 Open Access Article
Open Access ArticleA practical one-pot synthesis of dehydroalanine esters†
Qingyuan Shi‡
a,
Mengxiao Zhu‡a,
Zhenchu Liang‡a,
Xu Donga,
Minyue Zhanga,
Lili Yanga,
Pingping Suna,
Chuanyi Xinga,
Xiaohao Zhaa,
Fei Li*abc,
Hongliang He*d and
Dongyin Chen *abce
*abce
aMedical Basic Research Innovation Center for Cardiovascular and Cerebrovascular Diseases, Ministry of Education, International Joint Laboratory for Drug Target of Critical Illnesses, School of Pharmacy, Nanjing Medical University, Nanjing 211166, China. E-mail: chendongyin@njmu.edu.cn; kldlf@njmu.edu.cn
bInnovation Center of Suzhou Nanjing Medical University, Suzhou 215000, China
cNational Center of Technology Innovation for Biopharmaceuticals, Suzhou 215000, China
dDepartment of Pharmacy, Sir Run Run Hospital, Nanjing Medical University, Nanjing 211100, China. E-mail: hcm1004@163.com
eThe Affiliated Huaian No.1 People's Hospital of Nanjing Medical University, Northern Jiangsu Institute of Clinical Medicine, Huaian 223300, China
First published on 27th March 2025
Abstract
A practical one-pot synthesis of various dehydroalanine esters was realized via a Cs2CO3-mediated simultaneous esterification/elimination process, starting from commercially available N-protected serines and various haloalkanes. This protocol provided easy access to structurally diverse dehydroalanine-based building blocks, which were successfully applied to construct more complex dehydroalanine derived molecules.
Introduction
Dehydroalanine is an important structural motif that broadly occurs in many complex natural products,1 multifunctional probes,2 modified peptides and proteins.3 Especially, as a highly active α, β-unsaturated amino acid residue, it always appears in some natural polycyclic peptide toxins, such as microcystins1b and thiostrepton.1c Due to their good stability and reactivity under physiological conditions, dehydroalanine and its derivatives are highly efficient Michael acceptors, which have strong chemical utilities for the synthesis of modified peptides and proteins.3 For example, the dehydroalanine unit incorporated in proteins, acting as a “chemical tag”, can undergo conjugate addition reactions with versatile nucleophiles, which mimics posttranslational modification of proteins through a variety of β,γ-bond formation (C–S, C–N, C–Se, et al.).3a This dehydroalanine-modification strategy has also been applied to design novel dehydroalanine-based activity probes for biological research and drug development.2 In addition, dehydroalanine derivatives are the key building blocks that are widely utilized to synthesize various β-substituted α-amino acids via metal-catalyzed cross-coupling processes,4 Michael-type additions,5 catalytic tandem transformations,6 etc.In view of their wide applications in molecular biology and organic synthesis, much effort has been devoted to develop simple and efficient synthetic methods for dehydroalanine derivatives.7,8 Generally, serine and cysteine are the controllable dehydroalanine precursors,7 which first transformed into leaving groups, followed by elimination to yield dehydroalanine (Fig. 1). Unlike this typical method, Rivera and coworkers reported a two-step approach for the synthesis of dehydroalanine derivatives via a consecutive Ugi-4CR/elimination reaction.8 Herein, we described an efficient one-pot synthesis of dehydroalanine esters, starting from commercially available N-protected serines and various haloalkanes by a Cs2CO3-mediated simultaneous esterification/elimination process (Fig. 1). This protocol provided easy access to structurally diverse dehydroalanine esters, that has been successfully utilized to synthesize more complex dehydroalanine derived molecules.
Results and discussion
Under alkaline conditions, the elimination of β-hydroxyl groups to form α,β-unsaturated carbonyl compounds9 and the esterification of carboxylic acid with a haloalkane10 are general reactions in organic synthesis. Based on these two conventional reactions, commercially available N-phthaloylserine (1a) and 2-bromopropane were employed as model substrates to explore one-pot synthesis of dehydroalanine ester 2a. The results are presented in Table 1. The efficiency of various inorganic carbonates was first investigated for this reaction. Li2CO3, Na2CO3 and K2CO3 were inefficient for the generation of 2a, only affording N-phthaloylserine isopropyl ester 3a in moderate yields (52–79%) with DMF as solvent at 60 °C (entries 1–3). Due to an increase in the alkalinity, Rb2CO3 and Cs2CO3 utilized as base provided the desired N-phthaloyldehydroalanine isopropyl ester (2a) in 18% and 58% yields, respectively (entries 4 and 5). However, very large amounts of 3a were inevitably produced.| Entry | Base | Solvent | 4 Å zeolite | T (oC) | Time (h) | Yield of 2a (%)b | Yield of 3a (%)b |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 2-bromopropane (1.5 mmol), base (1.5 mmol), 4 Å zeolite (90 mg), solvent (5 mL).b Isolated yield.c 2-bromopropane: 2.0 mmol.d Cs2CO3: 1.0 mmol.e Cs2CO3: 2.0 mmol. | |||||||
| 1 | Li2CO3 | DMF | ☒ | 60 | 12 | 0 | 52 |
| 2 | Na2CO3 | DMF | ☒ | 60 | 12 | 0 | 60 |
| 3 | K2CO3 | DMF | ☒ | 60 | 12 | 0 | 79 |
| 4 | Rb2CO3 | DMF | ☒ | 60 | 12 | 18 | 65 |
| 5 | Cs2CO3 | DMF | ☒ | 60 | 12 | 58 | 38 |
| 6 | Cs2CO3 | DMF | ☒ | 40 | 12 | 25 | 40 |
| 7 | Cs2CO3 | DMF | ☒ | 80 | 12 | 46 | 40 |
| 8 | Cs2CO3 | DMF | ☑ | 60 | 12 | 70, 65 c | 24, 30 c |
| 9 | Cs2CO3 | DMF | ☑ | 60 | 24 | 68 | 20 |
| 10 | Cs2CO3 d | DMF | ☑ | 60 | 12 | 22 | 70 |
| 11 | Cs2CO3 e | DMF | ☑ | 60 | 12 | 35 | 9 |
| 12 | Cs2CO3 | THF | ☑ | 60 | 24 | 0 | 10 |
| 13 | Cs2CO3 | DMSO | ☑ | 60 | 12 | 10 | 20 |
| 14 | Cs2CO3 | NMP | ☑ | 60 | 12 | 62 | 29 |
| 15 | Cs2CO3 | DMAC | ☑ | 60 | 12 | 63 | 30 |
Next, we found that the reaction temperature had an important effect on the formation of 2a. The low reaction temperature was not conducive to the elimination of β-hydroxyl group of 1a (entry 6), and the high reaction temperature might lead to further ester hydrolysis of 2a (entry 7). It was sufficient for this reaction to proceed smoothly at 60 °C. Surprisingly, owing to its good water absorption characteristics, 4 Å zeolite was beneficial to improve the yield of 2a (68–70%), and 12 h was the appropriate reaction time (entries 8 and 9). In addition, increasing the amount of 2-bromopropane didn't improve the yield of 2a (entry 8).
Subsequently, increasing/reducing the amount of Cs2CO3 had no benefits for this reaction (entries 10 and 11), and provided 2a in low yields (22–35%). In addition, different solvents were evaluated aiming at increasing the yield of 2a (entries 12–15), but only N-methyl-2-pyrrolidone (NMP) and N,N-dimethylacetamide (DMAC) gave 2a in moderate yields (62–63%). Finally, the optimized reaction conditions were determined that 1a (1.0 equiv.) and 2-bromopropane (1.5 equiv.) were smoothly converted into dehydroalanine ester 2a in 70% yield with Cs2CO3 (1.5 equiv.) as base and DMF as solvent at 60 °C (entry 8).
With the optimal reaction conditions in hand, we further investigated the generality of Cs2CO3-mediated simultaneous esterification/elimination of various N-protected serine 1 with 2-bromopropane, to prepare N-substituted dehydroalanine isopropyl ester 2. As shown in Table 2, 1b–1e containing alkoxycarbonyl protecting group (R1 = Ethoxycarbonyl, Alloc, Boc and Teoc) provided the desired dehydroalanine esters 2b–2e in moderate to good yields (51–69%), with N-protected serine isopropyl esters 3b–3e in 19–35% yields. By contrast, N-Cbz serine 1f gave the desired dehydroalanine ester 2f in lower yield (25%), mainly affording the corresponding product 3f. When N-acetylserine 1h used as substrate, the corresponding dehydroalanine ester 2h was obtained in 52% yield. However, due to its instability under alkaline conditions, N-Fmoc serine 1g didn't yield the desired product under the standard conditions. In addition, some other types of N-protected serines 1i–1k (R1 = Tos, Bn and PMB) mainly produced N-protected serine isopropyl esters 3i–3k in 15–45% yields, without the generation of dehydroalanine esters. These results indicate that the carbonyl group on nitrogen is the key factor for Cs2CO3-mediated simultaneous esterification/elimination of 1 with haloalkanes.
| Entry | Substrate | R1 | Yield of 2 (%)b | Yield of 3 (%)b |
|---|---|---|---|---|
| a Reaction conditions: 1 (1.0 mmol), 2-bromopropane (1.5 mmol), Cs2CO3 (1.5 mmol) and 4 Å zeolite (90 mg) in DMF (5 mL) at 60 °C for 12 h.b Isolated yield. | ||||
| 1 | 1b |  |
59 | 30 |
| 2 | 1c | 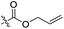 |
61 | 27 |
| 3 | 1d |  |
69 | 19 |
| 4 | 1e | 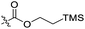 |
51 | 35 |
| 5 | 1f |  |
25 | 50 |
| 6 | 1g |  |
0 | 0 |
| 7 | 1h |  |
52 | 33 |
| 8 | 1i |  |
0 | 15 |
| 9 | 1j |  |
0 | 41 |
| 10 | 1k |  |
0 | 45 |
To further expand the structural diversity of dehydroalanine esters, N-Boc serine 1d and N-Boc-N-methyl serine 1l were selected to react with various haloalkanes. As depicted in Table 3, N-Boc serine 1d reacted with a series of primary haloalkanes under the standard conditions, to give the corresponding dehydroalanine esters 4a–4j in 24–68% yields. Unsatisfactorily, the greater steric hindrance of some haloalkanes containing cycloalkane and aromatic rings resulted in a significant decrease in the yields of target compounds. Similarly, the secondary haloalkane diphenylbromomethane as the substrate afforded dehydroalanine ester 4k in lower yield (19%). Interestingly, N-Boc-N-methyl serine 1l reacted well with less sterically hindered primary haloalkanes, to provide the corresponding dehydroalanine esters 4l–4o in good yields (60–71%). This result gives us more opportunities to construct more complex dehydroalanine derivatives.
| a Reaction conditions: 1 (1.0 mmol), haloalkane (1.5 mmol), Cs2CO3 (1.5 mmol) and 4 Å zeolite (90 mg) in DMF (5 mL) at 60 °C for 12 h. |
|---|
 |
In view of dehydroalanine derivatives having great potential for application in molecular biology and organic synthesis, we performed 100 gram-scale preparation of N-Boc dehydroalanine isopropyl ester 2d (63% yield), which was further employed as a key building block for the synthesis of highly functionalized molecules 5–8 in 46–83% yields (Fig. 2). 5a and 5b are the key medicine intermediates for the preparation of calcitonin gene-related peptide receptor antagonists.11 6a and 6b can serve as the precursors to synthesize a variety of unnatural amino acids.12 7a and 7b have the potential for application in molecular imaging13 and target identification.14 In addition, 8a–8d as the building blocks derived from 2d can be successfully prepared using conventional chemical methods such as Michael-type addition, halogenation and reduction reactions.
Conclusions
In summary, we establish a practical one-pot synthesis of structurally diverse dehydroalanine esters via a Cs2CO3-mediated simultaneous esterification/elimination process, starting from commercially available N-protected serines and various haloalkanes. Compared to traditional synthesis methods, this approach has many advantages, including simplified operation, reagent economy, large-scale preparation and wide application scenarios. Additionally, the newly synthesized dehydroalanine esters can serve as the key building blocks to prepare a series of highly functionalized molecules. Further applications of these dehydroalanine derived functionalized molecules are ongoing in our laboratory.Data availability
The authors confirm that the data supporting the findings of this study are all available within the article.Author contributions
D. C., H. H. and F. L. conceived the idea and designed the research. Q. S., M. Z., Z. L. and X. D. performed the research. M. Z., L. Y. and P. S. analyzed the data. D. C. and Q. S. wrote the original manuscript. C. X. and X. Z. reviewed the manuscript and suggested improvements. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82171224).Notes and references
- (a) I. Panina, A. Taldaev, R. Efremov and A. Chugunov, Micromachines, 2021, 12, 1169 Search PubMed; (b) N. Bouaïcha, C. O. Miles, D. G. Beach, Z. Labidi, A. Djabri, N. Y. Benayache and T. Nguyen-Quang, Toxins, 2019, 11, 714 Search PubMed; (c) R. J. Scamp, E. deRamon, E. K. Paulson, S. J. Miller and J. A. Ellman, Angew Chem. Int. Ed. Engl., 2020, 59, 890 CAS.
- (a) N. Haj-Yahya, H. P. Hemantha, R. Meledin, S. Bondalapati, M. Seenaiah and A. Brik, Org. Lett., 2014, 16, 540 CAS; (b) C. Li, T. Wang, L. Liang, G. Chu, J. Zhang, W. He, L. Liu and J. Li, Sci. China Chem., 2023, 66, 837 CAS.
- (a) J. Dadová, S. R. Galan and B. G. Davis, Curr. Opin. Chem. Biol., 2018, 46, 71 Search PubMed; (b) C. Bottecchia and T. Noël, Chem.–Eur. J., 2019, 25, 26 CrossRef CAS PubMed.
- A. D. de Bruijn and G. Roelfes, Chem.–Eur. J., 2018, 24, 12728 CrossRef CAS PubMed.
- R. Petracca, K. A. Bowen, L. McSweeney, S. O'Flaherty, V. Genna, B. Twamley, M. Devocelle and E. M. Scanlan, Org. Lett., 2019, 21, 3281 CrossRef CAS PubMed.
- L. Navarre, R. Martinez, J. P. Genet and S. Darses, J. Am. Chem. Soc., 2008, 130, 6159 CrossRef CAS PubMed.
- (a) R. Ramesh, K. De and S. Chandrasekaran, Tetrahedron, 2007, 63, 10534 CrossRef CAS; (b) Y.-Q. Yang, M.-C. Ji, Z. Lu, M. Jiang, W.-W. Huang and X.-Z. He, Synth. Commun., 2016, 46, 977 CrossRef CAS; (c) R. A. Croft, J. J. Mousseau, C. Choi and J. A. Bull, Chem.–Eur. J., 2018, 24, 818 CrossRef CAS PubMed; (d) J. A. Shin, J. Kim, H. Lee, S. Ha and H. Y. Lee, J. Org. Chem., 2019, 84, 4558 CrossRef CAS PubMed.
- K. Perez-Labrada, E. Florez-Lopez, E. Paz-Morales, L. D. Miranda and D. G. Rivera, Tetrahedron Lett., 2011, 52, 1635 CrossRef CAS.
- F. E. Medina, R. P. P. Neves, M. J. Ramos and P. A. Fernandes, ACS Catal., 2018, 8, 10267 CrossRef CAS.
- Z. Khan, F. Javed, Z. Shamair, A. Hafeez, T. Fazal, A. Aslam, W. B. Zimmerman and F. Rehman, J. Ind. Eng. Chem., 2021, 103, 80 CrossRef CAS.
- N. Yasuda, E. Cleator, B. Kosjek, J. Yin, B. Xiang, F. Chen, S. C. Kuo, K. Belyk, P. R. Mullens, A. Goodyear, J. S. Edwards, B. Bishop, S. Ceglia, J. Belardi, L. Tan, Z. J. Song, L. DiMichele, R. Reamer, F. L. Cabirol, W. L. Tang and G. Liu, Org. Process Res. Dev., 2017, 21, 1851 CrossRef CAS.
- (a) J. A. C. Delgado, J. T. M. Correia, E. F. Pissinati and M. W. Paixão, Org. Lett., 2021, 23, 5251 CrossRef CAS PubMed; (b) R. K. M. Khan, Y. Zhao, T. D. Scully and S. L. Buchwald, Chem.–Eur. J., 2018, 24, 15215 CAS; (c) S. Jiang, P. Li, C. C. Lai, J. A. Kelley and P. P. Roller, J. Org. Chem., 2006, 71, 7307 CAS.
- I. Benavides, E. D. Raftery, A. G. Bell, D. Evans, W. A. Scott, K. N. Houk and T. J. Deming, J. Am. Chem. Soc., 2022, 144, 4214 CAS.
- E. Oueis, C. Adamson, G. Mann, H. Ludewig, P. Redpath, M. Migaud, N. J. Westwood and J. H. Naismith, ChemBioChem, 2015, 16, 2646 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00035a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |




