Open Access Article
Open Access ArticleExploiting the inherent promiscuity of the acyl transferase of the stambomycin polyketide synthase for the mutasynthesis of analogues†
Li
Su
 abc,
Yaouba
Souaibou
abc,
Yaouba
Souaibou
 abd,
Laurence
Hôtel
b,
Christophe
Jacob
abd,
Laurence
Hôtel
b,
Christophe
Jacob
 a,
Peter
Grün
c,
Yan-Ni
Shi
a,
Peter
Grün
c,
Yan-Ni
Shi
 ce,
Alicia
Chateau
f,
Sophie
Pinel
ce,
Alicia
Chateau
f,
Sophie
Pinel
 f,
Helge B.
Bode
f,
Helge B.
Bode
 ceghi,
Bertrand
Aigle
ceghi,
Bertrand
Aigle
 *b and
Kira J.
Weissman
*b and
Kira J.
Weissman
 *a
*a
aUniversité de Lorraine, CNRS, IMoPA, F-54000 Nancy, France. E-mail: kira.weissman@univ-lorraine.fr
bUniversité de Lorraine, INRAE, DynAMic, F-54000 Nancy, France. E-mail: bertrand.aigle@univ-lorraine.fr
cMax-Planck-Institute for Terrestrial Microbiology, Department of Natural Products in Organismic Interactions, 35043 Marburg, Germany
dIPHC, UMR 7178, CNRS, Université de Strasbourg, Equipe de Chimie Analytique des Molécules Bioactives et Pharmacognosie, Illkirch, France
eMolecular Biotechnology, Department of Biosciences, Goethe University Frankfurt, Frankfurt am Main, Germany
fUniversité de Lorraine, CNRS, CRAN, F-54000 Nancy, France
gChemical Biology, Department of Chemistry, Philipps University of Marburg, 35043 Marburg, Germany
hSenckenberg Gesellschaft für Naturforschung, 60325 Frankfurt am Main, Germany
iCenter for Synthetic Microbiology (SYNMIKRO), University of Marburg, 35043 Marburg, Germany
First published on 22nd January 2025
Abstract
The polyketide specialized metabolites of bacteria are attractive targets for generating analogues, with the goal of improving their pharmaceutical properties. Here, we aimed to produce C-26 derivatives of the giant anti-cancer stambomycin macrolides using a mutasynthesis approach, as this position has been shown previously to directly impact bioactivity. For this, we leveraged the intrinsically broad specificity of the acyl transferase domain (AT12) of the modular polyketide synthase (PKS), which is responsible for the alkyl branching functionality at this position. Feeding of a panel of synthetic and commercially available dicarboxylic acid ‘mutasynthons’ to an engineered strain of Streptomyces ambofaciens (Sa) deficient in synthesis of the native α-carboxyacyl-CoA extender units, resulted in six new series of stambomycin derivatives as judged by LC-HRMS and NMR. Notably, the highest product yields were observed for substrates which were only poorly accepted when AT12 was transplanted into a different PKS module, suggesting a critical role for domain context in the overall functioning of PKS proteins. We also demonstrate the superiority of this mutasynthesis approach – both in terms of absolute titers and yields relative to the parental compounds – in comparison to the alternative precursor-directed strategy in which monoacid building blocks are supplied to the wild type strain. We further identify a malonyl-CoA synthetase, MatB_Sa, with specificity distinct from previously described promiscuous enzymes, making it a useful addition to a mutasynthesis toolbox for generating atypical, CoA activated extender units. Finally, we show that two of the obtained (deoxy)-butyl-stambomycins exhibit antibacterial and antiproliferative activities similar to the parental stambomycins, while an unexpected butyl-demethyl congener is less potent. Overall, this works confirms the interest of biosynthetic pathways which combine a dedicated route to extender unit synthesis and a broad specificity AT domain for producing bioactive derivatives of fully-elaborated complex polyketides.
Introduction
The reduced polyketide specialized metabolites of bacteria exhibit stunning structural diversity. One source of this architectural variety is the multiple acyl-CoA building blocks incorporated during chain building. This construction process is carried out by mega-enzyme assembly lines called type I polyketide synthases (PKSs), which comprise a series of functional modules distributed among multiple polypeptide subunits. The modules are composed of sets of catalytic domains (minimally ketosynthase (KS) and acyl transferase (AT)) which act in partnership with non-catalytic acyl carrier proteins (ACPs) to elongate the chains, followed by optional modification of each newly-incorporated monomer. Both the ‘starter units’ which initiate the biosynthesis and the ‘extender units’ used in chain elongation are selected by the AT domains. These domains thus govern to a large extent the branching functionality within polyketide structures. Correspondingly, manipulation of AT domains using genetic engineering offers a powerful potential means for the regioselective diversification of polyketide skeletons, especially in the case of ATs which exhibit unusual substrate specificity relative to the typical choice of malonyl-CoA or methylmalonyl-CoA1 (e.g. for propyl-, butyl-, allyl-malonyl-CoA, etc.2–4). To date, a variety of approaches including AT domain exchange, in trans AT complementation, and active site-directed mutagenesis, have been applied to polyketide structural engineering with varying levels of success.5Experimentally, AT swapping consists of exchanging only the AT domain within a module of interest for an AT of distinct substrate specificity from another module, either sourced from the same or a different PKS.6–10 This approach has resulted in various hybrid metabolites, with recent work revealing highly effective domain boundaries for such exchanges.11,12 Concerning the complementation strategy, it involves selectively inactivating the native AT within a particular PKS module and rescuing its activity via a co-expressed trans-acting AT.4,13,14 Drawbacks of this approach include that it depends on the ability of the trans-ATs to recognize noncognate ACPs as partners,14 and the typically limited substrate (for malonate)4 of these enzymes. Finally, AT active site mutagenesis has been used in efforts to redirect and/or broaden innate AT substrate selectivity.15–17 Despite the availability of several AT crystal structures,18–21 this approach remains hampered by the paucity of co-complex structures in the presence of native extender units. Indeed, all such attempts to date to switch AT specificity have only resulted in a shift in substrate preference,4,15–17,22 a result potentially explained by the currently insufficient predictive power of the identified AT substrate-binding motifs.12
An alternative approach towards polyketide structural diversification is to exploit the intrinsically broad substrate specificity of certain ATs, and challenge them with alternative building blocks via precursor-directed biosynthesis (PDB) and/or mutasynthesis.23–31 In the case of PDB (Fig. 1a), the supplied ‘mutasynthons’ must compete with the native substrates, while in a mutasynthetic strategy (Fig. 1b), precursor biosynthesis is disabled,2 and thus the pathway depends, at least in principle, on the presence of the exogenous building block in order to function. To date, multiple functional groups including several not found naturally in polyketides have been incorporated into polyketides in this manner, including alkyne, azide, and halogen moieties.24–31
 | ||
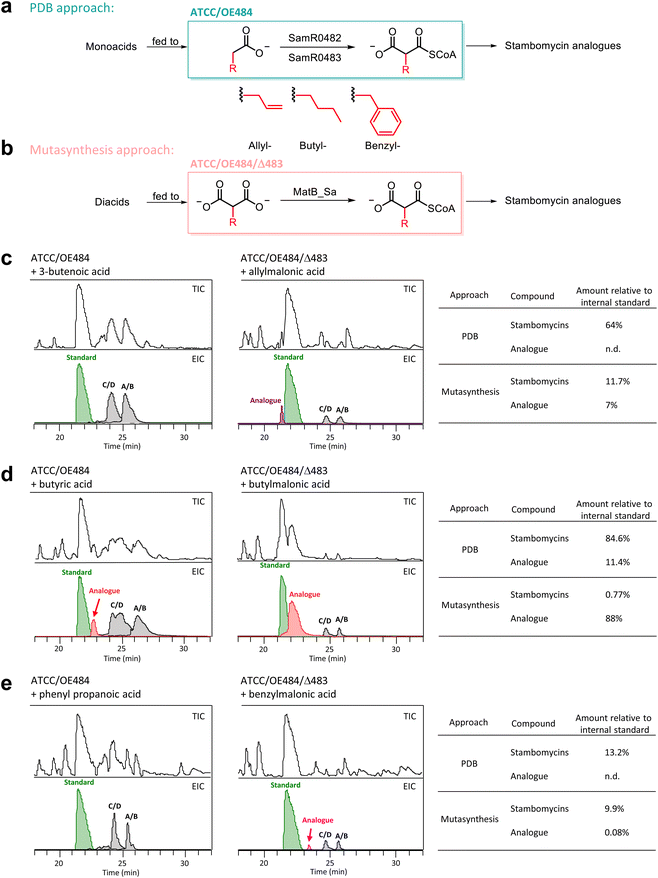
| Fig. 1 Schematic overview of (a) precursor-directed biosynthesis (PDB), (b) mutasynthesis approaches,23 (c) biosynthesis of CoA-linked polyketide synthase extender units via crotonyl-CoA carboxylase/reductase (CCR) and MatB enzymes (see details in Fig. S1†), and the (d) mutasynthesis strategy applied to stambomycin biosynthesis. Commercially sourced and synthetic malonic acids incorporating a wide variety of side chains were fed to the production culture of mutasynthesis mutant ATCC/OE484/Δ483. At least six of these alternative extender units were then successfully activated to their corresponding CoAs by the promiscuous MatB_Sa of S. ambofaciens, and incorporated into the pathway by AT12, resulting in stambomycin derivatives bearing modifications at C-26, a position which directly impacts the bioactivity.42 The hydroxylation at C-28 (red circle) was found to occur to variable extents, but the hydroxylation at C-50 and mycaminosylation went to completion. | ||
Evidently, a critical parameter for successful PDB and/or mutasynthesis is exogenous precursor supply. Previous in vitro studies have shown that commercially available malonyl-CoA derivatives can be used as mutasynthons.32 However, this method is not suitable for in vivo feeding studies due to poor acyl-CoA membrane permeability.33 This problem can be addressed by the use of synthetic N-acetylcysteamine thioesters (SNACs) which mimic the distal end of CoA, but leveraging them in large-scale applications is complicated by the need to synthesize the compounds, as well as their cellular toxicity.34 A promising alternative is a chemoenzymatic approach, in which a fed monomer (mono- or di-acid) is activated to its corresponding malonyl-CoA derivative in cellulo by an appropriate enzyme – either a crotonyl-CoA reductase/carboxylase (CCR)35–38 homologue or a broad-specificity malonyl-CoA synthetase (MatB)18,34,39–41 homologue (Fig. 1c and S1†).
ATs which recognize atypical extender units typically show broad substrate specificity relative to their malonate- or methylmalonate equivalents.12,38 Even among such domains, a particularly promiscuous AT participates in the biosynthesis of the stambomycin family of 51-membered macrolides in S. ambofaciens ATCC23877 (ref. 42–44). This domain (AT12) naturally recruits at least six atypical alkyl malonyl-CoA-derived extender units: (4-methylhexyl)malonyl-CoA, (5-methylhexyl)malonyl-CoA, (4-methylpentyl)malonyl-CoA, n-hexylmalonyl-CoA, n-pentylmalonyl-CoA and (4,5-dimethylhexyl)malonyl-CoA (Fig. 1d). This broad specificity results in six stambomycins (A–F, respectively) bearing distinct side chains at C-26. Notably, modifications at this position directly impact the biological properties of the metabolites, with stambomycins C/D showing improved antiproliferative activity towards a range of human cancer cell lines.42 In contrast to the typical CCR-mediated synthesis of polyketide extender units via reductive carboxylation of α,β-unsaturated acyl-thioesters,35–38 the stambomycin precursors are generated by direct carboxylation of medium chain acyl-CoA substrates catalyzed by the ATP-dependent enzyme SamR0483 (Fig. 1d).43 (Note: although SamR0483 was renamed MccB for medium chain acyl-CoA carboxylase β-subunit, we will refer to it here as SamR0483 in line with the gene nomenclature43). The acyl-CoAs derive either from the corresponding amino acids via the consecutive action of the branched-chain α-keto acid dehydrogenase complex and primary metabolic fatty acid synthase enzymes (FabH and FabF), or via direct conversion of the corresponding fatty acids to their CoA thioesters by SamR0482, a medium chain fatty acyl-CoA ligase homologue.43 Notably, SamR0482 and SamR0483 together were shown to generate the corresponding extender units from 6-azidohexanoic acid and 8-nonionic acid, resulting in stambomycin C-26 analogues bearing azide and alkyne groups43 (Fig. 1d). Thus, in addition to the intrinsically broad specificity of AT12 and all twelve downstream PKS modules, SamR0482 and SamR0483 exhibit useful substrate tolerance.
Here we aimed to further exploit the intrinsic flexibility of the stambomycin pathway to access a larger panel of analogues by mutasynthesis for biological testing (Fig. 1d). For this, we first disrupted the supply of the native extender units by mutational inactivation of samR0483. We then fed the resulting strains with 12 commercially-available or synthetic malonic acid derivatives, relying either on the intrinsic promiscuity of the S. ambofaciens MatB (MatB_Sa) or heterologously-expressed MatB from S. cinnamonensis45 for activation to their respective CoA thioesters. Six of the derivatives resulted in stambomycin analogues some of which were hydroxylated at C-28, including four at yields sufficient for structure elucidation by NMR and biological activity tests. We further show that MatB_cinna is more effective than the native S. ambofaciens homologue at monomer activation, while direct comparison of the yields obtained by PDB and mutasynthesis confirms the superiority of the latter approach for analogue generation.
Results
Construction of a mutant for stambomycin mutasynthesis
In order to disable the native pathway to the six extender units, the genes encoding SamR0482 and SamR0483 were deleted using PCR-targeting, resulting in the mutants ATCC/Δ482 (ref. 46) and ATCC/Δ483 (Tables S1–S4†). Subsequently, the LAL regulator overexpressing plasmid pOE484 was introduced into the mutants as previously described to trigger stambomycin biosynthesis,42 affording the mutants ATCC/OE484/Δ482 (ref. 46) and ATCC/OE484/Δ483 (Table S2†). Extracts of mutants ATCC/OE484/Δ482 and ATCC/OE484/Δ483 were then analyzed by LC-HRMS in comparison to the control strain ATCC/OE484. This analysis revealed that while production by ATCC/OE484/Δ483 was almost completely abolished (Fig. 2), the ATCC/OE484/Δ482 mutant continued to produce stambomycins at approximately 51% yield relative to the wild type strain (Fig. 2). BlastP analysis47 of the S. ambofaciens genome using SamR0482 as a query revealed several homologues, two of which are located in the antimycin and congocidine clusters: AntF (acyl-CoA synthetase, 29.8% amino acid identity to SamR0482) and Cgc22 (acyl-CoA synthetase, 34.5% amino acid identity to SamR0482). We thus propose that in addition to provision of the necessary acyl-CoAs via core metabolism, the loss-of-function of SamR0482 may be compensated by either AntF, Cgc22 or both (Table S1†). BlastP analysis using SamR0483 also revealed several homologues, notably including the duplicated enzyme AlpX (proposed to be involved in kinamycin biosynthesis48,49) and two propionyl-CoA carboxylase (PCC) β-subunits which show 59% and 52% amino acid identity to SamR0483 and high mutual similarity (Table S1†). However, PCCBs typically act on short chain acyl-CoAs,50 potentially explaining why only a low level of SamR0483 complementation was observed (0.3% stambomycin yield relative to wild type).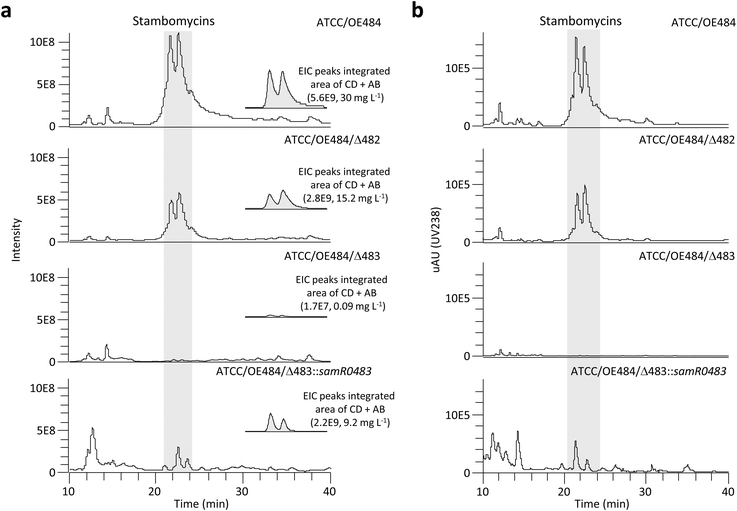 | ||
| Fig. 2 Analysis by (a) mass spectrometry and (b) UV at λmax 238 nm of ATCC/OE484 (control) and mutants for mutasynthesis. The TIC (total ion chromatogram) and UV spectrum of ATCC/OE484/Δ483 clearly show that production by the mutant of the stambomycins (peaks highlighted in grey) is almost abolished. The indicated yields of stambomycins (AB and CD) in the wild type and mutant strains are based on integration of the EIC (extracted ion chromatogram) peaks and a previously generated standard curve for stambomycin AB.44 The calculated areas represent the average of the analysis of at least two independent clones of each strain. | ||
To confirm that the substantial decrease in stambomycin production in the ATCC/OE484/Δ483 mutant was due to the loss function of SamR0483, we carried out a complementation experiment by integrating the samR0483 gene into the genome of ATCC/OE484/Δ483 mutant under the control of the constitutive promoter ermE*p.51 Although the observed complementation was incomplete (31% stambomycin yields relative to wt (Fig. 2)), restoration of biosynthesis nonetheless supports the proposed essential role for SamR0483 in the pathway.43 We therefore used mutant ATCC/OE484/Δ483 as our base strain for the mutasynthesis experiments.
Mutasynthesis of stambomycin analogues
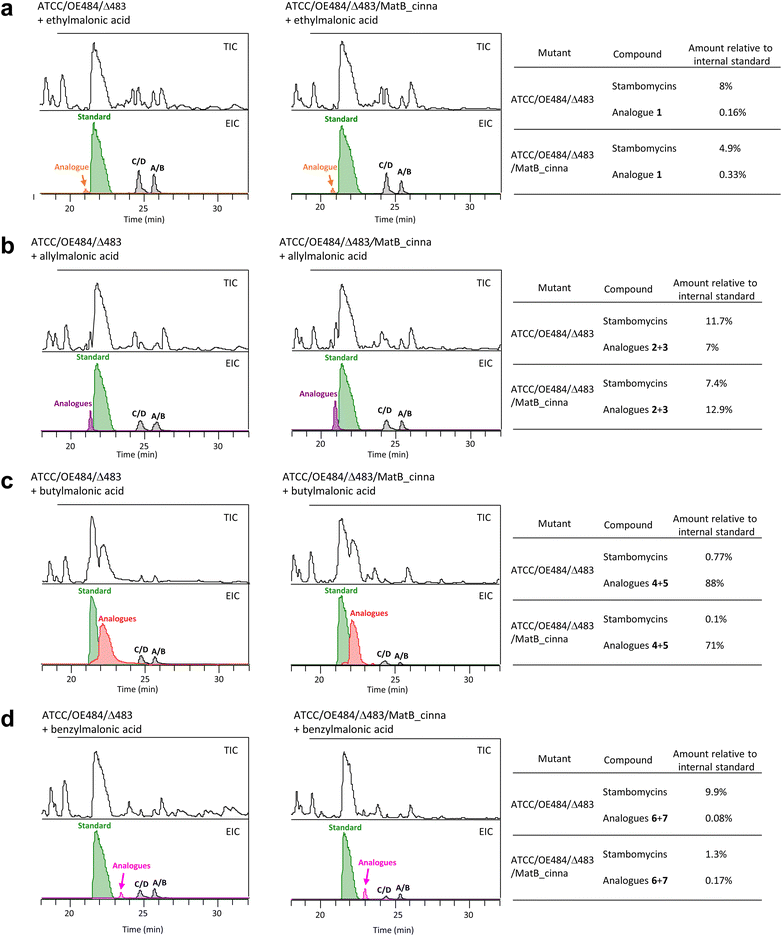
BlastP analysis against the genome sequence of S. ambofaciens using the MatB_Sc (S. coelicolor)40 as the query revealed an enzyme (referred to here as MatB_Sa) with amino acid identity of 90% to MatB_Sc. Sequence alignment also showed that MatB_Sa shares 75% and 39% amino acid identity with MatB_cinna (S. cinnamonensis)34 and MatB_Rt (Rhizobium trifolii),41 respectively (Fig. S2†). The high homology between MatB_Sa and the demonstrably promiscuous MatB_cinna34 suggested that MatB_Sa might also possess the necessary broad specificity to efficiently activate non-native malonate derivatives to their corresponding CoAs. On this basis, individual cultures of the ATCC/OE484/Δ483 mutant strain were supplemented with a wide panel of commercially sourced and synthetic diacids, including ethyl-, isopropyl-, allyl-, butyl-, benzyl-, octyl-, phenylpropyl-, phenoxypropyl-, thiophenyl-, thienylmethyl-, propargyl-, and 6-bromohexyl-malonic acids (Fig. 1d) at a final concentration of 10 mM, in parallel with a control (i.e. a culture of the mutant strain lacking added malonic acid derivatives).Analysis by LC-HRMS of cell pellets following extraction with methanol showed unexpectedly, that for all fed extracts with the exception of thiophenyl-malonic acid and thienylmethyl-malonic acid for which there was no change, peaks corresponding to stambomycins A/B and C/D were always present, and at yields 2–8 times higher than those from the unsupplemented ATCC/OE484/Δ483 mutasynthesis mutant (Tables 1, S7 and Fig. S3†). To explain this surprising observation, we propose that the fed malonic acid derivatives somehow induce expression of the identified SamR0483 homologues (Table S1†), which can then more substantially compensate for the loss of SamR0483. However, this mechanism remains to be verified directly by targeted inactivation of this set of genes.
| Malonic acid derivatives | Titers of stambomycins and their analogues (mg L−1) | ||
|---|---|---|---|
| Stambomycins (A–D) | Analogue (with C-28 OH) | Deoxy analogue (lacking C-28 OH) | |
| Control (no supplementation) | 0.09 | n.d | n.d |
| Ethyl- | 0.77 | n.d | 0.00077 (1) |
| Isopropyl- | 0.32 | n.d | n.d |
| Allyl- | 0.17 | 0.0018 (2) | 0.34 (3) |
| Butyl- | 0.17 | 3.9 (4) | 18.56 (5) |
| Benzyl- | 0.43 | 0.025 (6) | 0.049 (7) |
| Octyl- | 0.76 | 0.07 (8) | n.d |
| Phenylpropyl- | 0.53 | n.d | n.d |
| Phenoxypropyl- | 0.21 | 0.0098 (9) | 0.012 (10) |
| Thiophenyl- | 0.08 | n.d | n.d |
| Thienylmethyl- | 0.09 | n.d | n.d |
| Propargyl- | 0.41 | n.d | n.d |
| 6-Bromohexyl- | 0.55 | 0.025 (11) | n.d |
Nevertheless, we observed that supplementation with ethyl- (giving rise to compound 1), allyl- (2 and 3), butyl- (4 and 5), benzyl- (6 and 7), phenoxypropyl- (9 and 10), and 6-bromohexyl-malonic acids (11) yielded new peaks relative to the control, all with masses consistent with the expected C26-modified stambomycin analogues (Tables 1, S7 and Fig. S3†). It must nonetheless be noted that the exact mass of the 6-bromohexyl-malonic acid analogue corresponds to a structure lacking two of the expected hydrogens (Fig. S3†). This structural variation may correspond to a ketone at position C-28 instead of the expected hydroxyl functionality, due to over-oxidation by the P450 hydroxylase SamR0478.52 Indeed, SamR0478 belongs to the CYP107H family whose members have been shown to catalyse ketonization of C–H bonds, but this hypothesis remains to be verified. In contrast, the phenylpropyl-, thiophenyl-, thienylmethyl-, and propargyl-malonic acids failed to be incorporated (Tables 1, S5, S7 and Fig. S3†). A peak at 1390.9546 ([M + H]+, C74H130NO22+; r.t. 25.4 min) found in the octyl-fed extract (Table 1) requires further investigation in order to determine whether it corresponds to a novel octyl-stambomycin (8) or the native stambomycin F53 (r.t. 24.2 min), as the two masses are identical (Table S5†). Arguing for the formation of octyl-stambomycin, however, the observed yield of the metabolite is 9.2% relative to the stambomycins A–D (Fig. S3†), while the typical titers of stambomycin F are only 2.5% (Fig. S4†).
Biosynthesis of the parental stambomycins involves C-28 hydroxylation by the P450 hydroxylase SamR0478, as well as on-line C-50 hydroxylation by a second P450 SamR0479 to generate the nucleophile required for macrolide formation.54 For five of the successfully incorporated precursors (ethyl-, allyl-, butyl-, benzyl- and phenoxypropyl-malonate), the macrocyclic stambomycin analogues lacking C-28 hydroxylation (the ‘deoxy’ forms, 1, 3,5, 7 and 10) were the main or even sole products (i.e. no ethyl-stambomycin was detected), accounting for 55.3–99.5% of the total yields based on their relative abundance in the mass spectra. In contrast, 6-bromohexyl-stambomycin (11) is the sole analogue observed in the 6-bromohexyl feeding experiments. This observation suggests that SamR0478 strongly prefers its native substrates or at minimum closely similar chains (Fig. 1). In contrast, in no case were aglycone analogues detected in the fed extracts (data not shown), showing that the post-PKS glycosylase SamR0481, exhibits broad substrate tolerance, at least towards substrates of comparable size to the parental stambomycins44 (Fig. 1).
Quantitative analysis of the stambomycin derivatives produced by the mutasynthesis mutants ATCC/OE484/Δ483 and ATCC/OE484/Δ483/MatB_cinna
We next considered whether the lack of production of stambomycin derivatives in the presence of isopropyl-, phenylpropyl-, thiophenyl-, thienylmethyl-, and propargyl-malonic acid, might have resulted from poor acceptance of the modified polyketide chains by modules downstream of AT12. As such a mechanism could have resulted in release of stalled intermediates, we analyzed the extracts for the presence of intermediates corresponding to the products of modules 12 and 13. However, no convincing signal was detected (data not shown). Furthermore, we did not detect any of these predicted shunt products in extracts from feeding experiments where the corresponding stambomycin analogues were obtained, consistent with the idea that the modules downstream of module 12 are broadly tolerant to alternative chains. In light of this evidence, we hypothesized that incorporation of diacids failed either due to poor activation of the fed substrates to their CoA forms by MatB_Sa, or the inability of AT12 to recognize the modified malonates.To directly address the first possibility, we introduced promiscuous MatB_cinna45 into mutant ATCC/OE484/Δ483, resulting in mutant ATCC/OE484/Δ483/MatB_cinna. Mutants ATCC/OE484/Δ483 and ATCC/OE484/Δ483/MatB_cinna were then fed with ethyl-, allyl-, butyl- and benzyl-malonic acids, followed by quantification of the resulting analogues produced by the two strains. For this, erythromycin (1 mM added during the work-up step) was used as an internal standard for the LC-HRMS analyses to control for any differences in extraction efficiency. The obtained data demonstrated that the presence of MatB_cinna improved the incorporation of ethyl-, allyl- and benzyl-malonic acids (Fig. 3a, b, d and Table S8†), resulting in a nearly 2-fold increase relative to the parental strain which contains only MatB_Sa. Indeed, recent work has demonstrated that MatB_cinna efficiently converts ethyl-, allyl- and benzyl-malonic acid to their corresponding CoA forms,34 which is consistent with the results obtained here. In contrast, levels of incorporation of butyl-malonic acid by the two mutants were essentially identical (88% vs. 71%, Fig. 3c and Table S8†), reflecting the fact that MatB_cinna is poorly active with butyl-diacid.34
With the aim of elucidating the structures of certain analogues, we therefore used ATCC/OE484/Δ483/MatB_cinna to obtain allyl-incorporated stambomycin, and the original mutant ATCC/OE484/Δ483 to produce the butyl variant. This approach resulted in sufficient quantities of deoxy-allyl-stambomycin (3) (0.7 mg L−1) to allow for its purification (the yields of allyl-stambomycin (2) were inadequate), while three compounds (22.5 mg L−1 combined yield of the two most abundant compounds) were purified from extracts of ATCC/OE484/Δ483 fed with butyl-malonic acid (final yields, deoxy-allyl-stambomycin (3): 1.8 mg; butyl-stambomycin (4): 11.6 mg; deoxy-butyl-stambomycin (5): 36.3 mg; C-24-demethyl-butyl-stambomycin (12) (vide infra): 3.1 mg). The structures of these four compounds were elucidated by comprehensive NMR and HRMS analysis (Fig. S5–S29†), yielding data consistent with deoxy-allyl-stambomycin (3), butyl-stambomycin (4) and deoxy-butyl-stambomycin (5). Concerning the third butyl derivative, comparison of the obtained NMR data with that of butyl-stambomycin (4) revealed a methyl signal missing from the 1H NMR spectrum (δH 1.64) corresponding to the methyl at C-24, and the shift of an olefinic proton from δH 5.22 to δH 5.43. Additionally, coupling constants of 15.4 and 8.0 were observed for H-25 (Fig. S17–S22†). These data are all consistent with a C-24-demethyl derivative of butyl stambomycin (12). Such a compound would arise from alternative incorporation of malonyl-CoA instead of methylmalonyl-CoA by AT13. Given the intrinsically broad specificity of AT12, it is not unreasonable that a second AT within the same system (i.e. AT13) might also exhibit a certain level of promiscuity. In support of this idea, reinspection of wild type extracts revealed a peak with a mass of 1334.8911 (C70H128NO22+; m/z [M + H]+ = 1334.8922, Δppm = −0.8), which matches that of a demethyl derivative of stambomycin E (Fig. S4†) (note, it was not possible to conclusively identify analogous demethyl analogues of stambomycins AB, CD and F, as C-24-demethyl-stambomycins AB would have the same exact masses as stambomycins CD, C-24-demethyl-stambomycins CD, the same mass as stambomycin E, and C-24-demethyl-stambomycin F the same as stambomycins AB).
Quantitative comparative analysis of the PDB and mutasynthesis approaches
As we observed production of the parental stambomycins in the mutasynthesis strains which is the classical outcome of PDB experiments, it was of interest to directly compare the relative efficiency of mutasynthesis to PDB. In contrast to mutasynthesis, PDB in S. ambofaciens relies on fed mono-acids, which are then activated by the SamR0482/SamR0483 pair to generate the corresponding extender unit CoA thioesters. Thus, to judge the relative efficacies of the two approaches, we supplemented strain ATCC/OE484 with 3-butenoic acid, butyric acid and phenyl propanoic acid (Fig. 4a), and in parallel, mutant ATCC/OE484/Δ483 with the corresponding allyl-, butyl- and benzyl-malonic acids (Fig. 4b). Erythromycin (1 mM) was again used as internal standard in the LC-HRMS analyses to permit accurate quantification. The obtained data showed that butyric acid was activated by the SamR0482/SamR0483-mediated pathway in strain ATCC/OE484, resulting in butyl-incorporated stambomycin analogues (Fig. 4d), but that incorporation of 3-butenoic acid and benzyl-malonic acid failed (Fig. 4c and e). Moreover, the yield of butyl-incorporated analogues from mutasynthesis with the ATCC/OE484/Δ483 strain relative to PDB based on ATCC/OE484 (88% vs. 11%, Fig. 4d and Table S8†), confirmed that mutasynthesis was the superior approach, despite the continued biosynthesis of the wild type stambomycins.Biological activities of the stambomycin analogues
The stambomycins have moderate activity against Gram-positive bacteria but none against Gram-negative bacteria.42 More interestingly they were also shown to possess antiproliferative activity towards a range of human cancer cells, with potency comparable to several clinically used compounds.42 We therefore tested the bioactivity of the four purified stambomycin analogues (3, 4, 5 and 12). All four exhibited anti-bacterial activity, albeit variable, against both Gram-positive Bacillus subtilis ATCC6633 and Micrococcus luteus ATCC10240, but not against the Gram-negative Escherichia coli DH5α (Fig. S30†). The butyl- and deoxy-butyl-stambomycins (4 and 5) were the most active (with an efficiency similar to the original stambomycins), while the allyl form (3) showed only weak anti-bacterial activity. Interestingly, the C-24-demethyl derivative of butyl-stambomycin (12) was less active relative to the (deoxy-)butyl-stambomycins.Next, we tested the antiproliferative activities against two human cancer cell lines, U87-MG glioblastoma cells (brain cancer) (ATCC HTB-14) and MDA-MB-231 (ATCC HTB-26) cells, which are commonly used to model late-stage breast cancer. MTT assays revealed significant and similar antiproliferative activities against the two lines for the butyl-stambomycin and its deoxy form (Fig. S31†) with IC50 values around 1 μM for both the U87 and the MDA-MB-231 cells (Table S10†). These values are in the range of those observed for the parental stambomycins42 and are better than those measured for the clinical anticancer agent doxorubicin used as a control (Table S10†). Cell counting further confirmed that the inhibition observed in the MTT assays was related to cell death (Fig. S31†). As observed with the anti-bacterial assays, the C-24-demethyl derivative (12) of butyl-stambomycin and the allyl analogue (3) were significantly less active against the two cancer cell lines (Table S9 and Fig. S31†).
Discussion and conclusions
Many PKS ATs responsible for starter-unit selection show useful promiscuity, a property which has been extensively exploited for mutasynthesis of analogues.25,27,55–66 By contrast, the majority of extender-unit ATs exhibit strict substrate specificity,4 and so successful mutasynthesis based on these building blocks remains rare.2,16 In this context, the AT12 domain of the stambomycin biosynthetic pathway is remarkable, as it naturally recognizes at least six atypical malonate extender units, attesting to its innate broad substrate tolerance. This promiscuity in fact extends to all 12 downstream PKS modules and post-PKS enzymes, as the system generates a family of fully-elaborated stambomycins.42 These observations motivated us to carry out extender-unit based mutasynthesis towards expanding the library of stambomycin C-26 analogues43 available for biological evaluation. For this, we aimed to disable production of the native extender units by targeting the pathway-specific enzymes (SamR0482 and SamR0483) involved in their biosynthesis from medium-chain fatty acids.43 By deletion of the individual genes, we demonstrated that SamR0483 was critical for stambomycin biosynthesis in the absence of additional growth modification, but that SamR0482 could be complemented by other enzymes encoded in the genome. We therefore carried out mutasynthesis using the SamR0483 inactivation mutant.Supplementation of ATCC/OE484/Δ483 with suitable precursors yielded 6 novel series of stambomycin analogues at varying yields. Derivatives obtained in this way incorporated ethyl, butyl, allyl, benzyl, phenoxypropyl and 6-bromohexyl groups at C-26 instead of the native chains (Table 1) (initial evidence was also obtained for the C-26 octyl analogue). For each of the analogues except that bearing the 6-bromohexyl group which was fully modified, a major proportion of the compounds lacked the C-28 hydroxylation catalyzed by the P450 enzyme SamR0478. It is also notable that feeding of butylmalonic acid resulted in almost exclusive production of butyl-stambomycin relative to residual stambomycins A–D, whereas the native metabolites dominated in previous precursor-directed biosynthesis experiments.43 Indeed, in our hands, the PDB approach only yielded a single novel derivative when a limited series of monoacid substrates was fed. Overall, successful production of this panel of analogues depended on the intrinsic substrate tolerance of three distinct enzymatic events in the pathway: the MatB_Sa-catalyzed activation of the diacids to their CoA thioesters, substrate selection and ACP loading by AT12, and recognition of the modified intermediates by the downstream PKS modules, the C-50 hydroxylase SamR0479,54 and SamR0481-mediated mycaminosylation. It is also worth noting that producing comparable analogues using synthetic chemistry would require their total synthesis. In future, the approach described here could be coupled with metabolic engineering to introduce pathways dedicated to the synthesis of alternative extender units,67 thereby alleviating the dependence on costly commercial building blocks and/or chemical synthesis.
Nonetheless, the intrinsically broad specificity of AT12 and the overall PKS system did not translate into universal acceptance of all alternative building blocks. Intriguingly, despite the bulky nature of the (methyl)pentyl/(methyl)hexyl side chains of the native extender units, the non-native analogues showing the highest level of incorporation were significantly shorter (i.e. butyl and allyl). The efficient incorporation of butyl-malonic acid is also notable for a second reason, as it suggests that MatB_Sa has unusually high activity towards this substrate, which was not seen with MatB_cinna34 and MatB_Rt41 despite their intrinsic promiscuity. This hypothesis is also supported by the fact that expression of MatB_cinna alongside MatB_Sa did not improve incorporation of butyl-malonate by the strain. Concerning the substrates which were not accepted (isopropyl, phenylpropyl-, thiophenyl-, thienylmethyl- and propargylmalonic acid), it is not possible to conclusively identify the enzymatic bottleneck(s), as none of these substrates were directly evaluated in previous work with MatB_cinna,34 but the absence of prematurely released chain extension intermediates points to either AT12 or MatB.
Our positive incorporation results notably contrast with recent data obtained from an engineered swap of AT12 into module 6 of the erythromycin (DEBS) PKS.12 In this case, analysis in vitro with purified hybrid protein and a series of α-carboxyacyl-CoA substrates revealed essentially no activity towards ethyl- and allylmalonyl-CoA, comparable but low activity with propyl-, butyl-, pentyl-, hexylmalonyl-CoA, and a pronounced preference for isopentyl-CoA. This result taken alongside other obtained data was interpreted to show that atypical AT domains can incorporate extender units which are equal in size or slightly larger than their native substrates.12 Indeed, AT12 was predicted by AlphaFold2 (ref. 12 and 68) to possess one of the largest active site cavity volumes among the evaluated ATs, in accord with the high steric demands of its native substrates. At this stage, it is difficult to reconcile the two sets of data, particularly as incorporation of butyl-malonyl-CoA in our hands resulted in mature analogues at yields comparable to that of the parental stambomycins (20–30 mg L−1).42 Either the AT in its native context exhibits a more relaxed specificity, or the specificity of AT12 was unchanged, but the tolerance of the other DEBS module 6 domains was negatively impacted by introduction of this heterologous AT (in the presence of alternative non-native ATs, DEBS module 6 accepted both allyl- and butylmalonyl-CoA with good efficiency12). Another possible explanation, however, is that insertion of AT12 into the module caused structural perturbation, which impacted turnover. Indeed, the AT swapped modules which gave the lowest purification yields relative to wild type also exhibited the most restricted substrate specificities.12 In any case, the cumulative results point to a complex interplay between the AT and the remaining domains of the PKS in determining the efficacy with which alternative building blocks can be incorporated into engineered polyketides. This parameter will need to be considered in future PKS engineering experiments based on AT swapping.11,12
Our observation that the butyl (C4) congeners (4 and 5) retained good activity relative to the native metabolites (linear and branched C5 and C6 side chains) demonstrates a certain tolerance to the chain length and functionality at the C-26 position. However, the allyl analogue (C3) (3) was markedly less potent, an effect which may be due to the further reduced length, or the presence of the double bond. Our data also confirm an earlier observation made with the parental stambomycins46 that the hydroxylation at C-28 has no significant effect on the biological activities of the analogues. Interestingly, the methyl group at C-24 appears to contribute significantly to both the antibacterial and antiproliferative properties of the derivatives, as its absence (i.e.12) results in a sharp decrease in bioactivity. Potential reasons for this drop include reduced lipophilicity and thus biomembrane solubility, or the lack of an important conformational constraint on the macrolides. We note that the effect of methylation on biological interactions has strong precedence in the literature.69
Finally, given the observed specificity differences between MatB_Sa and MatB_cinna, it would be of interest to attempt to further broaden the substrate tolerance of MatB_Sa by structure-guided mutagenesis. Notably, an engineered version of MatB_Rt (T207G/M306I) showed improved catalytic activity toward ethyl-, propargyl- and allyl-malonic acids, as well some tolerance towards isopropyl-, butyl-, phenyl-, and azido-malonic acids.41 The two residues corresponding to T207 and M306 in MatB_Sa are V190 and M293 (Fig. S2†), which could be modified in attempts to similarly broaden the substrate scope of MatB_Sa. In parallel, it would be worth targeting for inactivation the identified homologues of SamR0483 in order to reduce the background synthesis of the parental stambomycins, potentially boosting the yields of the novel derivatives. We also envision that an analogous mutasynthesis strategy could be applied to other diversity oriented pathways but with supplementation using one of the native precursors – redirecting the pathway to production of a single natural compound which should simplify purification and subsequent structure elucidation.70 Together, such approaches should further expand the enzymatic toolbox available for efficient AT-based polyketide analogue generation.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
K. J. W. and B. A. designed and supervised the project, with initial assistance from C. J. K. J. W. and B. A. obtained the funding at the U. L. and H. B. B. at the MPI. L. S. constructed and fermented (together with L. H., Y. S., and C. J.) the mutasynthesis strains, and analyzed and interpreted the LC-MS data. Y. S. synthesized propargyl-malonic acid and interpreted the NMR data. P. G., Y-N. S., and H. B. B purified compounds and performed the NMR measurements. A. C. and S. P. carried out the biological testing. L. S., B. A. and K. J. W. wrote the manuscript, with contributions from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the Université de Lorraine, the Centre National de la Recherche Scientifique (CNRS), and the IMPACT Biomolecules project of the Lorraine Université d’Excellence (LUE) (Investissements d'avenir – ANR 15-004 to L.S., Y.S., C.J., B.A., and K.J.W.). Work in the Bode lab was supported by an ERC Advanced Grant (835108) and the Max Planck Society. We thank Prof. Yi Yu (Wuhan University, China) for the chemical synthesis of 6-bromohexyl-malonic acid and Prof. Frank Schulz (Ruhr-Universität Bochum, Germany) for the plasmid encoding MatB_cinna. We are grateful for access to the PASM platform of the Université de Lorraine, and the technical assistance provided by C. Paris.Notes and references
- C. A. Dejong, G. M. Chen, H. Li, C. W. Johnston, M. R. Edwards, P. N. Rees, M. A. Skinnider, A. L. Webster and N. A. Magarvey, Polyketide and nonribosomal peptide retro-biosynthesis and global gene cluster matching, Nat. Chem. Biol., 2016, 12, 1007–1014 CrossRef CAS PubMed.
- S. Mo, D. H. Kim, J. H. Lee, J. W. Park, D. B. Basnet, Y. H. Ban, Y. J. Yoo, S. W. Chen, S. R. Park, E. A. Choi, E. Kim, Y. Y. Jin, S. K. Lee, J. Y. Park, Y. Liu, M. O. Lee, K. S. Lee, S. J. Kim, D. Kim, B. C. Park, S. G. Lee, H. J. Kwon, J. W. Suh, B. S. Moore, S. K. Lim and Y. J. Yoon, Biosynthesis of the allylmalonyl-CoA extender unit for the FK506 polyketide synthase proceeds through a dedicated polyketide synthase and facilitates the mutasynthesis of analogues, J. Am. Chem. Soc., 2011, 133, 976–985 CrossRef CAS PubMed.
- M. Sandy, Z. Rui, J. Gallagher and W. Zhang, Enzymatic synthesis of dilactone scaffold of antimycins, ACS Chem. Biol., 2012, 7, 1956–1961 CrossRef CAS PubMed.
- B. J. Dunn and C. Khosla, Engineering the acyltransferase substrate specificity of assembly line polyketide synthases, J. R. Soc. Interface, 2013, 10, 20130297 CrossRef PubMed.
- K. J. Weissman, Genetic engineering of modular PKSs: from combinatorial biosynthesis to synthetic biology, Nat. Prod. Rep., 2016, 33, 203–230 RSC.
- L. Liu, A. Thamchaipenet, H. Fu, M. Betlach and G. Ashley, Biosynthesis of 2-Nor-6-deoxyerythronolide B by Rationally Designed Domain Substitution, J. Am. Chem. Soc., 1997, 119, 10553–10554 CrossRef CAS.
- X. Ruan, A. Pereda, D. L. Stassi, D. Zeidner, R. G. Summers, M. Jackson, A. Shivakumar, S. Kakavas, M. J. Staver, S. Donadio and L. Katz, Acyltransferase domain substitutions in erythromycin polyketide synthase yield novel erythromycin derivatives, J. Bacteriol., 1997, 179, 6416 CrossRef CAS PubMed.
- D. L. Stassi, S. J. Kakavas, K. A. Reynolds, G. Gunawardana, S. Swanson, D. Zeidner, M. Jackson, H. Liu, A. Buko and L. Katz, Ethyl-substituted erythromycin derivatives produced by directed metabolic engineering, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 7305–7309 CrossRef CAS PubMed.
- R. McDaniel, A. Thamchaipenet, C. Gustafsson, H. Fu, M. Betlach, M. Betlach and G. Ashley, Multiple genetic modifications of the erythromycin polyketide synthase to produce a library of novel “unnatural” natural products, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 1846–1851 CrossRef CAS PubMed.
- H. Petkovic, R. E. Lill, R. M. Sheridan, B. Wilkinson, E. L. McCormick, H. A. I. McArthur, J. Staunton, P. F. Leadlay and S. G. Kendrew, A novel erythromycin, 6-desmethyl erythromycin D, made by substituting an acyltransferase domain of the erythromycin polyketide synthase, J. Antibiot., 2003, 56, 543–551 CrossRef CAS PubMed.
- S. Yuzawa, K. Deng, G. Wang, E. E. K. Baidoo, T. R. Northen, P. D. Adams, L. Katz and J. D. Keasling, Comprehensive in Vitro Analysis of Acyltransferase Domain Exchanges in Modular Polyketide Synthases and Its Application for Short-Chain Ketone Production, ACS Synth. Biol., 2017, 6, 139–147 CrossRef CAS PubMed.
- E. Englund, M. Schmidt, A. A. Nava, A. Lechner, K. Deng, R. Jocic, Y. Lin, J. Roberts, V. T. Benites, R. Kakumanu, J. W. Gin, Y. Chen, Y. Liu, C. J. Petzold, E. E. K. Baidoo, T. R. Northen, P. D. Adams, L. Katz, S. Yuzawa and J. D. Keasling, Expanding Extender Substrate Selection for Unnatural Polyketide Biosynthesis by Acyltransferase Domain Exchange within a Modular Polyketide Synthase, J. Am. Chem. Soc., 2023, 145, 8822–8832 CrossRef CAS PubMed.
- B. J. Dunn, K. R. Watts, T. Robbins, D. E. Cane and C. Khosla, Comparative analysis of the substrate specificity of trans- versus cis-acyltransferases of assembly line polyketide synthases, Biochemistry, 2014, 53, 3796–3806 CrossRef CAS PubMed.
- M. Hans, A. Hornung, A. Dziarnowski, D. E. Cane and C. Khosla, Mechanistic analysis of acyl transferase domain exchange in polyketide synthase modules, J. Am. Chem. Soc., 2003, 125, 5366–5374 CrossRef CAS PubMed.
- U. Sundermann, K. Bravo-Rodriguez, S. Klopries, S. Kushnir, H. Gomez, E. Sanchez-Garcia and F. Schulz, Enzyme-Directed Mutasynthesis: A Combined Experimental and Theoretical Approach to Substrate Recognition of a Polyketide Synthase, ACS Chem. Biol., 2013, 8, 443–450 CrossRef CAS PubMed.
- K. Bravo-Rodriguez, S. Klopries, K. R. M. Koopmans, U. Sundermann, S. Yahiaoui, J. Arens, S. Kushnir, F. Schulz and E. Sanchez-Garcia, Substrate Flexibility of a Mutated Acyltransferase Domain and Implications for Polyketide Biosynthesis, Chem. Biol., 2015, 22, 1425–1430 CrossRef CAS PubMed.
- I. Koryakina, C. Kasey, J. B. McArthur, A. N. Lowell, J. A. Chemler, S. Li, D. A. Hansen, D. H. Sherman and G. J. Williams, Inversion of Extender Unit Selectivity in the Erythromycin Polyketide Synthase by Acyltransferase Domain Engineering, ACS Chem. Biol., 2017, 12, 114–123 CrossRef CAS PubMed.
- Y. Li, W. Zhang, H. Zhang, W. Tian, L. Wu, S. Wang, M. Zheng, J. Zhang, C. Sun, Z. Deng, Y. Sun, X. Qu and J. Zhou, Structural Basis of a Broadly Selective Acyltransferase from the Polyketide Synthase of Splenocin, Angew. Chem., Int. Ed., 2018, 57, 5823–5827 CrossRef CAS PubMed.
- F. Zhang, T. Shi, H. Ji, I. Ali, S. Huang, Z. Deng, Q. Min, L. Bai, Y. Zhao and J. Zheng, Structural Insights into the Substrate Specificity of Acyltransferases from Salinomycin Polyketide Synthase, Biochemistry, 2019, 58, 2978–2986 CrossRef CAS PubMed.
- C. Khosla, Y. Tang, A. Y. Chen, N. A. Schnarr and D. E. Cane, Structure and mechanism of the 6-deoxyerythronolide B synthase, Annu. Rev. Biochem., 2007, 76, 195–221 CrossRef CAS PubMed.
- P. Paiva, S. F. Sousa, M. J. Ramos and P. A. Fernandes, Understanding the Catalytic Machinery and the Reaction Pathway of the Malonyl-Acetyl Transferase Domain of Human Fatty Acid Synthase, ACS Catal., 2018, 8, 4860–4872 CrossRef CAS.
- E. E. Drufva, E. G. Hix and C. B. Bailey, Site directed mutagenesis as a precision tool to enable synthetic biology with engineered modular polyketide synthases, Synth. Syst. Biotechnol., 2020, 5, 62–80 CrossRef PubMed.
- K. J. Weissman, Mutasynthesis – uniting chemistry and genetics for drug discovery, Trends Biotechnol., 2007, 25, 139–142 CrossRef CAS PubMed.
- R. J. M. Goss, S. E. Lanceron, N. J. Wise and S. J. Moss, Generating rapamycin analogues by directed biosynthesis: starter acid substrate specificity of mono-substituted cyclohexane carboxylic acids, Org. Biomol. Chem., 2006, 4, 4071–4073 RSC.
- R. J. M. Goss, S. Lanceron, A. Deb Roy, S. Sprague, M. Nur-e-Alam, D. L. Hughes, B. Wilkinson and S. J. Moss, An Expeditious Route to Fluorinated Rapamycin Analogues by Utilising Mutasynthesis, ChemBioChem, 2010, 11, 698–702 CrossRef CAS PubMed.
- Y. H. Ban, J. H. Lee, G. R. Gu, B. Lee, S. Mo, H. J. Kwon and Y. J. Yoon, Mutational biosynthesis of a FK506 analogue containing a non-natural starter unit, Mol. BioSyst., 2013, 9, 944–947 RSC.
- D. H. Kim, J. H. Ryu, K. S. Lee, B. M. Lee, M. O. Lee, S.-K. Lim and P. J. Maeng, Mutational biosynthesis of tacrolimus analogues by fkbO deletion mutant of Streptomyces sp. KCTC 11604BP, Appl. Microbiol. Biotechnol., 2013, 97, 5881–5892 CrossRef CAS PubMed.
- O. Ghisalba, H. Fuhrer, W. J. Richter and S. Moss, A genetic approach to the biosynthesis of the rifamycin-chromophore in Nocardia mediterranei, J. Antibiot., 1981, 34, 58–63 CrossRef CAS PubMed.
- D. Hunziker, T. W. Yu, C. R. Hutchinson, H. G. Floss and C. Khosla, Primer unit specificity in rifamycin biosynthesis principally resides in the later stages of the biosynthetic pathways, J. Am. Chem. Soc., 1998, 120, 1092–1093 CrossRef CAS.
- C. J. Dutton, S. P. Gibson, A. C. Goudie, K. S. Holdom, M. S. Pacey, J. C. Ruddock, J. D. Bulock and M. K. Richards, Novel avermectins produced by mutational biosynthesis, J. Antibiot., 1991, 44, 357–365 CrossRef CAS PubMed.
- Y. Zhuo, T. Zhang, Q. Wang, P. Cruz-Morales, B. Zhang, M. Liu, F. Barona-Gómez and L. Zhang, Synthetic biology of avermectin for production improvement and structure diversification, Biotechnol. J., 2014, 9, 316–325 CrossRef CAS PubMed.
- J. Lau, D. E. Cane and C. Khosla, Substrate specificity of the loading didomain of the erythromycin polyketide synthase, Biochemistry, 2000, 39, 10514–10520 CrossRef CAS PubMed.
- J. Franke and C. Hertweck, Biomimetic Thioesters as Probes for Enzymatic Assembly Lines: Synthesis, Applications, and Challenges, Cell Chem. Biol., 2016, 23, 1179–1192 CrossRef CAS PubMed.
- M. Grote and F. Schulz, Exploring the Promiscuous Enzymatic Activation of Unnatural Polyketide Extender Units in Vitro and in Vivo for Monensin Biosynthesis, ChemBioChem, 2019, 20, 1183–1189 CrossRef CAS PubMed.
- T. J. Erb, I. A. Berg, V. Brecht, M. Müller, G. Fuchs and B. E. Alber, Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 10631–10636 CrossRef CAS PubMed.
- T. J. Erb, V. Brecht, G. Fuchs, M. Müller and B. E. Alber, Carboxylation mechanism and stereochemistry of crotonyl-CoA carboxylase/reductase, a carboxylating enoyl-thioester reductase, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 8871–8876 CrossRef CAS PubMed.
- R. G. Rosenthal, M. O. Ebert, P. Kiefer, D. M. Peter, J. A. Vorholt and T. J. Erb, Direct evidence for a covalent ene adduct intermediate in NAD(P)H-dependent enzymes, Nat. Chem. Biol., 2014, 10, 50–55 CrossRef CAS PubMed.
- M. C. Wilson and B. S. Moore, Beyond ethylmalonyl-CoA: the functional role of crotonyl-CoA carboxylase/reductase homologs in expanding polyketide diversity, Nat. Prod. Rep., 2012, 29, 72–86 RSC.
- Y. Yan, J. Chen, L. Zhang, Q. Zheng, Y. Han, H. Zhang, D. Zhang, T. Awakawa, I. Abe and W. Liu, Multiplexing of combinatorial chemistry in antimycin biosynthesis: expansion of molecular diversity and utility, Angew. Chem., Int. Ed., 2013, 52, 12308–12312 CrossRef CAS PubMed.
- A. J. Hughes and A. Keatinge-Clay, Enzymatic Extender Unit Generation for In Vitro Polyketide Synthase Reactions: Structural and Functional Showcasing of Streptomyces coelicolor MatB, Chem. Biol., 2011, 18, 165–176 CrossRef CAS PubMed.
- I. Koryakina, J. McArthur, S. Randall, M. M. Draelos, E. M. Musiol, D. C. Muddiman, T. Weber and G. J. Williams, Poly Specific trans-Acyltransferase Machinery Revealed via Engineered Acyl-CoA Synthetases, ACS Chem. Biol., 2013, 8, 200–208 CrossRef CAS PubMed.
- L. Laureti, L. Song, S. Huang, C. Corre, P. Leblond, G. L. Challis and B. Aigle, Identification of a bioactive 51-membered macrolide complex by activation of a silent polyketide synthase in Streptomyces ambofaciens, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 6258–6263 CrossRef CAS PubMed.
- L. Ray, T. R. Valentic, T. Miyazawa, D. M. Withall, L. J. Song, J. C. Milligan, H. Osada, S. Takahashi, S. C. Tsai and G. L. Challis, A crotonyl-CoA reductase-carboxylase independent pathway for assembly of unusual alkylmalonyl-CoA polyketide synthase extender units, Nat. Commun., 2016, 7, 13609 CrossRef CAS PubMed.
- L. Su, L. Hôtel, C. Paris, C. Chepkirui, A. O. Brachmann, J. Piel, C. Jacob, B. Aigle and K. J. Weissman, Engineering the stambomycin modular polyketide synthase yields 37-membered mini-stambomycins, Nat. Commun., 2022, 13, 515 CrossRef CAS PubMed.
- D. Möller, S. Kushnir, M. Grote, A. Ismail-Ali, K. R. M. Koopmans, F. Calo, S. Heinrich, B. Diehl and F. Schulz, Flexible enzymatic activation of artificial polyketide extender units by Streptomyces cinnamonensis into the monensin biosynthetic pathway, Lett. Appl. Microbiol., 2018, 67, 226–234 CrossRef PubMed.
- L. Laureti, PhD thesis, Université de Lorraine, 2010.
- S. F. Altschul, W. Gish, W. Miller, E. W. Myers and D. J. Lipman, Basic local alignment search tool, J. Mol. Biol., 1990, 215, 403–410 CrossRef CAS PubMed.
- X. Pang, B. Aigle, J. M. Girardet, S. Mangenot, J. L. Pernodet, B. Decaris and P. Leblond, Functional angucycline-like antibiotic gene cluster in the terminal inverted repeats of the Streptomyces ambofaciens linear chromosome, Antimicrob. Agents Chemother., 2004, 48, 575–588 CrossRef CAS PubMed.
- R. Bunet, L. Song, M. V. Mendes, C. Corre, L. Hotel, N. Rouhier, X. Framboisier, P. Leblond, G. L. Challis and B. Aigle, Characterization and manipulation of the pathway-specific late regulator AlpW reveals Streptomyces ambofaciens as a new producer of Kinamycins, J. Bacteriol., 2011, 193, 1142–1153 CrossRef CAS PubMed.
- Y. A. Chan, A. M. Podevels, B. M. Kevany and M. G. Thomas, Biosynthesis of polyketide synthase extender units, Nat. Prod. Rep., 2009, 26, 90–114 RSC.
- M. Myronovskyi and A. Luzhetskyy, Native and engineered promoters in natural product discovery, Nat. Prod. Rep., 2016, 33, 1006–1019 RSC.
- Y. Yan, J. Wu, G. Hu, C. Gao, L. Guo, X. Chen, L. Liu and W. Song, Current state and future perspectives of cytochrome P450 enzymes for C-H and C=C oxygenation, Synth. Syst. Biotechnol., 2022, 7, 887–899 CrossRef CAS PubMed.
- B. Aigle, G. L. Challis, L. Laureti, L. Song, P. Leblond, US Pat., 20120142622A1, 2012 Search PubMed.
- L. Song, L. Laureti, C. Corre, P. Leblond, B. Aigle and G. L. Challis, Cytochrome P450-mediated hydroxylation is required for polyketide macrolactonization in stambomycin biosynthesis, J. Antibiot., 2014, 67, 71–76 CrossRef CAS PubMed.
- S. Eichner, H. G. Floss, F. Sasse and A. Kirschning, New, highly active nonbenzoquinone geldanamycin derivatives by using mutasynthesis, ChemBioChem, 2009, 10, 1801–1805 CrossRef CAS PubMed.
- A. Miyanaga, R. Takaku, M. Takaishi, E. Tashiro, F. Kudo and T. Eguchi, Generation of incednine derivatives by mutasynthesis, J. Antibiot., 2020, 73, 794–797 CrossRef CAS PubMed.
- J. A. Kalaitzis, M. Izumikawa, L. Xiang, C. Hertweck and B. S. Moore, Mutasynthesis of enterocin and wailupemycin analogues, J. Am. Chem. Soc., 2003, 125, 9290–9291 CrossRef CAS PubMed.
- S. G. Kendrew, H. Petkovic, S. Gaisser, S. J. Ready, M. A. Gregory, N. J. Coates, E. A. M. Nur, T. Warneck, D. Suthar, T. A. Foster, L. McDonald, G. Schlingman, F. E. Koehn, J. S. Skotnicki, G. T. Carter, S. J. Moss, M. Q. Zhang, C. J. Martin, R. M. Sheridan and B. Wilkinson, Recombinant strains for the enhanced production of bioengineered rapalogs, Metab. Eng., 2013, 15, 167–173 CrossRef CAS PubMed.
- M. Werneburg, B. Busch, J. He, M. E. Richter, L. Xiang, B. S. Moore, M. Roth, H. M. Dahse and C. Hertweck, Exploiting enzymatic promiscuity to engineer a focused library of highly selective antifungal and antiproliferative aureothin analogues, J. Am. Chem. Soc., 2010, 132, 10407–10413 CrossRef CAS PubMed.
- J. Hermane, S. Eichner, L. Mancuso, B. Schröder, F. Sasse, C. Zeilinger and A. Kirschning, New geldanamycin derivatives with anti Hsp properties by mutasynthesis, Org. Biomol. Chem., 2019, 17, 5269–5278 RSC.
- M. A. Gregory, H. Petkovic, R. E. Lill, S. J. Moss, B. Wilkinson, S. Gaisser, P. F. Leadlay and R. M. Sheridan, Mutasynthesis of rapamycin analogues through the manipulation of a gene governing starter unit biosynthesis, Angew. Chem., Int. Ed., 2005, 44, 4757–4760 CrossRef CAS PubMed.
- M. G. Malmierca, I. Pérez-Victoria, J. Martín, F. Reyes, C. Méndez, J. A. Salas and C. Olano, New Sipanmycin Analogues Generated by Combinatorial Biosynthesis and Mutasynthesis Approaches Relying on the Substrate Flexibility of Key Enzymes in the Biosynthetic Pathway, Appl. Environ. Microbiol., 2020, 86, e02453 CrossRef CAS PubMed.
- H. B. Bode, P. Meiser, T. Klefisch, N. S. Cortina, D. Krug, A. Göhring, G. Schwär, T. Mahmud, Y. A. Elnakady and R. Müller, Mutasynthesis-derived myxalamids and origin of the isobutyryl-CoA starter unit of myxalamid B, ChemBioChem, 2007, 8, 2139–2144 CrossRef CAS PubMed.
- F. Taft, M. Brünjes, H. G. Floss, N. Czempinski, S. Grond, F. Sasse and A. Kirschning, Highly active ansamitocin derivatives: mutasynthesis using an AHBA-blocked mutant, ChemBioChem, 2008, 9, 1057–1060 CrossRef CAS PubMed.
- F. Kudo, S. Takahashi, A. Miyanaga, Y. Nakazawa, K. Nishino, Y. Hayakawa, K. Kawamura, F. Ishikawa, G. Tanabe, N. Iwai, Y. Nagumo, T. Usui and T. Eguchi, Mutational Biosynthesis of Hitachimycin Analogs Controlled by the β-Amino Acid-Selective Adenylation Enzyme HitB, ACS Chem. Biol., 2021, 16, 539–547 CrossRef CAS PubMed.
- J. F. Castro, V. Razmilic, J. P. Gomez-Escribano, B. Andrews, J. A. Asenjo and M. J. Bibb, Identification and Heterologous Expression of the Chaxamycin Biosynthesis Gene Cluster from Streptomyces leeuwenhoekii, Appl. Environ. Microbiol., 2015, 81, 5820–5831 CrossRef CAS PubMed.
- W. S. Jung, E. Kim, Y. J. Yoo, Y. H. Ban, E. J. Kim and Y. J. Yoon, Characterization and engineering of the ethylmalonyl-CoA pathway towards the improved heterologous production of polyketides in Streptomyces venezuelae, Appl. Microbiol. Biotechnol., 2014, 98, 3701–3713 CrossRef CAS PubMed.
- K. Tunyasuvunakool, J. Adler, Z. Wu, T. Green, M. Zielinski, A. Žídek, A. Bridgland, A. Cowie, C. Meyer, A. Laydon, S. Velankar, G. J. Kleywegt, A. Bateman, R. Evans, A. Pritzel, M. Figurnov, O. Ronneberger, R. Bates, S. A. A. Kohl, A. Potapenko, A. J. Ballard, B. Romera-Paredes, S. Nikolov, R. Jain, E. Clancy, D. Reiman, S. Petersen, A. W. Senior, K. Kavukcuoglu, E. Birney, P. Kohli, J. Jumper and D. Hassabis, Highly accurate protein structure prediction for the human proteome, Nature, 2021, 596, 590–596 CrossRef CAS PubMed.
- E. J. Barreiro, A. E. Kümmerle and C. A. Fraga, The methylation effect in medicinal chemistry, Chem. Rev., 2011, 111, 5215–5246 CrossRef CAS PubMed.
- G. Kosec, D. Goranovič, P. Mrak, S. Fujs, E. Kuščer, J. Horvat, G. Kopitar and H. Petković, Novel chemobiosynthetic approach for exclusive production of FK506, Metab. Eng., 2012, 14, 39–46 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06976e |
| This journal is © The Royal Society of Chemistry 2025 |