Open Access Article
Open Access ArticleRedox mediated dimerisation of a cyclo-As8 complex†
Christoph
Riesinger
 and
Manfred
Scheer
and
Manfred
Scheer
 *
*
Institute of Inorganic Chemistry, University of Regensburg, Universit, ä, tsstr 31, 93053 Regensburg, Germany. E-mail: manfred.scheer@chemie.uni-regensburg.de
First published on 8th April 2025
Abstract
Reduction as well as oxidation of the cyclo-As8 complex [{Cp′′Ta}2(μ,η2:2:2:2:1:1-As8)] (A, Cp′′ = 1,3-tBu2C5H3) are demonstrated to afford controlled dimerisation to unprecedented As16 species. The dication [{Cp′′Ta}4(μ4,η2:2:2:2:2:2:2:2:1:1:1:1-As16)]2+ slowly disproportionates in solution, yielding the largest polyarsenide species in a molecular complex known to date.
Compared to its lighter homologs in group 15, nitrogen and phosphorus, the chemistry of arsenic is highly underdeveloped.1,2 Although arsenic is crucial in applications such as semiconductor manufacturing (e.g. GaAs),3–5 arsines, for example, are far less utilised in fields like catalysis, when compared to ubiquitous phosphines.6 Of course, this is most notably due to the higher toxicity associated with arsenic compounds. Nevertheless, arsenic species often hold chemical advantages over their lighter homologs rendering their exploration of significant fundamental value.2 For example, transition metal stabilised polyarsenide (Asn) ligands7 can be employed in the preparation of organometallic coordination compounds.8–10 This often results in distinct structural motifs which drastically differ from those of their lighter polyphosphorus (Pn) analogues.11,12 However, these Asn ligand complexes are far less accessible due to the limited availability of starting materials (e.g. yellow arsenic, As4).2 Furthermore, their reactivity remains underexplored compared to the lighter Pn species, which have received considerable interest in the light of sustainable transformation of white phosphorus P4.13–15 Thus, the functionalisation and transformation of Asn ligand complexes offer potential routes towards novel Asn scaffolds.16,17 Most notably, the redox chemistry of those complexes may serve as a promising starting point for the preparation of unprecedented molecular polyarsenides. However, oxidation and reduction of end-deck Asn complexes have been demonstrated to result in more or less uncontrolled fragmentations,18,19 which can be attributed to the low As–As bond energy (146 kJ mol−1) compared to e. g. P–P bonds (220 kJ mol−1).20 A prominent example for such unselective reactivity is found in the reduction of [Cp*Fe(η5-As5)] (Cp* = C5Me5, Fig. 1a),21 which is in stark contrast to the redox chemistry of its lighter congener [Cp*Fe(η5-P5)].22 The associated difficulties in sample purification hamper the utilisation of the polyarsenide products in synthetic applications. Furthermore, the hardly accessible 75As (I = 3/2) NMR spectroscopy fails as a tool to analyse reaction mixtures when compared to the widely used 31P NMR spectroscopy. On the other hand, the redox chemistry of Asn triple decker complexes and cluster compounds generally resulted in only minor structural changes and did not afford new Asn scaffolds.23,24 Thus, the As2 ligands in [{Cp′′′Co}2(μ,η2:2-As2)2] (Cp′′′ = 1,2,4-tBu3C5H2) underwent only intramolecular bond formation upon oxidation as well as reduction (Fig. 1b).25 Similarly, oxidation of a hexaarsa-benzene ligand in [{Cp*Mo}2(μ,η6:6-As6)] leads to a bisallylic distortion and formation of the respective radical cation.26 Only when the bimetallic [{CpMo(CO)2}2(μ,η2:2-As2)] was oxidised a controlled intermolecular As–As bond formation to an As4 chain was observed.27
 | ||
| Fig. 1 Previously reported redox chemistry of Asn ligand complexes (a and b) and unprecedented dimerisation of [{Cp′′Ta}2(μ,η2:2:2:2:1:1-As8)] (A) to As16 species (c). | ||
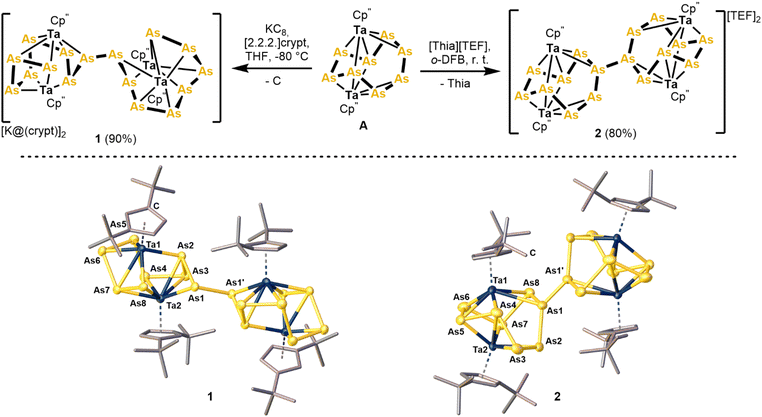
Expanding this reactivity to complexes with larger Asn ligands could enable the synthesis of yet unprecedented molecular polyarsenides but has not yet been realised. The cyclo-As8 ligand complex [{Cp′′Ta}2(μ,η2:2:2:2:1:1-As8)] (A, Cp′′ = 1,3-tBu2C5H2)28 appears to be a promising candidate for studying its redox behaviour. Herein we present the controlled and selective redox mediated dimerisation of this complex (Fig. 1c). In view of the recent preparation and study of the coordination behaviour as well as redox chemistry of the cyclo-P8 complex [{Cp′′′Ta}2(μ,η2:2:2:2:1:1-P8)] (B),29 starting with the cyclo-As8 analogue A could deliver unprecedented molecular polyarsenides.
Initially, the redox properties of A were assessed electrochemically by cyclic voltammetry (−2.7 V to 1.3 V, 30 mg in 10 mL of o-DFB with 1000 mg [nBu4N][PF6] as supporting electrolyte; see ESI for further details, Fig. S1†). This revealed two synthetically accessible, but irreversible, redox events at −1441 mV and 294 mV (vs. FcH/FcH+), respectively. Thus, the strong reducing agent KC8 and salts of the strongly oxidising [Thia]+ (Thia = thianthrene, C12H8S2) radical cation were chosen to investigate the reactivity of A experimentally. Exposing A to one equivalent of KC8 in THF at −80 °C leads to an immediate colour change from light brown to dark brownish black. As the product was expected to be highly sensitive, [2.2.2]-cryptand was added directly into the reaction mixture. After warming to room temperature, filtration and layering with n-hexane the product [K@crypt]2[{Cp''Ta}4(μ4,η2:2:2:2:2:2:2:2:1:1:1:1-As16)] (1) was isolated as nearly black crystalline blocks in yields of 90% (Fig. 2). The dianion in 1 features an unprecedented catena-As16 ligand arising from the dimerisation of A. Similarly, oxidation of A with one equivalent of [Thia][TEF] (Thia = thianthrene, [TEF]– = [Al{OC(CF3)3}4]–) in o-DFB (1,2-difluorobenzene) at −30 °C leads to a dimerisation and [{Cp''Ta}4(μ4,η2:2:2:2:2:2:2:2:1:1:1:1-As16)][TEF]2 (2) was isolated as a dark brown powder in 80% yield after precipitation from the reaction solution with n-pentane (Fig. 2). 2 can be stored as a solid indefinitely but slowly disproportionates, even at 4 °C, when kept in solution for several days. Notably, 1 and 2 display only the second and third example of controlled redox-mediated dimerisation of a polyarsenic ligand complex.27 The solid state structure of 1 reveals two mostly intact As8 ligands of A being dimerised via the former As4 atoms (in A, Fig. 2). However, the As1–As8 distance of 3.083(1) Å agrees with this bond being cleaved (the corresponding As4–As4′ bond in A is 2.537(2) Å long).28 In contrast, the newly formed As1–As1′ bond (2.441(1) Å) is in the range of a single bond (2.42 Å).30 The remaining As–As bonds (2.403(1)–2.458(1) Å) are similar in length implying no localised multiple bond character for any of them. Furthermore, the Ta1–Ta2 distance of 3.354(1) Å is identical to that found in A (3.353(1) Å) and confirms that a weak metal–metal interaction (vide infra) is present in 1. On the other hand, layering a concentrated solution of 2 in o-DFB with n-pentane and storage at 4 °C affords dark rod-shaped crystals of this compound within 1–2 weeks. 2 features two intact cyclo-As8 groups being linked by a central As1–As1′ bond. While the formal bond length of the latter (2.120(3) Å) is shorter than an expected single bond (2.42 Å),30 this can be attributed to packing effects but especially to a complex disorder within the cationic core of this compound (see ESI† and computational results, vide infra). In contrast to 1, the As5–As6 bond (2.621(2) Å) is only slightly elongated when compared to the respective As4–As4′ bond in A (2.537(2) Å). The other As–As bonds are similar in length (2.370(2) – 2.493(2) Å) and in the range of single bonds.30 Notably, the Ta1–Ta2 distance in 2 (3.253(3) Å) is about 0.1 Å shorter compared to A.28 Both compounds, 1 and 2, are isostructural to their lighter P analogues arising from reduction and oxidation of B, respectively.29 Nevertheless, the catena-As16 ligand in 1 is unprecedented and the dication in 2 represents the largest dicationic polyarsenide in general. The integrity of both compounds 1 and 2 was additionally verified by elemental analysis, 1H, and 19F NMR spectroscopy (see ESI†). Additionally, the ESI(+) mass spectrum of 2 reveals the molecular ion peak of its dication, further corroborating these results. Both compounds can be stored as solids under inert conditions for several months without showing signs of degradation. However, when 2 is kept in CH2Cl2 or o-DFB solution for more than a week it partly decomposes in a complex disproportionation reaction, affording two novel polyarsenide complexes 3 and 4. This disproportionation could not be circumvented by lowering the temperature, changing the solvent or the utilisation of other counter anions. On the one hand, the arsenic depleted complex [{Cp′′Ta}2(μ,η1:1:1:1:1:1-As6)][TEF] (3, Fig. 3) bears a central cyclo-As6 ligand bridging two Ta centers. It shows As–As bond lengths (2.380(3) – 2.545(3) Å) in the range of single bonds, with only the As1–As6 distance (2.728(2) Å) being significantly elongated.30 On the other hand, this disproportionation leads to the formation of the arsenic rich [{Cp′′Ta}6(μ6,η2:2:2:2:2:2:2:2:2:2:2:2:1:1:1:1:1:1-As25)][TEF]3 (4) representing the largest molecular polyarsenide known so far (see ESI† and Fig. 3). In 4 formally three units of A are linked by one central As atom, thus leading to the formation of the As25 ligand. The central As1/As1′–As9 bond lengths (2.533(2)–2.553(2) Å) in 4 are in the range of elongated single bonds, which may be a result of steric hindrance around the central As9 atom. The remaining As–As bonds (2.164(2) – 2.611(2) Å) are similar to those in A.28 Unfortunately, both 3 and 4 cannot be separated, neither from residual 2 nor from each other, as all three compounds co-crystallise, which hinders their isolation and spectroscopic characterisation. However, the integrity of the triple-decker cation in 3 and even the trication in 4 could be confirmed by ESI(+) mass spectrometry (see ESI†). To get deeper insight into the molecular and electronic structure of 1 and 2 computational investigations were carried out on the ωB97X-D4/def2-TZVP level of theory (Fig. 4). In general, the molecular structures of both compounds are well reproduced in these computations. Furthermore, the As1–As1′ bond in the computed structure of 2 (2.503 Å) is closer to the expected As–As single bond length compared to the crystallographic data (2.120(3) Å). This corroborates the assumption that the observed bond length in the solid state is a result of packing effects, as well as a complex disorder (vide supra). Furthermore, NBO analysis consolidates the bonding between
the monomeric subunits of 1 and 2 to occur via localised As–As single bonds with Wiberg bond indices close to unity (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.92, 2
0.92, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.84). Thus, potential multiple bonding, as suggested by the short As1–As1′ bond in the X-ray structure of 2, can be ruled out.
0.84). Thus, potential multiple bonding, as suggested by the short As1–As1′ bond in the X-ray structure of 2, can be ruled out.
 | ||
| Fig. 4 Computed molecular structures of the dianion [1]2– (left) and the dication [2]2+ (right) with the NBOs of the corresponding As1–As1′ bonds; cut-off at 0.4 a. u. | ||
Conclusions
In conclusion, contrasting known polyarsenic ligand complexes, the redox mediated dimerisation of the cyclo-As8 ligand complex A proceeds selectively and results in two unprecedented polyarsenide scaffolds. The dianion [K@crypt]2[{Cp′′Ta}4(μ4,η2:2:2:2:2:2:2:2:1:1:1:1-As16)] (1) features a novel As16 chain, while [{Cp′′Ta}4(μ4,η2:2:2:2:2:2:2:2:1:1:1:1-As16)][TEF]2 (2) represents the largest dicationic polyarsenide known to date. The latter is shown to be unstable towards disproportionation, when kept in solution for prolonged times. This results in the formation of the cyclo-As6 triple decker complex [{Cp′′Ta}2(μ,η1:1:1:1:1:1-As6)][TEF] (3) as well as the arsenic rich trication [{Cp′′Ta}6(μ6,η2:2:2:2:2:2:2:2:2:2:2:2:1:1:1:1:1:1-As25)][TEF]3 (4), which marks the largest molecular polyarsenide ligand isolated so far. Additional computational data elaborates the bonding situation in the dimeric complexes 1 and 2.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Author contributions
The conceptualisation (together with MS), experimental work and writing of the manuscript of this work were achieved by CR. CR accomplished the solution and refinement of X-ray structural data and performed the DFT calculations. The entire work was supervised, guided, and revised by MS, who also acquired funding for the project. The final manuscript was reviewed and edited by CR and MS.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) within the project Sche384/36-2. C. R. is grateful to the Studienstiftung des Deutschen Volkes for a PhD fellowship. The authors thank Benjamin Falge for assistance with conducting the cyclic voltammetry studies. Additional references are cited within the ESI.†27,28,31–51Notes and references
- A. F. Holleman, E. Wiberg and N. Wiberg, “Lehrbuch der Anorganischen Chemie”, Walter de Gruyter, Berlin (DE), 2007, pp. 822–860 Search PubMed.
- M. Seidl, G. Balázs and M. Scheer, Chem. Rev., 2019, 119, 8406 CrossRef CAS PubMed.
- R. L. Wells and W. L. Gladfelter, J. Cluster Sci., 1997, 8, 217 CrossRef CAS.
- S. Schulz in. “Advances in Organometallic Chemistry”, Elsevier, 2003, pp. 225–317 Search PubMed.
- B. Neumüller and E. Iravani, Coord. Chem. Rev., 2004, 248, 817 CrossRef.
- J. H. Downing and M. B. Smith in. “Comprehensive Coordination Chemistry II”, Elsevier, 2003, pp. 253–296 Search PubMed.
- O. J. Scherer, Angew Chem. Int. Ed. Engl., 1990, 29, 1104 CrossRef.
- M. Fleischmann, S. Welsch, H. Krauss, M. Schmidt, M. Bodensteiner, E. V. Peresypkina, M. Sierka, C. Gröger and M. Scheer, Chem.–Eur. J., 2014, 20, 3759 CrossRef CAS PubMed.
- M. E. Moussa, J. Schiller, E. Peresypkina, M. Seidl, G. Balázs, P. Shelyganov and M. Scheer, Chem.–Eur. J., 2020, 26, 14315 CrossRef CAS PubMed.
- M. E. Moussa, M. Fleischmann, G. Balázs, A. V. Virovets, E. Peresypkina, P. A. Shelyganov, M. Seidl, S. Reichl and M. Scheer, Chem.–Eur. J., 2021, 27, 9742 CrossRef CAS PubMed.
- M. Fleischmann, J. S. Jones, F. P. Gabbaï and M. Scheer, Chem. Sci., 2015, 6, 132 RSC.
- M. Fleischmann, L. Dütsch, M. E. Moussa, A. Schindler, G. Balázs, C. Lescop and M. Scheer, Chem. Commun., 2015, 51, 2893 RSC.
- B. M. Cossairt, N. A. Piro and C. C. Cummins, Chem. Rev., 2010, 110, 4164 CrossRef CAS PubMed.
- M. Caporali, L. Gonsalvi, A. Rossin and M. Peruzzini, Chem. Rev., 2010, 110, 4178 CrossRef CAS PubMed.
- C. M. Hoidn, D. J. Scott and R. Wolf, Chem. - Eur. J., 2021, 27, 1886 CrossRef CAS PubMed.
- S. Reichl, C. Riesinger and M. Scheer, Angew. Chem., Int. Ed., 2023, 62, e202307696 CrossRef CAS PubMed.
- S. Reichl, C. Riesinger, R. Yadav, A. Y. Timoshkin, P. W. Roesky and M. Scheer, Angew. Chem., Int. Ed., 2024, 63, e202316117 CrossRef CAS PubMed.
- M. Schmidt, A. E. Seitz, M. Eckhardt, G. Balázs, E. V. Peresypkina, A. V. Virovets, F. Riedlberger, M. Bodensteiner, E. M. Zolnhofer, K. Meyer and M. Scheer, J. Am. Chem. Soc., 2017, 139, 13981 CrossRef CAS PubMed.
- C. Riesinger, L. Zimmermann and M. Scheer, Organometallics, 2023, 42, 2065 CrossRef CAS.
- N. K. Kildahl, J. Chem. Educ., 1995, 72, 423 CrossRef CAS.
- M. Schmidt, D. Konieczny, E. V. Peresypkina, A. V. Virovets, G. Balázs, M. Bodensteiner, F. Riedlberger, H. Krauss and M. Scheer, Angew. Chem., Int. Ed., 2017, 56, 7307 CrossRef CAS PubMed.
- M. V. Butovskiy, G. Balázs, M. Bodensteiner, E. V. Peresypkina, A. V. Virovets, J. Sutter and M. Scheer, Angew. Chem., Int. Ed., 2013, 52, 2972 CrossRef CAS PubMed.
- M. Piesch, S. Reichl, C. Riesinger, M. Seidl, G. Balazs and M. Scheer, Chem.–Eur. J., 2021, 27, 9129 CrossRef CAS PubMed.
- C. Riesinger, L. Dütsch and M. Scheer, Z. Anorg. Allg. Chem., 2022, 648, e202200102 CrossRef CAS.
- M. Piesch, C. Graßl and M. Scheer, Angew. Chem., Int. Ed., 2020, 59, 7154 CrossRef CAS PubMed.
- M. Fleischmann, F. Dielmann, G. Balázs and M. Scheer, Chem.–Eur. J., 2016, 22, 15248 CrossRef PubMed.
- L. Dütsch, M. Fleischmann, S. Welsch, G. Balázs, W. Kremer and M. Scheer, Angew. Chem., Int. Ed., 2018, 57, 3256 CrossRef PubMed.
- K. Mast, J. Meiers, O. J. Scherer and G. Wolmershäuser, Z. Anorg. Allg. Chem., 1999, 625, 70 CrossRef CAS.
- C. Riesinger, F. Dielmann, R. Szlosek, A. V. Virovets and M. Scheer, Angew. Chem., Int. Ed., 2023, 62, e202218828 CrossRef CAS PubMed.
- P. Pyykkö, J. Phys. Chem. A, 2015, 119, 2326 CrossRef PubMed.
- https://omics.pnl.gov/software/molecular-weight-calculator, (22.01.2025).
- J.-M. Lalancette, G. Rollin and P. Dumas, Can. J. Chem., 1972, 50, 3058 CrossRef CAS.
- Agilent, CrysAlis PRO, Agilent Technologies Ltd, Yarnton, Oxfordshire, England, 2014 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A, 2015, 71, 3 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C:Struct. Chem., 2015, 71, 3 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A, 2008, 64, 112 CrossRef CAS PubMed.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73 CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, e1327 Search PubMed.
- F. Neese, F. Wennmohs, U. Becker and C. Riplinger, J. Chem. Phys., 2020, 152, 224108 CrossRef CAS PubMed.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2022, 12, e1606 Search PubMed.
- F. Neese, J. Comput. Chem., 2023, 44, 381 CrossRef CAS PubMed.
- A. D. Becke, Phys. Rev. A:At., Mol., Opt. Phys., 1988, 38, 3098 CrossRef CAS PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999 CrossRef CAS PubMed.
- E. D. Glendening, C. R. Landis and F. Weinhold, J. Comput. Chem., 2019, 40, 2234 CrossRef CAS PubMed.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615 RSC.
- J.-D. Chai and M. Head-Gordon, J. Chem. Phys., 2008, 128, 84106 CrossRef PubMed.
- Y.-S. Lin, G.-D. Li, S.-P. Mao and J. D. Chai, J. Chem. Theory Comput., 2013, 9, 263 CrossRef CAS PubMed.
- E. Caldeweyher, S. Ehlert, A. Hansen, H. Neugebauer, S. Spicher, C. Bannwarth and S. Grimme, J. Chem. Phys., 2019, 150, 154122 CrossRef PubMed.
- Chemcraft - graphical software for visualization of quantum chemistry computations, https://www.chemcraftprog.com, (22.01.2025) Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2421973–2421975. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc01685a |
| This journal is © The Royal Society of Chemistry 2025 |