Open Access Article
Open Access ArticleTherapeutic potential of betaine and its derivatives in cancer treatment: a comprehensive review
Muhammad Shahzaib Hassan†
 a,
Tanzila Khalid†a,
Maida Akhlaqa,
Abdul Hameedb,
Faiza Sharif
a,
Tanzila Khalid†a,
Maida Akhlaqa,
Abdul Hameedb,
Faiza Sharif c,
Sobia Ranad and
Maliha Uroos
c,
Sobia Ranad and
Maliha Uroos *a
*a
aSchool of Chemistry, University of the Punjab, Lahore-54590, Pakistan. E-mail: malihauroos.chem@pu.edu.pk
bDepartment of Chemistry, University of Sahiwal, Sahiwal 57000, Pakistan
cInterdisciplinary Research Centre in Biomedical Materials, COMSATS University Islamabad, Lahore Campus, Lahore, Pakistan
dDr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Karachi-75270, Pakistan
First published on 17th June 2025
Abstract
Bioactive natural compounds and their analogues play a significant role in the treatment of fatal diseases such as malaria, pneumonia, hepatic disorders, heart diseases and various types of cancer. Existing chemoprotective treatments for different types of cancer employ numerous natural compounds from medicinal plant extracts, fruits, vegetables, herbs, microorganisms and marine life. One of these bioactive compounds is betaine, which is a natural constituent with remarkable antioxidant and anticancer properties. The betaine scaffold and its derivatives have demonstrated suppressive effects on the progression, metastasis and angiogenesis of cancer cells. Although multiple studies have reported that betaine and its derivatives demonstrate remarkable efficiency in treating cancer or preventing tumor growth, a review summarizing the literature on this important anticancer agent is lacking to date. Thus, the present review article provides a detailed overview of the reported literature on the use of betaine and its derivatives in the prevention of different types of cancers and discusses the inhibitory activities of betaine against various types of tumorigenesis and its mechanism of action. The synergistic effects of betaine with phytochemicals and nanostructure-based medication delivery systems and its molecular modification for lowering the risk of cancer in humans are also discussed in detail.
1. Introduction
Cancer has become a major health concern in recent years as it is the second leading cause of mortality after heart disease.1,2 Cancer is defined by the uncontrolled growth of cells, which results in the formation of tumors.3 It can result from genetic anomalies in any gene that codes for a cell cycle protein or somatic mutations in upstream cell signalling pathways. Annually, millions of patients die from cancer-related causes, despite the increasing availability of traditional cancer treatment alternatives.4 Furthermore, many conventional therapeutic approaches are no longer effective against many malignant tumors because of the metastasis, recurrence, diversity, and resistance of cancer cells to chemotherapy and radiation.5 Conventional cancer therapy approaches cannot always yield adequate results, since numerous limitations have been identified. Chemotherapy is considered the most popular therapeutic modality and is employed in numerous cancer therapies.6 However, conventional chemotherapeutic drugs have been widely shown in the literature to have the drawback of nonspecific distribution, resulting in low bioavailability, quick blood clearance, and relatively poor solubility in bodily fluids.7,8 In addition, the emergence of drug resistance in tumor cells following repeated antitumor drug treatment significantly reduces the curative effect of chemotherapy.9 The ultimate objective of cancer therapy agents is to selectively kill tumor cells while preventing damage to the surrounding healthy cells and tissues, although this has not been accomplished yet.Herbs and botanical plants are natural medicines that have proven capacity and therapeutic qualities to inhibit cancer growth. Additionally, natural dietary ingredients such as fruits, vegetables, and spices have gained significant interest from the scientific community and general public,10 and some of these have been turned into functional foods.11 Dietary agents are made up of a broad range of physiologically active substances that are found in many plants and have been utilized for thousands of years in indigenous medicine owing to their strong anti-inflammatory, antifungal, antiviral and antibacterial properties.12 They are also beneficial for treating or preventing systemic and infectious disorders because of immunomodulatory processes such as altered cytokine secretion, histamine release, immunoglobulin synthesis, immunoglobulin class switching, expression of cellular co-receptors, lymphocyte proliferation and stimulation of phagocytosis.13
Generally, cancer occurs due to abnormalities in genetic and epigenetic processes. Epigenetic processes work by regulating gene expression through a variety of channels without changing the sequence of DNA. Cytosine residue methylation in cytosine–guanine (CG) pairs, which yields 5-methylcytosine, is one of the epigenetic techniques involved in DNA methylation. The loss of DNA integrity and genetic abnormalities can result from the hypomethylation of CG sites. Tumor cells may begin to grow and spread as a result of the inactivation of tumor suppressors such as p16 and activation of particular proto-oncogenes such as c-Myc.14 Attaining balance between DNA methylation and demethylation mechanisms sustain optimum methylation patterns under normal physiological settings. However, this balance is upset by several pathogenic phenomena such as cancer, inflammation and oxidative stress. Due to its reversible nature, the process of DNA methylation is gaining interest as a potential target for the treatment of cancer. According to certain research, nutrition and diet can influence DNA methylation by promoting the synthesis of methyl donors at particular CG sites. The field of nutri-epigenomics, the interface between nutrients and epigenetics, is one that shows promise for the application of bioactive foods in cancer treatment in the future. These bioactive foods are regarded as necessary methyl donors for DNA methylation and include vitamin B12, folic acid, choline and betaine.15 Therefore, they can have both curative and preventive effects on cancer.
In this review article, we first briefly discuss the anticarcinogenic action of betaine and its different derivatives and complexes (Fig. 1), particularly focusing on current investigations analysing their impact on the development of cancer and their possible underlying mechanisms. After that, we discuss novel approaches to treat cancer by combining betaine with anticancer medications, phytochemicals, nanotechnology and structurally modified betaine molecules.
 | ||
| Fig. 1 Some common (a) derivatives and (b) composites of betaine exhibiting potent anticancer activities. | ||
2. Production of betaine and its significance
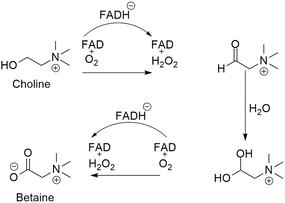
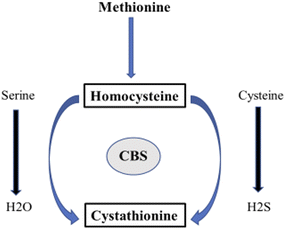
Betaine, also known as trimethyl glycine, was initially discovered in beets (Beta vulgaris) in the 19th century.16 It is a stable, harmless natural chemical found in plants, animals and microorganisms. In addition, it is present in high amounts in microbes, aquatic invertebrates and other dietary sources such as spinach, wheat bran and wheat germ. Choline metabolism produces betaine (Fig. 2) endogenously or it can be obtained exogenously through the diet.17 Betaine can prevent numerous diseases such as kidney malfunctioning, liver disorders, homocysteinemia and heart problems and has a wide range of physiological benefits on overall health. Betaine, similar to other electrolytes, functions as an osmolyte by donating methyl groups to maintain the intracellular osmotic pressure. The structure and function of proteins are stabilized when cells regulate the surface tension of water through the action of betaine, which exhibits minimal or no binding to protein surfaces. Thus, it defends against osmotic stress on proteins, cells, and enzymes. Because the kidney has a high concentration of urea and electrolytes, this is especially important. In terms of hydrating albumin, betaine is the most successful osmolyte that has been researched. It is capable of preserving haemoglobin solvation and creating nearly a full monolayer of water surrounding proteins. Betaine, being an osmolyte, prevents the immune system from being affected by the hyperosmolarity-induced inhibition of tumor necrosis factor α-release, prostaglandin synthesis, and cyclooxygenase-2 expression in liver-resident macrophages, known as Kupffer cells. The ability of betaine to donate its methyl group to the harmful metabolite homocysteine and convert it to methionine is one of its other significant roles. Betaine–homocysteine methyltransferase (BHMT), an enzyme first believed to be mostly found in the liver and kidneys, is responsible for catalyzing this process. A recent study has shown that BHMT is expressed in the gut and white adipose tissue, two significant organs. These results have encouraged further investigation into the potential benefits of betaine for both human wellness and the prevention of disease.3. Chemical aspects of betaine in different types of cancer treatment
Betaine is an inert, naturally occurring chemical that has three extra methyl groups. Studies on a variety of human illnesses such as cancer, Alzheimer's disease and metabolic syndrome revealed the advantageous and beneficial effects of betaine. Furthermore, in in vitro tests on cancer, betaine has been shown to suppress the proliferation of cancer cells. According to one hypothesis, betaine regulates oxidative stress, apoptosis and inflammation in single-carbon metabolism, which is effective in cancer treatment. Unusual alterations in DNA methylation contribute to the initiation and advancement of cancer by triggering the activation of specific proto-oncogenes such as c-Myc and the inactivation of specific tumor suppressors such as p16. BHMT catalyzes trans-methylation activity, which requires betaine as a methyl group donor. It was revealed that the methylation levels were influenced by the presence of methyl group donors. Additionally, the trans-sulfuration pathway, which changes homocysteine into cystathionine, is connected to the methyl group donors. The level of glutathione (GSH) is one of the key indicators of the oxidant-antioxidant state in these pathways.18 Trans-methylation and trans-sulfuration pathway metabolite level alterations have been identified as initiators of oxidative stress and apoptosis. The amounts of oxidant-antioxidant molecules and methionine cycle metabolites were altered by varying betaine doses. Low-dose betaine administration increased the GSH and S-adenosyl methionine (SAM) levels, while lowering the homocysteine levels. An essential transcription factor called nuclear factor-κB (NF-κB) regulates a large number of genes linked to oxidative damage-induced inflammation. Pro-inflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are encoded by these genes. When associated with certain cancer disorders, betaine can change these cytokine levels. Cancer is caused by unchecked cell division and the inability of cells to undergo apoptosis.19 Therefore, substances that can trigger the death of cancer cells can lead to the development of effective cancer treatment medications. Caspases, which are primarily engaged in the mechanism of apoptosis, are impacted by betaine. In following sections, the progress in exploring anticancer potential of betaine (Table 1) thus far is described in detail.| Sr. no. | Type of cancer | Betaine and its complexes | Cell line and animal model | Observed effects | Ref. |
|---|---|---|---|---|---|
| 1 | Breast cancer | Betaine gallium tetrachloride–ZnO NPs | Mammary tumors in female rats | Apoptosis increased | 20 |
| Pegylated chitosan nanoparticles loaded with betaine and nedaplatin | Mammary gland tumors in rats | Lowering of MCF-cell line viability and reduction in the size of tumors | 21 | ||
| Polyhexamethylene biguanide–betaine (PHMB–B) + silver sulfadiazine | Breast cancer wounds in woman | Biofilm treatment | 22 | ||
| Nanoemulsion of betaine and belinostat containing BGs | ER-positive ZR-75-1 and TNBC, MDA-MB-231 | Decrease in angiogenesis-related indicators, i.e. VEGF and TSP-1 | 23 | ||
| 2 | Lung cancer | Synergistic effect of betaine and C-phycocyanin | A549 cell line of nude rats | Decrease in NF-κB expression and increase in proapoptotic p38 MAPK and cell cycle arrest in the G2/M phase | 24 |
| Betaine and C-phycocyanin as micro nutrients | A549 cells in naked rats | Decrease in phosphorylated AKT/total AKT ratios, while increase in phosphorylated p38/total p38 ratio, increase in autophagy and apoptosis | 25 | ||
| 3 | Cervical cancer | Betaine | MCF-10A and HeLa cells | Interruption of cell cycle at the G1/S or S/G2 checkpoints, activation of p53, cyclin D1, and apoptosis | 26 |
| 4 | Colon cancer | Betaine | In vivo tests on mice | Reduction in inflammatory cytokines, including TNF-α, IL-6, iNOS, and COX-2 | 27 |
| 5 | Prostate cancer | Betaine | DU 145 cell line | Decrease in glutathione GSH and total antioxidant status (TAS), cell proliferation and increase in apoptosis | 28 |
| Betaine | C4-2B cell line | Upregulation of the protein expression of Bax, caspase-3 and decrease in phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT), and nuclear factor (NF-κB) | 29 | ||
| 6 | Oral squamous cell carcinoma | Synergistic effect of δ-valerobetaine and γ-butyrobetaine | CAL 27 cell line | Activation of SIRT1 and decrease of cyclin B1 and procaspase 3 | 30 |
| 7 | Hepatic cancer | Betaine | Rat liver cells | Activation of proto-oncogenes, i.e., c-myc and inactivation of tumor suppressors genes, i.e., p16 | 31 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Role of betaine in drug delivery systems | |||||
| i | Breast and cervical carcinoma | Betaine complex with p-SC4 | MCF-7 and HeLa cells | Cytotoxicity | 32 |
| ii | Chronic myeloid leukemia | Betaine–dasatinib complex | Decrease in pH of the gastrointestinal tract | 33 | |
| iii | Chronic lymphocytic leukemia and non-Hodgkin's lymphoma | Dual chlorambucil-tailed betaine conjugate | MCF-7, HeLa and HepG2 cancer cells | Cytotoxicity | 34 |
| iv | Multiple cancers | DOX–SPBB–siRNA | A549 cells | Serum tolerance, low cytotoxicity and impressive gene delivery | 35 |
3.1 Suppression of breast cancer
Breast cancer is the most common type of cancer in women worldwide. The World Health Organization (WHO) states that breast cancer is the most common proliferative disease among women globally, which accounts for up to one-third of newly diagnosed cases of women cancer in some countries.36 Annually, more than 1.5 million women (or 25% of all women with cancer) are diagnosed with breast cancer worldwide.37 It was estimated that breast cancer contributed 30% of all new cancer diagnoses among women in the United States in 2017.38 Breast cancer is usually metastatic because it spreads to distant organs such as the liver, brain, lungs, and bone, making it incurable. One of the biggest challenges in its effective treatment is the resistance of breast cancer cells to the current chemotherapy treatments. This disease, once it has spread to distant areas, is typically incurable with current systemic therapies including radiation, chemotherapy, immunotherapy, and hormone therapy. Thus, it is necessary to create novel approaches for the early identification, management, and elimination of cancer resistance.39,40Betaine, a metabolite of choline,41 has been found to be an effective cancer-preventive agent. Increased betaine and choline consumption have also been shown to decrease the mortality associated with breast cancer in a dose-dependent manner, suggesting that this might be a potential way to either prevent it from developing or to help lower its mortality once it has been identified.42 Some complexes of betaine, focusing on the recent developments in breast cancer therapy and its identification, are discussed below (Fig. 3).
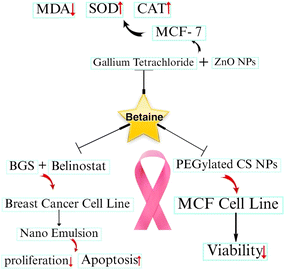 | ||
| Fig. 3 Some complexes of betaine, focusing on the recent developments in breast cancer therapy and its identification. | ||
Breast adenocarcinoma (MCF-7) cells, as the most commonly used breast cancer cell line43 in cancer research, and breast cancer-bearing rats were used to examine the anticancer effects of PEGylated chitosan nanoparticles (CS-NPs) coloaded with betaine (BT) and nedaplatin (ND). Nanoparticles of different polymers are used as vehicles for drug delivery due to their ability to encapsulate different active compounds and modify their surface.44,45 In this case, due to the bioavailability and biodegradability of chitosan, it is mainly used for the synthesis of nanoparticles as effective drug delivery vehicles.46 PEG-uncoated and PEG-coated CS NPs covering either BT, ND, or both (BT–ND) were synthesized using the ionotropic gelation technique. The produced nanoparticles had comparatively high surface charges. Due to their spherical shape, they showed high entrapment efficiency. The results of release studies showed that both PEGylated and non-PEGylated CS NPs could release their contents into the pH 5.5 micro-environment of tumor cells. Furthermore, the NPs showed exceptional capacity to reduce the viability of MCF-7 cells. It was discovered that BT–ND/PEG–CS NPs had the greatest potency among the NP preparations, resulting in a 90% reduction in the size of the tumors in the mammary gland of rats compared to the animals attached treated with the vehicle21 (Table 2).
| Polymeric nanoparticles (PNPs) | Liposomes | Metal–organic frameworks (MOFs) | |
|---|---|---|---|
| Physicochemical properties | Particles of solid colloids, ranging in size from 1 to 1000 nm, made of biodegradable polymers | Biocompatible and biodegradable spherical vesicles containing lipid bilayers, ranging in size from 50 to 450 nm | 90–300 nm in size, crystalline porous structures are made up of organic linkers and metal ions |
| Drug-loading capacity | High loading for hydrophobic medications: regulated release characteristics | Drugs that are hydrophilic or hydrophobic can be encapsulated with an efficiency of up to 92% | Significant drug loading is possible due to the high surface area; encapsulation efficiency is approximately 90% |
| Pharmacokinetics | Reduced rates of degradation and increased bioavailability | PEGylation enhances stability; the reticuloendothelial system (RES) clears it quickly; short half-life | Varies; relies on metal ions and linkers; some show quick clearance, while others show longer circulation |
| Toxicity profile | Low toxicity; depending upon breakdown products and polymer type | Generally safe, although susceptible to oxidation and leaks | The discharge of metal ions may be hazardous; composition affects biocompatibility |
| In Vitro efficacy | Elevated rates of apoptosis in cancer cell lines, long-term medication release | Increased cancer cell cytotoxicity and improved cellular uptake | Effective medication release in acidic environments with dual-responsive release (pH/ultrasound) |
| In Vivo efficacy | Longer survival rates and a notable reduction of tumor development in animal models | Enhanced permeability and retention (EPR) impact for better tumor targeting and decreased systemic toxicity | Quick absorption with a long half-life; biodegradable with low toxicity |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Examples | |||
| Betaine coloaded with PEGylated chitosan nanoparticles (CS NPs) | Liposome-like nanocapsules of dual-tailed betaine | Ag@NMOF nanocomposite | |
| Nanoparticles based on poly(t-butyl betaine carboxylate) | Liposomal form of beet root pigment | HAP/MIL-88(Fe) (HM) nanocomposite | |
| Ref. | 21, 47 and 48 | 34, 49 and 50 | 51 and 52 |
3.2 Suppression of lung cancer
Globally, lung cancer is the leading cause of cancer-related mortality.55 Lung cancer appears in two varieties, non-small cell lung cancer (NSCLC) and small cell lung cancer. Between them, the former is the most common,56 accounting for about 85% of lung cancer cases and surgery, immunotherapy, tyrosine kinase inhibitors, and platinum-based chemotherapy are all used to treat it.573.3 Suppression of cervical cancer
Cervical cancer accounted for 9% of all new cases and 8% of all female cancer deaths in 2008, making it the third most common cancer overall and the fourth leading cause of cancer-related death in women worldwide.59 The majority of these cases, i.e., more than 85%, occur in underdeveloped nations. Additionally, 27% of all cervical cancer fatalities occur in India, the second most populous nation in the world.60 The lack of screening for the early diagnosis of cervical cancer is a major contributing factor to the disproportionately high incidence of cervical cancer in medically underprivileged populations and developing nations.61,62 Cervical cancer has been treated with a comprehensive approach, which includes chemotherapy, radiation therapy, and surgery since 1999.63 Many phytonutrients extracted from edible plants are the most promising agents that have been shown to disrupt various phases of carcinogenesis, potentially reducing 7–31% of all cancer cases globally.64 One of them is betaine, which inhibits the methylation patterns of proto-oncogenes and tumor suppressors to control their expression and has an anticancer effect.65Originating from the biopsy of a tumor of cervical cancer, the first human cell line to be developed in culture was HeLa cells.66 The underlying molecular processes responsible for the impact of betaine on HeLa cell proliferation and apoptosis was studied. The anticancer activity of betaine in HeLa cells was assessed at concentrations of 0.1, 1.0, 5.0, 20.0, and 100.0 mg mL−1, respectively. MCF-10A was also found to be a normal diploid cell control. Using the MTT test, Yu et al. discovered that exposure to increasing betaine levels significantly decreased the growth of HeLa cells (p < 0.05). High concentrations of betaine (>5.0 mg mL−1) greatly accelerated the death of HeLa cells, and the percentage of S phase cells in the low dosage groups (<5 mg mL−1) was significantly higher than in the high dose groups, with the rates of the sub-G1 phase being the opposite (p < 0.01). The low-dose groups SOD activity was somewhat greater than that of the control group. The expression of the apoptosis-suppressive gene Bcl-2 and the apoptosis-promoting genes BAX, P53, and caspase-3 showed clear synchronization and association. When the cell cycle gets interrupted at the G1/S or S/G2 checkpoints, p53 and cyclin D1 may be activated in response to a stimulus that induces apoptosis.26
3.4 Suppression of colon cancer
Colon and rectal cancers are types of cancerous growth originating from the lining of the large intestine. Individuals who suffer from Crohn's disease (CD) and ulcerative colitis (UC) are those with inflammatory bowel disease (IBD) who are more susceptible to be diagnosed with colorectal cancer (CRC).67 CRC is a global health issue whose prevalence is steadily rising. Up to 20 years ago, IBD was thought to be rare in Asia.68 However, more recent cohorts from referral centres and population-based studies have revealed an increase in the incidence and prevalence of IBD in Asia.69 A defective immune response and luminal, environmental and genetic variables, such as tumor necrosis factor (TNF)-α, can contribute to the development of IBD. Particularly, the blood, intestinal mucosa, and faeces of IBD patients had the indicated level of TNF-α. Other pro-inflammatory mediators have also been found to be elevated in the faeces and rectal dialysates of IBD patients, in addition to TNF-α.70 Based on available data, there is a growing trend of TNF-α inhibitor use in IBD-related research and patient populations. TNF-α antibodies, such as chimeric IgG1 monoclonal antibody infliximab, human monoclonal IgG1 antibody adalimumab, and PEGylated Fab fragment of a humanized IgG4 isotype monoclonal antibody certolizumab pegol are the most often utilized biologics in IBD.71 However, these anti-TNF medications explicitly increase the overall risk of serious infections such as varicella, tuberculosis, and other diseases.72Researchers have demonstrated that betaine exhibits anti-inflammatory properties by suppressing reactive species (RS) and adjusting the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) during the aging process, as evidenced by both in vitro and in vivo investigations.73–75 Likewise, Dong et al. investigated the impact of the anti-inflammatory and tumor-preventive properties of betaine on mice with colitis-associated cancer. They used azoxymethane (AOM) and dextran sulfate sodium (DSS) to create colon cancers in mice during the in vivo tests, and they assessed the impact of betaine on tumor formation. When betaine was administered, the incidence of tumor formation was considerably reduced and inflammation was diminished. In the colonic mucosa, betaine treatment reduced the amount of GSSG and the production of ROS. The treatment of betaine reduced inflammatory cytokines, including TNF-α, IL-6, iNOS, and COX-2, according to the qPCR findings. Betaine administration reduced LPS-induced NF-κB and inflammatory-related cytokines in RAW 264.7 murine macrophage cells in vitro. Thus, it was proven that betaine is a potential treatment option for colon inflammation.27
3.5 Betaine for prostate cancer control
Prostate cancer (PCa) is the fifth most common cause of cancer-related mortality in males and the second most common solid tumor. Giorgio et al. profoundly investigated the epidemiology of this cancer worldwide and inferred its risk factors based on heredity, environmental factors, metabolic diseases, dietary factors and physical activities.76DU-145 is an important cell line for investigating prostate cancer, and the cell viability of DU-145 human prostate cancer cells was evaluated based on dose-dependent betaine.78
The treatment employing low doses of betaine concentrations statistically enhanced the cell viability. The betaine concentration of 50 mg mL−1 was the primary indicative concentration that exhibited a convincing decrease from untreated cells. Above this concentration, the cell viability greatly decreased.
Subsequently, Fatih Kar et al. demonstrated that betaine inhibited proliferation and caused oxidative stress and apoptosis, and its high dose treatment caused inflammation by increasing the total oxidant status (TOS) levels and decreasing the glutathione GSH and total antioxidant status (TAS) in the DU-145 prostate cancer cell line. Thus, the therapeutic effect of betaine is entirely based on its dosage and concentration.28
The Bax gene is an apoptosis-promoting constituent of the bcl-2 gene group. The Bax protein governs cell death through its cooperation in the disruption of mitochondria and successive cytochrome c release, and is also known to be one of the essential p53 targets. Caspase-3 is well-known as one of the primary regulators of apoptosis. The activation of caspase-3 demands proteolytic processing of its dormant and inactive zymogen state into activated p17 and p19 subunits. Bax initiates the release of cytochrome c from the mitochondria, and as a result cytochrome c release stimulates caspase-3. Cleaved caspase-3 is considered to play a fundamental role in apoptosis.79 This research group reported that a specific concentration of betaine upregulated the protein expression of Bax and caspase-3, and at the same time downregulated phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT) and nuclear factor (NF-κB).29
3.6 Betaine in oral squamous cell carcinoma
N D'Onofrio et al. substantially studied the effect of dietary betaine on the oral squamous cell carcinoma CAL-27 cell line. Oral and oropharynx cancer is challenging, precarious and escalating worldwide especially among young patients.80 Oral cancer is a protruding tumor, which undergoes rapid metastasis, and ultimately leads to death. Different oral squamous cell carcinoma (OSCC) cell lines have been employed as a tool to investigate the therapeutic treatment of oral cancer. CAL-27 is one of primary cell lines for the detailed inspection of oral cancer. This research group prudently scrutinized the synergistic role of δ-valerobetaine (δVB) and γ-butyrobetaine (γBB) (present in various diets) on the inhibition and suppression of cell proliferation and induction of apoptosis in the CAL-27 cell line. In addition, the level of sirtuins was also studied in the cell proliferation and inhibition of tumors. In mammals, there are seven members of sirtuins (SIRT1-SIRT7), among which SIRT1 plays important role in the deacetylation of histones and many nonhistone substrates. However, the complete role of SIRT1 in cancer is ambiguous in terms of whether it behaves as a tumor suppressor or tumor promoter.81 Nevertheless, in the case of oral cancer, SIRT1 is highly suggested to play a role in tumor suppression.82Furthermore, these combined betaines strikingly enhanced the generation of reactive oxygen species (ROS) as well as SIRT1, which caused deacetylation, and SIRT1 intervened in cell propagation, as clearly indicated by gene silencing with small interfering RNA. Betaines effectively induced apoptosis in CAL-27 by activating SIRT1 and degrading cyclin B1 and procaspase-3, reducing cell proliferation, and ultimately leading to apoptosis.30
3.7 Betaine-mediated inhibition of hepatocellular carcinoma
Hepatic cancer is one of the most common and deadliest types of malignancy, which is considered a global concern. Several factors cause liver cancer such as alcohol-induced cirrhosis, fatty liver, smoking, obesity, and hepatitis B and C virus.83 The mechanism of liver cancer involves the key role of tumor-associated macrophages (TAMs). These macrophages undergo compelling phenotypic changeover during carcinogenesis. Due to their high plasticity and heterogeneity, TAMs play a significant role in the proliferation and angiogenesis of cancer cells. In a normal liver, macrophages mainly exist in form of Kupffer cells. However, with the advancement of liver cancer, pro-tumorigenic factors aggravate tissue-resident macrophages, ultimately leading to TAM inflammation and tumor progression.84 A significant role of betaine was observed in the prevention of liver cancer. A study found that betaine improved liver diseases through various molecular mechanisms such as inhibiting inflammation, improving insulin resistance, reducing endoplasmic reticulum stress, decreasing oxidative stress, increasing autophagy, remodeling the intestinal flora, and regulating epigenetic alterations. Betaine can improve liver diseases by targeting signaling pathways such as NF-κB, AMPK, PPAR-α/γ, LXRα, Akt, TLR4, and caspase-3.85Inactivation of p16 and activation of c-myc are majorly induced by DNA methylation. The p16 gene curtails enzymatic activity, which induces the abnormal phosphorylation of another tumor suppressor Rb, leading to cell proliferation. Therefore, p16 silencing in rat liver cells was indicated by hypermethylation. Conversely, c-myc is crucial regulator of various cellular processes and studies revealed that the hypomethylation of c-myc gene results in its overexpression in hepatic cancer.86 As a methyl donor, betaine effectively regulated the degree of mRNA expression of both p16 and c-myc. Betaine supplementation substantially mitigated DEN-induced hepatic carcinoma.65
Moreover, a few studies investigated how BET affects the hepatitis B virus (HBV), which is the most common cause of liver illness. Southern blotting, quantitative polymerase chain reaction, and enzyme-linked immunosorbent assay were used to evaluate the anti-HBV activity of betaine both in vitro and in vivo. The therapeutic treatment of HBV is complicated by the resistance of this virus to lamivudine and interferon alpha. Thus, the impact of betaine on its resistance was examined. The findings showed that BET dramatically reduced the release of HBV DNA, HbeAg (HBV e antigen), and HBsAg (HBV surface antigen) in HepG2.2.15 cells by suppressing GRP78 expression. The serum DHBV DNA considerably decreased with betaine treatment in ducklings infected with HBV (DHBV). Betaine reduced the resistance mutation of HBV DNA (rtM204V/I) and inhibited the HBV DNA rebound caused by the resistance of this virus to lamivudine. Betaine supplementation may enhance the anti-HBV action of interferon alpha by boosting the production of antiviral dsRNA-dependent protein kinase, which is triggered by the JAK–STAT signaling pathway (JAK = Janus kinase; STAT = signal transducer and activator of transcription). These findings can offer valuable insights into the clinical utilization of betaine and HBV medication resistance solutions in anti-HBV treatment.9
3.8 Synthetic derivatives of betaine in chemotherapeutic transport and their molecular modeling
Chemotherapy has been employed in cancer treatment for many years, but together with this, it has many complications. If small doses of anticancer drugs are taken for treatment, they fail to get to the desired area and unable to reduce the tumor. On the contrary, high doses of chemotherapeutic drugs have been reported to damage healthy cells, making the patient susceptible to several other complications.87 Moreover, the drug resistance phenomenon is another challenge encountered during the chemotherapeutic mode of treatment. Thus, several protocols have been designed to tackle the systematic toxicity, early elimination, resistance and control the release of drugs. Strategies based on drug delivery systems (DDSs) are being manipulated to enhance the efficacy of chemotherapy with minimum side effects.88 The use of numerous drug delivery vehicles to encapsulate anticancer drugs is a promising way to enable the uptake these drugs for tumor alleviation, while at the same time prevent their degradation in the circulation.89–91 The host–guest complexation mode of drug delivery has attracted attention from researchers owing to its effective outcomes.92Betaine-based DDSs (Fig. 5) are being employed to enhance the ability of anticancer drugs to treat cancer at high concentrations. The host–guest complexation of betaine with a calixarene receptor was investigated successfully for cancer treatment. Particularly, calix[n]arenes (n = 4, 6, and 8) are a compelling class of macrocycles that have been widely applied for the delivery of anticancer drugs. Calixarenes are the optimal host molecules, which are composed of phenolic units associated with methylene bridges containing an upper periphery with a para-substituent of a phenolic ring, a lower periphery with a phenolic hydroxyl group, and a hydrophobic π electron-rich core cavity.
3.9 Drug conjugation chemistry of betaine in cancer therapy
Drug–drug interactions are a common phenomenon observed upon the simultaneous intake of different drugs. A drug inducing a change in gastric pH, particularly hypochlorhydria, results in the reduced solubility, absorption and exposure of the drug.93 Thus acid reducing agents (ARAs) or proton pump inhibitors (PPIs) are taken to counter the symptoms of gastroesophageal reflux disease (GERD) or peptic ulcer disease. These drugs alleviate the pH in the gastrointestinal tract, hence reducing acid reflux. However, the use of these PPIs significantly impact the solubility and bio-absorption of other essential drugs.94 For instance, famotidine is a PPI drug, and when it is administered with dasatinib it significantly reduces the area under the concentration versus time curve (AUC) and maximum plasma concentration (Cmax) of dasatinib by 60%. A similar phenomenon was observed in the case of rabeprazole-induced hypochlorhydria, where rabeprazole (20 mg b.i.d.) substantially lessened the Cmax and AUC0–∞ of dasatinib by 92% and 78%, respectively. Therefore, transitory gastric reacidification at the time of drug dosing may assist in alleviating gastric pH-drug–drug interactions.
Betaine hydrochloride (BHCl) is employed to mitigate this drug–drug interaction problem. BHCl is a natural supplement that is commonly used in digestive aids, which upon dosage in solid oral form gets dissociated into betaine and hydrochloric acid in gastric fluids, consequently decreasing the gastric pH and making it susceptible for dasatinib solubility. However, coadministration of BHCl predominantly enhanced the Cmax and AUC0–∞ of dasatinib by 15- and 6.7-fold, restoring them to 105% and 121%, respectively, compared to dasatinib alone. On account of this, BHCl enhanced the solubility and absorption of dasatinib with rabeprazole-induced hypochlorhydria.33
A dual chlorambucil-tailed betaine conjugate (DCBC) was assessed via both in vitro and in vivo analysis. In the in vitro analysis, the viability of MCF-7, HeLa and HepG2 cancer cells was evaluated using free chlorambucil and DCBC. It was reported that DCBC had high drug loading ability and enhanced cytotoxic effects compared to chlorambucil.
In the in vivo analysis, MCF-7 cell tumor-bearing mice were employed. After the intravenous administration of DCBC, it was reported that DCBC liposomes such as nanocapsules possess enhanced in vivo antitumor efficacy compared to the free drug. In addition, their high biocompatibility and safety were also reported.34
In this study, PEI–betaine (PB) was further combined with single-walled carbon nanotubes (CWCNT) to generate SWCNT–PB (SPB), which was proposed to exhibit pH-responsive lysosomal release efficacy. SPB was additionally modified by DOX, survivin siRNA and peptide BR2 (SPBB)23 to form the final product, DOX–SPBB–siRNA.
In vitro and in vivo experiments were performed using A549 cells. DOX–SPBB–siRNA considerably scaled down the tumor size in nude mice bearing A549 tumor cells, thereby exhibiting the synergistic effects of DOX and surviving siRNA without noticeable damage to normal tissues.35
3.10 Betaine as cytoprotective agents
A Jaiswal et al. hypothesized that the beneficial effects of betaine can play a key role in the prevention of DOX-induced cardiotoxicity given that betaine is a natural, safe (9–15 g) and non-toxic methyl donor in one-cycle. It is involved in the methylation of proteins and DNA. In addition, betaine diminishes autoimmune encephalomyelitis, cortical and hippocampal MDA grading in mice.109,110 It adequately reduces several brain disorders such as Alzheimer's disease. Moreover, betaine is also beneficial in metabolic disorders such as obesity by improving glucose homeostasis and decreasing hepatic steatosis. Alternatively, it plays a pivotal role in productively mitigating CCl4-induced liver fibrosis, atherosclerosis and colitis-associated cancer. Based on all these perquisites, this group hypothesized that betaine can be a potent natural compound to reduce DOX-induced cardiotoxicity by inhibiting ROS production, caspase 3, oxidative stress, apoptotic factors, and Ca2+ dysregulation and increasing the antioxidant level.111
Cisplatin is antineoplastic agent, which is widely being employed in the treatment of various cancers such as head and neck cancer, lung cancer, cervical cancer, ovarian cancer, bladder cancer and oesophageal cancer. However, this drug is responsible for many complexities such as DNA damage and cytoplasmic organelle dysfunction notably in the mitochondria and endoplasmic reticulum.113 In addition, cisplatin elevates the blood urea nitrogen and serum creatinine levels, promoting renal injury and decreases glomerular filtration rate.
Several methods are being employed to prevent or minimize cisplatin-induced nephrotoxicity. However, there is no clinically effective drug to treat this complexity, and thus many natural products such as curcumin, ginseng and pomegranate are being considered due to their anti-inflammatory and antioxidant properties.114 H Hagar et al. experimentally investigated the role of betaine in preventing cisplatin-induced nephrotoxicity. Betaine as a natural antioxidant substantially improved cisplatin-induced lipid peroxidation and prevented the continuous reduction in antioxidant enzymes. In addition, renal functional and morphological deterioration ineluctably improved upon betaine pre-treatment. A recent study examined cisplatin-induced DNA damage and cell apoptosis through the stimulation of reactive oxygen species. Further, betaine inhibited caspase 3 by receding signal production either via intrinsic membrane death receptor proteins such as Fas and TNFR1 or the mitochondrial cytochrome c release pathway. Similarly, the protein stabilizing effect of betaine can contribute to protection against cisplatin-induced DNA damage. Hence, betaine supplementation can be effectively used for protection and prevention against anticancer drug-induced nephrotoxicity.115
Cisplatin is a widely used chemotherapeutic drug, which is being employed for the treatment of various cancers but at the same time is responsible for hepatotoxicity. It rapidly penetrates body tissues by passive transport, increasing its concentration in liver. However, diverse methods are being employed to ameliorate cisplatin-induced hepatotoxicity, for instance, molsidomine (MOL) is a pro-drug and robust vasodilator, and it also exhibits antianginal effects, which can be effectively used against oxidative stress and hepatotoxicity.118 In addition, various natural antioxidants such as green tea, Acacia hydaspica, Ephedra alata and Curcuma longa have been experimentally investigated to mitigate cisplatin-caused hepatotoxicity.119 Similarly, betaine is also a natural and organic antioxidant, which can be utilized for analogous outcomes.
H Hagar et al. experimentally scrutinized the use of betaine to mitigate cisplatin-induced hepatotoxicity. Previously, the administration of betaine effectively decreased lipid peroxides and augmented the concentration of enzymatic and non-enzymatic antioxidants (SOD and GSH, respectively). This research group reported that the powerful reducing and direct antioxidation effect of betaine can substantially validate its hepatoprotective property. Further, oxidative hepatic injury caused by hepatotoxicants such as chloroform, ethanol and lipopolysaccharides can be prevented by the action of betaine. Moreover, the metabolism of sulfur-containing amino acids and methionine synthesis by betaine can enhance the production of reduced glutathione, which in turn supports the detoxification role of the liver. The expression of an oxidative stress-induced transcription factor (NF-kB) in hepatic tissues by the action of cisplatin was nullified by pre-treatment with betaine. Furthermore, the inhibitory effect of betaine on caspase-3 significantly contributed to the mitigation of hepatic injury (Table 3).
| Cancer type | Cell line/model system | Targeted genes | Experimental approach | Key findings | References |
|---|---|---|---|---|---|
| Prostate (DU-145) | DU-145 | Bax, caspase-3 | Overexpression/knockout | Apoptosis blocked with caspase-3 KO; affirms oxidative-stress-induced apoptosis | 28 |
| Prostate (C4-2B) | C4-2B | PI3K, AKT, NF-κB, Bax, caspase-3 | Overexpression/knockdown | Overexpression of AKT counteracts apoptosis; upregulated Bax/caspase-3 affirms mechanism | 29 |
| Cervical | HeLa | p53, cyclin D1, caspase-3 | Knockout/overexpression | p53 KO suppresses apoptosis; overexpression of cyclin D1 blocks cell cycle arrest | 26 |
| Oral | CAL-27 | SIRT1, cyclin B1, caspase-3 | siRNA knockdown | SIRT1 knockdown abolishes the betaine effect, which confirms its key role | 30 |
| Lung | A549 | NF-κB, p38 MAPK | Gene silencing/inhibitor | NF-κB silencing increases effect; p38 inhibition inhibits apoptosis | 24 |
| Colon | RAW 264.7 macrophages | NF-κB, IL-6, COX-2, TNF-α | Gene knockdown (siRNA), pharmacological inhibition | iRNA NF-κB or IL-6 establishes inflammatory cytokine function in CRC development | 27 |
| Liver | HepG2, rat liver cells | p16, c-Myc, caspase-3 | Methylation/epigenetic regulation, gene expression modulation | p16 hypermethylation and c-Myc hypomethylation reversed by betaine | 65 |
| Breast | MCF-7, BR-75-1, MDA-MB-231 | VEGF, TSP-1, caspase-3 | Protein expression modulation (transfection, siRNA) | VEGF/TSP-1 decreased after beta-derivative treatments; increase in caspase-3 confirms apoptosis | 23 |
| Drug toxicity (cardiac, kidney, and liver) | In vitro & in vivo mouse/rat models | PUMA, TNFR1, SOD, GSH, NF-κB | Gene/protein expression analysis (qPCR, western blot, siRNA) | Betaine overturns Dox-induced upregulation of PUMA/TNFR1; caspase-3 suppression maintains organs | 115 |
Owing to the anti-inflammatory and antioxidant properties of the non-toxic and stable betaine, its supplementation can alleviate cisplatin-induced hepatotoxicity and act as a hepatoprotective agent during chemotherapy (Fig. 8).120
3.11 Clinical trials of betaine
Direct clinical trials of betaine are limited; however, other observational and preclinical studies have provided insights into its benefits. In observational studies, the link between cancer risk and betaine level was explored. A cohort of studies presented a meta-analysis demonstrating that the betaine level is linked to a decrease in the risk of cancer cases, more specifically colorectal cancer. The shared relative risk by combining top and bottom levels of betaine was 0.93.121 Animal studies investigated the effect of betaine on tumor growth. In one study, rats were utilized and there was significant decrease in their tumor volume and weight compared to the control group. However, the linkage of betaine with C-phycocyanin (C-PC) failed to show any effective synergistic effects beyond C-PC alone.1223.12 Safety profile and dose limitations of betaine
Betaine has demonstrated potential therapeutic effects in cancer research given that it inhibits the growth of cancer cells by inflammation, oxidative stress and apoptosis. In its dose-dependent mechanism, betaine behaves as an oxidant at higher concentrations e.g., in human prostate cancer, increasing its concentration from 40 to 50 mg mL−1 resulted in a decrease in cell viability and increase in malondialdehyde (MDA), pro-inflammatory cytokines including IL-6, TNF-α and total oxidant status (TOS). Owing to these effects, there was reduction in antioxidant defenses such as total oxidant status (TOS), catalase (CAT) and superoxide dismutase (SOD).123In clinic, betaine has been used to target deficiencies in critical methyl donors involved in gene expression regulation and DNA methylation, that is, S-adenosylmethionine. In acute lymphoblastic leukemia patients, low SAM levels cause interruptions in chemotherapy, and in this case, betaine in supplement form, i.e., 25 mg kg−1 twice daily and gradually increasing to 150 mg−1 kg−1 day−1, increased the plasma SAM concentrations by facilitating chemotherapy and alleviating SAM-related metabolic challenges.124
In terms of safety, betaine has shown good tolerance in humans, where studies demonstrated no adverse effects with an oral dose 4 g day−1 in individuals. However, there may be mild side effects with a higher dose such as gastrointestinal discomfort. In animal trials, its low toxicity was further accentuated with lethal doses (LD50) in rats in the range of 10–11 g kg−1 by oral administration.125
4. Conclusion
Numerous types of cancers have been cured with natural components, many of which are included in everyday diets. They offer considerable defence against numerous malignancies. Antioxidant compounds shield cells from damage, combating cancer and other disorders. Betaine, the natural substance covered in this review, has anticancer properties. In this study, we discussed the diverse antitumor activities of betaine and its complexes, which can serve as a theoretical basis for their role as a natural defence against cancer. In this review, we evaluated the role of betaine in different types of cancers with different potential mechanisms such as breast cancer, liver cancer, lung cancer, prostate cancer, cervical cancer, and colon cancer. Studies have shown that betaine in combination with anticancer drugs exhibits striking aptitude to augment the chemotherapeutic action of drugs. Furthermore, betaine as a natural compound is quite compatible with living organisms, showing a favourable safety profile and minimal side effects. Thus, the use of betaine in the treatment of cancer is growing quickly with preclinical trials on animals and observations for the suppression of cancer.Data availability
No primary research results, software or codes has been included and no new data were generated or analysed as part of this review.Conflicts of interest
Authors declare no conflict of interest.Acknowledgements
The authors greatly acknowledge Higher Education Commision (HEC), Pakistan and the School of Chemistry, University of the Punjab, Lahore, for funding and support.References
- R. L. Siegel, et al., Colorectal cancer statistics, 2017, Ca-Cancer J. Clin., 2017, 67(3), 177–193 CrossRef PubMed.
- X. Yang, et al., Down-regulation of 14-3-3zeta reduces proliferation and increases apoptosis in human glioblastoma, Cancer Gene Ther., 2020, 27(6), 399–411 CrossRef CAS PubMed.
- Z. Shen, et al., Emerging strategies of cancer therapy based on ferroptosis, Adv. Mater., 2018, 30(12), 1704007 CrossRef PubMed.
- L. A. Torre, et al., Global cancer statistics, 2012, Ca-Cancer J. Clin., 2015, 65(2), 87–108 CrossRef PubMed.
- Y. Sun, Translational horizons in the tumor microenvironment: harnessing breakthroughs and targeting cures, Med. Res. Rev., 2015, 35(2), 408–436 CrossRef PubMed.
- D. S. Chi, et al., An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT), Gynecol. Oncol., 2012, 124(1), 10–14 CrossRef PubMed.
- K. Cho, et al., Therapeutic nanoparticles for drug delivery in cancer, Clin. Cancer Res., 2008, 14(5), 1310–1316 CrossRef CAS PubMed.
- K. Han, et al., Synergistic gene and drug tumor therapy using a chimeric peptide, Biomaterials, 2013, 34(19), 4680–4689 CrossRef CAS PubMed.
- C. Bock and T. Lengauer, Managing drug resistance in cancer: lessons from HIV therapy, Nat. Rev. Cancer, 2012, 12(7), 494–501 CrossRef CAS PubMed.
- P. Balachandran and R. Govindarajan, Cancer—an ayurvedic perspective, Pharmacol. Res., 2005, 51(1), 19–30 CrossRef PubMed.
- Y. Zhou, et al., Dietary natural products for prevention and treatment of liver cancer, Nutrients, 2016, 8(3), 156 CrossRef PubMed.
- R. Domitrović and I. Potočnjak, A comprehensive overview of hepatoprotective natural compounds: mechanism of action and clinical perspectives, Arch. Toxicol., 2016, 90, 39–79 CrossRef PubMed.
- W. R. Sawadogo, et al., Traditional West African pharmacopeia, plants and derived compounds for cancer therapy, Biochem. Pharmacol., 2012, 84(10), 1225–1240 CrossRef CAS PubMed.
- M. Kulis, et al., Intragenic DNA methylation in transcriptional regulation, normal differentiation and cancer, Biochim. Biophys. Acta, Gene Regul. Mech., 2013, 1829(11), 1161–1174 CrossRef CAS PubMed.
- S. H. Zeisel, Choline, other methyl-donors and epigenetics, Nutrients, 2017, 9(5), 445 CrossRef PubMed.
- S. A. Craig, Betaine in human nutrition, Am. J. Clin. Nutr., 2004, 80(3), 539–549 CrossRef CAS PubMed.
- B. D. Willingham, T. J. Ragland and M. J. Ormsbee, Betaine supplementation may improve heat tolerance: potential mechanisms in humans, Nutrients, 2020, 12(10), 2939 CrossRef CAS PubMed.
- E. Mosharov, M. R. Cranford and R. Banerjee, The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes, Biochemistry, 2000, 39(42), 13005–13011 CrossRef CAS PubMed.
- S. Huerta, et al., Screening and detection of apoptosis, J. Surg. Res., 2007, 139(1), 143–156 CrossRef CAS PubMed.
- A. Salem, et al., Crystal structure and chemotherapeutic efficacy of the novel compound, gallium tetrachloride betaine, against breast cancer using nanotechnology, Tumor Biol., 2016, 37, 11025–11038 CrossRef CAS PubMed.
- S. A. Fahmy, et al., PEGylated Chitosan Nanoparticles Loaded with Betaine and Nedaplatin Hamper Breast Cancer: In Vitro and In Vivo Studies, ACS Omega, 2023, 8(44), 41485–41494 CrossRef CAS PubMed.
- K. W. Kim, et al., Polyhexamethylene Biguanide-Betaine Dressing Combined with Silver Sulfadiazine for Biofilm Control in an Advanced Cancer Wound: A Case Report, Journal of Wound Management and Research, 2023, 19(2), 133–138 CrossRef.
- S. H. Deghedy, et al., Synergistic effect of bacterial ghosts loaded with belinostat and betaine nano-emulsion: a potential treatment strategy for breast cancer, Cancer Res., 2023, 83(suppl. 7), 834 CrossRef.
- R. Bingula, et al., Study of the Effects of Betaine and/or C-Phycocyanin on the Growth of Lung Cancer A549 Cells In Vitro and In Vivo, J. Oncol., 2016, 2016(1), 8162952 Search PubMed.
- C. Dupuis, et al., Effect of betaine, C-phycocyanin or physical activity on tumour growth of lung cancer in rats, in Fourth International Congress of Translational Research in Human Nutrition ICTRHN, 2017 Search PubMed.
- Y. Guo, et al., Betaine effects on morphology, proliferation, and p53-induced apoptosis of HeLa cervical carcinoma cells in vitro, Asian Pac. J. Cancer Prev., 2015, 16(8), 3195–3201 CrossRef PubMed.
- D. H. Kim, et al., Anti-inflammatory effects of betaine on AOM/DSS-induced colon tumorigenesis in ICR male mice, Int. J. Oncol., 2014, 45(3), 1250–1256 CrossRef CAS PubMed.
- F. Kar, et al., Betaine suppresses cell proliferation by increasing oxidative stress-mediated apoptosis and inflammation in DU-145 human prostate cancer cell line, Cell Stress Chaperones, 2019, 24, 871–881 CrossRef CAS PubMed.
- H. Zhang, et al., Betaine induces apoptosis of C4-2B prostate cancer cells via inhibiting PI3K/AKT/NF-κB signaling pathway, Chin. J. Cell. Mol. Immunol., 2021, 37(6), 513–519 Search PubMed.
- N. D'Onofrio, et al., Synergistic effect of dietary betaines on SIRT1-mediated apoptosis in human oral squamous cell carcinoma Cal 27, Cancers, 2020, 12(9), 2468 CrossRef PubMed.
- D. Anwanwan, et al., Challenges in liver cancer and possible treatment approaches, Biochim. Biophys. Acta, Rev. Cancer, 2020, 1873(1), 188314 CrossRef CAS PubMed.
- S. A. Fahmy, et al., Betaine host–guest complexation with a calixarene receptor: enhanced in vitro anticancer effect, RSC Adv., 2021, 11(40), 24673–24680 RSC.
- M. R. Yago, et al., The use of betaine HCl to enhance dasatinib absorption in healthy volunteers with rabeprazole-induced hypochlorhydria, AAPS J., 2014, 16, 1358–1365 CrossRef CAS PubMed.
- S. Fang, et al., Liposome-like nanocapsules of dual drug-tailed betaine for cancer therapy, Int. J. Pharm., 2015, 493(1–2), 460–465 CrossRef CAS PubMed.
- Y. Cao, et al., Enhanced lysosomal escape of pH-responsive polyethylenimine–betaine functionalized carbon nanotube for the codelivery of survivin small interfering RNA and doxorubicin, ACS Appl. Mater. Interfaces, 2019, 11(10), 9763–9776 CrossRef CAS PubMed.
- D. R. Youlden, et al., The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality, Cancer Epidemiol., 2012, 36(3), 237–248 CrossRef PubMed.
- B. W. Stewart and P. Kleihues, World cancer report, IARC Press, Lyon, 2003, vol. 57 Search PubMed.
- R. L. Siegel, K. D. Miller and A. Jemal, Cancer statistics, Ca-Cancer J. Clin., 2018, 68(1), 7–30 CrossRef PubMed.
- K. Aljarrah, et al., Magnetic nanoparticles sensitize MCF-7 breast cancer cells to doxorubicin-induced apoptosis, World Journal of Surgical Oncology, 2012, 10, 1–5 CrossRef PubMed.
- X.-H. Peng, et al., Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy, Int. J. Nanomed., 2008, 3(3), 311–321 CAS.
- R. W. Friesen, et al., Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants, J. Nutr., 2007, 137(12), 2641–2646 CrossRef CAS PubMed.
- X. Xu, et al., High intakes of choline and betaine reduce breast cancer mortality in a population-based study, Faseb. J., 2009, 23(11), 4022 CrossRef CAS PubMed.
- Ş. Comşa, A. M. Cimpean and M. Raica, The story of MCF-7 breast cancer cell line: 40 years of experience in research, Anticancer Res., 2015, 35(6), 3147–3154 Search PubMed.
- S. A. Fahmy, et al., Nanoenabled bioseparations: current developments and future prospects, BioMed Res. Int., 2019, 1, 4983291 Search PubMed.
- L. J. Mohan, et al., Optimising PLGA-PEG nanoparticle size and distribution for enhanced drug targeting to the inflamed intestinal barrier, Pharmaceutics, 2020, 12(11), 1114 CrossRef CAS PubMed.
- M. M. Silva, et al., Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration, Mar. Drugs, 2017, 15(12), 370 CrossRef PubMed.
- M. Pourmadadi, et al., Novel epirubicin-loaded nanoformulations: advancements in polymeric nanocarriers for efficient targeted cellular and subcellular anticancer drug delivery, Inorg. Chem. Commun., 2023, 155, 110999 CrossRef CAS.
- X. Hou, et al., Fabrication of poly(t-butyl betaine carboxylate)-based nanoparticles and study on their in vivo biosecurity, J. Biomater. Sci., Polym. Ed., 2021, 32(18), 2387–2401 CrossRef CAS PubMed.
- G. Kocic, et al., Antioxidative, membrane protective and antiapoptotic effects of melatonin, in silico study of physico-chemical profile and efficiency of nanoliposome delivery compared to betaine, RSC Adv., 2017, 7(3), 1271–1281 RSC.
- D. A. Fadeel, et al., Nano-liposomal beetroot phyto-pigment in photodynamic therapy as a prospective green approach for cancer management: in vitro evaluation and molecular dynamic simulation, Pharmaceutics, 2024, 16(8), 1038 CrossRef CAS PubMed.
- F. Mahboubi, J. Mohammadnejad and S. Khaleghi, Bifunctional folic acid targeted biopolymer Ag@NMOF nanocomposite [{Zn2(1,4-bdc)2(DABCO)}n] as a novel theranostic agent for molecular imaging of colon cancer by SERS, Heliyon, 2024, 10(8), 1–13 CrossRef PubMed.
- H. Poursadegh, et al., Incorporating mannose-functionalized hydroxyapatite/metal-organic framework into the hyaluronic acid hydrogel film: a potential dual-targeted oral anticancer delivery system, Int. J. Biol. Macromol., 2024, 274, 133516 CrossRef CAS PubMed.
- N. Rabin, et al., Biofilm formation mechanisms and targets for developing antibiofilm agents, Future Med. Chem., 2015, 7(4), 493–512 CrossRef CAS PubMed.
- G. Schultz, et al., Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds, Wound Repair and Regeneration, 2017, 25(5), 744–757 CrossRef PubMed.
- M. D. Swartz, et al., Investigating multiple candidate genes and nutrients in the folate metabolism pathway to detect genetic and nutritional risk factors for lung cancer, PLoS One, 2013, 8(1), e53475 CrossRef CAS PubMed.
- E. Filaire, et al., Lung cancer: what are the links with oxidative stress, physical activity and nutrition, Lung Cancer, 2013, 82(3), 383–389 CrossRef PubMed.
- X. Zhang, et al., Maintenance therapy with continuous or switch strategy in advanced non-small cell lung cancer: a systematic review and meta-analysis, Chest, 2011, 140(1), 117–126 CrossRef CAS PubMed.
- R. Bingula, et al., Study of the effects of betaine and/or C-phycocyanin on the growth of lung cancer A549 cells in vitro and in vivo, J. Oncol., 2016, 1, 8162952 Search PubMed.
- A. Jemal, et al., Global cancer statistics, Ca-Cancer J. Clin., 2011, 61(2), 69–90 CrossRef PubMed.
- D. M. Parkin, et al., Cancer in Africa 2012, Cancer Epidemiol., Biomarkers Prev., 2014, 23(6), 953–966 CrossRef PubMed.
- D. M. Parkin, et al., Burden and trends of type-specific human papillomavirus infections and related diseases in the Latin America and Caribbean region, Vaccine, 2008, 26, L1–L15 CrossRef PubMed.
- A. P. Vizcaino, et al., International trends in incidence of cervical cancer: II. Squamous-cell carcinoma, Int. J. Cancer, 2000, 86(3), 429–435 CrossRef CAS PubMed.
- A. H. Hamed, et al., Neoadjuvant chemotherapy followed by simultaneous robotic radical trachelectomy and reversal of tubal sterilization in stage IB2 cervical cancer, Journal of the Society of Laparoendoscopic Surgeons, 2012, 16(4), 650 CrossRef PubMed.
- S. Rupachandra and D. Sarada, Induction of apoptotic effects of antiproliferative protein from the seeds of Borreria hispida on lung cancer (A549) and cervical cancer (HeLa) cell lines, BioMed Res. Int., 2014, 1, 179836 Search PubMed.
- Y.-p. Du, et al., Assessment of the effect of betaine on p16 and c-myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model, BMC Cancer, 2009, 9, 1–9 CrossRef PubMed.
- J. J. Landry, et al., The genomic and transcriptomic landscape of a HeLa cell line, G3: Genes, Genomes, Genet., 2013, 3(8), 1213–1224 CrossRef PubMed.
- S. H. Itzkowitz and X. Yio, Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation, Am. J. Physiol.: Gastrointest. Liver Physiol., 2004, 287(1), G7–G17 CrossRef CAS PubMed.
- E. V. Loftus Jr, Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences, Gastroenterology, 2004, 126(6), 1504–1517 CrossRef PubMed.
- J. K. Hou, H. El-Serag and S. Thirumurthi, Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: a systematic review, Official Journal of the American College of Gastroenterology, 2009, 104(8), 2100–2109 CrossRef PubMed.
- J. Pedersen, et al., Inflammatory pathways of importance for management of inflammatory bowel disease, World J. Gastroenterol., 2014, 20(1), 64 CrossRef PubMed.
- O. Nielsen, et al., Use of biological molecules in the treatment of inflammatory bowel disease, J. Intern. Med., 2011, 270(1), 15–28 CrossRef CAS PubMed.
- L. E. Targownik and C. N. Bernstein, Infectious and malignant complications of TNF inhibitor therapy in IBD, Official Journal of the American College of Gastroenterology, 2013, 108(12), 1835–1842 CrossRef CAS PubMed.
- E. K. Go, et al., Betaine suppresses proinflammatory signaling during aging: the involvement of nuclear factor-κB via nuclear factor-inducing kinase/IκB kinase and mitogen-activated protein kinases, J. Gerontol., Ser. A, 2005, 60(10), 1252–1264 CrossRef PubMed.
- E. K. Go, et al., Betaine modulates age-related NF-κB by thiol-enhancing action, Biol. Pharm. Bull., 2007, 30(12), 2244–2249 CrossRef CAS PubMed.
- E. K. Lee, et al., Betaine attenuates lysophosphatidylcholine-mediated adhesion molecules in aged rat aorta: modulation of the nuclear factor-κB pathway, Exp. Gerontol., 2013, 48(5), 517–524 CrossRef CAS PubMed.
- G. Gandaglia, et al., Epidemiology and prevention of prostate cancer, European Urology Oncology, 2021, 4(6), 877–892 CrossRef PubMed.
- S. Ghafouri-Fard, et al., The interaction between miRNAs/lncRNAs and nuclear factor-κB (NF-κB) in human disorders, Biomed. Pharmacother., 2021, 138, 111519 CrossRef CAS PubMed.
- E.-H. Dakir, C. Gajate and F. Mollinedo, Antitumor activity of alkylphospholipid edelfosine in prostate cancer models and endoplasmic reticulum targeting, Biomed. Pharmacother., 2023, 167, 115436 CrossRef CAS PubMed.
- A. M. Roy, et al., Grape seed proanthocyanidins induce apoptosis through p53, Bax, and caspase 3 pathways, Neoplasia, 2005, 7(1), 24–36 CrossRef CAS PubMed.
- A. A. Hussein, et al., Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: a systematic review, Eur. J. Cancer, 2017, 82, 115–127 CrossRef PubMed.
- D. Ezhilarasan, et al., The ambiguous role of sirtuins in head and neck squamous cell carcinoma, Oral Dis., 2022, 28(3), 559–567 CrossRef PubMed.
- A. Hu, et al., Curcumin as therapeutics for the treatment of head and neck squamous cell carcinoma by activating SIRT1, Sci. Rep., 2015, 5(1), 13429 CrossRef CAS PubMed.
- D. Dhar, et al., Mechanisms of liver fibrosis and its role in liver cancer, Exp. Biol. Med., 2020, 245(2), 96–108 CrossRef CAS PubMed.
- K. Cheng, et al., Tumor-associated macrophages in liver cancer: from mechanisms to therapy, Cancer Commun., 2022, 42(11), 1112–1140 CrossRef PubMed.
- C. Wang, et al., Preventive and therapeutic role of betaine in liver disease: a review on molecular mechanisms, Eur. J. Pharmacol., 2021, 912, 174604 CrossRef CAS PubMed.
- H. Chen, et al., Association of c-myc overexpression and hyperproliferation with arsenite-induced malignant transformation, Toxicol. Appl. Pharmacol., 2001, 175(3), 260–268 CrossRef CAS PubMed.
- C. Pacheco, et al., Recent advances in long-acting drug delivery systems for anticancer drug, Adv. Drug Delivery Rev., 2023, 114724 CrossRef CAS PubMed.
- Y. Wang, et al., Overcoming cancer chemotherapy resistance by the induction of ferroptosis, Drug Resistance Updates, 2023, 66, 100916 CrossRef CAS PubMed.
- F. M. Kashkooli, M. Soltani and M. Souri, Controlled anti-cancer drug release through advanced nano-drug delivery systems: static and dynamic targeting strategies, J. Controlled Release, 2020, 327, 316–349 CrossRef PubMed.
- X. Qiu, et al., The development of multifunctional sulfated polyguluronic acid-based polymeric micelles for anticancer drug delivery, Carbohydr. Polym., 2023, 303, 120451 CrossRef CAS PubMed.
- M. Jain, et al., PVA/Guanidinium Oleate Transdermal Patch as a pH-responsive Drug Delivery System for the Localized and Targeted Delivery of Anticancer Drug, Mater. Adv., 2024, 5(5), 1998–2011 RSC.
- M. Yan, et al., Recent progress of supramolecular chemotherapy based on host–guest interactions, Adv. Mater., 2023, 2304249 Search PubMed.
- J. Hofmann, et al., Dasatinib anhydrate containing oral formulation improves variability and bioavailability in humans, Leukemia, 2023, 37(12), 2486–2492 CrossRef CAS PubMed.
- H. M. Fadda, P. M. Hellström and D.-L. Webb, Intra-and inter-subject variability in gastric pH following a low-fat, low-calorie meal, Int. J. Pharm., 2022, 625, 122069 CrossRef CAS PubMed.
- J. Pang, et al., Pharmacokinetics and absorption of the anticancer agents dasatinib and GDC-0941 under various gastric conditions in dogs–reversing the effect of elevated gastric pH with betaine HCl, Mol. Pharmaceutics, 2013, 10(11), 4024–4031 CrossRef CAS PubMed.
- M.-Q. Gong, et al., Self-assembled polymer/inorganic hybrid nanovesicles for multiple drug delivery to overcome drug resistance in cancer chemotherapy, Langmuir, 2015, 31(18), 5115–5122 CrossRef CAS PubMed.
- Z. Song, et al., Self-assembly of peptide amphiphiles for drug delivery: the role of peptide primary and secondary structures, Biomater. Sci., 2017, 5(12), 2369–2380 RSC.
- S. Pengnam, et al., Delivery of small interfering RNAs by nanovesicles for cancer therapy, Drug Metab. Pharmacokinet., 2022, 42, 100425 CrossRef CAS PubMed.
- T. L. Cuellar, et al., Systematic evaluation of antibody-mediated siRNA delivery using an industrial platform of THIOMAB–siRNA conjugates, Nucleic Acids Res., 2015, 43(2), 1189–1203 CrossRef CAS PubMed.
- N. Wang, P. Saidhareddy and X. Jiang, Construction of sulfur-containing moieties in the total synthesis of natural products, Nat. Prod. Rep., 2020, 37(2), 246–275 RSC.
- L. Bai and X. Jiang, Smelless/Stable/Sustainable Sulfur Chemistry, CCS Chem., 2025, 1–23 Search PubMed.
- M. Wang and X. Jiang, Prospects and Challenges in Organosulfur Chemistry, ACS Sustain. Chem. Eng., 2022, 10(2), 671–677 CrossRef CAS.
- K. Iwakura, Modulation of individual susceptibility to the no-reflow phenomenon after acute myocardial infarction, Curr. Pharm. Des., 2013, 19(25), 4519–4528 CrossRef CAS PubMed.
- Y. Wang, et al., Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling, J. Cell Biol., 2018, 217(6), 1915–1928 CrossRef CAS PubMed.
- M. C. H. Gruhlke and A. J. Slusarenko, The biology of reactive sulfur species (RSS), Plant Physiol. Biochem., 2012, 59, 98–107 CrossRef CAS PubMed.
- E. A. Ilardi, E. Vitaku and J. T. Njardarson, Data-mining for sulfur and fluorine: an evaluation of pharmaceuticals to reveal opportunities for drug design and discovery: miniperspective, J. Med. Chem., 2014, 57(7), 2832–2842 CrossRef CAS PubMed.
- Y. Octavia, et al., Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies, J. Mol. Cell. Cardiol., 2012, 52(6), 1213–1225 CrossRef CAS PubMed.
- M. Sheibani, et al., Doxorubicin-induced cardiotoxicity: an overview on pre-clinical therapeutic approaches, Cardiovasc. Toxicol., 2022, 22(4), 292–310 CrossRef CAS PubMed.
- D. Ibi, et al., Involvement of GAT2/BGT-1 in the preventive effects of betaine on cognitive impairment and brain oxidative stress in amyloid β peptide-injected mice, Eur. J. Pharmacol., 2019, 842, 57–63 CrossRef CAS PubMed.
- C. Yang, et al., Betaine ameliorates experimental autoimmune encephalomyelitis by inhibiting dendritic cell-derived IL-6 production and Th17 differentiation, J. Immunol., 2018, 200(4), 1316–1324 CrossRef CAS PubMed.
- A. Jaiswal, et al., Betaine Intervention as a Novel Approach to Preventing Doxorubicin-Induced Cardiotoxicity, Adv. Redox Res., 2023, 9, 100084 CrossRef CAS.
- C. Porta, et al., Renal effects of targeted anticancer therapies, Nat. Rev. Nephrol., 2015, 11(6), 354–370 CrossRef CAS PubMed.
- S. Manohar and N. Leung, Cisplatin nephrotoxicity: a review of the literature, J. Nephrol., 2018, 31(1), 15–25 CrossRef CAS PubMed.
- C.-y. Fang, et al., Natural products: potential treatments for cisplatin-induced nephrotoxicity, Acta Pharmacol. Sin., 2021, 42(12), 1951–1969 CrossRef CAS PubMed.
- H. Hagar, et al., Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats, Exp. Toxicol. Pathol., 2015, 67(2), 133–141 CrossRef CAS PubMed.
- C. Berasain, et al., Loss of liver function in chronic liver disease: an identity crisis, J. Hepatol., 2023, 78(2), 401–414 CrossRef CAS PubMed.
- M. Mihajlovic and M. Vinken, Mitochondria as the target of hepatotoxicity and drug-induced liver injury: molecular mechanisms and detection methods, Int. J. Mol. Sci., 2022, 23(6), 3315 CrossRef CAS PubMed.
- R. Bentli, et al., Molsidomine prevents cisplatin-induced hepatotoxicity, Arch. Med. Res., 2013, 44(7), 521–528 CrossRef CAS PubMed.
- N. Abd Rashid, et al., The role of natural antioxidants in cisplatin-induced hepatotoxicity, Biomed. Pharmacother., 2021, 144, 112328 CrossRef CAS PubMed.
- H. Hagar, et al., Inhibition of NF-κB and the oxidative stress-dependent caspase-3 apoptotic pathway by betaine supplementation attenuates hepatic injury mediated by cisplatin in rats, Pharmacol. Rep., 2019, 71(6), 1025–1033 CrossRef PubMed.
- J. Youn, E. Cho and J. E. Lee, Association of choline and betaine levels with cancer incidence and survival: a meta-analysis, Clin. Nutr., 2019, 38(1), 100–109 CrossRef CAS PubMed.
- R. Bingula, et al., Study of the Effects of Betaine and/or C-Phycocyanin on the Growth of Lung Cancer A549 Cells In Vitro and In Vivo, J. Oncol., 2016, 2016, 8162952 Search PubMed.
- F. Kar, et al., Betaine suppresses cell proliferation by increasing oxidative stress-mediated apoptosis and inflammation in DU-145 human prostate cancer cell line, Cell Stress Chaperones, 2019, 24(5), 871–881 CrossRef CAS PubMed.
- B. Bostrom, B. Sweta and S. J. James, Betaine for Patients with Acute Lymphoblastic Leukemia Intolerant of Maintenance Chemotherapy Due Deficiency of S-Adenosyl Methionine, Blood, 2015, 126(23), 1296 CrossRef.
- M. K. Arumugam, et al., Beneficial Effects of Betaine: A Comprehensive Review, Biology, 2021, 10(6), 1–24 CrossRef CAS PubMed.
Footnote |
| † Joint first authors. |
| This journal is © The Royal Society of Chemistry 2025 |