Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Halogenation of arenes using alkali metal halides/Fe(NO3)3·9H2O at room temperature†
Caicui Li‡
ab,
Yao Cheng‡b,
Fudan Pangb,
Xiushuo Yanb,
Zhengtao Huangb,
Xinmei Wangb,
Xiaodan Wang*ab,
Yiying Li*c,
Jinhui Wangab and
Huanjun Xu *b
*b
aDepartment of Medicinal Chemistry and Natural Medicine Chemistry, College of Pharmacy, Harbin Medical University, Harbin, China. E-mail: 15999290001@126.com
bSchool of Science, Qiongtai Normal University, Haikou, 571127, China. E-mail: 15798946232@163.com
cKey Laboratory of Tropical Translational Medicine of Ministry of Education, Key Laboratory of Brain Science Research Transformation in Tropical Environment of Hainan Province, School of Basic Medicine and Life Sciences, Hainan Medical University, Haikou, China. E-mail: liyiying_hy@163.com
First published on 19th March 2025
Abstract
A simple, efficient and environmentally friendly methodology for the halodecarboxylation of anisole analogues using Fe(NO3)3·9H2O/KBr or NaI at room temperature was developed. In this method, most substrates with an electron-donating group afforded corresponding products in good to excellent yields, whereas those with an electron-withdrawing group afforded low to moderate yields. More importantly, this protocol was also applicable for gram-scale synthesis. It is hoped that this methodology will be highly useful in organic synthesis.
Introduction
Aryl halides are very common structural units widely present in a large number of natural products, industrial chemicals and pharmaceuticals.1 Additionally, aryl halides are important synthetic blocks to construct various compounds.2 A series of cross-coupling reactions including the Heck reaction, Suzuki reaction, Ullmann reaction, Grignard reaction, Stille reaction, Sonogashira reaction, Negishi reaction, Kumada reaction, and Hiyama reaction can be conducted using aryl halides.However, hazardous, toxic and corrosive molecular halogens are usually used as halogenating agents in the laboratory,3 e.g., in bromination, where bromine is used as a direct reagent for the synthesis of bromoaromatic compounds, which causes environmental pollution and some undesirable side reactions such as the production of toxic HBr gas, uncontrolled polybromination and lower yields. Thus, many reagents have been developed to replace molecular halogens as the source of electrophilic halide cations. The most common alternative to Br2 is NBS,4 which is used as a “Br” source via electrophilic substitution to obtain brominated compounds, which is an important way to produce bromides. Essentially, bromide anions were reacted with oxidants to generate Br+. Further, co-oxidants such as PhI(OAc)2,5 H2O2 (ref. 6) and metal oxidants7 were developed. Meanwhile, quaternary ammonium salts,8 pyridinium salts,9 4-(dimethylamino)pyridine tribromide10 and N,N-dibromotosylamide (TsNBr2)11 were selected as bromination reagents. In recent years, Lewis or Brønsted acids and Lewis bases have been employed to boost the reactivity of bromination by different research groups.12 Nevertheless, most of these methods suffer from limitations such as the requirement for essential transition-metal catalysts, inevitable operational complexity, uncommon bromine sources, and a narrow substrate range. Therefore, efficient preparation of aryl halides under mild and green reaction conditions is a continuing goal for chemists in organic synthesis and several other research fields.
In recent years, considerable attention has been paid to the development of a new route for the construction of halogenated scaffolds by utilizing safe and readily available halide sources such as alkali metal halides (halide = I, Br, and Cl).13 A combination of oxidants and bromides such as oxone/NaBr,14 CAN/KBr,15 CAN/LiBr,16 Selectfluor®/KBr,17 NaBrO3–NaBr,18 and NaBr/NaNO3/TFA19 has been employed in these bromination reactions. We also noted that Fe(NO3)3·9H2O had relatively high Lewis acidity and great catalytic activity.20 Meanwhile, Fe(NO3)3·9H2O is a cheap, non-toxic and readily available inorganic oxidant and has been used as an efficient oxidant,21 nitro source22 and catalyst in cross-coupling reactions.23 We also found that I2 could be generated in situ from Fe(NO3)3·9H2O/NaI in DMSO to catalyze the oxidation process.24 Thus, we propose that Fe(NO3)3·9H2O competes with alkali metal halides, showing potential to induce halogenation of arenes. In this study, it was found that Fe(NO3)3·9H2O/KBr exhibits efficiency for the synthesis of p-bromoaromatic compounds at room temperature, while Fe(NO3)3·9H2O/KI is efficient for the synthesis of p-iodoaromatic compounds. Compared with the previous reports, a Fe(NO3)3·9H2O/alkali metal halide system could synthesize aryl halides at room temperature with high to excellent yields for substrates with an electron-donating group and low to moderate yields for substrates with an electron-withdrawing group. In addition, this protocol was readily scaled up to 15 mmol level without any loss of efficiency.25
Results and discussion
The initial step of the study was the optimization of reaction with Fe(NO3)3·9H2O(0.625 mmol)/KBr(0.625 mmol), using anisole (0.5 mmol) as a model substrate in various solvents for 2 h at room temperature (Table 1, entries 1–11). To our delight, para-bromination of anisole proceeded smoothly in CH3CN, giving the desired product in a high yield up to 99%. When the reaction was carried out in hexane, CH2Cl2, and EA, the brominated product was obtained in high yields up to 67%, 98% and 89%, respectively. Then, other types of Br sources such as NaBr, LiBr, CuBr2 or FeBr3 could also be applied in this reaction to give excellent yields (Table 1, entries 12–15). This indicated that Fe(NO3)3·9H2O combined with other bromine salts also successfully afforded 4-bromoanisole. In order to find the best method for the bromination of anisole, the reaction time and the ratio of Fe(NO3)3·9H2O/KBr were determined, which indicated that the optimum conditions are Fe(NO3)3·9H2O (0.625 mmol)/KBr(0.625 mmol) and a reaction time of 2 h (Table 1, entries 16–24). In addition, the reaction did not occur without Fe(NO3)3·9H2O (Table 1, entry 25). We also tested other types of Fe salts and nitrates, among which the desired product was offered by Cu(NO3)2 with 29% yield (Table 1, entries 26–31).| Entry | Salt | Br-source | T/h | Solvent | Yield (%) |
|---|---|---|---|---|---|
| a Anisole (0.5 mmol), salt (0.625 mmol), Br-source (0.625 mmol), rt, GC yield.b CuBr2 (0.313 mmol).c FeBr3 (0.208 mmol).d Fe(NO3)3·9H2O (0.5 mmol).e Fe(NO3)3·9H2O (0.375 mmol).f Fe(NO3)3·9H2O (0.25 mmol).g Fe(NO3)3·9H2O (0.125 mmol).h KBr (0.575 mmol).i KBr (0.525 mmol). | |||||
| 1 | Fe(NO3)3·9H2O | KBr | 2 h | H2O | 7 |
| 2 | Fe(NO3)3·9H2O | KBr | 2 h | DMSO | 9 |
| 3 | Fe(NO3)3·9H2O | KBr | 2 h | Acetone | <1 |
| 4 | Fe(NO3)3·9H2O | KBr | 2 h | DMF | 3 |
| 5 | Fe(NO3)3·9H2O | KBr | 2 h | NMP | <1 |
| 6 | Fe(NO3)3·9H2O | KBr | 2 h | Hexane | 67 |
| 7 | Fe(NO3)3·9H2O | KBr | 2 h | CH2Cl2 | 98 |
| 8 | Fe(NO3)3·9H2O | KBr | 2 h | EA | 87 |
| 9 | Fe(NO3)3·9H2O | KBr | 2 h | Methanol | <1 |
| 10 | Fe(NO3)3·9H2O | KBr | 2 h | Ethanol | 3 |
| 11 | Fe(NO3)3·9H2O | KBr | 2 h | CH3CN | >99 |
| 12 | Fe(NO3)3·9H2O | NaBr | 2 h | CH3CN | 98 |
| 13 | Fe(NO3)3·9H2O | LiBr | 2 h | CH3CN | 95 |
| 14 | Fe(NO3)3·9H2O | CuBr2 | 2 h | CH3CN | 96b |
| 15 | Fe(NO3)3·9H2O | FeBr3 | 2 h | CH3CN | 95c |
| 16 | Fe(NO3)3·9H2O | KBr | 1 h | CH3CN | 57 |
| 17 | Fe(NO3)3·9H2O | KBr | 3 h | CH3CN | 97 |
| 18 | Fe(NO3)3·9H2O | KBr | 4 h | CH3CN | 96 |
| 19 | Fe(NO3)3·9H2O | KBr | 2 h | CH3CN | 95d |
| 20 | Fe(NO3)3·9H2O | KBr | 2 h | CH3CN | 94e |
| 21 | Fe(NO3)3·9H2O | KBr | 2 h | CH3CN | 92f |
| 22 | Fe(NO3)3·9H2O | KBr | 2 h | CH3CN | 29g |
| 23 | Fe(NO3)3·9H2O | KBr | 2 h | CH3CN | 65h |
| 24 | Fe(NO3)3·9H2O | KBr | 2 h | CH3CN | 64i |
| 25 | — | KBr | 2 h | CH3CN | <1 |
| 26 | FeCl3 | KBr | 2 h | CH3CN | <1 |
| 27 | FeBr3 | KBr | 2 h | CH3CN | <1 |
| 28 | Fe2(SO4)3 | KBr | 2 h | CH3CN | <1 |
| 29 | Cu(NO3)2 | KBr | 2 h | CH3CN | 29 |
| 30 | Co(NO3)2 | KBr | 2 h | CH3CN | <1 |
| 31 | Ce(NO3)3·6H2O | KBr | 2 h | CH3CN | <1 |
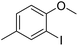
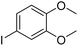
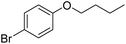
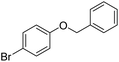
The obtained results for the reaction of para-bromination of anisole promoted our procedure to various anisole analogues (Table 2). Gratifyingly, the bromination of arenes containing electron-donating substituents such as alkoxy (Table 2, entries 1–5) and benzyloxy (Table 2, entry 6) proceeded smoothly to give the corresponding para-brominated products in good to excellent yields. Naphthyl substrates (Table 2, entries 7 and 8) were found to work well, giving the corresponding product in 86% and 97% yields. It is noteworthy that when 2-methoxynaphthalene reacted with multiple substrates under standard conditions but with the decreasing amount of Fe(NO3)3·9H2O to 0.25 mmol, the yield of desired product was increased to 87%. In addition, phenyl-, methyl-, and ethyl-substituted substrates also gave the corresponding products in high yields with monobromination at the para or ortho position with respect to the methoxyl substituent (Table 2, entries 9–13). Meanwhile, for weak electron-withdrawing substituted substrates, it offered the corresponding products in a relatively low yield, but when under adjusted conditions, the yield could be improved to a satisfactory value (Table 2, entries 14–19). However, for strong electron-withdrawing substituted substrates such as –CF3 and –CN, the reaction did not occur (Table 2, entries 20 and 21).
| Entry | Substrate | Product | Yield/% |
|---|---|---|---|
| a Anisole (0.5 mmol), Fe(NO3)3·9H2O (0.625 mmol), KBr (0.625 mmol), rt, 2 h, GC yield.b 60 °C.c 4 h.d Fe(NO3)3·9H2O (0.25 mmol).e Fe(NO3)3·9H2O (0.25 mmol), KBr (0.6 mmol).f Fe(NO3)3·9H2O (0.375 mmol), KBr (1.5 mmol). | |||
| 1 |  |
 |
>99 |
| 2 |  |
 |
96 |
| 3 |  |
 |
90 |
| 4 |  |
 |
83 |
| 5 |  |
 |
89 |
| 6 | 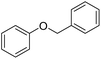 |
 |
85/99b |
| 7 | 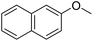 |
 |
97 |
| 8 | 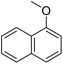 |
 |
86c |
| 9 |  |
 |
71/91b |
| 10 | 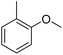 |
 |
98 |
| 11 | 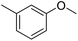 |
 |
99 |
| 12 |  |
 |
98 |
| 13 | 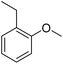 |
 |
97 |
| 14 |  |
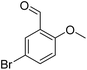 |
61/93b |
| 15 | 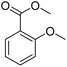 |
 |
79/96b |
| 16 | 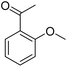 |
 |
77/91c |
| 17 |  |
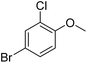 |
50/96b |
| 18 | 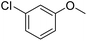 |
 |
64/96b |
| 19 |  |
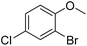 |
6/68b |
| 20 |  |
 |
<1 |
| 21 |  |
 |
<1 |
| 22 |  |
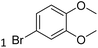 |
72/15 |
 |
94d/5d | ||
| 23 |  |
 |
>99e |
| 24 |  |
 |
84 |
| 25 | 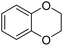 |
 |
82 |
| 26 | 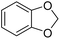 |
 |
79 |
| 27 |  |
 |
90f |
| 28 | 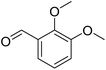 |
 |
33/65b |
| 29 | 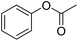 |
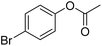 |
<1 |
| 30 |  |
 |
87 |
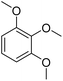
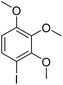
Meanwhile, the strong electron-donating groups on anisole should be reacted under optimized conditions, that is, some bromination products or nitrification products were obtained under standard conditions (Table 2, entries 22–26). For 1,2-dimethoxybenzene, the side product was a nitrification product (15%). While the amount of Fe(NO3)3·9H2O was decreased to 0.25 mmol, the yield of desired product was increased to 94%, and the yield of nitrification product was decreased to 5% (Table 2, entry 22). For 1,3-dimethoxybenzene, the desired product was obtained in 99% yield at the ratio of Fe(NO3)3·9H2O (0.25 mmol)/KBr(0.6 mmol) (Table 2, entry 23). For 1,4-dimethoxybenzene, the main product was a nitrification product with 83% yield (Table 2, entry 24). In addition, for 1,2,3-trimethoxybenzene, the highly electron-rich substrate afforded the desired product in 90% yield with a Fe(NO3)3·9H2O/KBr ratio of 0.375 mmol/1.5 mmol. It should note that 2,3-dimethoxybenzaldehyde could offer 65% yield at a reaction temperature of 60 °C. Moreover, acetyl aniline and phenyl ester were also found to be compatible with this catalytic protocol with high yields (Table 2, entries 29 and 30). We also tested toluene, aniline and phenol, but unfortunately, the reaction was sluggish.
Furthermore, it is notable that the present bromination could be scaled up to gram level: excellent yields of the corresponding products were obtained in one batch from 15 mmol of anisole and 3-methylanisole by using Fe(NO3)3·9H2O/KBr as an additive for 22 h (Scheme 1).
Inspired by the protocol of the bromination of anisole, the scope of the reaction was extended to iodination with Fe(NO3)3·9H2O/NaI (Table S1†). The results are provided in Table 3, which indicate that this protocol is suitable for the iodination of anisole analogues by Fe(NO3)3·9H2O/NaI with a ratio of 2 equiv./2 equiv. for 5 h at room temperature. In addition, the results were similar to those of the bromination process.
Moreover, possible mechanisms for the formation of the bromination are shown in Scheme 2. According to these results, that is, for 1,2-dimethoxybenzene, the side product was a nitro product (15%), and for 1,4-dimethoxybenzene, the main product was a nitrification product with 83% yield. Meanwhile, 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) and 2,6-di-tert-butyl-4-methylphenol (BHT) are the generally accepted radical scavengers in organic chemistry. It was successfully found that the addition of TEMPO and BHT could influence the nitrification process very significantly. For example, a low yield of 2 was obtained when TEMPO (2%, 0.5 equiv.) or BHT (7%, 0.25 equiv.) was added, and the bromination process was affected to a small extent under the reaction condition (Table 4).
From these results, we propose a similar ionic pathway for our bromination process since our results are comparable with some of those works, 25a–c (Scheme 2). First, Fe(NO3)3·9H2O was reacted with Fe(OH)3 and H+ (eqn (1)), and then NO3− was converted into +NO2 (eqn (2)). The bromide ion (Br−) from KBr would be reacted with −NO3 and H+ to generate intermediate Br2 (eqn (3)). Then, unstable nitryl bromide (BrNO2) was formed and depending on the substrate nature, it could act as electrophilic and radical species (eqn (4) and (5)). It was presumed that the bromination process involves typical electrophilic bromination to a large extent.
Conclusions
In conclusion, we have developed a simple, efficient and environmentally friendly methodology for the halogenation of anisole analogues using Fe(NO3)3·9H2O/KBr or NaI at room temperature. In this method, substrates with an electron-donating group afforded the corresponding product in good to excellent yields, while those with an electron-withdrawing group gave low to moderate yields. More importantly, this protocol is also applicable to gram-scale synthesis. It is hoped that this methodology will be very much useful in organic synthesis.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Author contributions
Conceptualization: Huanjun Xu and Yiying Li; methodology: Caicui Li, Yao Cheng and Fudan Pang; formal analysis: Xinmei Wang and Xiaodan Wang; investigation: Caicui Li, Xiushuo Yan, Zhengtao Huang and Yao Cheng; resources: Yiying Li, Huanjun Xu and Jinhui Wang; writing – original draft preparation: Xiaodan Wang and Huanjun Xu; writing – review and editing: Huanjun Xu and Jinhui Wang; supervision: Yiying Li and Huanjun Xu; project administration: Huanjun Xu; and funding acquisition: Yiying Li, Huanjun Xu and Jinhui Wang. All the authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Youth Project of Yazhou Bay Innovation Institute of Hainan Tropical Ocean University (No. 2022CXYQNXM01), the National Natural Science Foundation of China (22165009), the Hainan Provincial Natural Science Foundation of China (821QN260), the Project of Hainan Association for Science and Technology of Youth Science and Talents (QCXM202023), and the National Science and Technology Major Project of the Ministry of Science and Technology of China (2018ZX09735005).Notes and references
- (a) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 Search PubMed; (b) C. Cordovilla, C. Bartolomé, J. M. Martínez-Ilarduya and P. Espinet, ACS Catal., 2015, 5, 3040 CrossRef CAS; (c) M. Granda, C. Blanco, P. Alvarez, J. W. Patrick and R. Menéndez, Chem. Rev., 2014, 114, 1608 CrossRef CAS PubMed.
- (a) S. Indranireha, B. ArunJyothi and P. Pradeep, Chem. Rev., 2016, 116, 6837 CrossRef PubMed; (b) M. L. Tang and Z. Bao, Chem. Mater., 2011, 23, 446 CrossRef CAS; (c) J. C. Tellis, C. B. Kelly and D. N. Primer, et al, Acc. Chem. Res., 2016, 49, 1429 CrossRef CAS.
- (a) S. Indranireha, B. Arunjyothi and P. Pradeep, Chem. Rev., 2015, 116, 3364 Search PubMed; (b) B. Huang, Y. Zhao and C. Yang, et al., Org. Lett., 2017, 19, 3799 CrossRef CAS PubMed; (c) L. G. Voskressensky, N. E. Golantsov and A. M. Maharramov, Synthesis, 2016, 48, 615 CrossRef CAS; (d) S. Song, X. Sun and X. Li, et al., Org. Lett., 2015, 17, 2886 CrossRef CAS.
- (a) B. Das, K. Venkateswarlu, A. Majhi, V. Siddaiah and K. R. Reddy, J. Mol. Catal. A: Chem., 2007, 267, 30 CrossRef CAS; (b) V. Paul, A. Sudalai, T. Daniel and K. V. Srinivasan, Tetrahedron Lett., 1994, 35, 7055 CrossRef CAS.
- S. Kurbah and A. Lal Ram, New J. Chem., 2020, 44, 5410 RSC.
- J. Xiaochen, H. Huawen and X. Wenfang, et al., J. Org. Chem., 2014, 79, 7005 CrossRef.
- (a) Q. Yu, L. Hu and Y. Wang, et al., Angew. Chem., Int. Ed., 2015, 54, 15284 CrossRef CAS PubMed; (b) T. Zhang, X. Qi and S. Liu, et al., Chem.–Eur. J., 2017, 23, 2690 CrossRef CAS PubMed; (c) W. Liu, J. Chen and R. Jin, et al., Org. Chem. Front., 2016, 3, 852 RSC; (d) H. Y. Zhao, X. Y. Yang and H. Lei, et al., Synth. Commun., 2019, 49, 1406 CrossRef CAS.
- S. Gao, T. K. Bethel and T. Kaeshpour, et al., J. Org. Chem., 2018, 83, 9250 CrossRef CAS PubMed.
- D. Bilman, M. Pettersson and M. Bond, et al., Tetrahedron Lett., 2014, 55, 2929 CrossRef.
- A. Arrieta, I. Ganboa and C. Palomo, Synth. Commun., 1984, 14, 939 CrossRef CAS.
- S. Indranirekha, C. Pranitha and M. JyothiSarma, et al., Synth. Commun., 2015, 45, 211 CrossRef.
- (a) S. E. Denmark and M. T. Burk, Proc. Natl. Acad. Sci. U.S.A., 2010, 107, 20655 CrossRef CAS; (b) Y. A. Cheng, T. Chen and C. K. Tan, et al., J. Am. Chem. Soc., 2012, 134, 16492 CrossRef CAS PubMed.
- (a) D. Jiang, F. Wua and H. Cui, Org. Biomol. Chem., 2023, 21, 1571 RSC; (b) R. Van Kerrebroeck, T. Horsten and C. V. Stevens, Eur. J. Org Chem., 2022, 35, e202200310 CrossRef.
- R. K. Dieter, L. E. Nice and S. E. Velu, Tetrahedron Lett., 1996, 37, 2377 CrossRef CAS.
- S. C. Roy, C. Guin and K. K. Rana, et al., Tetrahedron Lett., 2001, 42, 6941 CrossRef CAS.
- V. Nair, S. B. Panicker and A. Augustine, et al., Tetrahedron, 2001, 57, 7417 CrossRef CAS.
- C. Ye and J. M. Shreeve, J. Org. Chem., 2004, 69, 8561 CrossRef CAS PubMed.
- A. Subbarayappa, S. Ghosh and P. U. Patoliya, et al., Green Chem., 2008, 10, 232 RSC.
- P. Chen, S. Hsu and D. Hou, Adv. Synth. Catal., 2024, 366, 1575 CrossRef CAS.
- L. Xu, Y. Chen and Z. Shen, et al., Tetrahedron Lett., 2019, 59, 4349 CrossRef.
- (a) K. Liu, J. Meng and X. Jiang, Org. Process Res. Dev., 2023, 27, 1198 CrossRef CAS; (b) Z. Jia, P. Zhang and Z. Guo, J. Organomet. Chem., 2024, 108, 123288 Search PubMed.
- (a) Y. Zheng, Q. Hu and Q. Huang, et al., Org. Lett., 2024, 26, 3316 Search PubMed; (b) Y. Gao, Y. Mao and B. Zhang, et al., Org. Biomol. Chem., 2018, 16, 3881 RSC; (c) A. Shao, Y. Li and M. Dong, et al., Tetrahedron, 2024, 168, 134329 CrossRef CAS.
- (a) N. Hazeri, M. T. Maghsoodlou and S. M. Hagbibi-Khorassani, et al., J. Chin. Chem. Soc., 2013, 60, 355 CrossRef CAS; (b) H. Kang, S. An and S. Lee, Org. Chem. Front., 2023, 10, 5151 RSC.
- X. Wang, H. Sun and C. Li, et al., Front. Chem., 2022, 10, 933763 CrossRef CAS PubMed.
- (a) V. D. Filimonov, Y. Y. Kulmanakova and M. S. Yusubov, et al., Russ. J. Org. Chem., 2004, 7, 917 Search PubMed; (b) M. S. Yusubov, I. A. Perederina and Y. Y. Kulmanakova, et al., Russ. J. Org. Chem., 1999, 35, 1264 CAS; (c) M. S. Yusubov, I. A. Perederina and V. D. Filimonov, et al., Russ. J. Org. Chem., 1999, 35, 1191 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00837a |
| ‡ These authors have contributed equally to this work and share first authorship. |
| This journal is © The Royal Society of Chemistry 2025 |