Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structural chemistry of intermetallic compounds for active site design in heterogeneous catalysis
Nilanjan
Roy†
 a,
Kathryn
MacIntosh†
a,
Kathryn
MacIntosh†
 a,
Mustafa
Eid
a,
Mustafa
Eid
 b,
Griffin
Canning
b,
Griffin
Canning
 a and
Robert M.
Rioux
a and
Robert M.
Rioux
 *ab
*ab
aDepartment of Chemical Engineering, Pennsylvania State University, University Park, PA 16802, USA. E-mail: rmr189@psu.edu
bDepartment of Chemistry, Pennsylvania State University, University Park, PA 16802, USA
First published on 21st April 2025
Abstract
Completely or partially ordered intermetallic compounds possess unique electronic structure and chemical bonding, establishing them as an emergent class of catalytic materials for selective hydrogenation reactions. In this review, we focus on the structural and chemical aspects of different classes of intermetallic compounds, followed by illustrative examples highlighting the impact of their structural/chemical features on catalytic hydrogenation. We limit the scope of our discussion to the selective hydrogenation of alkynes (acetylene). We focus our discussion on how the isolation of active sites, formation of defined surface ensembles, partial charge transfer between heteroatoms, and alteration of the surface electronic structure impact activity and selectivity toward the desired product(s), based on recent literature observations. This review contributes to informing the appropriate selection of intermetallic catalysts for hydrogenation reactions to achieve high selectivity.
1. Introduction
The well-defined and ordered structures of intermetallic compounds offer the opportunity to rationally design catalysts with specific active sites for various catalytic processes. The precise control over active ensembles establishes intermetallic compounds well-suited as model catalysts for gaining a deeper understanding of structure–activity–selectivity relationships. Comprehensive reviews on the broad field of intermetallic compounds in heterogeneous catalysis are already available in the literature.1–11 However, the correlation between the structural chemistry of intermetallic compounds and their application as model catalysts in heterogeneous catalysis is still lacking.The selective hydrogenation of acetylene is a catalytic process of significant industrial importance, as the production of polyethylene requires that acetylene impurities in the ethylene stream be reduced to less than 5–10 ppm.12,13 Polymerization catalysts are extremely sensitive to the presence of alkynes, with even low levels causing significant poisoning and loss of activity. Therefore, highly selective catalysts are required to hydrogenate low concentrations of alkynes in the presence of a large excess of alkenes while minimizing over-hydrogenation to the undesired alkane.
In this review, we introduce the ‘structural chemistry’ of intermetallic compounds and discuss how their structural features can be leveraged to develop selective and active catalysts for hydrogenation reactions. This article is broadly divided into two parts: part I discusses the fundamental aspects of intermetallic compounds, such as their definition, structural properties (including bulk and surface characterization), and their unique electronic structure and chemical bonding scenarios. Part II focuses on recent investigations into the selective hydrogenation of acetylene using intermetallics as model catalysts, with particular emphasis on the precise control over active site configuration and composition inherent to intermetallic compounds.
2. Part I: fundamental aspects of intermetallic compounds
2.1 Definitions: solid solutions vs. intermetallic compounds
The mixing of metals has played an important role in human society. For thousands of years, it has been known that combining hard and soft metals at elevated temperatures results in the formation of new materials with improved properties. Early examples include bronze (88% Cu + 12% Sn) and brass (Cu + Zn), with early steel variants emerging a couple thousand years later.14,15 These materials exhibit emergent properties relative to the constituent metals. These new materials and their corresponding emergent properties revolutionized human society at the time, and the discovery of new metal mixtures continues to drive technological advancement today. For example, Ni–Al based intermetallic compounds are widely used in furnace components, jet engines, power plants, and water turbines due to their significantly high strength, low density, excellent thermal conductivity, and resistance to corrosion and oxidation.16–18 Increasingly complex mixtures of a wide array of elements have been discovered with properties desirable for different applications, including heterogeneous catalysis. The optimization of these materials for catalytic applications requires an understanding of the fundamental principles behind their formation and structure–property behavior. | ||
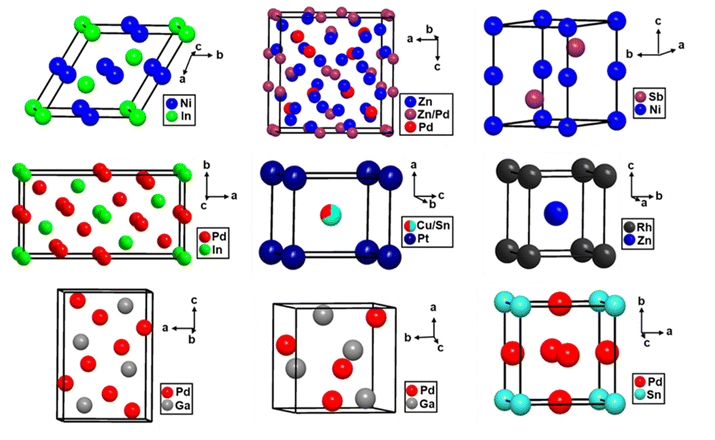
| Fig. 1 (a) Representative average structures of solid solutions and intermetallic compounds. In solid solutions, the structure type remains the same as at least one of the constituents, which distinguishes them from intermetallic compounds. (b) Structure of ideal γ-brass Cu5Zn8 (bcc),19 which differs from elemental Cu (fcc) and Zn (hcp), representing an intermetallic compound. All structural figures in this manuscript were drawn using DIAMOND 3 (ref. 20) and CIF files were collected from Pearson's Crystal Database.21 | ||
(a) The atomic size of the solute (minor component) in substitutional solid solutions should differ by less than 15% from that of the solvent (major component).
(b) For an interstitial solid solution, the solute atom's radius shouldn't be greater than 59% of the solvent.
(c) Electronegativities and valence electron counts (VEC) of the components must be comparable for the formation of solid solutions, whether substitutional or interstitial.
2.2 Challenging chemistry of intermetallic compounds: an inorganic chemist's perspective
Forty years ago, H. Schäfer stated “Our knowledge of the number of compounds in the intermetallic systems, their chemical properties, and most importantly, the bonding mechanism in these phases are far less developed than in other chemical fields”.35 Today, we ask the question, what is the current understanding of these materials, compared to other inorganic compounds? The Materials Genome and Materials Project database36–38 contains a vast collection of inorganic extended solids of already known structure types (most of them include ionic solids, thermoelectric materials, and perovskites), which has accelerated the discovery of new materials, but the prediction of the chemical properties of intermetallic compounds may be more difficult than for any other class of inorganic solids. Difficulty arises because intermetallic compounds have a large variety of compositions, crystal structures, charge transfer phenomena, and chemical bonding scenarios.26,34,39–41 As a result of these complexities, they are rarely the focus of inorganic chemists, compared to other solids e.g., oxides, halides, perovskites, and chalcogenides. Since several factors influence the chemistry of intermetallic compounds, only databases of known materials may not be helpful in the discovery of new compounds. One such factor is the assignment of the oxidation states of the constituent atoms in an intermetallic solid, which is not straightforward as in ionic and covalent solids, mostly due to partial charge transfer between constituent metals.26 Moreover, there is no specific electron counting scheme applicable to all classes of IMCs, making it a significantly more difficult task to comprehend this class of compounds compared to ionic solids. There have been some recent efforts to interconnect certain building blocks and polyhedra that occur in homologous intermetallic compounds and to determine the electronic stability of these phases by applying the 18-n rule.42,43 Predicting possible compositions that can exist in a particular system is also challenging. While many are likely able to rationalize the compositions of compounds such as KMnO4, HgI2, and Fe3O4, through simple oxidation state assignment and electron counting, it may not be straightforward for materials such as binary PtSb2, PtBi2, Pt3Co,44 PdSb2,45 Cu5Zn8,19 Pd2Zn11,46 Be21Pt5,31 and Sn3Sb2,;47 ternary ZrAl2.6Sn0.4,48 Ni3InSb,49 InPd2Cu,50 and many more. The situation becomes more complex for multinary systems like R2MoSi2C (R = Y, Gd)51 and Th2Au5Al8Si2.52For non-intermetallic compounds, when there is a significant covalent character or partial ionic character in bonding, charge transfer occurs between bonding atoms, which can be explained easily in terms of periodic table trends, whereas analysis of the electronic structure of intermetallic compounds is complicated due to the presence of delocalized electrons. Nevertheless, electronic structure calculations and detailed chemical bonding analysis are often required to explain structural features and phase stability.39,53 It is important to recognize the experimental reality of synthesizing these intermetallic compounds, specifically, the growth of single-crystalline and phase-pure materials is often itself a challenge. For example, the presence of oxophilic metals (e.g., Zn, Sn) as constituents may lead to oxide impurities along with the targeted intermetallic phase. If the targeted ternary intermetallic phase is a kinetic product, thermodynamically stable binary phases will interfere with the main phase, and in such an occasion, it is difficult to obtain phase-pure compounds suitable for measurements of physical and chemical properties.26,34
In solid-state chemistry, significant effort has been expended to develop structure–bonding–property relationships that connect fundamental variables like valence electron concentration (VEC, e/a), atomic size, and electronegativity. Due to the difficulties mentioned above, it is not a simple task to predict the crystal structure of intermetallic phases; even for the simplest equiatomic binary compounds, e.g., PdZn,54 RhCd,55,56 RhZn.57 Recently, machine learning approaches have been used to predict the structures of AB-type RhCd,55 Heusler compounds,58 binary rare-earth intermetallics,59 Laves phases,60,61 and a few other classes of inorganic extended solids.62 Machine learning models are trained based on the datasets of experimentally known compositions and several other geometrical and electronic factors alongside the reported structure type.63
Since the standard electron counting rules (e.g. the 18-electron rule, the octet rule, Wade's rules) do not universally apply to all intermetallic phases, chemical bonding analysis has been employed to uncover commonalities and trends within different intermetallic structures.39 This analysis positions various compounds along a continuum between structures governed by the octet rule and localized two-center, two-electron bonds at one extreme (the most basic Zintl compounds, VEC ≥ 4) and perturbations on the free electron (Hume-Rothery phases, VEC < 2) at the other, with the polar intermetallic compounds emerging in the middle (Fig. 2).53
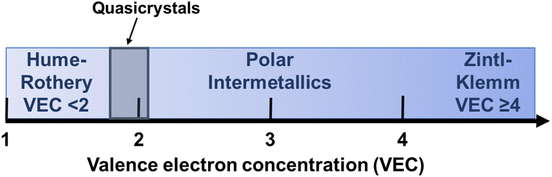 | ||
| Fig. 2 Trends of VEC for Hume-Rothery phases, quasicrystals (discussed later, grey zone), polar intermetallics, and Zintl–Klemm phases (left to right, respectively). | ||
Our limited knowledge of the properties of intermetallic compounds suggests we have only scratched the surface of their potential for advancing chemical applications. New compounds are frequently discovered by the trial-and-error approach of synthetic investigations and more knowledge-based approaches, such as machine learning and extensive phase diagram explorations.64 Improving our ability to discover new IMCs through knowledge-based approaches is the key to tailoring IMC properties to the desired applications. This will only be possible when we can establish rigorous structure–bonding–property correlations.26,39,53,65,66
2.3 Three broad classifications of IMCs
Based on the valence electron concentration (VEC), intermetallic phases can be broadly classified into three major groups: Hume-Rothery phases, polar intermetallic phases and Zintl–Klemm phases (Fig. 2). Hume-Rothery phases mainly consist of transition metal elements and post-transition metal main group elements. The average valence electron per atom (e/a), also known as the valence electron concentration (VEC), plays an important role in determining the structure of Hume-Rothery phases.24 Theoretical investigations suggest over a range of VEC values, the Fermi surface interacts with the first Brillouin zone, which leads to the formation of a “pseudogap” at the Fermi level (EF) in the electronic density of states (DOS).67,68 The result is a decrease in the total kinetic energy of the valence electrons, stabilizing phases with appropriate crystal structures to maximize the interactions between the Fermi surface and the first Brillouin zone. For this reason, alloys with similar VEC values exhibit similar crystal structures, even when their constituent elements differ. Other factors, such as the radius ratio and atomic size, also play a significant role alongside VEC in determining the crystal structure of these phases.68,69Zintl–Klemm phases are another class of intermetallic compounds consisting of electropositive (such as alkali and alkaline earth metals) and electronegative metals (such as p-block elements of group 13–15). In general, they occur at (e/a) ≥ 4. The large electronegativity difference between constituent elements leads to significant charge transfer between atoms, permitting the elements to obey the octet rule and behave like ionic salt-like compounds. The description of this class of compounds was developed by Zintl and later extended by Klemm.70,71 “This very simple idea, in my opinion, is the single most important theoretical concept (and how not very theoretical it is!) in solid-state chemistry of this century… it builds a bridge between solid-state chemistry and organic or main-group chemistry”, stated by Prof. Roald Hoffmann in 1987 regarding the charge transfer concept in Zintl–Klemm phases.65 NaTl (cF16, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, 227) was the first material that prompted Zintl's investigation into this class of intermetallic compounds.70–72
m, 227) was the first material that prompted Zintl's investigation into this class of intermetallic compounds.70–72
Polar intermetallic compounds fall between these two classes of compounds. They demonstrate partial charge transfer between their constituents (electropositive alkali, alkaline earth, or rare earth metals and more electronegative metals or metalloids), but complete valence electron transfer does not occur.73 Phase stabilization is conferred by the significant orbital overlap between the valence orbitals of the constituent elements. This class of compounds possesses both ionic and covalent interactions and, unlike Zintl phases, valence electron counting rules fail to justify the structure–composition relationship. Polar intermetallic compounds form a bridge between Hume-Rothery phases and Zintl phases.74
2.4 Other important classes of IMCs
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (227) space group in which Mg atoms form a cubic diamond net, while the Cu atoms form tetrahedral units. These tetrahedra share all the vertices in such a manner that each pair of fused tetrahedra adopts a staggered conformation (Fig. 3d). MgZn2 (C14) crystallizes in the hexagonal P63/mmc (194) space group where the Mg atoms form a hexagonal diamond net, and the Zn atoms are again described as forming tetrahedral units. These tetrahedra alternately share their faces along the c direction, forming chains of trigonal bipyramids joined at their apex. MgNi2 (C36) also crystallizes in the hexagonal P63/mmc (194) space group, where the Mg atoms form a hexagonal diamond net, and the nickel atoms form tetrahedral units, which are a combination of MgZn2 and MgCu2 type tetrahedral units. These structures can also be described by the stacking of 3.6.3.6 Kagomé nets and 36 triangular nets. The stacking sequence differs in different structure types. The coordination polyhedra of the A/B atoms in various Laves phases are shown in Fig. 3e. The A atoms lie at the center of the tetra-capped truncated tetrahedra with a coordination number of 16 (12B + 4A), which is the so-called Friauf polyhedra. The B atoms lie at the center of the distorted icosahedra, which comprises six A and six B atoms (Fig. 3e). The tetrahedral intersections in Laves phases could be a potential site to efficiently locate hydrogen atoms with optimal host–guest interactions. These Laves phase hydrides significantly contribute to atomic hydrogen-based energy storage.81
m (227) space group in which Mg atoms form a cubic diamond net, while the Cu atoms form tetrahedral units. These tetrahedra share all the vertices in such a manner that each pair of fused tetrahedra adopts a staggered conformation (Fig. 3d). MgZn2 (C14) crystallizes in the hexagonal P63/mmc (194) space group where the Mg atoms form a hexagonal diamond net, and the Zn atoms are again described as forming tetrahedral units. These tetrahedra alternately share their faces along the c direction, forming chains of trigonal bipyramids joined at their apex. MgNi2 (C36) also crystallizes in the hexagonal P63/mmc (194) space group, where the Mg atoms form a hexagonal diamond net, and the nickel atoms form tetrahedral units, which are a combination of MgZn2 and MgCu2 type tetrahedral units. These structures can also be described by the stacking of 3.6.3.6 Kagomé nets and 36 triangular nets. The stacking sequence differs in different structure types. The coordination polyhedra of the A/B atoms in various Laves phases are shown in Fig. 3e. The A atoms lie at the center of the tetra-capped truncated tetrahedra with a coordination number of 16 (12B + 4A), which is the so-called Friauf polyhedra. The B atoms lie at the center of the distorted icosahedra, which comprises six A and six B atoms (Fig. 3e). The tetrahedral intersections in Laves phases could be a potential site to efficiently locate hydrogen atoms with optimal host–guest interactions. These Laves phase hydrides significantly contribute to atomic hydrogen-based energy storage.81
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, Cu2MnAl-type) and half Heusler, XYZ, with the C1b structure type (F
m, Cu2MnAl-type) and half Heusler, XYZ, with the C1b structure type (F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m, MgAgAs-type), where X and Y are transition or rare-earth metals and Z is a main-group element (Fig. 4). The structure of full Heusler compounds can be described by four interpenetrating fcc sublattices of the constituent elements. The removal of X or X′ leads to half-Heusler structures.83 Tetragonally distorted, hexagonal and quaternary variants are also known in this class of intermetallic compounds.82,83 Although Heusler and half-Heusler phases have long been recognized as magnetic materials, they can also be heavy fermion systems and superconductors if rare-earth elements are included.84,85 Their potential in heterogeneous catalysis has been investigated more recently.86–89
3m, MgAgAs-type), where X and Y are transition or rare-earth metals and Z is a main-group element (Fig. 4). The structure of full Heusler compounds can be described by four interpenetrating fcc sublattices of the constituent elements. The removal of X or X′ leads to half-Heusler structures.83 Tetragonally distorted, hexagonal and quaternary variants are also known in this class of intermetallic compounds.82,83 Although Heusler and half-Heusler phases have long been recognized as magnetic materials, they can also be heavy fermion systems and superconductors if rare-earth elements are included.84,85 Their potential in heterogeneous catalysis has been investigated more recently.86–89
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m (217) with a lattice parameter, a ≈ 9 Å, and contains ∼52 atoms per unit cell. The γ-brass type structures can be described by a 26-atom cluster formed by four atomic shells. Moving outward from the center of the cluster, these shells can be described as follows: four atoms forming an inner tetrahedron (IT), an outer tetrahedron (OT) of four atoms sitting above the faces of the IT, six atoms forming an octahedron (OH) such that each OH atom lies above the edge of the OT, and a distorted cuboctahedron (CO) of twelve atoms placed above the edges of the OH. In γ-brass Cu5Zn8, Zn atoms occupied the IT and CO sites, whereas Cu atoms occupied the OT and OH sites (Fig. 5). Electronic structure calculations based on Mulliken population analyses demonstrated the less electronegative element in a γ-brass variant prefers the IT and CO sites, whereas the more electronegative element prefers the OT and OH sites.19 It is noteworthy that the atomic distributions in the homologous compound Cu4Cd9 are different from those of Cu5Zn8, where the difference between the atomic radius of Cu and Cd and the chemical bonding scenario play a decisive role (Fig. 5).91
3m (217) with a lattice parameter, a ≈ 9 Å, and contains ∼52 atoms per unit cell. The γ-brass type structures can be described by a 26-atom cluster formed by four atomic shells. Moving outward from the center of the cluster, these shells can be described as follows: four atoms forming an inner tetrahedron (IT), an outer tetrahedron (OT) of four atoms sitting above the faces of the IT, six atoms forming an octahedron (OH) such that each OH atom lies above the edge of the OT, and a distorted cuboctahedron (CO) of twelve atoms placed above the edges of the OH. In γ-brass Cu5Zn8, Zn atoms occupied the IT and CO sites, whereas Cu atoms occupied the OT and OH sites (Fig. 5). Electronic structure calculations based on Mulliken population analyses demonstrated the less electronegative element in a γ-brass variant prefers the IT and CO sites, whereas the more electronegative element prefers the OT and OH sites.19 It is noteworthy that the atomic distributions in the homologous compound Cu4Cd9 are different from those of Cu5Zn8, where the difference between the atomic radius of Cu and Cd and the chemical bonding scenario play a decisive role (Fig. 5).91
 | ||
| Fig. 5 (top) A 26-atom γ-brass cluster shown in the unit cell of typical gamma-brass; IT (yellow), OT (pink), OH (blue), and CO (green). (bottom) Comparison between the atomic distributions of two 26-atom γ-clusters, Cu5−xZn8+x (left)19 and Cu4Cd9 (right):91 brown spheres: Cu atoms; white spheres: Zn atoms; and black sphere: Cd atoms. | ||
One of the first examples of a complex IMC is NaCd2. Despite its simple stoichiometry, the diffraction patterns were found to be so complex that solving the structure was nearly impossible 100 years ago. It took more than 30 years before the structure was successfully solved; it crystallized in the high symmetry Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (227) space group with a unit cell of ∼30 Å that contains 1152 atoms.94 Our ability to accurately determine the crystal structure of complex intermetallic compounds has significantly improved, especially with the development of electron diffraction. Nevertheless, the complexity of these structures challenges our ability to understand their formation.
m (227) space group with a unit cell of ∼30 Å that contains 1152 atoms.94 Our ability to accurately determine the crystal structure of complex intermetallic compounds has significantly improved, especially with the development of electron diffraction. Nevertheless, the complexity of these structures challenges our ability to understand their formation.
The bulk electronic structures of many CMAs feature real or pseudogaps in the density of states at the Fermi level. The electronic stability of CMAs is often associated with a definite valence electron concentration (e/a), which is attributed to the previously mentioned Hume-Rothery mechanism.25 The complex bulk crystal structures of CMAs mean they often possess rather irregular and strongly corrugated surfaces, which leads to difficulties in the determination of the atomistic details of the surface. To deal with the complexity of these surfaces, a combination of STM and ab initio DFT calculations was used to unveil both the energetics and electronic and geometric structure of the stable surfaces of CMAs.95
Intermetallic carbides96 and hydrides,97 though less extensively studied than their binary and ternary counterparts, represent important subclasses of IMCs that exhibit unique structural motifs and offer intriguing properties related to heterogeneous catalysis and atomic hydrogen storage.
2.5 Coloring problem in intermetallic compounds: site preference in the bulk crystal structure
In solid-state chemistry, the ‘coloring problem’66 refers to the difficulties associated with determining the distribution of different atoms within a given crystal structure. The connectivity and arrangement of different atoms in extended solids strongly affect their physical and chemical properties. The fundamental question is “Given a molecular or extended network and several different types of atoms or chemical groups, in what arrangement is the energy of the system minimized for a fixed stoichiometry?” In other words, which ‘coloring scheme’ is favorable over another? It is also important to consider why one ‘coloring scheme’ is favorable over another. Determining the preferred ‘coloring scheme’ is key to establishing structure–property relationships in catalysis and other research areas.103The accurate assignment of constituent atoms among different sites is primarily investigated by X-ray diffraction (XRD). The assignment of atoms from XRD may not be accurately determined for elements that neighbor each other on the periodic table because they have similar X-ray scattering factors and therefore are indistinguishable using XRD. In these cases, neutron diffraction is usually employed to differentiate the neighboring elements under investigation. However, some elements commonly used in solid-state materials are neutron absorbers (e.g., Cd, B, Gd), which rules out the application of neutron diffraction to characterize intermetallics containing these elements or require the use of expensive isotopes. Additionally, neutron diffraction typically requires a beamline neutron source, complicating the accessibility of the measurement. Due to experimental challenges, the site distribution of atoms in structures is often addressed using first-principles calculations.
Density Functional Theory (DFT)-based104 total energy calculations are executed on various models to accurately determine the coloring scheme preferred by the constituent elements in a given structure. The factors influencing one coloring scheme over others are addressed by the energetic contributions from site potentials and pairwise interatomic potentials. Valence electron population analysis (Mulliken or Löwdin's population analysis)105,106 at each site of all configurations is often required to accurately determine the preferred coloring scheme. For site preference, site energy is an important contributing factor. The more electronegative atoms usually prefer sites with a higher electron population to lower the electronic energy of the system (topological charge stabilization).107,108 Conversely, bond energy factors can also contribute to the observed site distribution pattern in the same structure, which can be addressed by comparing the number of homoatomic and heteroatomic contacts in all possible coloring schemes. If a greater number of heteroatomic contacts lead to a lower total energy, the correct coloring scheme will likely contain a higher number of heteroatomic contacts. In many intermetallic structures, both the site energy and bond energy factors contribute to the observed distribution pattern of the constituents.
2.6 Relationship between crystal structure, electronic structure, partial charge transfer, and core chemical bonding scenario in the IMCs
To understand the electronic stability of any intermetallic compound from a chemical standpoint and specific site substitution patterns, electronic structure calculations were performed. The electronic density of states (DOS) uncovers ‘where the electrons are’ and provides an overall idea about the electronic environment of intermetallic compounds. The crystal orbital Hamilton population (COHP)109 method is a theoretical bond analysis tool for solids that ‘partitions the band-structure energy into orbital pair interactions’ (orbital-space bonding description). From a chemical point of view, COHP is a “bond-weighted density-of-states between two adjacent atoms within their interacting distance”.110 A COHP diagram is generally plotted alongside the DOS, which indicates the bonding and anti-bonding contributions to the band-structure energy. DOS and COHP provide a clear picture of the complete electronic structure of the material (i.e., where the electrons are and how they bond to each other). The integrated electronic DOS yields the total number of electrons in the system under investigation, whereas the integrated COHP (ICOHP) provides information regarding the bond strength (in eV or kJ mol−1). After the calculation of the self-consistent electronic wave function (SCF), both the DOS and COHP can be determined, allowing important chemical information to be extracted.111,112 Another method to quantify covalent bonding in solids, crystal orbital bond index (COBI), has been introduced recently.113 COBI is very similar to COHP and relates directly to the ‘bond order’. Additionally, COBI can identify multicenter interactions in periodic extended solids.53,113 TB-LMTO (tight binding linear muffin-tin orbital)114 developed by Andersen et al. and LOBSTER (local-orbital basis suite toward electronic structure reconstruction)110,115 developed by Dronskowski et al. are the two widely used open-source tools to calculate COHP in periodic solids. The latter method uses plane-wave basis sets and allows the extraction of a chemically useful projected COHP (pCOHP).For illustrative purposes, we calculated the DOS, COHP and COBI of a catalytically relevant intermetallic compound PdGa (FeSi-type, P213) (Fig. 7) using the crystal structure from ref. 118. The Fermi level of the density of states is significantly different from that of a pure metal with a hint of a pseudogap, and the overlap between the Pd 4d and Ga 4s, 4p states leads to strong and partially covalent Pd–Ga heteroatomic interactions. Among the three possible interactions in the structure of PdGa, the average integrated COHP (and COBI) values were highest for the Pd–Ga bonds, which contributed to the structural stability of PdGa.
 | ||
| Fig. 7 (top) Electronic density of states of the catalytically relevant intermetallic compound PdGa.27 (bottom) COHP and COBI plots of individual interactions in the structure of PdGa (with average integrated values). Adapted from ref. 27 and calculated by LOBSTER115 for illustrative purposes. Structural optimization was performed using Quantum Espresso,116 and plots were created using wxDragon.117 | ||
In the context of heterogeneous catalysis, these calculations extract the chemical bonding interactions, bond strengths, and structural stability of the catalyst,119,120 but can also reveal information about substrate–catalyst surface interactions and may provide insightful mechanistic details by revealing the different possible adsorption modes of a substrate on the surface of a catalyst.121
The Quantum Theory of Atoms in Molecules (QTAIM),122 developed by Richard Bader, is another intuitive method for breaking molecules down into atoms to understand their mutual interactions. The definition of an atom is based only on the density of charge and divides atoms using a technique known as zero flux surfaces.123 A definition of chemical bonding with numerical values for bond strength can also be provided by this theory. Henkelman's group developed a grid-based approach that can be successfully applied to extended solids, e.g., intermetallic compounds. This approach allows for the analysis of the large grids generated from plane wave-based density functional theory calculations.124 A similar approach is implemented in the novel functional ‘electron localizability indicator’ (ELI), which has been proven useful in investigating the core chemical bonding situation in periodic solids. ELI can be broken down into partial orbital contributions (unlike the classic ‘electron localization function’,125 ELF).126 A combination of the electron density (ED) and electron-localizability indicator (ELI-D) can reveal details about a larger range of interactions, from single pairs to multi-center bonds. Both the distinct core regions and the shared valence region can be identified in the ELI-D distribution. The distribution is spherically symmetric for non-interacting atoms, and the existence of atomic interactions is indicated by any divergence from the spherical distribution. Local ELI-D maxima (attractors) in the valence region help visualize bonds in real space.127
Unlike Bader's QTAIM,122 Mulliken106 and Löwdin105 population analyses are based on the electron density matrix, using a suitable and balanced basis set of atomic-like orbitals and considering the overlap population of the orbitals. Despite being a simplified approach, Mulliken populations are still widely used to determine the atomic charges of the constituent elements of non-molecular solids. For example, the ELF plot (Fig. 8) on the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) slice of cubic FeSi-type PdGa shows a significant electron density between Pd and Ga (partial covalent character), and Mulliken charges on Pd and Ga indicate the direction of charge transfer from Ga to Pd, which correctly aligns with the expected trend based on their electronegativity.27,118 Wider green regions between the Ga atoms indicate more profound covalency of Ga–Ga interactions. The implications of the electronic charge transfer, strong Pd–Ga heteroatomic bonds of intermetallic PdGa on its performance in the selective hydrogenation of acetylene are discussed in Section 3.4.
) slice of cubic FeSi-type PdGa shows a significant electron density between Pd and Ga (partial covalent character), and Mulliken charges on Pd and Ga indicate the direction of charge transfer from Ga to Pd, which correctly aligns with the expected trend based on their electronegativity.27,118 Wider green regions between the Ga atoms indicate more profound covalency of Ga–Ga interactions. The implications of the electronic charge transfer, strong Pd–Ga heteroatomic bonds of intermetallic PdGa on its performance in the selective hydrogenation of acetylene are discussed in Section 3.4.
 | ||
Fig. 8 (a) Unit cell of FeSi-type PdGa. (b) ELF plot on a (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) slice of PdGa [adapted from ref. 27 and 118]. The average Mulliken charge on the Pd site is −0.45 and on Ga site is +0.45, indicating partial charge transfer from Ga to Pd. The ELF was calculated using Quantum Espresso,116 and the plot was drawn using VESTA. ) slice of PdGa [adapted from ref. 27 and 118]. The average Mulliken charge on the Pd site is −0.45 and on Ga site is +0.45, indicating partial charge transfer from Ga to Pd. The ELF was calculated using Quantum Espresso,116 and the plot was drawn using VESTA. | ||
The contribution of the atomic sizes of the constituent elements in an intermetallic structure remains vague in the abovementioned experimental and theoretical approaches. An intuitive and relatively newer approach, called density functional theory-chemical pressure (DFT-CP) analysis, introduced by Fredrickson et al.128 considers the influence of atomic size as the ‘most pronounced factor’ while taking into account chemical interactions between the constituents. The tools developed by Fredrickson et al.129 can visualize the local chemical pressures on individual elements inside the unit cell of a periodic structure. This aids in understanding whether bond formation is inhibited by steric repulsion or by insufficient electronic support in homologous compounds. This method has been applied to relatively simple structure types to rather complicated structures exemplified by complex metallic alloys and quasicrystal approximants.130,131
Our understanding of the formation, structural stability, site preference of constituent elements, and differences in the structure types of homologous series of compounds in intermetallic solids remains incomplete despite the varied approaches used to characterize their electronic structure chemical bonding. As discussed above, this includes quantum theory of atoms in molecules (QTAIM),122 electron localization function125 (and electron localizability indicator),126 orbital space chemical bonding analysis (e.g., COHP,109,112 COBI113), DFT-CP analysis (chemical pressure).128 A single approach often fails to explain the intricate structural phenomenon in intermetallic solids. For example, the unprecedented site preference of Zn in the structure of ordered tetragonal Cu6Zn2Sb2 can be explained based on Mulliken's and Lowdin's charge population analysis (topological charge stabilization), but Bader's QTAIM failed to arrive at the same conclusion.132,133 A recent study of the CoSn-type intermetallic compound (P6/mmm) NiIn1−xSnx (x = 0.7–0.9)134 has shown the specific site-preference of Sn can be explained by Bader's charge, Mulliken's and Lowdin's charge population and real space charge density analyses. All these calculations led to the same conclusion (unlike the previously mentioned Cu6Zn2Sb2)133 regarding the experimentally established (based on X-ray and neutron diffraction) ‘coloring scheme’. However, one should be extremely careful regarding the choice of basis-sets (orbitals) in orbital-space calculations.
These different approaches can lead to significant differences in the perception of chemical bonding in extended inorganic solids. For example, Bader's density-based approach identifies a new bonding mechanism, i.e., metavalent bonding/electron-deficient bonds in phase change memory materials,135 topological insulators, and halide perovskites. On the other hand, the orbital-based approach finds this bonding scheme “unjustified” and identifies the bonding in these electron-rich materials, 3c–4e (‘hypervalent’); this approach believes ‘electron density’ alone is insufficient and requires analysis of the electronic wave functions (the orbitals).136
2.7 Knowledge of the bulk is not enough: importance of surface structure and composition in heterogeneous catalysis
The previous section discussed the structure(s) of different type(s) of intermetallic compounds and the fundamentals associated with them. However, when considering these materials from the perspective of heterogeneous catalysis, the surface and electronic structure are more important because they determine the nature of the active sites. From a heterogeneous catalysis perspective, site preferences (the ‘coloring scheme’ on the surface) and the most stable surface facets (under the influence of adsorbates) are important because they determine the local environment of the exposed active sites. Site preferences in the bulk can be found by completing bulk energy calculations for different possible configurations within the experimentally determined model structure; however, this does not provide information about surface site preferences. Therefore, surface energy calculations are needed to provide accurate predictions of the distribution of active and inactive constituent elements on the surface.95The importance of surface structure is highlighted with Ni–Zn γ-brass compounds that contain different Ni atomic percentages (Ni8Zn44, Ni9Zn43, Ni10Zn42, Ni11Zn41).137 While it was anticipated the catalyst would become more active with increasing Ni content, the rate of H–D exchange was invariant with the bulk composition. Neutron powder diffraction, in combination with bulk energy calculations, was used to investigate the bulk structure of the compounds, including site preferences, and additional Ni in the higher Ni content preferred octahedral sites (OH). Thus, the isolation of the Ni-sites was lost, and the bridging Ni atoms in the OH sites formed Ni–Ni–Ni trimers. The results of the bulk structure investigation do not provide any explanation for the insensitivity of the rate of H–D formation to Ni content, requiring consideration of the most stable surface facet(s) and the extent of Ni exposure on those facets. Surface energy calculations demonstrated that surface facets containing Ni–Ni–Ni trimer sites were energetically unfavorable and therefore not exposed (Fig. 9). Since the additional Ni in these compounds remains sub-surface, it has no measurable impact on catalysis.137
 | ||
Fig. 9 (left) Ni–Ni–Ni trimer present as determined by experiments in the bulk of Ni9Zn43. (right) The corresponding Wulff construction showing that the Ni–Ni–Ni trimer containing {1 ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0} facet is not energetically favored to be exposed. The orange and grey spheres represent Ni and Zn, respectively. Reprinted with permission from ref. 137, copyright 2017, the American Chemical Society. 0} facet is not energetically favored to be exposed. The orange and grey spheres represent Ni and Zn, respectively. Reprinted with permission from ref. 137, copyright 2017, the American Chemical Society. | ||
The homologous γ-brass compounds in the Pd–Zn system [Pd8Zn44, Pd9Zn43, Pd8MZn44 (M = Cu/Ag/Au)] differ significantly from each other (and from the Ni–Zn system) in terms of their calculated surface composition and surface ensembles. A combined experimental and theoretical effort has revealed that M (Cu/Ag/Au) has a strong substitution preference on the Zn atoms of the OH-site, whereas Pd exclusively occupies the OT-site.138 Surface energy calculations led to the conclusion that, different catalytic ensembles on the surface, such as monomer Pd1 (i.e., Pd–Zn–Pd) for Pd8Zn44, and trimer Pd3 (i.e., Pd–Pd–Pd) sites for Pd9Zn43, are responsible for at least six orders of magnitude difference in the rates of ethylene hydrogenation.139 A very specific site occupancy pattern of Pd and Zn in the Pd–Zn γ-brass IMCs lead to the transition from Pd1 monomer active sites in Pd8Zn44 (15.4 at% Pd) to Pd3 sites in Pd9Zn43 (17.3 at% Pd). When a third element was introduced with the nominal composition Pd8MZn44 (M = Cu/Ag/Au), the coinage metal showed a strong preference for the OH-site which modifies the catalytic active site ensemble to Pd–M–Pd (Fig. 11).138,140 These modifications have influenced the performance of these intermetallic catalysts for the selective hydrogenation of acetylene, and these aspects are discussed in Section 3.2.
Alongside the DFT-based energy calculations, experimental surface characterization techniques are extremely important to establish a complete understanding of the structure (surface)–catalytic property relationship of IMCs. A detailed surface characterization of PdGa (FeSi-type) by Kovnir et al.141 compared the bulk structure of PdGa with its surface structure. In situ XRD and in situ EXAFS (Extended X-ray Absorption Fine Structure) proved the structural stability of PdGa during the selective hydrogenation of acetylene. XPS (X-ray photoelectron spectroscopy) measurements confirmed the electronic structure of the surface closely resembled the bulk. Comparative CO adsorption experiments (Fourier transform infrared spectroscopic measurements, CO-FTIR) of PdGa and a commercially available reference catalyst, Pd/Al2O3, revealed the isolated nature of the active Pd sites on the surface of PdGa.141 The aspects of this catalyst related to the selective hydrogenation of acetylene are discussed in Section 3.3.
3. Part II: recent investigations of intermetallic compounds as selective hydrogenation catalysts
3.1 Alloying introduces limited flexibility in active site composition
Noble metals such as rhodium, ruthenium, palladium, and platinum are frequently employed as heterogeneous catalysts for various hydrogenation reactions because of their high activity. However, these metals are expensive, and their high activity is typically achieved at the expense of poor selectivity. Deactivation of these expensive materials via coking imposes the need for catalyst regeneration or replacement.142 The inherent lack of flexibility in the active site composition of monometallic catalysts implies a reliance on the intrinsic properties of the metal surface, which rarely results in the optimum adsorbate binding energy, as described by the Sabatier principle.143Alloying these noble metals, typically with a less catalytically active metal, offers the ability to modify the active sites and, as such, the potential to improve catalytic properties, particularly selectivity and stability. For instance, AgxPd1−x alloys have been employed industrially for many years in the selective hydrogenation of acetylene to ethylene due to a higher selectivity compared to pure Pd.144–147 X-ray absorption spectroscopy studies, in combination with classic electronic structure calculations, have demonstrated the selectivity improvement is largely due to an electronic effect; charge transfer from Ag to Pd results in an increase in filled Pd 4d-states.148–150 The increased electron density in the Pd 4d-states results in weaker binding energies of unsaturated hydrocarbons (ethylene and acetylene) since they are electronically rich and typically donate electron density to Pd. Although this adsorption behavior may lead to decreased rates of acetylene hydrogenation, the weakened ethylene adsorption decreases the propensity for over-hydrogenation and therefore improves selectivity.
The presence of electronic modifications does not eliminate the possibility of structural changes to the surface of the active metal, which may also contribute to improved catalyst performance. Infrared studies using CO as a probe molecule demonstrated an increase in the isolation of Pd sites upon alloying with Ag correlated with improved selectivity in acetylene hydrogenation.151 It was proposed that an increase in the isolation of Pd sites may result in a decrease in hydrogen concentration around the active site, and therefore allow ethylene to desorb before over-hydrogenation. This highlights one of the key challenges in understanding the catalytic behavior of these materials: distinguishing the contribution of electronic and geometric effects on catalytic performance. The origin of the increased selectivity over Ag–Pd alloys is further complicated because these alloys are randomly substituted (see substitutional alloys in Section 2.1 above), implying that several possible active site configurations contribute to the observed catalytic behavior. Additionally, the lack of any significant preference for a particular lattice position by the constituent elements means catalyst pre-treatment (e.g., calcination, reduction) or changes in the process conditions may result in significant restructuring of the catalyst.147,151–153
Nørskov and Studt et al.154,155 established scaling relationships between acetylene and ethylene adsorption energies on transition metal surfaces as a function of the adsorption energy of the methyl group. Acetylene and ethylene form four and two σ-bonds, respectively, with the surface of the transition metal and these adsorption energies scale with respect to the adsorption energy of a methyl group. A catalyst that weakly binds a methyl group, compared with pure Pd, will be more selective than pure Pd. Their prediction identified several alloys potentially active and selective catalysts, e.g., Pd–Au, Pd–Ag, Pd–Ga, and Pd–Sb.155 The same work identified alloys of Ni, a cheaper alternative to Pd, as selective catalysts. The results were experimentally verified for Ni–Zn alloys, and it was found the alloy with the highest Zn content had a higher selectivity to ethylene than the widely used Pd–Ag alloys.154
Intermetallic compounds offer the opportunity for significantly greater control over the electronic and geometric structure of active sites, largely due to specific site preferences in the crystal structure, which allows for the development of rationally designed catalysts for selective hydrogenation. This strong site preference also has implications regarding stability since it can mitigate the issue of segregation under pre-treatment and/or reaction conditions, which is often a challenge with random alloys. The complex bonding and electronic structure of intermetallic compounds suggest they can have very different catalytic properties compared to the parent metals, which allows for the potential to develop active and selective catalysts with cheaper and more abundant elements. There is an increasing interest in the use of intermetallic compounds as heterogeneous catalysts; the next section of this review focuses on their use as model catalysts in selective alkyne hydrogenation reactions. The crystal structures of a few catalytically relevant intermetallic compounds are depicted in Fig. 10.
3.2 Controlling active site composition and configuration using intermetallic compounds for the rational design of catalysts for selective hydrogenation reactions
The specific site preferences found in intermetallic compounds allow precise control and accurate prediction of the composition and configuration of active sites present on the surface of the catalyst. The superior control offered by intermetallic compounds was exemplified in a recent study that explored Pd–Zn γ-brass intermetallics for the selective hydrogenation of acetylene to ethylene.140 The lowest energy facet, {110}, exposes the OT–OH–OT ensemble (see Section 2.7 above), with the composition of this ensemble being highly dependent on the stoichiometry of the intermetallic compound. For instance, in the case of Pd8Zn44, the ensemble consists of Pd–Zn–Pd resulting in Pd sites completely isolated by Zn, denoted as Pd “monomers”. Subtle increases in the stoichiometric Pd content lead to the introduction of Pd–Pd–Pd ensembles, denoted as Pd “trimers”, while Pd9Zn43 consisting of a mix of Pd trimers and monomers and Pd10Zn42 consisting of only Pd trimers (Fig. 11). Pd8Zn44, composed of completely isolated Pd monomer sites (Pd–Zn–Pd), was highly selective for ethylene but with a low rate of acetylene hydrogenation. The Pd3-trimer sites (Pd–Pd–Pd) on Pd9Zn43 were found to be significantly more active for the hydrogenation of acetylene; however, they were also at least ∼106 times more active for ethylene hydrogenation, resulting in a significant decrease in selectivity. The di-σ-binding of acetylene across a Pd monomer “pair” permits H co-adsorption followed by C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C hydrogenation. However, π-bound ethylene prevents H2 dissociative adsorption, H co-adsorption, and C
C hydrogenation. However, π-bound ethylene prevents H2 dissociative adsorption, H co-adsorption, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogenation at the isolated Pd-sites. The nuclearity of the active sites (i.e., Pd monomers versus trimers) is directly correlated to both the activity and selectivity of the catalyst during the selective hydrogenation of acetylene.
C hydrogenation at the isolated Pd-sites. The nuclearity of the active sites (i.e., Pd monomers versus trimers) is directly correlated to both the activity and selectivity of the catalyst during the selective hydrogenation of acetylene.
 | ||
| Fig. 11 (top) Visualization of Pd–Zn–Pd, Pd–Pd–Pd, and Pd–M–Pd (M = Cu/Ag/Au) trimers in the {110} facet of Pd–Zn γ-brass intermetallic compounds.140 (bottom) The activity (left) and selectivity (middle) show strong agreement between experimental kinetics and DFT. Reprinted with permission from ref. 140 copyright 2022, Nature. | ||
Further modification of the active site was also found to be possible through the inclusion of small amounts of a coinage metal to produce intermetallic compounds with a composition of Pd8MZn43 (M = Cu, Ag, Au). With this stoichiometry, Pd was found to preferentially occupy the OT sites and the coinage metal occupied OH sites,138 resulting in Pd–M–Pd ensembles. The activity and selectivity of the Pd–M–Pd ensembles were intermediate between the Pd monomers and the Pd trimers in the binary system. Therefore, both the nuclearity and composition of the active site ensemble were found to determine the overall catalyst activity and selectivity in acetylene hydrogenation, which can be controlled with the careful and rational design of intermetallic compounds.140
The configuration of the active site was also found to be crucial to achieve high selectivity in acetylene hydrogenation with Pd–In intermetallic compounds.156 PdIn (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) has isolated Pd sites on the lowest energy {110} surface, however Pd3In (P4/mmm) has Pd3-trimer sites on the lowest energy {111} surface. These intermetallic catalysts displayed notably different selectivity in acetylene hydrogenation; PdIn was found to have a selectivity of 92%, whereas Pd3In had a selectivity of only 21%. DFT calculations for PdIn showed the desorption energy for ethylene was lower than the hydrogenation barrier because of the isolation of the Pd sites by In. However, for Pd3In, the ethylene desorption energy was similar to the hydrogenation barrier, consistent with the increased rate of ethylene hydrogenation and poor ethylene selectivity. The distance between adjacent Pd atoms was therefore identified as a structural descriptor of ethylene formation during the selective hydrogenation of acetylene.156
m) has isolated Pd sites on the lowest energy {110} surface, however Pd3In (P4/mmm) has Pd3-trimer sites on the lowest energy {111} surface. These intermetallic catalysts displayed notably different selectivity in acetylene hydrogenation; PdIn was found to have a selectivity of 92%, whereas Pd3In had a selectivity of only 21%. DFT calculations for PdIn showed the desorption energy for ethylene was lower than the hydrogenation barrier because of the isolation of the Pd sites by In. However, for Pd3In, the ethylene desorption energy was similar to the hydrogenation barrier, consistent with the increased rate of ethylene hydrogenation and poor ethylene selectivity. The distance between adjacent Pd atoms was therefore identified as a structural descriptor of ethylene formation during the selective hydrogenation of acetylene.156
Modification of the active site configuration in intermetallic compounds has implications not only for selectivity but also stability. Spanjers et al.157 explored the effect of the Zn content in Ni-based intermetallics on the active site geometry for the selective hydrogenation of acetylene. Monometallic Ni catalysts are prone to oligomerization during acetylene hydrogenation, which leads to the formation of by-products which contribute to catalyst deactivation.158 Ni–Zn intermetallic catalysts were proposed to significantly reduce the formation of these oligomeric species, which should limit catalyst deactivation and improve the selectivity toward ethylene.157 Three intermetallic systems were chosen in this study: cubic Ni4Zn (fcc-Cu type, with 2.4 Ni neighbors replaced by Zn), tetragonal NiZn (AuCu-type, with 8 Zn nearest neighbors) and Ni5Zn21 (gamma-brass type, with the Ni sites completely isolated by Zn). The amount of observed oligomerization during acetylene hydrogenation was found to decrease with increasing Zn content (and increasing Ni isolation); however, DFT calculations initially appeared to contradict this result since the barrier for C–C bond formation was found to be lower for NiZn compared to monometallic Ni. The inclusion of Zn was also found to lower the acetylene binding energy, and the authors demonstrated through the implementation of a Langmuir–Hinshelwood model that the lower acetylene binding energy would result in significantly lower acetylene surface coverage. Since oligomerization occurs through the reaction of an adsorbed acetylene molecule with a partially hydrogenated acetylene intermediate, the lower surface coverage of acetylene would drastically lower the rate of oligomerization. Therefore, the lower acetylene binding energy observed for the NiZn intermetallic compound explains the decrease in oligomerization and the resultant increase in both selectivity and stability.157
An extreme case of site isolation was reported by Furukawa et al.159 by the introduction of multiple metals to CsCl-type NiGa. The Ni and Ga sites were partially substituted by Fe/Cu and Ge, respectively, to form a high entropy intermetallic (HEI) of (NiFeCu)(GaGe). This HEI material was used as a catalyst for the hydrogenation of acetylene and demonstrated high selectivity for ethylene compared to the parent NiGa catalyst. These site-specific substitutions lowered the energy barrier of acetylene hydrogenation and weakened acetylene adsorption, facilitating hydrogen adsorption and activation. DFT studies demonstrated the most stable facet of the HEI was (110), because of the relaxation of the surface upon site-specific substitution. The lower surface energy of (110) promotes the desorption of ethylene, inhibiting undesired over-hydrogenation. DOS calculations indicated there was a negligible change in the electronic structure of Ni upon substitution with Fe and Cu, suggesting the enhanced catalytic performance was due to geometric effects (site isolation) rather than electronic effects.159
Furukawa et al. also demonstrated the extreme site isolation achieved in HEIs through the site-specific substitution of the FeAs-type PtGe intermetallics.160 The Pt site in the binary PtGe was partially substituted by catalytically less active metals (Co and Cu), whereas the Ge site was partially replaced by inert metals (Ga and Sn) to form a senary HEI of the form, (PtCoCu)(GeGaSn) (Fig. 12). This HEI catalyst was used for propane dehydrogenation, demonstrating high selectivity to propylene by inhibiting unwanted side reactions. X-ray absorption near-edge structure (XANES) confirmed the d-band of Pt did not significantly change between PtGe and the HEI, indicating negligible electronic effects upon Pt substitution, consistent with DFT calculations (a very similar observation was reported by the authors in the case of (NiFeCu)(GaGe)).159 The high selectivity of the HEI was attributed to geometric effects due to the dilution of Pt with less active metals, which reduced the concentration of surface Pt–Pt ensembles and hence favored the desorption of propylene as a consequence of the limited number of sites available for binding propylene. The authors compared the HEI to other quaternary catalysts to demonstrate the significance of the high entropy effect on the catalyst selectivity and stability. However, it is very challenging to attribute such improvement to the high entropy effect and/or site isolation since the composition of the active metal (Ni/Pt) was not constant across these catalysts.
 | ||
| Fig. 12 Schematic of the site-specific substitution of PtGe to form HEIs, and the Pt site isolation in HEIs compared with HEAs. Reprinted with permission from ref. 160, copyright 2022, the American Chemical Society. | ||
3.3 Role of electronic modification on the performance of intermetallic catalysts in selective hydrogenation reactions
The previous section discussed the precise control of the active site configuration and composition that can be achieved using intermetallic compounds and how this control affects the catalyst performance in selective hydrogenation reactions. However, intermetallic compounds also offer the opportunity to modify the electronic properties of active sites. While there are several examples of intermetallic compounds in which the dominant effect is geometric, there are few examples in which the dominant effect is electronic. Instead, the improvement in the catalytic performance of these intermetallic compounds can be attributed to a combination of electronic and geometric modifications. The ordered structures inherent to intermetallic compounds allow the contributions of these two effects to be distinguished in many cases, as discussed in the following section.Pd–Ga ordered intermetallic compounds (e.g. GaPd2, GaPd, Ga7Pd3) were proven to be highly selective for acetylene hydrogenation compared to pure Pd.27,28,141,161 The increased selectivity to ethylene over these intermetallic catalysts was largely explained based on the modification to the electronic structure of Pd through Pd–Ga interactions (Pd 4d–Ga 4p hybridization) (Section 2.6, Fig. 7); however, the isolation of Pd active sites by Ga may also play a role, and it is difficult to decouple these effects. Electronic structure calculations coupled with X-ray photoelectron spectroscopy (XPS) demonstrated a lower DOS at the Fermi level in PdGa compared to monometallic Pd, indicating an induced directionality in Pd–Ga interactions, i.e., partial covalent character in the heteroatomic interactions. QTAIM analysis suggested the electronegativity difference between Pd and Ga is largely responsible for the electronic modification observed (Fig. 8). The partially negative Pd sites formed in intermetallic Pd–Ga enhance the tendency of acetylene to form a donor complex without being activated by backdonation through the Pd 4d-states (confirmed through infrared spectroscopic studies of CO adsorption), which leads to the superior selectivity to ethylene.27,141 The directionality in the Pd–Ga bonds is also an important factor in the increased stability observed for these catalysts and accounts for the increased resistance to segregation that frequently occurs in solid solution alloys. Despite the strong evidence that the electronic modifications significantly improved the selectivity and stability in Pd–Ga intermetallics, the Pd sites were completely isolated (Fig. 13). DFT studies revealed the configuration of the Pd active sites (Pd trimers or Pd monomers) affected the selectivity to ethylene.162 Both configurations were found to have similar barriers for acetylene hydrogenation; however, the Pd trimer sites were found to have a significantly higher barrier for ethylene hydrogenation, largely due to the hydrogen diffusion barrier to the active site, which would result in improved selectivity for Pd trimer sites.162 Therefore, the geometry of the active sites present in the different Pd–Ga intermetallic compounds also contributes to the selectivity of the catalyst during acetylene hydrogenation, meaning any improvements are likely due to a combination of electronic and geometric effects.
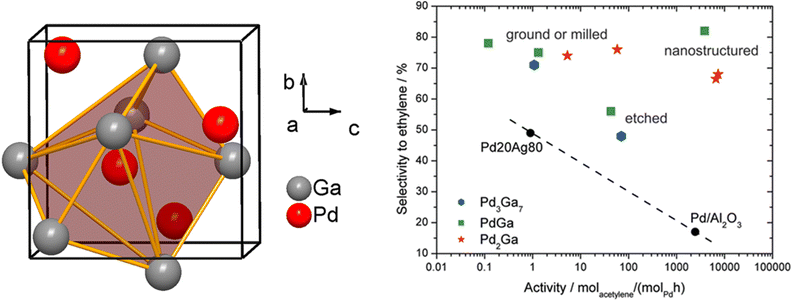 | ||
| Fig. 13 Isolation of Pd-sites in the structure of PdGa (left, made by DIAMOND 3, adapted from ref. 27), selectivity to ethylene in % over the rate of acetylene hydrogenation for PdGa, Pd2Ga, Pd3Ga7, and other industrially relevant catalysts (right, reprinted with permission from ref. 27, copyright 2010, the American Chemical Society). | ||
In the search for alternatives to expensive noble metals (e.g., Pd), the unique bonding and control over the active site configuration suggest intermetallic compounds offer the opportunity to develop such catalysts. Armbrüster et al.163 introduced a novel PGM-free catalyst, Al13Fe4, for the selective hydrogenation of acetylene. Al13Fe4 crystallizes with the monoclinic space group C2/m with more than 100 atoms per unit cell. All Fe sites were coordinated by Al or consisted of Fe–Al–Fe ensembles embedded in the three-dimensional Al-network (Fig. 14). The covalent Fe–Al and Fe–Al–Fe multi-centered interactions lead to the site-isolation of Fe, which alters the electronic structure of the compound with respect to pure Fe and provides thermodynamic stability. The increased thermodynamic stability prevents segregation under the reaction conditions.163
 | ||
| Fig. 14 Isolation of Fe sites by Al in the bulk structure of Al13Fe4. Conversion (C) and selectivity (S) to ethylene of unsupported Al13Fe4, 5 wt% Pd/Al2O3 and an industrial benchmark catalyst for the selective hydrogenation of acetylene over 20 h time on stream. Adapted from ref. 163. | ||
An in-depth DFT (including surface energy calculations) and STM study of Al13Fe4 revealed the active sites of Al13Fe4 likely comprised isolated Fe atoms protruding from a pentagonal ensemble of Al atoms (i.e. Al5Fe), which were found to appear on the Al13Fe4 (010) surface.164 In other intermetallic catalysts, site isolation is crucial to achieving high selectivity in the hydrogenation of acetylene to ethylene. On completely isolated sites, acetylene adsorbs to the transition metal atoms within these ensembles via a di-σ configuration, but ethylene can only form a weak π-bond.27,28,141,157,161 The weaker binding of ethylene is crucial to preventing over-hydrogenation to ethane, while active site isolation minimizes oligomerization since the greater distances between active sites prevents coupling of hydrocarbon adsorbates. By taking advantage of the unique control over the active site composition through the use of intermetallic compounds, Armbrüster et al. demonstrated the development of noble metal free catalysts for selective hydrogenation reactions.163
Heusler alloys have recently attracted the attention of the catalysis community. Kojima et al.86 found that Co2MnGe and Co2FeGe alloys exhibit high alkene selectivity during alkyne hydrogenation. The catalytic performance of the alloys was tuned through the substitution of different elements in the parent compounds. The substitution of Ga into the Ge position of Co2FeGe led to the formation of isostructural Co2FeGa0.5Ge0.5, which showed high rates of acetylene hydrogenation with low ethylene selectivity. The authors proposed that based on apparent activation energies and DFT-based binding energy calculations, Ga has a higher propensity to adsorb small molecules (e.g., C2H4, C2H2) than Ge, resulting in increased activity but lower selectivity. The substitution of Mn for Fe (Co2MnxFe1−xGe) resulted in very high alkene selectivity even at complete conversion. The authors hypothesized this was largely due to a change in the electronic structure due to site-specific substitution. The results of this study highlight the flexibility and tunability of Heusler compounds, allowing for the alteration of the geometric ensembles and/or electronic structure to optimize catalytic performance.
Another strategy for the modification of the electronic structure of active sites was demonstrated by Niu et al.165 and Chen et al.96 through the introduction of carbon into the lattices of cubic Ni3Zn and Pd3Zn to form intermetallic carbides. The overall structure of the intermetallic compounds remained the same, and carbon atoms occupied interstitial sites. Through combined DFT and XANES experiments, the authors showed the C atom coordinates with the active metal site and neutralizes its positive charge. This process facilitates ethylene desorption and enhances acetylene hydrogenation selectivity.
Liu et al.166 identified a clear difference in acetylene hydrogenation activity between atomically ordered CsCl-type PdCu intermetallic nanoparticles and an fcc bimetallic solid solution of PdCu. The specific activity of CsCl-type PdCu was one order of magnitude greater than that of fcc-PdCu. CsCl-type PdCu is characterized by more densely populated and weaker Pd–Cu bonds than fcc-PdCu, along with a lower coordination number and higher-lying d-states. The authors argued the high-lying d-band centers facilitated hydrogen dissociation on the isolated Pd surface, leading to much higher activity for CsCl-type PdCu than for the substitutional solid solution (fcc-PdCu).166
3.4 Increasing the surface area of intermetallic compounds via alternative synthetic techniques
While the previous section mostly deals with the utilization of bulk intermetallic catalysts for the selective hydrogenation of acetylene, one of the challenges associated with bulk intermetallic compounds is their low surface area (typically <0.5 m2 g−1), which results in several fundamental and practical challenges, including limitations on able characterization techniques. While the preparation of nanoparticulate intermetallic catalysts can have significant downsides (e.g. homogeneity, support effects), they open the possibility to utilize additional characterization methods that can provide further experimental information regarding the active sites present in IMCs. The following section discusses intermetallic nanoparticle systems used for selective alkyne hydrogenation, focusing on the benefits and challenges associated with nanoparticulate IMCs.Zhang et al.167 prepared ZnO-supported intermetallic PdZn nanoparticles (P4/mmm, CuTi-type), in which the active site was shown to consist of Pd–Zn–Pd ensembles. The authors studied the active site configuration using infrared spectroscopy of CO adsorption and measured the adsorption energies of relevant molecules on the active sites using microcalorimetry. Compared to the CO-FTIR spectrum obtained for a monometallic Pd catalyst, the spectrum for the PdZn catalyst showed a significant decrease in bridging CO, with the vast majority present as the linear bound form. This is consistent with the isolation of Pd sites since the increased distance between Pd sites in the Pd–Zn–Pd ensembles disfavors the formation of bridging CO. Additionally, a red shift in the wavenumber for the linear bound CO indicates increased electron density on Pd due to electron transfer from Zn to Pd. XPS analysis of the Pd–Zn catalyst was consistent with the IR results, finding a lower binding energy for Pd 3d5/2 in Pd–Zn compared to monometallic Pd. The higher surface area of the nanoparticulate catalyst also allowed the adsorption energy of relevant species, such as acetylene and ethylene, to be measured. The authors found the binding energies of acetylene on monometallic Pd and the Pd–Zn catalysts were comparable; however, the binding energy of ethylene was significantly lower on Pd–Zn/ZnO. The suppressed adsorption of ethylene on the Pd–Zn intermetallic catalyst was consistent with the experimentally observed improvement in ethylene selectivity during acetylene hydrogenation. DFT results suggest the site isolation of Pd in the Pd–Zn–Pd ensembles resulted in weakly π-bound ethylene, leading to improved ethylene selectivity. Electronic modifications may also contribute because the increased electron density on Pd is likely to result in weakened binding of electron-rich unsaturated molecules. Therefore, the improved selectivity observed during acetylene hydrogenation with the Pd–Zn intermetallic catalyst is likely due to a combination of geometric and electronic effects. Preparation of nanoparticulate intermetallic compounds has allowed for additional characterization techniques that are inaccessible with the low surface area of bulk intermetallics; however, it is important to be aware that confirming phase purity becomes more challenging since the smaller grain sizes broadened Bragg reflections in powder XRD.167
Another approach to increase the surface area of intermetallic compounds is through colloidal synthesis. Liu et al.168 synthesized NixMy (M = Ga, Sn) intermetallic nanocrystals with various compositions and ordered crystal structures. Most of these compounds showed high alkene selectivity for alkyne hydrogenation, including acetylene. The authors reported the improvement was due to a combination of electronic modification and site isolation (i.e., geometric effect); however, the origin of these effects and the direct implications of these modifications on catalysis were not explored in detail. Additionally, the presence of a residual capping agent (oleylamine, a long chain amine) has the potential to contribute to changes in the catalytic properties, including selectivity. Removal of oleylamine (and similar capping agents) from the surface of colloidal nanoparticles is known to be challenging, with the most successful methods often having inadvertent and potentially problematic consequences, e.g., calcination,169 which is likely to result in oxidation of intermetallic compounds. Any changes in the measured catalytic behavior of intermetallic nanoparticles produced via colloidal methods may be due to the inherent properties of IMC or the presence of capping agents on the surface of the particles.
Furukawa et al.170 recently prepared supported nanoparticles of Cu-substituted L12-type pseudo-binary compounds (Ni1−xCux)3Ga/TiO2 (x = 0.2, 0.25, 0.33, 0.5, 0.6, and 0.75) by conventional wet impregnation. The catalysts were characterized with STEM-EDX (scanning transmission electron microscopy coupled with energy-dispersive X-ray spectroscopy), pXRD and EXAFS to confirm the formation of the desired crystallographic phase. However, as discussed above, small impurities may be missed due to the broadened and weak signals in the pXRD patterns inherent to the small particles and/or low metal content of the supported catalysts. The catalysts were evaluated for the selective hydrogenation of acetylene and (Ni0.8Cu0.2)3Ga/TiO2 was found to be the optimum catalyst in terms of activity and selectivity. This highlights a key benefit of preparing intermetallic nanoparticles: high selectivity due to the controlled electronic and geometric modifications associated with IMC and high activity due to the increased metal surface area. DFT calculations indicated the increased selectivity was due to the formation of Ni2Cu hollow sites on the lowest energy (111) surface that destabilized the multifold coordination of adsorbates, leading to significantly higher adsorption energy of ethylidene compared to the Ni3 sites present on Ni3Ga and therefore prevented over-hydrogenation to ethane. Overall, intermetallic nanoparticles were found to be highly active and selective in acetylene hydrogenation; however, concerns over homogeneity implies the implementation of these catalysts as models to understand the fundamental origins of structure–activity–selectivity relationships is challenging.170
The difficulties in synthesizing phase-pure intermetallic nanoparticles are highlighted by Dasgupta et al., who investigated the preparation of supported Pd–Zn, Cu–Zn and Ni–Zn intermetallic nanoparticles.171 The catalysts were synthesized by first preparing a M/SiO2 (where M = Pd, Ni or Cu) catalyst via standard impregnation techniques, followed by the vapor deposition of Zn, with the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn molar ratio set to match the γ-brass phase. Powder XRD patterns of the supported nanoparticulate M–Zn catalysts confirmed the formation of the γ-brass phase, with no residual reflections corresponding to the parent metal observable. However, the atomic ratio of Pd
Zn molar ratio set to match the γ-brass phase. Powder XRD patterns of the supported nanoparticulate M–Zn catalysts confirmed the formation of the γ-brass phase, with no residual reflections corresponding to the parent metal observable. However, the atomic ratio of Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn in individual nanoparticles obtained through STEM-EDX analysis varied, with occasional particles having Pd
Zn in individual nanoparticles obtained through STEM-EDX analysis varied, with occasional particles having Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn atomic ratios outside the compositional bounds of the γ-brass phase. While these outliers may be in the minority, if the impurity phase is significantly more active than the target compound (i.e., γ-brass phase), even a minority concentration would be expected to dominate the catalytic behavior. Additionally, as discussed above, small changes in the composition of Pd–Zn γ-brass compounds (i.e., even changes to the Pd
Zn atomic ratios outside the compositional bounds of the γ-brass phase. While these outliers may be in the minority, if the impurity phase is significantly more active than the target compound (i.e., γ-brass phase), even a minority concentration would be expected to dominate the catalytic behavior. Additionally, as discussed above, small changes in the composition of Pd–Zn γ-brass compounds (i.e., even changes to the Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn atomic ratio within the compositional bounds) have been shown to have dramatic effects on the activity and selectivity in acetylene hydrogenation.140 The authors used ethylene hydrogenation as a probe reaction for active site modification due to the formation of Pd–Zn γ-brass and found the apparent activation energy increased to 63 kJ mol−1, as compared to 38 kJ mol−1 for monometallic Pd.171 While the formation of Pd–Zn intermetallic compounds impacted the catalytic behavior, the variation in the composition of the Pd–Zn nanoparticles demonstrates it is not possible to relate this change to specific active site modifications, like in the case of bulk Pd–Zn γ-brass. The success of this method was largely dependent on the identity of M, with only M = Pd producing small nanoparticles (<10 nm) of the desired intermetallic compound. Additionally, the low boiling point of metallic Zn is crucial to this approach.171
Zn atomic ratio within the compositional bounds) have been shown to have dramatic effects on the activity and selectivity in acetylene hydrogenation.140 The authors used ethylene hydrogenation as a probe reaction for active site modification due to the formation of Pd–Zn γ-brass and found the apparent activation energy increased to 63 kJ mol−1, as compared to 38 kJ mol−1 for monometallic Pd.171 While the formation of Pd–Zn intermetallic compounds impacted the catalytic behavior, the variation in the composition of the Pd–Zn nanoparticles demonstrates it is not possible to relate this change to specific active site modifications, like in the case of bulk Pd–Zn γ-brass. The success of this method was largely dependent on the identity of M, with only M = Pd producing small nanoparticles (<10 nm) of the desired intermetallic compound. Additionally, the low boiling point of metallic Zn is crucial to this approach.171
Other approaches rely on the use of expensive and/or highly air sensitive reagents, e.g., the co-reduction of ionic metal precursors to form GaPd and GaPd2 (ref. 172) or the thermal reduction of a zerovalent organozinc precursor in hot organoamine solvent to produce various M–Zn based intermetallics (PdZn, AuZn and Au3Zn, as well as several γ-brasses).173 Chemical vapor deposition of the organotin compound Sn(CH3)4 onto Ni/SiO2 was performed by Onda et al.174 to synthesize catalytically relevant Ni–Sn intermetallic nanocrystals. Furukawa et al.175 applied a rather simple co-impregnation method to synthesize SiO2-supported Heusler compound Co2FeGe, which was explored as a selective hydrogenation catalyst.
4. Conclusive remarks: a perspective on the future of intermetallics in heterogeneous catalysis
The most significant benefit offered by intermetallic compounds is the precise control over the active site, both in terms of structure and composition (geometric effects) and electronic structure modifications. Isolation of active sites by a more catalytically ‘inert’ element can result in active sites with ensembles of a specific geometry, while minute adjustments in the bulk stoichiometry of the material can vary the composition of exposed active sites. Differences in the coordination environment of catalytically active elements can result in changes to the bulk and surface electronic structure, with some systems displaying partial charge transfer or covalent and partially ionic type interactions in an overall metallic system. This control over active sites is what makes intermetallic compounds ideal as model catalysts; it enables in-depth structure–activity relationships to be established and facilitates detailed kinetics studies, such that it is possible to produce fully coverage-enumerated microkinetic models.139 Intermetallic compounds offer the unique opportunity to rationally design active, selective and stable catalysts.However, there are limitations on the scope of reactions where intermetallic compounds are likely to remain active and stable, largely due to their susceptibility to oxidation. While this review has focused on the use of intermetallic compounds as catalysts for selective alkyne hydrogenations, there are many examples of their use in other reaction types;176–179 however, all of these reactions are carried out in reducing environments. When intermetallic compounds are used in processes with potentially oxidizing environments, such as the steam reforming of methanol, the catalyst is likely to be at least partially oxidized. Armbrüster et al.180 found the most active and selective PdZn-based catalyst consists of a combination of reduced and oxidized species. Therefore, although there are several processes in which intermetallic compounds can be employed as model catalysts, even mildly oxidizing environments can modify the catalyst and either introduce additional complexity or render it inactive.
One consequence of the unique bonding and interactions present in intermetallic compounds is their potential to act as ‘pseudoelements’,11 where the electronic structure of the intermetallic compound mimics that of another element. This opens opportunities to develop catalysts made of cheaper and more abundant elements to replace current catalysts that often consist of rarer and more expensive elements such as Pd, Pt and other noble metals. A proof-of-concept example explored PdZn and PdCd intermetallic compounds for the steam reforming of methanol and found that both the electronic structure and catalytic performance of the materials were similar to those of a monometallic copper catalyst.11 While this is a potentially exciting avenue to explore in the effort to develop active and stable noble-metal-free catalysts, it is important to consider the challenges associated with the use of intermetallic compounds for large-scale industrial processes; their low surface area (and therefore poor metal utilization) and the feasibility of scaling up solid state synthetic methods (synthesis under vacuum at high temperatures for extended periods). Therefore, if the full potential of intermetallic compounds is to be realized, substantial effort would be required to develop simple and reliable methods for the synthesis of supported versions of these compounds, which would make them more viable as catalysts for industrial processes.
Ultimately, bulk intermetallic compounds allow significant control and flexibility over the active sites, which makes it possible to rationally design specific active sites for a chosen reaction, making them ideal as model catalysts. If the challenges associated with low surface area and challenging synthetic methods can be overcome, this would expand the scope of applications for intermetallic compounds and allow their unique properties to be fully leveraged.
Data availability
This review utilized data available and cited in the literature. New data were generated solely for illustrative purposes (including all structural figures, particularly Fig. 7 and 8). Data will be made available upon request.Author contributions
N. R. and G. C. conceptualized the initial draft of the manuscript. N. R. and K. M. co-wrote the original draft, prepared the structural figures for visualization, and conducted the formal analysis. M. E. contributed to writing, reviewing, editing, and visualization. G. C. revised and edited the manuscript. R. M. R. revised and edited the manuscript and supervised the entire project.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This material is based upon work supported by the National Science Foundation under grant number CHE-2247797, and the Department of Energy (DOE), Basic Energy Sciences, via award no. DE-SC0020147. N. R. would like to thank his PhD supervisor, Prof. Partha Pratim Jana (Department of Chemistry, IIT Kharagpur, India), and former colleagues of the PPJ lab for their training in solid-state and structural chemistry.References
- M. Armbrüster, Intermetallic compounds in catalysis – a versatile class of materials meets interesting challenges, Sci. Technol. Adv. Mater., 2020, 21, 303–322 CrossRef.
- M. Armbrüster, R. Schlögl and Y. Grin, Intermetallic compounds in heterogeneous catalysis—a quickly developing field, Sci. Technol. Adv. Mater., 2014, 15, 034803 CrossRef.
- J. C. Bauer, X. Chen, Q. Liu, T.-H. Phan and R. E. Schaak, Converting nanocrystalline metals into alloys and intermetallic compounds for applications in catalysis, J. Mater. Chem., 2008, 18, 275–282 RSC.
- A. Dasgupta and R. M. Rioux, Intermetallics in catalysis: An exciting subset of multimetallic catalysts, Catal. Today, 2019, 330, 2–15 CrossRef CAS.
- S. Furukawa and T. Komatsu, Intermetallic Compounds: Promising Inorganic Materials for Well-Structured and Electronically Modified Reaction Environments for Efficient Catalysis, ACS Catal., 2017, 7, 735–765 CrossRef CAS.
- J. Li and S. Sun, Intermetallic Nanoparticles: Synthetic Control and Their Enhanced Electrocatalysis, Acc. Chem. Res., 2019, 52, 2015–2025 CrossRef CAS PubMed.
- Y. Nakaya and S. Furukawa, High-entropy intermetallics: emerging inorganic materials for designing high-performance catalysts, Chem. Sci., 2024, 15, 12644–12666 RSC.
- S. Penner and P. D. Kheyrollahi Nezhad, Steering the Catalytic Properties of Intermetallic Compounds and Alloys in Reforming Reactions by Controlled in Situ Decomposition and Self-Activation, ACS Catal., 2021, 11, 5271–5286 CrossRef CAS.
- C. Quilis, N. Mota, E. Millán, B. Pawelec and R. M. Navarro Yerga, Application of Intermetallic Compounds as Catalysts for the Selective Hydrogenation of CO2 to Methanol, ChemCatChem, 2024, 16, e202301496 CrossRef CAS.
- L. Rößner and M. Armbrüster, Electrochemical Energy Conversion on Intermetallic Compounds: A Review, ACS Catal., 2019, 9, 2018–2062 CrossRef.
- A. P. Tsai, S. Kameoka, K. Nozawa, M. Shimoda and Y. Ishii, Intermetallic: A Pseudoelement for Catalysis, Acc. Chem. Res., 2017, 50, 2879–2885 CrossRef CAS.
- A. N. R. Bos and K. R. Westerterp, Mechanism and kinetics of the selective hydrogenation of ethyne and ethene, Chem. Eng. Process. Process Intensif., 1993, 32, 1–7 CrossRef CAS.
- M. Takht Ravanchi, S. Sahebdelfar and S. Komeili, Acetylene selective hydrogenation: a technical review on catalytic aspects, Rev. Chem. Eng., 2018, 34(2), 215–237 CrossRef CAS.
- L. Gardner, The use of stainless steel in structures, Prog. Struct. Eng. Mater., 2005, 7, 45–55 CrossRef.
- S. S. Naboychenko, I. B. Murashova and O. D. Neikov, in Handbook of Non-Ferrous Metal Powders, ed. O. D. Neikov, S. S. Naboychenko, I. V. Murashova, V. G. Gopienko, I. V. Frishberg and D. V. Lotsko, Elsevier, Oxford, 2009, pp. 331–368, DOI:10.1016/B978-1-85617-422-0.00016-1.
- P. Jozwik, W. Polkowski and Z. Bojar, Applications of Ni3Al based intermetallic alloys—current stage and potential perceptivities, Materials, 2015, 8, 2537–2568 CrossRef CAS.
- A. R. Paul, M. Mukherjee and D. Singh, A Critical Review on the Properties of Intermetallic Compounds and Their Application in the Modern Manufacturing, Cryst. Res. Technol., 2022, 57, 2100159 CrossRef CAS.
- R. Darolia, NiAl alloys for high-temperature structural applications, JOM, 1991, 43, 44–49 CrossRef CAS.
- O. Gourdon, D. Gout, D. J. Williams, T. Proffen, S. Hobbs and G. J. Miller, Atomic Distributions in the γ-Brass Structure of the Cu−Zn System: A Structural and Theoretical Study, Inorg. Chem., 2007, 46, 251–260 CrossRef CAS PubMed.
- K. Brandenburg and H. Putz, Crystal Impact GbR, Bonn, Germany, 2005 Search PubMed.
- P. Villars and K. Cenzual, Pearson's crystal data: crystal structure database for inorganic compounds, 2007 Search PubMed.
- G. Krauss, Steels: processing, structure, and performance, ASM international, 2015 Search PubMed.
- W. Hume-Rothery, Researches on the nature, properties, and conditions of formation of intermetallic compounds, with special reference to certain compounds of tin, 1926 Search PubMed.
- W. Hume-Rothery, Phase stability in metals and alloys, Springer Ser. Biomater. Sci. Eng., 1967, 3–23 CAS.
- U. Mizutani, Hume-Rothery rules for structurally complex alloy phases, MRS Bull., 2012, 37, 169 CrossRef.
- M. G. Kanatzidis, R. Pöttgen and W. Jeitschko, The Metal Flux: A Preparative Tool for the Exploration of Intermetallic Compounds, Angew. Chem., Int. Ed., 2005, 44, 6996–7023 CrossRef CAS PubMed.
- M. Armbrüster, K. Kovnir, M. Behrens, D. Teschner, Y. Grin and R. Schlögl, Pd−Ga Intermetallic Compounds as Highly Selective Semihydrogenation Catalysts, J. Am. Chem. Soc., 2010, 132, 14745–14747 CrossRef PubMed.
- J. Osswald, R. Giedigkeit, R. E. Jentoft, M. Armbrüster, F. Girgsdies, K. Kovnir, T. Ressler, Y. Grin and R. Schlögl, Palladium–gallium intermetallic compounds for the selective hydrogenation of acetylene: Part I: Preparation and structural investigation under reaction conditions, J. Catal., 2008, 258, 210–218 CrossRef CAS.
- A. Werwein, F. Maaß, L. Y. Dorsch, O. Janka, R. Pöttgen, T. C. Hansen, J. Kimpton and H. Kohlmann, Hydrogenation Properties of Laves Phases LnMg2 (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Ho, Er, Tm, Yb), Inorg. Chem., 2017, 56, 15006–15014 CrossRef CAS.
- W. G. Zeier, J. Schmitt, G. Hautier, U. Aydemir, Z. M. Gibbs, C. Felser and G. J. Snyder, Engineering half-Heusler thermoelectric materials using Zintl chemistry, Nat. Rev. Mater., 2016, 1, 16032 CrossRef CAS.
- A. Amon, A. Ormeci, M. Bobnar, L. G. Akselrud, M. Avdeev, R. Gumeniuk, U. Burkhardt, Y. Prots, C. Hennig, A. Leithe-Jasper and Y. Grin, Cluster Formation in the Superconducting Complex Intermetallic Compound Be21Pt5, Acc. Chem. Res., 2018, 51, 214–222 CrossRef CAS PubMed.
- R. J. Cava, H. Takagi, H. W. Zandbergen, J. J. Krajewski, W. F. Peck, T. Siegrist, B. Batlogg, R. B. van Dover, R. J. Felder, K. Mizuhashi, J. O. Lee, H. Eisaki and S. Uchida, Superconductivity in the quaternary intermetallic compounds LnNi2B2C, Nature, 1994, 367, 252–253 CrossRef CAS.
- A. Provino, V. Smetana, D. Paudyal, K. A. Gschneidner, A.-V. Mudring, V. K. Pecharsky, P. Manfrinetti and M. Putti, Gd3Ni2 and Gd3CoxNi2−x: magnetism and unexpected Co/Ni crystallographic ordering, J. Mater. Chem. C, 2016, 4, 6078–6089 RSC.
- W. Steurer and J. Dshemuchadse, Intermetallics: structures, properties, and statistics, Oxford University Press, 2016 Search PubMed.
- H. Schäfer, On the Problem of Polar Intermetallic Compounds: The Stimulation of E. Zintl's Work for the Modern Chemistry of Intermetallics, Annu. Rev. Mater. Sci., 1985, 15, 1–42 CrossRef.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. A. Persson, Commentary: The Materials Project: A materials genome approach to accelerating materials innovation, APL Mater., 2013, 1, 011002 CrossRef.
- A. Merchant, S. Batzner, S. S. Schoenholz, M. Aykol, G. Cheon and E. D. Cubuk, Scaling deep learning for materials discovery, Nature, 2023, 624, 80–85 CrossRef CAS.
- L. Chanussot, A. Das, S. Goyal, T. Lavril, M. Shuaibi, M. Riviere, K. Tran, J. Heras-Domingo, C. Ho, W. Hu, A. Palizhati, A. Sriram, B. Wood, J. Yoon, D. Parikh, C. L. Zitnick and Z. Ulissi, Open Catalyst 2020 (OC20) Dataset and Community Challenges, ACS Catal., 2021, 11, 6059–6072 CrossRef CAS.
- R. Nesper, Bonding patterns in intermetallic compounds, Angew Chem. Int. Ed. Engl., 1991, 30, 789–817 CrossRef.
- A. Iandelli and A. Palenzona, Crystal chemistry of intermetallic compounds, Handbook on the physics and chemistry of rare earths, 1979, vol. 2, pp. 1–54 Search PubMed.
- R. Ferro and A. Saccone, Structure of intermetallic compounds and phases, Phys. Metall., 1996, 1, 205–369 Search PubMed.
- J. D. Kraus, J. S. Van Buskirk and D. C. Fredrickson, The Zintl Concept Applied to Intergrowth Structures: Electron-Hole Matching, Stacking Preferences, and Chemical Pressures in Pd5InAs, Z. Anorg. Allg. Chem., 2023, 649, e202300125 CrossRef CAS.
- A. Lim and D. C. Fredrickson, Entropic Control of Bonding, Guided by Chemical Pressure: Phase Transitions and 18-n+m Isomerism of IrIn3, Inorg. Chem., 2023, 62, 10833–10846 CrossRef CAS.
- W. Zhang, F. Li, Y. Li, A. Song, K. Yang, D. Wu, W. Shang, Z. Yao, W. Gao, T. Deng and J. Wu, The role of surface substitution in the atomic disorder-to-order phase transition in multi-component core–shell structures, Nat. Commun., 2024, 15, 9762 CrossRef CAS.
- N. E. Brese and H. G. von Schnering, Bonding trends in pyrites and a reinvestigation of the structures of PdAs2, PdSb2, PtSb2 and PtBi2, Z. Anorg. Allg. Chem., 1994, 620, 393–404 CrossRef CAS.
- O. Gourdon and G. J. Miller, Intergrowth Compounds in the Zn-Rich Zn−Pd System: Toward 1D Quasicrystal Approximants, Chem. Mater., 2006, 18, 1848–1856 CrossRef CAS.
- S. Lidin and L. C. Folkers, In Situ Synthesis and Single Crystal Synchrotron X-ray Diffraction Study of ht-Sn3Sb2: An Example of How Complex Modulated Structures Are Becoming Generally Accessible, Acc. Chem. Res., 2018, 51, 223–229 CrossRef CAS PubMed.
- K. R. Kamp and D. C. Fredrickson, Frustrated Packing in Simple Structures: Chemical Pressure Hindrance to Isolobal Bonds in the TiAl3 type and ZrAl2.6Sn0.4, Inorg. Chem., 2021, 60, 4779–4791 CrossRef CAS.
- S. K. Kuila, Harshit, N. Roy, S. Ghanta, R. Pan, K. Buxi, P. Pramanik, A. K. Bera, B. Saha, S. M. Yusuf, V. Petříček, A. Roy and P. P. Jana, Ni3InSb: Synthesis, Crystal Structure, Electronic Structure, and Magnetic Properties, Inorg. Chem., 2023, 62, 7304–7314 CrossRef CAS.
- N. Roy, S. K. Kuila, Harshit, P. Pramanik and P. P. Jana, Selective Chemical Substitution of Cu in the Structure of TiAl3 Type InPd3: Experimental and Theoretical Studies, Eur. J. Inorg. Chem., 2022, 2022, e202200309 CrossRef CAS.
- A. Verniere, L. V. B. Diop, I. Sarr, T. Schweitzer and B. Malaman, Crystal structure of new quaternary intermetallic compounds R2MoSi2C (R = Y, Gd), Acta Crystallogr., Sect. B, 2024, 80, 504–508 CrossRef CAS.
- S. E. Latturner, D. Bilc, S. D. Mahanti and M. G. Kanatzidis, Quaternary Intermetallics Grown from Molten Aluminum: The Homologous Series Th2(AuxSi1-x)[AuAl2]nSi2 (n = 1, 2, 4), Chem. Mater., 2002, 14, 1695–1705 CrossRef CAS.
- L. S. Reitz, J. Hempelmann, P. C. Müller, R. Dronskowski and S. Steinberg, Bonding Analyses in the Broad Realm of Intermetallics: Understanding the Role of Chemical Bonding in the Design of Novel Materials, Chem. Mater., 2024, 36, 6791–6804 CrossRef CAS.
- M. Friedrich, A. Ormeci, Y. Grin and M. Armbrüster, PdZn or ZnPd: Charge Transfer and Pd–Pd Bonding as the Driving Force for the Tetragonal Distortion of the Cubic Crystal Structure, Z. Anorg. Allg. Chem., 2010, 636, 1735–1739 CrossRef CAS.
- A. O. Oliynyk and A. Mar, Discovery of Intermetallic Compounds from Traditional to Machine-Learning Approaches, Acc. Chem. Res., 2018, 51, 59–68 CrossRef CAS.
- N. Roy and S. Giri, Influence of the Homoatomic and Multicenter Bonding on the Formation of CsCl-type RhCd, Z. Anorg. Allg. Chem., 2024, 650, e202300232 CrossRef CAS.
- N. Gross, G. Kotzyba, B. Künnen and W. Jeitschko, Binary Compounds of Rhodium and Zinc: RhZn, Rh2Zn11, and RhZn13, Z. Anorg. Allg. Chem., 2001, 627, 155–163 CrossRef CAS.
- A. O. Oliynyk, E. Antono, T. D. Sparks, L. Ghadbeigi, M. W. Gaultois, B. Meredig and A. Mar, High-Throughput Machine-Learning-Driven Synthesis of Full-Heusler Compounds, Chem. Mater., 2016, 28, 7324–7331 CrossRef CAS.
- V. Gvozdetskyi, B. Selvaratnam, A. O. Oliynyk and A. Mar, Revealing Hidden Patterns through Chemical Intuition and Interpretable Machine Learning: A Case Study of Binary Rare-Earth Intermetallics RX, Chem. Mater., 2023, 35, 879–890 CrossRef CAS.
- R. Sikdar, B. Selvaratnam, V. Mishra and A. Mar, Is There a Simple Descriptor to Predict Laves Phases?, Cryst. Growth Des., 2025, 25, 849–857 CrossRef CAS.
- R. Sikdar, N. Roy, B. Selvaratnam, V. Mishra, D. Mumbaraddi, A. Mondal, K. Buxi, P. P. Jana and A. Mar, Ahead by a Century: Discovery of Laves Phases Assisted by Machine Learning, Inorg. Chem., 2024, 63, 5972–5981 CrossRef CAS.
- B. Selvaratnam, A. O. Oliynyk and A. Mar, Interpretable Machine Learning in Solid-State Chemistry, with Applications to Perovskites, Spinels, and Rare-Earth Intermetallics: Finding Descriptors Using Decision Trees, Inorg. Chem., 2023, 62, 10865–10875 CrossRef CAS PubMed.
- F. Oviedo, J. L. Ferres, T. Buonassisi and K. T. Butler, Interpretable and Explainable Machine Learning for Materials Science and Chemistry, Acc. Mater. Res., 2022, 3, 597–607 CrossRef CAS.
- D. C. Fredrickson and G. J. Miller, Intermetallic Chemistry: New Advances in Humanity’s Age-Old Exploration of Metals and Alloys, Acc. Chem. Res., 2018, 51, 213 CrossRef CAS PubMed.
- R. Hoffmann, How Chemistry and Physics Meet in the Solid State, Angew Chem. Int. Ed. Engl., 1987, 26, 846–878 CrossRef.
- G. J. Miller, The “Coloring Problem” in Solids: How It Affects Structure, Composition and Properties, Eur. J. Inorg. Chem., 1998, 1998, 523–536 CrossRef.
- U. Mizutani, H. Sato and T. B. Massalski, The original concepts of the Hume-Rothery rule extended to alloys and compounds whose bonding is metallic, ionic, or covalent, or a changing mixture of these, Prog. Mater. Sci., 2021, 120, 100719 CrossRef CAS.
- U. Mizutani and H. Sato, The Physics of the Hume-Rothery Electron Concentration Rule, Crystals, 2017, 7, 9 CrossRef.
- L. M. Hoistad and S. Lee, The Hume-Rothery electron concentration rules and second moment scaling, J. Am. Chem. Soc., 1991, 113, 8216–8220 CrossRef CAS.
- E. Zintl and W. Dallenkopf, Über den Gitterbau von NaTl und seine Beziehung zu den Strukturen vom Typus des β-Messings, 4. Mitteilung über Metalle und Legierungen, Z. Phys. Chem., 1932, 16B, 195–205 CrossRef.
- F. Wang and G. J. Miller, Revisiting the Zintl–Klemm Concept: Alkali Metal Trielides, Inorg. Chem., 2011, 50, 7625–7636 CrossRef CAS.
- S. Tiefenthaler, N. Korber and S. Gärtner, Synthesis of the Tetragonal Phase of Zintl’s NaTl and Its Structure Determination from Powder Diffraction Data, Materials, 2019, 12, 1356 CrossRef CAS.
- V. Smetana, M. Rhodehouse, G. Meyer and A.-V. Mudring, Gold Polar Intermetallics: Structural Versatility through Exclusive Bonding Motifs, Acc. Chem. Res., 2017, 50, 2633–2641 CrossRef CAS.
- Q. Lin and G. J. Miller, Electron-Poor Polar Intermetallics: Complex Structures, Novel Clusters, and Intriguing Bonding with Pronounced Electron Delocalization, Acc. Chem. Res., 2018, 51, 49–58 CrossRef CAS.
- R. L. Johnston and R. Hoffmann, Structure-Bonding Relationships in the Laves Phases, Z. Anorg. Allg. Chem., 1992, 616, 105–120 CrossRef CAS.
- F. Laves and K. Löhberg, Die Kristallstruktur von intermetallischen Verbindungen der Formel AB− 2, von, Weidmann, 1934 Search PubMed.
- J. B. Friauf, The crystal structures of two intermetallic compounds, J. Am. Chem. Soc., 1927, 49, 3107–3114 CrossRef CAS.
- F. Stein, M. Palm and G. Sauthoff, Structure and stability of Laves phases part II—structure type variations in binary and ternary systems, Intermetallics, 2005, 13, 1056–1074 CrossRef CAS.
- F. Stein, M. Palm and G. Sauthoff, Structure and stability of Laves phases. Part I. Critical assessment of factors controlling Laves phase stability, Intermetallics, 2004, 12, 713–720 CrossRef CAS.
- A. Ormeci, A. Simon and Y. Grin, Structural topology and chemical bonding in Laves phases, Angew. Chem., 2010, 49, 8997–9001 CrossRef CAS.
- V. A. Yartys and M. V. Lototskyy, Laves type intermetallic compounds as hydrogen storage materials: A review, J. Alloys Compd., 2022, 916, 165219 CrossRef CAS.
- F. Heusler, Über magnetische manganlegierungen, Verh. Dtsch. Phys. Ges., 1903, 5, 219 CAS.
- J. K. Kawasaki, S. Chatterjee, P. C. Canfield and E. Guest, Full and half-Heusler compounds, MRS Bull., 2022, 47, 555–558 CrossRef CAS.
- K. Manna, Y. Sun, L. Muechler, J. Kübler and C. Felser, Heusler, Weyl and Berry, Nat. Rev. Mater., 2018, 3, 244–256 CrossRef CAS.
- K. Elphick, W. Frost, M. Samiepour, T. Kubota, K. Takanashi, H. Sukegawa, S. Mitani and A. Hirohata, Heusler alloys for spintronic devices: review on recent development and future perspectives, Sci. Technol. Adv. Mater., 2021, 22, 235–271 CrossRef CAS PubMed.
- T. Kojima, S. Kameoka, S. Fujii, S. Ueda and A.-P. Tsai, Catalysis-tunable Heusler alloys in selective hydrogenation of alkynes: A new potential for old materials, Sci. Adv., 2018, 4, eaat6063 CrossRef CAS PubMed.
- T. Kojima, S. Kameoka and A.-P. Tsai, The emergence of Heusler alloy catalysts, Sci. Technol. Adv. Mater., 2019, 20, 445–455 CrossRef CAS.
- Z. Wu, S. Ma, S. Weng, H. Liu, Z. Jin and X. Jiang, Mechanistic Insights into the Direct Deoxygenation of Phenolic Compounds over Novel Heusler Alloy Catalysts, ACS Appl. Mater. Interfaces, 2024, 16, 38111–38123 CrossRef CAS.
- T. Jin and Y. Jung, Classifying Intermetallic Tetragonal Phase of All-d-Metal Heusler Alloys for Catalysis Applications, Top. Catal., 2022, 65, 208–214 CrossRef CAS.
- U. Mizutani, T. Noritake, T. Ohsuna and T. Takeuchi, Hume-Rothery electron concentration rule across a whole solid solution range in a series of gamma-brasses in Cu–Zn, Cu–Cd, Cu–Al, Cu–Ga, Ni–Zn and Co–Zn alloy systems, Philos. Mag., 2010, 90, 1985–2008 CrossRef CAS.
- N. Roy, Harshit, A. Mondal, F. Wang and P. P. Jana, Structural and Theoretical Investigations on the Unique Coloring Scheme of the γ-Brass Type Phase: Cu5+δCd8-δ (−1.0≤δ≤0.1), Z. Anorg. Allg. Chem., 2022, 648, e202100354 CrossRef CAS.
- K. Urban and M. Feuerbacher, Structurally complex alloy phases, J. Non-Cryst. Solids, 2004, 334, 143–150 CrossRef.
- J. W. Cahn, D. Gratias and D. Shechtman, Indexing of icosahedral quasiperiodic crystals, J. Mater. Res., 1986, 1, 13–26 CrossRef CAS.
- S. Samson, Crystal Structure of NaCd2, Nature, 1962, 195, 259–262 CrossRef CAS.
- J. Hafner and M. Krajčí, Surfaces of Complex Intermetallic Compounds: Insights from Density Functional Calculations, Acc. Chem. Res., 2014, 47, 3378–3384 CrossRef CAS.
- H. Chen, L. Li, Z.-J. Zhao, B. Yang, Y. Zhang, X. Liu, Q. Gu, Z. Yu, X. Yang, J. Gong, A. Wang and T. Zhang, Co-infiltration and dynamic formation of Pd3ZnCx intermetallic carbide by syngas boosting selective hydrogenation of acetylene, Nat. Commun., 2024, 15, 9850 CrossRef CAS.
- S. F. Matar, Intermetallic hydrides: A review with ab initio aspects, Prog. Solid State Chem., 2010, 38, 1–37 CrossRef CAS.
- M.-H. Tsai, Three Strategies for the Design of Advanced High-Entropy Alloys, Entropy, 2016, 18, 252 CrossRef.
- J. W. Yeh, S. K. Chen, S. J. Lin, J. Y. Gan, T. S. Chin, T. T. Shun, C. H. Tsau and S. Y. Chang, Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes, Adv. Eng. Mater., 2004, 6, 299–303 CrossRef CAS.
- B. Cantor, I. T. H. Chang, P. Knight and A. J. B. Vincent, Microstructural development in equiatomic multicomponent alloys, Mater. Sci. Eng., A, 2004, 375–377, 213–218 CrossRef.
- E. P. George, D. Raabe and R. O. Ritchie, High-entropy alloys, Nat. Rev. Mater., 2019, 4, 515–534 CrossRef CAS.
- Y. Nakaya and S. Furukawa, Catalysis of Alloys: Classification, Principles, and Design for a Variety of Materials and Reactions, Chem. Rev., 2023, 123, 5859–5947 CrossRef CAS PubMed.
- J. K. Burdett, S. Lee and T. J. McLarnan, Coloring problem, J. Am. Chem. Soc., 1985, 107, 3083–3089 CrossRef CAS.
- P. Hohenberg and W. Kohn, Density functional theory (DFT), Phys. Rev., 1964, 136, B864 CrossRef.
- P. O. Löwdin, On the non-orthogonality problem connected with the use of atomic wave functions in the theory of molecules and crystals, J. Chem. Phys., 1950, 18, 365–375 CrossRef.
- R. S. Mulliken, Electronic population analysis on LCAO–MO molecular wave functions. I, J. Chem. Phys., 1955, 23, 1833–1840 CrossRef CAS.
- B. M. Gimarc, Topological charge stabilization, J. Am. Chem. Soc., 1983, 105, 1979–1984 CrossRef CAS.
- N. Roy, A Theoretical Study on the Unique Site Preference and Atomic Ordering of Hexagonal CoSn-type Ternary Intermetallic Compound Co3Ge2Sn, Z. Anorg. Allg. Chem., 2023, 649, e202300127 CrossRef CAS.
- R. Dronskowski and P. E. Bloechl, Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations, J. Phys. Chem., 1993, 97, 8617–8624 CrossRef CAS.
- https://www.cohp.de/ .
- S. Maintz, V. L. Deringer, A. L. Tchougréeff and R. Dronskowski, Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids, J. Comput. Chem., 2013, 34, 2557–2567 CrossRef CAS.
- V. L. Deringer, A. L. Tchougréeff and R. Dronskowski, Crystal Orbital Hamilton Population (COHP) Analysis As Projected from Plane-Wave Basis Sets, J. Phys. Chem. A, 2011, 115, 5461–5466 CrossRef CAS PubMed.
- P. C. Müller, C. Ertural, J. Hempelmann and R. Dronskowski, Crystal Orbital Bond Index: Covalent Bond Orders in Solids, J. Phys. Chem. C, 2021, 125, 7959–7970 CrossRef.
- G. Krier, O. Jepsen, A. Burkhardt and O. K. Andersen, The TB-LMTO-ASA program, Stuttgart, 1995 Search PubMed.
- R. Nelson, C. Ertural, J. George, V. L. Deringer, G. Hautier and R. Dronskowski, LOBSTER: Local orbital projections, atomic charges, and chemical-bonding analysis from projector-augmented-wave-based density-functional theory, J. Comput. Chem., 2020, 41, 1931–1940 CrossRef CAS PubMed.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- B. Eck, wxDragon 2.2.2, RWTH Aachen University, Germany, 2020 Search PubMed.
- M. Armbrüster, H. Borrmann, M. Wedel, Y. Prots, R. Giedigkeit and P. Gille, Refinement of the crystal structure of palladium gallium (1:1), PdGa, Z. Kristallogr. New Cryst. Struct., 2010, 225, 617–618 Search PubMed.
- D. Chen, R. Yu, H. Zhao, J. Jiao, X. Mu, J. Yu and S. Mu, Boron-Induced Interstitial Effects Drive Water Oxidation on Ordered Ir−B Compounds, Angew. Chem., Int. Ed., 2024, 63, e202407577 CrossRef CAS PubMed.
- J. Liang, X. Pan, B. Zeng, C. Zhong, L. Zhang, J. Zhang, H. Song, L. Du, S. Liao and Z. Cui, Achieving Highly Durable Intermetallic PtMn3N Electrocatalyst via the Strong Metal-N Bonds toward Oxygen Reduction Reaction, Adv. Funct. Mater., 2025, 35, 2414790 CrossRef CAS.
- H. Wang, L. Zou, M. Li and L. Zhang, Identification of linear scaling relationships in polysulfide conversion on α-In2Se3-supported single-atom catalysts, Phys. Chem. Chem. Phys., 2023, 25, 16968–16978 RSC.
- R. F. W. Bader and T. T. Nguyen-Dang, in Adv. Quantum Chem., Elsevier, 1981, vol. 14, pp. 63–124 Search PubMed.
- https://www.chemistry.mcmaster.ca/bader/ .
- W. Tang, E. Sanville and G. Henkelman, A grid-based Bader analysis algorithm without lattice bias, J. Phys.: Condens. Matter, 2009, 21, 084204 CrossRef CAS.
- A. Savin, R. Nesper, S. Wengert and T. F. Fässler, ELF: The Electron Localization Function, Angew Chem. Int. Ed. Engl., 1997, 36, 1808–1832 CrossRef CAS.
- M. Kohout, A measure of electron localizability, Int. J. Quantum Chem., 2004, 97, 651–658 CrossRef CAS.
- L. Agnarelli, Y. Prots, R. Ramlau, M. Schmidt, U. Burkhardt, A. Leithe-Jasper and Y. Grin, Mg29–xPt4+y: Chemical Bonding Inhomogeneity and Structural Complexity, Inorg. Chem., 2022, 61, 16148–16155 CrossRef CAS PubMed.
- D. C. Fredrickson, DFT-Chemical Pressure Analysis: Visualizing the Role of Atomic Size in Shaping the Structures of Inorganic Materials, J. Am. Chem. Soc., 2012, 134, 5991–5999 CrossRef CAS.
- https://www2.chem.wisc.edu/%7Edanny/software/dftcpp/ .
- A. Lim and D. C. Fredrickson, Navigating the 18-n+m Isomers of PdSn2: Chemical Pressure Relief through Isolobal Bonds and Main Group Clustering, Inorg. Chem., 2024, 63, 11726–11736 CrossRef CAS.
- K. M. Sanders, D. G. Gressel, R. T. Fredrickson and D. C. Fredrickson, Toward Predicting the Assembly of Modular Intermetallics from Chemical Pressure Analysis: The Interface Nucleus Approach, Inorg. Chem., 2024, 63, 6626–6637 CrossRef CAS.
- N. Roy, A Fundamental Perspective on the Selective Zn Substitution in Ternary Ordered Intermetallic Compound Cu6Zn2Sb2, Z. Anorg. Allg. Chem., 2024, 650, e202400030 CrossRef.
- S. Misra, S. Mallick, B. Koley, F. Wang, S. Chatterjee and P. P. Jana, Chemical substitution of Zn in the structure of ordered Cu6Zn2Sb2: A structural and theoretical study, Solid State Sci., 2020, 107, 106333 CrossRef CAS.
- S. K. Kuila, P. Pramanik, N. Roy, K. Buxi, A. K. Bera and P. P. Jana, Unprecedented Site Preference of Sn in the Structure of CoSn-Type NiIn1–xSnx (x < 0.7), Inorg. Chem., 2025, 64, 1798–1807 CrossRef CAS PubMed.
- M. Wuttig, C.-F. Schön, D. Kim, P. Golub, C. Gatti, J.-Y. Raty, B. J. Kooi, Á. M. Pendás, R. Arora and U. Waghmare, Metavalent or Hypervalent Bonding: Is There a Chance for Reconciliation?, Adv. Sci., 2024, 11, 2308578 CrossRef CAS.
- P. C. Müller, S. R. Elliott, R. Dronskowski and R. O. Jones, Chemical bonding in phase-change chalcogenides, J. Phys.: Condens. Matter, 2024, 36, 325706 CrossRef.
- C. S. Spanjers, A. Dasgupta, M. Kirkham, B. A. Burger, G. Kumar, M. J. Janik and R. M. Rioux, Determination of bulk and surface atomic arrangement in Ni–Zn γ-brass phase at different Ni to Zn ratios, Chem. Mater., 2017, 29, 504–512 CrossRef CAS.
- R. Gong, A. Dasgupta, S.-L. Shang, H. He, M. Kirkham, E. K. Zimmerer, G. A. Canning, M. J. Janik, R. M. Rioux and Z.-K. Liu, Determination of Site Occupancy in the M–Pd–Zn (M = Cu, Ag, and Au) γ-Brass Phase by CALculation of PHAse Diagrams Modeling and Rietveld Refinement, Inorg. Chem., 2025, 64, 1690–1701 CrossRef CAS.
- H. He, G. A. Canning, A. Nguyen, A. Dasgupta, R. J. Meyer, R. M. Rioux and M. J. Janik, Active-site isolation in intermetallics enables precise identification of elementary reaction kinetics during olefin hydrogenation, Nat. Catal., 2023, 6, 596–605 CrossRef CAS.
- A. Dasgupta, H. He, R. Gong, S.-L. Shang, E. K. Zimmerer, R. J. Meyer, Z.-K. Liu, M. J. Janik and R. M. Rioux, Atomic control of active-site ensembles in ordered alloys to enhance hydrogenation selectivity, Nat. Chem., 2022, 14, 523–529 CrossRef CAS PubMed.
- K. Kovnir, M. Armbrüster, D. Teschner, T. V. Venkov, L. Szentmiklósi, F. C. Jentoft, A. Knop-Gericke, Y. Grin and R. Schlögl, In situ surface characterization of the intermetallic compound PdGa – A highly selective hydrogenation catalyst, Surf. Sci., 2009, 603, 1784–1792 CrossRef CAS.
- Á. Molnár, A. Sárkány and M. Varga, Hydrogenation of carbon–carbon multiple bonds: chemo-, regio- and stereo-selectivity, J. Mol. Catal. A: Chem., 2001, 173, 185–221 CrossRef.
- P. Sabatier, La catalyse en chimie organique, ed. C. Béranger, 1920 Search PubMed.
- L. K. Frevel and L. J. Kressley, US Pat., US2802889A, 1957 Search PubMed.
- M. M. Johnson, D. W. Walker and G. P. Nowack, US Pat., US4404124A, 1983 Search PubMed.
- M. M. Johnson, D. W. Walker and G. P. Nowack, US Pat., US4484015A, 1984 Search PubMed.
- H. Zea, K. Lester, A. K. Datye, E. Rightor, R. Gulotty, W. Waterman and M. Smith, The influence of Pd–Ag catalyst restructuring on the activation energy for ethylene hydrogenation in ethylene–acetylene mixtures, Appl. Catal., A, 2005, 282, 237–245 CrossRef CAS.
- D. C. Huang, K. H. Chang, W. F. Pong, P. K. Tseng, K. J. Hung and W. F. Huang, Effect of Ag-promotion on Pd catalysts by XANES, Catal. Lett., 1998, 53, 155–159 CrossRef CAS.
- G. Meitzner and J. H. Sinfelt, X-ray absorption studies of the electronic structures of Pd-Ag and Pd-Au alloys, Catal. Lett., 1994, 30, 1–10 CrossRef CAS.
- B. Cordts, D. Pease and L. V. Azároff, Difference between the local density of unoccupied states in Ag-Pd and Cu-Ni alloys, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 24, 538–543 CrossRef CAS.
- Y. Jin, A. K. Datye, E. Rightor, R. Gulotty, W. Waterman, M. Smith, M. Holbrook, J. Maj and J. Blackson, The Influence of Catalyst Restructuring on the Selective Hydrogenation of Acetylene to Ethylene, J. Catal., 2001, 203, 292–306 CrossRef CAS.
- C. R. O'Connor, M. A. van Spronsen, T. Egle, F. Xu, H. R. Kersell, J. Oliver-Meseguer, M. Karatok, M. Salmeron, R. J. Madix and C. M. Friend, Hydrogen migration at restructuring palladium–silver oxide boundaries dramatically enhances reduction rate of silver oxide, Nat. Commun., 2020, 11, 1844 CrossRef PubMed.
- E. Vignola, S. N. Steinmann, B. D. Vandegehuchte, D. Curulla and P. Sautet, C2H2-Induced Surface Restructuring of Pd–Ag Catalysts: Insights from Theoretical Modeling, J. Phys. Chem. C, 2016, 120, 26320–26327 CrossRef CAS.
- F. Studt, F. Abild-Pedersen, T. Bligaard, R. Z. Sørensen, C. H. Christensen and J. K. Nørskov, Identification of Non-Precious Metal Alloy Catalysts for Selective Hydrogenation of Acetylene, Science, 2008, 320, 1320–1322 CrossRef CAS.
- F. Studt, F. Abild-Pedersen, T. Bligaard, R. Z. Sørensen, C. H. Christensen and J. K. Nørskov, On the Role of Surface Modifications of Palladium Catalysts in the Selective Hydrogenation of Acetylene, Angew. Chem., Int. Ed., 2008, 47, 9299–9302 CrossRef CAS.
- Q. Feng, S. Zhao, Y. Wang, J. Dong, W. Chen, D. He, D. Wang, J. Yang, Y. Zhu, H. Zhu, L. Gu, Z. Li, Y. Liu, R. Yu, J. Li and Y. Li, Isolated Single-Atom Pd Sites in Intermetallic Nanostructures: High Catalytic Selectivity for Semihydrogenation of Alkynes, J. Am. Chem. Soc., 2017, 139, 7294–7301 CrossRef CAS.
- C. S. Spanjers, J. T. Held, M. J. Jones, D. D. Stanley, R. S. Sim, M. J. Janik and R. M. Rioux, Zinc inclusion to heterogeneous nickel catalysts reduces oligomerization during the semi-hydrogenation of acetylene, J. Catal., 2014, 316, 164–173 CrossRef CAS.
- D. L. Trimm, I. O. Y. Liu and N. W. Cant, The oligomerization of acetylene in hydrogen over Ni/SiO2 catalysts: Product distribution and pathways, J. Mol. Catal. A: Chem., 2008, 288, 63–74 CrossRef CAS.
- J. Ma, F. Xing, Y. Nakaya, K.-i. Shimizu and S. Furukawa, Nickel-Based High-Entropy Intermetallic as a Highly Active and Selective Catalyst for Acetylene Semihydrogenation, Angew. Chem., Int. Ed., 2022, 61, e202200889 CrossRef CAS PubMed.
- Y. Nakaya, E. Hayashida, H. Asakura, S. Takakusagi, S. Yasumura, K.-i. Shimizu and S. Furukawa, High-Entropy Intermetallics Serve Ultrastable Single-Atom Pt for Propane Dehydrogenation, J. Am. Chem. Soc., 2022, 144, 15944–15953 CrossRef CAS PubMed.
- J. Osswald, K. Kovnir, M. Armbrüster, R. Giedigkeit, R. E. Jentoft, U. Wild, Y. Grin and R. Schlögl, Palladium–gallium intermetallic compounds for the selective hydrogenation of acetylene: Part II: Surface characterization and catalytic performance, J. Catal., 2008, 258, 219–227 CrossRef CAS.
- J. Prinz, C. A. Pignedoli, Q. S. Stöckl, M. Armbrüster, H. Brune, O. Gröning, R. Widmer and D. Passerone, Adsorption of Small Hydrocarbons on the Three-Fold PdGa Surfaces: The Road to Selective Hydrogenation, J. Am. Chem. Soc., 2014, 136, 11792–11798 CrossRef CAS.
- M. Armbrüster, K. Kovnir, M. Friedrich, D. Teschner, G. Wowsnick, M. Hahne, P. Gille, L. Szentmiklósi, M. Feuerbacher, M. Heggen, F. Girgsdies, D. Rosenthal, R. Schlögl and Y. Grin, Al13Fe4 as a low-cost alternative for palladium in heterogeneous hydrogenation, Nat. Mater., 2012, 11, 690–693 CrossRef.
- J. Ledieu, É. Gaudry, L. N. S. Loli, S. A. Villaseca, M. C. de Weerd, M. Hahne, P. Gille, Y. Grin, J. M. Dubois and V. Fournée, Structural Investigation of the (010) Surface of the Al13Fe4 Catalyst, Phys. Rev. Lett., 2013, 110, 076102 CrossRef CAS PubMed.
- Y. Niu, X. Huang, Y. Wang, M. Xu, J. Chen, S. Xu, M.-G. Willinger, W. Zhang, M. Wei and B. Zhang, Manipulating interstitial carbon atoms in the nickel octahedral site for highly efficient hydrogenation of alkyne, Nat. Commun., 2020, 11, 3324 CrossRef CAS PubMed.
- S. Liu, Y. Li, X. Yu, S. Han, Y. Zhou, Y. Yang, H. Zhang, Z. Jiang, C. Zhu, W.-X. Li, C. Wöll, Y. Wang and W. Shen, Tuning crystal-phase of bimetallic single-nanoparticle for catalytic hydrogenation, Nat. Commun., 2022, 13, 4559 CrossRef CAS PubMed.
- H. Zhou, X. Yang, L. Li, X. Liu, Y. Huang, X. Pan, A. Wang, J. Li and T. Zhang, PdZn Intermetallic Nanostructure with Pd–Zn–Pd Ensembles for Highly Active and Chemoselective Semi-Hydrogenation of Acetylene, ACS Catal., 2016, 6, 1054–1061 CrossRef CAS.
- Y. Liu, X. Liu, Q. Feng, D. He, L. Zhang, C. Lian, R. Shen, G. Zhao, Y. Ji, D. Wang, G. Zhou and Y. Li, Intermetallic NixMy (M = Ga and Sn) Nanocrystals: A Non-precious Metal Catalyst for Semi-Hydrogenation of Alkynes, Adv. Mater., 2016, 28, 4747–4754 CrossRef CAS PubMed.
- D. Li, C. Wang, D. Tripkovic, S. Sun, N. M. Markovic and V. R. Stamenkovic, Surfactant Removal for Colloidal Nanoparticles from Solution Synthesis: The Effect on Catalytic Performance, ACS Catal., 2012, 2, 1358–1362 CrossRef CAS.
- J. Ma, F. Xing, K.-I. Shimizu and S. Furukawa, Active site tuning based on pseudo-binary alloys for low-temperature acetylene semihydrogenation, Chem. Sci., 2024, 15, 4086–4094 RSC.
- A. Dasgupta, E. K. Zimmerer, R. J. Meyer and R. M. Rioux, Generalized approach for the synthesis of silica supported Pd-Zn, Cu-Zn and Ni-Zn gamma brass phase nanoparticles, Catal. Today, 2019, 334, 231–242 CrossRef CAS.
- M. Armbrüster, G. Wowsnick, M. Friedrich, M. Heggen and R. Cardoso-Gil, Synthesis and Catalytic Properties of Nanoparticulate Intermetallic Ga–Pd Compounds, J. Am. Chem. Soc., 2011, 133, 9112–9118 CrossRef.
- R. E. Cable and R. E. Schaak, Solution Synthesis of Nanocrystalline M−Zn (M = Pd, Au, Cu) Intermetallic Compounds via Chemical Conversion of Metal Nanoparticle Precursors, Chem. Mater., 2007, 19, 4098–4104 CrossRef CAS.
- A. Onda, T. Komatsu and T. Yashima, Preparation and Catalytic Properties of Single-Phase Ni–Sn Intermetallic Compound Particles by CVD of Sn(CH3)4 onto Ni/Silica, J. Catal., 2001, 201, 13–21 CrossRef CAS.
- T. Kojima, Y. Nakaya, H. Ham, S. Kameoka and S. Furukawa, Synthesis of Co2FeGe Heusler alloy nanoparticles and catalysis for selective hydrogenation of propyne, RSC Adv., 2021, 11, 18074–18079 RSC.
- X. Chen, C. Kong, H. Huang, D.-L. Chen, F.-F. Wang, F. Zhang and W. Zhu, Mechanism of Selective Hydrogenation of 4-Nitrophenylacetylene Using Pt–Zn Intermetallic Nanoparticles: The Role of Hydrogen Coverage, J. Phys. Chem. C, 2021, 125, 23803–23812 CrossRef CAS.
- M. Miyazaki, S. Furukawa and T. Komatsu, Regio- and Chemoselective Hydrogenation of Dienes to Monoenes Governed by a Well-Structured Bimetallic Surface, J. Am. Chem. Soc., 2017, 139, 18231–18239 CrossRef CAS.
- H. Sugiyama, M. Miyazaki, M. Sasase, M. Kitano and H. Hosono, Room-Temperature CO2 Hydrogenation to Methanol over Air-Stable hcp-PdMo Intermetallic Catalyst, J. Am. Chem. Soc., 2023, 145, 9410–9416 CrossRef CAS PubMed.
- T. Takayama, R. Kariya, Y. Nakaya, S. Furukawa, S. Yamazoe and T. Komatsu, Hydrosilylation of carbonyls over electron-enriched Ni sites of intermetallic compound Ni3Ga heterogeneous catalyst, Chem. Commun., 2021, 57, 4239–4242 RSC.
- M. Friedrich, D. Teschner, A. Knop-Gericke and M. Armbrüster, Influence of bulk composition of the intermetallic compound ZnPd on surface composition and methanol steam reforming properties, J. Catal., 2012, 285, 41–47 CrossRef CAS.
Footnote |
| † Equal contribution. |
| This journal is © The Royal Society of Chemistry 2025 |