 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Self-assembled molecular hybrids comprising lacunary polyoxometalates and multidentate imidazole ligands†
Haoran
Sun
,
Atsuhiro
Jimbo
,
Chifeng
Li
,
Kentaro
Yonesato
 ,
Kazuya
Yamaguchi
,
Kazuya
Yamaguchi
 and
Kosuke
Suzuki
and
Kosuke
Suzuki
 *
*
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: ksuzuki@appchem.t.u-tokyo.ac.jp
First published on 16th May 2024
Abstract
Self-assembly via coordination bonding facilitates the creation of diverse inorganic–organic molecular hybrids with distinct structures and properties. Recent advances in this field have been driven by the versatility of organic ligands and inorganic units. Lacunary polyoxometalates are a class of well-defined metal-oxide clusters with a customizable number of reactive sites and bond directions, which make them promising inorganic units for self-assembled molecular hybrids. Herein, we report a novel synthesis method for self-assembled molecular hybrids utilizing the reversible coordination of multidentate imidazole ligands to the vacant sites of lacunary polyoxometalates. We synthesized self-assembled molecular hybrids including monomer, dimers, and tetramer, demonstrating the potential of our method for constructing intricate hybrids with tailored properties and functionalities.
Introduction
Self-assembly via coordination bonding is a powerful approach for constructing various inorganic–organic molecular hybrids with interesting applications such as catalysis, reactivity control, molecular recognition, and separation.1 Recent remarkable advances in this field have been enabled by the diversity and design possibilities of both organic ligands and inorganic units. Polyoxometalates (POMs) are a class of structurally well-defined inorganic metal-oxide clusters, where metals are typically in high oxidation states such as Mo6+, W6+, and V5+.2 Their structures and constituent elements can be tailored to facilitate the modification of their chemical and physical properties for various applications, such as in catalysis, photocatalysis, sensors, batteries, and energy conversion.2 Integrating organic ligands into POM systems enables further control over their stabilities, structures, and functionalities.3,4 In particular, lacunary POMs, where one or more [MOx] units are removed from the plenary structure, possess vacant reactive sites for metal atoms or organic ligands.2,5 Moreover, the number of reactive sites and bond directions can be tailored depending on the lacunary structure; therefore, these lacunary POMs are promising inorganic units for constructing coordination-driven inorganic–organic molecular hybrids. Various types of organic ligands such as phosphates, amines, silanols, and alcohols have been used for constructing POM–organic hybrids via coordination with the vacant sites of lacunary POMs3,6 or additionally incorporated metal sites at these vacant sites.7Recently, our research group reported that pyridine-based ligands are effective for constructing POM–organic hybrids via reversible coordination bonds between pyridine and the vacant sites of lacunary POMs.8 Three pyridine molecules can coordinate with the Mo atoms at the vacant sites of a trivacant lacunary Keggin-type phosphomolybdate (TBA3H6[A-α-PMo9O34], TBA = tetra-n-butylammonium) to form TBA3[A-α-PMo9O31(pyridine)3]. Moreover, we found that this pyridine-coordinated hybrid can be reacted with multidentate pyridine-based ligands in organic solvents to synthesize more complex molecular hybrids via ligand exchange reactions.8
We hypothesized that imidazole is a promising ligand to expand this approach because it has a stronger coordination ability with metal ions compared with pyridine and its five-membered ring structure offers coordination directions that differ from the six-membered ring structure of pyridine-based ligands.9 To date, various imidazole-based metal–organic structures such as cages and capsules have been synthesized using metal ions and imidazole ligands as building units.10 Although several hybrids of POMs and imidazole ligands synthesized via hydrogen bonding and electronic interaction as well as coordination to the substituted metals in POMs have been reported,11 a system directly coordinating imidazole to lacunary POMs has not yet been reported.
Herein, for the first time, we present a synthesis method for fabricating self-assembled molecular hybrids comprising lacunary POMs and multidentate imidazole ligands. By reacting a pyridine-coordinated trivacant lacunary phosphomolybdate TBA3[A-α-PMo9O31(pyridine)3] with four types of multidentate imidazole ligands in organic solvents, we successfully synthesized a series of self-assembled molecular POM–organic hybrids: a capped monomer I, pillared dimer II, sandwich-like dimer III, and tetrahedron tetramer IV through the direct coordination of imidazole ligands to lacunary POMs (Fig. 1).
 | ||
| Fig. 1 Self-assembly of molecular hybrids I, II, III, and IV using multidentate imidazole ligands and pyridine-coordinated trivacant lacunary phosphomolybdate. | ||
Results and discussion
To synthesize I, 1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene (L1), which possesses three imidazole groups on a benzene ring through flexible methylene groups, was used as a ligand and reacted with TBA3[A-α-PMo9O31(pyridine)3] in acetonitrile at 80 °C for 10 min (L1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBA3[A-α-PMo9O31(pyridine)3] = 2
TBA3[A-α-PMo9O31(pyridine)3] = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This was followed by recrystallization in a mixed solvent of 1,2-dichloroethane and diethyl ether, which yielded single crystals of I. X-ray crystallographic analysis revealed that the anion part of I adopted a capped monomer structure comprising one [α-PMo9] unit and one L1 ligand, wherein three imidazole groups of L1 coordinated to three Mo atoms at the vacant site of the [α-PMo9] unit (Fig. 2a, Table S1†). The electrospray ionization (ESI) mass spectrum of I in acetonitrile exhibited sets of signals at m/z 2437.0 and 2678.3, which were attributed to [TBA3H(PMo9O31)(L1)]+ and [TBA4(PMo9O31)(L1)]+, respectively (Fig. 2b). The 31P nuclear magnetic resonance (NMR) spectrum of I in acetonitrile-d3 (CD3CN) exhibited a signal at −2.06 ppm (Fig. S1a†), which was observed up-field compared with TBA3[A-α-PMo9O31(pyridine)3] (−0.68 ppm).8a The 1H NMR spectrum of I in CD3CN showed the splitting of the signals of the methylene H atoms in the ligand at 5.11 ppm (d, J = 13.5 Hz) and 4.88 ppm (d, J = 13.5 Hz), which can be ascribed to the geminal H–H spin–spin coupling on the same methylene C atom (Fig. S1b†). This observation of 1H NMR spectrum agrees with the rigid structure of I, where two H atoms in a methylene group exist in different chemical environments. We also confirmed that the 31P NMR spectrum remained almost unchanged after 3 days, demonstrating the stability of I in the solvent (Fig. S1c†). Based on these results as well as elemental analysis and thermogravimetry differential thermal analysis (TG-DTA) results (Fig. S2a†), the formula of I was determined to be TBA3[(PMo9O31)(L1)](C2H4Cl2)2.
1). This was followed by recrystallization in a mixed solvent of 1,2-dichloroethane and diethyl ether, which yielded single crystals of I. X-ray crystallographic analysis revealed that the anion part of I adopted a capped monomer structure comprising one [α-PMo9] unit and one L1 ligand, wherein three imidazole groups of L1 coordinated to three Mo atoms at the vacant site of the [α-PMo9] unit (Fig. 2a, Table S1†). The electrospray ionization (ESI) mass spectrum of I in acetonitrile exhibited sets of signals at m/z 2437.0 and 2678.3, which were attributed to [TBA3H(PMo9O31)(L1)]+ and [TBA4(PMo9O31)(L1)]+, respectively (Fig. 2b). The 31P nuclear magnetic resonance (NMR) spectrum of I in acetonitrile-d3 (CD3CN) exhibited a signal at −2.06 ppm (Fig. S1a†), which was observed up-field compared with TBA3[A-α-PMo9O31(pyridine)3] (−0.68 ppm).8a The 1H NMR spectrum of I in CD3CN showed the splitting of the signals of the methylene H atoms in the ligand at 5.11 ppm (d, J = 13.5 Hz) and 4.88 ppm (d, J = 13.5 Hz), which can be ascribed to the geminal H–H spin–spin coupling on the same methylene C atom (Fig. S1b†). This observation of 1H NMR spectrum agrees with the rigid structure of I, where two H atoms in a methylene group exist in different chemical environments. We also confirmed that the 31P NMR spectrum remained almost unchanged after 3 days, demonstrating the stability of I in the solvent (Fig. S1c†). Based on these results as well as elemental analysis and thermogravimetry differential thermal analysis (TG-DTA) results (Fig. S2a†), the formula of I was determined to be TBA3[(PMo9O31)(L1)](C2H4Cl2)2.
To construct oligomeric hybrid structures, we investigated the use of rigid multidentate ligands. TBA3[A-α-PMo9O31(pyridine)3] was reacted with 1,4-di(1H-imidazol-1-yl)benzene (L2), which is a linear ligand possessing two imidazole groups, in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in acetonitrile at 50 °C for 10 min. By recrystallization of the crude product in 1,2-dichloroethane, single crystals of II suitable for X-ray crystallographic analysis were obtained. The anion part of II had a pillared dimer structure where two [PMo9] units were bridged by three L2 ligands (Fig. 3a, Table S1†). Some of the [α-PMo9] units were transformed into [β-PMo9] units via rotation of the [Mo3O13] unit underneath by approximately 60° from the original orientation (Fig. S3†). X-ray crystallographic analysis results showed that the ratio of [α-PMo9] and [β-PMo9] was 1
1 ratio in acetonitrile at 50 °C for 10 min. By recrystallization of the crude product in 1,2-dichloroethane, single crystals of II suitable for X-ray crystallographic analysis were obtained. The anion part of II had a pillared dimer structure where two [PMo9] units were bridged by three L2 ligands (Fig. 3a, Table S1†). Some of the [α-PMo9] units were transformed into [β-PMo9] units via rotation of the [Mo3O13] unit underneath by approximately 60° from the original orientation (Fig. S3†). X-ray crystallographic analysis results showed that the ratio of [α-PMo9] and [β-PMo9] was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.07. The 31P NMR spectrum of II in CD3CN exhibited two signals at −1.34 and −0.01 ppm with a signal integral ratio of 1
0.07. The 31P NMR spectrum of II in CD3CN exhibited two signals at −1.34 and −0.01 ppm with a signal integral ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.09, which were attributed to the [α-PMo9] and [β-PMo9] isomers, respectively (Fig. S4†). The ESI mass spectrum of II in acetonitrile exhibited a set of signals at m/z = 2434.0, which was assignable to [TBA6H2(PMo9O31)2(L2)3]2+ (Fig. 3b). These NMR and ESI mass spectra showed that II maintained its structure in the solvent. The 31P NMR spectrum of II in CD3CN after 3 days showed only two signals at −1.34 and −0.01 ppm, but the integral ratio of these signals changed from 1
0.09, which were attributed to the [α-PMo9] and [β-PMo9] isomers, respectively (Fig. S4†). The ESI mass spectrum of II in acetonitrile exhibited a set of signals at m/z = 2434.0, which was assignable to [TBA6H2(PMo9O31)2(L2)3]2+ (Fig. 3b). These NMR and ESI mass spectra showed that II maintained its structure in the solvent. The 31P NMR spectrum of II in CD3CN after 3 days showed only two signals at −1.34 and −0.01 ppm, but the integral ratio of these signals changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.09 to 1
0.09 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.27 (Fig. S4c†). This result showed that although the ratio of [α-PMo9] and [β-PMo9] in II changes after several days, the structure of II is stable and kept in the solvent. Based on these results as well as the elemental analysis and TG-DTA results (Fig. S2b†), the formula of II was determined to be TBA5.7H0.3[(PMo9O31)2(L2)3](C2H4Cl2).
0.27 (Fig. S4c†). This result showed that although the ratio of [α-PMo9] and [β-PMo9] in II changes after several days, the structure of II is stable and kept in the solvent. Based on these results as well as the elemental analysis and TG-DTA results (Fig. S2b†), the formula of II was determined to be TBA5.7H0.3[(PMo9O31)2(L2)3](C2H4Cl2).
 | ||
| Fig. 3 (a) Crystal structure of the anion part of II. (b) ESI mass spectrum of II in acetonitrile. (Inset): enlarged spectrum and simulated pattern for m/z 2434.0 ([TBA6H2(PMo9O31)2(L2)3]2+). | ||
The structure of II is similar to that of our previously reported hybrid dimer comprising three 4,4′-bipyridine ligands (L5) and two [PMo9] units ([(A-α-PMo9O31)2(L5)3]6−) (Fig. 4).8a Changing the pyridine-based ligand to the imidazole-based ligand shortened the Mo–N bond lengths in II (2.26 Å on average, Mo–imidazole coordination bonds) compared with those in [(A-α-PMo9O31)2(L5)3]6− (2.32 Å on average, Mo–pyridine coordination bonds) (Fig. 4). Although only [α-PMo9] units were observed in [(A-α-PMo9O31)2(L5)3]6−, isomerized [β-PMo9] units were also observed in II. To investigate the formation of these imidazole- and pyridine-based hybrids as well as the isomerization of [α-PMo9] to [β-PMo9] units, we performed density functional theory calculations for these reactions. The standard Gibbs energy of the reaction 2[A-α-PMo9O31(pyridine)3]3− + 3L2 → [(A-α-PMo9O31)2(L2)3]6− (II) + 3pyridine was −69.2 kJ mol−1, while that for the reaction 2[A-α-PMo9O31(pyridine)3]3− + 3L5 → [(A-α-PMo9O31)2(L5)3]6− + 3pyridine was −23.2 kJ mol−1. These results indicate that the formation of II was considerably more favorable than that of [(A-α-PMo9O31)2(L5)3]6−, which is likely owing to the stronger coordination of imidazole ligands to Mo atoms when compared with pyridine ligands. In addition, we investigated the isomerization from [α-PMo9] to [β-PMo9] units in the aforementioned hybrids. The standard Gibbs energies for isomerization from [α-PMo9] to [β-PMo9] units in [(A-α-PMo9O31)2(L5)3]6− and II were 15.58 and 0.38 kJ mol−1, respectively. These results support the partial isomerization of [β-PMo9] in the imidazole-based II.
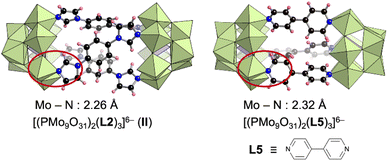 | ||
| Fig. 4 Structures of [(PMo9O31)2(L2)3]6− (II) and [(PMo9O31)2(L5)3]6− and their average Mo–N bond lengths.8a | ||
To construct a more complex structure, we next utilized 2,4,6-(1H-imidazol-yl)-1,3,5-triazine (L3), which possesses three imidazole groups at the 1,3,5-positions of the triazine ring, as a ligand. Reacting TBA3[A-α-PMo9O31(pyridine)3] and L3 in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in acetonitrile at room temperature (∼25 °C) for 30 min followed by pouring the solution into an excess amount of diethyl ether obtained III as a crude powder. Recrystallization of the crude product in 1,2-dichloroethane obtained single crystals of III suitable for X-ray crystallographic analysis, which revealed that the anion part of III comprised two [PMo9] units and three L3 ligands lying in parallel (Fig. 5a, Table S1†). For each L3 ligand, two of three imidazole units coordinated to Mo atoms, and the other imidazole unit remained unreacted. All [PMo9] units in the crystalline product of III were [β-PMo9] units. The 31P NMR spectrum of III in CD3CN exhibited a signal at 0.36 ppm, which was attributed to the [β-PMo9] isomer (Fig. S5a†). The ESI mass spectrum of III in acetonitrile exhibited a set of signals at m/z 2537.9, 2658.5, and 2779.1, which could be assigned to [TBA6H2(PMo9O31)2(L3)3)]2+, [TBA7H(PMo9O31)2(L3)3]2+, and [TBA8(PMo9O31)2(L3)3]2+, respectively (Fig. 5b). Based on these results as well as elemental analysis, TG-DTA, and 31P NMR results (Fig. S2c and S5a†), the formula of III was determined as TBA6[(PMo9O31)2(L3)3](C2H4Cl2)2.
1 ratio in acetonitrile at room temperature (∼25 °C) for 30 min followed by pouring the solution into an excess amount of diethyl ether obtained III as a crude powder. Recrystallization of the crude product in 1,2-dichloroethane obtained single crystals of III suitable for X-ray crystallographic analysis, which revealed that the anion part of III comprised two [PMo9] units and three L3 ligands lying in parallel (Fig. 5a, Table S1†). For each L3 ligand, two of three imidazole units coordinated to Mo atoms, and the other imidazole unit remained unreacted. All [PMo9] units in the crystalline product of III were [β-PMo9] units. The 31P NMR spectrum of III in CD3CN exhibited a signal at 0.36 ppm, which was attributed to the [β-PMo9] isomer (Fig. S5a†). The ESI mass spectrum of III in acetonitrile exhibited a set of signals at m/z 2537.9, 2658.5, and 2779.1, which could be assigned to [TBA6H2(PMo9O31)2(L3)3)]2+, [TBA7H(PMo9O31)2(L3)3]2+, and [TBA8(PMo9O31)2(L3)3]2+, respectively (Fig. 5b). Based on these results as well as elemental analysis, TG-DTA, and 31P NMR results (Fig. S2c and S5a†), the formula of III was determined as TBA6[(PMo9O31)2(L3)3](C2H4Cl2)2.
Despite three imidazole groups in L3, the obtained III was a dimer structure in which one of the three imidazole units remained uncoordinated in each ligand. The distance between the L3 ligand layers in III was approximately 3.3 Å and the centers of the parallel ligands were split by approximately 2.9 Å (Fig. 5a), indicating the existence of [π–σ]2 interactions between the three L3 ligands.12 To avoid such interactions, we next used 1,3,5-tri(1H-imidazol-1-yl)benzene (L4), which possesses a benzene ring instead of a triazine at the center, as a ligand. Reacting L4 with TBA3[PMo9O31(pyridine)3] in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in N,N-dimethylformamide at 50 °C afforded IV as a crude powder. Recrystallization of the crude product in a mixed solvent of N,N-dimethylacetamide and 1,4-dioxane afforded single crystals of IV. X-ray crystallographic analysis revealed that the anion part of IV was a tetrahedral tetramer cage structure where four [PMo9] units and four L4 ligands were placed at the vertices and faces, respectively (Fig. 6a, Table S1†). The ESI mass spectrum of IV in CH3CN exhibited signals at m/z 2641.1, 2701.5, and 3440.7, which could be assigned to [TBA16(PMo9O31)4(L4)4(H2O)]4+, [TBA17(PMo9O31)4(L4)4(OH)]4+, and [TBA15(PMo9O31)4(L4)4(H2O)]3+, respectively (Fig. 6b).
1 ratio in N,N-dimethylformamide at 50 °C afforded IV as a crude powder. Recrystallization of the crude product in a mixed solvent of N,N-dimethylacetamide and 1,4-dioxane afforded single crystals of IV. X-ray crystallographic analysis revealed that the anion part of IV was a tetrahedral tetramer cage structure where four [PMo9] units and four L4 ligands were placed at the vertices and faces, respectively (Fig. 6a, Table S1†). The ESI mass spectrum of IV in CH3CN exhibited signals at m/z 2641.1, 2701.5, and 3440.7, which could be assigned to [TBA16(PMo9O31)4(L4)4(H2O)]4+, [TBA17(PMo9O31)4(L4)4(OH)]4+, and [TBA15(PMo9O31)4(L4)4(H2O)]3+, respectively (Fig. 6b).
The tetrahedral structure of IV had an inner cavity, where the distance between the P atom of the [PMo9] unit and the center of the opposite ligand L4 was about 12.9 Å (Fig. S6†). Some of the [α-PMo9] units were transformed into [β-PMo9] units, and the X-ray crystallographic analysis was performed with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of these units. Based on the above results as well as elemental analysis, TG-DTA, and 31P NMR spectra (Figs. S2d and S5b†), the formula of IV was determined to be TBA12[(PMo9O31)4(L4)4](C4H9NO)4.
1 ratio of these units. Based on the above results as well as elemental analysis, TG-DTA, and 31P NMR spectra (Figs. S2d and S5b†), the formula of IV was determined to be TBA12[(PMo9O31)4(L4)4](C4H9NO)4.
Conclusions
We report the first method for synthesizing self-assembled molecular hybrid structures via direct coordination of imidazole ligands to lacunary POMs. In particular, using the pyridine-coordinated trivacant lacunary polyoxomolybdate TBA3[A-α-PMo9O31(pyridine)3] as a precursor, we successfully synthesized several hybrids: the capped monomer I, pillar dimer II, sandwich-like dimer III, and tetrahedron tetramer IV. Because this synthesis method is applicable to various types of lacunary POMs and imidazole ligands, we expect it to help increase the number of POM–organic hybrids as well as their potential applications, such as in catalysis, photocatalysis, energy conversion, and sensors.Data availability
The data supporting this manuscript is available in the ESI† and available on request.Author contributions
H. S., A. J., C. L. performed the synthesis and characterizations. H. S., A. J., C. L., K. Yo. performed the crystallographic analysis. A. J. and K. S. performed the DFT calculations. All authors analyzed and discussed the results. K. S. conceived and directed the project. H. S., K. Yo., K. Ya., K. S. co-wrote the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was supported in part by JST FOREST (JPMJFR213M), JSPS KAKENHI (22H04971), and the JSPS Core-to-Core program. Synchrotron X-ray diffraction measurements were conducted at SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (proposal numbers: 2023B1842, 2022B1605, 2022B1859). A part of computations was performed using Research Center for Computational Science, Okazaki, Japan (Project: 23-IMS-C106, 24-IMS-C101).Notes and references
- (a) S. R. Seidel and P. J. Stang, Acc. Chem. Res., 2002, 35, 972 CrossRef CAS PubMed; (b) K. Harris, D. Fujita and M. Fujita, Chem. Commun., 2013, 49, 6703 RSC; (c) S. H. A. M. Leenders, R. Gramage-Doria, B. de Bruin and J. N. H. Reek, Chem. Soc. Rev., 2015, 44, 433 RSC; (d) Y. Fang, J. A. Powell, E. Li, Q. Wang, Z. Perry, A. Kirchon, X. Yang, Z. Xiao, C. Zhu, L. Zhang, F. Huang and H.-C. Zhou, Chem. Soc. Rev., 2019, 48, 4707 RSC; (e) X.-Z. Li, C.-B. Tian and Q.-F. Sun, Chem. Rev., 2022, 122, 6374 CrossRef CAS PubMed; (f) H. Takezawa, K. Shitozawa and M. Fujita, Nat. Chem., 2020, 12, 574 CrossRef CAS PubMed; (g) P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, Science, 2009, 324, 1697 CrossRef CAS PubMed.
- (a) M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer, Berlin, 1983 CrossRef; (b) N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199 CrossRef CAS PubMed; (c) C. L. Hill, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier Pergamon, Amsterdam, 2003, vol. 4, p. 679 Search PubMed; (d) H. N. Miras, J. Yan, D.-L. Long and L. Cronin, Chem. Soc. Rev., 2012, 41, 7403 RSC; (e) S.-S. Wang and G.-Y. Yang, Chem. Rev., 2015, 115, 4893 CrossRef CAS PubMed; (f) N. I. Gumerova and A. Rompel, Nat. Rev. Chem, 2018, 2, 0112 CrossRef CAS; (g) K. Suzuki, N. Mizuno and K. Yamaguchi, ACS Catal., 2018, 8, 10809 CrossRef CAS; (h) A. Misra, K. Kozma, C. Streb and M. Nyman, Angew. Chem., Int. Ed., 2020, 59, 596 CrossRef CAS PubMed.
- (a) A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009 CrossRef CAS PubMed; (b) J. M. Cameron, G. Guillemot, T. Galambos, S. S. Amin, E. Hampson, K. M. Haidaraly, G. N. Newton and G. Izzet, Chem. Soc. Rev., 2022, 51, 293 RSC; (c) F. Li and L. Xu, Dalton Trans., 2011, 40, 4024 RSC; (d) A. V. Anyushin, A. Kondinski and T. N. Parac-Vogt, Chem. Soc. Rev., 2020, 49, 382 RSC; (e) A. Proust, B. Matt, R. Villanneau, G. Guillemot, P. Gouzerh and G. Izzet, Chem. Soc. Rev., 2012, 41, 7605 RSC.
- (a) J. M. Breen and W. Schmitt, Angew. Chem., Int. Ed., 2008, 47, 6904 CrossRef CAS PubMed; (b) M. B. Mahimaidoss, S. A. Krasnikov, L. Reck, C. I. Onet, J. M. Breen, N. Zhu, B. Marzec, I. V. Shvets and W. Schmitt, Chem. Commun., 2014, 2265 RSC; (c) Z. Zhang, L. Wojtas and M. J. Zaworotko, Chem. Sci., 2014, 927 RSC; (d) Y. Zhang, H. Gan, C. Qin, X. Wang, Z. Su and M. J. Zaworotko, J. Am. Chem. Soc., 2018, 17365 CrossRef CAS PubMed; (e) N. Xu, H. Gan, C. Qin, X. Wang and Z. Su, Angew. Chem., Int. Ed., 2019, 58, 4649 CrossRef CAS PubMed; (f) Y. Gong, C. Qin, Y. Zhang, C. Sun, Q. Pan, X. Wang and Z. Su, Angew. Chem., Int. Ed., 2020, 59, 22034 CrossRef CAS PubMed.
- (a) L.-L. Liu, L. Wang, X.-Y. Xiao, P. Yang, J. Zhao and U. Kortz, Coord. Chem. Rev., 2024, 506, 215687 CrossRef CAS; (b) O. Oms, A. Dolbecq and P. Mialane, Chem. Soc. Rev., 2012, 41, 7497 RSC; (c) K. Suzuki, N. Mizuno and K. Yamaguchi, J. Jpn. Petrol. Inst., 2020, 63, 258 CrossRef CAS.
- (a) G. S. Chorghade and M. T. Pope, J. Am. Chem. Soc., 1987, 109, 5134 CrossRef CAS; (b) C. R. Mayer and R. Thouvenot, J. Chem. Soc., Dalton Trans., 1998, 7 RSC; (c) S. Fujimoto, J. M. Cameron, R.-J. Wei, K. Kastner, D. Robinson, V. Sans, G. N. Newton and H. Oshio, Inorg. Chem., 2017, 56, 12169 CrossRef CAS PubMed; (d) T. Minato, K. Suzuki, K. Yamaguchi and N. Mizuno, Chem. - Eur. J., 2017, 23, 14213 CrossRef CAS PubMed; (e) A. Jimbo, C. Li, K. Yonesato, T. Ushiyama, K. Yamaguchi and K. Suzuki, Chem. Sci., 2023, 14, 10280 RSC.
- (a) S.-T. Zheng, J. Zhang, X.-X. Li, W.-H. Fang and G.-Y. Yang, J. Am. Chem. Soc., 2010, 132, 15102 CrossRef CAS PubMed; (b) Q. Chang, X. Meng, W. Ruan, Y. Feng, R. Li, J. Zhu, Y. Ding, H. Lv, W. Wang, G. Chen and X. Fang, Angew. Chem., Int. Ed., 2022, 61, e202117637 CrossRef CAS PubMed; (c) J. Guo, Q. Chang, Z. Liu, Y. Wang, C. Liu, M. Wang, D. Huang, G. Chen, H. Zhao, W. Wang and X. Fang, Chem. Sci., 2021, 12, 7361 RSC.
- (a) C. Li, N. Mizuno, K. Yamaguchi and K. Suzuki, J. Am. Chem. Soc., 2019, 141, 7687 CrossRef CAS PubMed; (b) C. Li, K. Yamaguchi and K. Suzuki, Angew. Chem., Int. Ed., 2021, 60, 6960 CrossRef CAS PubMed; (c) M. Yamaguchi, K. Shioya, C. Li, K. Yonesato, K. Murata, K. Ishii, K. Yamaguchi and K. Suzuki, J. Am. Chem. Soc., 2024, 146, 4549 CrossRef CAS PubMed.
- (a) A. Richaud, N. Barba-Behrens and F. Méndez, Org. Lett., 2011, 13, 972 CrossRef CAS PubMed; (b) R. J. Sundberg and R. B. Martin, Chem. Rev., 1974, 74, 471 CrossRef CAS.
- (a) X.-W. Zhu, D. Luo, X.-P. Zhou and D. Li, Coord. Chem. Rev., 2022, 455, 214354 CrossRef CAS; (b) P. Kukkar, K.-H. Kim, D. Kukkar and P. Singh, Coord. Chem. Rev., 2021, 446, 214109 CrossRef CAS; (c) S.-S. Chen, CrystEngComm, 2016, 18, 6543 RSC; (d) J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Chem. Rev., 2012, 112, 1001 CrossRef CAS PubMed; (e) J. R. Galán-Mascarós and K. R. Dunbar, Angew. Chem., Int. Ed., 2003, 42, 2289 CrossRef PubMed; (f) Q. Chen, Z. Chang, W.-C. Song, H. Song, H.-B. Song, T.-L. Hu and X.-H. Bu, Angew. Chem., Int. Ed., 2013, 52, 11550 CrossRef CAS PubMed.
- (a) H.-J. Pang, H.-Y. Ma, J. Peng, C.-J. Zhang, P.-P. Zhang and Z.-M. Su, CrystEngComm, 2011, 13, 7079 RSC; (b) H. Zhao, L. Tao, F. Zhang, Y. Zhang, Y. Lui, H. Xu, G. Diao and L. Ni, Chem. Commun., 2019, 55, 1096 RSC; (c) X.-J. Yang, M. Sun, H.-Y. Zang, Y.-Y. Ma, X.-J. Feng, H.-Q. Tan, Y.-H. Wang and Y.-G. Li, Chem.–Asian J., 2016, 11, 858 CrossRef CAS PubMed; (d) J. Du, J. Yu, J. Tang, J. Wang, W. Zhang, W. R. Thiel and M. Jia, Eur. J. Inorg. Chem., 2011, 15, 2361 CrossRef.
- T. J. Mooibroek and P. Gamez, Inorg. Chim. Acta, 2007, 360, 381 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, Fig. S1–S7, Table S1. CCDC 2345494–2345497. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc02384f |
| This journal is © The Royal Society of Chemistry 2024 |



