Open Access Article
Open Access ArticleThe preparation of titanium adipate and optimization of its catalytic ester exchange reaction for the synthesis of diisooctyl adipate
Linlin Zhao,
Guoliang Shen *,
Tiejun Xu,
Ruiyang Wen and
Sijin Jiang
*,
Tiejun Xu,
Ruiyang Wen and
Sijin Jiang
School of Petrochemical Engineering, Shenyang University of Technology, Liaoyang 111003, China. E-mail: 13869119262@163.com; petrochem@126.com; xtjltlt@126.com; Wenruiyang6@163.com; 2093894641@qq.com
First published on 4th March 2025
Abstract
Chelated titanium adipate with stable properties was prepared via an ester-exchange reaction using low-carbon alkyl titanate, and it was characterized and analyzed using infrared spectroscopy, thermogravimetry, and scanning electron microscopy. Diisooctyl adipate (DOA) was synthesized via an ester-exchange method using the synthesized novel titanium adipate as a catalyst and isooctanol and dimethyl adipate as raw materials, where dimethyl adipate is the methyl esterification product of the by-product (mixed dicarboxylic acid) in industrial adipic acid production. The process is easy to carry out, green and environmentally friendly, and the methanol by-product can be recycled and utilized. With the objective of optimizing the DOA ester exchange rate, the effects of three factors, namely, catalyst dosage, the molar ratio of isooctanol to dimethyl adipate and the reaction temperature at catalyst addition, on the optimization objective were systematically investigated via one-way and response surface tests. Analysis of variance (ANOVA) and parameter optimization were conducted using Design-Expert software to obtain the optimal parameter combinations and verify the accuracy of the experimental results. The results showed that the optimal reaction conditions were as follows: a catalyst dosage of 2.39%, an isooctanol to dimethyl adipate molar ratio of 2.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a reaction temperature of 117 °C at the time of catalyst addition; this resulted in a high ester exchange rate of 94.23%. Due to the high catalytic efficiency, environmental friendliness and energy saving, and recyclable catalyst, this study provides a feasible process for the effective synthesis of DOA using industrial by-products, which is of significance.
1, and a reaction temperature of 117 °C at the time of catalyst addition; this resulted in a high ester exchange rate of 94.23%. Due to the high catalytic efficiency, environmental friendliness and energy saving, and recyclable catalyst, this study provides a feasible process for the effective synthesis of DOA using industrial by-products, which is of significance.
1 Introduction
Diisooctyl adipate (DOA) is an excellent cold-resistant plasticizer.1 It can significantly improve the flexibility of polymers, does not easily change color, and has good water resistance and impact resistance, alongside other characteristics.2–4 It is widely used in the processing of polyvinyl chloride, vinyl chloride copolymers, polystyrene and other plastics.5 It is also used as a cold-resistant plasticizer in synthetic rubber, paints and coatings, and as an additive in lubricating oil. In modern industry, the synthesis of DOA is mainly conducted via the direct esterification of adipic acid and isooctanol under the action of a catalyst; a large amount of water will be produced in the reaction process, which requires the addition of a water-carrying agent to remove the water.6 It is necessary to add a water-carrying agent to remove the water to improve the reaction efficiency, however, the water produced as a by-product cannot be reused and can only be discharged as wastewater after proper treatment. Concentrated sulfuric acid, p-toluenesulfonic acid, solid acids and other acidic catalysts are widely used in industry. Among them, sulfuric acid, despite its good catalytic effects, strongly corrodes equipment, generates more side reactions, has complicated post-treatment steps, shows low esterification rates during direct esterification reactions, and makes the recovery and utilization of catalysts difficult, causing serious pollution to the environment.7–12 If it enters water sources, it will also threaten the aquatic ecosystem and human health.13–15In the large-scale production of adipic acid, a large amount of mixed dibasic acid by-product is inevitably generated during the industrial production process.16,17 This mixed dibasic acid has a complex composition, and mainly contains adipic acid, glutaric acid, and succinic acid.18,19 So far, it is impossible to separate these directly and effectively via physical separation methods.20 To overcome this problem, researchers have explored an effective solution, methyl esterification distillation, which can easily separate mixed diacids from the mixture as dimethyl esters. Dimethyl adipate is often used as a high-boiling solvent.21,22 The added value of product utilization is low.
Alkyl titanates are metal titanium organic compounds, which are green and environmentally friendly, with high catalytic activity for esterification and transesterification reactions; however, alkyl titanates are particularly easy to hydrolyze and not easy to separate from reactants. Chelated titanates have significant advantages, such as good stability, not being easy to hydrolyze, needing only mild catalytic reaction conditions, and high selectivity, while they are also non-toxic and harmless, showing great potential for application in organic synthesis, the chemical industry and other fields.
In this study, the chelated titanium adipate catalyst was first prepared from adipic acid and low-carbon alkyl titanates, and the titanium adipate salt was solid. Then, the chelated titanium adipate was used to catalyze the direct transesterification reaction between dimethyl adipate and isooctanol for the synthesis of DOA, and the optimal reaction conditions for the synthesis of DOA were determined using a combination of one-factor and response surface methods. The study not only meets the requirements of green catalysis, easy separation, and recyclability but it also significantly improves the utilization value of dimethyl adipate. Meanwhile, it provides new ideas for related DOA preparation studies and helps to solve existing technical challenges.
2 Materials and methods
2.1 Materials and instruments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×. The mass spectrometry (MS) analyzer used was a 6540 UHD Q-TOF, Agilent Technologies, USA; the test conditions were as follows: number of injections – 1, injection volume – 20 μL, running time – 1 min.
000×. The mass spectrometry (MS) analyzer used was a 6540 UHD Q-TOF, Agilent Technologies, USA; the test conditions were as follows: number of injections – 1, injection volume – 20 μL, running time – 1 min.2.2 Preparation of titanium adipate
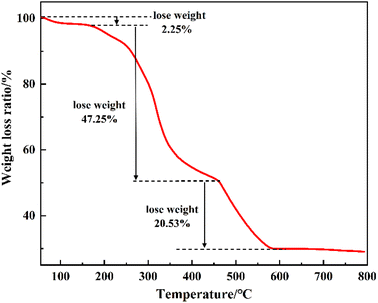
7.0 g of adipic acid and 8.0 g of high-temperature solvent were weighed and added into a 150 mL three-necked flask. After stirring and warming up to complete dissolution, 6.8 g of titanate was added dropwise under nitrogen protection and warming was continued; the color of the reaction solution gradually changed from transparent to milky white to a rice-white turbid slurry. The by-product alcohol was evaporated under reduced pressure until no distillate flowed out and the reaction was stopped after 2 h. The reaction product was cooled to room temperature, and the reaction then stopped. The reaction product was transferred to a beaker, washed with anhydrous ethanol three times, and filtered to remove raw materials and solvent that had not completely reacted. The filter cake was dried in a vacuum drier at 90 °C for 3 h to produce a highly active hydrolysis-resistant chelated titanium adipate solid catalyst, as shown in Fig. 1 and 2.2.3 Synthesis of DOA
In a reaction flask with a stirring device, 5.0 g of dimethyl adipate and 9.3 g of isooctanol were added sequentially under stirring. After heating, when it reached a certain temperature, 2% titanium adipate catalyst (in terms of the total mass of dimethyl adipate + isooctanol) was added, and the temperature of the reaction solution was further elevated until methanol flowed out; the reaction system was then maintained at this temperature for a certain period. When there was no further obvious outflow of methanol, the temperature of the reaction liquid was gradually increased to remove all methanol and stop the reaction. The by-product methanol was recovered and stored for later use in the methyl esterification of mixed dicarboxylic acid to produce the raw material dimethyl adipate. Subsequently, the reaction solution was transferred to a beaker, and the titanium adipate catalyst in the system was separated via hot filtration. The catalyst can be recycled after washing and drying. Then the filtrate was distilled under reduced pressure to evaporate unreacted raw materials and by-products, and finally pure DOA was obtained, as shown in Fig. 3.In this case, the ester exchange ratio (Y) is calculated as follows (eqn (1)):
 | (1) |
3 Results and discussion
3.1 Catalyst characterization analysis
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and –C–O bending vibration in –COO–, respectively. The absence of a large broad peak from –OH near 3000 cm−1 indicates that the hydroxyl group in –COOH forms coordination bonds with Ti ions. The absorption vibration peaks of Ti–O at 598.50 cm−1 and 540.29 cm−1 further confirm the successful coordination of titanium with oxygen. In addition, vibrational peaks from –CH2 at 2930.59 cm−1 and 2852.95 cm−1 were observed. As a result, the product can be initially identified as a titanium adipate catalyst.
O stretching vibration and –C–O bending vibration in –COO–, respectively. The absence of a large broad peak from –OH near 3000 cm−1 indicates that the hydroxyl group in –COOH forms coordination bonds with Ti ions. The absorption vibration peaks of Ti–O at 598.50 cm−1 and 540.29 cm−1 further confirm the successful coordination of titanium with oxygen. In addition, vibrational peaks from –CH2 at 2930.59 cm−1 and 2852.95 cm−1 were observed. As a result, the product can be initially identified as a titanium adipate catalyst.
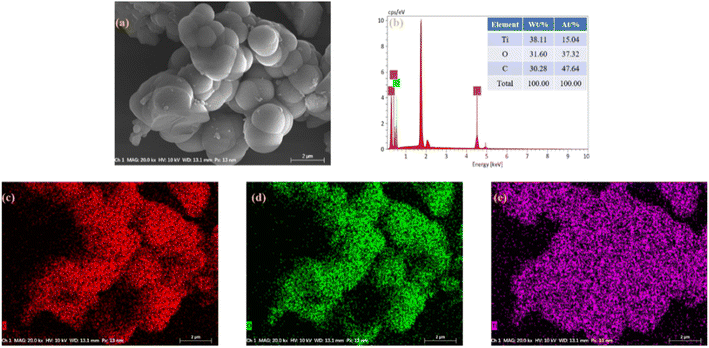
The morphology and elemental composition of titanium adipate recovered after use were analyzed, and it can be seen from Fig. 7(a) that the morphology of the recovered titanium adipate changed little; it was still made up of spherical-like particles. According to the EDS analysis shown in Fig. 7(b–e), the Ti, O and C elements were evenly distributed on the surface of the catalyst, while the Ti and C elemental mass fractions decreased.
3.2 Characterization and analysis of diisooctyl adipate
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O condensation vibrational absorption peak,23 and those at 2957.41 cm−1 and 2929.10 cm−1 are attributed to the asymmetric stretching vibrational absorption peaks of –CH3 and –CH2, respectively.24 The peaks at 2873.23 cm−1 and 2859.45 cm−1 are attributed to –CH3 and –CH2 -symmetric stretching vibrational absorption peaks, respectively. The peak at 1459.88 cm−1 is attributed to the C–H bond deformation vibration absorption peaks.23 No –OH absorption peaks or absorption peaks from other substances appeared, indicating that there are no raw materials, water or other substances in the product; it can be initially deduced that the obtained product is high-purity DOA.
O condensation vibrational absorption peak,23 and those at 2957.41 cm−1 and 2929.10 cm−1 are attributed to the asymmetric stretching vibrational absorption peaks of –CH3 and –CH2, respectively.24 The peaks at 2873.23 cm−1 and 2859.45 cm−1 are attributed to –CH3 and –CH2 -symmetric stretching vibrational absorption peaks, respectively. The peak at 1459.88 cm−1 is attributed to the C–H bond deformation vibration absorption peaks.23 No –OH absorption peaks or absorption peaks from other substances appeared, indicating that there are no raw materials, water or other substances in the product; it can be initially deduced that the obtained product is high-purity DOA.
3.3 Effects of factors on the esterification rate
In general, the factors influencing a transesterification reaction include the reactant molar ratio, catalyst dosage, reaction temperature, temperature upon catalyst addition, and reaction time. In this experiment, the controllable main influencing factors are the reactant molar ratio, catalyst dosage, and temperature upon catalyst addition. In this regard, the effects of catalyst dosage (1.0%, 1.5%, 2.0%, 2.5%, and 3.0%), reactant molar ratio (2.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.25
1, 2.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.50
1, 2.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.75
1, 2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3.00
1, and 3.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and reaction temperature at the time of catalyst incorporation (80 °C, 90 °C, 100 °C, 110 °C, and 120 °C) on the transesterification rate were investigated in this study. The results are shown in Fig. 11.
1), and reaction temperature at the time of catalyst incorporation (80 °C, 90 °C, 100 °C, 110 °C, and 120 °C) on the transesterification rate were investigated in this study. The results are shown in Fig. 11.
It can be seen from Fig. 11 that the ester exchange rate initially increased with an increase in catalyst dosage, indicating that the titanium adipate catalyst has a good catalytic effect on this reaction. When the catalyst dosage was increased to 2.0%, the number of side reactions increased, resulting in a decrease in the ester exchange rate. The effect of the molar ratio of reactants on the transesterification reaction showed a similar trend; within a certain range, increasing the dosage of isooctanol was favorable to the reaction, and when n(isooctanol)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(dimethyl adipate) = 2.50
n(dimethyl adipate) = 2.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the transesterification rate reached 93.77%. Upon continuing to increase the dosage of isooctanol, the concentration of the reaction system decreased, which led to a gradual decrease in the yield. When the catalyst addition temperature was low, the activation energy of the reactant molecules was low, and the catalytic effect of the catalyst was not obvious. As the temperature increases, the activation energy of the reactant molecules increases, and the catalyst can reduce the activation energy of the reaction more effectively. However, when the addition temperature is too high, the catalyst may undergo physical or chemical changes, resulting in a decrease in its activity.
1, the transesterification rate reached 93.77%. Upon continuing to increase the dosage of isooctanol, the concentration of the reaction system decreased, which led to a gradual decrease in the yield. When the catalyst addition temperature was low, the activation energy of the reactant molecules was low, and the catalytic effect of the catalyst was not obvious. As the temperature increases, the activation energy of the reactant molecules increases, and the catalyst can reduce the activation energy of the reaction more effectively. However, when the addition temperature is too high, the catalyst may undergo physical or chemical changes, resulting in a decrease in its activity.
At a catalyst dosage of 2%, a reactant molar ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a catalyst addition temperature of 110 °C, the ester exchange rate reached relatively high values; this provided coded-level central values for the establishment of the response surface below.
1, and a catalyst addition temperature of 110 °C, the ester exchange rate reached relatively high values; this provided coded-level central values for the establishment of the response surface below.
3.4 Response surface optimization
| Independent variable | Unit | Coded levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A: reaction temperature when the catalyst is added | °C | 100 | 110 | 120 |
| B: catalyst amount | % | 1.5 | 2.0 | 2.5 |
| C: raw material alcohol:ester molar ratio | mol mol−1 | 2.25 | 2.50 | 2.75 |
| Number | A | B | C | Ester exchange ratio/% |
|---|---|---|---|---|
| 1 | 100 | 2.0 | 2.75 | 93.02 |
| 2 | 110 | 2.0 | 2.50 | 93.35 |
| 3 | 120 | 2.0 | 2.25 | 90.12 |
| 4 | 110 | 2.5 | 2.75 | 94.16 |
| 5 | 110 | 1.5 | 2.25 | 80.84 |
| 6 | 120 | 2.5 | 2.50 | 94.78 |
| 7 | 120 | 1.5 | 2.50 | 89.78 |
| 8 | 110 | 1.5 | 2.75 | 89.95 |
| 9 | 100 | 2.0 | 2.25 | 83.93 |
| 10 | 100 | 1.5 | 2.50 | 84.49 |
| 11 | 100 | 2.5 | 2.50 | 92.35 |
| 12 | 110 | 2.0 | 2.50 | 92.96 |
| 13 | 110 | 2.5 | 2.25 | 92.89 |
| 14 | 110 | 2.0 | 2.50 | 92.57 |
| 15 | 120 | 2.0 | 2.75 | 94.58 |
| Y (ester exchange ratio) = 92.96 + 1.93A + 3.64B + 2.99C − 0.715AB − 1.16AC − 1.96BC − 0.8287A2 − 1.78B2 − 1.72C2 | (2) |
It can be seen that eqn (2) has great value and can be optimized for the parameters involved. To further examine the significance of the model, the simulated regression equation was subjected to ANOVA and significance test, and the results are shown in Table 3.
| Source | Sum of squares | df | Mean square | F-value | P-value | Significance |
|---|---|---|---|---|---|---|
| a 0.01 < P < 0.05 – significant; P < 0.01 – very significant. | ||||||
| Model | 252.52 | 9 | 28.06 | 46.68 | 0.0003 | Significant |
| A | 29.92 | 1 | 29.92 | 49.77 | 0.0009 | |
| B | 106.00 | 1 | 106.00 | 176.34 | < 0.0001 | |
| C | 71.58 | 1 | 71.58 | 119.09 | 0.0001 | |
| AB | 2.04 | 1 | 2.04 | 3.40 | 0.1244 | |
| AC | 5.36 | 1 | 5.36 | 8.92 | 0.0306 | |
| BC | 15.37 | 1 | 15.37 | 25.56 | 0.0039 | |
| A2 | 2.54 | 1 | 2.54 | 4.22 | 0.0952 | |
| B2 | 11.72 | 1 | 11.72 | 19.49 | 0.0069 | |
| C2 | 10.91 | 1 | 10.91 | 18.15 | 0.0080 | |
| Residual | 3.01 | 5 | 0.6011 | |||
| Lack of fit | 2.70 | 3 | 0.9004 | 5.92 | 0.1479 | Not significant |
| Pure error | 0.3042 | 2 | 0.1521 | |||
| Cor total | 255.53 | 14 | ||||
| R2 | 0.9882 | C.V.% | 0.8553 | |||
| Adjusted R2 | 0.9671 | Adeq. precision | 22.6531 | |||
As shown in Table 3, the model P-value of 0.0003 is less than 0.01, indicating that the model as a whole has good significance and regressivity. By analyzing the F-values, it can be determined that the three factors affect the model in the following order of importance: the catalyst dosage is the most significant, followed by the molar ratio of the reactants, and lastly, the reaction temperature at the time of catalyst addition. The lack-of-fit term of the model has a P-value of 0.1479, which is greater than 0.05, indicating that the lack-of-fit of the response values is not significant and that the model suitably describes the relationship between the independent variables and the response value. In addition, the correlation coefficient of the model, R2, is 0.9882, the adjusted coefficient of determination, RAdj2, is 0.9671, and the coefficient of variation, C.V., is 0.8553%, which is less than 10%, indicating that it has a sufficiently strong signal and the model is ideal.
Combining Fig. 12(a1) and (a2), it can be seen that the transesterification rate is less than 80% when the catalyst dosage is low and the addition temperature is low, but with an increase in dosage and temperature, the yield can be increased to more than 95%. Combining Fig. 12(b1) and (b2), it can be seen that the transesterification rate becomes higher and higher with the elevation of the alcohol: ester molar ratio and the catalyst addition temperature. Combining Fig. 12(c1) and (c2), it can be seen that with an increase in the alcohol: ester molar ratio and catalyst dosage, the ester exchange rate also showed an increasing trend, and the effects of the three factors on the ester exchange rate were positively correlated. Among them, the interaction of the alcohol:ester molar ratio and catalyst dosage had the greatest effect on the ester exchange rate, and the interaction of the alcohol:ester molar ratio and catalyst addition temperature had the second greatest effect on the ester exchange rate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; the model response value under these conditions was 95.3345%. For the convenience of practical operation, the optimal process conditions were modified: the catalyst dosage was 2.39%, the catalyst temperature was 117 °C, and the reactant molar ratio was 2.55
1; the model response value under these conditions was 95.3345%. For the convenience of practical operation, the optimal process conditions were modified: the catalyst dosage was 2.39%, the catalyst temperature was 117 °C, and the reactant molar ratio was 2.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Under these conditions, three sets of repeat validation tests were conducted; the average ester exchange rate obtained in the tests was 94.23%, and the relative error of the predicted value was −1.1045%, which indicated that the results of this model were reliable and had excellent predictive ability.28,35
1. Under these conditions, three sets of repeat validation tests were conducted; the average ester exchange rate obtained in the tests was 94.23%, and the relative error of the predicted value was −1.1045%, which indicated that the results of this model were reliable and had excellent predictive ability.28,35
3.5 Catalyst reuse effects
The reaction was carried out under the optimal conditions. At the end of the reaction, the catalyst was filtered and separated, washed and dried with anhydrous ethanol, and used repeatedly to study the service life of the catalyst. The results are shown in Fig. 14.It is obvious from Fig. 14 that the ester exchange rate decreased when the catalyst was reused, but the decrease was not obvious, and the ester exchange rate was still above 85% after four cycles of recycling. Therefore, the catalyst prepared in this study has high activity and stability and can be reused.
4 Conclusions
Novel titanium adipate catalysts, which are non-toxic and harmless, with high catalytic activity and thermal stability, were prepared via an exchange reaction using titanate and adipic acid as raw materials. The prepared titanium adipate catalysts had a regular spherical structure with uniform size.Titanium adipate was used to catalyze the ester exchange reaction for the synthesis of diisooctyl adipate, and the effects of factors such as the reaction temperature, catalyst dosage, and alcohol to ester molar ratio at the time of catalyst addition on the ester exchange reaction were investigated. Based on one-way experiments, response surface methodology was used to optimize the reaction conditions for the synthesis of DOA, and the optimal process conditions were obtained as follows: a catalyst dosage of 2.39%, a catalyst addition temperature of 117 °C, and a molar ratio of the reactants isooctanol to dimethyl adipate of 2.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Validation experiments were carried out several times under these experimental conditions, and the average yield of DOA obtained was 94.23%, which was similar to that predicted by the model. As the titanium adipate catalyst is solid, it is easy to separate from the product and can be reused many times without impurities in the product. The method creates little environmental pollution, uses mild reaction conditions, is simple to operate, can effectively reduce production costs, and meets the requirements of green chemistry; thus, it has good prospects for industrial application.
1. Validation experiments were carried out several times under these experimental conditions, and the average yield of DOA obtained was 94.23%, which was similar to that predicted by the model. As the titanium adipate catalyst is solid, it is easy to separate from the product and can be reused many times without impurities in the product. The method creates little environmental pollution, uses mild reaction conditions, is simple to operate, can effectively reduce production costs, and meets the requirements of green chemistry; thus, it has good prospects for industrial application.
Data availability
All data supporting the findings of this study are presented in the article.Author contributions
Guoliang Shen and Tiejun Xu conceived, planned and supervised the experiment. Linlin Zhao and Ruiyang Wen were responsible for the main part of the experiment, including the preparation of catalysts and the synthesis of DOA. Ruiyang Wen and Sijin Jiang were responsible for the characterization and analysis of the catalyst. Linlin Zhao and Sijing Jiang wrote the first draft of the manuscript, and all the authors reviewed the manuscript.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
Thanks to all the authors for their efforts in completing this manuscript.Notes and references
- W. Fletcher, A. Gent and R. Wood, Rubber Chem. Technol., 1957, 30, 652–666 CrossRef.
- H.-J. Kim, Y. Kwon and C. K. Kim, J. Nanosci. Nanotechnol., 2013, 13, 577–581 CrossRef CAS PubMed.
- X. Li, X. Nie, J. Chen and Y. Wang, Polym. Int., 2017, 66, 443–449 CrossRef CAS.
- V. Najafi and H. Abdollahi, Eur. Polym. J., 2020, 128, 109620 CrossRef CAS.
- Z. Y. Guo, P. P. Gai, J. Duan, J. X. Zhai, S. S. Zhao, S. Wang and D. Y. Wei, Biomed. Chromatogr., 2010, 24, 1094–1099 CrossRef CAS PubMed.
- A. Sahu and A. B. Pandit, Ind. Eng. Chem. Res., 2019, 58, 2672–2682 CrossRef CAS.
- A. H. M. Fauzi and N. A. S. Amin, Energy Convers. Manage., 2013, 76, 818–827 CrossRef.
- N. Muhammad, Y. A. Elsheikh, M. I. A. Mutalib, A. A. Bazmi, R. A. Khan, H. Khan, S. Rafiq and Z. Man, J. Ind. Eng. Chem., 2015, 21, 1–10 CrossRef CAS.
- P. Fan, S. Xing, J. Wang, J. Fu, L. Yang, G. Yang, C. Miao and P. Lv, Fuel, 2017, 188, 483–488 CrossRef CAS.
- Y. Feng, T. Qiu, J. Yang, L. Li, X. Wang and H. Wang, Chin. J. Chem. Eng., 2017, 25, 1222–1229 CrossRef CAS.
- A. P. da Luz Corrêa, R. R. C. Bastos, G. N. da Rocha Filho, J. R. Zamian and L. R. V. da Conceição, RSC Adv., 2020, 10, 20245–20256 RSC.
- D. Procopio and M. L. Di Gioia, Catalysts, 2022, 12, 50 CrossRef CAS.
- X. Xu, S. Zhang, Y. Wang, N. Wang, Q. Jiang, X. Liu, Q. Guan and W. Zhang, Appl. Catal., B, 2024, 345, 123701 CrossRef CAS.
- N. Wang, Z. Zhang, Y. Zhang, X. Xu and Q. Guan, Sep. Purif. Technol., 2025, 355, 129566 CrossRef CAS.
- Z. Shan, Y. Yang, H. Shi, J. Zhu, X. Tan, Y. Luan, Z. Jiang, P. Wang and J. Qin, Front. Chem., 2021, 9, 755836 CrossRef CAS PubMed.
- P. Vogtel and G. Steinhoff, Process for the recovery of adipic acid, US Pat., 5264624, 1993 Search PubMed.
- J. Lu, H. Chen, Y. Huang, L. Zhang, Z. Zhao, W. Du and X. Wang, Fluid Phase Equilib., 2019, 485, 1–15 CrossRef CAS.
- B. C. Sutradhar, Crystallization of adipic acid from its solution in aqueous nitric acid, US Pat., 6946572, 2005 Search PubMed.
- F. Wang, Y. Li, Z. Ning, C. Jiang and X. Wang, J. Chem. Eng. Data, 2016, 61, 3059–3068 CrossRef CAS.
- R. Li, L. Lin, W. Feng, J. Xu, C. Du and H. Zhao, J. Chem. Thermodyn., 2017, 107, 8–17 CrossRef CAS.
- A. M. Silva, M. A. Morales, E. M. Baggio-Saitovitch, E. Jordao and M. A. Fraga, Appl. Catal., A, 2009, 353, 101–106 CrossRef CAS.
- A. Gorak and A. Stankiewicz, Annu. Rev. Chem. Biomol. Eng., 2011, 2, 431–451 CrossRef CAS PubMed.
- Y. Sun, Y. Yan, X. Zheng, J. Han, B. Wang, Q. Wu and G. Bai, Mol. Catal., 2024, 553, 113732 CrossRef CAS.
- Z. Fu, K. Shi, F. Ma, R.-m. Song, L. Chen, J.-s. Dai and W.-q. Shen, Int. J. Pavement Eng., 2022, 23, 2644–2653 CrossRef CAS.
- A. K. Domingos, E. B. Saad, H. M. Wilhelm and L. P. Ramos, Bioresour. Technol., 2008, 99, 1837–1845 CrossRef CAS PubMed.
- Y. Kim, M. Kim, J. Hwang, E. Im and G. D. Moon, Polymers, 2022, 14, 656 CrossRef CAS PubMed.
- R. Wen, G. Shen, J. Zhai, L. Meng and Y. Bai, New J. Chem., 2023, 47, 14646–14655 RSC.
- R. Wen, G. Shen, M. Zhang, Y. Yu and S. Xu, New J. Chem., 2024, 48, 17254–17260 RSC.
- N. Altunay, Y. Unal and A. Elik, Food Addit. Contam.,: Part A, 2020, 37, 869–881 CrossRef CAS PubMed.
- C. Fanali, V. Gallo, S. Della Posta, L. Dugo, L. Mazzeo, M. Cocchi, V. Piemonte and L. De Gara, Molecules, 2021, 26, 2652 CrossRef CAS PubMed.
- P. Yu, Q. Li, Y. Feng, S. Ma, Y. Chen and G. Li, Molecules, 2021, 26, 1310 CrossRef CAS PubMed.
- Y.-T. Ao, Y.-C. Chen and W.-H. Ding, J. Hazard. Mater., 2021, 401, 123383 CrossRef CAS PubMed.
- C. Xing, W.-Q. Cui, Y. Zhang, X.-S. Zou, J.-Y. Hao, S.-D. Zheng, T.-T. Wang, X.-Z. Wang, T. Wu and Y.-Y. Liu, Ultrason. Sonochem., 2022, 83, 105946 CrossRef CAS PubMed.
- M. Shahedi, Z. Habibi, M. Yousefi, J. Brask and M. Mohammadi, Int. J. Biol. Macromol., 2021, 170, 490–502 CrossRef CAS PubMed.
- P. Chen, X. Liu, L. Wang, C. Wang and J. Fu, J. Sep. Sci., 2021, 44, 585–599 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |