Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring the catalytic activity of –NHSO3H functionalized natural asphalt: a sustainable and efficient catalyst for condensation reactions in water†
Sahar Abdolahi and
Mohammad Soleiman-Beigi *
*
Department of Chemistry, Faculty of Basic Sciences, Ilam University, P.O. Box 69315516, Ilam, Iran. E-mail: SoleimanBeigi@yahoo.com; m.soleimanbeigi@ilam.ac.ir
First published on 26th March 2025
Abstract
Herein, we report the synthesis of natural asphalt sulfamic acid (NA-NHSO3H) via functionalization of natural asphalt (NA). The –SO3H groups on the surface of natural asphalt function as acid catalytic sites. This configuration not only enhances the acidity but also increases the surface area available for catalytic activity, making NA-NHSO3H a promising candidate for various acid-catalyzed reactions. The synthesized catalyst was characterized using Fourier Transform Infrared (FT-IR) spectroscopy, Thermogravimetric Analysis (TGA), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Energy Dispersive X-ray (EDX) and X-ray mapping analysis. The catalyst in question has been previously explored for its role in synthesizing various heterocyclic compounds such as polyhydroquinolines, tetrahydrobenzo[b]pyran, and 3,4-dihydropyrimidine-2(1H)-one/thiones. The NA-NHSO3H demonstrated good to excellent yields in the Knoevenagel and Claisen–Schmidt condensations in water at room temperature, indicating its efficiency in these reactions. Conducting these reactions at room temperature and in water can be particularly advantageous, as it enhances safety and sustainability by minimizing the use of hazardous solvents and heat. Conducting these reactions in water at room temperature in the presence of a reusable catalyst aligns with green chemistry principles, which favor the use of benign solvents and milder conditions. This enhances the appeal of NA-NHSO3H for industry and academia by reducing environmental impact and energy usage. Furthermore, the catalyst's heterogeneity was evidenced by its excellent reusability and results from hot-filtration tests.
1. Introduction
Green chemistry plays a vital role in promoting sustainability within the chemical industry. By focusing on principles like minimizing hazardous substances and reducing waste, it aims to create processes that are not only efficient but also environmentally friendly. The ongoing research into new catalysts and synthesis methods is essential for advancing green chemistry. Heterogeneous Brønsted acids are particularly interesting because they offer several advantages over traditional liquid acids, such as easier separation from products, reusability, and a reduced risk of corrosion. These solid acid catalysts can enhance reaction efficiency and help in achieving higher selectivity, which aligns perfectly with the goals of green chemistry. The development of these solid catalysts and other innovative approaches is crucial for the transition to a more sustainable chemical production process.1,2Carbon nanoallotropes such as fullerene, carbon nanotubes (CNTs), carbon nanofibers (CNFs), graphene and graphene oxide (GO) have attracted significant interest in catalysis due to several beneficial properties including surface chemistry, chemical stability, high surface area, electrical conductivity, and cost-effectiveness. These characteristics make carbon nanomaterials promising candidates for a range of catalytic processes, including fuel cells, environmental remediation, and various organic transformations.3,4 The carbon nanoallotropes share a common structural feature – they are all primarily composed of sp2-hybridized carbon atoms arranged in a hexagonal network. This shared structural motif is what allows them to be considered part of the same general group or family of carbon nanomaterials. Despite their common hexagonal carbon framework, these nanoallotropes can exhibit quite different properties and behaviors due to factors like their dimensionality, size, and topology. While carbon nanoallotropes like graphene, carbon nanotubes, and fullerenes do share some fundamental properties such as electrical conductivity, mechanical strength, chemical reactivity, and optical characteristics, they differ significantly in terms of their dispersibility in organic solvents. While the raw materials for fullerene synthesis, such as graphite, are inexpensive and abundant, the methods to produce fullerenes often face issues that can make the process costly and environmentally burdensome. The main disadvantages of fullerene preparation methods are low yields, environmental impacts, cost implications, and complicated isolating and purifying. While CNT preparation techniques have advanced significantly, the presence of metallic and amorphous carbon, impurities and synthesis method limitations continues to be a major challenge. The CNFs can indeed be synthesized using methods that are quite similar to those employed for CNTs, particularly through chemical vapor deposition (CVD) and catalytic plasma-enhanced chemical vapor deposition (C-PECVD) techniques. CNFs typically have a more varied structure compared to CNTs. They can be either relatively straight or convoluted and often feature a larger diameter than CNTs. CNFs may also have a more significant presence of graphene-like planes. CNTs, on the other hand, are characterized by their cylindrical nanostructure and can exhibit specific chiralities (which affect their electrical properties). Different preparation methods mentioned have varied efficiencies and effectiveness in purifying CNTs, and each method has its own set of associated costs and scalability challenges. The simultaneous formation of multilayered structures during the production of graphene is indeed a significant hurdle. Isolating pure monolayers requires advanced techniques like chemical vapor deposition (CVD) or liquid-phase exfoliation, which can be intricate and costly. Additionally, the small size of the graphene nanoplatelets, often less than 1 μm, can limit their application in certain fields where larger surfaces are required for optimal performance, such as in composites or conductive materials. This duality of producing high-quality graphene while dealing with size constraints and purity issues poses a complex challenge for researchers and manufacturers. GO is primarily produced by oxidizing graphite to introduce functional groups, which increases its solubility and processability in various solvents. The methods of Brodie, Staudenmaier, and Hummers & Offeman are the most widely cited approaches for synthesizing GO. Both common methods for producing graphene from graphite (direct liquid exfoliation and GO reduction) are challenging due to high solvent consumption, low yield in terms of usable graphene, and time-consuming processes.3,5–8
Terrestrial biomass, also known as a carbon source, can be used as a renewable feedstock for the production of biofuels and valuable chemicals. Lignocellulosic biomass is indeed the most abundant organic carbon source on Earth, comprising a significant portion of the world's biomass. Lignocellulosic biomass is widely available from agricultural residues, wood, and dedicated energy crops, making it a promising feedstock. Using lignocellulosic biomass can contribute to a circular economy by utilizing waste materials and reducing reliance on fossil fuels. This source can be converted into a variety of products, including bioethanol, biobutanol, phenolic compounds, and other specialty chemicals.9,10
Ongoing research continues to optimize carbon-based materials properties for specific catalytic applications. There are several Brønsted acid catalysts, including p-toluene sulfonic acid (p-TsOH),11 silica-sulfuric acid (SSA),12 PPF-SO3H,13 sulfonic MCM-41 (MCM-SO3H),14 chitosan (CS)-derived magnetic solid acid catalyst (CS-Fe3O4@SO3H),15 organosilane sulfonated graphene oxide (SSi-GO)16 and cellulose sulfuric acid (CSA),17 which have advantages such as reusability and recyclability, good yield, short reaction time, and easy separation. However, they suffer from expensive starting materials and reagents, complicated synthesis, low catalytic activity and product separation with toxic solvents.
Discovering new supports for Brønsted acid catalysts is essential for overcoming current limitations and advancing the field of catalysis. The discovery and utilization of natural resources have profoundly transformed global energy. Natural asphalt (NA) is a naturally occurring substance primarily composed of hydrocarbons. It can be found in the form of deposits, often in locations like natural seeps or tar pits. When mined and processed, it typically has a shiny, black appearance but can break down into a powdery consistency under certain conditions. NA is known for its durability and resistance to water, making it a valuable resource in many industries. Unlike carbon nanomaterials, this natural material is available, non-toxic and cheap. It has high carbon content and can be an excellent candidate as a catalyst support in organic reactions.18–20 NA is characterized by its high melting point, typically between 180 to 245 °C, which makes it suitable for various applications requiring thermal stability. Research indicates that NA exhibits high solubility in several organic solvents, including toluene, xylene, and carbon disulfide. NA consists of hydrocarbons, which can include polycyclic aromatic structures, saturated hydrocarbons and functional groups. The structure of NA is complex, but its analysis shows that it contains 70–80% of carbon, 15% of hydrogen and other elements including traces of nitrogen, sulfur, oxygen and various metals.19–21
Furthermore, it is eco-friendly and can enhance the sustainability of chemical processes. The functional groups present on the surface of NA can significantly influence its interaction properties, making it a valuable substrate for supported catalyst. It enhancing catalytic efficiency and selectivity in various chemical reactions. This attribute is particularly advantageous in processes like heterogeneous catalysis, where effective dispersion and stabilization of active sites are crucial.22
Traditional liquid-acid catalysts, like sulfuric acid (H2SO4), hydrochloric acid (HCl), hydrobromic acid (HBr) and trifluoroacetic acid (CF3COOH) often provide high efficiency in various chemical reactions, particularly in processes. Indeed, the challenges associated with liquid-acid catalysts in homogeneous reactions can lead to significant environmental and economic concerns. The difficulties in separation and recovery often result in large quantities of non-recyclable acid waste, which not only increases disposal costs but also poses environmental risks. To mitigate these issues, several strategies can be applied, including use of solid acid catalysts, alternative reaction conditions, immobilization techniques, continuous flow systems, and continuous flow systems. Solid acid catalysts gained significant attention in recent years due to their numerous advantages such as non-toxicity, reusability, lower costs, milder reaction conditions, environmental benefits, simple handling, stability, and diverse applications in various chemical reactions. They facilitate reactions by providing a pathway with lower activation energy, thus increasing reaction rates. The ability to recover and reuse solid catalysts significantly contributes to the principles of green chemistry by minimizing waste and reducing the need for additional resources. Solid catalysts often allow for easier separation from reaction mixtures compared to homogeneous catalysts, leading to less environmental impact. Their reusability not only enhances cost-effectiveness but also decreases the overall energy and material consumption associated with chemical processes. This aligns well with the goals of sustainable development in chemical manufacturing.2,23–26
The promotion of simple and neat protocols in synthetic chemistry aligns closely with the principles of green chemistry, which emphasize sustainability and minimizing environmental impact. The evolution of synthetic chemistry is increasingly focusing on atom-economy, cost-effectiveness, and environmental sustainability. This shift is driven by the need to minimize waste and reduce the environmental impact of chemical processes, which is crucial for both industrial applications and academic research. Accordingly, Claisen–Schmidt and Knoevenagel condensations have garnered significant interest due to their ability to produce target products in a single synthetic step. This approach eliminates the need to separate intermediates, which contributes to reduced energy consumption, shorter reaction times, and minimized waste materials. As a result, these methods promote environmentally friendly processes in organic synthesis.27–30
The Knoevenagel condensation is a key reaction in organic synthesis, valued for its efficiency in forming carbon–carbon double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The products of the Knoevenagel condensation serve as valuable organic intermediates that can be further transformed into a wide variety of natural compounds with significant medicinal and physical properties. In industry, Knoevenagel products are essential intermediates in the synthesis of pharmaceuticals, agrochemicals, and fine chemicals. The ongoing research into the Knoevenagel condensation reflects both its fundamental importance in organic synthesis and its adaptability to modern sustainable chemistry practices. The continuous exploration in this area is likely to lead to more efficient, cost-effective, and environmentally friendly synthetic methodologies.31
C). The products of the Knoevenagel condensation serve as valuable organic intermediates that can be further transformed into a wide variety of natural compounds with significant medicinal and physical properties. In industry, Knoevenagel products are essential intermediates in the synthesis of pharmaceuticals, agrochemicals, and fine chemicals. The ongoing research into the Knoevenagel condensation reflects both its fundamental importance in organic synthesis and its adaptability to modern sustainable chemistry practices. The continuous exploration in this area is likely to lead to more efficient, cost-effective, and environmentally friendly synthetic methodologies.31
The Claisen–Schmidt (CS) condensation is a specific type of cross-aldol condensation, especially for forming α,β-unsaturated carbonyl compounds. This reaction is pivotal in organic synthesis for creating α,β-unsaturated carbonyl compounds, which are important in various chemical applications. Many drugs and drug precursors are synthesized using CS condensation. Compounds such as flavonoids and their derivatives, which often exhibit anti-inflammatory, antioxidant, anti-viral, and anti-cancer properties, are commonly produced via this reaction. Chalcones, a class of compounds derived from CS condensation, are extensively studied for their potential therapeutic benefits. The reaction is utilized in the synthesis of flavor compounds and food additives, enhancing the sensory attributes of food products. Colorants derived from chalcones and flavonoids are also important in food and beverage applications for enhancing color without synthetic dyes. The CS reaction is valuable in the production of specialty chemicals used in perfumes and fragrances.32,33
The CS reaction can be catalyzed by bases or acids, and the choice of catalyst can significantly influence the reaction conditions and outcomes. However, this process is plagued by reverse reactions and side reactions that lower overall yields. In the past few decades, various metal(II) ion complexes such as Yb(OTf)3, RuCl3, FeCl3, Cp2ZrH2, TMSCl/NaI, KF-Al2O3, SmI3, InCl3, BF3·OEt2, TiCl3(SO3CF3) and BMPTO have been introduced as catalysts. However, the yields were not satisfactory (<38%). However, in most cases, good yields are achieved at high temperatures, but these conditions can be accompanied by challenges such as longer reaction times and complex and tedious purification procedures.34
Herein, the NA-NHSO3H catalyst is being applied for Knoevenagel and Claisen–Schmidt condensations. These condensation reactions are carried out in water as the solvent and at room temperature. The use of NA-NHSO3H as a solid acid catalyst in the Knoevenagel and Claisen–Schmidt condensations is an interesting approach, as it combines the advantages of NA (easy separation and recovery) with the catalytic activity of the –SO3H groups. This type of heterogeneous, recoverable catalyst can potentially offer improved efficiency, sustainability, and recycling capabilities compared to traditional homogeneous catalysts.
2. Experimental
2.1. Materials and instruments
All solvents and chemicals utilized in this study were obtained from Sigma-Aldrich and Merck chemical companies, and NA was obtained from the natural bitumen mines in west of Iran. Fourier Transform Infrared (FT-IR) spectroscopy was conducted using a VERTEX 70 FT-IR spectrometer from Bruker, Germany. The samples were prepared as KBr pellets for analysis. Scanning Electron Microscopy (SEM) imaging was performed using a TESCAN MIRA III Field Emission Scanning Electron Microscope (FE-SEM) from the Czech. Energy-dispersive X-ray spectroscopy (EDX) mapping was performed using a TESCAN instrument from the Czech to analyze the elemental composition of the samples. Additionally, the nitrogen adsorption–desorption isotherms were measured at 77 K using the BET method with a Micromeritics Asap 2020 instrument from the USA. Thermogravimetric analysis (TGA) was conducted using a NETZSCH TGA instrument, measuring temperature ranges from 25 °C to 800 °C to assess the thermal stability of the samples. Transmission Electron Microscopy (TEM) imaging of NA-NHSO3H was carried out using a Philips EM 208S TEM to investigate the morphology and size of the particles. 1HNMR and 13CNMR spectra was performed on Bruker Avance DPX-400 and DPX-500 spectrometers. Chemical shifts were reported in parts per million (ppm) relative to tetramethylsilane (TMS), which served as the internal standard.2.2. Preparation and characterization of NA-NHSO3H
2.2.2.1. Catalyst preparation for reduction reaction. In a 50 mL flask, 0.02 mol of thiourea was dissolved in a mixture of ethanol and water (20 mL each). The solution was heated to 50 °C to ensure complete dissolution of thiourea. Once dissolved, 0.005 mol of nickel(II) chloride hexahydrate (NiCl2·6H2O) was added. The reaction mixture was stirred at 80 °C for 4 hours. After the reaction was complete, the mixture was allowed to stand at room temperature for one week to facilitate the evaporation of ethanol, resulting in the formation of the NiII(Thiourea)2Cl2 complex.21 The product was then washed several times with ethanol to purify it. The final yield of the pure complex was 95%.
2.2.2.2. Convert the –NO2 group to –NH2. 1.0 g of NA-NO2 and 1.0 g of NiII(Thiourea)2Cl2 complex were added to a 100 mL two-neck round-bottom flask. The reaction mixture was stirred for 20 minutes. To manage the production of hydrogen gas (H2), the temperature of the reaction was lowered to 0 °C. Then, 0.7 g of sodium borohydride (NaBH4) was added to the flask. After 2 hours, the reaction mixture was allowed to stand at room temperature for 24 hours to dry. Ultimately, 0.8 g of product was obtained.
Method I: 0.5 g of NA-NH2 was added to 5 mL of liquid SO3 into a round-bottom flask at 0 °C. The reaction mixture was stirred at room temperature for 4 hours. The reaction mixture, then, was poured to cold water slowly, precipitate was filtered, washed with water and dried. Finally 0.92 g of the product was obtained.
Method II: After pouring 1.5 g of NA-NH2 and 15 mL of chloroform into a round-bottom flask, 0.5 mL of chlorosulfonic acid was added dropwise to the mixture for 15 minutes at −5 °C. The reaction was then continued for 4 hours at room temperature.
The reaction mixture, then, was poured to cold water slowly, the resulting precipitate was washed with water and dried. After drying, 0.9 g of the product was prepared.18
3. Results and discussion
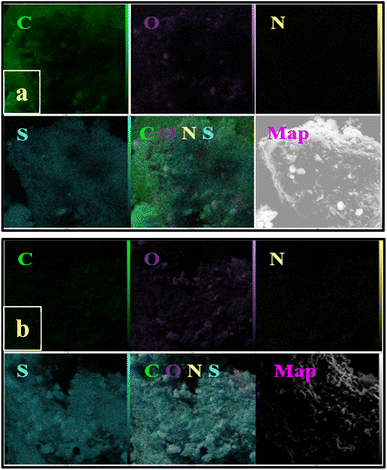
NA-NHSO3H was characterized using Fourier transform infrared (FT-IR) spectroscopy, thermogravimetric analysis (TGA), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and energy-dispersive X-ray spectroscopy (EDX) mapping.3.1. Characterization of NA-NHSO3H
| Functional group | NA | NA-NO2 | NA-NH2 | NA-NHSO3H |
|---|---|---|---|---|
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic C aromatic |
1450–1630 cm−1 | 1446–1638 cm−1 | 1408–1620 cm−1 | 1426–1635 cm−1 |
| C–H aliphatic | 2850–2918 cm−1 | 2852–2922 cm−1 | 1857–2921 cm−1 | Covered |
| OH/NH | 3450 cm−1 | 3440 cm−1 | Covered | 2700–3500 cm−1 |
| NO2 | — | 1340–1535 cm−1 | — | — |
| NH2 | — | — | 3182–3395 cm−1 | — |
| S–O | — | — | — | 560–680 cm−1 |
| SO2 | — | — | — | 1058–1196 cm−1 |
The presence of metal sulfides and SiO2 in NA and their subsequent removal in an acidic environment can provide valuable insights into the catalyst's performance and the stability of the materials involved. The acidic environment likely promotes the dissolution or transformation of the metal sulfides and SiO2. Understanding the specific reactions that occur in NA-NHSO3H could help in optimizing the catalyst further. The removal of these materials might enhance the catalytic properties of the remaining structure, possibly by increasing the availability of active sites or improving the overall morphology of the catalyst.
3.2. Catalytic studies
For this purpose, the condensation of malononitrile with 4-chlorobenzaldehyde was used as a model reaction. Then, different reaction conditions were screened to optimize the process. The results of the optimization experiments are summarized in Table 2. The results showed that 10 mg of catalyst, water and room temperature were selected as the best reaction conditions among different amounts, solvents and temperatures (Table 2, entry 4)
| Entry | NA-NHSO3H (mg) | Solvent | Temperature (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1 mmol, 140 mg), malononitrile (1.05 mmol, 69 mg), NA-NHSO3H (mg) and solvent (1 mL).b Isolated yield.c The reaction catalyzed by NA-NH2. | |||||
| 1 | — | Water | r.t. | 270 | 36 |
| 2 | 20 | Water | r.t. | 20 | 90 |
| 3 | 15 | Water | r.t. | 18 | 93 |
| 4 | 10 | Water | r.t. | 18 | 99 |
| 5 | 5 | Water | r.t. | 30 | 91 |
| 6 | 10 | Ethanol | r.t. | 84 | 86 |
| 7 | 10 | Water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1 ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
r.t. | 55 | 88 |
| 8 | 10 | Water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (2 ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
r.t. | 35 | 90 |
| 9 | 10 | Acetonitrile | r.t. | 300 | 48 |
| 10 | 10 | Water | 50 | 28 | 92 |
| 11 | 10 | Water | 80 | 34 | 90 |
| 12 | 10 | Water | r.t. | 150 | 31c |
The observed decrease in reaction yield with increasing temperature can be attributed to the potential hydrolysis of nitrile groups within the product. This hydrolysis, occurring at higher temperatures, generates by-products and intermediates that interfere with the intended Knoevenagel reaction pathway. Furthermore, competing side reactions, including Diels–Alder and hetero-Diels–Alder additions, contribute to the formation of various by-products.35 These side reactions effectively compete with the formation of the desired Knoevenagel product, leading to a dilution of its concentration and a subsequent reduction in both the overall yield and reaction rate (Tables 2, 4 and 5). In many chemical reactions, especially reactions involving organic substrates, the strength of the acid can affect the reaction rate. Acids can act as catalysts and lower the activation energy by protonating the reactants. This causes the reaction to proceed more quickly. The pH scale is logarithmic and measures the concentration of hydrogen ions (H+) in a solution. As the temperature rises, the ionization of water increases, leading to a higher concentration of H+ ions. This can cause the pH value to decrease (become more acidic), even in pure water (eqn (1)).
pH = −log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H+ H+
| (1) |
To investigate the effect of temperature on the reaction rate and acidity, a glass electrode was used. Three different temperatures (r.t., 50 °C, 80 °C) were investigated for the reaction of model 2a. First, 10 mg of the catalyst was dispersed in 5 mL of deionized water at room temperature. Then, the pH at room temperature, 50 and 80 °C showed the values of 2.10, 2.21 and 2.33, respectively. The effect of temperature on the reaction rate was also evaluated for reactions 4a and 6a at r.t., 45, and 70 °C, respectively. The results indicated that for reaction 4a, pH values of 2.11, 2.23, and 2.36 were obtained, respectively. Furthermore, for reaction 6a, values of 2.11, 2.24, and 2.37 were measured, respectively. The observed relationship between increasing temperature, decreasing pH, increasing acid strength, and accelerating reaction rate is a coherent and well-supported pattern in chemistry. As temperature rises, changes in high pH cause a decrease in acid strength, which ultimately slows down the reaction (Tables 2, 4 and 5).
The relationship between the Nernst equation, acid strength, reaction rate, and the effect of decreasing temperature is examined. The Nernst equation describes how the potential of an electrode (E) changes with the concentration of the reactants and products involved in an electrochemical reaction (eqn (2)).36
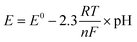 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485C mol−1).
485C mol−1).
The acid strength of a solution is determined by its pH. Acidic solutions have a lower pH and a higher concentration of hydrogen ions (H+). A decrease in temperature directly reduces the magnitude of the (RT/nF) term. Smaller (RT/nF) means it will have less effect on the cell potential (E). In other words, the cell potential becomes less sensitive to changes in concentration as the temperature decreases. In addition, if the acid strength decreases (pH increases, [H+] decreases), it will also decrease the cell potential (E). A lower cell potential generally translates to a slower reaction rate. The driving force for the electron transfer is reduced (Tables 2, 4 and 5).
The generality of the catalytic system was evaluated by reacting different aldehydes (both electron-donating and electron-withdrawing substituted aromatic aldehydes) with malononitrile (Scheme 2). According to the results, the desired products were obtained in satisfactory to excellent yields, suggesting a high degree of efficiency in the reaction process (Table 3).
| Entry | Aldehyde | Product | Time (min) | Yield (%) | Mp (°C) measured | Mp (°C) literature |
|---|---|---|---|---|---|---|
| a Reaction conditions: aldehyde (1 mmol), malononitrile (1.05 mmol, 69 mg), 10 mg of NA-NHSO3H in 1 mL of water at room temperature. | ||||||
| 1 | 4-ClC6H4CHO | 2a | 18 | 99 | 160–162 | 162–164 (ref. 37) |
| 2 | C6H5CHO | 2b | 24 | 97 | 80 | 80–82 (ref. 37) |
| 3 | 4-MC6H4CHO | 2c | 38 | 94 | 128–130 | 130–133 (ref. 37) |
| 4 | 4-MeOC6H4CHO | 2d | 54 | 91 | 116–118 | 114–116 (ref. 37) |
| 5 | 3,4-(MeO)2C6H3CHO | 2e | 64 | 88 | 130–132 | 132–135 (ref. 38) |
| 6 | 4-BrC6H4CHO | 2f | 72 | 89 | 150–152 | 153–156 (ref. 38) |
| 7 | 3-O2NC6H4CHO | 2g | 45 | 93 | 102–104 | 106–108 (ref. 37) |
| 8 | 4-O2NC6H4CHO | 2h | 40 | 95 | 158–160 | 159–161 (ref. 30) |
| 9 | 4-HOC6H4CHO | 2i | 50 | 90 | 186–188 | 188–190 (ref. 38) |
| 10 | 4-Me2NC6H4CHO | 2j | 28 | 98 | 181–183 | 180–182 (ref. 39) |
Green chemistry emphasizes the development of new synthesis methods and biocompatible catalysts to prevent negative effects on human health and the environment. High atom economy, energy efficiency of processes, low amount of waste products, avoiding the formation of byproducts, renewable materials and replacing hazardous reagents or catalysts with less harmful materials are important and significant criteria in green chemistry. After completing the reaction, separating out the catalyst emphasizes ease of recovery, which is key in reducing waste and enhancing sustainability. Using crystallization with ethanol for product purification is a good choice, as ethanol is often regarded as a greener solvent compared to others, especially if it's derived from renewable sources. Knoevenagel and Claisen–Schmidt condensation reactions performed at room temperature using water align well with green chemistry principles, as they minimize energy consumption and use non-toxic solvents. The adoption of green chemistry principles during catalyst synthesis and product preparation indicates a commitment to reducing environmental impact. This includes minimizing hazardous substances, using renewable resources, and maximizing atom economy.
| Entry | NA-NHSO3H (mg) | Solvent | Temperature (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1 mmol, 140 mg), acetophenone (1 mmol, 120 mg), NA-NHSO3H (mg) and solvent (1 mL).b Isolated yield.c The reaction catalyzed by NA-NH2. | |||||
| 1 | — | Water | r.t. | 110 | Trace |
| 2 | 20 | Water | r.t. | 40 | 92 |
| 3 | 15 | Water | r.t. | 20 | 95 |
| 4 | 10 | Water | r.t. | 20 | 97 |
| 5 | 5 | Water | r.t. | 30 | 90 |
| 6 | 10 | — | r.t. | 65 | 81 |
| 7 | 10 | Ethanol | r.t. | 55 | 88 |
| 8 | 10 | Water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (2 ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
r.t. | 50 | 89 |
| 9 | 10 | Tetrahydrofuran | r.t. | 80 | 20 |
| 10 | 10 | Water | 45 | 35 | 87 |
| 11 | 10 | Water | 70 | 40 | 85 |
| 12 | 10 | Water | r.t. | 170 | Tracec |
| Entry | NA-NHSO3H (mg) | Solvent | Temperature (°C) | Time (min) | Yieldb (%) | ||
|---|---|---|---|---|---|---|---|
| 6a | 6c | 6a | 6c | ||||
| a Reaction conditions: 4-chlorobenzaldehyde (2 mmol, 240 mg), ketone (1 mmol, 58 mg), NA-NHSO3H (mg) and solvent (1 mL).b Isolated yield.c The reaction catalyzed by NA-NH2. | |||||||
| 1 | — | Water | r.t. | 90 | 150 | 21 | 11 |
| 2 | 20 | Water | r.t. | 42 | 90 | 93 | 86 |
| 3 | 15 | Water | r.t. | 26 | 70 | 91 | 90 |
| 4 | 10 | Water | r.t. | 28 | 50 | 96 | 96 |
| 5 | 5 | Water | r.t. | 36 | 60 | 91 | 91 |
| 6 | 10 | — | r.t. | 68 | 72 | 80 | 84 |
| 7 | 10 | Ethanol | r.t. | 58 | 65 | 87 | 92 |
| 8 | 10 | Water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (2 ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
r.t. | 54 | 63 | 86 | 90 |
| 9 | 10 | Toluene | r.t. | 116 | 110 | 18 | 15 |
| 10 | 10 | Water | 45 | 44 | 58 | 83 | 85 |
| 11 | 10 | Water | 70 | 50 | 64 | 81 | 80 |
| 12 | 10 | Water | r.t. | 188 | 225 | Tracec | Tracec |
Solvents can significantly influence reaction kinetics, yield, and selectivity. Protic solvents, like water, often provide better results due to their ability to stabilize charged intermediates and facilitate proton transfer, which can enhance reaction rates and yields. In contrast, aprotic solvents (like tetrahydrofuran) and nonpolar solvents (like toluene) may not stabilize charged species as effectively, leading to longer reaction times and lower yields. This can be particularly important in reactions involving ionic intermediates or mechanisms that rely on hydrogen bonding (Tables 4 and 5).
According to the results, it was found that the use of protic solvents has a positive effect on the reaction and the selectivity towards the Knoevenagel and Claisen–Schmidt products is significantly affected. Because with decreasing temperature, the pH decreases, which increases the acidic strength and the reaction rate.
In order to assess the generality of the catalytic protocol, the reaction was expanded to a wide range of aromatic aldehydes (Scheme 3). The outcomes are detailed in Table 6. The results indicate that the reactions proceeded cleanly, yielding the corresponding products with exceptional yields ranging from 90% to 97%. This high degree of yield across various substrates highlights the robustness and versatility of the NA-NHSO3H catalyst in facilitating the cyclocondensation process under the optimized conditions.
| Entry | Aldehyde | Ketone | Product | Time (min) | Yield (%) | Mp (°C) Measured | Mp (°C) Literature |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: aldehyde (1 mmol), acetophenone (1 mmol, 120 mg), 10 mg of NA-NHSO3H in 1 mL of water at room temperature. | |||||||
| 1 | 4-ClC6H4CHO |  |
4a | 20 | 97 | 110–112 | 110–112 (ref. 37) |
| 2 | C6H5CHO |  |
4b | 28 | 96 | 50–52 | 54–56 (ref. 37) |
| 3 | C6H5CHO | 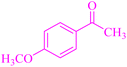 |
4c | 34 | 93 | 104–106 | 102–105 (ref. 37) |
| 4 | 4-ClC6H4CHO |  |
4d | 42 | 91 | 144–146 | 143–144 (ref. 37) |
| 5 | 4-ClC6H4CHO |  |
4e | 36 | 93 | 120–124 | 125–126 (ref. 39) |
| 6 | 4-ClC6H4CHO |  |
4f | 34 | 94 | 150–152 | 148–150 (ref. 39) |
| 7 | 4-MeOC6H4CHO |  |
4g | 50 | 90 | 118–121 | 119–120 (ref. 37) |
Also, the synthesis of electron-donating and electron-withdrawing aromatic aldehydes was studied using model reactions 6a and 6c (Scheme 4). The results showed that the desired products were synthesized with good to excellent yields regardless of the electronic nature of the substituent in the aldehydes. This suggests that the 6a and 6c model reactions accommodated a range of electron-donating and electron-withdrawing groups on the aromatic aldehydes, producing the desired products in satisfactory to high yields (Table 7).
 | ||
| Scheme 4 Synthesis of dibenzalacetone derivates and α,α′-bis(substituted-benzylidene) cycloalkanones from the reaction between various aldehydes with ketones catalyzed by NA-NHSO3H. | ||
| Entry | Aldehyde | Ketone | Product | Time (min) | Yield (%) | Mp (°C) measured | Mp (°C) literature |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: aldehyde (2 mmol), ketone (1 mmol, 58 mg), 10 mg of NA-NHSO3H in 1 mL of water at room temperature. | |||||||
| 1 | C6H5CHO |  |
6a | 48 | 94 | 40–42 | 41–43 (ref. 37) |
| 2 | 4-MeC6H4CHO |  |
6b | 55 | 88 | 122–124 | 126–129 (ref. 39) |
| 3 | 4-ClC6H4CHO | 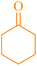 |
6c | 50 | 96 | 144–146 | 145–147 (ref. 34) |
| 4 | 4-MeC6H4CHO |  |
6d | 70 | 93 | 199–201 | 199–200 (ref. 34) |
| 5 | 4-MeOC6H4CHO |  |
6e | 75 | 90 | 168–170 | 169–171 (ref. 34) |
| 6 | C6H5CHO |  |
6f | 60 | 95 | 116–118 | 115–117 (ref. 34) |
| 7 | 3-O2NC6H4CHO |  |
6g | 80 | 91 | 198–200 | 200–202 (ref. 34) |
The reaction yields were calculated using eqn (3):
 | (3) |
For further confirmation, 13CNMR, 1HNMR and FT-IR analysis were applied for some products and the results were displayed in Fig. S1–S21† which confirmed the formation of products.
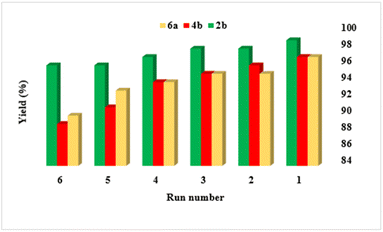
3.3. Reusability of the catalyst leaching test
The reusability of catalysts is crucial for economic and environmental sustainability in catalytic processes. To investigate the reusability of this catalyst, reactions that produce products 2b, 4b, and 6a were evaluated (Fig. 7). After each cycle of the reaction, the catalyst can be easily recovered using hot ethanol, which minimizes loss and facilitates the reusability assessment. The recovered catalyst can then be washed and reused in subsequent reaction cycles. In summary, the catalytic activity was assessed over six cycles, demonstrating consistent performance with no significant loss in the original catalytic activity. Furthermore, The FT-IR spectra, SEM images and TG curves taken before and after recovery (Fig. 8–10) showed strong similarities, indicating that the catalyst maintained its stability throughout the testing. In addition, the solubility of the NA-NHSO3H catalyst before and after recovery in organic solvents including toluene, xylene and carbon disulfide was tested. The results confirmed that, unlike natural asphalt, NA-NHSO3H is not soluble in the mentioned solvents. Catalyst recovery studies shows a decline in product yield across successive cycles. It can be attributed to several factors. In the initial cycle, the catalyst typically displays its highest activity and selectivity, promoting efficient conversion to the desired product. Over subsequent cycles, catalyst deactivation, potentially due to structural changes or active site poisoning, leads to reduced selectivity and activity. Additionally, the accumulation of reactants, by-products, and residual solvents on catalyst can physically block active sites, thereby limiting access for reactants and reducing the overall catalytic activity.The evaluation of leaching for the supported catalyst using a glass electrode indicates that there is no significant difference in the loading amount of acid groups on the surface of the catalyst even after six cycles. This suggests that the catalyst demonstrates good stability and retention of its –SO3H groups, which is crucial for maintaining its catalytic activity over multiple uses. The consistent loading of acid groups implies that the catalyst can be effectively reused without substantial loss of its active sites.
Dispersing 3 mg of the NA-NHSO3H catalyst in 3 mL of deionized water at room temperature is a standard method for assessing the acidity of solid catalysts. This ensures that the catalyst is adequately suspended for accurate pH measurement. The initial pH value of 2.02 indicates a strongly acidic environment, which is consistent with the presence of –SO3H groups on the catalyst. This acidity is crucial for the catalyst's performance in acid-catalyzed reactions. Continuing pH measurements for six more steps likely involves observing changes in pH over time with adding catalyst. This could help in understanding how the acidity of the catalyst evolves during the evaluation period. Monitoring pH can give insights into the stability of the acidic properties of the catalyst before and after recycling. Since acidity directly influences the catalytic activity of acid catalysts, maintaining low pH levels after recycling is desirable for consistent performance in catalytic reactions (Table 8).
| Entry | NA-NHSO3H | Reusability | ||
|---|---|---|---|---|
| Amount (mg) | pH | Run | pH | |
| 1 | 3 | 2.02 | — | 2.02 |
| 2 | 6 | 2.04 | 1 | 2.03 |
| 3 | 9 | 2.07 | 2 | 2.06 |
| 4 | 12 | 2.10 | 3 | 2.08 |
| 5 | 15 | 2.12 | 4 | 2.11 |
| 6 | 18 | 2.14 | 5 | 2.12 |
| 7 | 21 | 2.15 | 6 | 2.13 |
Comparison of the catalyst before and after recovery with NA indicates that the recovering process effectively eliminates excess ash and minerals from the catalyst. This is significant because impurities can affect the catalyst's performance, possibly leading to decreased activity and selectivity. The TG curves showing around 70% decomposition for the catalyst before recovery and 34% for NA imply that the recovery process not only enhances the purity of the catalyst but also significantly impacts its thermal stability. The higher decomposition rate in the catalyst suggests that it contained more volatile components or impurities initially. The reduction of these impurities likely contributes to improved catalytic performance since a purer catalyst is often more efficient. Removing unwanted materials can lead to better access to active sites for the reactants and minimize side reactions. The successful removal of ash and minerals through recovery processes could improve the recyclability of the catalyst, making it more economical for industrial applications. Ensuring high purity can help maintain consistent catalytic activity over multiple reaction cycles.
3.4. Hot filtration
The hot filtration test aimed to demonstrate the heterogeneous nature of the catalyst and to assess the leaching of acidic sites during the reaction. For this purpose, reaction 2a was conducted at room temperature in 1 mL of water. The catalyst was removed after 9 minutes, at which point the yield was 57%. The reaction mixture was stirred for an additional 9 minutes without the catalyst, resulting in a final yield of 60%. The catalyst removal showed that the reaction yielded 57% in the presence of the catalyst. The stability of the –SO3H groups on the NA support surface has been confirmed. This confirms that the –SO3H groups are covalently bonded to the catalytic support material. The results show that the acidic –SO3H groups are not leaching into the reaction solution. Additionally, the –SO3H groups are not degrading under the reaction conditions. The stability of the covalent attachment of the –SO3H groups and the lack of leaching are significant findings. This indicates that the catalyst design is effective at maintaining the integrity of the active sites and preventing contamination of the reaction mixture. These results suggest the catalyst has good long-term stability and recyclability, which are important practical considerations for industrial applications. The covalent bonding also likely improves the overall performance and efficiency of the catalytic system. The data supports the heterogeneous nature of the catalyst, as significant leaching was not observed. This allows for potential reusability without significant loss of active sites.3.5. Comparison
The study compares NA-NHSO3H with various reported catalysts for specific reactions (2b and 4b) in the Knoevenagel and Claisen–Schmidt condensations. NA-NHSO3H can function effectively at lower temperatures and pressures, which is crucial for reactions involving sensitive substrates that might degrade or react undesirably under harsher conditions (milder reaction conditions). The catalyst enables faster reactions, which can increase efficiency in synthetic processes. The catalyst demonstrated superior performance in terms of yield compared to other catalysts, indicating its effectiveness (Table 9).| Entry | Catalyst | Conditions | Yield (%) | Product | [Ref.] |
|---|---|---|---|---|---|
| 1 | Imine-linked COFs (TaDA) | 12 h/100 °C, toluene | 97 | 2b | 40 |
| 2 | Zn/Cd-metal–organic frameworks | 6 h/60 °C | 99 | 2b | 41 |
| 3 | Calix[4]arene-based polyoxometalate | 1 h/60 °C | 99 | 2b | 42 |
| 4 | Li-zagronas | 90 min/r.t., ethanol | 90 | 2b | 39 |
| 5 | Chitosan | 6 h/40 °C, ethanol | 99 | 2b | 43 |
| 6 | BCN | 30 min/80 °C, acetonitrile | 92 | 2b | 44 |
| 7 | NA-NHSO3H | 24 min/r.t., water | 96 | 2b | This work |
| 8 | Cs-zagronas | 30 min/r.t., ethanol | 95 | 4b | 39 |
| 9 | GO | 16 h/100 °C, TBAB, solvent-free | 84 | 4b | 45 |
| 10 | K-NAS | 210 min/r.t., ethanol | 95 | 4b | 37 |
| 12 | NA-NHSO3H | 28 min/r.t., water | 96 | 4b | This work |
The characteristics of NA-NHSO3H position it as a user-friendly and environmentally benign catalyst, aligning with the principles of green chemistry. This is particularly important in modern chemistry, where sustainability and reduced environmental impact are priorities. The superior performance of NA-NHSO3H suggests it may be a more viable option for industrial applications or in laboratories seeking efficient and eco-friendly methodologies.
4. Conclusion
In summary, a metal-free solid acid catalyst, NA-NHSO3H, has been introduced for the synthesis of α,β-unsaturated compounds, dibenzalacetone derivatives and α,α′-bis(substituted-benzylidene) cycloalkanones. Notable advantages of this protocol are good to excellent yields, rapid reaction times, simplified one-pot synthesis, use of environmentally-friendly “green” reaction conditions, reduced number of synthetic steps, minimal waste generation, easy product separation, good reaction conditions and low operational costs. Additionally, the catalyst demonstrates excellent reusability without significant loss of activity. These are all highly desirable features for an efficient and sustainable chemical process or synthesis. The combination of high productivity, resource efficiency and cost-effectiveness could make this a very attractive approach. The recovered catalyst was characterized using FT-IR, SEM and TGA techniques, confirming its structural integrity and performance.Data availability
The data that support the findings of this study are available in ESI.†Author contributions
Sahar Abdolahi: validation, methodology, investigation, writing – original draft, conceptualization. Mohammad Soleiman-Beigi: funding acquisition, supervision, project administration, conceptualization, editing, resources.Conflicts of interest
All authors declare that there are no competing interests.Acknowledgements
We would like to thank the Iran National Science Foundation (INSF) for financial support of this research project (Grant No. 4020787).References
- M.-Y. Li, A. Gu, J. Li and Y. Liu, Green Synth. Catal., 2025, 6, 36–66 CrossRef CAS.
- A. Munyentwali, H. Li and Q. Yang, Appl. Catal., A, 2022, 633, 118525 CrossRef CAS.
- V. Georgakilas, J. A. Perman, J. Tucek and R. Zboril, Chem. Rev., 2015, 115, 4744–4822 CAS.
- H. Kohzadi and M. Soleiman-Beigi, Sci. Rep., 2021, 11, 24508 CrossRef CAS PubMed.
- M. Pagliaro, M. V. Pagliaro, R. Caliandro, C. Giannini, R. Ciriminna and A. Lavacchi, Mater. Adv., 2024, 5, 2759–2766 RSC.
- M. Raji, N. Zari and R. Bouhfid, in Functionalized graphene nanocomposites and their derivatives, Elsevier, 2019, pp. 1–20 Search PubMed.
- A. Mehmood, N. M. Mubarak, M. Khalid, R. Walvekar, E. C. Abdullah, M. T. H. Siddiqui, H. A. Baloch, S. Nizamuddin and S. Mazari, J. Environ. Chem. Eng., 2020, 8, 103743 CrossRef CAS.
- N. Iqbal, A. Afzal, N. Cioffi, L. Sabbatini and L. Torsi, Sens. Actuators, B, 2013, 181, 9–21 CrossRef CAS.
- H. Li, S. Saravanamurugan, S. Yang and A. Riisager, ACS Sustainable Chem. Eng., 2015, 3, 3274–3280 Search PubMed.
- H. Li, A. Riisager, S. Saravanamurugan, A. Pandey, R. S. Sangwan, S. Yang and R. Luque, ACS Catal., 2018, 8, 148–187 Search PubMed.
- S. Pal, D. Das and S. Bhunia, Org. Biomol. Chem., 2024, 22, 1527–1579 RSC.
- P. Salehi, M. Dabiri, M. A. Zolfigol and M. A. Bodaghi Fard, Tetrahedron Lett., 2003, 44, 2889–2891 CAS.
- X. L. Shi, H. Yang, M. Tao and W. Zhang, RSC Adv., 2013, 3, 3939–3945 RSC.
- P. Polikarpova, A. Akopyan, A. Shlenova and A. Anisimov, Catal. Commun., 2020, 146, 106123 CrossRef CAS.
- P. R. Thombal and S. S. Han, Biofuel Res. J., 2018, 5, 886–893 CrossRef CAS.
- J. Safari, S. Gandomi-Ravandi and S. Ashiri, New J. Chem., 2016, 40, 512–520 RSC.
- A. Rajack, K. Yuvaraju, C. Praveen and Y. L. N. Murthy, J. Mol. Catal. A:Chem., 2013, 370, 197–204 CrossRef CAS.
- S. Abdolahi and M. Soleiman-Beigi, RSC Adv., 2024, 14, 35971–35979 RSC.
- H. Kohzadi and M. Soleiman-Beigi, New J. Chem., 2020, 44, 12134–12142 Search PubMed.
- T. Zolfaghari, M. Soleiman-Beigi and H. Kohzadi, ACS Omega, 2023, 8, 36152–36161 CrossRef CAS PubMed.
- S. Abdolahi and M. Soleiman-Beigi, Heliyon, 2024, 11, e41492 CrossRef PubMed.
- M. Soleiman-Beigi, H. Kohzadi and S. Toolabi, in Asphalt Materials – Recent Developments and New Perspective, IntechOpen, 2024 Search PubMed.
- W. Gong, Y. Liu, H. Li and Y. Cui, Coord. Chem. Rev., 2020, 420, 213400 CrossRef CAS.
- P. Noppawan, S. Sangon, N. Supanchaiyamat and A. J. Hunt, Green Chem., 2021, 23, 5766–5774 RSC.
- F. Sajjad and M.-H. Xu, Green Synth. Catal., 2025, 6, 86–90 CrossRef CAS.
- K. Nakajima and M. Hara, ACS Catal., 2012, 2, 1296–1304 Search PubMed.
- J. Fessler, K. Junge and M. Beller, Chem. Sci., 2023, 14, 11374–11380 RSC.
- V. G. Zuin, I. Eilks, M. Elschami and K. Kümmerer, Green Chem., 2021, 23, 1594–1608 RSC.
- S. S. Choudhury, S. Mahapatra, A. K. Sahu, P. Hembram, S. Jena and H. S. Biswal, ACS Sustain. Chem. Eng., 2022, 10, 14271–14279 CrossRef CAS.
- H. Chen, L. Fan and X. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 54884–54892 CrossRef CAS PubMed.
- P. X. Kolagkis, S. K. Serviou, N. A. Stini, V. P. Demertzidou, E. T. Poursaitidis, E. M. Galathri, O. G. Mountanea, E. Skolia and C. G. Kokotos, Org. Biomol. Chem., 2024, 22, 8293–8299 Search PubMed.
- R. Sun, C. Han and J. Xu, RSC Adv., 2022, 12, 29240–29245 RSC.
- H. Annath, J. C. Manayil, J. Thompson, A. C. Marr and R. Raja, Appl. Catal., A., 2021, 627, 118376 Search PubMed.
- B. Das, P. Thirupathi, I. Mahender and K. R. Reddy, J. Mol. Catal. A:Chem., 2006, 247, 182–185 CrossRef CAS.
- S. Yamazaki, K. Katayama, Z. Wang, Y. Mikata, T. Morimoto and A. Ogawa, ACS Omega, 2021, 6, 28441–28454 CrossRef CAS PubMed.
- Y.-J. Shih, Z.-L. Wu and S.-K. Lin, Sens. Actuators, B., 2024, 409, 135600 CrossRef CAS.
- S. Falah, M. Soleiman-Beigi and H. Kohzadi, Appl. Organomet. Chem., 2020, 34, e5840 CrossRef CAS.
- M. Saghian, S. Dehghanpour and Z. Bayatani, Sci. Rep., 2023, 13, 15563 CrossRef CAS PubMed.
- M. Soleiman-Beigi, S. Ghalavand, H. G. Venovel and H. Kohzadi, J. Iran. Chem. Soc., 2021, 18, 3267–3279 CrossRef CAS.
- X. Wu, B. Wang, Z. Yang and L. Chen, J. Mater. Chem. A, 2019, 7, 5650–5655 RSC.
- Z.-W. Zhai, S.-H. Yang, Y.-R. Lv, C.-X. Du, L.-K. Li and S.-Q. Zang, Dalt. Trans., 2019, 48, 4007–4014 RSC.
- L.-J. Yue, Y.-Y. Liu, G.-H. Xu and J.-F. Ma, New J. Chem., 2019, 43, 15871–15878 Search PubMed.
- B. Sakthivel and A. Dhakshinamoorthy, J. Colloid Interface Sci., 2017, 485, 75–80 CrossRef CAS PubMed.
- X. Li, B. Lin, H. Li, Q. Yu, Y. Ge, X. Jin, X. Liu, Y. Zhou and J. Xiao, Appl. Catal., B, 2018, 239, 254–259 CrossRef CAS.
- D. Khalili, S. Lavian and M. Moayyed, Tetrahedron Lett., 2020, 61, 151470 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00870k |
| This journal is © The Royal Society of Chemistry 2025 |