsorption energies of the inhibitors over the surface of the metal, highlighting the promising prospects of exploiting natural plant extracts, such as Chrysanthemum coronarium leaves extract, as effective corrosion inhibitors, paving the way for sustainable solutions in corrosion control and mitigation.
Data availability
The data supporting this article have been included as part of the ESI.†
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the High-Performance Computing Centre (Aziz Supercomputer) at King Abdulaziz University (http://hpc.kau.edu.sa/) for assisting with the calculations for this study. This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (GPIP-732-247-2024); the authors, therefore, gratefully acknowledge DSR's technical and financial support. The authors would like to thank the National Center for Scientific and Technical Research (CNRST) of Morocco for putting at their disposal the technical facilities of the UATRS division.
References
- IndustryARC, Corrosion Monitoring Market – Forecast (2024–2030).
- G. Koch, J. Varney, N. Thompson, O. Moghissi, M. Gould and J. Payer, NACE Int., 2016, 1–216 Search PubMed.
- S. H. Alrefaee, K. Y. Rhee, C. Verma, M. A. Quraishi and E. E. Ebenso, J. Mol. Liq., 2021, 321, 114666 CrossRef CAS.
- R. Aslam, G. Serdaroglu, S. Zehra, D. Kumar Verma, J. Aslam, L. Guo, C. Verma, E. E. Ebenso and M. A. Quraishi, J. Mol. Liq., 2022, 348, 118373 CrossRef CAS.
- Q. H. Zhang, N. Xu, Z. N. Jiang, H. F. Liu and G. A. Zhang, J. Colloid Interface Sci., 2023, 640, 1052–1067 CrossRef CAS PubMed.
- C. Verma, E. E. Ebenso, M. A. Quraishi and C. M. Hussain, Mater. Adv., 2021, 2, 3806–3850 RSC.
- Q. Wang, Q. Zhang, H. Zheng, L. Liu, X. Wu, C. Zhao, X. Zhou, Y. Sun, Z. Yan and X. Li, Sustainable Chem. Pharm., 2023, 34, 101177 CrossRef CAS.
- M. Akbari Shahmirzadi and M. Azadi, Heliyon, 2024, 10, e29962 CrossRef CAS.
- T. Rajachandrasekar, I. Muthuvel, K. Kavitha, S. R. Anishia, S. Sasikruba and S. Sujatha, Mater. Today: Proc., 2024 DOI:10.1016/j.matpr.2024.04.024.
- B. Lin, X. Zhou, T. Duan, C. Zhao, J. Zhu and Y. Xu, Arabian J. Chem., 2024, 17, 105410 CrossRef CAS.
- D. I. Udunwa, O. D. Onukwuli, M. C. Menkiti, S. C. Nwanonenyi, C. B. Ezekannagha and C. O. Aniagor, J. Mol. Struct., 2024, 1302, 137508 CrossRef CAS.
- G. D. Pai, M. R. Rathod, R. S K and A. A. Kittur, Results Surf. Interfaces, 2024, 15, 100203 CrossRef.
- A. Kumar and C. Das, Sci. Total Environ., 2024, 929, 172569 CrossRef CAS.
- F.-Z. Eddahhaoui, A. Najem, M. Elhawary, M. Boudalia, O. S. Campos, M. Tabyaoui, A. José Garcia, A. Bellaouchou and H. M. A. Amin, J. Alloys Compd., 2024, 977, 173307 CrossRef CAS.
- Q. Wang, C. Zhao, R. Wang, R. Aslam, X. Zhou, Q. Zhang, Z. Yan, Y. Sun, X. Li and H. Zheng, Colloids Surf., A, 2024, 682, 132904 CrossRef CAS.
- B. Tan, Y. Liu, Z. Gong, X. Zhang, J. Chen, L. Guo, J. Xiong, J. Liu, R. Marzouki and W. Li, J. Mol. Liq., 2024, 397, 124117 CrossRef CAS.
- R. Kellal, D. Benmessaoud Left, Z. S. Safi, N. Wazzan, O. S. Al-Qurashi and M. Zertoubi, J. Ind. Eng. Chem., 2023, 125, 370–389 CrossRef CAS.
- O. Dagdag, Z. Safi, H. Erramli, N. Wazzan, I. B. Obot, E. D. Akpan, C. Verma, E. E. Ebenso, O. Hamed and A. El Harfi, J. Mol. Liq., 2019, 287, 110977 CrossRef CAS.
- M. Tang, X. Li, S. Deng and R. Lei, J. Mol. Liq., 2021, 344, 117926 CrossRef CAS.
- Ecocrop, Chrysanthemum coronarium Var. Coronarium, Rome,Italie., 2007 Search PubMed.
- N. Hossain, M. Aminul Islam and M. Asaduzzaman Chowdhury, Results Chem., 2023, 5, 100883 CrossRef.
- K. Hosni, I. Hassen, H. Sebei and H. Casabianca, Ind. Crops Prod., 2013, 44, 263–271 CrossRef CAS.
- L. Sulas, G. L. Petretto, G. Pintore and G. Piluzza, Nat. Prod. Res., 2017, 31, 2941–2944 CrossRef CAS PubMed.
- J. P. Lai, Y. H. Lim, J. Su, H. M. Shen and C. N. Ong, J. Chromatogr. B:Anal. Technol. Biomed. Life Sci., 2007, 848, 215–225 CrossRef CAS.
- Y. Chuda, H. Ono, M. Ohnishi-Kameyama, T. Nagata and T. Tsushida, J. Agric. Food Chem., 1996, 44, 2037–2039 CrossRef CAS.
- T. Murayama, H. Yada, M. Kobori, H. Shinmoto and T. Tsushida, J. Jpn. Soc. Hortic. Sci., 2002, 71, 236–242 CrossRef CAS.
- Z. Ait EL Caid, D. Benmessaoud Left, R. Kellal, Z. S. Safi, A. Thoume, N. A. Wazzan and M. Zertoubi, Mater. Chem. Phys., 2024, 316, 129081 CrossRef CAS.
- B. lan Lin, T. hu Duan and Y. ye Xu, J. Mol. Struct., 2024 DOI:10.1016/j.molstruc.2024.140351.
- Z. Jebali, H. Ferkous, M. Zerroug, A. Boublia, A. Delimi, A. Bouzid, H. Majdoub, B. Ernst, N. Elboughdiri and Y. Benguerba, J. Environ. Chem. Eng., 2024, 12, 112374 CrossRef CAS.
- A. Ehsani, M. G. Mahjani, M. Hosseini, R. Safari, R. Moshrefi and H. Mohammad Shiri, J. Colloid Interface Sci., 2017, 490, 444–451 CrossRef CAS PubMed.
- J. Dai and X. An, Int. J. Electrochem. Sci., 2023, 18, 100080 CrossRef.
- B. Cherinka, B. H. Andrews, J. Sánchez-Gallego, J. Brownstein, M. Argudo-Fernández, M. Blanton, K. Bundy, A. Jones, K. Masters and D. R. Law, Astron. J., 2019, 158, 74 CrossRef.
- D. Roy, T. A. Keith, and J. M. Millam, Current version: GaussView, version 6, Semichem Inc., 2016 Search PubMed.
- R. A., Gaussian09, Inc., Wallingford CT, 2009, vol. 121, pp. 150–166 Search PubMed.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1996, 104, 1040–1046 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- Ş. Erdoğan, Z. S. Safi, S. Kaya, D. Ö. Işın, L. Guo and C. Kaya, J. Mol. Struct., 2017, 1134, 751–761 CrossRef.
- Z. S. Safi and A. M. Lamsabhi, J. Phys. Org. Chem., 2010, 23, 751–758 CrossRef CAS.
- R. Hsissou, K. Dahmani, A. El Magri, A. Hmada, Z. Safi, N. Dkhireche, M. Galai, N. Wazzan and A. Berisha, Polymers, 2023, 15, 1967 CrossRef CAS.
- B. Mennucci, J. Tomasi, R. Cammi, J. R. Cheeseman, M. J. Frisch, F. J. Devlin, S. Gabriel and P. J. Stephens, J. Phys. Chem. A, 2002, 106, 6102–6113 CrossRef CAS.
- A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS.
- S. H. Kouadri I, Ind. Crops Prod., 2018, 124, 787–796 CrossRef.
- I. Kouadri, A. Layachi, A. Makhlouf and H. Satha, Int. J. Polym. Anal. Charact., 2018, 23, 362–375 CrossRef CAS.
- V. S. Sastri and J. R. Perumareddi, Corrosion, 1997, 53, 617–622 CrossRef CAS.
- R. G. Parr and R. G. Pearson, J. Am. Chem. Soc., 1983, 105, 7512–7516 CrossRef CAS.
- R. G. Parr, L. v Szentpály and S. Liu, J. Am. Chem. Soc., 1999, 121, 1922–1924 CrossRef CAS.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- W. Yang and R. G. Parr, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 6723–6726 CrossRef CAS.
- T. Koopmans, Physica, 1933, 1, 104–113 CrossRef CAS.
- L. Guo, Z. S. Safi, S. Kaya, W. Shi, B. Tüzün, N. Altunay and C. Kaya, Front. Chem., 2018, 6, 155 CrossRef.
- B. Gómez, N. V. Likhanova, M. A. Domínguez-Aguilar, R. Martínez-Palou, A. Vela and J. L. Gázquez, J. Phys. Chem. B, 2006, 110, 8928–8934 CrossRef.
- K. Fukui, T. Yonezawa and H. Shingu, J. Chem. Phys., 1952, 20, 722–725 CrossRef CAS.
- A. K. Chandra and M. T. Nguyen, in Chemical Reactivity Theory: A Density-Functional View, ed. P. K. Chattaraj, Taylor and Francis, New York, 2008 Search PubMed.
- J. Hafner and G. Kresse, The Vienna AB-Initio Simulation Program VASP: An Efficient and Versatile Tool for Studying the Structural, Dynamic, and Electronic Properties of Materials, in Properties of Complex Inorganic Solids, ed. A. Gonis, A. Meike, P. E. A. Turchi, 1997, Springer, Boston, MA, DOI:10.1007/978-1-4615-5943-6_10.
- M. Hao, W. Zeng and Y. Li, Phys. E, 2022, 135, 114938 CrossRef CAS.
- L. Xu, D. Kirvassilis, Y. Bai and M. Mavrikakis, Surf. Sci., 2018, 667, 54–65 CrossRef CAS.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B, 1976, 13, 5188–5192 CrossRef.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- K. Mathew, R. Sundararaman, K. Letchworth-Weaver, T. A. Arias and R. G. Hennig, J. Chem. Phys., 2014, 140, 84106 CrossRef.
- V. Vorobyova and M. Skiba, Waste Biomass Valorization, 2021, 12, 4623–4641 CrossRef CAS.
- A. Thakur, S. Kaya, A. S. Abousalem, S. Sharma, R. Ganjoo, H. Assad and A. Kumar, Process Saf. Environ. Prot., 2022, 161, 801–818 CrossRef CAS.
- H. Yang, S. Deng and X. Li, Int. J. Electrochem. Sci., 2024, 19, 100790 CrossRef CAS.
- B. Tamilselvi, D. S. Bhuvaneshwari, S. Padmavathy and P. B. Raja, J. Mol. Liq., 2022, 359, 119359 CrossRef CAS.
- Z. Shahryari and K. Gheisari, Mater. Chem. Phys., 2023, 299, 127474 CrossRef CAS.
- A. Batah, A. Chaouiki, O. I. El Mouden, M. Belkhaouda, L. Bammou and R. Salghi, Sustainable Chem. Pharm., 2022, 27, 100677 CrossRef CAS.
- R. H. Ellerbrock and H. H. Gerke, J. Plant Nutr. Soil Sci., 2021, 184, 388–397 CrossRef CAS.
- M. Iranpour, A. Babaei and M. Bagherzadeh, Results Surf. Interfaces, 2023, 13, 100163 CrossRef.
- H. Mobtaker, M. Azadi and M. Rassouli, Heliyon, 2022, 8, e12297 CrossRef CAS.
- A. Berrissoul, A. Ouarhach, F. Benhiba, A. Romane, A. Guenbour, B. Dikici and F. Bentiss, Ind. Crops Prod., 2022, 187, 115310 CrossRef CAS.
- Z. Ait El Caid, D. Benmessaoud Left, A. Thoume, R. Kellal and M. Zertoubi, J. Bio- Tribo-Corros., 2024, 10, 9 CrossRef.
- M. Errami, A. EL-Asri, S. Fdil, S. Ourouadi, O. Iddelmouiden, A. Jmiai, L. Bazzi, A. Hadfi and R. Ait Akbour, Colloids Surf., A, 2024, 689, 133684 CrossRef CAS.
- R. Kellal, D. Benmessaoud Left, M. Azzi and M. Zertoubi, J. Appl. Electrochem., 2023, 53, 811–832 CrossRef CAS.
- Z. Ait El Caid, D. Benmessaoud Left, A. Thoume, R. Kellal and M. Zertoubi, J. Bio- Tribo-Corros., 2023, 9, 83 CrossRef.
- M. Faustin, Etude de l’effet des alcaloïdes sur la corrosion de l’acier C38 en milieu acide chlorhydrique 1M : Application à Aspidosperma album et Geissospermum laeve (Apocynacées), Université des Antilles et de la Guyane, 2013 Search PubMed.
- H. Khoshsang and A. Ghaffarinejad, Chem. Data Collect., 2022, 37, 100799 CrossRef CAS.
- B. Fan, X. Zhao, Z. Liu, Y. Xiang and X. Zheng, Sustainable Chem. Pharm., 2022, 29, 100821 CrossRef CAS.
- G. K. Shamnamol, P. Rugma, S. John and J. Mary Jacob, Results Chem., 2023, 5, 100728 CrossRef CAS.
- R. Tolulope Loto, Mater. Today: Proc., 2023, 80, 1519–1524 CAS.
- A. Kumar and C. Das, Sustainable Chem. Pharm., 2023, 36, 101261 CrossRef CAS.
- X. Liu, Y. Gao, J. Guan, Q. Zhang, Y. Lin, C. Shi, Y. Wang, J. Du and N. Ma, Arabian J. Chem., 2023, 16, 105066 CrossRef CAS.
- R. Kellal, D. Benmessaoud Left, Z. S. Safi, A. Thoume, N. A. Wazzan, O. S. AL-Qurashi and M. Zertoubi, Mater. Chem. Phys., 2024, 314, 128846 CrossRef CAS.
- M. Faustin, A. Maciuk, P. Salvin, C. Roos and M. Lebrini, Corros. Sci., 2015, 92, 287–300 CrossRef CAS.
- J. P. P. Thanikaivelan, V. Subramanian and T. Ramasami, Theor. Chem. Acc., 2002, 107, 326–335 Search PubMed.
- N. O. Obi-Egbedi, I. B. Obot and S. A. Umoren, Arabian J. Chem., 2012, 5, 361–373 Search PubMed.
- R. González Gómez, I. del Rosal, K. Philippot and R. Poteau, Theor. Chem. Acc., 2019, 138, 1–10 Search PubMed.
- S. Martinez, Mater. Chem. Phys., 2003, 77, 97–102 CrossRef CAS.
- M. J. S. Dewar and W. Thiel, J. Am. Chem. Soc., 1977, 99, 4899–4907 CrossRef CAS.
- A. Kokalj, Chem. Phys., 2012, 393, 1–12 CrossRef CAS.
- H. Babas, M. Khachani, I. Warad, S. Ajebli, A. Guessous, A. Guenbour, Z. Safi, A. Berisha, A. Bellaouchou and Z. Abdelkader, J. Mol. Liq., 2022, 356, 119019 CrossRef CAS.
- I. Lukovits, K. Palfi, I. Bako and E. Kalman, Corrosion, 1997, 53, 915–919 CrossRef CAS.
- I. Lukovits, E. Kálmán and F. Zucchi, Corrosion, 2001, 57, 3–8 CrossRef CAS.
- P. Geerlings, F. De Proft and W. Langenaeker, Chem. Rev., 2003, 103, 1793–1874 CrossRef CAS.
- S.-B. Liu, Acta Phys.-Chim. Sin., 2009, 25, 590–600 CAS.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS.
- A. D. S. K. Saha, P. Ghosh, D. Sukul and P. Banerjee, Phys. Chem. Chem. Phys., 2016, 18, 17898–17911 RSC.
- C. Morell, A. Grand and A. Toro-Labbé, J. Phys. Chem. A, 2005, 109, 205–212 CrossRef CAS.
- C. Cárdenas, N. Rabi, P. W. Ayers, C. Morell, P. Jaramillo and P. Fuentealba, J. Phys. Chem. A, 2009, 113, 8660–8667 CrossRef.
- H. Hashimoto, T. Fukuda, H. Tobita, M. Ray and S. Sakaki, Angew. Chem., Int. Ed., 2012, 51, 2930–2933 CrossRef CAS PubMed.
- M. Ray, Y. Nakao, H. Sato, S. Sakaki, T. Watanabe, H. Hashimoto and H. Tobita, Organometallics, 2010, 29, 6267–6281 CrossRef CAS.
- R. Kellal, Z. Ait El Caid, A. Thoume, M. Zertoubi and D. Benmessaoud Left, J. Bio- Tribo-Corros., 2024, 10, 69 CrossRef.
- Z. A. El Caid, D. Benmessaoud Left and M. Zertoubi, J. Mol. Struct., 2024, 1300, 137218 CrossRef CAS.
- A. Thoume, D. Benmessaoud Left, A. Elmakssoudi, Z. S. Safi, N. Benzbiria, A. Berisha, R. Kellal and M. Zertoubi, Mater. Chem. Phys., 2023, 310, 128487 CrossRef CAS.
- L. Chahir, M. El Faydy, N. Abad, F. Benhiba, I. Warad, D. Benmessaoud Left, M. Zertoubi, M. Allali, G. Kaichouh, B. Dikici, A. Bellaouchou, Y. Ramli and A. Zarrouk, J. Bio- Tribo-Corros., 2024, 10, 36 CrossRef.
- Z. A. El Caid, D. B. Left, A. Thoume, R. Kellal and M. Zertoubi, J. Bio- Tribo-Corros., 2023, 9, 83 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Mustapha Zertoubi
a,
Mustapha Zertoubi a,
Zaki S. Safi
a,
Zaki S. Safi b,
Nuha A. Wazzan
b,
Nuha A. Wazzan c,
Ohoud S. Al-qurashid and
Driss Benmessaoud Left
c,
Ohoud S. Al-qurashid and
Driss Benmessaoud Left *a
*a

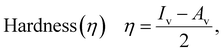





![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations.63–66 The peak at 1413 cm−1 was linked to the skeletal vibration of C–O–O bonds, while the peaks at 1269 cm−1 and 1107 cm−1 were attributed to C–O bonds in esters and alcohol derivatives.67 The FTIR analysis confirmed the presence of phenolic compounds in the aqueous extract of Chrysanthemum coronarium leaves, as indicated by the phytochemical analysis. These results further substantiate the presence of complex functional groups in the Chrysanthemum coronarium leaf extract, highlighting the potential therapeutic properties of its compounds.
O stretching vibrations.63–66 The peak at 1413 cm−1 was linked to the skeletal vibration of C–O–O bonds, while the peaks at 1269 cm−1 and 1107 cm−1 were attributed to C–O bonds in esters and alcohol derivatives.67 The FTIR analysis confirmed the presence of phenolic compounds in the aqueous extract of Chrysanthemum coronarium leaves, as indicated by the phytochemical analysis. These results further substantiate the presence of complex functional groups in the Chrysanthemum coronarium leaf extract, highlighting the potential therapeutic properties of its compounds.

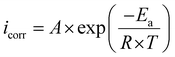













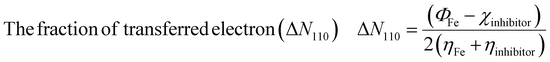
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –OH functional groups that are chemically bonded to the surface. According to the calculations, the process of forming bonds between Fe and inhibitors is exothermic. As well, all inhibitors exhibited negative adsorption energies between −108 and −275 kJ mol−1.
O and –OH functional groups that are chemically bonded to the surface. According to the calculations, the process of forming bonds between Fe and inhibitors is exothermic. As well, all inhibitors exhibited negative adsorption energies between −108 and −275 kJ mol−1.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the hydrogen of hydroxyl groups is transferred to bond with Fe atoms. Those Fe–H bonds have bond lengths between 1.57 to 1.79 Å. On the other hand, the other systems Chlorogenic acid@Fe, Rutin@Fe, and 4s35dca@Fe formed one or two non-classical Fe–H bonds. Their bond lengths range between 2.98 to 1.61 Å, which is different from the standard (1.50 Å) about 1.48 to 0.11 Å. Several studies have demonstrated nonclassical interactions between the H atoms in inhibitors and the transition metal center.99,100
O and the hydrogen of hydroxyl groups is transferred to bond with Fe atoms. Those Fe–H bonds have bond lengths between 1.57 to 1.79 Å. On the other hand, the other systems Chlorogenic acid@Fe, Rutin@Fe, and 4s35dca@Fe formed one or two non-classical Fe–H bonds. Their bond lengths range between 2.98 to 1.61 Å, which is different from the standard (1.50 Å) about 1.48 to 0.11 Å. Several studies have demonstrated nonclassical interactions between the H atoms in inhibitors and the transition metal center.99,100










