Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
CO2 hydrosilylation catalyzed by an N-heterocyclic carbene (NHC)-stabilized stannyliumylidene†
Dechuang
Niu
 ,
Arseni
Kostenko
,
Arseni
Kostenko
 ,
John A.
Kelly
,
John A.
Kelly
 ,
Debotra
Sarkar
,
Debotra
Sarkar
 ,
Huihui
Xu
,
Huihui
Xu
 and
Shigeyoshi
Inoue
and
Shigeyoshi
Inoue
 *
*
TUM School of Natural Sciences, Department of Chemistry, Institute of Silicon Chemistry and Catalysis Research Center, Technische Universität München, Lichtenbergstraße 4, 85748 Garching bei München, Germany. E-mail: s.inoue@tum.de
First published on 22nd January 2025
Abstract
The di-N-heterocyclic carbene (NHCs) stabilized stannyliumylidene, [MesTerSn(IMe4)2][BArF], (MesTer = 2,6-Mes2C6H3, Mes = 2,4,6-Me3-C6H2, IMe4 = 1,3,4,5-tetramethylimidazol-2-ylidene, BArF = (3,5-(CF3)2-C6H5)4B), was isolated from the reaction of (MesTer)SnCl with two equivalents of IMe4, followed by one equivalent of Na[BArF]. This stannyliumylidene acts as a precatalyst for the homogeneous hydrosilylation of CO2. Experimental mechanistic studies and quantum chemical calculations have been conducted to elucidate the catalytically active species and the mechanism for the transformation, revealing the stannyliumylidene [MesTerSn(CO2IMe4)2][BArF], which is formed in the presence of CO2, as the catalytically active species.
Introduction
A major goal in contemporary chemical research is the utilization of carbon dioxide in the creation of fine chemicals, due to its high production/abundance and its status as a greenhouse gas. Recent advances in main group chemistry have seen the successful isolation of highly reactive compounds with unprecedented oxidation states and bonding modes, which in turn have demonstrated the remarkable capability of activating CO2.1–5 For example, silylenes can react with CO2 yielding the corresponding siliconIV carbonates [C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(O–)2]2−.6,7 Germylene and stannylene species tend to afford EII (E = Ge and Sn) carboxylate compounds, owing to the weak nucleophilicity of their lone pair of electrons.8,9
O)(O–)2]2−.6,7 Germylene and stannylene species tend to afford EII (E = Ge and Sn) carboxylate compounds, owing to the weak nucleophilicity of their lone pair of electrons.8,9
While main group-mediated CO2 activation is becoming more commonplace, the catalytic transformation of carbon dioxide is still rare, especially when it comes to hydrosilylation reactions. Main-group catalysis has attracted tremendous attention recently, due to the higher abundance and lower toxicity of such elements. Currently, the most potent catalysts for the hydrosilylation of CO2 using main group compounds are: Lewis bases (LBs), Lewis acids (LAs), and frustrated Lewis pairs (FLPs).1 The use of N-heterocyclic carbenes (NHCs) as catalysts for the hydrosilylation of CO2 was first reported by Zhang, Ying and coworkers (Fig. 1, A).10 Later, experimental and theoretical studies showed that the NHC–CO2 adduct acts as the active catalytic species in the hydrosilylation of CO2, wherein the Si–H bond is activated via coordination of the oxygen atom of the NHC–CO2 adduct (Fig. 1, B).11,12
 | ||
| Fig. 1 (1) Proposed mechanism for NHC-catalyzed hydrosilylation of CO2; (2) LA systems effective in CO2 hydrosilylation. | ||
Main-group Lewis acids have been shown to be capable of the catalytic hydrosilylation of CO2, examples include the silylium cation, C13 and two-coordinate group 13 cations D, E, and F (Fig. 1(2)).14–16 Okuda and coworkers found BPh3 could catalyze the hydrosilylation of CO2 to form silyl formate, but only in highly polar, aprotic solvents, such as acetonitrile.17 They posit the mechanism involves weak/dynamic coordination of BPh3 to CO2, as well as to the Si–H moiety, generating two partially polarized transient species.
Another pertinent contribution to main-group based catalysis was reported in 2009 by the Piers group, in which amine/borane FLP (Fig. 2, G) was effective in the hydrosilylation of CO2.18 Since then, a multitude of various FLP catalytic systems have been reported, such as {[TismPribenz]M} [HB(C6F5)3](M = Zn, Mg; TismPribenz = tri[(1-isopropylbenzimidazol-2-yl)-dimethylsilyl]methyl)19,20 and [(Dippnacnac)Ga(Ad)][HB(C6F5)3](Dippnacnac = [{N(Dipp)CMe}2CH]−, Dipp = 2,6-diisopropylphenyl, Ad = 1-adamantyl).21 A noteworthy example from the Kato group is an N,P-heterocyclic germylene/B(C6F5)3 Lewis pair that can be used as a catalyst for the selective CO2 hydrosilylation to H2C(OSiEt3)2, through Si–H bond activation to give the highly reactive cationic (amino)(phosphino) germylene with HB(C6F5)3− as a counter anion, followed by activation of CO2 (Fig. 2, H).22
An emerging class of compounds that is of high interest to main group chemists, from the point of view of their catalytic capabilities, are the tetryliumylidenes. Tetryliumylidenes (RE:+), are mono-substituted EII (E = Si, Ge, Sn, Pb) atoms with a lone pair of electrons and two vacant orbitals, that exhibit high reactivity due to their unsaturated and amphiphilic nature.4,23,24 There has been a growing number of reports of these compounds ever since the first was reported by Jutzi and coworkers,23,25 whereby they used pentamethyl-cyclopentadienyl to stabilize the heavier tetryliumylidenes (Fig. 2, I, Cp*E:+, E = Si,26 Ge,27 Sn,27 Pb;28 Cp* = Me5C5). These were shown to be able to activate small molecules (e.g., CO2, H2, N2O, alkenes, ketones, etc.).24 Silyliumylidene ions stabilized by Lewis bases (e.g., N-heterocyclic carbene (NHC) (J),29 PMe3) could reduce CO2, resulting in the silaacylium ions, [RSi(O)(IMe4)2]Cl (R = MesTer or Tipp; MesTer = 2,6-Mes2-C6H3, Mes = 2,4,6-Me3-C6H2, Tipp = 2,4,6-iPr3-C6H2, IMe4 = 1,3,4,5-tetramethylimidazol-2-ylidene),30,31via the initial coordination of the C atom to the Si center. We reported on the CO2 insertion into the Si–CNHC bond of the dicationic siliconIV compound [MesTer(IMe4)2SiH][OTf]2 (OTf = CF3SO3−).32
Recently, our group has isolated the germyliumylidene ion (K, Fig. 2), which was shown to react with N2O to produce a germa-acylium ion [(MesTer)Ge(O)(IMe4)2]X (X = Cl or BArF, BArF = (3,5-(CF3)2C6H5)4B).33 This germa-acylium ion could then be utilized in the catalytic functionalization of CO2, forming the catalytically active germylene, (MesTer)Ge(OSiHPh2)(IMe4). It is also worth noting that K catalyzes the reduction of CO2 with silane via a three-component transition state (Fig. 2, L).34 The mechanism is analogous to the cooperative silane/CO2 mechanism for the hydrosilylation of CO2 catalyzed by NHC (Fig. 1, B).12
The heavier tetryliumylidene analogues, i.e. stannyliumylidene cations, are also an emerging class of low valent main group species that that may have the potential to be used in catalysis. However, examples of the utilization of stannyliumylidene in the activation of small molecules are rare. Herein, we present the first substantiated example of stannyliumylidene acting as a catalyst for CO2 hydrosilylation. We report the synthesis of the NHC-stabilized stannyliumylidene [2]+ – the tin analogue of J and K, which reacts with CO2 to form the catalytically active stannyliumylidene [4]+. We present the mechanism of conversion of [2]+ to the catalytically active stannyliumylidene species, isolating and fully describing the reactive intermediates of this process, as well as the catalytically active species. Additionally, we examine the catalytic performance of the stannyliumylidene species in the catalytic hydrosilylation CO2 using various silanes and conditions. Based on these studies and quantum chemical calculations we propose a mechanism for the catalytic reaction.
Results and discussion
Synthesis of stannyliumylidene
NHC-stabilized stannyliumylidene [MesTerSn(IMe4)2][BArF] ([2][BArF]) was obtained by the reaction of chlorostannylene, MesTerSnCl (1) with two equivalents of IMe4 in toluene, followed by adding one equivalent of Na[BArF] in a one-pot procedure (Scheme 1). It was isolated as a colorless crystalline solid in a 72% yield. [2][BArF] has poor solubility in nonpolar organic solvents, e.g., benzene and toluene, but it is soluble in fluorobenzene and THF. In acetonitrile, substitution occurs between the IMe4 and solvent molecules leading to a mixture of coordination compounds. This is in stark contrast to silyliumylidene (Fig. 2, J) and germyliumylidene ions (Fig. 2, K), where ligand substitution does not occur.29,33 In the 119Sn{1H} NMR spectrum, one sharp signal was observed at δ = −235.72 ppm in THF-d8, similar to the cationic stannylene, [TippTerSn(IMe2)2][HB(C6F5)3](TippTer = 2,6-Tipp2-C6H3) (δ = −234 ppm), reported by the Wesemann group.35 The 13C{1H} NMR spectrum displays a resonance at δ = 170.18 ppm for the carbon of IMe4, which is downshifted compared to that of the germyliumylidene (K) (δ = 164 ppm) and the silyliumylidene (J) (δ = 160 ppm).29,33X-ray quality crystals of [2][BArF] were obtained from a toluene/fluorobenzene (C6H5F) solution stored at −35 °C. Single crystal X-ray diffraction (SC-XRD) analysis revealed that the Sn central is tricoordinate with two IMe4 moieties and one MesTer-substituent (Fig. 3). The sum of the bond angles around the Sn1 atom is 300.4°. The Sn1–B1 distance is 8.587 Å, indicating no interaction between the cation and anion. Additionally, we found the shortest distance between the Sn atom and a fluorine atom at 4.032 Å. This also indicates there is no Sn–F interaction as the sum of the van der Waals radii of these atoms amounts to 3.56 Å (2.16 Å for Sn and 1.40 Å for F). The Sn–CNHC bond distances are Sn1–C25 = 2.339(4) Å and Sn1–C32 = 2.308(4) Å. They are similar to that found in IPrMeSnCl2 (2.290(5) Å) (IPrMe = 1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene),36 IPrMeSn[C(Cl)PMes*]2 (Mes* = 2,4,6-tri-tert-butylphenyl) (2.316(2) Å)37 and slightly longer than (DippTer)Sn(IMe4)CH2P(CH3)2 = P(MesTer) (2.274(3) Å) (DippTer = 2,6-Dipp2-C6H3, Dipp = 2,6-iPr2-C6H3).38 As expected, they are longer than the observed distances in the NHC-stabilized silyliumylidene ion (J) (1.948(19) Å, 1.966(19) Å) and NHC-stabilized germyliumylidene ion (K) (2.063(3) Å, 2.093(3) Å).
To gain further insight into the electronic structure of [2]+, as well as to analyze the thermodynamics and kinetics in the processes involving this species, quantum chemical calculations were carried out. For details regarding the computational methods see the ESI.† Natural Bond Orbital (NBO) analysis of the optimized structure of [2]+ (Fig. 4) shows that the stannyliumylidene cation exhibits the expected bonding situation around the tin center with a σ-type lone pair of electrons at Sn (NBO 55), and a polarized Sn–CAr bond (NBO 57) with Wiberg Bond Index (WBI) of 0.67. The bonding between the Sn1 and C25 (of IMe4) where the WBI = 0.58, indicates a very polarized bonding interaction with electron density of 18.25% on Sn and 81.75% on C (NBO 55). This type of polarization, as well as the sp1.45 hybridization of the carbon, which interacts with the almost pure p orbital of Sn (sp19.72), can be interpreted as carbene coordination to the empty p-orbital of the Sn center. The situation is almost identical to that of the second carbene (Sn1–C32, NBO 56), in terms of bond polarization, hybridization of the interacting orbitals and the WBI (0.58) (Fig. 5). The Sn center of [2]+ is positively charged by 0.79 el. The aryl moiety of [2]+ withdraws substantial electron density being negatively charged with −0.48 el., while the Sn center is compensated by the donation from the NHC moieties with the sum of NPA charges of +0.34 and +0.35 el. In addition to the [2]+ representation as a bis(NHC)-stabilized stannyliumylidene – with dative bonds between the NHC ligands and the Sn atom – the NBO depiction may also hint at the stannyl anion character of the Sn center. In this case, the ionic representation of [2]+, where the negative charge is located on the Sn atom to which the two positively charged NHC moieties are bound covalently, should be considered. A detailed discussion of such stannyl-anion-type species in given in the ESI (Fig. S55 and S56†).
 | ||
| Fig. 4 NBOs of [2]+ (iso = 0.04) at the PBE0/def2-TZVP//r2SCAN-3c level of theory. The mesityl substituents and the methyl substituents are shown as wireframes for clarity. | ||
The frontier orbitals of [2]+ are presented in Fig. 6. According to the NBO analysis of the canonical MOs, the HOMO has 52% non-bonding and 43% bonding character and consists mostly of the non-bonding lone pair of electrons at Sn (51%). Additional important contributions arise from the Sn–Ar and Sn–IMe4 bonding interactions (Sn–C1 (16%) and Sn–C25 (7%)). The LUMO is 73% anti-bonding and 19% non-bonding with the most significant contributions from π*(N3–C25) 25%, π*(N1–C32) 21%, σ*(Sn1–C1) 11%, σ*(Sn1–C32) 7%.
 | ||
| Fig. 6 Frontier molecular orbitals of [2]+ (iso = 0.04) at the PBE0/def2-TZVP//r2SCAN-3c level of theory. The mesityl substituents and the methyl substituents are shown as wireframes for clarity. | ||
Calculations at the PW6B95-D4/def2-QZVPP(CPCM)/r2SCAN-3c level of theory show that the anion–cation interaction in [2][BArF] is weak, and only 10.4 and 0.0 kcal mol−1 are required to fully separate the ions in benzene and difluorobenzene, respectively (Scheme 2). The charge transfer in [2][BArF] between [2]+ cation (ΣqNPA = + 0.98 el.) and the [BArF]− anion (ΣqNPA = −0.98 el.) is negligible. The Sn–IMe4 bonding is also quite labile, with a dissociation free energy for [2][BArF] to form [2a][BArF] and free NHC of 12.0 and 9.3 kcal mol−1 in benzene and difluorobenzene, respectively (Scheme 2). This bond lability may imply that [2][BArF] may be capable of reacting as a two coordinated stannyliumylidene, upon dissociation of an IMe4 ligand in solution.
 | ||
| Scheme 2 Calculated free energies for anion cation interaction and for NHC dissociation in [2][BArF]. | ||
Hydrosilylation of CO2 by stannyliumylidene
Inspired by the successful hydrosilylation of CO2 catalyzed by germyliumylidene (K),34 we attempted to investigate the catalytic potential of [2][BArF] in the same reaction.We found the optimal conditions for the catalytic reaction of CO2 and diphenylsilane at room temperature in the presence of 5 mol% [2][BArF] in the C6D6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6H5F (4
C6H5F (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent mixture. According to the 1H NMR spectrum, the consumption of the majority (94%) of the Ph2SiH2 was observed within 25 hours, resulting in silyl formate, Ph2SiH(OCHO), which is then transformed into silylated methanol (Table 1, Entry 1) (Fig. S35†). The catalyst [2][BArF] exhibited poor solubility in benzene, which led to lower catalytic efficiency (Table, Entry 2). To further investigate the impact of polar solvents on the CO2 hydrosilylation catalyzed by [2][BArF], 1,2-difluorobenzene (C6H4F2) (Table 1, Entry 4) in different ratios with C6D6 (Table 1, Entry 5) were selected as reaction solvents. The results indicated that polar solvents did not participate in the hydrosilylation reaction mechanism; their role was limited to enhancing the solubility of [2][BArF]. In comparison to fluorobenzene, the use of THF, a donor solvent, resulted in lower conversion (Table 1, Entry 3). Elevated temperature accelerated the catalytic rate, achieving 64% conversion in 2 hours (Table 1, Entry 6). However, the catalyst decomposed rapidly under these conditions (Fig. S40†). Furthermore, to examine the effect the counter anion has on the conversion we performed this reaction under the same conditions with [2][Al(OC(CF3)3)4], and a decrease in activity was observed (Table 1, Entry 10). This decrease in catalytic activity could indicate that the very weak coordination strength between Al(ORf)4− and the Sn atom increases the electrophilicity of the Sn center, which suppresses NHC dissociation from Sn center, compared to BArF−.39 Furthermore, the hydrosilylation of CO2, catalyzed by the [2][BArF], exhibited minimal perturbation in the presence of excess mercury (Hg), strongly suggesting the nature of the catalytic cycle is homogeneous (Table 1, Entry 15).
1) solvent mixture. According to the 1H NMR spectrum, the consumption of the majority (94%) of the Ph2SiH2 was observed within 25 hours, resulting in silyl formate, Ph2SiH(OCHO), which is then transformed into silylated methanol (Table 1, Entry 1) (Fig. S35†). The catalyst [2][BArF] exhibited poor solubility in benzene, which led to lower catalytic efficiency (Table, Entry 2). To further investigate the impact of polar solvents on the CO2 hydrosilylation catalyzed by [2][BArF], 1,2-difluorobenzene (C6H4F2) (Table 1, Entry 4) in different ratios with C6D6 (Table 1, Entry 5) were selected as reaction solvents. The results indicated that polar solvents did not participate in the hydrosilylation reaction mechanism; their role was limited to enhancing the solubility of [2][BArF]. In comparison to fluorobenzene, the use of THF, a donor solvent, resulted in lower conversion (Table 1, Entry 3). Elevated temperature accelerated the catalytic rate, achieving 64% conversion in 2 hours (Table 1, Entry 6). However, the catalyst decomposed rapidly under these conditions (Fig. S40†). Furthermore, to examine the effect the counter anion has on the conversion we performed this reaction under the same conditions with [2][Al(OC(CF3)3)4], and a decrease in activity was observed (Table 1, Entry 10). This decrease in catalytic activity could indicate that the very weak coordination strength between Al(ORf)4− and the Sn atom increases the electrophilicity of the Sn center, which suppresses NHC dissociation from Sn center, compared to BArF−.39 Furthermore, the hydrosilylation of CO2, catalyzed by the [2][BArF], exhibited minimal perturbation in the presence of excess mercury (Hg), strongly suggesting the nature of the catalytic cycle is homogeneous (Table 1, Entry 15).
| Entry | Deviation from the standard conditionsa | Conversionb (%) |
|---|---|---|
a Standard reaction conditions: H2SiPh2 (80 μmol), 1,3,5-trimethoxylbenzene (8 μmol, internal standard), and 5 mol% [2][BArF] catalysis in C6D6 (0.32 mL) and C6H5F (0.08 mL) under 1 bar CO2 at RT for 25 hours.
b NMR yield was monitored by 1H NMR with methoxybenzene as an internal standard.
c Refer to Scheme 3 for the synthesis of [3][BArF] and [4][BArF].
d Hg to catalyst ratio 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
||
| 1 | None | 94 |
| 2 | C6D6 | 78 |
| 3 | THF-d8 | 75 |
| 4 | C6D6/C6H4F2 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
97 |
| 5 | C6D6/C6H4F2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
94 |
| 6 | 50 °C | 64 (2 hours) |
| 7 | 0.5 mol% | 4 |
| 8 | 1 mol% | 11 |
| 9 | 2.5 mol% | 74 |
| 10 | [2][Al(OC(CF3)3)4] | 70 |
| 11 | [3][BArF]c | 92 |
| 12 | [4][BArF]c | 93 |
| 13 | IMe4 (10 mol%) | 51 |
| 14 | IMe4–CO2 (10 mol%), C6D6/C6H4F2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
51 |
| 15 | Hgd | 89 |
In addition to diphenylsilane we tested the catalytic performance of [2][BArF] toward a few other silanes (Table 2). In the case of HSiMe2Ph (Table 2, Entry 2), the corresponding silyl formate is formed with a 47% conversion within 25 hours. The catalyst deactivated after consumption of 71% of phenylsilane within 12 hours (Entry 5). The more sterically demanding silanes, HSiPh3 and HSiEt3, were less effective in the hydrosilylation of CO2 even at elevated temperatures (Table 2, Entries 4 and 5).
| Entry | Silanesa | T (°C) | Time (h) | Conversionb (%) |
|---|---|---|---|---|
a Standard reaction conditions: H2SiPh2 (80 μmol), 1,3,5-trimethoxylbenzene (8 μmol, internal standard), and 5 mol% [2][BArF] catalysis in C6D6 (0.32 mL) and C6H5F (0.08 mL) under 1 bar CO2 at RT for 25 hours.
b NMR yield was monitored by 1H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NMR with methoxybenzene as an internal standard.
c Refer to Scheme 3 for the synthesis of [3][BArF] and [4][BArF].
d Hg to catalyst ratio 250 NMR with methoxybenzene as an internal standard.
c Refer to Scheme 3 for the synthesis of [3][BArF] and [4][BArF].
d Hg to catalyst ratio 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
||||
| 1 | H2SiPh2 | RT | 25 | 94 |
| 2 | HSiMe2Ph | RT | 25 | 47 |
| 3 | H3SiPh | RT | 12c | 71 |
| 4 | HSiEt3 | 60 | 25 | 29 |
| 5 | HSiPh3 | 80 | —d | — |
[2][BArF] demonstrated superior performance in CO2 hydrosilylation compared to certain Lewis acids, such as [Et2Al]+ and [Et3Si]+.14 However, it was slightly less effective than [(2,6-Mes2C6H3O)2Al]+, [2,6-Ph2C6H3AlEt]+, and [(2,6-Dipp2C6H3)GaEt]+ at elevated temperatures, which produced methane, toluene, and diphenylmethane as products.15,16 [2][BArF] was less effective than some FLP catalytic systems, such as the N,P-heterocyclic germylene/B(C6F5)3 pair (H),22 and {[TismPribenz]M}[HB(C6F5)3] (M = Zn, Mg),19 which selectively produced bis(silyl)acetals or methane. Its performance was comparable to that of germa-acylium ions and germyliumylidene (L), which yielded silyl formate, bis(silyl)acetal, and silylated methanol.33,34
Although [2][BArF] exhibits moderate catalytic ability for CO2 hydrosilylation, its ability to selectively reduce CO2 to silyl formate in a one-step process is valuable for studying the mechanism of CO2 hydrosilylation.
Mechanistic studies
To study the mechanism of the hydrosilylation of CO2 catalyzed by [2][BArF], stoichiometric reactivity studies were conducted. Exposing [2][BArF] to 1 equivalent of CO2 in toluene:C6H5F (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature, afforded the mono-insertion product [MesTerSn(CO2IMe4)IMe4][BArF], [3][BArF], after 4 hours. It bears one CO2 molecule inserted between the Sn center and one of the IMe4 ligands. It has increased solubility in nonpolar organic solvents such as benzene and toluene compared to [2][BArF]. The 119Sn{1H} NMR spectrum of [3][BArF] shows a resonance at δ = −10.61 ppm, which is downfield shifted compared to [2][BArF], due to the Sn being bound to the more electronegative oxygen atom. The 13C{1H} resonance of the corresponding carboxylate (IMe4–C(O)OSn) appears at δ = 157.48 ppm (THF-d8), which is comparable to the reported NHC–CO2 adducts, i.e., IPrCO2 (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazole-2-ylidene) (δ = 152.3 ppm, CD2Cl2), IMeCO2 (IMe = 1,3-dimethylimidazolium) (δ = 158 ppm, D2O),40 ItBuCO2 (ItBu = 1,3-di-tert-butyl-4,5-dimethylimidazolin-2-ylidene) (δ = 160 ppm, CD2Cl2), IMe4CO2 (δ = 155.9 ppm, CD2Cl2).41
1) at room temperature, afforded the mono-insertion product [MesTerSn(CO2IMe4)IMe4][BArF], [3][BArF], after 4 hours. It bears one CO2 molecule inserted between the Sn center and one of the IMe4 ligands. It has increased solubility in nonpolar organic solvents such as benzene and toluene compared to [2][BArF]. The 119Sn{1H} NMR spectrum of [3][BArF] shows a resonance at δ = −10.61 ppm, which is downfield shifted compared to [2][BArF], due to the Sn being bound to the more electronegative oxygen atom. The 13C{1H} resonance of the corresponding carboxylate (IMe4–C(O)OSn) appears at δ = 157.48 ppm (THF-d8), which is comparable to the reported NHC–CO2 adducts, i.e., IPrCO2 (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazole-2-ylidene) (δ = 152.3 ppm, CD2Cl2), IMeCO2 (IMe = 1,3-dimethylimidazolium) (δ = 158 ppm, D2O),40 ItBuCO2 (ItBu = 1,3-di-tert-butyl-4,5-dimethylimidazolin-2-ylidene) (δ = 160 ppm, CD2Cl2), IMe4CO2 (δ = 155.9 ppm, CD2Cl2).41
The SC-XRD analysis of [3][BArF] revealed that the central Sn is tricoordinate with an IMe4 ligand, a terphenyl substituent, and an imidazolium carboxylate (Fig. 7(a)). The Sn1–O1 bond length of 2.231(2) Å is longer than that of the reported Gibson's (Dippnacnac)SnO(iPr), (2.000(5) Å),42 tin-carbamate complex ((Dippnacnac)SnOC(O)N(iPr2)) (2.1346(16) Å)43 and shorter than (ArNEt2Ge)(μ-k1(C)-k2(O,O′)-CO2)(SnArNEt2) (2.301(3), 2.336(3) Å), (ArNEt2 = [2,6-(Et2NCH2)2C6H3]).44 The C–O bond lengths (C39–O1 = 1.263(4), C39–O2 = 1.211(4) Å) with the C–O moiety bound to the Sn center being the longer of the two, are longer than those in free CO2 (1.16 Å). This is also in the same range as other imidazolium carboxylates,45 IPrCO2 (1.221(4) and 1.225(4) Å)46 and ItBuMe-CO2-B(C6F5)3 (ItBuMe = 1,3-di-tert-butyl-4,5-dimethylimidazolin-2-ylidene) (1.302(3) and 1.211(3) Å).46 The C–C bond length between the IMe4 and CO2 is 1.508(5) Å in [3][BArF], which is close to the reported imidazolium carboxylates,41,45,46 IPrCO2 (1.511(4) Å), IMe4CO2 (1.521 Å) and IEtMeCO2 (IEtMe = 1,3-diethyl-4,5-methyl-imidazolium) (1.535(15) Å). In addition, the carboxylic carbon atoms exhibit a trigonal-planar coordination environment, with the bond angles of 126.5(3)° for O1–C32–O2, 116.3(3)° for C33–C32–O2 and 117.0(3)° for C33–C32–O1.
The addition of excess CO2 (1 bar) to a solution of [2][BArF] in Et2O and pentane at room temperature results in the doubly CO2 inserted product [MesTerSn(CO2IMe4)2][BArF], [4][BArF], after 21 hours. Yellow crystals suitable for SC-XRD were obtained from an Et2O/pentane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) solution stored at −35 °C under a CO2 atmosphere. The 119Sn{1H} NMR spectrum of [4][BArF], shows a sharp singlet signal at δ = 55.9 ppm, which is shifted downfield compared to [2][BArF] and [3][BArF], due to the Sn center now being bound to two electronegative O atoms. The resonance of the carboxyl carbon atom appears at δ = 157.48 ppm in the 13C{1H} NMR spectrum, which is consistent with [3][BArF]. The SC-XRD of [4][BArF] (Fig. 7(b)) shows that the bond lengths of Sn1–O1 and Sn1–O3 are 2.168(2) Å and 2.230(2) Å, respectively. The C–O bond lengths are as follows: C39–O1 (1.270(3) Å), C40–O3 (1.279(2) Å) and C39–O2 (1.223(3) Å), C40–O4 (1.220(3) Å). The lengths of C–C bonds between the IMe4 carbon and the CO2 group C39–C25 (1.450(1) Å) and C40–C32 (1.522(5) Å) are different compared to [3][BArF] (1.508(5) Å), but comparable to imidazolium carboxylates.41,45,46
2) solution stored at −35 °C under a CO2 atmosphere. The 119Sn{1H} NMR spectrum of [4][BArF], shows a sharp singlet signal at δ = 55.9 ppm, which is shifted downfield compared to [2][BArF] and [3][BArF], due to the Sn center now being bound to two electronegative O atoms. The resonance of the carboxyl carbon atom appears at δ = 157.48 ppm in the 13C{1H} NMR spectrum, which is consistent with [3][BArF]. The SC-XRD of [4][BArF] (Fig. 7(b)) shows that the bond lengths of Sn1–O1 and Sn1–O3 are 2.168(2) Å and 2.230(2) Å, respectively. The C–O bond lengths are as follows: C39–O1 (1.270(3) Å), C40–O3 (1.279(2) Å) and C39–O2 (1.223(3) Å), C40–O4 (1.220(3) Å). The lengths of C–C bonds between the IMe4 carbon and the CO2 group C39–C25 (1.450(1) Å) and C40–C32 (1.522(5) Å) are different compared to [3][BArF] (1.508(5) Å), but comparable to imidazolium carboxylates.41,45,46
The isolation of large quantities of pure solid [4][BArF] is not possible as it decomposes to [3][BArF] under reduced pressure via the release of one molecule of CO2.
To study the stability of [4][BArF] in solution, 1H NMR-monitoring experiments were conducted in a toluene-d8/C6H5F solution under a CO2 atmosphere (Fig. S29†). At 100 °C, [4][BArF] slowly converts to [3][BArF], releasing one equivalent of CO2. Leaving the sample at ambient temperature for 15 hours resulted in the reformation of [4][BArF].
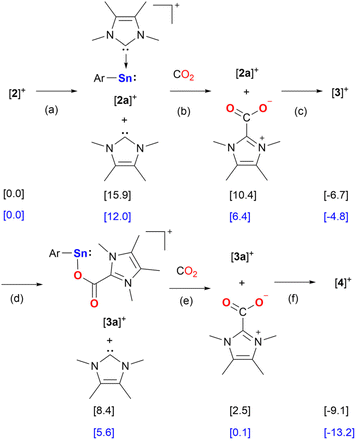
Calculations at the PW6B95-D4/def2-QZVPP(CPCM)/r2SCAN-3c level of theory show that the likely scenario for the formation of [3][BArF] and [4][BArF] is a stepwise reaction, in which the consecutive dissociation of a IMe4 ligands from the Sn center of [2][BArF] occurs (Scheme 4). The IMe4 ligands react with carbon dioxide to form the imidazolium carboxylates and reassociate with the Sn complex (Scheme 4). The IMe4 dissociation from [2][BArF] is endergonic by 12.0 kcal mol−1 and proceeds without a barrier yielding [2a][BArF] and free IMe4 (Scheme 4, path (a)). The IMe4 reacts with a molecule of CO2 to form the IMe4–CO2 imidazolium carboxylate at 6.4 kcal mol−1 (Scheme 4, path (b)). The barrier for the reaction of IMe4 with CO2 is only 9.3 kcal mol−1. [3][BArF] forms upon barrierless association of IMe4–CO2 with [2a][BArF] at −4.8 kcal mol−1 (Scheme 4, path (c)). We also considered a mechanism in which [2][BArF] reacts with CO2 concertedly, without IMe4 dissociation, to give [3][BArF]. However, this scenario requires overcoming a barrier of 29.6 kcal mol−1 (Fig. S57†). The formation of [4][BArF] proceeds via similar steps from [3][BArF], i.e. dissociation of the IMe4 from [3][BArF] giving [3a][BArF] + IMe4 at 5.6 kcal mol−1 (Scheme 4, path (d)), followed by the formation of the imidazolium carboxylate at 0.1 kcal mol−1 (Scheme 4, path (e)), and finally association of [3a][BArF] with IMe4–CO2 to give [4][BArF] at −13.2 kcal mol−1 (Scheme 4, path (f)).
Notably, comparison of the spectroscopic data indicated the formation of [3][BArF] and [4][BArF] during the [2][BArF] catalyzed hydrosilylation of CO2 (Fig. S35†). Furthermore, [4][BArF] could be converted to [3][BArF] by adding Ph2SiH2 under an argon atmosphere, with the simultaneous formation of silyl formate (Fig. S31†). DFT calculations for the proposed mechanism of this transformation were performed (see Fig. S58†). Conversely, [3][BArF] decomposed to unidentified compounds when treated with Ph2SiH2 at room temperature. Both [3][BArF] and [4][BArF] are efficient catalysts for CO2 hydrosilylation (Table 1, Entries 11 and 12), comparable to the performance of [2][BArF]. Thus, we propose [4][BArF] as the resting state of the active cycle. Furthermore, control experiments using IMe4 and IMe4–CO2 as catalysts both show lower conversion (Table 1, Entries 13 and 14), suggesting that these compounds are not the catalytically active species.
Based on our reactivity studies and the quantum chemical calculations (Scheme 4, Fig. S57 and S58†) a mechanism for the catalytic hydrosilylation of CO2 using [2][BArF] is proposed (Scheme 5). Under catalytic conditions, the stannyliumylidene [2]+ reacts with two equivalents of carbon dioxide to form the double insertion product, [4]+, via [3]+, as described above (Scheme 5, step (a)). Compound [4]+ reacts with a molecule of diphenylsilane to form intermediate INT_A (Scheme 5, step (b)), which dissociates to the ((silylcarbonyl)oxy)imidazolium intermediate (INT_B) and the stannyl hydride (INT_C). The former can abstract a hydride from the stannyl hydride, releasing the tin complex [3a]+ and forming a zwitterionic adduct, INT_D (Scheme 5, step (d)). This type of adduct is well known in NHC organocatalysis, as similar species can form upon the reaction of NHC with an aldehyde, which can later convert to amino enols, known as Breslow intermediates.47 In this case the reverse reaction of the zwitterionic adduct releasing the diphenylsilyl formate product, and the NHC (Scheme 5, step (e)). The NHC reacts with an additional equivalent of CO2 forming the imidazolium carboxylate (Scheme 5, step (f)), which reassociates with tin complex [3a]+ to regenerate the starting compound [4]+ (Scheme 5, step (g)).
 | ||
| Scheme 5 Proposed catalytic cycle of hydrosilylation of carbon dioxide by diphenylsilane in the presence of stannyliumylidene [2]+. | ||
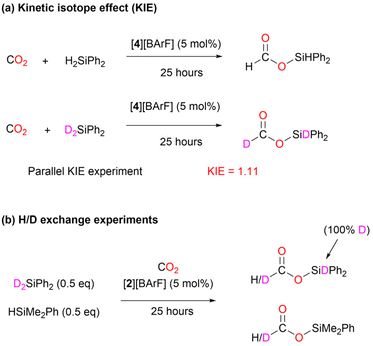
In addition to the isolation of the two key intermediates of the catalytic cycle ([3][BArF] and [4][BArF]) and the quantum chemical calculations, we carried out additional experiments (Scheme 6), using deuterated silanes (D2SiPh2), to support the reaction mechanism proposed. Under standard conditions, the rate of the reactions with H2SiPh2 and D2SiPh2 were compared, giving a kinetic isotope effect (KIE) of 1.11 (Scheme 6, (a)). This value indicates a secondary isotope effect, which is consistent with the rate-determining transition state TS4 in the proposed mechanism (Fig. S58†), in which the H/D atom is a substituent on carbon from which the NHC moiety is dissociated.48 Furthermore, CO2 hydrosilylation catalyzed by [2][BArF] was performed in the presence of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of D2SiPh2 and HSiMe2Ph (Scheme 6, (b)). H/D exchange was observed at the carbon center, supporting the formation of the tin-hydride intermediate INT_C (Scheme 5) in the proposed catalytic cycle.49 This intermediate can form either via deuterium abstraction from D2SiPh2 or via hydrogen abstraction from HSiMe2Ph. Subsequently, INT_C, whether D/H-substituted, can transfer deuterium or hydrogen to the ((silylcarbonyl)oxy)imidazolium INT_B, which can also from in the previous step from either D2SiPh2 or HSiMe2Ph. The H/D transfer from INT_C to INT_B will ultimately produce Ph2SiD(OCDO), Ph2SiD(OCHO), Me2PhSi(OCDO), and Me2PhSi(OCHO).
1 mixture of D2SiPh2 and HSiMe2Ph (Scheme 6, (b)). H/D exchange was observed at the carbon center, supporting the formation of the tin-hydride intermediate INT_C (Scheme 5) in the proposed catalytic cycle.49 This intermediate can form either via deuterium abstraction from D2SiPh2 or via hydrogen abstraction from HSiMe2Ph. Subsequently, INT_C, whether D/H-substituted, can transfer deuterium or hydrogen to the ((silylcarbonyl)oxy)imidazolium INT_B, which can also from in the previous step from either D2SiPh2 or HSiMe2Ph. The H/D transfer from INT_C to INT_B will ultimately produce Ph2SiD(OCDO), Ph2SiD(OCHO), Me2PhSi(OCDO), and Me2PhSi(OCHO).
Conclusions
In summary, we reported on the synthesis of a di-NHCs-stabilized stannyliumylidene [2][BArF], which can act as an efficient carbon dioxide hydrosilylation catalyst under ambient conditions. Both experimental and computational studies have been performed to elucidate the reaction mechanism, revealing it to be distinct from those previously proposed for the lighter germyliumylidenes.34 In the latter case, [MesTerGe(IMe4)2]+ (K, Fig. 2) was identified as the active catalytic species, with the Si–H bond activation occurring through coordination with the germanium lone pair (L, Fig. 2), which constitutes the rate-determining step. In comparison to K, [2][BArF] captures CO2via dissociation of NHC, followed by reassociation of the NHC–CO2 moiety to the Sn center, forming the catalytically active species, [4][BArF]. This divergent mechanism is another example of how the lighter and heavier congeners can behave differently and is something we hope to exploit in our further studies in applying tetryliumylidene cations to catalytic transformations.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
D. N. and D. S. carried out the synthetic and reaction studies, and D. N. wrote the original draft. A. K. carried out the computational studies. J. A. K. conducted the crystallographic studies. A. K., J. A. K, H. X., and D. S. reviewed and edited the draft. S. I. managed the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. N. gratefully acknowledges financial support from the China Scholarship Council (CSC). We thank Dr Franziska Hanusch and Fiona Kiefer for SC-XRD measurements. We appreciate Ivan Antsiburov (Prof. Roland A. Fischer) and Tobias Weng for the Measurement of LIFDI-MS. The authors gratefully acknowledge the computational and data resources provided by the Leibniz Supercomputing Centre.Notes and references
- J. Chen, M. McGraw and E. Y. Chen, ChemSusChem, 2019, 12, 4543–4569 CrossRef CAS PubMed
.
- S. Fujimori and S. Inoue, Eur. J. Inorg. Chem., 2020, 2020, 3131–3142 CrossRef CAS PubMed
.
- J. A. Kelly, F. J. Kiefer, A. Kostenko and S. Inoue, Adv. Inorg. Chem., 2023, 82, 157–187 CrossRef CAS
.
- R. Akhtar, K. Gaurav and S. Khan, Chem. Soc. Rev., 2024, 53, 6150–6243 RSC
.
- M. He, C. Hu, R. Wei, X. F. Wang and L. L. Liu, Chem. Soc. Rev., 2024, 53, 3896–3951 RSC
.
- S. Yadav, S. Saha and S. S. Sen, ChemCatChem, 2016, 8, 486–501 CrossRef CAS
.
- C. Shan, S. Yao and M. Driess, Chem. Soc. Rev., 2020, 49, 6733–6754 RSC
.
- D. Sarkar, L. Groll, D. Munz, F. Hanusch and S. Inoue, ChemCatChem, 2022, 14, e202201048 CrossRef CAS
.
- L. Groll, J. A. Kelly and S. Inoue, Chem.–Asian J., 2023, 19, e202300941 CrossRef PubMed
.
- S. N. Riduan, Y. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2009, 48, 3322–3325 CrossRef CAS PubMed
.
- F. Huang, G. Lu, L. Zhao, H. Li and Z.-X. Wang, J. Am. Chem. Soc., 2010, 132, 12388–12396 CrossRef CAS PubMed
.
- Q. Zhou and Y. Li, J. Am. Chem. Soc., 2015, 137, 10182–10189 CrossRef CAS PubMed
.
- A. Schafer, W. Saak, D. Haase and T. Muller, Angew. Chem., Int. Ed., 2012, 51, 2981–2984 CrossRef PubMed
.
- M. Khandelwal and R. J. Wehmschulte, Angew. Chem., Int. Ed., 2012, 51, 7323–7326 CrossRef CAS PubMed
.
- M. Saleh, D. R. Powell and R. J. Wehmschulte, Organometallics, 2017, 36, 4810–4815 CrossRef CAS
.
- R. J. Wehmschulte, M. Saleh and D. R. Powell, Organometallics, 2013, 32, 6812–6819 CrossRef CAS
.
- D. Mukherjee, D. F. Sauer, A. Zanardi and J. Okuda, Chem.–Eur. J., 2016, 22, 7730–7733 CrossRef CAS PubMed
.
- A. Berkefeld, W. E. Piers and M. Parvez, J. Am. Chem. Soc., 2010, 132, 10660–10661 CrossRef CAS PubMed
.
- M. Rauch and G. Parkin, J. Am. Chem. Soc., 2017, 139, 18162–18165 CrossRef CAS PubMed
.
- M. Rauch, Z. Strater and G. Parkin, J. Am. Chem. Soc., 2019, 141, 17754–17762 CrossRef CAS PubMed
.
- A. Caise, J. Hicks, M. Angeles Fuentes, J. M. Goicoechea and S. Aldridge, Chem.–Eur. J., 2021, 27, 2138–2148 CrossRef CAS PubMed
.
- N. Del Rio, M. Lopez-Reyes, A. Baceiredo, N. Saffon-Merceron, D. Lutters, T. Muller and T. Kato, Angew. Chem., Int. Ed., 2017, 56, 1365–1370 CrossRef CAS PubMed
.
- V. S. Swamy, S. Pal, S. Khan and S. S. Sen, Dalton Trans., 2015, 44, 12903–12923 RSC
.
- S. Stigler, S. Fujimori, A. Kostenko and S. Inoue, Chem. Sci., 2024, 15, 4275–4291 RSC
.
- S. L. Powley and S. Inoue, Chem. Rec., 2019, 19, 2179–2188 CrossRef CAS PubMed
.
- P. Jutzi, A. Mix, B. Rummel, W. W. Schoeller, B. Neumann and H.-G. Stammler, Science, 2004, 305, 849–851 CrossRef CAS PubMed
.
- P. Jutzi, F. Kohl, P. Hofmann, C. Krüger and Y. H. Tsay, Chem. Ber., 1980, 113, 757–769 CrossRef CAS
.
- P. Jutzi, R. Dickbreder and H. Nöth, Chem. Ber., 2006, 122, 865–870 CrossRef
.
- S. U. Ahmad, T. Szilvasi and S. Inoue, Chem. Commun., 2014, 50, 12619–12622 RSC
.
- S. U. Ahmad, T. Szilvasi, E. Irran and S. Inoue, J. Am. Chem. Soc., 2015, 137, 5828–5836 CrossRef CAS PubMed
.
- R. Nougue, S. Takahashi, A. Dajnak, E. Maerten, A. Baceiredo, N. Saffon-Merceron, V. Branchadell and T. Kato, Chem.–Eur. J., 2022, 28, e202202037 CrossRef CAS PubMed
.
- S. Stigler, M. Park, A. Porzelt, A. Kostenko, D. Henschel and S. Inoue, Organometallics, 2022, 41, 2088–2094 CrossRef CAS
.
- D. Sarkar, C. Weetman, S. Dutta, E. Schubert, C. Jandl, D. Koley and S. Inoue, J. Am. Chem. Soc., 2020, 142, 15403–15411 CrossRef CAS PubMed
.
- D. Sarkar, S. Dutta, C. Weetman, E. Schubert, D. Koley and S. Inoue, Chem.–Eur. J., 2021, 27, 13072–13078 CrossRef CAS PubMed
.
- C. P. Sindlinger, F. S. Aicher and L. Wesemann, Inorg. Chem., 2017, 56, 548–560 CrossRef CAS PubMed
.
- N. Kuhn, T. Kratz, D. Bläser and R. Boese, Chem. Ber., 1995, 128, 245–250 CrossRef CAS
.
- T. G. Kocsor, D. Matioszek, G. Nemes, A. Castel, J. Escudie, P. M. Petrar, N. Saffon and I. Haiduc, Inorg. Chem., 2012, 51, 7782–7787 CrossRef CAS PubMed
.
- T. Ochiai, D. Franz and S. Inoue, Chem. Soc. Rev., 2016, 45, 6327–6344 RSC
.
- I. M. Riddlestone, A. Kraft, J. Schaefer and I. Krossing, Angew. Chem., Int. Ed., 2018, 57, 13982–14024 CrossRef CAS PubMed
.
- J. D. Holbrey, W. M. Reichert, I. Tkatchenko, E. Bouajila, O. Walter, I. Tommasi and R. D. Rogers, Chem. Commun., 2003, 28–29 RSC
.
- E. Theuergarten, T. Bannenberg, M. D. Walter, D. Holschumacher, M. Freytag, C. G. Daniliuc, P. G. Jones and M. Tamm, Dalton Trans., 2014, 43, 1651–1662 RSC
.
- A. P. Dove, V. C. Gibson, E. L. Marshall, A. J. P. White and D. J. Williams, Chem. Commun., 2001, 283–284 RSC
.
- L. A. M. Harris, M. P. Coles and J. R. Fulton, Inorg. Chim. Acta, 2011, 369, 97–102 CrossRef CAS
.
- A. Caise, L. P. Griffin, C. McManus, A. Heilmann and S. Aldridge, Angew. Chem., Int. Ed., 2022, 61, e202117496 CrossRef CAS PubMed
.
- B. R. Van Ausdall, J. L. Glass, K. M. Wiggins, A. M. Aarif and J. Louie, J. Org. Chem., 2009, 74, 7935–7942 CrossRef CAS PubMed
.
- H. A. Duong, T.
N. Tekavec, A. M. Arif and J. Louie, Chem. Commun., 2004, 112–113 RSC
.
- M. Pareek, Y. Reddi and R. B. Sunoj, Chem. Sci., 2021, 12, 7973–7992 RSC
.
- D. Bai, F. Wu, L. Chang, M. Wang, H. Wu and J. Chang, Angew. Chem., Int. Ed., 2022, 61, e202114918 CrossRef CAS PubMed
.
- Z. Cheng, M. Li, X. Y. Zhang, Y. Sun, Q. L. Yu, X. H. Zhang and Z. Lu, Angew. Chem., 2023, 135, e202215029 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2255434, 2255353 and 2255355. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc07116f |
| This journal is © The Royal Society of Chemistry 2025 |