Open Access Article
Open Access ArticleEnhanced electrocatalytic nitrate-to-ammonia performance from Mott–Schottky design to induce electron redistribution†
Ruikai
Qi‡
a,
Qiuling
Jiang‡
bd,
Li
Deng
a,
Xianqiang
Yu
a,
Bingyan
Shi
a,
Mengxiao
Zhong
*c,
Ying
Wang
*b and
Xiaofeng
Lu
 *a
*a
aAlan G. MacDiarmid Institute, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: xflu@jlu.edu.cn
bState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, China. E-mail: ywang_2012@ciac.ac.cn
cState Key Laboratory of Integrated Optoelectronics, Key Laboratory of Advanced Gas Sensors, College of Electronic Science and Engineering, Jilin University, 2699 Qianjin Street, Changchun, Jilin Province 130012, P. R. China. E-mail: zhongmx@jlu.edu.cn
dSchool of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, 230026, China
First published on 18th November 2024
Abstract
Constructing highly efficient electrocatalysts via interface manipulation and structural design to facilitate rapid electron transfer in electrocatalytic nitrate-to-ammonia conversion is crucial to attaining superior NH3 yield rates. Here, a Mott–Schottky type electrocatalyst of Co/In2O3 with a continuous fiber structure has been designed to boost the electrocatalytic nitrate-to-ammonia performance. The optimized Co/In2O3-1 catalyst exhibits an impressive NH3 yield rate of 70.1 mg cm−2 h−1 at −0.8 V vs. the reversible hydrogen electrode (RHE), along with an NH3 faradaic efficiency (FE) of 93.34% at 0 V vs. RHE, greatly outperforming the single-component Co and In2O3 samples. The yield rate of Co/In2O3-1 is also superior to that of most currently reported Co-based catalysts and heterostructured ones. Evidence from experiments and theoretical results confirms the formation of a Mott–Schottky heterojunction, which achieves a Co site enriched with electrons, coupled with an In2O3 facet enriched with holes, inducing an electron redistribution to promote the utilization of electroactive sites. Consequently, the reaction energy barrier for nitrate-to-ammonia conversion is significantly reduced, further enhancing its yield efficiency.
1. Introduction
NH3 serves a variety of purposes, including fertilization, chemical production, and fuel.1 In recent years, scientific research on electrocatalytic nitrate-to-ammonia conversion has gained extensive interest due to its advantages of safety, energy conservation, and eco-friendliness for NH3 production.2–10 However, the electrocatalytic nitrate-to-ammonia process involves complex multiple electron transfer and pathways, posing a great challenge to rational design and fabrication of efficient catalysts for electrochemical NH3 generation.11–19 Fortunately, some cost-effective transition metal-based materials hold promise as nitrate-to-ammonia electrocatalysts, particularly cobalt-based catalysts such as FeCoNiAlTi,20 Co2Mo6S8 (ref. 21) and Ru15Co85 (ref. 22) due to their superior intrinsic activities. Despite significant improvement in NH3 faradaic efficiency (FE) that has been achieved at lower potentials, the NH3 yield rates of those electrocatalysts are still unsatisfactory because of the low current density. Therefore, researchers relentlessly strive for exploring efficient nitrate reduction catalysts with high NH3 yield.Heterojunction structures significantly optimize the electronic structure and energy bands at catalyst interfaces, essential for rapid electron transfer. Consequently, at heterojunction interfaces, the adsorption of reactants, desorption of products, and the activity of active sites are promoted.23–25 Typically, according to the Mott–Schottky effect, electrons in metal/semiconductor heterojunctions tend to spontaneously traverse interfaces until the work functions on both sides balance. This continuous adjustment at the interfaces alters the work function of the Mott–Schottky barrier, regulates the electron cloud density of the catalyst, and induces negative charge accumulation on the side with a higher work function.26,27 Hence, creating Mott–Schottky junctions is a reliable strategy for modulating electron density at electrocatalyst interfaces. The construction of metal/semiconductor heterostructures with Mott–Schottky rectification effects holds potential for boosting the efficiency of the nitrate-to-ammonia reaction.
Indium oxide (In2O3) is a common n-type semiconductor with a wide bandgap, low resistivity, and high catalytic activity, which is widely used in electrocatalysis.28–30 Therefore, we have demonstrated the clever construction of a continuous fibrous Mott–Schottky type Co/In2O3 heterostructure by combining Co with In2O3 through a simple electrospinning-calcination-partial reduction strategy. The experimental results demonstrate that the long-range ordered fiber network presents numerous active sites, and the formation of a Co/In2O3-1 heterojunction with the Mott–Schottky effect induces an electron redistribution at the interface, benefitting to reduce the reaction barrier of the catalytic process. Density functional theory (DFT) analysis indicates an augmentation of the density of states (DOS) near the Fermi level for the Co/In2O3-1 catalyst, and the d-band center (εd) of Co in Co/In2O3-1 aligns nearer to the Fermi level compared to Co (111). Therefore, Co/In2O3-1 exhibits the smallest thermodynamic reaction energy barrier (ΔG(PDS)) for the potential-determining step with the formation of 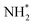 from NH* protonation, suggesting that the Mott–Schottky type Co/In2O3 heterostructure significantly enhances the electrocatalytic nitrate-to-ammonia activity. Therefore, the optimized Co/In2O3-1 catalyst presents a high NH3 yield rate of 70.1 mg cm−2 h−1 at −0.8 V vs. RHE, outperforming most currently reported nitrate-to-ammonia electrocatalysts.
from NH* protonation, suggesting that the Mott–Schottky type Co/In2O3 heterostructure significantly enhances the electrocatalytic nitrate-to-ammonia activity. Therefore, the optimized Co/In2O3-1 catalyst presents a high NH3 yield rate of 70.1 mg cm−2 h−1 at −0.8 V vs. RHE, outperforming most currently reported nitrate-to-ammonia electrocatalysts.
2. Results and discussion
2.1 Synthesis and characterization of the Mott–Schottky type Co/In2O3 heterostructure
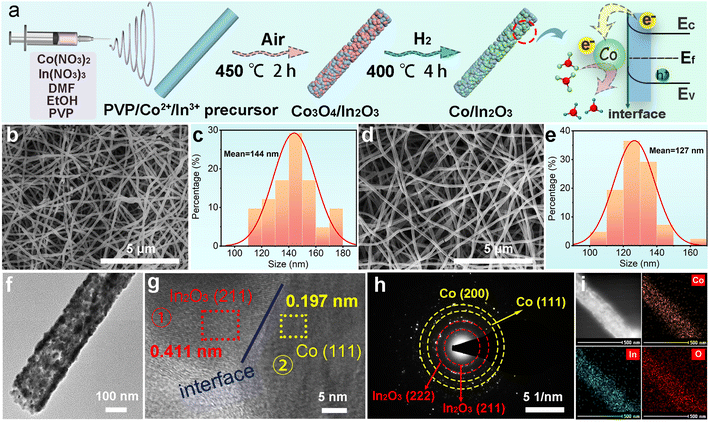
The nanofibrous Co/In2O3 heterostructure is synthesized using an electrospinning, calcination, and partial reduction process (Fig. 1a). Initially, a poly(vinylpyrrolidone) (PVP)/Co2+/In3+ precursor nanofibrous membrane is prepared via an electrospinning technique and then immediately calcined in air to yield continuous and uniform Co3O4/In2O3 nanofibers with a rough surface and an average diameter of 144 nm (Fig. 1b and c). After being reduced in a H2/Ar atmosphere at 400 °C for 4 h, Co3O4 can be selectively reduced, and then Co/In2O3 is obtained, and its fibrous morphology remains basically unchanged (Fig. 1d). However, the diameter of the Co/In2O3 nanofibers slightly decreases due to oxygen elimination (Fig. 1e). The transmission electron microscopy (TEM) image shows that Co/In2O3 nanofibers are composed of particles stacked together and have obviously porous characteristic (Fig. 1f). The high-resolution TEM (HRTEM) image (Fig. 1g) reveals 0.197 and 0.411 nm periodicities, corresponding to the (111) plane of Co and (211) plane of In2O3, respectively, and discernible grain boundaries can be observed, demonstrating the successful formation of the Co/In2O3 heterostructure. Fig. 1h exhibits the selected area electron diffraction (SAED) pattern, presenting legible rings attributed to the crystal planes of Co and In2O3, suggesting a polycrystalline characteristic of Co and In2O3. Moreover, the energy dispersive X-ray (EDX) spectrum presents strong Co, In and O signals in Co/In2O3-1 (Fig. S1, ESI†). Elemental mapping displays homogeneous distribution of these elements throughout the Co/In2O3-1 nanofiber (Fig. 1i). In addition, a series of control catalysts with diverse contents of Co are prepared using the same synthesis method, named In2O3-H, Co/In2O3-0.5, and Co/In2O3-2 (Fig. S2 and S3, ESI†), which exhibit similar nanofibrous morphologies and average diameters. And the inductively coupled plasma-optical emission spectrometry (ICP-OES) results confirm that the molar ratio and feeding amount of Co and In are almost the same (Table S1, ESI†). After bare Co3O4 is reduced to Co metal, the fiber morphology collapses, reflecting that the presence of In2O3 can maintain the stability of the structure (Fig. S4, ESI†).X-ray diffraction (XRD) analysis identifies the obtained Co3O4/In2O3 products (Fig. 2a), exhibiting both In2O3 (PDF #06-0416) and Co3O4 (PDF #42-1467) phases. After H2/Ar reduction treatment at 400 °C, XRD analysis exhibits the disappearance of Co3O4 peaks, replaced by those of Co (PDF #15-0806), while In2O3 is still in the oxide phase, demonstrating the formation of the Co/In2O3 heterostructure (Fig. 2b). Here, the effect of the H2/Ar reduction temperature on Co/In2O3 synthesis is investigated. From Fig. S5 (ESI),† it can be seen that when the temperature is below 400 °C, Co3O4 cannot be completely reduced, while a high temperature can destroy the In2O3 crystal structure. Therefore, the temperature of 400 °C is chosen as the optimal experimental condition. Fig. 2c displays the Fourier transform infrared (FTIR) spectra of In2O3-H, Co/In2O3-0.5, Co/In2O3-1, and Co/In2O3-2 samples. The peaks at 472 and 606 cm−1 of all the as-prepared Co/In2O3 samples correspond to the In–O bond vibration, while those at 1398 and 1621 cm−1 are related to adsorbed water molecules. Due to the metallic properties of Co, the characteristic peaks of Co/In2O3 composites are completely consistent with those of In2O3-H.31
The X-ray photoelectron spectroscopy (XPS) test further assesses valence states of the components in the Co/In2O3-1 sample. Fig. 2d shows that Co/In2O3-1 nanofibers discern the presence of Co, In and O elements. The narrow scan Co 2p XPS spectrum yields six distinct peaks (Fig. 2e),32,33 notably at 778.1 eV and 795.7 for Co nanoparticles, aligning with the XRD results. The spin–orbit peaks at 781.8 and 798.6 eV correspond to Co 2p3/2 and Co 2p1/2, respectively, and the peaks at 786.3 and 803.2 eV are satellite peaks. In the In 3d core-level spectrum, In 3d5/2 and In 3d3/2 spin orbit peaks appear at 444.9 and 452.5 eV (Fig. 2f).34,35 Notably, the Co 2p3/2 binding energy (BE) of Co/In2O3-1 presents a negative shift of 1.4 eV relative to Co, while In 2p5/2 undergoes a positive shift of 0.28 eV compared with In2O3-H, signifying electron transfer from In2O3 to Co in the heterostructure. Fig. 2g presents the O 1s XPS spectra of Co/In2O3-1 and In2O3-H, identifying two dominant peaks at a BE of 530.4 and 532.2 eV, indicating the lattice (OLatt) and the surface adsorbed oxygen (Oads), respectively. The ratio of Oads/(Oads + Olatt) in Co/In2O3-1 is higher than that in In2O3-H, suggesting more surface oxygen defects accessible in Co/In2O3-1.36
Rationally engineered Mott–Schottky interfaces stimulate spontaneous electron transfer, substantially enhancing charge transfer efficiency. The Mott–Schottky data for In2O3-H material are depicted in Fig. 2h, where the positive slope and the x-intercept suggest n-type In2O3-H possessing a flat band potential of 0.19 V (vs. RHE). The Tauc plot (Fig. 2i) derived from the UV-vis result (Fig. S6a, ESI†) indicates a bandgap of 2.76 eV. It is generally accepted that the flat band potential of n-type semiconductors is usually employed to approximate the conduction band, typically lying between 0.1 and 0.3 eV below the Fermi level. Therefore, the band relationship suggests that the heterojunction formed by Co and In2O3 conforms to the Mott–Schottky model (Fig. S6b, ESI†).
2.2 Evaluation of electrocatalytic nitrate-to-ammonia performances
The electrocatalytic nitrate-to-ammonia performance is evaluated in 1.0 M KOH with 0.1 M KNO3. Initially, a substantial augmentation of current density occurs in the presence of KNO3, demonstrating that the electrocatalytic nitrate-to-ammonia reaction possesses higher activity than the hydrogen evolution reaction (HER). The current density and onset potential of the obtained Co/In2O3 is also larger than that of individual Co, In2O3-H, and Co3O4/In2O3, demonstrating that the catalyst with the Mott–Schottky heterostructure exhibits a higher catalytic activity (Fig. S7, ESI†). Furthermore, the effect of the feeding ratio of Co to the In precursor on the electrocatalytic activity has also been revealed. By comparing the LSV curves of varied catalysts with 80% iR-compensation, it is preliminarily believed that Co/In2O3-1 has the highest ammonia production activity due to its largest current density (Fig. 3a). Afterwards, using electrochemical impedance spectroscopy (EIS), we evaluate the charge-transfer kinetics (Fig. S8, ESI†). After calculation and fitting, the charge transfer resistance of Co/In2O3-1 is calculated to be 6.89 Ω, which is lower than that of other control samples including In2O3-H (246 Ω), Co (14.14 Ω), Co/In2O3-0.5 (57.67 Ω), and Co/In2O3-2 (9.61 Ω). This signifies faster charge transfer and optimal electrocatalytic kinetics of Co/In2O3-1, benefitting the enhanced nitrate-to-ammonia performance. Cyclic voltammetry (CV) is executed, with the double-layer capacitance (Cdl) determined through potential scanning in the non-faraday region (Fig. S9, ESI†). The Cdl of Co/In2O3-1 is 3.2 mF cm−2, larger than those of In2O3-H (1.7 mF cm−2), Co/In2O3-0.5 (3.1 mF cm−2), Co/In2O3-2 (1.8 mF cm−2) and Co (2.0 mF cm−2), showcasing more active sites to boost the nitrate-to-ammonia efficiency (Fig. S10, ESI†).Chronoamperometry and UV-vis examinations are performed to determine the NH3 yield rate and FE (Fig. S11–S14, ESI†). Initially, the NH3 FE exceeds that of NO2− across diverse catalyst electrodes at different electrolysis voltages, indicating domination of NH3 as the electrolysis product (Fig. S15, ESI†). As depicted in Fig. 3b, the NH3 FE of Co/In2O3-1 reaches 93.34% at 0 V, surpassing that of In2O3-H (79.67% at 0 V), Co/In2O3-0.5 (82.16% at −0.2 V), Co/In2O3-2 (73.53% at −0.2 V) and Co (69.41% at −0.2 V). Concurrently, Co/In2O3-1 exhibits higher NH3 partial current densities (jNH3) across various potentials, suggesting its superior electrocatalytic nitrate-to-ammonia activity over the entire potential range (Fig. 3c). Fig. 3d reveals an augmentation in the NH3 yield rate with a decrease in applied potential for the as-synthesized catalysts. Notably, the NH3 yield rate of Co/In2O3-1 outperforms that of In2O3-H, Co/In2O3-0.5, Co/In2O3-2 and Co over the entire potential range, thanks to its exceptional NH3 FE and jNH3. Specifically, at a potential of −0.8 V, the NH3 yield rate of Co/In2O3-1 reaches 70.1 mg cm−2 h−1, superior to that of In2O3-H (12.7 mg cm−2 h−1), Co/In2O3-0.5 (33.7 mg cm−2 h−1), Co/In2O3-2 (35.1 mg cm−2 h−1) and Co (26.1 mg cm−2 h−1). Additionally, as depicted in Fig. 3e and Table S2 (ESI),† the NH3 yield rate of Co/In2O3-1 also surpasses that of many Co-based and other heterostructured nitrate-to-ammonia catalysts reported so far, suggesting that this cost-effective and efficient Co/In2O3-1 has the potential for large-scale NH3 production. To evaluate the efficiency of Co/In2O3-1 in nitrate removal, a batch experiment is conducted with an initial NO3−–N concentration of around 1800 ppm to detect the remaining products (Fig. 3f). Remarkably, nearly all NO3−–N sources are reduced within 6 h, with an impressive NH3–N selectivity of 88.7%. Following 6 h of electrolysis, NO3− is almost removed and there is nearly no obvious NO2− formation. These findings demonstrate that Co/In2O3-1 achieves an outstanding activity and NH3 FE, showing potential for nitrate removal/conversion in wastewater. Subsequently, isotope labeling experiments ascertain the source of NH3 (Fig. 3g). Compared to the negligible peak of NH4+ before electrolysis, the 1H NMR spectrum of the 14NO3− solution reveals three different 14NH4+ peaks, while the 15NO3− solution features a notable 15NH4+ double peak after 1 h of electrolysis at −0.8 V, demonstrating the origination of the produced ammonia from KNO3 reduction.
Next, the concentration of KNO3 in the electrolyte is adjusted to range from 0.02 M to 0.2 M to evaluate the environmental compatibility of Co/In2O3-1 for the electrocatalytic nitrate-to-ammonia reaction. First, as the KNO3 concentration increases, the onset potential and current density increase as expected (Fig. S16, ESI†), accompanied by a progressive increase in the NH3 yield rate, reaching 83.3 mg cm−2 h−1 at a KNO3 concentration of 0.2 M (Fig. 3h). Second, the NH3 FE consistently remains above 80%, indicating that Co/In2O3-1 maintains efficient electrocatalytic nitrate-to-ammonia activity across a broad concentration range. To assess the stability of Co/In2O3-1 during nitrate-to-ammonia electrocatalysis, we conducted three cycle tests with a total 24-h electrocatalysis at −0.8 V (Fig. 3i). The catalytic current density remains steady throughout the test. Moreover, the NH3 FE and yield rate remain consistent in the different cycles, with average values of 69.24% and 58.3 mg cm−2 h−1, affording evidence for the ideal durability of the Co/In2O3-1 catalyst for electrocatalytic nitrate-to-ammonia conversion (Fig S17, ESI†).
2.3 The investigations of the nitrate-to-ammonia reaction mechanism
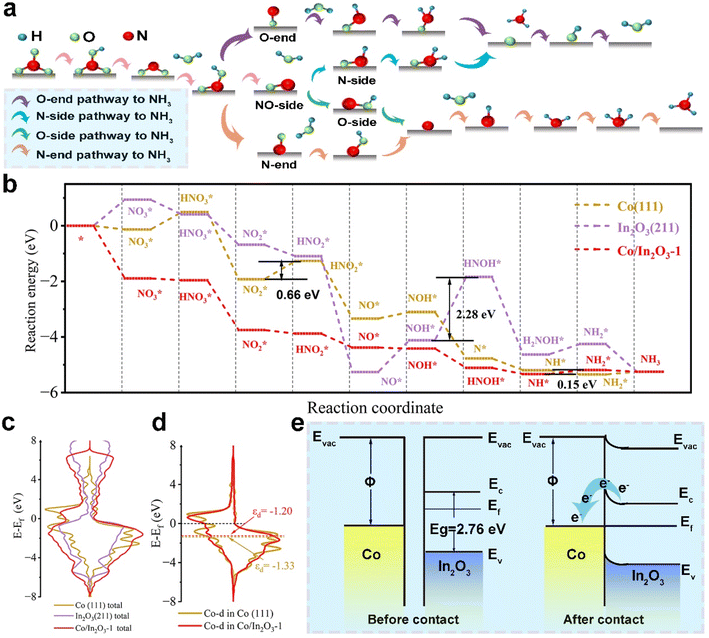
Owing to the multiple reactive sites on the Co/In2O3-1 catalyst, the charge density difference is determined prior to investigating the nitrate-to-ammonia reaction mechanism. The red circle in the charge density difference in Fig. S18 (ESI)† highlights the Co/In2O3-1 interface with a relatively enhanced charge density, suggesting these locations as preferential adsorption sites for nitrate reduction. According to the previous study,37 the possible reaction pathways of the nitrate reduction process are illustrated in Fig. 4a. Fig. S19–S21 (ESI)† illustrate the optimal structures of reaction intermediates on Co (111), In2O3 (211), and Co/In2O3-1, respectively, along the most conductive reaction pathway. The free energy diagrams for nitrate reduction are depicted in Fig. 4b, revealing varied favorable pathways for nitrate reduction on Co (111), In2O3 (211), and Co/In2O3-1, predominantly arising from the NOH* hydrogenation step. Upon protonating NOH* on the Co (111) surface, we observe its tendency towards forming N* and producing H2O, with a reaction energy of −1.67 eV. However, on both In2O3 (211) and Co/In2O3-1, the formation of HNOH* is energetically favored after NOH* protonation. Notably, this process on In2O3 (211) is endothermic with an energy requirement of 2.28 eV, and it becomes spontaneous on Co/In2O3-1, exhibiting an exothermic reaction energy of −0.69 eV. The PDS analysis reveals the catalytic potential for nitrate-to-ammonia conversion on the three catalysts. On Co (111), the PDS is identified as the formation of , with a corresponding reaction energy (ΔG(PDS)) of 0.66 eV. On In2O3 (211), NOH* hydrogenation is regarded as the PDS, exhibiting the highest ΔG(PDS) value of 2.28 eV. The high thermodynamic energy barriers on both Co (111) and In2O3 (211) suggest that these two catalysts are unlikely to facilitate nitrate-to-ammonia efficiently. However, on Co/In2O3-1, the protonation of NH* to form
, with a corresponding reaction energy (ΔG(PDS)) of 0.66 eV. On In2O3 (211), NOH* hydrogenation is regarded as the PDS, exhibiting the highest ΔG(PDS) value of 2.28 eV. The high thermodynamic energy barriers on both Co (111) and In2O3 (211) suggest that these two catalysts are unlikely to facilitate nitrate-to-ammonia efficiently. However, on Co/In2O3-1, the protonation of NH* to form 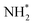 appears as the PDS, with a ΔG(PDS) of merely 0.15 eV. This remarkably small ΔG(PDS) signifies the significant synergistic enhancement of nitrate-to-ammonia catalytic activity of Co/In2O3-1 by the strong interaction between Co and In2O3. This result is corroborated by studying the electronic attributes of these three catalysts. As shown in Fig. 4c, the density of states (DOS) near the Fermi level for Co/In2O3-1 noticeably increases compared to the In2O3 (211) substrate, suggesting improved electrical conductivity of Co/In2O3. Moreover, as shown in Fig. 4d, the d band center (εd) of Co in Co/In2O3-1 is −1.20 eV, situated 0.13 eV above the Fermi level compared to the value of Co in Co (111) (−1.33 eV). This shift not only strengthens the catalyst-intermediate interaction, but also further validates the beneficial impact of the Co–In2O3 interaction on the enhanced electrocatalytic activity of Co/In2O3-1.
appears as the PDS, with a ΔG(PDS) of merely 0.15 eV. This remarkably small ΔG(PDS) signifies the significant synergistic enhancement of nitrate-to-ammonia catalytic activity of Co/In2O3-1 by the strong interaction between Co and In2O3. This result is corroborated by studying the electronic attributes of these three catalysts. As shown in Fig. 4c, the density of states (DOS) near the Fermi level for Co/In2O3-1 noticeably increases compared to the In2O3 (211) substrate, suggesting improved electrical conductivity of Co/In2O3. Moreover, as shown in Fig. 4d, the d band center (εd) of Co in Co/In2O3-1 is −1.20 eV, situated 0.13 eV above the Fermi level compared to the value of Co in Co (111) (−1.33 eV). This shift not only strengthens the catalyst-intermediate interaction, but also further validates the beneficial impact of the Co–In2O3 interaction on the enhanced electrocatalytic activity of Co/In2O3-1.
Fig. 4e presents the energy band diagram of the Co/In2O3 heterojunction, illustrating the mechanism behind its promoted nitrate-to-ammonia performance. This enhancement is attributed to the prompt and spontaneous electron transfer from In2O3 to Co, facilitated by the elevated Fermi level of In2O3. Upon intimate contact, the In2O3 band drops, achieving equilibrium between Fermi levels of Co and In2O3. This forms a rectifying Schottky junction in the depletion region to generate a charge density gradient and result in an electron-rich zone at the Co side and a hole-rich region at the In2O3 side, creating a built-in electric field to provide a directed electron flow path. This electron reallocation is forecast to augment electroactive site utilization, lower reaction energy thresholds, and speed up the overall reaction kinetics. Therefore, the Co/In2O3-1 heterojunction electrocatalyst exhibits a superior NH3 FE and high NH3 yield rate during the nitrate-to-ammonia process.
2.4 Zn–NO3− battery
Finally, an aqueous Zn–NO3− battery is constructed with Co/In2O3-1 as the cathode and Zn foil as the anode (Fig. S22a, ESI†), achieving both energy production and NH3 manufacturing. Due to the excellent electrocatalytic nitrate-to-ammonia performance of Co/In2O3-1, the Zn–NO3− battery exhibits a consistent open circuit voltage of 1.388 V vs. Zn within 1000 s (Fig. S22b, ESI†). In addition, a peak power density of 2.15 mW cm−2 is achieved (Fig. S22c, ESI†). Fig. S23a (ESI)† illustrates the discharging curves of the Zn–NO3− battery at different current densities for 1 h, demonstrating stable battery voltage. An NH3 yield rate of 1.36 mg h−1 cm−2 at 20 mA cm2 is observed (Fig. S23b, ESI†). This suggests that the nitrate-to-ammonia process has outstanding application prospects in practical power generation devices, concurrently producing valuable NH3 industrial products.3. Conclusion
In summary, a Co/In2O3 heterostructure with the Mott–Schottky effect is ingeniously constructed through electrospinning, calcination, and partial reduction techniques. The experimental results reveal the spontaneous electron flow from In2O3 to Co at the interface, achieving an electron redistribution in the Co/In2O3 heterojunction to improve the utilization of active sites. Theoretical findings further prove that the εd of Co in Co/In2O3-1 is closer to the Fermi level than that of Co in Co (111), with Co/In2O3-1 exhibiting the smallest ΔG(PDS). This suggests that the remarkable synergistic interaction between Co and In2O3 significantly boosts the nitrate-to-ammonia catalytic activity of Co/In2O3-1. This work offers fresh perspectives for the thoughtful design of efficient Mott–Schottky heterojunction electrocatalysts for electrocatalytic nitrate-to-ammonia conversion.Data availability
Data supporting the findings of this study are available within the article ESI.†Author contributions
R. Qi and Q. Jiang contributed equally to this work. X. Lu, Y. Wang and M. Zhong conceived the experiments and supervised this project. R. Qi performed the experiments. R. Qi, L. Deng, X. Yu, and B. Shi characterized the catalysts and analyzed the data. Q. Jiang and Y. Wang carried out the theoretical calculation. R. Qi and Q. Jiang wrote the manuscript. All authors have approved the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (52273056 and 22373097) and the Jilin Province Science and Technology Development Program (20220101056JC). Part of the computational time is supported by the High Performance and Computing Center of Jilin Province, Network and Computing Center in Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, and Computing Center in Jilin Normal University.References
- J. Galloway, A. Townsend, J. Erisman, M. Bekunda, Z. Cai, J. Freney, L. Martinelli, S. Seitzinger and M. Sutton, Science, 2008, 320, 889 Search PubMed.
- H. Xu, Y. Ma, J. Chen, W. Zhang and J. Yang, Chem. Soc. Rev., 2022, 51, 2710 Search PubMed.
- J. Liang, Z. Li, L. Zhang, X. He, Y. Luo, D. Zheng, Y. Wang, T. Li, H. Yan, B. Ying, S. Sun, Q. Liu, M. Hamdy, B. Tang and X. Sun, Chem, 2023, 9, 1768 Search PubMed.
- Y. Xiong, Y. Wang, J. Zhou, F. Liu, F. Hao and Z. Fan, Adv. Mater., 2023, 36, 2304021 CrossRef.
- H. Zhang, H. Wang, X. Cao, M. Chen, Y. Liu, Y. Zhou, M. Huang, L. Xia, Y. Wang, T. Li, D. Zheng, Y. Luo, S. Sun, X. Zhao and X. Sun, Adv. Mater., 2024, 36, 2312746 CrossRef CAS PubMed.
- L. Ouyang, J. Liang, Y. Luo, D. Zheng, S. Sun, Q. Liu, M. Hamdy, X. Sun and B. Ying, Chin. J. Catal., 2023, 50, 6 CrossRef CAS.
- T. Xie, X. He, L. He, K. Dong, Y. Yao, Z. Cai, X. Liu, X. Fan, T. Li, D. Zheng, S. Sun, L. Li, W. Chu, A. Farouk, M. Hamdy, C. Xu, Q. kong and X. Sun, Chin. Chem. Lett., 2024, 35, 110005 CrossRef CAS.
- J. Miao, Q. Hong, L. Liang, G. Li, Z. Liu, S. Yin and Y. Chen, Chin. Chem. Lett., 2024, 35, 108935 CrossRef CAS.
- C. Cang and H. Zheng, Chin. J. Struct. Chem., 2023, 42, 100143 CrossRef.
- Y. Kim, J. Ko, M. Shim, J. Park, H. Shin, Z. Kim, Y. Jung and H. Byon, Chem. Sci., 2024, 15, 2578 RSC.
- Q. Hong, B. Miao, T. Wang, F. Li and Y. Chen, Energy Lab., 2023, 1, 220022 Search PubMed.
- K. Dong, Y. Yao, H. Li, H. Li, S. Sun, X. He, Y. Wang, Y. Luo, D. Zheng, Q. Liu, Q. Liu, D. Ma, X. Sun and B. Tang, Nat. Synth., 2024, 3, 763 CrossRef CAS.
- Y. Wang, C. Wang, M. Li, Y. Yu and B. Zhang, Chem. Soc. Rev., 2021, 50, 6720 RSC.
- J. Zhou, M. Wen, R. Huang, Q. Wu, Y. Luo, Y. Tian, G. Wei and Y. Fu, Energy Environ. Sci., 2023, 16, 2611 RSC.
- Z. Y. Wu, M. Karamad, X. Yong, Q. Huang, D. A. Cullen, P. Zhu, C. Xia, Q. Xiao, M. Shakouri, F. Y. Chen, J. Kim, Y. Xia, K. Heck, Y. Hu, M. Wong, Q. Li, L. Gates, S. Siahrostami and H. Wang, Nat. Commun., 2021, 12, 2870 CrossRef CAS PubMed.
- F. Y. Chen, Z. Y. Wu, S. Gupta, D. J. Rivera, S. V. Lambeets, S. Pecaut, J. Y. T. Kim, P. Zhu, Y. Z. Finfrock, D. M. Meira, G. King, G. Gao, W. Xu, D. Cullen, H. Zhou, Y. Han, D. Perea, C. Muhich and H. Wang, Nat. Nanotechnol., 2022, 17, 759 CrossRef CAS PubMed.
- Y. Wang, M. Sun, J. Zhou, Y. Xiong, Q. Zhang, C. Ye, X. Wang, P. Lu, T. Feng, F. Hao, F. Liu, J. Wang, Y. Ma, J. Yin, S. Chu, L. Gu, B. Huang and Z. Fan, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2306461120 CrossRef CAS PubMed.
- Z. Zhang, A. Niu, Y. Lv, H. Guo, J. S. Chen, Q. Liu, K. Dong, X. Sun and T. Li, Angew. Chem., Int. Ed., 2024, 63, e202406441 CrossRef CAS PubMed.
- J. Liang, P. Liu, Q. Li, T. Li, L. Yue, Y. Luo, Q. Liu, N. Li, B. Tang, A. A. Alshehri, I. Shakir, P. Agboola, C. Sun and X. Sun, Angew. Chem., Int. Ed., 2022, 61, e202202087 CrossRef CAS.
- R. Zhang, Y. Zhang, B. Xiao, S. Zhang, Y. Wang, H. Cui, C. Li, Y. Hou, Y. Guo, T. Yang, J. Fan and C. Zhi, Angew. Chem., Int. Ed., 2024, 63, e202407589 CrossRef CAS PubMed.
- B. Li, F. Xia, Y. Liu, H. Tan, S. Gao, J. Kaelin, Y. Liu, K. Lu, T. J. Marks and Y. Cheng, Nano Lett., 2023, 23, 1459 CrossRef CAS PubMed.
- S. Han, H. Li, T. Li, F. Chen, R. Yang, Y. Yu and B. Zhang, Nat. Catal., 2023, 6, 402 CrossRef CAS.
- Z. Huang, J. Song, Y. Du, S. Xi, S. Dou, J. M. V. Nsanzimana, C. Wang, Z. J. Xu and X. Wang, Nat. Energy, 2019, 4, 329 CrossRef CAS.
- R. Qi, L. Zhang, S. Ren, B. Shi, M. Zhong, Z. Chen and X. Lu, Nano Lett., 2024, 24, 8964 CrossRef CAS PubMed.
- Z. Sun, Y. Wang, L. Zhang, H. Wu, Y. Jin, Y. Li, Y. Shi, T. Zhu, H. Mao, J. Liu, C. Xiao and S. Ding, Adv. Funct. Mater., 2020, 30, 1910482 CrossRef CAS.
- X. Li, Y. Pan, H. Yi, J. Hu, D. Yang, F. Lv, W. Li, J. Zhou, X. Wu, A. Lei and L. Zhang, ACS Catal., 2019, 9, 4632 CrossRef CAS.
- Y. Li, W. Wang, B. Zhang, L. Fu, M. Wan, G. Li, Z. Cai, S. Tu, X. Duan, Z. W. Seh, J. Jiang and Y. Sun, Nano Lett., 2021, 21, 6656 CrossRef CAS.
- Y. Zhang, Z. Li, C. Qiang, K. Chen, Y. Guo and K. Chu, ACS Nano, 2024, 18, 25316 CrossRef CAS PubMed.
- H. Sun, S. Lee, R. Tang, L. Wang, C. Yang, W. Liang, S. Zhao, C. Dong, A. Soon and J. Huang, Adv. Funct. Mater., 2024, 2415859 CrossRef.
- D. Deng, Y. Wang, J. Jiang, Y. Bai, Y. Chen, H. Zheng, H. Ou and Y. Lei, Chem. Commun., 2024, 60, 9364 RSC.
- Q. Liang, S. Zhao, Z. Li, Z. Wu, H. Shi, H. Huang and Z. Kang, ACS Appl. Mater. Interfaces, 2021, 13, 40754 CrossRef CAS.
- C. Liu, Y. Li, T. Peng, S. Luo, Y. Feng, W. Xie, D. Lu and W. Sun, J. Power Sources, 2020, 468, 228393 CrossRef CAS.
- W. Li, S. Chen, M. Zhong, C. Wang and X. Lu, Chem. Eng. J., 2021, 415, 128879 CrossRef CAS.
- X. Zhou, J. Wu, Q. F. Li, T. Zeng, Z. Ji, P. He, W. G. Pan, X. M. Qi, C. Y. Wang and P. K. Liang, J. Catal., 2017, 355, 26 CrossRef CAS.
- Q. Wang, Y. J. Chen, X. Liu, L. G. Li, L. Z. Du and G. H. Tian, Chem. Eng. J., 2021, 421, 129968 CrossRef CAS.
- J. Ding, Z. Geng, L. Li, Y. Wang, Y. Zuo, H. Li, M. Yang and G. Li, J. Mater. Chem. A, 2021, 9, 12623 RSC.
- L. Lv, Y. Shen, J. Liu, X. Meng, X. Gao, M. Zhou, Y. Zhang, D. Gong, Y. Zheng and Z. Zhou, J. Phys. Chem. Lett., 2021, 12, 11143 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06818a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |