 Open Access Article
Open Access ArticleSynthesis, structural characterization, and antimicrobial activity of Zn(cloxyquin)2: towards harnessing zinc intoxication and immune response restoration to combat Staphylococcus aureus and Mycobacterium tuberculosis†
Khalil Mudarmahad,
Nalin Abeydeerab,
Guanyu Chena,
Wjdan Jogadia,
Jeanette A. Krausec,
Jonathan M. Budzik*b and
Songping D. Huang *a
*a
aDepartment of Chemistry and Biochemistry, Kent State University, Kent, OH 44240, USA. E-mail: shuang1@kent.ed
bDivision of Pulmonary, Critical Care, Allergy and Sleep Medicine, Department of Medicine, University of California San Francisco, San Francisco, CA 94143, USA. E-mail: jonathan.budzik2@ucsf.edu
cDepartment of Chemistry, University of Cincinnati, Cincinnati, OH 45221-0172, USA
dDepartment of Chemistry, Jazan University, Jazan, 45142, Saudi Arabia
First published on 4th June 2025
Abstract
Zinc is both essential and potentially toxic to microorganisms including pathogenic bacteria. To harness the antimicrobial activity of Zn, use of a suitable Zn ionophore is necessary to facilitate its penetration of the bacterial cell membrane. On the other hand, 5-chloro-8-hydroxyquinoline, also known as cloxyquin, has known antibacterial, anti-fungal and anti-protozoal activity and can act as a Zn ionophore. When cloxyquin is repurposed as a chelating agent to form the Zn complex Zn(cloxyquin)2, the antimicrobial activity is enhanced by approximately 1000 times owing to a dual mode of action (MOA) by this Zn complex as opposed to cloxyquin itself. Specifically, the measured minimum inhibitory concentration (MIC) values of Zn(cloxyquin)2 against five strains of pathogenic bacteria, including Staphylococcus aureus (SA) bacteria including methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate Staphylococcus aureus (VISA) and Erdman strain of Mycobacterium tuberculosis (Erdman Mtb), range from 2.5 to 9.5 μM, making it one of the most potent Zn-based antimicrobial metallodrugs reported in the literature thus far. Furthermore, drug resistance development by SA bacteria toward Zn(cloxyquin)2 is considerably delayed when compared with ciprofloxacin and cloxyquin, respectively.
1. Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) represents a significant threat to healthcare systems worldwide, as it is resistant to multiple antibiotics and can lead to severe and even life-threatening infections.1,2 While treatment options for MRSA infections are now very limited, ongoing research into innovative antimicrobial agents that possess different mechanisms than those of existing antibiotics has yet to produce new therapeutic alternatives.3,4 The pursuit of new antibiotic classes has reached a period marked by a historic low rate of success.5,6 For instance, since the introduction of linezolid in 2000, no new classes of antimicrobials have been developed and commercialized. Most existing antibiotics target approximately 40 out of the 200 essential bacterial proteins that are highly conserved.6 The present pipeline for antibiotic development is scarce, comprising only 43 compounds. Among these, only 11 are distinguished as representing entirely new classes of antibiotics.7 Furthermore, none of these compounds are specifically tailored for treating MRSA infection. On the other hand, after suitable lead compounds are discovered, the development of one into a clinical drug is a substantial investment, potentially costing up to one billion dollars and requiring approximately 10 to 15 years of dedicated research and development.8 As a result, the strategy of repurposing nonantibacterial drugs to treat MRSA infections offers a promising alternative to mitigate the risks posed by this bacterial pathogen.9Cloxyquin (i.e., 5-chloro-8-hydroxyquinoline or 5-chloro-8-quinolinol), a mono-halogenated 8-hydroxyquinoline with known antibacterial, anti-fungal and anti-protozoal activity has been used in the treatment of dermatological infections including crusted eczematous dermatoses and acne vulgaris.10 The inhibitory effect on growth exhibited by cloxyquin is due to its chelating properties that can dislodge divalent metal ions, such as Mn(II), Fe(II), Zn(II), and Cu(II) from their respective enzymes and proteins.11 Additionally, in a recent drug repurposing study, cloxyquin has been reported to exhibit potent in vitro activity against 9 standard strains and 150 clinical isolates of Mycobacterium tuberculosis (Mtb), including 20 drug-resistant and 30 multidrug-resistant clinical isolates.12 Furthermore, another drug repurposing study has shown that cloxyquin is a novel activator of the two-pore domain potassium channel TRESK – a channel linked to causing migraines and controlling the sensitivity of pain nerves in the brain, suggesting a possible therapeutic role for cloxyquin in certain neonatal neurological conditions, particularly those involving excitotoxicity and neuroinflammation.13 Hence, cloxyquin represents a promising drug to explore metal chelation as a means to enhance drug repurposing efficacy.
Among many antimicrobial metals studied for therapeutic use, such as Cu, Ag, Zn, Cd, Bi and Ga,14,15 Zn stands out for its broad-spectrum antimicrobial properties against a variety of pathogenic bacteria. Although the precise mechanisms underlying its antimicrobial effects are not fully understood, but they are likely multifaceted including destabilizing the membrane integrity to increase permeability16 and/or interacting with nucleic acids and deactivating respiratory enzymes.17 As an essential metal, the cellular acquisition, transport and metabolism of Zn is tightly regulated since both deficiency and overload of Zn is detrimental to metal homeostasis of the cell.18,19 Consequently, while Zn is effectively utilized in treating dermatological infections, treating systemic infections with Zn necessitates the use of a suitable Zn ionophore to facilitate its penetration of the bacterial cell membrane.20 This is particularly important because the Zn2+ ion cannot easily pass through the hydrophobic lipid bilayer of cell membranes on its own. One of the widely studied Zn ionophores is clioquinol (i.e., 5-chloro-7-iodo-8-hydroxyquinoline; abbreviated as CQ hereafter), first marketed in the 1930s as an antibiotic for treating a range of intestinal diseases such as shigellosis, nonspecific chronic diarrhea, and traveler's diarrhea.21 In the 1970s, this drug was withdrawn from the market due to its neurotoxicity, which is presumably attributable to its ability to cross the blood–brain barrier (BBB) to chelate metal ions.22,23 These very same properties have later led researchers to successfully repurpose CQ as a potential treatment for Alzheimer's disease (AD), Parkinson's disease (PD), and Huntington's disease (HD).24 A pilot phase II clinical trial of oral CQ showed that the drug improved cognition and behavior in these patients. However further development of CQ as a treatment for neurodegenerative disorders was curtailed due to mutagenicity and neurotoxicity caused by the presence of di-iodo-8-hydroxyquinoline impurities introduced during the manufacturing process.24,25 Considering that cloxyquin is a mono-halogenated analogue of CQ and poses no risk of mutagenicity and neurotoxicity, we chose the former as a more suitable ligand for our investigations into drug repurposing in combination with metal complexation to avoid potential mutagenicity and neurotoxicity in the development of new metal complexes for our drug repurposing studies.
In this publication, we report on the synthesis, structural characterization, and antimicrobial activity of Zn(5-chloro-8-hydroxyquinoline)2 (abbreviated as Zn(cloxyquin)2 hereafter) that harnesses a dual mode of action (MOA) against five different strains of pathogenic bacteria including methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate Staphylococcus aureus (VISA) and Erdman strain of Mycobacterium tuberculosis (Erdman Mtb). Specifically, the measured minimum inhibitory concentration (MIC) values of Zn(cloxyquin)2 against these strains of pathogenic bacteria range from 2.5 to 9.5 μM, making it one of the most potent antimicrobial metallodrugs reported in the literature thus far. Furthermore, drug resistance development by SA bacteria toward Zn(cloxyquin)2 is considerably delayed when compared with ciprofloxacin and cloxyquin, respectively.
2. Results and discussion
2.1. Synthesis and characterization of Zn(cloxyquin)2
When zinc chloride was reacted with two molar equivalents of 5-chloro-8-hydroxyquinolinol in an ethanolic solution containing Na2CO3 at room temperature, a yellow precipitate was formed after vigorous stirring for 3 hours (Scheme S1†). The product was isolated by filtration and washed with water–ethanol three times. Characterization by UV-Vis (Fig. S1†), FT-IR (Fig. S2†), 1H NMR (Fig. S3†), high resolution electrospray ionization mass spectrometry (ESI-HRMS; Fig. S4†), and elemental analysis (Table S1†) unequivocally established the identity of the obtained product to be Zn(cloxyquin)2 with purity >97%. In order to determine its solid-state structure, single crystals were grown in a mixture of THF and hexane using the solvent evaporation method to afford a THF/methylcyclopentane solvate. The latter is a common minor component in the typical lab-grade hexane. The crystal data and the results of the structure refinement are listed in Table S2,† while atomic coordinates, bond lengths and angles, anisotropic displacement parameters, and torsion angles are given as Tables S3 through S6 in the ESI.† The single-crystal X-ray structure analysis showed that this compound crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with the asymmetric unit containing a [Zn(cloxyquin)2]2 moiety positioned near the center of inversion. As a result, the solid-state molecular structure of Zn(cloxyquin)2 manifests as a tetramer, comprising two distinct types of Zn ions based on their coordination environments (see Fig. 1). The two inner Zn ions, Zn1 and Zn1A, are related by inversion and exhibit a six-coordinate pseudo-octahedral geometry. In contrast, the two outer Zn ions, Zn2 and Zn2A, also inversion-related, display a five-coordinate distorted square pyramidal geometry. The ligand molecules, totaling eight, are categorized into two sets. At each cluster terminus, there are two ligand molecules, both functioning as typical bidentate ligands to simultaneously chelate to Zn2 or Zn2A. Meanwhile, the six inner cloxyquin molecules bridge adjacent Zn ions using their O atoms, in addition to their bidentate chelation to specific Zn atoms. This bridging action elevates the coordination numbers of Zn ions from the expected four to five and six, respectively. In solution, the complex exists as a monomer, as evidenced by the 1H NMR and ESI-HRMS studies of the complex in DMSO solution. Specifically, the 1H NMR spectrum clearly indicates that, in solution, each H atom of the coordinated ligand molecules of cloxyquin is in a unique environment, rather than in two different environments. This suggests that the Zn complex exists as a monomer in solution. Conversely, if the complex remained as a tetramer in solution, each H atom would be in two distinct environments due to their different coordination modes (see Fig. S3†). Additionally, the ESI-HRMS measurements consistently failed to show any fragment peak with a mass exceeding that of the monomeric entity (Fig. S4†). It appears to be common for some Zn complexes with tetrahedral coordination to increase their coordination number in the solid state by oligomerization. For instance, zinc pyrithione (i.e., a Zn complex formed with 2-mercaptopyridine-N-oxide), a board-spectrum anti-fungal and antibacterial agent used as an over-the-counter medication to treat psoriasis, eczema, ringworm, and athlete's foot, exists as a monomer in solution, and as a dimer in the solid-state through Zn coordination to the O atom from the neighboring ligand to form an O-bridge.26
with the asymmetric unit containing a [Zn(cloxyquin)2]2 moiety positioned near the center of inversion. As a result, the solid-state molecular structure of Zn(cloxyquin)2 manifests as a tetramer, comprising two distinct types of Zn ions based on their coordination environments (see Fig. 1). The two inner Zn ions, Zn1 and Zn1A, are related by inversion and exhibit a six-coordinate pseudo-octahedral geometry. In contrast, the two outer Zn ions, Zn2 and Zn2A, also inversion-related, display a five-coordinate distorted square pyramidal geometry. The ligand molecules, totaling eight, are categorized into two sets. At each cluster terminus, there are two ligand molecules, both functioning as typical bidentate ligands to simultaneously chelate to Zn2 or Zn2A. Meanwhile, the six inner cloxyquin molecules bridge adjacent Zn ions using their O atoms, in addition to their bidentate chelation to specific Zn atoms. This bridging action elevates the coordination numbers of Zn ions from the expected four to five and six, respectively. In solution, the complex exists as a monomer, as evidenced by the 1H NMR and ESI-HRMS studies of the complex in DMSO solution. Specifically, the 1H NMR spectrum clearly indicates that, in solution, each H atom of the coordinated ligand molecules of cloxyquin is in a unique environment, rather than in two different environments. This suggests that the Zn complex exists as a monomer in solution. Conversely, if the complex remained as a tetramer in solution, each H atom would be in two distinct environments due to their different coordination modes (see Fig. S3†). Additionally, the ESI-HRMS measurements consistently failed to show any fragment peak with a mass exceeding that of the monomeric entity (Fig. S4†). It appears to be common for some Zn complexes with tetrahedral coordination to increase their coordination number in the solid state by oligomerization. For instance, zinc pyrithione (i.e., a Zn complex formed with 2-mercaptopyridine-N-oxide), a board-spectrum anti-fungal and antibacterial agent used as an over-the-counter medication to treat psoriasis, eczema, ringworm, and athlete's foot, exists as a monomer in solution, and as a dimer in the solid-state through Zn coordination to the O atom from the neighboring ligand to form an O-bridge.26
2.2. Antibacterial activity of Zn(cloxyquin)2 against different strains of pathogenic bacteria
To evaluate the antimicrobial potency of Zn(cloxyquin)2, we determined its MIC values against five different strains of pathogenic bacteria in comparison with cloxyquin and ciprofloxacin. The latter is one of the widely prescribed clinical antibiotics for treating bacterial infections and its inclusion in these measurements would provide the proper perspective with respect to the antimicrobial potency of Zn(cloxyquin)2. On the other hand, our selection of different strains of SA bacteria included both methicillin-susceptible and methicillin-resistant bacteria to reveal its full antimicrobial potential. The chosen strains are ATCC 6538 referred to as MSSA – a methicillin-susceptible strain of SA, ATCC BAA-44 referred to as MRSAα and ATCC BAA-1717 referred to as MRSAβ – both methicillin-resistant strains of SA, ATCC 700699 – referred to as (VISA) – a vancomycin-intermediate strain of SA, and ATCC 35801 – referred to as Erdman Mtb – a wild-type strain of Mycobacterium tuberculosis. As shown in Table 1, all five strains of bacteria tested in our studies are susceptible to both Zn(cloxyquin)2 and cloxyquin with the MICs in the lower μM range, while ZnCl2 shows no antimicrobial activity against these bacteria. On the other hand, only one strain, i.e., MSSA is susceptible to ciprofloxacin, confirming that drug-resistance of SA bacteria to ciprofloxacin is widespread. Additionally, results of the MIC measurements seem to indicate that there is a slight enhancement of antimicrobial activity in Zn(cloxyquin)2 as compared to cloxyquin. However, given that each Zn(cloxyquin)2 contains two cloxyquin molecules, the enhancement may not be statistically significant (see Table 1). On the other hand, the rather high activity of Zn(cloxyquin)2 against the intracellular pathogen Mycobacterium tuberculosis Erdman strain is particularly noticeable as zinc deficiency is known to severely impair both innate and adaptive immune functions. In other words, the proper functioning of macrophages and other innate immune cells is highly dependent on zinc. Consequently, zinc supplementation has been proven beneficial for maintaining a healthy immune system. Recently, it has been shown that several transition metals including zinc can be harnessed by different innate immunity cells as a distinct antimicrobial mechanism to combat bacterial infections.27 As part of the innate immune response towards pathogens, macrophages can deploy phagosomal zinc intoxication defense mechanisms to defend against multiple pathogens28,29 leading to impaired intracellular growth specifically of Mtb.30,31 Additionally, adaptive immunity is also known to play a crucial role in the treatment and control of Mtb infection. For example, CD4+ T cells are essential for orchestrating the immune response against Mtb by producing cytokines like interferon-gamma (IFN-γ), which promotes the microbicidal functions of macrophages to kill Mtb bacteria.32 Additionally, CD8+ T cells can directly kill infected cells and also produce IFN-γ.33 All this offers a unique opportunity to design and synthesize novel Zn-based anti-TB agents that can directly kill Mtb bacteria as well as stimulate both innate and adaptive immune responses to battle the multi-drug resistant Mtb infection.| Bacteria strain | Zn(cloxyquin)2 | Cloxyquin | ZnCl2 | Ciprofloxacin |
|---|---|---|---|---|
| MSSA (ATCC 6538) | 9.5 | 22.50 | >1000 | 0.75 |
| MRSAα (ATCC BAA – 44) | 4.75 | 22.50 | >1000 | 48 |
| MRSAβ (USA 300, ATCC BAA – 1717) | 9.5 | 45 | >1000 | 48 |
| VISA (ATCC 700699) | 9.5 | 22.5 | >1000 | 48–96 |
| Erdman Mtb (ATCC 35801) | 2.5 | 5.5 | Not tested | Not tested |
To further investigate the enhanced antimicrobial potency of Zn(cloxyquin)2, we employed the colony-forming unit (CFU) enumeration technique to evaluate the dose-dependent response of MRSAα (ATCC BAA-44) to Zn(cloxyquin)2 and cloxyquin. The results, as depicted in Fig. 2, clearly demonstrate that Zn(cloxyquin)2 exhibits significantly enhanced antimicrobial activity when compared with cloxyquin itself. For instance, at a concentration of 20 μM, Zn(cloxyquin)2 achieves a remarkable 5-log reduction in bacterial colonies, resulting in complete eradication of bacteria, whereas, at the same concentration, cloxyquin can only achieve a modest 1-log reduction in bacterial colonies, indicating that Zn(cloxyquin)2 possesses approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times higher antimicrobial potency compared to cloxyquin. Moreover, at a concentration of 40 μM, Zn(cloxyquin)2 can reach an impressive 7-log reduction in bacterial colonies, while cloxyquin only achieves a 3-log reduction.
000 times higher antimicrobial potency compared to cloxyquin. Moreover, at a concentration of 40 μM, Zn(cloxyquin)2 can reach an impressive 7-log reduction in bacterial colonies, while cloxyquin only achieves a 3-log reduction.
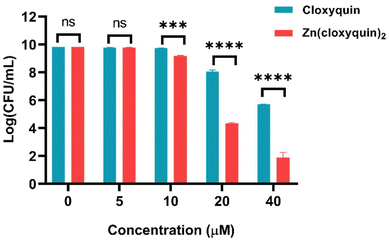 | ||
| Fig. 2 Growth-inhibitory effect of Zn(cloxyquin)2 against MRSAα in comparison with cloxyquin (mean ± s.d, n = 3 replicates; ***p < 0.001, ****p < 0.0001 and ns = not significant). | ||
2.3. Measurements of bacterial Zn uptake and intracellular generation of ROS
To investigate whether the enhanced antimicrobial activity of Zn(cloxyquin)2 is attributable to an additional mode of action induced by the delivery of Zn into the bacterial cells, we measured the intracellular concentration of Zn in the cells that were treated with varying doses of Zn(cloxyquin)2 using atomic absorption spectrometry (AAS) on MSSA cell lysates. Such measurements were performed side by side with the cells that were treated with ZnCl2 in order to determine the role of Zn(cloxyquin)2 in facilitating the cell membrane penetration of Zn. To ensure that a sufficient number of bacterial cells could survive the treatment in order to uptake Zn(cloxyquin)2, they were incubated with the Zn complex at sub-inhibitory concentrations (i.e., 8 μM) and for a shorter time period (i.e., 6 hours). Furthermore, the intracellular Zn concentrations were normalized with the population of live cells. As shown in Fig. 3a, the results reveal a dose-dependent elevation of intracellular Zn concentrations in MSSA bacterial cells treated with different concentrations of Zn(cloxyquin)2. Notably, the observed intracellular zinc levels are more than doubled in the cells treated with Zn(cloxyquin)2 at a concentration of 8 μM and higher, while there is only a slight increase of intracellular Zn levels in the ZnCl2 treated cells due to the ionic nature of Zn(II), attesting to the important role played by the lipophilic nature and its ability to form an electrically neutral complex with Zn(II). In general, Zn compounds are known to trigger the intracellular production of reactive oxygen species (ROS) in bacterial cells, a key MOA behind their antibacterial activity as ROS can cause significant damage to cellular components including cell membranes, lipids, proteins, and DNA, leading to bacterial cell death. To assess whether a similar MOA is operative with Zn(cloxyquin)2, we measured the intracellular ROS production in MSSA cells using the cellular ROS assay based on DCFH-DA (2′,7′-dichlorofluorescein diacetate). The results, when normalized with the live cell population, reveal dose-dependent ROS generation accompanied by simultaneous decrease of live cells (Fig. 3b), indicating that the intracellular ROS generation is another MOA responsible for the bacterial cell death. Please note that cloxyquin alone does not trigger any intracellular ROS production.2.4. Evaluation of resistance development
Our results clearly show that Zn(cloxyquin)2 possesses a dual antimicrobial mechanism of action. We hypothesized that Zn(cloxyquin)2 might be able to avoid resistance development that is so prevalent toward conventional antibiotics. To investigate this potential, we conducted an in vitro evaluation of resistance development by subjecting MSSA (ATCC 6538) bacteria to repeated exposure to Zn(cloxyquin)2 at a sub-lethal concentration for a series of 30 passages. We also performed similar assays with ciprofloxacin and cloxyquin for comparison. At each new passage, we determined the inhibitory concentrations of Zn(cloxyquin)2, ciprofloxacin, and cloxyquin to assess any development of resistance (Fig. 4). Our results demonstrate that repeated exposure of MSSA bacteria to ciprofloxacin can result in a progressive increase in MIC values. For example, by day 13, the MIC of ciprofloxacin has risen 32-fold, followed by 64-fold by day 18 and 128-fold by day 20, indicating a cumulative effect of five mutational events that activate various resistance mechanisms. In contrast, the repeated exposure of MSSA bacteria to cloxyquin resulted in a 4-fold increase in MIC for both strains. However, the MIC values of bacteria exposed to the Zn(cloxyquin)2 exhibited only a 2-fold increase and fluctuated between 2-fold and 4-fold throughout the 30-day exposure period. These findings highlight the potential of Zn(cloxyquin)2 to mitigate the development of resistance compared to ciprofloxacin and cloxyquin. The relatively limited increase in MIC values observed with the Zn(cloxyquin)2 suggests a reduced propensity for resistance development.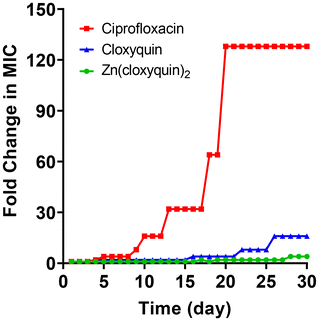 | ||
| Fig. 4 Development of bacterial resistance of MSSA towards ciprofloxacin, cloxyquin and Zn(cloxyquin)2. | ||
2.5. Evaluation of cytotoxicity in mammalian cells
The cytotoxicity of Zn(cloxyquin)2 in the concentration range of 2.5 μM to 160 μM was evaluated in human embryonic kidney cells (HEK 293) using the MTT assay. For comparison, both cloxyquin and ZnC2 were included in the cytotoxicity studies as references. The results show that both Zn(cloxyquin)2 and cloxyquin are toxic to HEK 293 cells, with IC50 values of 32.3 ± 0.59 μM and 103.2 ± 0.11 μM, respectively (see Fig. 5). Meanwhile, ZnCl2 was found to exhibit no cytotoxicity in these cells (IC50 > 160 μM). The slight increase in cell viability in the Zn-treated cells indicates that Zn can promote cell growth. These findings suggest that Zn(cloxyquin)2, as an antimicrobial agent, has a relatively small therapeutic window, likely due to the damaging effect of ROS production triggered by Zn(cloxyquin)2 in mammalian cells. | ||
| Fig. 5 Results of cell viability studies of Zn(cloxyquin)2, cloxyquin and ZnCl2 human embryonic kidney cells (HEK 293). | ||
3. Conclusions
The ability of cloxyquin to act as a Zn ionophore as well as a chelating agent for other divalent metal ions to disrupt metal homeostasis in bacterial cells, while harnessing Zn intoxication and restoring immune response, highlights a promising strategy for combating multidrug-resistant bacteria such as MRSA and Mtb. The markedly enhanced antibacterial potency of Zn(cloxyquin)2 over cloxyquin, reduced propensity for resistance development compared to ciprofloxacin, and selective cytotoxicity profile highlight its potential as a robust antimicrobial agent. These results support the continued development of Zn(cloxyquin)2 as a dual-action metallodrug capable of overcoming key limitations of conventional antibiotics. Overall, the approach of drug repurposing combined with metal chelation could be a significant step forward in addressing the urgent need for new antimicrobial agents.4. Materials and methods
Chemicals and reagents used in this work were purchased from commercial sources without further purification. Ciprofloxacin (≥ 98%), ZnCl2, 5-chloro-8-hydroxyquinoline, ethanol, THF, and DMSO were all obtained from MilliporeSigma. Bacterial strains, (MSSA; ATCC 6538, MRSAα; ATCC BAA-44, MRSAβ; USA 300 -ATCC BAA-1717, VISA; ATCC 700699) and Erdman Mtb (ATCC 35801) were purchased from American Type Culture Collection (ATCC). Tryptic Soy Broth powder (TSB), Tryptic Soy Agar (TSA) were purchased from Fisher Scientific. Middlebrook 7H9 broth (BCCD9911) and Middlebrook 7H10 Agar (BCCK5994) were purchased from Millipore Sigma, BBL Middlebrook OADC Enrichment media (212351) were purchased from Becton Dickinson Microbiology System.4.1. Synthesis of Zn(cloxyquin)2
First, cloxyquin (2.0 mmol) was dissolved in a 50 mL beaker containing a 10 mL ethanolic solution of Na2CO3 (1.5 mmol). A solution of zinc chloride (1.0 mmol) in 5 mL of ethanol was added and stirred at room temperature for 3 hours. The resulting pale-yellow precipitate was filtered and washed three times with ethanol. The crude product was dried in a vacuum oven overnight resulting in 79% yield. Elemental analysis and 1H NMR measurements showed that the purity of the product was ≥98%. Results of elemental analysis: calcd for C18H10Cl2N2O2Zn: C 51.16, H 2.39, N 6.63; found: C 51.182, H 2.324, N 6.702. Results of 1H NMR measurements: (400 MHz, DMSO-d6) δ = 8.69 (s, 1H, H–A), 8.52 (d, J = 7.6 Hz, 1H, H–B), 7.71 (dd, J = 7.5, 1.4 Hz, 1H, H–C), 7.48 (d, J = 8.1 Hz, 1H, H–D), 6.79 (d, J = 8.1 Hz, 1H, H–E), 3.38 (s, HDO), 2.50 (s, DMSO-d6), 1.06 (s, residual/impurity). Results of high resolution electrospray ionization mass spectrometry (ESI-HRMS): m/z calcd for [C18H10Cl2N2O2Zn + H]+: 422.9446; found: 420.9484. The product was further characterized by UV-Vis and FTIR spectroscopic techniques and single-crystal X-ray structure determination.4.2. Evaluation of minimum inhibitory concentrations (MICs)
We determined the minimum inhibitory concentrations (MICs) of both Zn(cloxyquin)2 and cloxyquin against four strains of SA bacteria using the standard microdilution method as described in our previous publications.34–38 Briefly, bacteria (1 × 106 CFU mL−1) were treated side by side with varying concentrations of Zn(cloxyquin)2 or cloxyquin. Subsequently, 200 μL of the treated bacterial suspension was transferred into a 96-well plate and incubated at 37 °C for 24 hours. The MIC value was identified as the lowest concentration at which no visible bacterial growth was observed. On the other hand, the MIC value against Mycobacterium tuberculosis was determined using the broth microdilution method.39 The Mtb bacteria culture was grown in the Middlebrook 7H9 media supplemented with Tween 80 and OADC up to OD600 of 0.2–0.3. A 2-fold dilution series (50 μL) of the Zn(cloxyquin)2 in 7H9/OADC/Tween 80 was placed in each well of a sterile 96-well round bottom plate, and then 50 μL of the bacterial suspension diluted to OD600 of 0.0002 was added to each well. The plates were incubated at 37 °C for 7 days. The MIC value was determined as the lowest drug concentration that inhibited visible growth of the Mtb bacteria with unaided eyes.4.3. Investigation of antibacterial activity of Zn(cloxyquin)2 and cloxyquin
A single colony of bacteria was first cultured for 24 hours in 5 mL of tryptic soy broth (TSB) at 37 °C and 180 rpm. The bacterial suspension was then diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in a new medium and incubated at 180 rpm for 4 hours at 37 °C to establish a bacterial density of approximately 1 × 109 CFU mL−1. An aliquot (100 μL) of this suspension was mixed with 890 μL of medium and treated with different amounts of Zn(cloxyquin)2 or cloxyquin in 10 μL of DMSO. The mixture was then incubated for 24 hours in an Incu-Shaker at 180 rpm and 37 °C. The number of colonies on the agar plate was counted to obtain the CFUs. All measurements were carried out in triplicate.
100 in a new medium and incubated at 180 rpm for 4 hours at 37 °C to establish a bacterial density of approximately 1 × 109 CFU mL−1. An aliquot (100 μL) of this suspension was mixed with 890 μL of medium and treated with different amounts of Zn(cloxyquin)2 or cloxyquin in 10 μL of DMSO. The mixture was then incubated for 24 hours in an Incu-Shaker at 180 rpm and 37 °C. The number of colonies on the agar plate was counted to obtain the CFUs. All measurements were carried out in triplicate.
4.4. Measurements of intracellular Zn concentrations
Three groups of bacterial cells at a density of 1 × 109 CFU mL−1 were each treated with three different doses (i.e., 2 μM, 4 μM, and 8 μM) of Zn(cloxyquin)2 or ZnCl2 and incubated for 6 hours. Next, 10 μL of each treated bacterial suspension was withdrawn for CFU counting on an agar plate. The rest of the bacterial suspension was centrifuged at 25 °C and 3750 rpm for 7 minutes to obtain a solid pellet, which was collected by removing the supernatant and then washed three times with deionized water. The harvested cells were digested with concentrated HNO3 and calcined at 620 °C in air for 5 hours to obtain zinc oxide. The latter was treated with aqua regia and diluted to the required volume for the determination of Zn concentration using AAS. All measurements were carried out in triplicate.4.5. Determination of intracellular ROS generation
The experimental procedure to measure intracellular ROS generation was adopted from our previously published protocol.35,40,41 MSSA bacteria were first cultured overnight, collected by centrifugation at 3750 rpm for 7 minutes, and then suspended in fresh TSB (400 μL). Each 100 μL of this suspension was mixed with 890 μL TSB and treated with 10 μL of either Zn(cloxyquin)2 or cloxyquin at three different concentrations. After incubation at 37 °C for 1 hour with gentle agitation, bacterial pellets were harvested by centrifugation, washed with Hank's balanced salt solution (HBSS 1×), and incubated at 37 °C with 20 μM of DCFH-DA (2′,7′-dichlorofluorescein diacetate) for 30 minutes in the dark. The fluorescence intensity of the bacterial suspension was determined using a SpectraMax M4 microplate reader, with excitation/emission wavelengths set at 497/529 nm.4.6. In vitro assays of resistance development
We conducted resistance development assays by successive passaging of bacteria, following a procedure previously reported by our group.42,43 MSSA bacteria were serially exposed to Zn(cloxyquin)2, cloxyquin, and ciprofloxacin for 30 days to compare the rate of resistance development. The change in MICs was progressively evaluated from the initial MICs of each respective drug. Each day, bacteria were treated with the drug at half-MIC concentration and inoculated in fresh TSB. After incubation at 37 °C for 24 hours, bacteria from growth treated with the highest concentration of each drug were collected to determine the MIC. This experiment lasted 30 days, with results plotted as the fold change of MIC over multiple passages.4.7. Cell viability (MTT) assay
The MTT assay was employed to evaluate cytotoxicity of Zn(cloxyquin)2, cloxyquin and ZnCl2 in the mammalian cell line HEK293. First, cells were seeded into 96-well plates at a density of 2 × 105 cells per mL in 100 μL per well. The plates were incubated for 24 hours at 37 °C with 5% CO2 to allow cell attachment. Subsequently, the medium was replaced with 100 μL of fresh medium containing various concentrations of cloxyquin or Zn(cloxyquin)2, and the plates were incubated for an additional 24 hours under the same conditions. After the treatment period, 10 μL of MTT reagent was added to each well, and the plates were incubated for 2 hours at 37 °C. Following this, 100 μL of DMSO was added to dissolve the formazan crystals formed, and the plates were kept in the dark for another 2 hours. Absorbance was then measured at 570 nm using a SpectraMax M4 microplate reader. The experiments were performed in triplicate. Data analysis was conducted using Origin and GraphPad Prism software to determine IC50 values. The results were expressed as a percentage of viable cells relative to the untreated control group.4.8. Statistical analysis
Statistical analysis was performed using GraphPad Prism version 8.0 software. A statistical significance among multiple groups was analyzed using one-way ANOVA followed by Holm–Sidak comparisons test. Data were presented as mean ± standard deviation (mean ± s.d).Data availability
All data generated or analyzed during this study are included in this published article and its ESI† are available free of charge. CCDC 2383653† contains the supplementary crystallography data for this paper.Conflicts of interest
Authors declare no competing interests.Acknowledgements
Funding for the University of Cincinnati Bruker APEX-II diffractometer was through NSF-MRI grant CHE-0215950.References
- A. S. Lee, H. De Lencastre, J. Garau, J. Kluytmans, S. Malhotra-Kumar, A. Peschel and S. Harbarth, Methicillin-resistant Staphylococcus aureus, Nat. Rev. Dis. Primers, 2018, 4, 1–23 CrossRef PubMed.
- M. A. Blaskovich, The fight against antimicrobial resistance is confounded by a global increase in antibiotic usage, ACS Infect. Dis., 2018, 4, 868–870 CrossRef CAS PubMed.
- M. N. Al-Hasan, H. R. Winders, P. B. Bookstaver and J. A. Justo, Direct measurement of performance: a new era in antimicrobial stewardship, Antibiotics, 2019, 8, 127 CrossRef PubMed.
- A. Khan, B. Wilson and I. Gould, Current and future treatment options for community-associated MRSA infection, Expert Opin. Pharmacother., 2018, 19, 457–470 Search PubMed.
- N. K. Boyd, C. Teng and C. R. Frei, Brief overview of approaches and challenges in new antibiotic development: a focus on drug repurposing, Front. Cell. Infect. Microbiol., 2021, 11, 684515 Search PubMed.
- K. Lewis, Platforms for antibiotic discovery, Nat. Rev. Drug Discovery, 2013, 12, 371–387 Search PubMed.
- M. S. Butler, I. R. Henderson, R. J. Capon and M. A. T. Blaskovich, Antibiotics in the clinical pipeline as of December 2022, J. Antibiot., 2023, 76, 431–473 CrossRef CAS PubMed.
- D. Sun, W. Gao, H. Hu and S. Zhou, Why 90% of clinical drug development fails and how to improve it?, Acta Pharm. Sin. B, 2022, 12, 3049–3062 Search PubMed.
- S. Thangamani, H. Mohammad, W. Younis and M. N. Seleem, Drug repurposing for the treatment of staphylococcal infections, Curr. Pharm. Des., 2015, 21, 2089–2100 Search PubMed.
- M. Bortolin, A. Bidossi, E. De Vecchi, M. Avveniente and L. Drago, In vitro antimicrobial activity of chlorquinaldol against microorganisms responsible for skin and soft tissue infections: comparative evaluation with gentamicin and fusidic acid, Front. Microbiol., 2017, 8, 1039 Search PubMed.
- A. R. Joaquim, M. P. Gionbelli, G. Gosmann, A. M. Fuentefria, M. S. Lopes and S. Fernandes de Andrade, Novel antimicrobial 8-hydroxyquinoline-based agents: Current development, structure–activity relationships, and perspectives, J. Med. Chem., 2021, 64, 16349–16379 Search PubMed.
- P. Hongmanee, K. Rukseree, B. Buabut, B. Somsri and P. Palittapongarnpim, In vitro activities of cloxyquin (5-chloroquinolin-8-ol) against Mycobacterium tuberculosis, Antimicrob. Agents Chemother., 2007, 51, 1105–1106 CrossRef CAS PubMed.
- J. A. Schreiber, A. Derksen, G. Goerges, S. Schütte, J. Sörgel, A. K. Kiper, N. Strutz-Seebohm, T. Ruck, S. G. Meuth and N. Decher, Cloxyquin activates hTRESK by allosteric modulation of the selectivity filter, Commun. Biol., 2023, 6, 745 CrossRef CAS PubMed.
- J. A. Lemire, J. J. Harrison and R. J. Turner, Antimicrobial activity of metals: mechanisms, molecular targets and applications, Nat. Rev. Microbiol., 2013, 11, 371–384 CrossRef CAS PubMed.
- A. Frei, A. D. Verderosa, A. G. Elliott, J. Zuegg and M. A. Blaskovich, Metals to combat antimicrobial resistance, Nat. Rev. Chem., 2023, 7, 202–224 CrossRef CAS PubMed.
- J. C. Ballin, Evaluation of a new topical agent for burn therapy: Silver sulfadiazine (Silvadene), J. Am. Med. Assoc., 1974, 230, 1184–1185 CrossRef CAS.
- M. Fang, J.-H. Chen, X.-L. Xu, P.-H. Yang and H. F. Hildebrand, Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests, Int. J. Antimicrob. Agents, 2006, 27, 513–517 CrossRef CAS PubMed.
- D. J. Eide, Zinc transporters and the cellular trafficking of zinc, Biochim. Biophys. Acta, Mol. Cell Res., 2006, 1763, 711–722 CrossRef CAS PubMed.
- N. Abeydeera, I. C. Perera and T. Perera, Synthesis, Characterization, and BSA–Binding Studies of Novel Sulfonated Zinc–Triazine Complexes, Bioinorg. Chem. Appl., 2018, 2018, 7563820 Search PubMed.
- S. Scavo and V. Oliveri, Zinc ionophores: chemistry and biological applications, J. Inorg. Biochem., 2022, 228, 111691 CrossRef CAS PubMed.
- Z. You, X. Ran, Y. Dai and Y. Ran, Clioquinol, an alternative antimicrobial agent against common pathogenic microbe, J. Mycol. Med., 2018, 28, 492–501 CrossRef CAS PubMed.
- S. R. Bareggi and U. Cornelli, Clioquinol: review of its mechanisms of action and clinical uses in neurodegenerative disorders, CNS Neurosci. Ther., 2012, 18, 41–46 CrossRef CAS PubMed.
- D. R. Perez, L. A. Sklar and A. Chigaev, Clioquinol: To harm or heal, Pharmacol. Ther., 2019, 199, 155–163 CrossRef CAS PubMed.
- L. W. Hung and K. J. Barnham, Modulating metals as a therapeutic strategy for Alzheimer's disease, Future Med. Chem., 2012, 4, 955–969 CrossRef CAS PubMed.
- V. Oliveri and G. Vecchio, 8-Hydroxyquinolines in medicinal chemistry: A structural perspective, Eur. J. Med. Chem., 2016, 120, 252–274 CrossRef CAS PubMed.
- B. Barnett, H. Kretschmar and F. Hartman, Structural characterization of bis (N-oxopyridine-2-thionato) zinc(II), Inorg. Chem., 1977, 16, 1834–1838 CrossRef CAS.
- S. L. Stafford, N. J. Bokil, M. E. Achard, R. Kapetanovic, M. A. Schembri, A. G. McEwan and M. J. Sweet, Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper, Biosci. Rep., 2013, 33, e00049 CrossRef PubMed.
- C. J. Stocks, J. B. von Pein, J. E. Curson, J. Rae, M.-D. Phan, D. Foo, N. J. Bokil, T. Kambe, K. M. Peters and R. G. Parton, Frontline Science: LPS-inducible SLC30A1 drives human macrophage-mediated zinc toxicity against intracellular Escherichia coli, J. Leukoc. Biol., 2021, 109, 287–297 CrossRef CAS PubMed.
- A. Maure, E. Lawarée, F. Fiorentino, A. Pawlik, S. Gona, A. Giraud-Gatineau, M. J. Eldridge, A. Danckaert, D. Hardy and W. Frigui, A host-directed oxadiazole compound potentiates antituberculosis treatment via zinc poisoning in human macrophages and in a mouse model of infection, PLoS Biol., 2024, 22, e3002259 CrossRef CAS PubMed.
- H. Botella, P. Peyron, F. Levillain, R. Poincloux, Y. Poquet, I. Brandli, C. Wang, L. Tailleux, S. Tilleul and G. M. Charrière, Mycobacterial P1-type ATPases mediate resistance to zinc poisoning in human macrophages, Cell Host Microbe, 2011, 10, 248–259 CrossRef CAS PubMed.
- D. G. Russell, The galvanizing of Mycobacterium tuberculosis: an antimicrobial mechanism, Cell Host Microbe, 2011, 10, 181–183 CrossRef CAS PubMed.
- M. M. Ravesloot-Chávez, E. Van Dis and S. A. Stanley, The innate immune response to Mycobacterium tuberculosis infection, Annu. Rev. Immunol., 2021, 39, 611–637 CrossRef PubMed.
- K. D. Mayer-Barber and D. L. Barber, Innate and adaptive cellular immune responses to Mycobacterium tuberculosis infection, Cold Spring Harbor Perspect. Med., 2015, 5, a018424 CrossRef PubMed.
- N. Abeydeera, B. M. Benin, K. Mudarmah, B. D. Pant, G. Chen, W. S. Shin, M.-H. Kim and S. D. Huang, Harnessing the Dual Antimicrobial Mechanism of Action with Fe (8-Hydroxyquinoline) 3 to Develop a Topical Ointment for Mupirocin-Resistant MRSA Infections, Antibiotics, 2023, 12, 886 CrossRef CAS PubMed.
- N. Abeydeera, B. Yu, B. D. Pant, M.-H. Kim and S. D. Huang, Harnessing the toxicity of dysregulated iron uptake for killing Staphylococcus aureus: reality or mirage?, Biomater. Sci., 2022, 18(10), 474–484 RSC.
- Z. Wang, J. Li, B. M. Benin, B. Yu, S. D. Bunge, N. Abeydeera, S. D. Huang and M.-H. Kim, Lipophilic Ga Complex with Broad-Spectrum Antimicrobial Activity and the Ability to Overcome Gallium Resistance in both Pseudomonas aeruginosa and Staphylococcus aureus, J. Med. Chem., 2021, 64(13), 9381–9388 CrossRef CAS PubMed.
- Z. Wang, B. Yu, H. Alamri, S. Yarabarla, M. H. Kim and S. D. Huang, KCa(H2O)2[FeIII (CN)6]·H2O Nanoparticles as an Antimicrobial Agent against Staphylococcus aureus, Angew. Chem., 2018, 130, 2236–2240 CrossRef.
- K. Mudarmah, B. Bagale, G. Chen, J. A. Krause, J. D. Mighion and S. D. Huang, Harnessing the dual antimicrobial mode of action with a lipophilic Mn(II) complex using the principle of the Irving–Williams Series to completely eradicate Staphylococcus aurous, Dalton Trans., 2023, 52, 12203–12207 RSC.
- J. E. Hugonnet, L. W. Tremblay, H. I. Boshoff, C. E. 3rd Barry and J. S. Blanchard, Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis, Science, 2009, 323(3235918), 1215–1218 CrossRef CAS PubMed.
- B. D. Pant, B. M. Benin, N. Abeydeera, M.-H. Kim and S. D. Huang, Bi2O3 nanoparticles exhibit potent broad-spectrum antimicrobial activity and the ability to overcome Ag-, ciprofloxacin-and meropenem-resistance in P. aeruginosa: the next silver bullet of metal antimicrobials?, Biomater. Sci., 2022, 10(6), 1523–1531 RSC.
- B. D. Pant, N. Abeydeera, R. Dubadi, M.-H. Kim and S. D. Huang, Broad-Spectrum Antimicrobial Activity of Ultrafine (BiO)2CO3 NPs Functionalized with PVP That Can Overcome the Resistance to Ciprofloxacin, AgNPs and Meropenem in Pseudomonas aeruginosa, Antibiotics, 2023, 12, 753 CrossRef CAS PubMed.
- R. Song, B. Yu, D. Friedrich, J. Li, H. Shen, H. Krautscheid, S. D. Huang and M.-H. Kim, Naphthoquinone-derivative as a synthetic compound to overcome the antibiotic resistance of methicillin-resistant S. aureus, Commun. Biol., 2020, 3, 1–11 CrossRef PubMed.
- T. M. Dassanayake, A. C. Dassanayake, N. Abeydeera, B. D. Pant, M. Jaroniec, M.-H. Kim and S. D. Huang, An aluminum lining to the dark cloud of silver resistance: harnessing the power of potent antimicrobial activity of γ-alumina nanoparticles, Biomater. Sci., 2021, 9, 7996–8006 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: All data generated or analyzed during this study are included in this published article and its supplementary information files are available free of charge. CCDC 2383653. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt02895c |
| This journal is © The Royal Society of Chemistry 2025 |


