Open Access Article
Open Access ArticleColloidally uniform single-crystal precursors enable uniform FAPbI3 films for efficient perovskite submodules†
Yugang
Liang
a,
Yingping
Fan
a,
Zhixiao
Qin
b,
Lei
Lu
a,
Haifei
Wang
a,
Meng
Ren
a,
Fang
Liu
a,
Yanfeng
Miao
ac,
Yuetian
Chen
 *ac and
Yixin
Zhao
*ac and
Yixin
Zhao
 *acd
*acd
aSchool of Environmental Science and Engineering, Frontiers Science Center for Transformative Molecules, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: yixin.zhao@sjtu.edu.cn; yuetian.chen@sjtu.edu.cn
bShanghai Perovskite Technology Co., Ltd, Shanghai 201109, China
cShanghai Non-carbon Energy Conversion and Utilization Institute, Shanghai 200240, China
dState Key Lab of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200240, China
First published on 20th January 2025
Abstract
With the unprecedented research development on lead halide perovskite photovoltaics, scaling up fabrication while comprehensively understanding the properties of cost-effective and highly uniform precursor films has become critical for their practical application. When enlarging the device area, good precursor purity serves as the first step in ensuring the uniformity of the perovskite film. Chemical purity and colloidal uniformity in the precursor solution both play important roles in dictating film uniformity and defect density. Here, for the first time, we explored the colloidal behavior of FAPbI3 precursors using different preparatory materials of varied costs but with similar metal purity. As the colloidal size of regular PbI2 + FAI powder precursors increased compared to that of PbI2 colloids, the FAPbI3 single-crystal precursor synthesized from low-purity chemicals exhibited a generally smaller and more uniform colloidal size, which yielded perovskite films with improved uniformity and reduced defect density at lower cost. The colloidally uniform single-crystal precursors led to photovoltaics with higher power conversion efficiency and better long-term operational stability. More importantly, the uniformity in the precursor and film was found to be beneficial for large-area fabrication, where the scaling-up production of 30 cm × 30 cm perovskite submodules based on single-crystal precursors achieved an impressive 20.7% efficiency.
Introduction
As a new generation of solar cells with bright industrialization prospects, perovskite solar cells have reached efficiency milestones comparable to crystalline silicon cells.1–4 Up to now, the solution-based method, with its low manufacturing cost and high processability, has enabled the power conversion efficiency (PCE) of perovskite solar cells to reach 26.7%.5–9 Specifically, the material formamidinium lead iodide (FAPbI3) exhibits an optimal bandgap close to the Shockley–Queisser limit, showing great potential and excellent stability.10,11 Unfortunately, for scaling-up manufacturing, the current heavy reliance on expensive high-purity lead halide and alkylammonium halide precursors could be a burden.12–14 Meanwhile, using low-purity precursors, such as 98% lead iodide, is considered to have adverse effects on the crystallinity and charge carrier lifetime of the prepared perovskite films.15–17 Moreover, efficiency loss from small-scale perovskite devices to large-area modules, along with poor reproducibility, is often reported even when using high-purity precursors.18–22 Previous studies have also identified that good control of precursor chemistry enables the fabrication of pinhole-free and compact large-area perovskite films.23–26 However, achieving spatial uniformity of perovskite films using precursor powder mixtures remains challenging as the area increases.27 The obstacle to scalability lies in the inhomogeneous distribution of impurities, composition variations and defect density across different regions within the perovskite film.26,28 Therefore, elucidating the relationship between solution chemistry and film uniformity is crucial for ensuring scalability in industrial applications.Perovskite single crystals (SCs) of various compositions, such as MAPbI3,27,29 FAPbI3,30,31 FASnI3,32 and even two-dimensional perovskites,33,34 with high purity and accurate stoichiometric ratios, have been used as precursor materials to ensure the reproducibility and cost-effectiveness of the fabricated photovoltaic devices.6,35–37 The films prepared from perovskite SCs inherit the materials' notable characteristics, such as high crystallinity, high purity, accurate stoichiometry, low trap density, and good stability.28,32,38,39 However, a fundamental understanding of the solution chemistry of perovskite single-crystal precursors has not yet been well explored, which fails to provide constructive guidance for further improving film quality and device performance.
In this work, uniform FAPbI3 perovskite films and devices of large areas are well achieved based on colloidally uniform single-crystal precursors. The economic viabilities of different FAPbI3 precursors, including the PbI2 + FAI powder and FAPbI3 SCs, are investigated. The photovoltaic performance of the FAPbI3 devices prepared using low-cost single crystals is compared to those prepared using halide salts with a purity exceeding 99.999%. The colloidal and chemical properties of these FAPbI3 perovskite precursors are studied to elucidate the key factors contributing to the observed variations in the photovoltaic performance. The reproducibility and scalability of the FAPbI3 perovskite films and devices based on these precursor materials are also explored. The challenge of inhomogeneity that arises during the scaling up of perovskite film fabrication is addressed by utilizing FAPbI3 perovskite single-crystal precursors with the underlying colloidal behavior elucidated. More encouragingly, the feasibility of the colloidally uniform single-crystal precursor for scale-up promotion is demonstrated with an impressive 20.7% efficiency achieved on 30 cm × 30 cm (aperture area: 663 cm2) perovskite submodules.
Results and discussion
Performance of FAPbI3 single crystal-based films and devices
First, black-phase (α-) and yellow-phase (δ-) FAPbI3 SCs were synthesized by inverse temperature crystallization (scheme in Fig. S1†) using just low-purity materials of 98% PbI2 + synthesized FAI.40,41 No impurities peaks were detected in the XRD patterns for both the δ-FAPbI3 and α-FAPbI3 SCs, confirming the high purity of the synthesized single crystals (Fig. S2†). To further assess the metal impurities in PbI2 + FAI and single-crystal precursors, inductively coupled plasma optical emission spectrometry (ICP-OES) was utilized for the quantitative analysis of metal elements, such as Ca, K, and Na, which have been found to exert adverse effects on perovskite solar cell.42 As shown in Fig. 1a, the feasibility of producing high-purity perovskite single crystals from low-purity preparatory materials is demonstrated by the significant inhibition of metallic residues. The amounts of these impurities in both the synthesized yellow- and black-phase perovskite single crystals are only subtly different from those found in the high-purity materials of 99.999% PbI2 + 99.5% FAI, indicating the high purity of the single crystals. The nature of the perovskite SC synthesis can be regarded as a thermodynamically driven self-purification process.43,44 Moreover, the mother liquor's high tolerance to impurities enables its recyclability for the production of single crystals. The purity of single crystals obtained from the circulating mother liquor was found to remain consistent with those obtained from the fresh solution (Fig. S3†). Cost analysis was conducted to assess the suitability of this SC-based method for commercial scale-up production, where the approximate cost difference when using single-crystal precursors was compared to the existing precursors (Tables S1 and S2†). Considering the effect of temperature on the life of mother liquor, we estimated FAPbI3 SCs at $0.2478 per gram (δ-FAPbI3 SCs for 15 cycles) and $0.2492 per gram (α-FAPbI3 SCs for 10 cycles), while low- and high-purity PbI2 + FAI are $0.196 and $37.7 per gram, respectively (Fig. 1a). The implementation of the single-crystal precursor strategy has resulted in a remarkable reduction of about 150-fold in the manufacturing costs of PV devices.To ascertain the comparability of the FAPbI3 single-crystal precursor prepared from low-purity materials with conventional high-purity PbI2 + FAI precursors, we prepared perovskite solar cells as the n–i–p configuration illustrated in Fig. S4.† The performance of perovskite solar cells based on traditional precursors PbI2 + FAI is severely limited by the purity of lead iodide, which undoubtedly increases production costs (Fig. S5†). The presence of residual elements, such as Ca, K and Na, in the precursor can significantly compromise the performance of the device, which aligns with previous findings.42 The encouraging discovery is that the FAPbI3 SC-based perovskite solar cells with an area of 0.085 cm2 exhibited greatly enhanced performance compared to those utilizing PbI2 + FAI despite possessing similar purity of the precursors. Fig. 1b shows the current density (J)–voltage (V) curves of the PV devices based on α-FAPbI3 and δ-FAPbI3 SCs, where higher PCEs of 25.0% and 23.8% are exhibited when compared to 22.7% of the device based on high-purity PbI2 + FAI. Moreover, the α-FAPbI3 SC-based devices exhibit the lowest hysteresis index (HI), with a device Voc of 1.17 V, short-circuit current density (Jsc) of 26.0 mA cm−2, and fill factor (FF) of 82.4% (Fig. S6†). Better reproducibility of the devices fabricated from FAPbI3 SCs is also revealed herein (Fig. S7†). The averaged open-circuit voltage (Voc) value of these typical PV devices increases from 1.11 V for PbI2 + FAI, to 1.14 V and 1.15 V for δ-FAPbI3 and α-FAPbI3 SCs, respectively (Fig. S8†). By integrating the external quantum efficiency (EQE) over the AM 1.5G standard spectrum, the projected Jsc of the PbI2 + FAI, δ-FAPbI3 and α-FAPbI3 SC-based PSCs are 25.00, 25.38 and 25.46 mA cm−2, respectively, which match well with the measured Jsc (Fig. S9†). Considering the manufacturing cost that relates to chemical purity, it is also worth noting that the PCE of the FAPbI3 SC-based device does not significantly improve when using lead iodides of higher purity for the preparation of FAPbI3 SCs (Fig. S10†). Moreover, Fig. S11† shows the stabilized power output (SPO) of the test device at the maximum power point. The same trend is that the maximum SPO value for the α-FAPbI3 SC-based device is 24.66%, which is higher than that of 23.57% for the δ-FAPbI3 SC-based device or 22.40% for the PbI2 + FAI-based device. We monitored the operational stability of the devices using maximum power point (MPP) tracking under simulated 1-sun illumination, as depicted in Fig. 1c. The PCEs of devices based on α-FAPbI3 and δ-FAPbI3 SCs retained about 94% and 81% of their initial PCE after 500 h of MPP tracking, respectively, while that of PbI2 + FAI rapidly declined to 75%.
The improvement in Voc and stability of the devices could be ascribed to the reduction of defects in the perovskite film. Therefore, steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) measurements were conducted to examine the trap density of the FAPbI3 perovskite films prepared from different precursors. As shown in Fig. S12† and 1d, for the low- and high-purity PbI2 + FAI precursors, the impurity concentration in the precursor significantly impacts both the PL intensity and carrier lifetime of the film. The reduction in calcium and sodium ions in the conventional PbI2 + FAI precursor leads to fewer carrier trap states.42 For the FAPbI3 films based on both δ-FAPbI3 and α-FAPbI3 SC precursors, the PL intensities of the peak at around 810 nm are much stronger than those of high-purity PbI2 + FAI. According to the TRPL data in Fig. 1d and Table S3,† the PL lifetime (τ) of α-FAPbI3 SC-based film is much longer (122.82 ns) than that of δ-FAPbI3 SC (88.89 ns) and high-purity PbI2 + FAI (73.58 ns). The enhancement of PL intensity and lifetime indicates suppressed non-radiative recombination and reduced defect density in the FAPbI3 SC precursor films. These results suggest that, apart from the metal ion purity, there must be some other differences in the properties between PbI2 + FAI and FAPbI3 single-crystal precursors to cause discrepancies in performance.
Origin of different FAPbI3 precursor properties
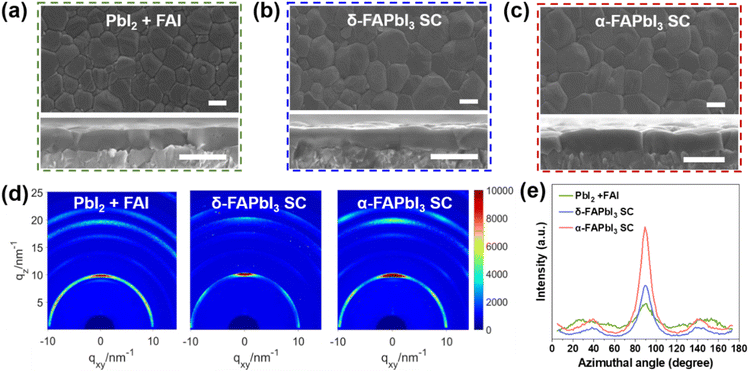
To investigate the factors that contribute to the superiority of single-crystal-based device over traditional high-purity PbI2 + FAI device, structural properties of the deposited perovskite films were analyzed by X-ray diffraction (XRD), scanning electron microscopy (SEM) and grazing-incidence wide-angle X-ray scattering (GIWAXS). Compared with the one based on PbI2 + FAI precursor, the films prepared by δ-FAPbI3 and α-FAPbI3 SC precursors exhibit better crystallinity and higher phase purity (Fig. S13†). In addition, the residual lattice strain of the film can be evaluated by applying the Williamson–Hall (W–H) method45,46 with data extracted from the XRD patterns. As the plots shown in Fig. S14,† the δ-FAPbI3 and α-FAPbI3 SC-based films exhibit lower lattice strain compared to the film based on PbI2 + FAI. Moreover, the SEM images in Fig. 2a–c demonstrate that the FAPbI3 SC precursor films exhibit larger and more uniform grain size (Fig. S15†), as well as smoother surface morphology compared to the PbI2 + FAI precursor. Cross-sectional images also demonstrate that the single-crystal precursor can enable films with larger, intact and vertically grown grains. The GIWAXS results are shown in Fig. 2d. While PbI2 residue (scattering vector of q = 9 nm−1) is observed in the sample of PbI2 + FAI film, the films based on single-crystal precursors exhibit a pure α-FAPbI3 phase from either δ-FAPbI3 or α-FAPbI3 SCs. Fig. 2e shows that the single-crystal precursor films display a sharper peak near the azimuth angle of 90° after integrating the (100) diffraction intensity from the GIWAXS data, suggesting a more preferred crystallization along the vertical direction in these perovskite films. The results suggest that the different FAPbI3 perovskite precursors, although with similarly high purity, could lead to the deposition of perovskite films exhibiting diverse structural profiles.Comprehending the crystallization pathways of the different precursors is essential for identifying the causes of the distinct properties. As previously reported, the perovskite precursor solution is essentially a colloidal solution.47 Therefore, to better study the FAPbI3 single-crystal precursor and the traditional Pb2 + FAI precursor, the high-purity single crystals of δ-FAPbI3 and α-FAPbI3 were dissolved in solvents for use. Fig. 3a shows the UV-vis absorption spectra of the diluted precursor solutions that were prepared from PbI2, PbI2 + FAI mixture, δ-FAPbI3 and α-FAPbI3 SCs. The characterization was conducted immediately following the complete dissolution of different FAPbI3 materials. The PbI2 precursor exhibits a unique absorption peak at around 325 nm, which is considered the signature of low-valent iodoplumbate.48,49 However, in the PbI2 + FAI precursors, a gradual reduction in the intensity of this peak is observed with the increase in the FAI ratio (Fig. S16†), while the intensity on the characteristic peak of high-valent iodoplumbate at around 370 nm increases owing to the stronger coordination between Pb2+ and the incoming I− ions.49,50 Nevertheless, PbI2 colloid with lower valence is still the dominant type of iodoplumbate in PbI2 + FAI precursor. Interestingly, the absorption spectra of both δ-FAPbI3 and α-FAPbI3 SC precursor solutions exhibit only one signature peak of high-valent iodoplumbate at 370 nm, indicating that the chemical composition of the colloids in the precursor is highly uniform. Moreover, some studies have suggested that a high percentage of high-valent iodoplumbate in the precursor could act as the structural framework of the perovskite during crystallization, which would lead to better morphology and higher crystallinity of the deposited film.27,29,32,33,51 Therefore, we then explored whether the FAPbI3 precursors based on the PbI2 + FAI mixture and FAPbI3 SCs exhibit different colloidal behavior.
Dynamic light scattering (DLS) was used to measure the particle size distribution of the precursor. As shown in the profiles in Fig. 3b, the colloid size in the PbI2 precursor has a limited range of 682–1398 nm, while that of the PbI2 + FAI precursor varies significantly from 630 nm to 7000 nm, indicating an increase in the size of colloids in the PbI2 + FAI precursor compared to PbI2. The increase in colloid size and distribution range can be ascribed to the coordination between I− and PbI2 colloids to form iodoplumbate clusters. Different from the PbI2 + FAI precursor, the δ-FAPbI3 and α-FAPbI3 SC precursors generally exhibit smaller colloids and a much narrower size distribution. The smaller colloid size is attributed to the dominance of the high-valent iodoplumbate with the highly uniform chemical composition,52 suggesting that the dissolution of SCs into precursor solution exhibits a different colloidal behavior from the PbI2 + FAI precursor.
These results confirm that uniform colloids containing high-valent iodoplumbate in FAPbI3 SC precursor solutions serve as the structural framework for perovskite growth and facilitate the film formation of higher crystallinity. Meanwhile, the uniform distribution of colloids could yield crystal nuclei with similar growth kinetics during perovskite film formation.53 Based on these results, it is concluded that in the FAPbI3 SC precursors, the highly crystalline colloids composed of high-valent iodoplumbate could better promote the growth of highly ordered and uniform crystal grains, as revealed by the GIWAXS results and SEM images. Therefore, we propose a crystallization mechanism based on the behaviors of different FAPbI3 precursors, as shown in Fig. 3c. For the PbI2 + FAI, the precursor behavior leads to increased colloidal size and the formation of a random Pb–I skeleton with varied stoichiometry, which makes the assembled colloid clusters and the perovskite films have less crystallization orientation and poor phase purity. In contrast, the δ-FAPbI3 and α-FAPbI3 SC precursor solutions retain their pristine high-valent and uniform Pb–I skeleton colloid originated from single crystals, which yields the films with well-preserved highly oriented crystallinity.
The device was subjected to a series of characterizations to further assess the impact of different colloidal behaviors on performance. The non-radiative recombination caused by defects in the device can be quantitatively evaluated using the external quantum efficiency of electroluminescence (EQEEL).54 Fig. S17† shows an increasing trend in the electroluminescence (EL) intensity of the device based on PbI2 + FAI, δ-FAPbI3 and α-FAPbI3 SCs. As revealed in Fig. 4a, under the injection current density corresponding to Jsc, the peak EQEEL values of the device based on α-FAPbI3 and δ-FAPbI3 SCs were measured to be 6.08% and 2.53%, respectively, while that of the PbI2 + FAI sample was 1.05%. We calculated the voltage loss due to non-radiative recombination (ΔVoc, non-rad), where the devices based on α-FAPbI3 and δ-FAPbI3 SCs exhibited lower ΔVoc, non-rad of 0.069 V and 0.095 V than the PbI2 + FAI (0.118 V), respectively, which is consistent with the Voc difference in the J–V curves. Mott–Schottky plots (Fig. 4b) were used to further evaluate the charge-carrier dynamics in the prepared devices. The PbI2 + FAI-based device reveals a low built-in potential (0.79 V), while the values for the δ-FAPbI3 and α-FAPbI3 SC-based devices increase to 0.87 V and 1.00 V, respectively. As shown in the space-charge limited current (SCLC) measurement in Fig. 4c, the trap-filled limit voltage (VTFL) decreased from 0.508 V for PbI2 + FAI to 0.413 V for δ-FAPbI3 SC and 0.223 V for α-FAPbI3 SC. The trap-state densities (ntrap) are calculated to be 3.54 × 1015, 2.88 × 1015 and 1.55 × 1015 cm−3 for PbI2 + FAI, δ-FAPbI3 and α-FAPbI3 SC-based devices, respectively. To better understand the origin of the enhanced Voc in FAPbI3 single crystal-based devices, a series of device comparisons were conducted with the results shown in Fig. 4d, S18 and S19.† As the relationship between effective voltage (Veff) and photocurrent density (Jph) shown in Fig. S18,† in the low Veff region where Jph is not saturated,55,56 PbI2 + FAI-based devices exhibit a lower photocurrent density than that of δ-FAPbI3 and α-FAPbI3 SC-based devices. The ideal factor (n) calculated from the relationship between −dV/dJ and (Jsc–J)−1 in Fig. 4d also indicates that the δ-FAPbI3 and α-FAPbI3 SC-based devices have higher charge-carrier transport capability. In addition, compared to conventional powder mixture precursors, the lowest saturation recombination current density (J0) of the device based on α-FAPbI3 SC precursor (Fig. S19†) further confirms its ability to suppress the non-radiative recombination that is associated with voltage loss. Transient photovoltage (TPV) decay (Fig. S20†) and electrochemical impedance spectroscopy (EIS) curves (Fig. 4e) also indicate that the devices based on single-crystal precursors have fewer defects than the PbI2 + FAI device.
Single-crystal precursor for high-performance modules
To achieve scalable fabrication of perovskite devices with high performance, the uniformity of the films needs to be ensured. The PL mapping results of 2 cm × 2 cm FAPbI3 perovskite films show that there is no significant difference in uniformity between perovskite films derived from single-crystal precursors and those derived from powder mixture precursors, except for the advantage of PL intensity (Fig. S21†). However, the differences between these perovskite films become more pronounced as the area increases. The FAPbI3 single crystal-based samples also show more uniform UV-vis absorption profiles (Fig. 5a) than the PbI2 + FAI mixture, regardless of different locations on the same 30 cm × 30 cm film. The variation of PL mapping intensity of PbI2 + FAI and δ-FAPbI3 SC precursor films in different locations is visualized in Fig. 5b and S22–S24.† The presence of spatial inhomogeneity is observed in large-area films based on PbI2 + FAI precursors, which can be attributed to the random nature of the precursor colloid (Fig. S22†). The uniformity of the precursor colloid size influences the crystallization rate of films in different regions. Such comparison also indicates that the single crystal-based films possess greater scalability than the PbI2 + FAI mixture (Fig. S23 and S24†). In addition, α-FAPbI3 SC-based perovskite films exhibit the narrowest distribution of PL mapping intensity, which is attributed to the homogeneous control of the colloidal properties in the precursor. The comparison of the total PL intensity distribution between these perovskite films (Fig. S25†) shows that the chemical homogeneity of precursor colloid is of great significance for the overall unity and photoelectric properties of large-area films.Owing to the high uniformity of the single-crystal precursor, we fabricated 30 cm × 30 cm sized perovskite modules with an aperture area of 663 cm2 (structure illustrated in Fig. 5c). Table S4† presents the device parameters of PbI2 + FAI, δ-FAPbI3 and α-FAPbI3 SC-based submodules under reverse scans. As shown in the J–V curves in Fig. 5d, PCEs of 19.7% and 20.7% were achieved using δ-FAPbI3 and α-FAPbI3 SCs, respectively. The submodule efficiency based on α-FAPbI3 SCs has been certified to be 20.4% with a Voc of 57.5 V, Isc of 0.302 A, and FF of 77.8% (Fig. S26 and S27†). Meanwhile, the PCEs of the submodules based on PbI2 + FAI were only 17.6%. More importantly, the α-FAPbI3 SCs used to fabricate the highest module PCE originated from low-purity and low-cost lead iodide, which greatly reduced the commercial cost of the manufacturing process.
Conclusions
In summary, in this work, we investigated and revealed that the colloid behavior of the perovskite precursor varies with the starting materials and can greatly affect photovoltaic performance. The FAPbI3 single-crystal precursor's behavior could exert good control over the chemical composition and colloidal properties, which is more favorable than the traditional PbI2 + FAI precursor's behavior in terms of phase purity, crystallization orientation, reduced defect density and large-area uniformity for film deposition. By preparing the perovskite single crystals, low-purity materials can also achieve high-performance perovskite photovoltaics, which exhibit an impressive efficiency of 20.7% even in large-area perovskite submodules with a 663 cm2 aperture area. These findings provide a fundamental understanding of the single-crystal precursor engineering of not only FAPbI3 perovskite but also other solution-based soft-lattice materials in various optoelectronic applications. More importantly, understanding such solution chemistry is beneficial for the scaling-up fabrication of perovskite solar cells, especially for low-cost flow-line production.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Yugang Liang: data curation, formal analysis, investigation, methodology, writing the original draft, review and editing. Yingping Fan: data curation, formal analysis, investigation. Zhixiao Qin: data curation, investigation. Lei Lu: data curation, validation. Haifei Wang: investigation. Meng Ren: review and editing. Fang Liu: formal analysis. Yanfeng Miao: investigation, review. Yuetian Chen: funding acquisition, methodology, writing and editing. Yixin Zhao: conceptualization, funding acquisition, methodology, supervision, review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (NSFC, Grant Numbers 22220102002, 22025505, 22209111, 22479098) and the Natural Science Foundation of Shanghai (Grant Number 23ZR1428000). The authors gratefully thank Shanghai Synchrotron Radiation Facility of CAS for the assistance with GIWAXS analysis, and the Instrumental Analysis Centers in Shanghai Jiao Tong University and School of Environmental Science and Engineering for assistance with characterization.Notes and references
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
- M. A. Green, A. Ho-Baillie and H. J. Snaith, Nat. Photonics, 2014, 8, 506–514 CrossRef CAS.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- Z. Xiao, C. Bi, Y. Shao, Q. Dong, Q. Wang, Y. Yuan, C. Wang, Y. Gao and J. Huang, Energy Environ. Sci., 2014, 7, 2619–2623 RSC.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- J. Park, J. Kim, H.-S. Yun, M. J. Paik, E. Noh, H. J. Mun, M. G. Kim, T. J. Shin and S. I. Seok, Nature, 2023, 616, 724–730 CrossRef CAS PubMed.
- National Renewable Energy Laboratory (NREL), Photovoltaic Research, Best Research-Cell Efficiency Chart, 2024, https://www.nrel.gov/pv/cell-efficiency.html, accessed 11 October, 2024 Search PubMed.
- H. Chen, C. Liu, J. Xu, A. Maxwell, W. Zhou, Y. Yang, Q. Zhou, A. S. R. Bati, H. Wan, Z. Wang, L. Zeng, J. Wang, P. Serles, Y. Liu, S. Teale, Y. Liu, M. I. Saidaminov, M. Li, N. Rolston, S. Hoogland, T. Filleter, M. G. Kanatzidis, B. Chen, Z. Ning and E. H. Sargent, Science, 2024, 384, 189–193 CrossRef CAS PubMed.
- S. Liu, J. Li, W. Xiao, R. Chen, Z. Sun, Y. Zhang, X. Lei, S. Hu, M. Kober-Czerny, J. Wang, F. Ren, Q. Zhou, H. Raza, Y. Gao, Y. Ji, S. Li, H. Li, L. Qiu, W. Huang, Y. Zhao, B. Xu, Z. Liu, H. J. Snaith, N.-G. Park and W. Chen, Nature, 2024, 632, 536–542 CrossRef PubMed.
- G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 982–988 RSC.
- H. Lu, Y. Liu, P. Ahlawat, A. Mishra, W. R. Tress, F. T. Eickemeyer, Y. Yang, F. Fu, Z. Wang, C. E. Avalos, B. I. Carlsen, A. Agarwalla, X. Zhang, X. Li, Y. Zhan, S. M. Zakeeruddin, L. Emsley, U. Rothlisberger, L. Zheng, A. Hagfeldt and M. Grätzel, Science, 2020, 370, eabb8985 CrossRef CAS PubMed.
- L. Chao, T. Niu, W. Gao, C. Ran, L. Song, Y. Chen and W. Huang, Adv. Mater., 2021, 33, 2005410 CrossRef CAS PubMed.
- M. Jung, S.-G. Ji, G. Kim and S. I. Seok, Chem. Soc. Rev., 2019, 48, 2011–2038 RSC.
- T. D. Siegler, A. Dawson, P. Lobaccaro, D. Ung, M. E. Beck, G. Nilsen and L. L. Tinker, ACS Energy Lett., 2022, 7, 1728–1734 CrossRef CAS.
- J. Xu, A. Maxwell, M. Wei, Z. Wang, B. Chen, T. Zhu and E. H. Sargent, ACS Energy Lett., 2021, 6, 4220–4227 CrossRef CAS.
- J. Chang, H. Zhu, B. Li, F. H. Isikgor, Y. Hao, Q. Xu and J. Ouyang, J. Mater. Chem. A, 2016, 4, 887–893 RSC.
- J. Yao, L. Yang, F. Cai, Y. Yan, R. S. Gurney, D. Liu and T. Wang, Sustainable Energy Fuels, 2018, 2, 436–443 RSC.
- P. Fassl, V. Lami, A. Bausch, Z. Wang, M. T. Klug, H. J. Snaith and Y. Vaynzof, Energy Environ. Sci., 2018, 11, 3380–3391 RSC.
- B.-w. Park, N. Kedem, M. Kulbak, D. Y. Lee, W. S. Yang, N. J. Jeon, J. Seo, G. Kim, K. J. Kim, T. J. Shin, G. Hodes, D. Cahen and S. I. Seok, Nat. Commun., 2018, 9, 3301 CrossRef PubMed.
- Y. Rong, Y. Hu, A. Mei, H. Tan, M. I. Saidaminov, S. I. Seok, M. D. McGehee, E. H. Sargent and H. Han, Science, 2018, 361, eaat8235 CrossRef PubMed.
- J. W. Yoo, E. Noh, J. Jang, K. S. Lee, J. Byeon, M. Choi, J. Im and S. I. Seok, Joule, 2023, 7, 797–809 CrossRef CAS.
- X. Liu, J. Zhang, H. Wang, Y. Miao, T. Guo, L. K. Ono, S. Yuan, Y. Wang, P. Ji, H. Chen, C. Zhang, T. Li, C. Ding, S. Mariotti, X. Huo, I.-N. Rabehi, H. Wang, Y. Zhao and Y. Qi, Joule, 2024, 8, 2851–2862 CrossRef CAS.
- T. Bu, J. Li, H. Li, C. Tian, J. Su, G. Tong, L. K. Ono, C. Wang, Z. Lin, N. Chai, X.-L. Zhang, J. Chang, J. Lu, J. Zhong, W. Huang, Y. Qi, Y.-B. Cheng and F. Huang, Science, 2021, 372, 1327–1332 CrossRef CAS PubMed.
- T. Bu, L. K. Ono, J. Li, J. Su, G. Tong, W. Zhang, Y. Liu, J. Zhang, J. Chang, S. Kazaoui, F. Huang, Y.-B. Cheng and Y. Qi, Nat. Energy, 2022, 7, 528–536 CrossRef CAS.
- Y. Miao, M. Ren, Y. Chen, H. Wang, H. Chen, X. Liu, T. Wang and Y. Zhao, Nat Sustainability, 2023, 6, 1465–1473 CrossRef.
- G. Zhang, B. Ding, Y. Ding, Y. Liu, C. Yu, L. Zeng, Y. Wang, X. Zhang, M. Liu, Q. Tian, B. Fan, Q. Liu, G. Yang, M. K. Nazeeruddin and B. Chen, Sci. Adv., 2024, 10, eadl6398 CrossRef CAS PubMed.
- D.-N. Jeong, D.-K. Lee, S. Seo, S. Y. Lim, Y. Zhang, H. Shin, H. Cheong and N.-G. Park, ACS Energy Lett., 2019, 4, 1189–1195 CrossRef CAS.
- W. Feng, J. Tao, G. Liu, G. Yang, J.-X. Zhong, Y. Fang, L. Gong, S. Yang and W.-Q. Wu, Angew. Chem., Int. Ed., 2023, 62, e202300265 CrossRef CAS PubMed.
- K. Wang, C. Wu, Y. Hou, D. Yang, T. Ye, J. Yoon, M. Sanghadasa and S. Priya, Energy Environ. Sci., 2020, 13, 3412–3422 RSC.
- J. Jeong, M. Kim, J. Seo, H. Lu, P. Ahlawat, A. Mishra, Y. Yang, M. A. Hope, F. T. Eickemeyer, M. Kim, Y. J. Yoon, I. W. Choi, B. P. Darwich, S. J. Choi, Y. Jo, J. H. Lee, B. Walker, S. M. Zakeeruddin, L. Emsley, U. Rothlisberger, A. Hagfeldt, D. S. Kim, M. Grätzel and J. Y. Kim, Nature, 2021, 592, 381–385 CrossRef CAS PubMed.
- M. Kim, I.-w. Choi, S. J. Choi, J. W. Song, S.-I. Mo, J.-H. An, Y. Jo, S. Ahn, S. K. Ahn, G.-H. Kim and D. S. Kim, Joule, 2021, 5, 659–672 CrossRef CAS.
- L. He, H. Gu, X. Liu, P. Li, Y. Dang, C. Liang, L. K. Ono, Y. Qi and X. Tao, Matter, 2020, 2, 167–180 CrossRef.
- Y. Qin, H. Zhong, J. J. Intemann, S. Leng, M. Cui, C. Qin, M. Xiong, F. Liu, A. K. Y. Jen and K. Yao, ACS Energy Lett., 2020, 10, 1904050 CAS.
- S. Sidhik, W. Li, M. H. K. Samani, H. Zhang, Y. Wang, J. Hoffman, A. K. Fehr, M. S. Wong, C. Katan, J. Even, A. B. Marciel, M. G. Kanatzidis, J.-C. Blancon and A. D. Mohite, Adv. Mater., 2021, 33, 2007176 CrossRef CAS PubMed.
- E. H. Jung, N. J. Jeon, E. Y. Park, C. S. Moon, T. J. Shin, T.-Y. Yang, J. H. Noh and J. Seo, Nature, 2019, 567, 511–515 CrossRef CAS PubMed.
- G. Kim, H. Min, S. Lee Kyoung, Y. Lee Do, M. Yoon So and I. Seok Sang, Science, 2020, 370, 108–112 CrossRef CAS PubMed.
- X. Zheng, Y. Hou, C. Bao, J. Yin, F. Yuan, Z. Huang, K. Song, J. Liu, J. Troughton, N. Gasparini, C. Zhou, Y. Lin, D.-J. Xue, B. Chen, A. K. Johnston, N. Wei, M. N. Hedhili, M. Wei, A. Y. Alsalloum, P. Maity, B. Turedi, C. Yang, D. Baran, T. D. Anthopoulos, Y. Han, Z.-H. Lu, O. F. Mohammed, F. Gao, E. H. Sargent and O. M. Bakr, Nat. Energy, 2020, 5, 131–140 CrossRef CAS.
- Y. Zhang, S. Seo, S. Y. Lim, Y. Kim, S.-G. Kim, D.-K. Lee, S.-H. Lee, H. Shin, H. Cheong and N.-G. Park, ACS Energy Lett., 2020, 5, 360–366 CrossRef CAS.
- G. Tong, D.-Y. Son, L. K. Ono, H.-B. Kang, S. He, L. Qiu, H. Zhang, Y. Liu, J. Hieulle and Y. Qi, Nano Energy, 2021, 87, 106152 CrossRef CAS.
- T. Zhang, M. Yang, E. E. Benson, Z. Li, J. van de Lagemaat, J. M. Luther, Y. Yan, K. Zhu and Y. Zhao, Chem. Commun., 2015, 51, 7820–7823 RSC.
- M. I. Saidaminov, A. L. Abdelhady, B. Murali, E. Alarousu, V. M. Burlakov, W. Peng, I. Dursun, L. Wang, Y. He, G. Maculan, A. Goriely, T. Wu, O. F. Mohammed and O. M. Bakr, Nat. Commun., 2015, 6, 7586 CrossRef PubMed.
- P. Zhu, D. Wang, Y. Zhang, Z. Liang, J. Li, J. Zeng, J. Zhang, Y. Xu, S. Wu, Z. Liu, X. Zhou, B. Hu, F. He, L. Zhang, X. Pan, X. Wang, N.-G. Park and B. Xu, Science, 2024, 383, 524–531 CrossRef CAS PubMed.
- Q. Gao, J. Qi, K. Chen, M. Xia, Y. Hu, A. Mei and H. Han, Adv. Mater., 2022, 34, 2200720 CrossRef CAS PubMed.
- H. Li, N. Shen, S. Chen, F. Guo and B. Xu, Adv. Funct. Mater., 2023, 33, 2214339 CrossRef CAS.
- J. T.-W. Wang, Z. Wang, S. Pathak, W. Zhang, D. W. deQuilettes, F. Wisnivesky-Rocca-Rivarola, J. Huang, P. K. Nayak, J. B. Patel, H. A. Mohd Yusof, Y. Vaynzof, R. Zhu, I. Ramirez, J. Zhang, C. Ducati, C. Grovenor, M. B. Johnston, D. S. Ginger, R. J. Nicholas and H. J. Snaith, Energy Environ. Sci., 2016, 9, 2892–2901 RSC.
- J.-W. Lee, S. Tan, T.-H. Han, R. Wang, L. Zhang, C. Park, M. Yoon, C. Choi, M. Xu, M. E. Liao, S.-J. Lee, S. Nuryyeva, C. Zhu, K. Huynh, M. S. Goorsky, Y. Huang, X. Pan and Y. Yang, Nat. Commun., 2020, 11, 5514 CrossRef CAS PubMed.
- K. Yan, M. Long, T. Zhang, Z. Wei, H. Chen, S. Yang and J. Xu, J. Am. Chem. Soc., 2015, 137, 4460–4468 CrossRef CAS PubMed.
- R. J. Stewart, C. Grieco, A. V. Larsen, G. S. Doucette and J. B. Asbury, J. Phys. Chem. C, 2016, 120, 12392–12402 CrossRef CAS.
- K. G. Stamplecoskie, J. S. Manser and P. V. Kamat, Energy Environ. Sci., 2015, 8, 208–215 RSC.
- A. Sharenko, C. Mackeen, L. Jewell, F. Bridges and M. F. Toney, Chem. Mater., 2017, 29, 1315–1320 CrossRef CAS.
- Y. Deng, C. H. Van Brackle, X. Dai, J. Zhao, B. Chen and J. Huang, Sci. Adv., 2019, 5, eaax7537 CrossRef CAS PubMed.
- J. Kim, B.-w. Park, J. Baek, J. S. Yun, H.-W. Kwon, J. Seidel, H. Min, S. Coelho, S. Lim, S. Huang, K. Gaus, M. A. Green, T. J. Shin, A. W. Y. Ho-baillie, M. G. Kim and S. I. Seok, J. Am. Chem. Soc., 2020, 142, 6251–6260 CrossRef CAS PubMed.
- L. Chao, Y. Xia, B. Li, G. Xing, Y. Chen and W. Huang, Chem, 2019, 5, 995–1006 CAS.
- H. Min, S.-G. Ji and S. I. Seok, Joule, 2022, 6, 2175–2185 CrossRef CAS.
- F.-C. Chen, J.-L. Wu, C.-L. Lee, Y. Hong, C.-H. Kuo and M. H. Huang, Appl. Phys. Lett., 2009, 95, 013305 CrossRef.
- Y. Wang, M. Feng, H. Chen, M. Ren, H. Wang, Y. Miao, Y. Chen and Y. Zhao, Adv. Mater., 2024, 36, 2305849 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, experimental methods, and additional J–V curves, XRD, ICP-OES, SPO curves, PL, UV-vis, PL mapping, IPCE, TPV, EL spectra, dark current–voltage curves, certified I–V curves and statistical device parameters. See DOI: https://doi.org/10.1039/d4sc07759h |
| This journal is © The Royal Society of Chemistry 2025 |