Open Access Article
Open Access ArticleIridium-catalyzed reductive sulfonamidation of alkoxy aryl alkynes†
Yuqiu Lianga,
Chengxiu Liua,
Penghao Weia,
Lu Ouyang *a and
Youchun Li*b
*a and
Youchun Li*b
aSchool of Pharmacy, Gannan Medical University, Ganzhou 341000, Jiangxi Province, P. R. China. E-mail: oyl3074@163.com
bThe Affiliated Ganzhou Hospital, Jiangxi Medical College, Nanchang University, Ganzhou 341000, Jiangxi Province, P. R. China. E-mail: liyouchun2007@163.com
First published on 2nd December 2024
Abstract
Sulfonamides are valuable structural building blocks, bioactives, and pharmaceuticals. While there have been great achievements in the sulfonamidation of alkyl and alkenyl carbon, the sulfonamidation of alkynyl carbon has not been studied. Herein, we report the synthesis of N-benzylated sulfonamides from alkoxy aryl alkynes and sulfonamides enabled by Ir-catalyzed reductive sulfonamidation using HCO2H as a hydrogen donor. This process was performed under mild conditions, resulting in the transformation of a variety of substituted benzene, heteroaromatic, and aliphatic sulfonamides. Particularly, the structural diversification of valdecoxib and zonisamide showcased the utility of this protocol.
Introduction
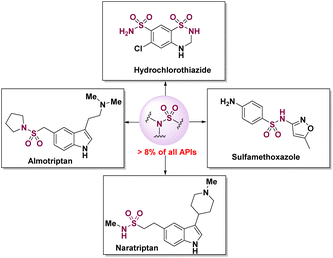
Sulfonamides are not only valuable structural building blocks in synthetic intermediates, but are also commonly found in biological and pharmaceutical fields.1 For instance, sulfonamides of almotriptan,2 sulfamethoxazol,3 hydrochlorothiazide,4 and naratriptan5 have found applications in the treatments of heavy migraine headache, urinary tract infections, and high blood pressure, respectively (Scheme 1). The latest statistics show that over 8% active pharmaceutical ingredients (APIs) contain sulfonamide skeletons, which have special physicochemical properties of metabolic stability.6 Therefore, the extensive application of sulphonamides in medicinal chemistry has attracted the attention of chemists in the synthesis and functionalization of sulfonamides in recent decades.7Reactions of primary sulfonamides with aliphatic halides,8 alcohols,9 and carbonyls10 present classical strategies for the synthesis of sulfonamides, in which the organic,11 inorganic,12 Ir,13 Ru,14 Rh,15 and other metal16 catalysts are employed. Coupling of primary sulfonamides with aryl halides,17 boronic acids,18 and diaryliodonium triflate19 constitutes another efficient approach to sulphonamide synthesis, where Cu,20 Pd,21 and Ni22 metals are commonly utilized as catalysts. Direct sulfonamidation of alkyl carbon provides an atom- and step economy strategy for sulfonamide synthesis, with commendable substrate scope and efficiency (Scheme 2a).23 However, the inevitable use of hypervalent iodine reagents or strong oxidation,24 excessive equivalents of oxidants,25 and poor regioselectivity26 limit the application of this strategy. Approaches to sulfonamide synthesis based on the sulfonamidation of alkenyl carbon via classical hydroamination or hydrogen atom transfer of alkenes are attractive alternatives (Scheme 2b).27 Notably, asymmetric sulfonamidation of alkenyl carbon for the synthesis of enantioenriched sulfonamides via hydrogen atom transfer has also be established.28 While there have been great achievements with regards to sulfonamidation of alkyl and alkenyl carbon, the sulfonamidation of alkynyl carbon has not been studied.
With our continuous research on transfer hydrogenation with Cp*Ir complexes,29 N-alkylation30 or para-Friedel–Crafts alkylation31 were achieved from alkynes via hydration and transfer hydrogenation. In a previous work, a relatively stable benzyl carbocation was generated from alkynes via a hydration, transfer hydrogenation, and successive dehydroxylation process, which might be captured by primary or secondary sulfonamides to deliver a variety of N-benzylated sulfonamides. Using alkynes as substrates comes with various challenges: (a) although hydration of alkynes had been developed in our previous work,31 there is a risk of deactivation of hydration using primary or secondary sulfonamides as nucleophilic reagents; (b) a similar outcome of poisoning subsequent transfer hydrogenation is possible under these reaction conditions; (c) a weaker nucleophilic property is noted while using nitrogen atoms as a nucleophilic reagent under acidic conditions, and using sulfonamides as nucleophilic agents will likely have similar related issues, hindering the final cross nucleophilic coupling process. Despite these difficulties, through protracted and unremitting efforts, herein, we realized the reductive sulfonamidation of alkynes with primary and secondary sulfonamides using metal catalysis, which provides inspiration for the synthesis of diversified sulfonamides (Scheme 2c).
Results and discussion
We initially examined the reductive sulfonamidation of alkynes by employing 4-ethynylanisole 1a and benzene sulfonamide 2a as model substrates, Cp*Ir complexes as catalyst,32 and HCO2H as a hydrogen donor (Table 1). Interestingly, the desired product 3aa was produced at a 38% yield using H2O as a solvent and TsOH as an additive (Table 1, entry 1). Screening of further reaction parameters indicated that the H2O and Cp*Ir catalyst were essential for successful reductive sulfonamidation (Table 1, entries 2–11). Increasing the ratio of TFEA would decrease the yield of 3aa (Table 1, entries 7–10). For instance, the yield of 3aa was reduced to trace even though the ratio of TFEA and H2O was loaded over 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entry 10). Additionally, catalyst optimization (Table 1, entries 12–17) showed that the Cp*Ir complex C5 could slightly enhance the sulfonamidation process leading to a 45% yield of the product 3aa (Table 1, entry 16). Satisfyingly, increasing the loading of 2a would sharply improve the yield of 3aa (Table 1, entries 18–24). Of note, the control experiment demonstrated that a Lewis acid was crucial for this reductive sulfonamidation process (Table 1, entries 25–35) and decreasing the loading of TsOH to 0.2 equiv. resulted in the best yield of 3aa (Table 1, entry 30). However, decreasing or increasing the reaction temperature was harmful to the production of 3aa (Table 1, entries 36 and 37). Control experiment showed that HCO2H was the essential hydrogen donor in this transformation, indicating that H2O only act as a reaction media (Table 1, entry 38).
1 (Table 1, entry 10). Additionally, catalyst optimization (Table 1, entries 12–17) showed that the Cp*Ir complex C5 could slightly enhance the sulfonamidation process leading to a 45% yield of the product 3aa (Table 1, entry 16). Satisfyingly, increasing the loading of 2a would sharply improve the yield of 3aa (Table 1, entries 18–24). Of note, the control experiment demonstrated that a Lewis acid was crucial for this reductive sulfonamidation process (Table 1, entries 25–35) and decreasing the loading of TsOH to 0.2 equiv. resulted in the best yield of 3aa (Table 1, entry 30). However, decreasing or increasing the reaction temperature was harmful to the production of 3aa (Table 1, entries 36 and 37). Control experiment showed that HCO2H was the essential hydrogen donor in this transformation, indicating that H2O only act as a reaction media (Table 1, entry 38).
| Entry | Cat. | Additive (equiv.) | 2a (x equiv.) | Solvent | Yield 3aab (%) |
|---|---|---|---|---|---|
a Reaction conditions: 1a (0.5 mmol), Cat. (1.0 mol%), HCO2H (10.0 equiv.), and solvent (1.5 mL) for 12 hours at 80 °C (under air).b Yield was determined by NMR with dimethyl terephthalate as the internal standard.c The ratio of the mixed solvent was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (v/v).d The ratio of the mixed solvent was 1 8 (v/v).d The ratio of the mixed solvent was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (v/v).e The ratio of the mixed solvent was 1 4 (v/v).e The ratio of the mixed solvent was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v).f The ratio of the mixed solvent was 1 2 (v/v).f The ratio of the mixed solvent was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v).g R1 = 4-Cl-Ph; R2 = 2-naphthyl.h 60 °C.i 100 °C.j Without HCO2H. 1 (v/v).g R1 = 4-Cl-Ph; R2 = 2-naphthyl.h 60 °C.i 100 °C.j Without HCO2H. |
|||||
| 1 | C3 | TsOH (0.6) | 1.0 | H2O | 38 |
| 2 | C3 | TsOH (0.6) | 1.0 | DMF | N.D. |
| 3 | C3 | TsOH (0.6) | 1.0 | TFEA | N.D. |
| 4 | C3 | TsOH (0.6) | 1.0 | MeCN | N.D. |
| 5 | C3 | TsOH (0.6) | 1.0 | p-Xylene | N.D. |
| 6 | C3 | TsOH (0.6) | 1.0 | Dioxane | N.D. |
| 7 | C3 | TsOH (0.6) | 1.0 | TFEA/H2Oc | 36 |
| 8 | C3 | TsOH (0.6) | 1.0 | TFEA/H2Od | 42 |
| 9 | C3 | TsOH (0.6) | 1.0 | TFEA/H2Oe | 22 |
| 10 | C3 | TsOH (0.6) | 1.0 | TFEA/H2Of | Trace |
| 11 | C3 | TsOH (0.6) | 1.0 | p-Xylene/H2Od | 30 |
| 12 | — | TsOH (0.6) | 1.0 | TFEA/H2Od | N.D. |
| 13 | C1 | TsOH (0.6) | 1.0 | TFEA/H2Od | 40 |
| 14 | C2 | TsOH (0.6) | 1.0 | TFEA/H2Od | 37 |
| 15 | C4 | TsOH (0.6) | 1.0 | TFEA/H2Od | 37 |
| 16 | C5 | TsOH (0.6) | 1.0 | TFEA/H2Od | 45 |
| 17 | C6 | TsOH (0.6) | 1.0 | TFEA/H2Od | 41 |
| 18 | C5 | TsOH (0.6) | 1.2 | TFEA/H2Od | 45 |
| 19 | C5 | TsOH (0.6) | 1.4 | TFEA/H2Od | 46 |
| 20 | C5 | TsOH (0.6) | 1.6 | TFEA/H2Od | 46 |
| 21 | C5 | TsOH (0.6) | 1.8 | TFEA/H2Od | 48 |
| 22 | C5 | TsOH (0.6) | 2.0 | TFEA/H2Od | 77 |
| 23 | C5 | TsOH (0.6) | 2.5 | TFEA/H2Od | 70 |
| 24 | C5 | TsOH (0.6) | 3.0 | TFEA/H2Od | 76 |
| 25 | C5 | — | 2.0 | TFEA/H2Od | N.D. |
| 26 | C5 | PhSO3H (0.6) | 2.0 | TFEA/H2Od | 52 |
| 27 | C5 | R1SO3H (0.6)g | 2.0 | TFEA/H2Od | 44 |
| 28 | C5 | MSA (0.6) | 2.0 | TFEA/H2Od | 59 |
| 29 | C5 | R2SO3H (0.6)g | 2.0 | TFEA/H2Od | 35 |
| 30 | C5 | TsOH (0.2) | 2.0 | TFEA/H2Od | 81 (79) |
| 31 | C5 | TsOH (0.4) | 2.0 | TFEA/H2Od | 80 |
| 32 | C5 | TsOH (0.8) | 2.0 | TFEA/H2Od | 46 |
| 33 | C5 | TsOH (1.0) | 2.0 | TFEA/H2Od | 43 |
| 34 | C5 | TsOH (1.5) | 2.0 | TFEA/H2Od | 29 |
| 35 | C5 | TsOH (2.0) | 2.0 | TFEA/H2Od | 18 |
| 36h | C5 | TsOH (0.2) | 2.0 | TFEA/H2Od | 63 |
| 37i | C5 | TsOH (0.2) | 2.0 | TFEA/H2Od | 42 |
| 38j | C5 | TsOH (0.2) | 2.0 | TFEA/H2Od | N.D. |
With the successfully optimized conditions, the substrate scope with respect to aryl alkynes and aryl sulfonamides was investigated (Table 2). As anticipated, aryl sulfonamides with electron-donating groups at differential positions were well tolerated, including methyl, ethyl, tert-butyl, hydroxyl, and methoxy, delivering the corresponding products (3ab–3ag) in good to excellent yields. Additionally, di-substituted aryl sulfonamide (2h) also performed well in this system. Aryl sulfonamides with electron-withdrawing groups, such as fluorine (2i), chlorine (2j, 2n), nitrile (2k, 2l), and trifluoromethyl (2m), were also efficient substrates to afford similar yields of the desired products (3ai–3an). However, significantly different yields were achieved with heteroaromatic sulfonamides (2o–2s) as substrates. For instance, low yield (15–26%) of corresponding products 3ao, 3ap were obtained using pyridine sulfonamides as substrates, while 2-thiophene-sulfonamides delivered the desired products 3aq–3as in good yields (69–77%). This difference could be attributed to the difference in the density of π electrons. Interestingly, switching the primary aryl sulfonamides to secondary aryl sulfonamides also allowed the formation of the desired products (3at–3av) in moderate yields. Furthermore, other alkoxy-substituted aryl alkynes (2b–2d) were also good substrates in the iridium catalyzed reductive sulfonamidation process.
| a Reaction conditions: 1 (0.5 mmol), 2 (1.0 mmol), C5 (1.0 mol%), HCO2H (10.0 equiv.), and TFEA (0.3 mL), H2O (1.2 mL) for 12 hours at 80 °C, and isolated yield. |
|---|
 |
Having established the conversion of aromatic sulfonamides into diversified sulfonamides, this method was extended to employ alkyl sulfonamides as substrates (Table 3). In comparison with aryl sulfonamides, the corresponding products (5aa–5ah, 5aj) were delivered in relatively lower yields using differential primary alkyl sulfonamides as substrates. Surprisingly, a better yield (66%) of sulfonamide 5ai was delivered by using secondary cyclic aliphatic sulfonamide of 1,3-propanesultam as a substrate.
| a Reaction conditions: 1a (0.5 mmol), 4 (1.0 mmol), C5 (1.0 mol%), HCO2H (10.0 equiv.), and TFEA (0.3 mL), H2O (1.2 mL) for 12 hours at 80 °C, and isolated yield. |
|---|
 |
Following success in developing a broad range of sulfonamides, we then explored the synthetic applications of this method. First, we investigated the derivatization of drugs containing the sulfonamide scaffold, which are of interest in medicinal chemistry. As shown in Scheme 3a, valdecoxib (COX-2 inhibitor)33 and zonisamide (used as an adjunctive therapy in adults with partial-onset of seizures)34 could be easily converted into N-benzyl sulfonamides. Moreover, the model reaction was scaled to a 10.0 mmol reaction and it delivered 2.5 g of the sulfonamide 3aa in 86% yield, which exhibited potential synthetic application in the organic chemistry industry (Scheme 3b).
To gain more insights into the reaction mechanism, control experiments were performed. According to our previous work,35 hydration of alkynes proceeded smoothly under acidic conditions to generate ketone and alcohol intermediates. Therefore, the ketone 6a and sulfonamide 2a were employed as substrates under standard conditions, resulting in a 70% yield of 3aa (Scheme 4a). Moreover, subjecting alcohol 7a and sulfonamide 2a to the standard conditions in the absence of C5 resulted in a 73% yield of 3aa (Scheme 4b).
Based on the reaction result and control experiments (Scheme 4), a possible mechanism was proposed (Scheme 5). The mechanism is characterized by a catalytic cycle that includes hydration and a transfer hydrogenation process that was completed to generate the intermediate alcohol 7a. Subsequently, carbocation occurred by dehydroxylation of 7a under acidic conditions, which was followed by cross-nucleophile coupling with sulfonamide to produce the desired product 3aa.
Conclusions
In conclusion, we have shown the sulfonamidation of alkoxy aryl alkynes with viable sulfonamides for the synthesis of diverse N-benzylated sulfonamides. This modular Cp*Ir complex-catalyzed reductive sulfonamidation synthesis was achieved under mild conditions. The reaction can be conducted at gram scale in air. Sulfonamide drugs of valdecoxib and zonisamide could also be employed as substrates and converted into N-benzyl sulfonamides. The good substrate suitability, wide range of functional group tolerance, scale-up performance, and mild reaction conditions provide evidence of the potential for the application of this reductive sulfonamidation transformation in rapid structural diversification of bioactive molecules.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Yuqiu Liang, Chengxiu Liu, and Penghao Wei: investigation, data curation, validation, visualization, manuscript, and ESI† writing and editing. Youchun Li and Lu Ouyang: conceptualization, funding acquisition, project administration, resources, supervision, visualization, revising the manuscript and the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ganzhou Bureau of Science and Technology (2022-YB1402), the Fundamental Research Funds for Gannan Medical University (QD201810, QD202106, TD2021YX05, TD202310) for financial support.Notes and references
- (a) J. M. Dorn, M. Alpern, C. McNulty and G. W. Volcheck, Sulfonamide Drug Allergy, Curr. Allergy Asthma Rep., 2018, 18, 38 CrossRef PubMed; (b) Q. Zhang, Z. Xia, S. Joshi, V. E. Scott and M. F. Jarvis, Optimization of ADME properties for sulfonamides leading to the discovery of a T-type calcium channel blocker, ABT-639, ACS Med. Chem. Lett., 2015, 6, 641 CrossRef CAS PubMed; (c) P. Devendar and G. Yang, Sulfur-containing agrochemicals, Top. Curr. Chem., 2017, 375, 1 CrossRef CAS PubMed; (d) K. A. Scott and J. T. Njardarson, Analysis of US FDA-Approved Drugs Containing Sulfur Atoms, Top. Curr. Chem., 2018, 376, 5 CrossRef PubMed.
- (a) J. Pascual and C. Vila, Almotriptan: a review of 20 years' clinical experience, Expert Rev. Neurother., 2019, 19, 759 CrossRef CAS PubMed; (b) S. Gupta, A. Perla, A. Roy, J. G. Vitore, K. Bharathi, S. Salave, D. Rana, A. Sharma, R. Rathod, H. Kumar and D. Benival, In Vivo Evaluation of Almotriptan malate Formulation through Intranasal Route for the Treatment of Migraine: Systematic Development and Pharmacokinetic Assessment, AAPS PharmSciTech, 2023, 24, 32 CrossRef CAS PubMed.
- (a) A. Stern, H. Green, M. Paul, L. Vidal and L. Leibovici, Prophylaxis for pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients, Cochrane Database Syst. Rev., 2014, 10, 1465–1858 Search PubMed; (b) Y. Duan, X. Zhang, W. Deng, S. Wang, J. Hu, X. Wang, W. Li and B. Chen, The first reported pulmonary nocardiosis caused by Nocardia gipuzkoensis resisted to trimethoprim/sulfamethoxazol (TMP-SMZ) in an immunocompetent patient, J. Glob. Antimicrob. Resist., 2004, 37, 214 CrossRef PubMed.
- (a) B.-J. H. van den Born, R. Olde-Engberink and L. Vogt, Hydrochlorothiazide and the Risk of Malignant Melanoma, JAMA Intern. Med., 2018, 178, 1425 CrossRef PubMed; (b) H. Sternlicht and G. L. Bakris, Hydrochlorothiazide as the Diuretic of Choice for Hypertension, J. Am. Coll. Cardiol., 2016, 67, 390 CrossRef CAS PubMed.
- (a) P. Tfelt-Hansen, Naratriptan is as effective as sumatriptan for the treatment of migraine attacks when used properly. A mini-review, Cephalalgia, 2021, 41, 1499 CrossRef PubMed; (b) N. T. Mathew, Naratriptan: a review, Expert Opin. Inv. Drug., 1999, 8, 687 CrossRef CAS PubMed.
- (a) C. Zhao, K. P. Rakesh, L. Ravidar, W.-Y. Fang and H.-L. Qin, Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review, Eur. J. Med. Chem., 2019, 162, 679 CrossRef CAS PubMed; (b) A. S. Kalgutkar, R. Jones and A. Sawant, in Metabolism, Pharmacokinetics and Toxicity of Functional Groups: Impact of the Building Blocks of Medicinal Chemistry on ADMET, ed. Smith, D. A., Royal Society of Chemistry, Cambridge, UK, 2010, p. 210 Search PubMed.
- (a) Y. Chen, Recent Functionalizations of Primary Sulfonamides, Synthesis, 2016, 48, 2483 CrossRef CAS; (b) D. Joseph, M. A. Idris, J. Chen and S. Lee, Recent Advances in the Catalytic Synthesis of Arylsulfonyl Compounds, ACS Catal., 2021, 11, 4169 CrossRef CAS; (c) T. C. Das, S. A. Quadri and M. Farooqui, Recent advances in synthesis of sulfonamides: A review, J. Chem. Biol. Interfaces, 2018, 8, 194 CAS; (d) O. M. Mulina, A. I. Ilovaisky and A. O. Terent’ev, Oxidative Coupling with S–N Bond Formation, Eur. J. Org Chem., 2018, 2018, 4648 CrossRef CAS; (e) S. Ghosh, P. P. Pal and A. Hajraa, N-Heteroarylation of Sulfonamides: An Overview, Adv. Synth. Catal., 2023, 365, 3020 CrossRef CAS.
- (a) V. A. Azov, D. Janott, D. Schlüter and M. Zeller, Tuning of tetrathiafulvalene properties: versatile synthesis of N-arylated monopyrrolotetrathiafulvalenes via Ullmann-type coupling reactions, Beilstein J. Org. Chem., 2015, 11, 860 CrossRef CAS PubMed; (b) S. J. Gharpure, D. Anuradha, J. V. Prasad and P. Srinivasa Rao, Stereoselective Synthesis of cis-2,6-Disubstituted Morpholines and 1,4-Oxathianes by Intramolecular Reductive Etherification of 1,5-Diketones, Eur. J. Org Chem., 2015, 2015, 86 CrossRef CAS.
- M. Dryzhakov, E. Richmond and J. Moran, Recent advances in direct catalytic dehydrative substitution of alcohols, Synthesis, 2016, 48, 935 CrossRef CAS.
- (a) L. Li, B. Zhou, Y. H. Wang, C. Shu, Y. F. Pan, X. Lu and L. W. Ye, Zinc-Catalyzed Alkyne Oxidation/C–H Functionalization: Highly Site-Selective Synthesis of Versatile Isoquinolones and β-Carbolines, Angew. Chem., Int. Ed., 2015, 54, 8245 CrossRef CAS PubMed; (b) Y.-M. Wang, N. C. Bruno, Á. L. Placeres, S. Zhu and S. L. Buchwald, Enantioselective synthesis of carbo-and heterocycles through a CuH-catalyzed hydroalkylation approach, J. Am. Chem. Soc., 2015, 137, 10524 CrossRef CAS PubMed; (c) H. Alinezhad, H. Yavari and F. Salehian, Recent advances in reductive amination catalysis and its applications, Curr. Org. Chem., 2015, 19, 1021 CrossRef CAS.
- (a) Q. Xu, Q. Li, X. Zhu and J. Chen, Green and Scalable Aldehyde-Catalyzed Transition Metal-Free Dehydrative N-Alkylation of Amides and Amines with Alcohols, Adv. Synth. Catal., 2013, 355, 73 CrossRef CAS; (b) S.-S. Weng, K. Hsieh and Z. Zeng, PhI(OCOCF3)2-catalyzed nucleophilic substitution of aromatic propargyl alcohols, Tetrahedron, 2015, 71, 2549 CrossRef CAS; (c) M. Zhuang and H. Du, Chiral Brønsted acid catalyzed enantioselective intermolecular allylic aminations, Org. Biomol. Chem., 2014, 12, 4590 RSC.
- Q.-Q. Li, Z.-F. Xiao, C.-Z. Yao, H.-X. Zheng and Y.-B. Kang, Direct alkylation of amines with alcohols catalyzed by base, Org. Lett., 2015, 17, 5328 CrossRef CAS PubMed.
- (a) P. Qu, C. Sun, J. Ma and F. Li, The N-alkylation of sulfonamides with alcohols in water catalyzed by the water-soluble iridium complex {Cp*Ir[6, 6′-(OH)2bpy](H2O)}[OTf]2, Adv. Synth. Catal., 2014, 356, 447 CrossRef CAS; (b) L. Lu, J. Ma, P. Qu and F. Li, Effective recognition of different types of amino groups: from aminobenzenesulfonamides to amino-(N-alkyl) benzenesulfonamides via iridium-catalyzed N-alkylation with alcohols, Org. Lett., 2015, 17, 2350 CrossRef CAS PubMed; (c) S. Kerdphon, X. Quan, V. S. Parihar and P. G. Andersson, C–N Coupling of Amides with Alcohols Catalyzed by N-Heterocyclic Carbene–Phosphine Iridium Complexes, J. Org. Chem., 2015, 80, 11529 CrossRef CAS PubMed; (d) Q. Zou, C. Wang, J. Smith, D. Xue and J. Xiao, Alkylation of amines with alcohols and amines by a single catalyst under mild conditions, Chem.–Eur. J., 2015, 21, 9656 CrossRef CAS PubMed.
- T. T. Dang, B. Ramalingam and A. M. Seayad, Efficient ruthenium-catalyzed N-methylation of amines using methanol, ACS Catal., 2015, 5, 4082 CrossRef CAS.
- P. Satyanarayana, G. M. Reddy, H. Maheswaran and M. L. Kantam, Tris (Acetylacetonato) Rhodium (III)-catalyzed α-alkylation of Ketones, β-alkylation of Secondary Alcohols and Alkylation of Amines with Primary Alcohols, Adv. Synth. Catal., 2013, 355, 1859 CrossRef CAS.
- (a) H. Hikawa, N. Matsuda, H. Suzuki, Y. Yokoyama and I. Azumaya, N-Benzylation/Benzylic C–H Amidation Cascade by the (η3-Benzyl) palladium System in Aqueous Media: An Effective Pathway for the Direct Construction of 3-Phenyl-3,4-dihydro-(2H)-1,2,4-benzothiadiazine 1,1-Dioxides, Adv. Synth. Catal., 2013, 355, 2308 CrossRef CAS; (b) M. Sharif, J. Opalach, P. Langer, M. Beller and X.-F. Wu, Oxidative synthesis of quinazolinones and benzothiadiazine 1,1-dioxides from 2-aminobenzamide and 2-aminobenzenesulfonamide with benzyl alcohols and aldehydes, RSC Adv., 2014, 4, 8 RSC; (c) P. Trillo, A. Baeza and C. Najera, Direct Nucleophilic Substitution of Free Allylic Alcohols in Water Catalyzed by FeCl3·6H2O: Which is the Real Catalyst, ChemCatChem, 2013, 5, 1538 CrossRef CAS; (d) X. Xu, H. Wu, Z. Li, X. Sun and Z. Wang, Iron oxide-silver magnetic nanoparticles as simple heterogeneous catalysts for the direct inter/intramolecular nucleophilic substitution of π-activated alcohols with electron-deficient amines, Tetrahedron, 2015, 71, 5254 CrossRef CAS.
- (a) J. Baffoe, M. Y. Hoe and B. B. Touré, Copper-mediated N-heteroarylation of primary sulfonamides: synthesis of mono-N-heteroaryl sulfonamides, Org. Lett., 2010, 12, 1532 CrossRef CAS PubMed; (b) R. Hosseinzadeh, M. Tajbakhsh, M. Mohadjerani and M. Alikarami, Copper-catalysed N-arylation of arylsulfonamides with aryl bromides and aryl iodides using KF/Al2O3, J. Chem. Sci., 2021, 122, 143 CrossRef; (c) X. Han, Cross coupling of 3-bromopyridine and sulfonamides (R1NHSO2R2· R1= H, Me, alkyl; R2= alkyl and aryl) catalyzed by CuI/1,3-di (pyridin-2-yl) propane-1,3-dione, Tetrahedron Lett., 2010, 51, 360 CrossRef CAS; (d) X. Wang, A. Guram, M. Ronk, J. E. Milne, J. S. Tedrow and M. M. Faul, Copper-catalyzed N-arylation of sulfonamides with aryl bromides under mild conditions, Tetrahedron Lett., 2012, 53, 7 CrossRef CAS; (e) B. Tan, Y. Teo and A. H. Seow, Low catalyst loadings for ligand-free copper (I)-oxide-catalyzed N-arylation of methanesulfonamide in water, Eur. J. Org Chem., 2014, 2014, 1541 CrossRef CAS.
- (a) M. Nasrollahzadeh, A. Rostami-Vartooni, A. Ehsani and M. Moghadam, Fabrication, characterization and application of nanopolymer supported copper (II) complex as an effective and reusable catalyst for the CN bond cross-coupling reaction of sulfonamides with arylboronic acids in water under aerobic conditions, J. Mol. Catal. A, 2014, 387, 123 CrossRef CAS; (b) S. Y. Moon, J. Nam, K. Rathwell and W. Kim, Copper-catalyzed Chan-Lam coupling between sulfonyl azides and boronic acids at room temperature, Org. Lett., 2014, 16, 338 CrossRef CAS PubMed; (c) J. C. Vantourout, L. Li, E. Bendito-Moll, S. Chabbra, K. Arrington, B. E. Bode and A. J. Watson, Mechanistic insight enables practical, scalable, room temperature Chan-Lam N-arylation of N-aryl sulfonamides, ACS Catal., 2018, 8, 9560 CrossRef CAS.
- S. Y. Moon, M. Koh, K. Rathwell, S. H. Jung and W. S. Kim, Copper-catalyzed N-arylation of tert-butyl N-sulfonylcarbamates with diaryliodonium salts at room temperature, Tetrahedron, 2015, 71, 1566 CrossRef CAS.
- Q. Li, L. Xu and D. Ma, Cu-Catalyzed coupling reactions of sulfonamides with (hetero) aryl chlorides/bromides, Angew. Chem., Int. Ed., 2022, 134, e202210483 CrossRef.
- (a) P. Innocenti, H. Woodward, L. O'Fee and S. Hoelder, Expanding the scope of fused pyrimidines as kinase inhibitor scaffolds: synthesis and modification of pyrido [3,4-d] pyrimidines, Org. Biomol. Chem., 2015, 13, 893 RSC; (b) A. J. DeAngelis, P. G. Gildner, R. Chow and T. J. Colacot, Generating active “L-Pd (0)” via neutral or cationic π-allylpalladium complexes featuring biaryl/bipyrazolylphosphines: synthetic, mechanistic, and structure-activity studies in challenging cross-coupling reactions, J. Org. Chem., 2015, 80, 6794 CrossRef CAS PubMed; (c) M. Khalaj, M. Ghazanfarpour-Darjani, M. R. Talei Bavil Olyai and S. F. Shamami, Palladium nanoparticles as reusable catalyst for the synthesis of N-aryl sulfonamides under mild reaction conditions, J. Sulfur Chem., 2016, 37, 211 CrossRef CAS; (d) S. S. Chourasiya, A. A. Wani, C. M. Nagaraja, A. K. Chakraborti and P. V. Bharatam, N-(acridin-9-yl) arenesulfonamides: Synthesis, quantum chemical studies and crystal structure analysis to establish the tautomeric preferences, Tetrahedron, 2018, 74, 3634 CrossRef CAS; (e) J. Becica, D. P. Hruszkewycz, J. E. Steves, J. M. Elward, D. C. Leitch and G. E. Dobereiner, High-throughput discovery and evaluation of a general catalytic method for N-arylation of weakly nucleophilic sulfonamides, Org. Lett., 2019, 21, 8981 CrossRef CAS PubMed.
- (a) T. Kim, S. J. McCarver, C. Lee and D. W. MacMillan, Sulfonamidation of aryl and heteroaryl halides through photosensitized nickel catalysis, Angew. Chem., Int. Ed., 2018, 130, 3546 CrossRef; (b) J. M. Blackburn, A. L. Gant Kanegusuku, G. E. Scott and J. L. Roizen, Photochemically-mediated, nickel-catalyzed synthesis of N-(hetero) aryl sulfamate esters, Org. Lett., 2019, 21, 7049 CrossRef CAS PubMed; (c) R. T. McGuire, C. M. Simon, A. A. Yadav, M. J. Ferguson and M. Stradiotto, Nickel-Catalyzed Cross-Coupling of Sulfonamides With (Hetero) aryl Chlorides, Angew. Chem., Int. Ed., 2020, 59, 8952 CrossRef CAS PubMed; (d) R. T. Simons, G. E. Scott, A. G. Kanegusuku and J. L. Roizen, Photochemically mediated nickel-catalyzed synthesis of N-(Hetero) aryl sulfamides, J. Org. Chem., 2020, 85, 6380 CrossRef CAS PubMed; (e) T. T. Zhao, H. N. Qin and P. F. Xu, Light-Promoted Nickel-Catalyzed C–O/C–N Coupling of Aryl Halides with Carboxylic Acids and Sulfonamides, Org. Lett., 2023, 25, 636 CrossRef CAS PubMed.
- (a) Y. Chen, Recent Functionalizations of Primary Sulfonamides, Synthesis, 2016, 48, 2483 CrossRef CAS; (b) S. Ghosh, P. Paramita Pal and A. Hajra, N-Heteroarylation of Sulfonamides: An Overview, Adv. Synth. Catal., 2023, 365, 1 CrossRef; (c) H. Wu, X. Chen, N. Sun and A. Sanchez-Mendoza, Recent developments in the synthesis of N-aryl sulfonamides, Synth. Commun., 2021, 51, 2287 CrossRef CAS.
- (a) G. Pelletier and D. A. Powell, Copper-Catalyzed Amidation of Allylic and Benzylic CsH Bonds, Org. Lett., 2006, 8, 6031 CrossRef CAS PubMed; (b) Z. Wang, Y. Zhang, H. Fu, Y. Jiang and Y. Zhao, Efficient Intermolecular Iron-Catalyzed Amidation of C–H Bonds in the Presence of N-Bromosuccinimide, Org. Lett., 2008, 10, 1863 CrossRef CAS PubMed; (c) J. A. Halfen, Recent Advances in Metal-Mediated Carbon-Nitrogen Bond Formation Reactions: Aziridination and Amidation, Curr. Org. Chem., 2005, 9, 657 CrossRef CAS; (d) H. Lai, J. Xu, X. Liu and D. Zha, A dual strategy of direct C(sp3)-H sulfonamidation by cross-dehydrogenative coupling and oxidative hydroxylation -condensation, Tetrahedron, 2024, 160, 134022 CrossRef CAS.
- (a) P. Becker, T. Duhamel, C. Martínez and K. Muñiz, Designing Homogeneous Bromine Redox Catalysis for Selective Aliphatic C–H Bond Functionalization, Angew. Chem., Int. Ed., 2018, 57, 5166 CrossRef CAS PubMed; (b) D. A. Powell and H. Fan, Copper-catalyzed amination of primary benzylic C–H bonds with primary and secondary sulfonamides, J. Org. Chem., 2010, 75, 2726 CrossRef CAS PubMed; (c) S. R. Guo, P. S. Kumar and M. Yang, Recent advances of oxidative radical cross-coupling reactions: direct α-C(sp3)–H bond functionalization of ethers and alcohols, Adv. Synth. Catal., 2017, 359, 2 CrossRef CAS.
- (a) A. M. Kazerouni, T. A. F. Nelson, S. W. Chen, K. R. Sharp and S. B. Blakey, Regioselective Cp*Ir (III)-catalyzed Allylic C–H Sulfamidation of Allylbenzene Derivatives, J. Org. Chem., 2019, 84, 13179 CrossRef CAS PubMed; (b) R. J. Harris, J. Park, T. A. F. Nelson, N. Iqbal, D. C. Salgueiro, J. Bacsa, C. E. MacBeth, M.-H. Baik and S. B. Blakey, The Mechanism of Rhodium Catalyzed Allylic C–H Amination, J. Am. Chem. Soc., 2020, 142, 5842 CrossRef CAS PubMed; (c) W. Pin Teh, D. C. Obenschain, B. M. Black and F. E. Michael, Catalytic Metal-free Allylic C–H Amination of Terpenoids, J. Am. Chem. Soc., 2020, 142, 16716 CrossRef PubMed.
- (a) H. Qin, N. Yamagiwa, S. Matsunaga and M. Shibasaki, Bismuth- and Hafnium-Catalyzed Hydroamination of Vinyl Arenes with Sulfonamides, Carbamates, and Carboxamides, Chem.–Asian J., 2007, 2, 150 CrossRef CAS PubMed; (b) G. Richard, O. Justin, L. Joo Ho, K. C. Michael and J. Kyung Woon, Chemoselective hydroamination of vinyl arenes catalyzed by an NHC-amidate-alkoxide Pd (II) complex and p-TsOH, Tetrahedron Lett., 2013, 54, 4083 CrossRef PubMed; (c) Z. S. Qureshi, K. M. Deshmukh, P. J. Tambade, K. P. Dhake and B. M. Bhanage, Amberlyst-15 in Ionic Liquid: An Efficient and Recyclable Reagent for Nucleophilic Substitution of Alcohols and Hydroamination of Alkenes, Eur. J. Org Chem., 2010, 2010, 6233 CrossRef.
- (a) C. B. Roos, J. Demaerel, D. E. Graff and R. R. Knowles, Enantioselective Hydroamination of Alkenes with Sulfonamides Enabled by Proton-Coupled Electron Transfer, J. Am. Chem. Soc., 2020, 142, 5974 CrossRef CAS PubMed; (b) Y.-X. Ji, J. Li, C.-M. Li, S. Qu and B. Zhang, Manganese-Catalyzed N–F Bond Activation for Hydroamination and Carboamination of Alkenes, Org. Lett., 2021, 23, 207 CrossRef CAS PubMed; (c) B. G. Hejna, J. M. Ganley, H. Shao, H. Tian, J. D. Ellefsen, N. J. Fastuca, K. N. Houk, S. J. Miller and R. R. Knowles, Catalytic Asymmetric Hydrogen Atom Transfer: Enantioselective Hydroamination of Alkenes, J. Am. Chem. Soc., 2023, 145, 16118 CrossRef CAS PubMed.
- (a) Y. Wei, Y. Liang, R. Luo and L. Ouyang, Recent advances of Cp*Ir complexes for transfer hydrogenation: focus on formic acid/formate as hydrogen donors, Org. Biomol. Chem., 2023, 21, 7484 RSC; (b) L. Ouyang, R. Miao, Z. Yang and R. Luo, Iridium-catalyzed Reductive Amination of Carboxylic Acids, J. Catal., 2023, 418, 283 CrossRef CAS; (c) Y. Xia, S. Wang, R. Miao, J. Liao, L. Ouyang and R. Luo, Synthesis of N-alkoxy Amines and Hydroxylamines via the Iridium-catalyzed Transfer Hydrogenation of Oximes, Org. Biomol. Chem., 2022, 20, 6394 RSC; (d) L. Ouyang, Y. Xia, R. Miao, J. Liao and R. Luo, Iridium-catalyzed reductive etherification of α,β-unsaturated ketones and aldehydes with alcohols, Org. Biomol. Chem., 2022, 20, 2621 RSC; (e) N. Luo, Y. Zhong, H. Shui and R. Luo, pH-Mediated Selective Synthesis of N-Allylic Alkylation or N-Alkylation Amines with Allylic Alcohols via an Iridium Catalyst in Water, J. Org. Chem., 2021, 86, 15509 CrossRef CAS PubMed.
- L. Ouyang, Y. Liang, S. Wang, R. Miao, J. Liao, Z. Yang and R. Luo, Iridium-catalyzed reductive hydroamination of terminal alkynes in water, J. Catal., 2023, 427, 115096 CrossRef CAS.
- (a) R. Luo, Y. Liang, S. Wang, J. Liao and L. Ouyang, Iridium-catalyzed selective para-C-alkylation of anilines/phenols with aryl alkynes, J. Catal., 2023, 428, 115184 CrossRef CAS; (b) L. Ouyang, Y. Liang, S. Wang, J. Liao and R. Luo, Access of arylmethanes via iridium-catalyzed deoxygenative cross-coupling of aryl ketones with anilines/phenols, J. Catal., 2024, 433, 115492 CrossRef CAS.
- Z. Yang, Q. Zhu, R. Luo, Q. Xiang, J.-T. Liu, J.-K. Yang and W. Tang, Iridium-catalyzed highly efficient chemoselective reduction of aldehydes in water using formic acid as the hydrogen source, Green Chem., 2017, 19, 3296–3301 RSC.
- (a) D. Ormrod, K. Wellington and A. J. Wagstaff, Valdecoxib, Drugs, 2002, 62, 2059 CrossRef CAS PubMed; (b) Y. Bluhm, R. Raudazus, A. Wagner, N. Urban, M. Schaefer and K. Hill, Valdecoxib blocks rat TRPV2 channels, Eur. J. Pharmacol., 2022, 915, 174702 CrossRef CAS PubMed.
- (a) J. C. Martínez-Ávila, A. G. Bartolomé, I. García, I. Dapía, H. Y. Tong, L. Díaz, L. Puerra, J. Frías, A. J. Carcás Sansuan and A. M. Borobia, Pharmacometabolomics applied to zonisamide pharmacokinetic parameter prediction, Metabolomics, 2018, 14, 70 CrossRef PubMed; (b) C. Li, L. Xue, Y. Liu, Z. Yang, S. Chi and A. Xie, Zonisamide for the Treatment of Parkinson Disease: A Current Update, Front. Neurosci., 2020, 14, 574652 CrossRef PubMed.
- N. Luo, Y. Zhong, J.-T. Liu, L. Ouyang and R. Luo, An Efficient Hydration and Tandem Transfer Hydrogenation of Alkynes for the Synthesis of Alcohol in Water, Synthesis, 2020, 52, 3439 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07579j |
| This journal is © The Royal Society of Chemistry 2024 |