Open Access Article
Open Access ArticleMechanistic pathway and optimization of rhodamine B degradation using Mn3O4/ZnO nanocomposite on microalgae-based carbon
Hoc Thang Nguyena,
Van-Dat Doan b,
Thi Lan Huong Nguyenc,
Anh-Tien Nguyend,
Quang- Hieu Tran
b,
Thi Lan Huong Nguyenc,
Anh-Tien Nguyend,
Quang- Hieu Tran e,
Vy Anh Tran*f and
Van Thuan Le
e,
Vy Anh Tran*f and
Van Thuan Le *gh
*gh
aFaculty of Chemical Technology, Ho Chi Minh City University of Industry and Trade, 140 Le Trong Tan, Ho Chi Minh City, 700000, Vietnam
bFaculty of Chemical Engineering, Industrial University of Ho Chi Minh City, Ho Chi Minh City, 700000, Vietnam
cInstitute of Biotechnology and Food Technology, Industrial University of Ho Chi Minh City, Ho Chi Minh City, 700000, Vietnam
dFaculty of Chemistry, Ho Chi Minh City University of Education, 280 An Duong Vuong, Ho Chi Minh City, 700000, Vietnam
eBasic Sciences Department-Saigon Technology University, 180 Cao Lo, Ho Chi Minh City 700000, Vietnam
fDeparment of Material Science, Institute of Applied Technology and Sustainable Development, Nguyen Tat Thanh University, Ho Chi Minh City, 700000, Vietnam. E-mail: tavy@ntt.edu.vn
gCenter for Advanced Chemistry, Institute of Research & Development, Duy Tan University, 03 Quang Trung, Da Nang City, 550000, Vietnam. E-mail: levanthuan3@duytan.edu.vn
hFaculty of Natural Sciences, Duy Tan University, 03 Quang Trung, Da Nang City, 550000, Vietnam
First published on 25th February 2025
Abstract
The development of cost-effective and eco-friendly photocatalysts for wastewater treatment is crucial for addressing environmental pollution challenges. In this study, we report a novel Mn3O4/ZnO nanocomposite supported on microalgae-derived activated carbon (AC) for the efficient photocatalytic degradation of rhodamine B (RhB) under visible light. FTIR analysis suggested interactions between the metal oxides and AC, while UV-vis DRS and PL studies revealed a reduced band gap and enhanced charge transfer in the composite, minimizing electron–hole recombination. The Mn3O4/ZnO/AC composite exhibited over 95.85% RhB removal efficiency and 80.56% mineralization after 420 min of irradiation. Stability tests showed over 88% degradation efficiency after four cycles, with no significant structural changes, as confirmed by XRD and SEM. Leaching tests demonstrated low Zn2+ (0.88 mg L−1) and Mn2+ (0.26 mg L−1) concentrations, well within WHO limits, indicating the composite's safety and structural integrity. Mechanistic studies identified hydroxyl and superoxide radicals as the main reactive species responsible for RhB degradation, with pathway analysis revealing the stepwise breakdown of the dye. This work highlights the potential of microalgae-derived AC as a sustainable support for photocatalysts, positioning the Mn3O4/ZnO/AC composite as a promising candidate for scalable wastewater treatment applications. The integration of green synthesis, high photocatalytic efficiency, and excellent safety profiles positions this composite as a valuable candidate for environmental remediation applications.
1. Introduction
Water pollution is a critical environmental issue, intensified by industrial activities that discharge various contaminants into aquatic ecosystems. Among them, synthetic dyes, particularly rhodamine B (RhB), pose significant ecological and health risks due to their chemical stability and resistance to degradation. RhB, a cationic xanthene dye widely used in textiles, printing, and coatings, is known for its persistence in wastewater and potential carcinogenic effects.1 Its removal from water bodies remains a pressing challenge, necessitating the development of efficient and sustainable treatment methods.Photocatalysis has gained significant attention as a sustainable approach for eliminating organic pollutants under mild conditions while minimizing secondary pollution.2 Zinc oxide (ZnO) is a widely studied photocatalyst due to its tunable band gap and high exciton binding energy.3 However, its wide band gap (3.37 eV) restricts absorption primarily to the ultraviolet (UV) region, limiting its practical application.4 To enhance its photocatalytic efficiency, ZnO is frequently modified through metal doping or heterojunction formation with other semiconductors.5–7
Recently, manganese oxide (Mn3O4), an emerging p-type semiconductor, has gained attention for its significant photocatalytic properties, effectively degrading various inorganic and organic pollutants in water. Its effectiveness is further enhanced when combined with other metal oxides, where it contributes to improved catalytic activity through synergetic effects. Moreover, Mn3O4 is economical, non-toxic, and can be synthesized using simple protocols, making it an attractive and sustainable choice for environmental remediation applications.8 The previous studies have demonstrated that the synergy between ZnO and Mn3O4 can enhance catalytic activity through improved surface area, facilitating the adsorption of pollutants and promoting the separation of photogenerated electron–hole pairs.9 The hierarchical nanostructures formed by the Mn3O4/ZnO composite are expected to improve electron mobility and extend the light-harvesting range, leading to more efficient photocatalytic degradation of organic contaminants. Despite these advantages, practical application of Mn3O4/ZnO nanocomposites is hindered by issues such as nanoparticle aggregation and challenges in catalyst recovery. To address these limitations, carbon-based materials, particularly activated carbon (AC), have been integrated with photocatalysts to improve dispersion, enhance adsorption, and facilitate electron transfer due to its high surface area, tunable porosity, and eco-friendly nature.10 In the search for sustainable and eco-friendly sources of AC, algae have emerged as a promising alternative. Several studies have highlighted the significant potential of algae-based AC in water treatment applications. For instance, Shoaib et al. utilized green algae Ulva lactuca to synthesize AC, achieving an impressive adsorption capacity of 303.78 mg g−1 for methylene blue dye from aqueous solutions.11 Similarly, Djezzar et al. produced AC from the green algae Spirogyra utilized it as an economical adsorbent for efficiently capturing copper(II) ions in industrial wastewater treatment.12 Additionally, the presence of nitrogen-functional groups in algae-based AC has also been shown to improve its surface chemistry and adsorption capacity, further enhancing its effectiveness as a pollutant adsorbent.11 These findings underscore the potential of using algae-derived AC, especially when modified, in environmental remediation applications.
In this study, we synthesized and characterized a novel Mn3O4/ZnO nanocomposite supported on microalgae-derived AC (Mn3O4/ZnO/AC) for the photocatalytic degradation of RhB. This composite aims to leverage the synergistic effects of ZnO and Mn3O4 while utilizing algae-derived AC to enhance stability and adsorption capabilities. Although Mn3O4/ZnO composites have been explored, the integration of algae-derived AC as a support material has not been reported. This study pioneers a novel Mn3O4/ZnO/AC nanocomposite, aiming to leverage the synergistic effects of ZnO, Mn3O4, and AC for enhanced photocatalytic activity and catalyst stability. The photocatalytic performance of Mn3O4/ZnO/AC was systematically evaluated under varying conditions, including catalyst dosage, pH, temperature, and dye concentration. Additionally, catalyst reusability and stability were assessed to determine its potential for practical water treatment applications.
2. Materials and methods
2.1. Materials and equipment
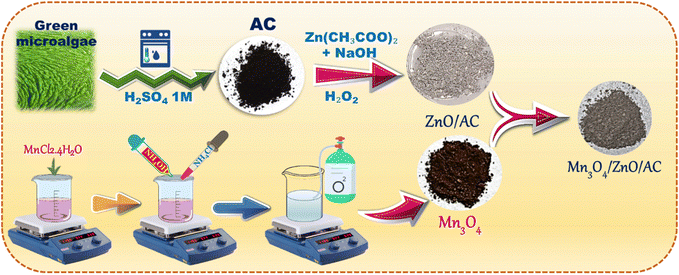
In this study, various chemicals were utilized for material synthesis, photocatalytic experiments, and scavenger tests. Zinc acetate dihydrate (Zn(CH3COO)2·2H2O, 99.5%) and manganese(II) chloride tetrahydrate (MnCl2·4H2O, ≥98%) were employed as precursor materials for catalyst synthesis. Rhodamine B (C28H31ClN2O3, 90%) served as the model organic pollutant to evaluate photocatalytic performance. For pH adjustment, hydrochloric acid (HCl, 37%) and sodium hydroxide (NaOH, ≥98.0%) were used. In the scavenger study, sodium formate (HCOONa, ≥99.0%), potassium dichromate (K2Cr2O7, ≥99.5%), p-benzoquinone (p-BQ, C6H4(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2, ≥98%), and isopropanol (IPA, (CH3)2CHOH, ≥98%) were employed to identify reactive species. Additional reagents, including ammonium chloride (NH4Cl, ≥99.5%), hydrogen peroxide (H2O2, 30%), aqueous ammonia solution (NH3, 30%), and ethanol (C2H5OH, 99.7%), were used in the synthesis and experimental processes. All chemicals were purchased from Sigma-Aldrich and used as received without further purification. The green microalgae (Chlamydomonas reinhardtii) used as the precursor for AC were collected from a fish pond in Bến Tre Province, Vietnam. After collection, the algae underwent thorough washing and screening to remove impurities, resulting in a pure culture of green microalgae for further experimentation.
O)2, ≥98%), and isopropanol (IPA, (CH3)2CHOH, ≥98%) were employed to identify reactive species. Additional reagents, including ammonium chloride (NH4Cl, ≥99.5%), hydrogen peroxide (H2O2, 30%), aqueous ammonia solution (NH3, 30%), and ethanol (C2H5OH, 99.7%), were used in the synthesis and experimental processes. All chemicals were purchased from Sigma-Aldrich and used as received without further purification. The green microalgae (Chlamydomonas reinhardtii) used as the precursor for AC were collected from a fish pond in Bến Tre Province, Vietnam. After collection, the algae underwent thorough washing and screening to remove impurities, resulting in a pure culture of green microalgae for further experimentation.
For the characterization of the synthesized materials, several instruments were utilized. UV-vis absorption spectra were recorded using a Metash UV-vis 5100 spectrophotometer, while FT-IR spectra were obtained with a Tensor 27 spectrometer from Bruker, Germany. The crystalline structures of the samples were analyzed using a Shimadzu 6100 X-ray diffractometer (XRD). The UV-vis diffuse reflectance spectra (DRS) were measured using a Cary 4000 Eclipse Fluorescence Spectrometer (Agilent, USA). The morphology and detailed structure of the samples were examined using field emission scanning electron microscopy (FE-SEM) with a model S-4800 and high-resolution transmission electron microscopy (HR-TEM) with a JEM 2100 from JEOL (Japan). The elemental composition was determined by energy-dispersive X-ray spectroscopy (EDX) using an S-4800 FE-SEM. Photoluminescence (PL) spectra were also recorded using the Cary 4000 Eclipse Fluorescence Spectrometer. Additionally, the specific surface area and porosity of the samples were analyzed using a NOVA 1200e surface area analyzer (Quantachrome Instruments, USA). The degree of contaminant mineralization was assessed by determining the total organic carbon (TOC) content with a Multi N/S 2100S TOC analyzer (Analytik Jena, Germany). The identification and analysis of organic intermediates and byproducts generated during the reaction were conducted using gas chromatography-mass spectrometry (GC-MS) on an Agilent 7890A/5975C system (USA). To evaluate the leaching of Zn and Mn ions throughout the photocatalytic process, inductively coupled plasma mass spectrometry (ICP-MS) was carried out on an Agilent 7700 series instrument (USA), ensuring accurate quantification of metal ion release and providing valuable information on the catalyst's stability over time.
2.2. Synthesis of catalyst materials
2.3. Catalytic study
The catalytic performance of the synthesized Mn3O4/ZnO/AC composite was evaluated through the degradation of RhB under visible light irradiation. The experiments were designed to investigate the effects of catalyst dosage, pH, RhB concentration, temperature, and the presence of radical scavengers on the degradation efficiency, as well as the catalyst's reusability. The photocatalytic activity was assessed by monitoring the degradation of RhB in an aqueous solution. In a typical experiment, 100 mg of the Mn3O4/ZnO/AC catalyst was dispersed in 50 mL of 10 mg L−1 RhB solution. To ensure adsorption–desorption equilibrium between RhB and the catalyst, the suspension was stirred in the dark for 1 hour before initiating the photocatalytic reaction. Afterward, the solution was irradiated using a 250 W Xenon lamp equipped with a UV cutoff filter (λ > 420 nm) to ensure visible light irradiation. During irradiation, 2–3 mL aliquots of the suspension were withdrawn at 30 minute intervals. The aliquots were centrifuged to remove the catalyst particles, and the remaining RhB concentration was measured using UV-vis spectrophotometry at 553 nm, which corresponds to the characteristic absorption peak of RhB.The removal efficiency, mineralization rate, and degradation kinetics were calculated using eqn (1)–(3), respectively:
 | (1) |
 | (2) |
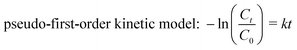 | (3) |
The effect of catalyst dosage on the degradation efficiency was studied by varying the amount of Mn3O4/ZnO/AC in the reaction mixture. Experiments were conducted with catalyst dosages of 0.05 g, 0.075 g, 0.1 g, and 0.125 g, using 50 mL of 10 mg L−1 RhB solution. The reaction conditions, including stirring at 200 rpm and room temperature, were kept constant. Post-reaction, the remaining RhB concentration was determined as described above. The relationship between catalyst dosage and degradation efficiency was plotted to identify the optimal catalyst amount for maximum degradation. The degradation efficiency was also evaluated across different pH levels to determine the optimal pH for RhB degradation. The pH of the RhB solution (10 mg L−1) was adjusted to pH 3, 5, 7, 9, and 11 using either HCl or NaOH before adding the catalyst. The same photocatalytic procedure was followed, and the degradation efficiency was calculated to assess the influence of pH on the catalyst's performance. The impact of initial RhB concentration on the degradation efficiency was investigated by preparing RhB solutions with concentrations of 5, 10, 15, and 20 mg L−1. The catalyst dosage was fixed at the optimal amount determined from previous experiments. The degradation process was monitored, and the efficiency was calculated for each concentration to determine the concentration that results in the highest degradation rate. The influence of temperature on the photocatalytic activity was examined by performing the degradation reactions at different temperatures: 25 °C, 35 °C, and 45 °C. The catalyst dosage and RhB concentration were kept constant, and the degradation efficiency was monitored to evaluate the effect of temperature on the reaction kinetics.
To identify the active species involved in the degradation process, experiments were conducted in the presence of different radical scavengers. p-BQ for superoxide radicals (˙O2−), IPA for hydroxyl radicals (˙OH), HCOONa for holes (h+), and K2Cr2O7 for electrons (e−) were added to the reaction mixture at a concentration of 10 mM. The impact of each scavenger on the degradation efficiency was analyzed to elucidate the mechanism of RhB degradation by the Mn3O4/ZnO/AC catalyst.
The reusability of the Mn3O4/ZnO/AC catalyst was assessed over four consecutive cycles of RhB degradation under the same conditions. After each cycle, the catalyst was recovered by centrifugation, washed with ethanol and distilled water, and dried at 60 °C before being reused. The degradation efficiency was measured after each cycle to evaluate the stability and reusability of the catalyst. All experiments were repeated independently three times, and the results are presented as the mean with standard deviation to ensure accuracy and reliability. Error bars are included in all figures to represent the variation across the different trials.
3. Results and discussion
3.1. Characterization of catalysts
The crystallite sizes (denoted as D (in nm)) of Mn3O4 and ZnO were estimated using the Scherrer equation (eqn (4)):
 | (4) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, characteristic of carbonyl groups, which may result from surface oxidation during the activation process. Peaks observed in the region of 1600–1650 cm−1 are assigned to C
O stretching, characteristic of carbonyl groups, which may result from surface oxidation during the activation process. Peaks observed in the region of 1600–1650 cm−1 are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations, suggesting the presence of aromatic structures. Additionally, the peaks around 1100 cm−1 can be assigned to C–O stretching, likely from ether or ester functionalities.16,17
C stretching vibrations, suggesting the presence of aromatic structures. Additionally, the peaks around 1100 cm−1 can be assigned to C–O stretching, likely from ether or ester functionalities.16,17The FTIR spectrum of Mn3O4 displays several characteristic absorption bands corresponding to Mn–O vibrations, which are indicative of its spinel structure. Specifically, strong absorption bands are observed at 623 cm−1 and 521 cm−1, which can be attributed to the stretching vibrations of Mn–O bonds in the tetrahedral and octahedral sites within the spinel lattice. Additionally, a weak band at 1086 cm−1 is observed, which may be attributed to Mn–O–H vibrations, likely due to surface hydroxylation or minor moisture absorption.18 Peaks at 1400 cm−1 and 1480 cm−1 are attributed to various vibrational modes of O–H groups from adsorbed water on the ZnO surface. The peak at 1632 cm−1 is assigned to the asymmetric stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, indicative of CO2 adsorption from ambient air. Finally, the broad absorption band near 3432 cm−1 is associated with O–H stretching vibrations, confirming the presence of adsorbed water, which is attributed to the hygroscopic nature of ZnO.19
O, indicative of CO2 adsorption from ambient air. Finally, the broad absorption band near 3432 cm−1 is associated with O–H stretching vibrations, confirming the presence of adsorbed water, which is attributed to the hygroscopic nature of ZnO.19
For the Mn3O4/ZnO/AC composite, the FTIR spectrum shows a combination of features from its individual components. The broad O–H stretching band around 3440 cm−1, which remains consistent with the intensity observed in pure AC, indicates the presence of hydroxyl groups. The Zn–O stretching band at approximately 430 cm−1 is still present, confirming the incorporation of ZnO into the composite. Additionally, the Mn–O stretching bands from Mn3O4 are evident in the region of 500–600 cm−1, further verifying the presence of manganese oxide. The composite spectrum also shows a slight shift or broadening of the Zn–O and Mn–O peaks compared to the pure materials, suggesting possible interactions between Mn3O4, ZnO, and AC in the composite. These interactions may enhance the material's structural stability and photocatalytic properties by promoting better electron transfer and reducing recombination of electron–hole pairs during photocatalysis.
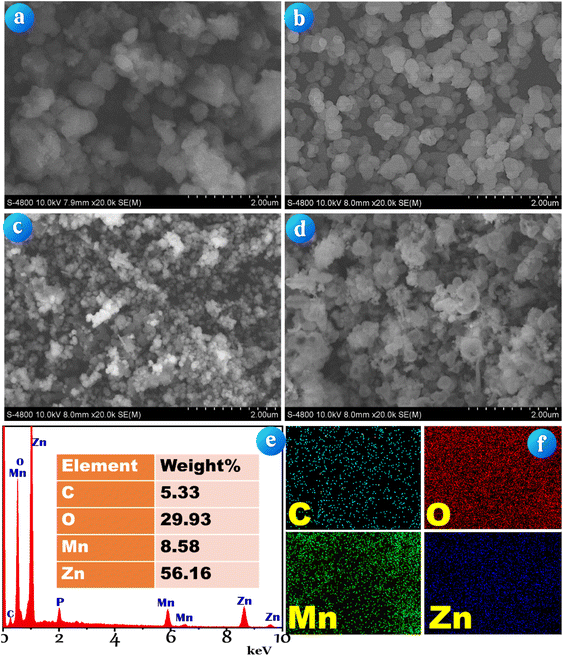 | ||
| Fig. 2 SEM images of (a) AC derived from green microalgae, (b) ZnO/AC, (c) Mn3O4 and (d) Mn3O4/ZnO/AC composites; (e) EDX spectrum and (f) element mapping of the Mn3O4/ZnO/AC composite. | ||
Fig. 2c presents the SEM image of Mn3O4, where the nanoparticles exhibit a spherical morphology with relatively uniform sizes, ranging from 100 to 200 nm. This consistency in particle size suggests a well-controlled synthesis process, which is critical for ensuring uniform catalytic activity. Fig. 2d captures the Mn3O4/ZnO/AC composite. The image reveals a complex structure where Mn3O4 and ZnO particles are dispersed within the AC matrix. The spherical Mn3O4 particles are clearly visible, while the ZnO particles remain too small to be detected by SEM, suggesting successful integration within the AC matrix.
The EDX spectrum in Fig. 2e complements the SEM images by confirming the elemental composition of the Mn3O4/ZnO/AC composite. Peaks corresponding to carbon (5.33%), oxygen (29.93%), manganese (8.58%), and zinc (56.16%) are clearly observed, indicating the successful incorporation of both Mn3O4 and ZnO onto the AC substrate. Additionally, a peak for phosphorus (P) is also detected, which is likely due to the inherent phosphorus content present in the microalgae. Fig. 2f provides elemental mapping images that visually represent the distribution of these elements within the composite. The uniform distribution of carbon, oxygen, manganese, and zinc throughout the material highlights the homogeneous integration of the active components, which is vital for consistent catalytic performance.
Further insights into the nanostructure of the ZnO/AC and Mn3O4/ZnO/AC composites are provided by the TEM images in Fig. 3. Fig. 3a shows the TEM image of the ZnO/AC composite, where ZnO nanoparticles are observed as small, evenly distributed particles on the AC matrix. The small size of these nanoparticles, consistent with the in situ synthesis, contributes to a high surface area for catalytic reactions. Fig. 3b offers a higher-resolution TEM image of the ZnO/AC composite, confirming that ZnO nanoparticles are well-dispersed within the matrix. The particles range from 5 to 10 nm, explaining their invisibility in SEM images. Fig. 3c provides an HR-TEM image of the ZnO/AC composite, with clear lattice fringes corresponding to ZnO. The d-spacing values, including d(101) = 0.236 nm, d(002) = 0.282 nm, and d(100) = 0.275 nm, affirm the crystalline nature of ZnO and confirm its successful synthesis within the AC matrix.20 Fig. 3d displays the TEM image of the Mn3O4/ZnO/AC composite, where spherical Mn3O4 particles are well-dispersed within the ZnO/AC matrix. The clear distinction between Mn3O4 and ZnO phases indicates that both materials are effectively integrated into the composite, enhancing its multifunctional catalytic properties.
Most notably, the Mn3O4/ZnO/AC nanocomposite exhibits a significantly lower PL intensity compared to pure ZnO. This suggests that the incorporation of Mn3O4 and AC into the ZnO matrix effectively suppresses the recombination of electron–hole pairs. The reduction in PL intensity indicates enhanced charge separation within the composite, likely due to the synergistic interaction between Mn3O4 and ZnO, as well as the conductive properties of AC, which may facilitate electron transfer and further reduce recombination. Consequently, the Mn3O4/ZnO/AC composite is expected to exhibit superior photocatalytic performance compared to the individual components, as the lower PL intensity correlates with improved charge separation and a longer lifetime of the photogenerated charge carriers.
The analysis of bandgap energies is crucial as it directly influences the photocatalytic activity by determining the material's light absorption range. The DRS data (Fig. 4b) for ZnO, Mn3O4, and Mn3O4/ZnO/AC composite were analyzed to determine the bandgaps using the Tauc formula (eqn (5)):23
| (αhν)n = A(hν − Eg) | (5) |
Fig. 4b indicates an absorption edge for ZnO at around 390 nm, corresponding to a bandgap energy (Eg) of around 3.22 eV. Meanwhile Mn3O4 displays an absorption edge near 600 nm, suggesting a narrower bandgap of approximately 1.87 eV. A wider bandgap like that of ZnO is beneficial for UV-light-driven photocatalysis, while Mn3O4's narrower bandgap allows for visible light absorption, making the Mn3O4/ZnO/AC composite potentially active under both UV and visible light. The energy level alignment allows for efficient electron transfer from Mn3O4 to ZnO in the composite under visible light excitation, promoting effective charge separation and reducing electron–hole recombination. The incorporation of AC further enhances charge separation by facilitating electron transport, which is expected to improve the composite's photocatalytic activity in applications like pollutant degradation. This synergistic effect, derived from the complementary band structure and light absorption properties, underscores the importance of determining bandgap energies and energy levels for designing efficient photocatalytic materials.
To estimate the energy band positions of ZnO and Mn3O4, Mulliken's electronegativity theory was applied using the eqn (6) & (7):
| ECB = χ − Ee − 0.5Eg | (6) |
| EVB = ECB + Eg | (7) |
Fig. 4d depicts the energy band structure of Mn3O4 and ZnO before contact, the formation of the p–n junction, and the charge separation mechanism in the Mn3O4/ZnO heterostructure under visible light irradiation. Mn3O4, a p-type semiconductor, has its Fermi level positioned near the valence band, whereas ZnO, an n-type semiconductor, features a Fermi level closer to the conduction band.25,26 When Mn3O4 and ZnO come into contact, they form a p–n junction. At this junction, electrons move from the n-ZnO region to the p-Mn3O4 region, causing a buildup of negative charges in p-Mn3O4 near the interface. At the same time, holes from p-Mn3O4 migrate into n-ZnO, creating a region with positive charges in n-ZnO close to the junction. Once the Fermi levels of Mn3O4 and ZnO balance, an internal electric field is created, pointing from n-ZnO to p-Mn3O4. This field prevents further movement of charges across the junction. During this process, the energy levels of ZnO shift downward, while those of Mn3O4 shift upward to reach equilibrium. Under visible-light irradiation, Mn3O4 generates electron–hole pairs, which contribute to the photocatalytic activity.27
| Sample | SBET (m2 g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) |
|---|---|---|---|
| AC | 8.04 | 0.011 | 1.69 |
| ZnO/AC | 69.49 | 0.125 | 1.01 |
| Mn3O4/ZnO/AC | 75.70 | 0.199 | 1.50 |
The green microalgae-derived AC exhibits a relatively low specific surface area of 8.04 m2 g−1, with a pore volume of 0.011 cm3 g−1 and an average pore diameter of 1.69 nm. This indicates a modest porosity, primarily consisting of micropores, which is consistent with the inherent structure of the microalgae. Notably, the specific surface area of our AC is comparable to that of the Ulva lactuca-derived biochar-sulfur reported in the literature, which has an SBET of 6.26 m2 g−1.11 This similarity suggests that the surface area of microalgae-derived carbons is relatively consistent across different species, underscoring the effectiveness of microalgae as a precursor for producing activated carbons with modest but functional porosity. Upon incorporation of ZnO onto the AC matrix, a significant enhancement in the textural properties is observed. The SBET increases markedly to 69.49 m2 g−1, along with a substantial rise in pore volume to 0.125 cm3 g−1. Interestingly, the average pore diameter decreases to 1.01 nm, suggesting that ZnO nanoparticles contribute to a denser packing within the pore structure, which enhances the surface area by opening up more micropores or creating new mesopores. Further modification by introducing Mn3O4 into the ZnO/AC composite results in a further increase in the specific surface area to 75.70 m2 g−1. The pore volume also increases to 0.199 cm3 g−1, and the average pore diameter expands to 1.50 nm. This increase in both surface area and pore volume can be attributed to the additional porosity introduced by Mn3O4, which likely generates new mesopores or enlarges existing ones, thus optimizing the material for catalytic applications where a high surface area and accessible pore structure are essential. These findings indicate that the sequential modification of AC with ZnO and Mn3O4 significantly enhances its textural properties, making the Mn3O4/ZnO/AC composite a promising candidate for applications that require high surface areas and well-distributed porosity, such as in catalysis and adsorption processes.
3.2. Photocatalytic evolution
The photocatalytic activity of the Mn3O4/ZnO/AC composite for the degradation of RhB under visible light irradiation was investigated and compared to that of individual and binary catalyst systems, including ZnO, Mn3O4, Mn3O4/AC, and ZnO/AC. Fig. 6a displays the UV-vis spectra of the RhB solution at different intervals during the photocatalytic reaction with the Mn3O4/ZnO/AC composite. The intensity of the absorption peak at 553 nm, characteristic of RhB, gradually decreased over time, indicating a progressive degradation of the dye. A nearly complete degradation was observed after 420 min of reaction, demonstrating the high photocatalytic efficiency of the Mn3O4/ZnO/AC composite.To further understand the efficiency of each catalyst, Fig. 6b compares the photocatalytic degradation profiles of RhB using various catalysts. A control experiment without any catalyst (photolysis) showed that only 4.23% of RhB was degraded after 7 h of illumination, highlighting the dye's stability under light and confirming that the observed RhB degradation in the presence of catalysts is primarily due to their photocatalytic activity.29 Before the photocatalytic tests, the adsorption capacity of each material in the dark was evaluated. After 60 min, adsorption equilibrium was reached, with the adsorption capacity following this trend: ZnO (3.60%) < Mn3O4 (3.66%) < Mn3O4/AC (5.11%) < ZnO/AC (20.40%) < Mn3O4/ZnO/AC (22.96%). The Mn3O4/ZnO/AC composite demonstrated the highest adsorption capacity, likely due to its larger BET surface area and the presence of AC, which is known for its excellent adsorption properties. Although the total adsorption capacity of the catalysts remained below 25%, the significant enhancement in adsorption due to AC was evident.
When exposed to light, the photocatalytic performance of the catalysts differed significantly. Among the tested materials, the Mn3O4/ZnO/AC composite exhibited the highest photocatalytic efficiency, achieving 95.85% degradation of RhB within 420 min of illumination. In contrast, ZnO and Mn3O4 alone showed significantly lower degradation efficiencies of 16.46% and 33.17%, respectively. The poor performance of these single-component systems can be attributed to their wide band gaps, limiting their activity under visible light.8,30 ZnO/AC showed only a slight improvement in RhB degradation under light compared to its dark adsorption capacity, with an additional 6% degradation. This suggests that ZnO/AC has limited photocatalytic activity, possibly due to its strong adsorption, which may prevent sufficient light from reaching the catalytic sites.31 Meanwhile, Mn3O4/AC exhibited a much greater photocatalytic effect, degrading nearly 40% of RhB under light, highlighting its superior photocatalytic performance compared to ZnO/AC. Both binary systems, ZnO/AC and Mn3O4/AC, outperformed their single-component counterparts in RhB degradation. However, the photocatalytic efficiency of these binary catalysts was still lower than that of the Mn3O4/ZnO/AC composite. The remarkable enhancement in the Mn3O4/ZnO/AC system can be attributed to the synergistic interactions between Mn3O4, ZnO, and AC, which likely promoted more efficient charge separation, improved light absorption, and increased RhB adsorption onto the catalytic surface.
In comparison to other catalysts presented in the literature (Table 2), the Mn3O4/ZnO/AC composite, despite a relatively longer degradation time of 7 h, still demonstrated a respectable RhB removal efficiency of 95.85%. For instance, ZnO NPs showed a similar efficiency of 95% within 70 min, and Bi2MoO6/g-C3N4 achieved 94.6% removal in 3 h under UV-light. Although these systems exhibit faster degradation rates, it's important to note that the Mn3O4/ZnO/AC composite operates under visible light, which is more relevant for practical environmental applications where UV light sources are less common. Other systems, such as the ZnO/MIL-101(Fe)[A], CuO/CdO, and HA-Ag3PO4 composites, reached higher efficiencies (97.1%, 99.5% and 99.87%, respectively), but they rely on expensive or less abundant materials, which may limit their large-scale applications. The Mn3O4/ZnO/AC composite's performance, combined with its ease of synthesis and operation under visible light, remains competitive, especially in applications where visible light activation is crucial.
| Materials | Conditions | Removal efficiency (%) | Ref. |
|---|---|---|---|
| ZnO NPs | [RhB] = 25 mg L−1, pH 7, catalyst dosage of 2 g L−1, UV light, 70 min | 95.0 | 32 |
| ZnO/NiFe2O4 | [RhB] = 10 mg L−1, catalyst dosage of 0.75 g L−1, sunlight, 3 h | 95.7 | 33 |
| ZnO/MIL-101(Fe)[A] | [RhB] = 10 mg L−1, catalyst dosage of 0.5 g L−1, vis. light, 5 h | 97.1 | 34 |
| Cu–ZnO/rGO | [RhB] = 5 mg L−1, catalyst dosage of 2 g L−1, sunlight, 7 h | 95.0 | 35 |
| CuO/CdO | [RhB] = 20 mg L−1, pH 7, catalyst dosage of 0.8 g L−1, vis. light, 90 min | 99.5 | 23 |
| HA-Ag3PO4 | [RhB] = 20 mg L−1, catalyst dosage of 1.0 g L−1, vis. light, 2 h | 99.87 | 36 |
| TiO2/CuFe2O4 | [RhB] = 5 mg L−1, pH 10, catalyst dosage of 1 g L−1, UV-vis. light, 2.5 h | 91.2 | 29 |
| Bi2MoO6/g-C3N4 | [RhB] = 1 mg L−1, catalyst dosage of 1 g L−1, pH = 6.9, UV light, 3 h | 94.6 | 37 |
| Mn3O4/ZnO/AC | [RhB] = 10 mg L−1, pH 7, catalyst dosage of 2 g L−1, vis. light, 7 h | 95.85 | This study |
To evaluate the extent of contaminant mineralization, TOC measurements were performed, providing a comprehensive assessment of the photocatalyst's ability to fully degrade RhB into inorganic products like CO2 and H2O. As depicted in Fig. 6c, the mineralization efficiency progressively increased with reaction time, demonstrating the composite's strong photocatalytic performance. Initially, the mineralization rate was modest (∼17%) due to the predominance of RhB adsorption and the gradual initiation of photocatalytic reactions. However, as the reaction proceeded, the mineralization rate accelerated. This enhancement was attributed to the generation of reactive species, such as hydroxyl radicals (˙OH) and superoxide anions (O2˙−), which effectively broke down the dye's aromatic structure into smaller intermediates and eventually converted them into inorganic end-products. After 420 min of visible light irradiation, the mineralization efficiency reached an impressive 80.56%. This result highlights the Mn3O4/ZnO/AC composite's capability to degrade RhB beyond mere decolorization, ensuring the removal of potentially toxic intermediates. Such a high level of mineralization underscores the photocatalyst's potential for practical wastewater treatment applications, where comprehensive pollutant removal is crucial for environmental safety.
The kinetic analysis of RhB degradation, as shown in Fig. 6d, was conducted using a pseudo-first-order model, which is commonly used to describe the photocatalytic degradation of organic pollutants. The model is represented by the linear relationship of −ln(Ct/C0) versus time, where C0 is the initial concentration of RhB, and Ct is the concentration at a given time. From the slope of the linear plots, the rate constant (k, min−1) for each catalyst was determined, offering a quantitative measure of their photocatalytic performance.29 The Mn3O4/ZnO/AC composite demonstrated the highest rate constant (k = 6.4 × 10−3 min−1), which was significantly higher than that of the Mn3O4/AC (k = 1.3 × 10−3 min−1) and ZnO/AC (k = 0.2 × 10−3 min−1) binary systems, as illustrated in Fig. 6e. Meanwhile, the individual catalysts, ZnO and Mn3O4, exhibited low rate constants of 0.4 × 10–3 min−1 and 0.7 × 10–3 min−1, respectively, further underscoring the synergistic effect observed in the ternary Mn3O4/ZnO/AC composite.
The superior kinetic performance of the Mn3O4/ZnO/AC composite can be attributed to several key factors. First, the presence of AC significantly increases the overall surface area, thereby enhancing the adsorption of RhB molecules and bringing them closer to the catalytic active sites. This improved adsorption, combined with the excellent light absorption properties of AC, allows for more efficient utilization of the visible light spectrum. Additionally, the heterojunction formed between Mn3O4 and ZnO is known to facilitate better charge separation, effectively suppressing electron–hole recombination, which is one of the primary factors limiting photocatalytic efficiency in semiconductor-based systems. Moreover, the hierarchical structure of the Mn3O4/ZnO/AC composite may promote more effective diffusion of RhB molecules to the catalytic sites, further enhancing the overall degradation rate. This suggests that the integration of AC with both Mn3O4 and ZnO not only improves adsorption but also significantly boosts the photocatalytic activity through enhanced light-harvesting and charge–transfer properties. In terms of practical implications, the high rate constant of the Mn3O4/ZnO/AC composite suggests its potential as an effective photocatalyst for the degradation of organic dyes and possibly other pollutants under visible light irradiation. This observation is consistent with other studies that report enhanced photocatalytic activity in multi-component systems, where a combination of semiconductor materials and a carbon-based support leads to superior performance in environmental remediation applications.10,28,38
3.3. Effect of the operating parameters
At more acidic conditions (pH 3 and 5), the degradation efficiency significantly decreases, with only 27.91% removal at pH 3 and 66.74% at pH 5. The reduced activity at low pH can be attributed to the protonation of the catalyst's surface, which, while increasing the positive charge density, may lead to electrostatic repulsion with the cationic form of RhB. This repulsion reduces the adsorption of RhB on the catalyst, lowering the degradation efficiency. Additionally, under strongly acidic conditions, excess H+ ions can interfere with the formation of reactive oxygen species, further hindering the photocatalytic process. At pH values above 7.8 (the isoelectric point of Mn3O4/ZnO/AC), the surface of the catalyst becomes negatively charged. Meanwhile, RhB remains positively charged (RhB+) in mildly basic environments. This creates an electrostatic attraction between the catalyst and RhB+, which may lead to strong adsorption of RhB onto the catalyst surface. While this could initially seem beneficial, it can, in fact, hinder the photocatalytic efficiency by excessively blocking the active sites, limiting light penetration and preventing effective generation of reactive species. Moreover, at very high pH (≥9), RhB may undergo partial deprotonation, leading to the formation of carboxylate groups (–COO−). This introduces electrostatic repulsion between the carboxylate anions and the negatively charged catalyst surface, further reducing RhB's adsorption and decreasing the photocatalytic degradation efficiency.39 Another possible factor contributing to the reduced performance at high pH is the transformation of ZnO and Mn3O4 into their respective hydroxide complexes, such as Zn(OH)2−4 and Mn(OH)2−4.40 These complexes are less effective as photocatalysts compared to the oxides, further limiting the system's overall catalytic activity. Thus, the decrease in photocatalytic activity at pH values above 9 is likely due to a combination of strong adsorption that limits light absorption, electrostatic repulsion between RhB and the catalyst, and the formation of inactive hydroxide species.
Initially, the photocatalytic degradation efficiency increased markedly as the catalyst dosage was raised from 1.0 g L−1 to 2.0 g L−1. At 1.0 g L−1, the degradation efficiency was 77.5%, which increased significantly to 89.1% at 1.5 g L−1 and reached the maximum of 95.85% at 2.0 g L−1. This improvement can be attributed to the increased availability of active sites on the catalyst surface, allowing for more photon absorption and enhanced generation of reactive species responsible for RhB degradation.29 However, a further increase in catalyst dosage beyond 2.0 g L−1 did not result in improved performance. In fact, the degradation efficiency slightly decreased to 92.5% at 2.5 g L−1. This reduction in efficiency at higher catalyst dosages can be explained by the screening effect: when the catalyst concentration is too high, excess catalyst particles can aggregate or scatter the incident light, reducing the overall light penetration through the suspension. As a result, fewer photons reach the active sites, and the rate of reactive species generation is diminished. Additionally, high catalyst concentrations may lead to excessive adsorption of RhB on the surface, further limiting its photocatalytic breakdown.41 Therefore, while increasing catalyst dosage enhances the degradation up to an optimal point (2.0 g L−1), higher amounts may negatively impact the process due to light shielding and particle agglomeration effects. Hence, an optimal balance of catalyst dosage must be maintained to maximize the photocatalytic activity of Mn3O4/ZnO/AC.
As the RhB concentration increased to 10 and 15 mg L−1, the removal efficiency dropped slightly to 95.85% and 88.07%, respectively. This reduction can be attributed to the saturation of active sites on the Mn3O4/ZnO/AC surface, leading to decreased accessibility for the dye molecules.42 At higher RhB concentrations, competition for available active sites intensifies, resulting in a lower photocatalytic efficiency. Furthermore, the higher concentration of RhB may limit the penetration of light into the solution due to increased absorption, which in turn reduces the generation of reactive oxygen species required for the degradation process.29 When the RhB concentration reached 20 mg L−1, a more significant decline in removal efficiency was observed, dropping to 67.74%. This pronounced decrease can be explained by the excessive amount of dye in the system, which not only overwhelms the catalyst's surface but also causes a shielding effect that inhibits effective light absorption. The high concentration of RhB molecules can absorb a significant portion of the incident light, reducing the energy available for the catalyst to initiate the photocatalytic reactions. Moreover, the increased number of RhB molecules leads to higher competition for photon absorption, further slowing the degradation rate.43 Thus, at higher concentrations of RhB, the catalytic efficiency of Mn3O4/ZnO/AC is hindered by both the limited availability of active sites and the obstruction of light, emphasizing the importance of optimizing dye concentrations in practical applications. In this study, 10 mg L−1 was selected as the representative RhB concentration for further experiments because it provides a balanced assessment of catalytic performance. At lower concentrations (e.g., 5 mg L−1), nearly complete removal (∼98.89%) was achieved, making it difficult to observe significant variations in degradation rates and reaction kinetics. Conversely, at higher concentrations (≥15 mg L−1), the degradation efficiency decreased substantially due to active site saturation and light shielding effects. Thus, 10 mg L−1 serves as an optimal midpoint, allowing for both sufficient degradation efficiency (∼95.85%) and measurable catalytic differences under varying conditions.
| T (°C) | Rate constant (min−1) | R2 | Ea (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | ΔG (kJ mol−1) |
|---|---|---|---|---|---|---|
| 25 | 0.0064 | 0.974 | −45.88 | −48.36 | −449.03 | 85.45 |
| 35 | 0.0025 | 0.952 | −48.44 | −444.30 | 88.41 | |
| 45 | 0.0014 | 0.943 | −48.52 | −439.89 | 91.36 | |
| 55 | 0.0012 | 0.978 | −48.61 | −435.74 | 94.32 |
From a practical application perspective, the significant decline in photocatalytic efficiency at elevated temperatures poses challenges for real-world deployment, especially in outdoor or industrial wastewater treatment systems where temperature fluctuations are common. However, this temperature-dependent behavior also presents certain advantages. The catalyst maintains high efficiency within the temperature range commonly found in natural and industrial wastewater settings (25–35 °C), reducing the need for external thermal regulation. Additionally, operation at lower temperatures may help preserve the structural integrity of the catalyst, preventing thermal degradation and extending its operational lifespan. These findings suggest that while improvements in thermal stability could enhance large-scale applicability, Mn3O4/ZnO/AC remains a promising material for moderate-temperature environments, where its efficiency and durability can be maximized.
The activation energy (Ea) and thermodynamic parameters such as enthalpy (ΔH), entropy (ΔS), and Gibbs free energy (ΔG) were also calculated (using eqn (8)–(11) (ref. 45)) to better understand the effect of temperature on the reaction mechanism.
 | (8) |
| ΔH = Ea − RT | (9) |
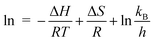 | (10) |
| ΔG = ΔH − TΔS | (11) |
The results of the thermodynamic parameters are presented in Table 3. The activation energy was calculated as −45.88 kJ mol−1, indicating an unusual reaction pathway where an increase in temperature results in a reduction in the reaction rate. The negative Ea observed in the degradation of RhB by the Mn3O4/ZnO/AC composite suggests a more complex reaction mechanism than typical elementary reactions. In simpler, single-step reactions, the activation energy is generally positive because increasing the temperature provides more molecules with enough energy to overcome the activation barrier, thus accelerating the reaction rate. However, in complex reactions involving intermediates, the situation changes. In this case, the reaction likely involves intermediates that play a crucial role in the overall degradation process. As temperature increases, the equilibrium between the reactants and these intermediates shifts, resulting in a decrease in the concentration of the intermediates. Even though the subsequent reaction step (involving the conversion of the intermediate to the final product) might proceed faster with increasing temperature due to a typical positive activation energy, the overall rate of RhB degradation can still decrease if the reduction in intermediate concentration outweighs the increased rate of the subsequent step. This interplay between the equilibrium shift and the reaction kinetics can create the appearance of a negative activation energy. The overall reaction slows down at higher temperatures not because the intrinsic activation energy is negative, but due to the diminished availability of key intermediates necessary for the reaction to proceed efficiently.46 In the case of RhB degradation, as temperature rises, the Mn3O4/ZnO/AC photocatalyst may experience structural or surface changes that limit the formation or effectiveness of intermediates, further reducing the reaction rate.
The ΔH values, ranging from −48.36 to −48.61 kJ mol−1, confirm that the degradation process is exothermic, releasing energy during the reaction. However, as the temperature increases, this energy release becomes less favorable, aligning with the observed decline in degradation efficiency at higher temperatures. The negative ΔS values, between −449.03 and −435.74 J mol−1 K−1, suggest a decrease in disorder during the reaction, possibly due to the organized adsorption of RhB molecules onto the catalyst's surface, where they align in a structured manner, reducing the overall randomness of the system. Additionally, the positive Gibbs free energy values, ranging from 85.45 to 94.32 kJ mol−1, indicate that the process is non-spontaneous without the presence of external energy, such as light.47 While light drives the photocatalytic degradation, the increase in ΔG at higher temperatures suggests that more energy is required to sustain the reaction, further explaining the drop in efficiency at elevated temperatures.
3.4. Degradation pathway of RhB
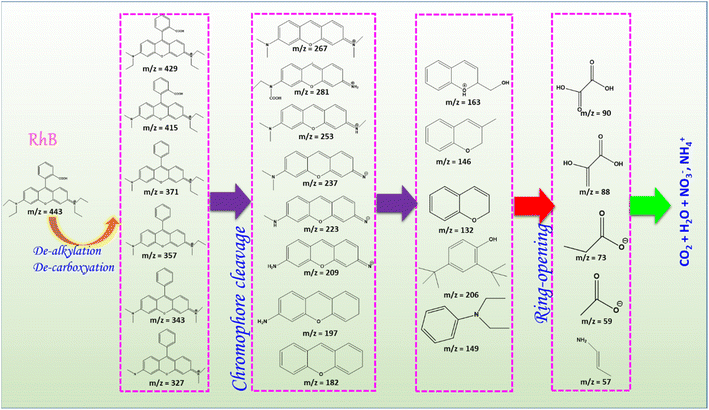
Understanding the degradation pathway of organic pollutants is crucial for evaluating the efficiency and mechanisms of photocatalytic processes. The identification of intermediate products not only provides insights into the stepwise degradation but also ensures the potential environmental safety of the final products. Catalyzed by Mn3O4/ZnO/AC under visible light irradiation was examined through GC-MS analysis. The GC-MS spectrum (Fig. 8) revealed several intermediate compounds with different mass-to-charge ratios (m/z), providing insights into the stepwise degradation of RhB. | ||
| Fig. 8 GC-MS spectrum of photodegradation products of RhB over Mn3O4/ZnO/AC catalyst under visible light after 3 h. Experiment condition: [RhB] = 10 mg L−1, pH 7, catalyst dosage of 2 g L−1, 3 h. | ||
Based on the GC-MS spectrum of photodegradation products (Fig. 8) and supported by previous studies,48,49 the plausible degradation pathway of RhB under visible light irradiation in the Mn3O4/ZnO/AC catalytic system is proposed (Fig. 9). Initially, RhB (m/z = 443) undergoes dealkylation and decarboxylation reactions, leading to the formation of partially degraded intermediates with m/z values of 429, 415, 371, 357, 343, and 327. These reactions involve the cleavage of alkyl and carboxyl groups, gradually reducing the complexity of the RhB molecule. Following these initial reactions, the chromophore structure of RhB is broken down, producing smaller aromatic compounds with m/z values of 267, 281, 253, 237, 223, 209, 197, and 182. The cleavage of the chromophore is a key step, as it results in the decolorization of the dye, effectively diminishing its environmental impact.
 | ||
| Fig. 9 Proposed degradation pathway of RhB over Mn3O4/ZnO/AC catalyst under visible light irradiation. | ||
Further degradation involves the ring-opening of aromatic intermediates into smaller organic molecules such as carboxylic acids, evidenced by the detection of compounds with m/z values of 163, 146, 132, 149, and 206. These reactions mark the transition from complex aromatic structures to simpler and more oxidized products. Ultimately, the degradation process culminates in mineralization, yielding end products such as CO2, H2O, NO3−, and NH4+. The presence of small organic acids, indicated by m/z values of 90, 88, 73, and 59, supports the complete breakdown of RhB into non-toxic compounds. This proposed degradation pathway highlights the high efficiency of the Mn3O4/ZnO/AC catalyst under visible light irradiation in decomposing RhB. These results not only validate the effectiveness of the catalyst but also provide a foundation for its application in real-world wastewater treatment systems.
3.5. Plausible mechanistic photodegradation
To understand the dominant reactive species responsible for RhB degradation over the Mn3O4/ZnO/AC composite, a series of radical trapping experiments were conducted using various scavengers to inhibit specific reactive species. The results, displayed in Fig. 10a, indicate the impact of each scavenger on the photocatalytic degradation efficiency of RhB. In the blank test (without any scavenger), the composite achieved a degradation efficiency of 95.85%. However, the introduction of scavengers significantly altered this efficiency, suggesting the primary active species involved in the photodegradation mechanism. In the presence of p-BQ, a superoxide radicals (O2˙−) scavenger, the degradation efficiency dropped drastically to 21.43%, indicating that O2˙− radicals play a crucial role in the degradation process. Similarly, the addition of IPA, a scavenger for hydroxyl radicals (˙OH), led to a reduction in degradation efficiency to 29.06%, suggesting that ˙OH radicals also significantly contribute to the photodegradation mechanism. When HCOONa was used as a hole (h+) scavenger, the efficiency decreased to 66.49%, suggesting that h+ also participates in the degradation process, though to a lesser extent than O2˙− and ˙OH. In contrast, the presence of K2Cr2O7, an electron scavenger, only slightly affected the efficiency, with a degradation rate of 91.85%, implying that photogenerated electrons play a minimal direct role in RhB degradation. These results reinforce the conclusion that O2˙− and ˙OH are the dominant reactive species driving the photocatalytic degradation mechanism on the Mn3O4/ZnO/AC composite. | ||
| Fig. 10 (a) Effect of different scavengers on the RhB degradation, and (b) proposed photodegradation mechanism of RhB over the Mn3O4/ZnO/AC composite. | ||
Based on the scavenger test results and the proposed charge separation mechanism in the Mn3O4/ZnO heterostructure under visible light (Fig. 4d), the plausible mechanism for RhB photodegradation over Mn3O4/ZnO/AC is illustrated in Fig. 10b. Upon irradiation, ZnO and Mn3O4 in the composite absorb light, promoting electrons from the valence band (VB) to the conduction band (CB) and generating electron–hole pairs (e−/h+) in both semiconductors (eqn (12) & (13)). The excited electrons in the CB of Mn3O4 migrate to the ZnO, which enhances the charge separation and reduces recombination (eqn (14)). The photogenerated electrons in the CB of ZnO react with O2 molecules in the surrounding medium to produce O2˙− (eqn (15)). Subsequently, O2˙− can further react with H2O to generate additional ˙OH radicals, as shown in eqn (16). The holes in the VB of Mn3O4, on the other hand, oxidize H2O or OH− to generate ˙OH, as shown in the eqn (17) & (18). The generated ROS contribute to the degradation of RhB, as described in eqn (19). Therefore, the in-depth understanding of the degradation mechanism further enhances the potential for optimizing this photocatalyst for targeted pollutant degradation in diverse environmental settings.
| ZnO + hν → e(CB)− + h(VB)+ | (12) |
| Mn3O4 + hν → e(CB)− + h(VB)+ | (13) |
| eMn3O4(CB)− → eZnO(CB)− | (14) |
| eZnO(CB)− + O2 → ˙O2− | (15) |
| ˙O2− + H2O → ˙OH + OH− | (16) |
| hMn3O4(VB)+ + OH− → ˙OH | (17) |
| hMn3O4(VB)+ + H2O → ˙OH + H+ | (18) |
| RhB + h+/˙OH/˙O2− → degradation products | (19) |
3.6. Reusability and stability study
The stability and reusability of the Mn3O4/ZnO/AC catalyst were assessed over four successive photocatalytic degradation cycles of RhB under the same experimental conditions. As shown in Fig. 11a, the removal efficiency of the catalyst gradually decreased with each recycling run, starting at 95.85% in the first cycle and dropping to 91.9%, 89.1%, and 88.7% in the second, third, and fourth cycles, respectively. Although there was a slight decline in efficiency, the Mn3O4/ZnO/AC composite maintained over 88% degradation performance after four cycles, indicating strong reusability and stable catalytic activity. The decrease in photocatalytic efficiency can be attributed to minor surface fouling and possible loss of active sites over repeated cycles. However, this drop in performance was minimal, highlighting the robustness of the Mn3O4/ZnO/AC material in maintaining catalytic function even after multiple uses. Such stability is essential for practical applications where long-term catalyst reusability is crucial for cost-effective and sustainable pollutant treatment.The structural stability of the catalyst after reuse was confirmed by XRD analysis (Fig. 11b). The XRD patterns of the Mn3O4/ZnO/AC composite after four cycles showed no significant shifts in peak positions, suggesting that the crystal structure of the catalyst remained intact. This indicates that no significant phase transformation or structural degradation occurred during the photocatalytic process, further confirming the material's stability. Additionally, SEM images (Fig. 11c) of the Mn3O4/ZnO/AC composite after reuse revealed no major morphological changes. The catalyst retained its original surface texture and structure, without noticeable agglomeration or particle deterioration. These observations suggest that the Mn3O4/ZnO/AC composite exhibits excellent mechanical and structural stability, which is critical for maintaining consistent catalytic activity over extended operational cycles.
To further assess the stability and environmental safety of the Mn3O4/ZnO/AC composite, leaching experiments were conducted to quantify the release of Zn2+ and Mn2+ ions by ICP-MS during the photocatalytic degradation process. The results showed that the concentrations of leached Zn2+ and Mn2+ ions were 0.88 mg L−1 and 0.26 mg L−1, respectively. These values were significantly below the maximum permissible limits set by the World Health Organization (WHO) for drinking water, which are 3 mg L−1 for Zn2+ and 0.4 mg L−1 for Mn2+.50 This indicates that the Mn3O4/ZnO/AC composite exhibits excellent chemical stability during operation, with minimal release of its constituent metal ions into the treated solution. The low leaching levels suggest that the material retains its structural integrity and minimizes the risk of secondary contamination, which is critical for the safe application of photocatalysts in real-world water treatment scenarios. Moreover, the observed ion concentrations were not only within safe limits but also reflected the robust bonding and interactions within the Mn3O4/ZnO/AC composite, which likely reduce the solubility of ZnO and Mn3O4 phases under photocatalytic conditions. An additional contributing factor is the neutral pH (pH 7) of the reaction environment, which helps stabilize the composite and prevents excessive ion dissolution. At pH 7, the solubility of metal oxides is minimized, reducing the likelihood of leaching and ensuring the material's chemical stability during the photocatalytic process. Overall, the reusability and stability tests confirm that the Mn3O4/ZnO/AC catalyst exhibits both high initial photocatalytic activity and outstanding durability, highlighting its potential for long-term environmental remediation applications.
4. Conclusions
This study successfully developed a Mn3O4/ZnO nanocomposite supported on microalgae-derived AC for efficient RhB degradation under visible light. The incorporation of Mn3O4 and ZnO into the AC matrix significantly enhanced photocatalytic efficiency by improving light absorption, charge carrier separation, and reactive oxygen species generation. Under optimal conditions ([RhB] = 10 mg L−1, catalyst dosage = 2 g L−1, pH = 7, T = 25 °C), the composite achieved over 95% RhB removal and 80.56% mineralization within 420 min of irradiation. Mechanistic investigations confirmed that hydroxyl radicals and superoxide radicals played dominant roles in RhB degradation. Stability tests revealed that the composite maintained 88.7% degradation efficiency after four cycles, with XRD and SEM analyses indicating minimal structural changes post-reuse. Furthermore, metal leaching tests confirmed the environmental compatibility of the composite, with Zn2+ (0.88 mg L−1) and Mn2+ (0.26 mg L−1) concentrations remaining well within WHO safety limits. These findings highlight the potential of Mn3O4/ZnO/AC as a cost-effective, sustainable photocatalyst for organic pollutant removal in wastewater treatment. The use of microalgae-derived AC aligns with green chemistry principles, transforming biomass waste into a value-added material. However, challenges remain, particularly regarding efficiency loss at elevated temperatures, which could limit large-scale outdoor applications. Future research should focus on enhancing thermal stability, integrating co-catalysts, and optimizing reactor design to improve practical performance and scalability.Data availability
All data generated or analyzed during this study are included in this published article.Author contributions
Conceptualization: Hoc Thang Nguyen, Van-Dat Doan, and Van Thuan Le; investigation and formal analysis: Hoc Thang Nguyen, Thi Lan Huong Nguyen, Anh-Tien Nguyen, and Quang-Hieu Tran; visualization and writing—original draft preparation: Hoc Thang Nguyen, Anh-Tien Nguyen, and Vy Anh Tran; writing—review and editing: Van-Dat Doan, Vy Anh Tran, and Van Thuan Le.Conflicts of interest
There are no conflicts to declare.References
- C. Benbrika, H. Menasra, A. Kularkar, L. Smaili and A. Sbaihi, J. Phys. Chem. Solids, 2024, 184, 111702 CrossRef CAS.
- A. Krishnan, A. Swarnalal, D. Das, M. Krishnan, V. S. Saji and S. M. A. Shibli, J. Environ. Sci., 2024, 139, 389–417 CrossRef CAS PubMed.
- F. Güell, A. Galdámez-Martínez, P. R. Martínez-Alanis, A. C. Catto, L. F. da Silva, V. R. Mastelaro, G. Santana and A. Dutt, Mater. Adv., 2023, 4, 3685–3707 RSC.
- A. R. Bhapkar and S. Bhame, J. Environ. Chem. Eng., 2024, 12, 112553 CrossRef CAS.
- A. Ashpak Shaikh, M. Rajendra Patil, B. Sonu Jagdale and V. Ashok Adole, Inorg. Chem. Commun., 2023, 151, 110570 CrossRef CAS.
- P. Akhter, S. Nawaz, I. Shafiq, A. Nazir, S. Shafique, F. Jamil, Y.-K. Park and M. Hussain, Mol. Catal., 2023, 535, 112896 CrossRef CAS.
- P. Jansanthea, N. Inyai, W. Chomkitichai, J. Ketwaraporn, P. Ubolsook, C. Wansao, A. Wanaek, A. Wannawek, S. Kuimalee and P. Pookmanee, Chemosphere, 2024, 351, 141212 CrossRef CAS PubMed.
- S. Khan, A. Hussain, K. He, B. Liu, Z. Imran, J. Ambreen, S. Hassan, M. Ahmad, S. S. Batool and C. Li, J. Environ. Manage., 2021, 293, 112854 CrossRef CAS PubMed.
- A. Gupta, Kajal, K. Ajravat, L. K. Brar, O. P. Pandey and P. Rajagopalan, Mater. Chem. Phys., 2024, 313, 128698 CrossRef CAS.
- T. Rungsawang, S. Krobthong, K. Paengpan, N. Kaewtrakulchai, K. Manatura, A. Eiad-Ua, C. Boonruang and S. Wongrerkdee, Radiat. Phys. Chem., 2024, 223, 111924 CrossRef CAS.
- A. G. M. Shoaib, H. T. Van, D. T. Tran, A. El Sikaily, M. A. Hassaan and A. El Nemr, Sci. Rep., 2024, 14, 11583 CrossRef CAS PubMed.
- Z. Djezzar, A. Aidi, H. Rehali, S. Ziad and T. Othmane, RSC Adv., 2024, 14, 5276–5289 RSC.
- V. T. Le, T. K. N. Tran, N. K. Dang, V. D. Doan, V. A. Tran, Y. Vasseghian and T. M. Aminabhavi, Chem. Eng. J., 2023, 474, 145903 CrossRef CAS.
- I. K. Tetteh, I. Issahaku and A. Y. Tetteh, Carbon Trends, 2024, 14, 100328 CrossRef CAS.
- J. Chen, K. Chu, S. Sun, H. Chen, B. Song, J. Wang, Z. Liu and L. Zhu, J. Environ. Chem. Eng., 2023, 11, 109230 CrossRef CAS.
- M. U. Dao, H. S. Le, H. Y. Hoang, V. A. Tran, V. D. Doan, T. T. N. Le, A. Sirotkin and V. T. Le, Environ. Res., 2021, 198, 110481 CrossRef CAS PubMed.
- V. T. Le, M. U. Dao, H. S. Le, D. L. Tran, V. D. Doan and H. T. Nguyen, Environ. Technol., 2020, 41, 2817–2832 CrossRef CAS PubMed.
- J. A. Lett, S. F. Alshahateet, I. Fatimah, R. P. Sivasankaran, A. K. Sibhatu, M.-V. Le and S. Sagadevan, Top. Catal., 2023, 66, 126–138 CrossRef CAS.
- M. L. de Peres, R. de A. Delucis, S. C. Amico and D. A. Gatto, Nanomater. Nanotechnol., 2019, 9 DOI:10.1177/1847980419876201.
- X. Zhang, S. Luo, X. Wu, M. Feng, Y. Li, H. Han and W. Li, Open Chem., 2021, 19, 377–384 CrossRef CAS.
- D. Anbuselvan, S. Nilavazhagan, A. Santhanam, N. Chidhambaram, K. V. Gunavathy, T. Ahamad and S. M. Alshehri, Phys. E, 2021, 129, 114665 CrossRef CAS.
- F. Mouzaia, D. Djouadi, A. Chelouche, L. Hammiche and T. Touam, Arab J. Basic Appl. Sci., 2020, 27, 423–430 Search PubMed.
- E. Arulkumar, S. Santhosh Shree and S. Thanikaikarasan, Results Chem., 2023, 6, 101169 CrossRef CAS.
- R. Rahimi, A. Mehrehjedy and S. Zargari, Environ. Prog. Sustainable Energy, 2017, 36, 1439–1448 CrossRef CAS.
- L. Bigiani, D. Zappa, C. Maccato, A. Gasparotto, C. Sada, E. Comini and D. Barreca, Nanomaterials, 2020, 10, 511 CrossRef CAS PubMed.
- K. D. Jayeola, D. S. Sipuka, T. I. Sebokolodi, O. V. Nkwachukwu, C. Muzenda, B. A. Koiki, J. O. Babalola, M. Zhou and O. A. Arotiba, Chem. Eng. J., 2024, 479, 147482 CrossRef CAS.
- J. Jiang, X. Zhang, P. Sun and L. Zhang, J. Phys. Chem. C, 2011, 115, 20555–20564 CrossRef CAS.
- N. S. M. Sayed, A. S. A. Ahmed, M. H. Abdallah and G. A. Gouda, Sci. Rep., 2024, 14, 5384 CrossRef CAS.
- M. Osanloo, F. Khorasheh and A. Larimi, J. Mol. Liq., 2024, 407, 125242 CrossRef CAS.
- S. Abou Zeid and Y. Leprince-Wang, Crystals, 2024, 14, 611 CrossRef CAS.
- V. T. Le, H. S. Le, V. A. Tran, L. Sang-Wha, V.-D. Doan, S.-W. Joo and Y. Vasseghian, J. Ind. Eng. Chem., 2022, 115, 345–354 CrossRef CAS.
- M. Lal, P. Sharma, L. Singh and C. Ram, Results Eng., 2023, 17, 100890 CrossRef CAS.
- Y. Stiadi, T. P. Wendari, Zilfa, Zulhadjri and Rahmayeni, Environ. Eng. Res., 2022, 28, 220074 CrossRef.
- E. Amdeha and R. S. Mohamed, Environ. Technol., 2021, 42, 842–859 CrossRef CAS PubMed.
- P. G. Ramos, J. Espinoza, L. A. Sánchez and J. Rodriguez, J. Alloys Compd., 2023, 966, 171559 CrossRef CAS.
- I. Fatimah, R. Audita, G. Purwiandono, H. Hidayat, S. Sagadevan, W.-C. Oh and R. Doong, Case Stud. Chem. Environ. Eng., 2024, 10, 100797 CrossRef CAS.
- A. Masoud, M. A. Ahmed, F. Kühn and G. Bassioni, Heliyon, 2023, 9, e22342 CrossRef CAS PubMed.
- V. T. Le, V. A. Tran, D. L. Tran, T. L. H. Nguyen and V.-D. Doan, Chemosphere, 2021, 270, 129417 CrossRef CAS PubMed.
- F. Laatar, H. Moussa, H. Alem, L. Balan, E. Girot, G. Medjahdi, H. Ezzaouia and R. Schneider, Beilstein J. Nanotechnol., 2017, 8, 2741–2752 CrossRef PubMed.
- A. T. Le, N. S. B. Samsuddin, S.-L. Chiam and S.-Y. Pung, Bull. Mater. Sci., 2021, 44, 5 CrossRef CAS.
- H. T. Nguyen, M. T. Truong, V.-D. Doan, T. L. H. Nguyen, V. H. Hoang, V. A. Tran, A.-T. Nguyen and V. T. Le, Chem. Eng. Sci., 2024, 284, 119487 CrossRef CAS.
- N. Mzimela, S. Tichapondwa and E. Chirwa, RSC Adv., 2022, 12, 34652–34659 RSC.
- V. Doan, T. Thanh, N. Nguyen, H. Ai, L. Pham, T. Lan, H. Nguyen and V. T. Le, J. Mol. Liq., 2024, 398, 124261 CrossRef CAS.
- I. Groeneveld, M. Kanelli, F. Ariese and M. R. van Bommel, Dyes Pigm., 2023, 210, 110999 CrossRef CAS.
- V. T. Le, T. G. Duong, V. T. Le, T. L. Phan, T. L. Huong Nguyen, T. P. Chau and V.-D. Doan, RSC Adv., 2021, 11, 15438–15448 RSC.
- Y.-H. Chiu, T.-F. Chang, C.-Y. Chen, M. Sone and Y.-J. Hsu, Catalysts, 2019, 9, 430 CrossRef CAS.
- W. R. Abd-Ellatif, N. G. Mahmoud, A. A. Hashem, M. K. El-Aiashy, E. M. Ezzo and S. A. Mahmoud, Environ. Technol. Innovation, 2022, 27, 102393 CrossRef CAS.
- Y. Ren, J. Wang, G. Qu, N. Ren, P. Lu, X. Chen, Z. Wang, Y. Yang and Y. Hu, Sep. Purif. Technol., 2023, 314, 123616 CrossRef CAS.
- N. A. Chopan and H.-T.-N. Chishti, Mater. Today Chem., 2023, 32, 101643 CrossRef CAS.
- M. Braga, R. Jaimes, W. Borysow, O. Gomes and W. Salcedo, Sensors, 2017, 17, 1730 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |