 Open Access Article
Open Access ArticleCatalytic reduction of nitrophenols and dyes by HKUST-1/hydrogel composite
Atipong Nachaichot a,
Orrawin Kenviseda,
Sirikanlaya Chorama,
Supinya Nijpanich
a,
Orrawin Kenviseda,
Sirikanlaya Chorama,
Supinya Nijpanich b and
Surangkhana Budsombat
b and
Surangkhana Budsombat *a
*a
aDepartment of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand. E-mail: surama@kku.ac.th; Fax: +66 432 02373; Tel: +66 432 02222-12243
bSynchrotron Light Research Institute (Public Organization), Nakhonratchasima 30000, Thailand
First published on 4th March 2025
Abstract
The effective removal of nitrophenols from wastewater is crucial due to their high carcinogenic risk. This research presents the development of a copper-based metal–organic framework (HKUST-1) integrated into a chitosan-graft-poly(acrylic acid) hydrogel. The hydrogel composite was evaluated as a catalyst for reducing nitrophenols and dyes using sodium borohydride (NaBH4) as a reducing agent. Various conditions were investigated for the reduction of 4-nitrophenol (4-NP) to the less harmful 4-aminophenol (4-AP), including catalyst dosage, NaBH4 concentration, initial 4-NP concentration, temperature, and pH. The catalyst was able to completely reduce 4-NP within 25 minutes at a dosage of 3 g L−1 and a NaBH4 concentration of 300 mM. The reduction rate increased with higher temperatures, with an Arrhenius activation energy of 54.4 kJ mol−1. Common anions found in surface water, such as Cl−, NO3−, SO42−, and HCO3−, had a slight impact on the reduction rate of 4-NP. When tested in real water environments, the reduction rate decreased, but complete conversion was still achieved. Additionally, the composite successfully reduced 100% of 2-nitrophenol, 100% of methyl orange, and 69% of Congo red. Overall, the hydrogel composite has shown significant potential as a catalyst for reducing various organic pollutants with high efficiency and easy separation through filtration.
1. Introduction
Due to the expansion of urbanization and industrialization, hazardous substances have been released into water environments. Nitrophenols, which are used in pesticides, explosives, pharmaceuticals, and dyes,1 are categorized as toxic pollutants by the United States Environmental Protection Agency (US EPA).2 The discharge of nitrophenols can be harmful to human health due to their high carcinogenic potential.3 As a result, developing effective methods for removing nitrophenols has become a significant area of interest. Additionally, the reduction of nitrophenols yields valuable aminophenols, which are useful in the pharmaceutical industry.Several approaches, including photodegradation and Fenton oxidation, have been employed to remove nitrophenols.4 Recently, 4-nitrophenol (4-NP) has been successfully reduced to 4-aminophenol (4-AP) using metal catalysts such as silver, gold, nickel, platinum, cobalt, and copper in combination with sodium borohydride (NaBH4) as a reducing agent.5–8 These metal catalysts facilitate the electron transfer from borohydride (BH4−) as an electron donor to nitrophenol as an electron acceptor.9 Copper (Cu) is considered a promising catalyst for the reduction of 4-NP due to its low cost, lower toxicity, and effectiveness.10,11 However, the use of homogeneous catalysts is limited because of the leaching of metal ions.
HKUST-1 is a copper-based metal–organic framework (MOF) composed of benzene-1,3,5-tricarboxylic acid (BTC) and dimeric cupric tetracarboxylate units. It was first synthesized at the Hong Kong University of Science and Technology.12 HKUST-1 has been utilized as a catalyst in chemical processes such as esterification, hydrogenation, and reduction. Additionally, it has proven to be an effective adsorbent for 4-NP with high water stability.13 In previous studies, HKUST-1 was embedded in functionalized SBA-15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 and mesoporous silica nanospheres,15 resulting in composite materials that exhibited high catalytic activity for the reduction of 4-NP.
14 and mesoporous silica nanospheres,15 resulting in composite materials that exhibited high catalytic activity for the reduction of 4-NP.
In this work, chitosan-graft-poly(acrylic acid) (CS-graft-PAA) hydrogel was synthesized and used as an adsorbent for Cu2+ ions due to its high adsorption capacity attributed to its multifunctional groups.16 To stabilize the Cu2+ ions adsorbed in the hydrogel, the Cu2+ ions were converted into HKUST-1. The HKUST-1/hydrogel composite was then used as a catalyst for reducing 4-NP. The three-dimensional network of the hydrogel was designed to provide excellent dispersion of the catalyst, thereby enhancing its catalytic performance. Moreover, the heterogeneous catalyst can be easily recovered through filtration. Various reduction parameters were investigated, including catalyst dosage, NaBH4 concentration, initial concentration of 4-NP, temperature, and solution pH. The performance of the catalyst was evaluated in the presence of common anions and in real water environments. Additionally, the catalyst was tested for the reduction reaction of 2-nitrophenol (2-NP) and two dye models—methyl orange and Congo red. This study represents the first example of the in situ synthesis of HKUST-1 within a Cu2+-containing hydrogel and demonstrates the circular reutilization of secondary waste for catalytic reduction.
2. Experimental
2.1 Chemicals
4-NP (98%) was sourced from Acros Organics (Belgium). 2-NP (99%), chitosan (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–190
000–190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da), and acrylic acid (AA, 99%) were purchased from Sigma-Aldrich (USA). BTC (98%) was obtained from TCI (Japan). Copper(II) nitrate trihydrate (Cu(NO3)2·3H2O, 99.5%), NaBH4 (95%), and Congo red were supplied by Loba Chemie (India). Methyl orange was acquired from Carlo Erba Reagents (Thailand). Potassium persulfate (99%) was purchased from VWR (Thailand). N,N′-Methylenebis(acrylamide) (MBA, 98%) was acquired from Fluka (USA).
000 Da), and acrylic acid (AA, 99%) were purchased from Sigma-Aldrich (USA). BTC (98%) was obtained from TCI (Japan). Copper(II) nitrate trihydrate (Cu(NO3)2·3H2O, 99.5%), NaBH4 (95%), and Congo red were supplied by Loba Chemie (India). Methyl orange was acquired from Carlo Erba Reagents (Thailand). Potassium persulfate (99%) was purchased from VWR (Thailand). N,N′-Methylenebis(acrylamide) (MBA, 98%) was acquired from Fluka (USA).
2.2 Preparation of HKUST-1 composite
The CS-graft-PAA hydrogel was synthesized following previously reported procedures.16 Initially, partial neutralization of 10.00 g of AA was conducted using a sodium hydroxide solution. Then, 0.45 g of chitosan, 0.02 g of MBA, and 0.04 g of potassium persulfate were added to the solution. The mixture was stirred and purged with nitrogen. Polymerization took place at 50 °C for 2.5 hours. Afterward, the hydrogel was cut into small pieces, thoroughly washed with water to remove residual chemicals, and then dried in an oven.HKUST-1 was synthesized in situ within the hydrogel as follows: first, 3.77 g of Cu(NO3)2·3H2O was dissolved in 151 mL of deionized water. The hydrogel (9.97 g) was immersed in the Cu2+ solution for 5 hours. The hydrogel was taken out and immersed in a 200 mL solution of BTC for 12 hours. The molar ratio of BTC to Cu(NO3)2·3H2O was 1.2. Finally, the hydrogel was removed from the BTC solution and washed with water. The resulting composite was then dried in an oven.
2.3 Catalytic reduction experiment
A typical catalytic reduction experiment for 4-NP was conducted as follows: a composite catalyst (1–3 g L−1) was added to a solution of 4-NP (10–20 mM). The mixture was stirred for 30 minutes to reach adsorption–desorption equilibrium. The reduction reaction was initiated by adding a 300 mM NaBH4 solution. At specific time intervals, samples were withdrawn from the solution, diluted with deionized water, and then analyzed using ultraviolet-visible (UV-vis) spectroscopy. The conversion of 4-NP to 4-AP was calculated using the absorbances at times 0 (A0) and t (At) using eqn (1).
 | (1) |
Additionally, the catalytic performance of the catalyst was evaluated in the presence of 5 mM common anions and in actual water environments. The pond water used in the experiments was collected from a wastewater treatment pond at Khon Kaen University. The reduction of other organic pollutants was monitored at their respective maximum wavelengths.
2.4 Characterizations
The surface morphology of the HKUST-1 composite was examined using a field emission scanning electron microscope equipped with an energy-dispersive X-ray spectroscope (FESEM-EDS, Helios NanoLab G3 CX, FEI, Australia). The functional groups present in the CS-graft-PAA and HKUST-1 composite were analyzed using a Bruker attenuated total reflection Fourier transform infrared spectrometer (ATR-FTIR, Tensor 27, Germany). Thermal analysis was performed under a nitrogen atmosphere using a thermogravimetric analyzer (TGA, STA7200, Hitachi, Japan) with a heating rate of 10 °C per minute. An X-ray diffractometer (XRD, EMPYREAN, PANalytical, United Kingdom) was used to examine the crystallinity of the samples. A UV-vis spectrophotometer (Agilent 8453, USA) was used to analyze the concentration of 4-NP and other pollutants. Liquid chromatography-mass spectrometry (LC-MS, Waters, Xevo TQ-XS, USA) was performed for mass analysis. The method utilized a gradient of methanol and a 2.5 mM ammonium acetate solution, with a flow rate of 0.3 mL per minute. The electronic properties of the HKUST-1 composite were studied using an X-ray photoelectron spectroscope (XPS, PHI5000 VersaProbe II, ULVAC-PHI, Japan).3. Results and discussions
3.1 Preparation of HKUST-1 composite
The CS-graft-PAA hydrogel was first synthesized via free radical grafting of AA onto chitosan, using MBA as a crosslinking agent. Subsequently, an in situ synthesis of HKUST-1 was conducted within the hydrogel. The chemical structures of the CS-graft-PAA hydrogel and HKUST-1 are shown in Scheme 1(a) and (b), respectively. Moreover, the schematic representation of the in situ synthesis of HHKUST-1 in the hydrogel is provided in Scheme 1(c). As a result, the yellow color of the hydrogel changed to blue. Both the pristine hydrogel and the HKUST-1 composite were characterized using ATR-FTIR, FESEM with EDX, XPS, and TGA.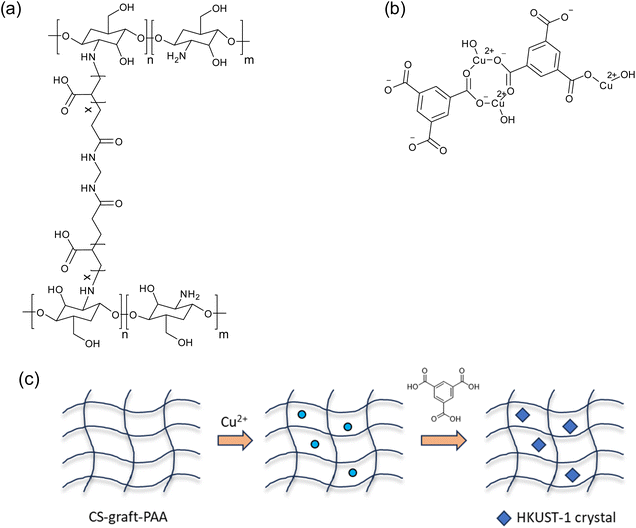 | ||
| Scheme 1 Chemical structures of CS-graft-PAA hydrogel (a) and HKUST-1 (b). (c) Schematic representation of the in situ synthesis of HHKUST-1 in the hydrogel. | ||
Fig. 1 illustrates the FTIR spectra of the pristine hydrogel and the composite. The pristine hydrogel displays characteristic bands for N–H bending and in-plane CH2 bending or scissoring of MBA at 1554 cm−1 and 1403 cm−1, respectively.17 Additionally, the O–H bending of a primary alcoholic group in chitosan is evident at 1057 cm−1, along with the –CH2 stretching observed at 2936 cm−1.18 For the composite, bands corresponding to the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and COO− of BTC in HKUST-1 are located at 1622 cm−1 and 1707 cm−1, respectively.13
C and COO− of BTC in HKUST-1 are located at 1622 cm−1 and 1707 cm−1, respectively.13
Fig. 2(a)–(d) present FESEM images with elemental mappings and compositions of the pristine hydrogel and the HKUST-1 composite. The non-uniform size and irregular shape of HKUST-1 possibly result from competitive covalent bonding between Cu(NO3)2 and CS-graft-PAA and BTC.19 The elemental mapping indicates that carbon, oxygen, and Cu are dispersed throughout the composite surface, with the weight percentage of Cu determined to be 62.0%.
 | ||
Fig. 2 FESEM images at (a) 5000× and (b) 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnifications. (c) Elemental mappings and (d) compositions of CS-graft-PAA hydrogel and HKUST-1 composite (80 000× magnifications. (c) Elemental mappings and (d) compositions of CS-graft-PAA hydrogel and HKUST-1 composite (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×). 000×). | ||
The survey XPS spectra of the pristine hydrogel and the HKUST-1 composite are presented in Fig. 3(a). For the pristine hydrogel, the C 1s peak and the O 1s peak, originating from chitosan and PAA, appear at 284.3 eV and 532.4 eV, respectively.16 The Cu 2p spectrum confirms the presence of HKUST-1 in the composite. As illustrated in Fig. 3(b), the 953.0 eV and 933.2 eV peaks corresponded to the Cu 2p1/2 and Cu 2p3/2 levels, respectively. Each peak has been deconvoluted into two forms of copper ions: monovalent (Cu+) and divalent (Cu2+).20 These results indicate the existence of both Cu2+/Cu2+ paddle-wheel arrangements and Cu+/Cu2+ paddle-wheel defects.21 Additionally, a small Cu2+ shake-up satellite peak is observed at 944.1 eV.22
 | ||
| Fig. 3 (a) Survey XPS spectra of CS-graft-PAA hydrogel and HKUST-1 composite and (b) Cu 2p spectra of HKUST-1 composite. | ||
TGA thermograms of the pristine hydrogel and the composite are shown in Fig. 4(a). Below 120 °C, both samples exhibit slight weight loss due to the loss of absorbed water. The pristine hydrogel displays a complex thermogravimetric profile, with a primary weight loss occurring around 450 °C, attributed to the decomposition of poly(acrylic acid) within the hydrogel.16 The HKUST-1 composite shows thermal stability up to approximately 200 °C, followed by a sharp weight loss at 300 °C, which is attributed to the transformation of Cu3(BTC)2 to CuO.23 The content of HKUST-1 in the hydrogel was calculated based on the difference in residual weights, yielding a value of 11.2%.
XRD was used to examine the crystallinity of the pristine hydrogel and the HKUST-1 composite. As shown in Fig. 4(b), the XRD pattern of the pristine hydrogel was broad between 15–45°, which was due to a random crosslinking network.24 No sharp characteristic peaks of HKUST-1 at 16.1°, 16.9°, 18.8°, 19.5°, 21.3°, 22.8°, 25.2°, 26.1°, 30.3°, and 33.8° were observed for the composite.25 This could be attributed to the relatively low HKUST-1 content and the peak overlap with those of the pristine hydrogel.
3.2 Reduction of 4-NP
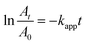 | (2) |
 | ||
| Fig. 7 (a) Effect of NaBH4 concentration and (b) rate constants for 4-nitrophenol reduction. Condition: [cat.] = 3 g L−1; [4-NP] = 15 mM. | ||
As illustrated in Fig. 7(b), the kapp value increased with rising NaBH4 concentrations and eventually leveled off at 300 mM. This indicates that a NaBH4 concentration of 300 mM is sufficient for the reduction of 4-NP and was therefore used in subsequent investigations.
| H2O ↔ H+ + OH− | (3) |
| H+ + BH4− + 3H2O ↔ H3BO3 + 2H2 | (4) |
The hydrolysis of borohydride ions occurs more slowly than the hydrolysis of water molecules, making it the rate-determining step in the reaction. At lower pH values, protons in solution readily bond with the hydrogen atoms in borohydride ions, resulting in a faster hydrolysis of the borohydride ions and an accelerated reduction of 4-NP.31 Under alkaline conditions, the available protons for reactions were reduced; therefore, the hydrolysis rate decreased. A similar observation has been reported for the catalytic reduction of 4-NP using heterostructured gold-magnetite nanocatalysts.4
| Catalyst | Reaction condition [4-NP]; [catalyst]; [NaBH4] | Performance conversion; time | References |
|---|---|---|---|
| Co-BDC | 20 ppm; 5 mg; 0.13 mM | 99.25%; 2 min | 33 |
| Carbonized Fe-BDC | 20 ppm; 5 mg/4 mL; 0.5 M | 100%; 4 min | 34 |
| NH2-MIL-101(Fe) | 20 ppm; 0.5 g L−1; 0.5 M | 100%; 4 min | 35 |
| HKUST-1@SBA-15C (1/8) | 0.09 mM; 1 mg; 30 mM | 100%; 7.5 min | 14 |
| HKUST-1@MSN-NH2 | ∼50 μM; 2.5 g L−1; 30 mM | 100%; 10 min | 15 |
| C-HK(Cu) | 2 mM; 0.1 g L−1; 40 mM | ∼65%; 40 min | 36 |
| HKUST-1/hydrogel | 15 mM; 3 g L−1; 300 mM | 100%; 25 min | This work |
3.3 Reductions of other organic pollutants
The composite catalyst was also evaluated for the reduction of 2-NP and two azo dye models: methyl orange and Congo red. As displayed in Fig. 13(a), the absorbance of 2-NP at 415 nm declined over time. The UV-vis spectra for methyl orange are presented in Fig. 13(b). A reduction in the peak intensity at 465 nm of methyl orange, along with an increase in the peak intensity at 247 nm corresponding to sulfanilic acid (the reduction product), confirmed the occurrence of the reduction reaction.40 The UV-vis spectra depicting the reduction of Congo red are illustrated in Fig. 13(c), where the peak intensity at 498 nm diminished over time due to the cleavage of the azo group, resulting in the formation of aromatic amines.41 The decrease in the peak intensity was not attributed to the dye adsorption as the adsorption–desorption equilibrium was established before initiating the reduction process using NaBH4. Fig. 13(d) shows the conversion rates of 4-NP, 2-NP, methyl orange, and Congo red over time. The reduction of 2-NP was slightly slower than that of 4-NP, with complete reduction achieved within 40 minutes. Furthermore, the composite catalyst successfully reduced 100% of methyl orange and 69% of Congo red. The kapp values for the reductions of 4-NP, 2-NP, methyl orange, and Congo red by the composite catalyst were 0.1008, 0.0845, 0.414, and 0.245, respectively (Fig. 13(e)).4. Conclusions
In this study, we demonstrated the reutilization of Cu2+-adsorbed hydrogel as a catalyst for the reduction of 4-NP to 4-AP. The Cu2+ ions adsorbed in the hydrogel were transformed into HKUST-1 through an in situ synthesis process. The synthesis of HKUST-1 was characterized by FTIR, FESEM, and XPS analyses. TGA determined that the HKUST-1 content in the hydrogel was 11.2%. The HKUST-1 composite completely reduced the 4-NP solution within 25 minutes. The reduction rates increased at elevated temperatures, with the activation energy calculated to be 54.4 kJ mol−1. When compared to other MOF-based catalysts for 4-NP reduction, the performance of our composite catalyst was competitive, even when evaluated at a higher concentration of 4-NP. Furthermore, the catalyst proved effective under ambient conditions, achieving a complete conversion of 4-NP. Finally, the composite catalyst demonstrated its capability to reduce other target organic pollutants, including 2-NP, methyl orange, and Congo red, and it could be easily separated via filtration.Data availability
The manuscript includes all data supporting this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Fundamental Fund of Khon Kaen University, the National Science Research and Innovation Fund (NSRF), and Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation. The authors thank Panjalak Meetam and Jiraporn Pimphumee for their assistance with UV-vis and LC-MS measurements.References
- K. Zhao, J. Wang, W. Kong and P. Zhu, J. Environ. Chem. Eng., 2020, 8, 103517 CrossRef CAS.
- J. Shen, R. He, L. Wang, J. Zhang, Y. Zuo, Y. Li, X. Sun, J. Li and W. Han, J. Hazard. Mater., 2009, 167, 193–198 CrossRef CAS PubMed.
- A. B. Azzam, R. Djellabi, S. M. Sheta and S. M. El-Sheikh, RSC Adv., 2021, 11, 18797–18808 RSC.
- F. Lin and R. Doong, Appl. Catal., A, 2014, 486, 32–41 CrossRef CAS.
- Z.-S. Lv, X.-Y. Zhu, H.-B. Meng, J.-J. Feng and A.-J. Wang, J. Colloid Interface Sci., 2019, 538, 349–356 CrossRef CAS PubMed.
- T. M. Ansari, M. Ajmal, S. Saeed, H. Naeem, H. B. Ahmad, K. Mahmood and Z. H. Farooqi, J. Iran. Chem. Soc., 2019, 16, 2765–2776 CrossRef CAS.
- Y. R. Mejía and N. K. Reddy Bogireddy, RSC Adv., 2022, 12, 18661–18675 RSC.
- P. Boonying, S. Martwiset and S. Amnuaypanich, Appl. Nanosci., 2018, 8, 475–488 CrossRef CAS.
- S. Jana, S. Ghosh, S. Nath, S. Pande, S. Praharaj, S. Panigrahi, S. Basu, T. Endo and T. Pal, Appl. Catal., A, 2006, 313, 41–48 CrossRef CAS.
- M. Ajmal, M. Siddiq, H. Al-Lohedan and N. Sahiner, RSC Adv., 2014, 4, 59562–59570 RSC.
- N. Sahiner and S. Sagbas, Colloids Surf., A, 2013, 418, 76–83 CrossRef CAS.
- S. S.-Y. Chui, S. M.-F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS PubMed.
- K.-Y. Andrew Lin and Y.-T. Hsieh, J. Taiwan Inst. Chem. Eng., 2015, 50, 223–228 CrossRef CAS.
- Y. Zhang, C. Qian, J. Duan, Y. Liang, J. Luo, Y. Han, J. Hu and F. Shi, Appl. Surf. Sci., 2022, 573, 151558 CrossRef CAS.
- J. Yu and Y. Sun, J. Environ. Chem. Eng., 2024, 12, 113051 CrossRef CAS.
- P. Meetam, K. Phonlakan, S. Nijpanich and S. Budsombat, Int. J. Biol. Macromol., 2024, 255, 128261 CrossRef CAS PubMed.
- K. Phonlakan, S. Pornsuwan, S. Nijpanich and S. Budsombat, Int. J. Biol. Macromol., 2024, 265, 130922 CrossRef CAS PubMed.
- A. Nachaichot, K. Phonlakan, S. Nijpanich, S. Pornsuwan and S. Budsombat, RSC Adv., 2024, 14, 35628–35637 RSC.
- X. Cui, X. Sun, L. Liu, Q. Huang, H. Yang, C. Chen, S. Nie, Z. Zhao and Z. Zhao, Chem. Eng. J., 2019, 369, 898–907 CrossRef CAS.
- B. Di Credico, M. Redaelli, M. Bellardita, M. Calamante, C. Cepek, E. Cobani, M. D'Arienzo, C. Evangelisti, M. Marelli, M. Moret, L. Palmisano and R. Scotti, Catalysts, 2018, 8, 353 CrossRef.
- L. Zhang, J. Zheng, C. Zhang, Y. Wang, J. Zeng, H. He, T. Yuan, Y. Qin and Y. Zheng, Trans. Nonferrous Met. Soc. China, 2022, 32, 251–261 CrossRef CAS.
- P. Jagódka, K. Matus and A. Łamacz, Molecules, 2022, 27, 7082 CrossRef PubMed.
- Y. Wang, Y. Lü, W. Zhan, Z. Xie, Q. Kuang and L. Zheng, J. Mater. Chem. A, 2015, 3, 12796–12803 RSC.
- Y. Wang, J. Wang, Z. Yuan, H. Han, T. Li, L. Li and X. Guo, Colloids Surf., B, 2017, 152, 252–259 CrossRef CAS PubMed.
- R. Shi, C. Liu, W. Wang, N. Wang, P. Shen, Z. Liu, J. Hu and F. Shi, Mater. Lett., 2023, 352, 135177 CrossRef CAS.
- E. Aykut, M. Sert and E. Sert, J. Water Process Eng., 2023, 54, 103970 CrossRef.
- S. Wunder, F. Polzer, Y. Lu, Y. Mei and M. Ballauff, J. Phys. Chem. C, 2010, 114, 8814–8820 CrossRef CAS.
- A. Feng, C. Lin, H. Zhou, W. Jin, Y. Hu, D. Li and Q. Li, Green Chem. Eng., 2024, 5, 205–212 CrossRef.
- A. Venkateshaiah, D. Silvestri, R. K. Ramakrishnan, S. Wacławek, V. V. T. Padil, M. Černík and R. S. Varma, Molecules, 2019, 24, 3643 CrossRef CAS PubMed.
- A. V. Churikov, I. M. Gamayunova, K. V. Zapsis, M. A. Churikov and A. V. Ivanishchev, Int. J. Hydrogen Energy, 2012, 37, 335–344 CrossRef CAS.
- T. K. Das and N. Ch. Das, Int. Nano Lett., 2022, 12, 223–242 CrossRef CAS.
- G. K. Parshetti and R. Doong, Chemosphere, 2012, 86, 392–399 CrossRef CAS PubMed.
- A. Ehsani, S. Nejatbakhsh, A. M. Soodmand, M. E. Farshchi and H. Aghdasinia, Environ. Res., 2023, 227, 115736 CrossRef CAS PubMed.
- Md. A. Ahsan, E. Deemer, O. Fernandez-Delgado, H. Wang, M. L. Curry, A. A. El-Gendy and J. C. Noveron, Catal. Commun., 2019, 130, 105753 CrossRef CAS.
- P. Karthik, A. Pandikumar, M. Preeyanghaa, M. Kowsalya and B. Neppolian, Microchim. Acta, 2017, 184, 2265–2273 CrossRef CAS.
- J. Li, X. Sun, S. Subhan, W. Gong, W. Li, W. Sun, Y. Zhang, M. Lu, H. Ji, Z. Zhao and Z. Zhao, Chem. Eng. J., 2022, 446, 137314 CrossRef CAS.
- S. Bo, X. Zhao, Q. An, J. Luo, Z. Xiao and S. Zhai, RSC Adv., 2019, 9, 5009–5024 RSC.
- T. Aditya, J. Jana, N. K. Singh, A. Pal and T. Pal, ACS Omega, 2017, 2, 1968–1984 CrossRef CAS PubMed.
- L. Sun, J. He, S. An, J. Zhang, J. Zheng and D. Ren, Chin. J. Catal., 2013, 34, 1378–1385 CrossRef CAS.
- A. Mondal, B. Adhikary and D. Mukherjee, Colloids Surf., A, 2015, 482, 248–257 CrossRef CAS.
- E. A. Bakr, M. N. El-Nahass, W. M. Hamada and T. A. Fayed, RSC Adv., 2021, 11, 781–797 RSC.
| This journal is © The Royal Society of Chemistry 2025 |










