Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermometer or freezer: dual functionality in a 2D mixed-anion terbium(III) oxide carbodiimide†
Juan
Medina-Jurado
a,
YiXu
Wang
a,
Hicham
Bourakhouadar
a,
Moritz
Köller
a,
Alex J.
Corkett
b,
David
Enseling
c,
Thomas
Jüstel
 c and
Richard
Dronskowski
c and
Richard
Dronskowski
 *a
*a
aChair of Solid-State and Quantum Chemistry, Institute of Inorganic Chemistry, RWTH Aachen University, 52056 Aachen, Germany. E-mail: drons@HAL9000.ac.rwth-aachen.de; Web: https://www.ssc.rwth-aachen.de
bJülich Center for Neutron Science-2 (JCNS), Forschungszentrum Jülich GmbH, 52425 Jülich, Germany
cDepartment of Chemical Engineering, FH Münster University of Applied Sciences, 48565 Steinfurt, Germany
First published on 26th May 2025
Abstract
Advances in the chemistry of compounds containing the NCN2− complex anion are leading to the discovery of new materials with interesting and promising properties. Here we present a comprehensive investigation of the optical and magnetic properties of the 2D phosphor Tb2O2NCN whose structure presents a double layer of Tb-triangular lattices separated from each other by the NCN2− anion, offering a conjunction of luminescent thermometry and magnetocaloric effects. Temperature-dependent luminescence studies reveal the typical behavior of thermal quenching, but when the temperature increases, a mechanism of direct relaxation to the 5D4 levels of Tb3+ ions is activated, thereby giving rise to an anti-thermal quenching effect. This peculiar feature has prompted us to explore the characteristics of Tb2O2NCN in temperature sensing, using the classical approach of optical ratiometric thermometry as well as an approach based on linear transformations (i.e., principal component analysis). The latter, by employing the entire emission spectrum, offers better sensitivity leading to more accurate temperature values. The magnetic properties of Tb2O2NCN reveal a long-range antiferromagnetic ordering below TN = 6.5 K as well as first-order field-induced metamagnetic transitions. The absence of hysteresis and its high magnetic density make Tb2O2NCN a fine candidate for magnetic refrigeration, with a surprisingly large value of −ΔSM = 11.7 J kg−1 K−1 at 5 T, much more promising than those reported for other Tb-comprising compounds.
Introduction
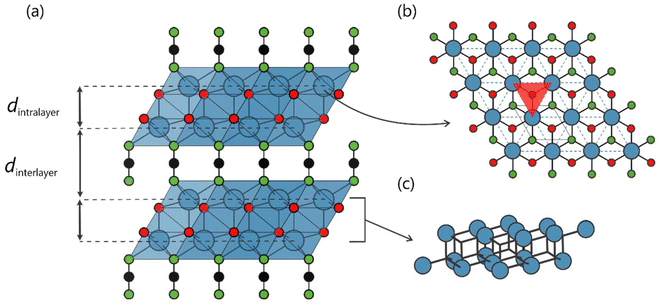
In recent years, layered crystal structures incorporating the NCN2− anion have been shown to display a wide variety of desirable optical properties, as well as finding applications in electrochemistry and photochemistry.1 Among the most prominent examples are the MNCN (M = Zn, Mn, Fe, Co) binary carbodiimide compounds, which exhibit outstanding performance as anodes in lithium- and sodium-ion batteries, and also silver cyanamide, Ag2NCN, which has been used as a catalyst for water oxidation.2–8 One area in which NCN-based compounds stand out is that of luminescence, however, as they are able to incorporate many of the most common activators into their crystal structure, such as lanthanide ions Ln3+ (Ln = Ce, Tb, Pr, Eu) or Mn2+ and Eu2+ divalent cations.9–20 Several phosphor materials have been synthesized by substitution using as host lattice NCN-based structures such as those based on binary carbodiimides, for example CaNCN:Mn2+, SrNCN:Eu2+, and Gd2NCN3:Tb3+, or those synthesized based on ternary cyanamides, such as LiIn(NCN)2:Tb3+ or the recent work on the alkaline-mixed compound LixNa1−xTb(NCN)2.21,22 Nonetheless, the mixed-anion oxide carbodiimides, Ln2O2NCN, currently constitute the largest family of host lattices suitable for the preparation of luminescent materials.The crystal structure of Ln2O2NCN (Ln = Sm–Yb, P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1) is composed of alternating layers of [Ln2O2]2+ and NCN2− stacked along the c-axis as shown in Fig. 1.23,24 The structure of the cationic layer is formed by a block (or double-layer) of Ln3+ ions composed of two sheets with a triangular Ln–Ln network extending throughout the ab-plane. The geometry of the crystal sublattice of the Ln3+ ions is such that the distance between sheets within the same block, dintra, is much smaller than the distance between sheets of different blocks, dinter, so that the distance arrangement between the Ln3+ ionic planes is dintra–dinter–dintra–dinter–… along the stacking axis, making it a structure that can be understood as being two-dimensional. Luminescent materials of this family have been developed, such as the substituted compounds Y2O2NCN:Eu3+ and Gd2O2NCN:Eu3+,Tb3+.25,26 As in the case of the other luminescent compounds based on the NCN group, however, the description of the optical properties is limited to the study of the excitation/emission spectra and radiative decay curves but potential uses as a functional material have not yet been explored in depth.
m1) is composed of alternating layers of [Ln2O2]2+ and NCN2− stacked along the c-axis as shown in Fig. 1.23,24 The structure of the cationic layer is formed by a block (or double-layer) of Ln3+ ions composed of two sheets with a triangular Ln–Ln network extending throughout the ab-plane. The geometry of the crystal sublattice of the Ln3+ ions is such that the distance between sheets within the same block, dintra, is much smaller than the distance between sheets of different blocks, dinter, so that the distance arrangement between the Ln3+ ionic planes is dintra–dinter–dintra–dinter–… along the stacking axis, making it a structure that can be understood as being two-dimensional. Luminescent materials of this family have been developed, such as the substituted compounds Y2O2NCN:Eu3+ and Gd2O2NCN:Eu3+,Tb3+.25,26 As in the case of the other luminescent compounds based on the NCN group, however, the description of the optical properties is limited to the study of the excitation/emission spectra and radiative decay curves but potential uses as a functional material have not yet been explored in depth.
An application field receiving increased attention recently is that of optical thermometry, where the temperature can be determined by the emission characteristics of a luminescent center present in the material hence called “thermometric”.27–37 Thus, by analyzing thermally induced changes in specific spectroscopic parameters of the phosphor, a direct correlation between said parameters and temperature can be established. Accordingly, luminescent thermometry has been developed on the basis of photoluminescent properties such as the intensity of an emission band, spectral position, bandwidth, radiative decay time, or, most importantly, the ratio of the emission intensity (dubbed “ratiometric”) of two different bands.27,28,38,39 The latter is the most frequently employed approach, since this type of thermometry is not affected by changes in the experimental conditions under which the emission intensities are obtained.28,40–42
Typically, in a ratiometric-thermometric material, two luminescent centers with different temperature response are required.39,43,44 The design usually involves a lanthanide ion (Ln3+) acting as a reference and a transition-metal ion (M2+) to detect the signal. This type of configuration is particularly effective given the difference in the responsible ions’ thermal quenching mechanism (i.e., how the emission of the centers is affected with respect to temperature).45–48 In this way, one will obtain thermometers with good accuracy (δT) and thermal sensitivity (Sr). This technique still has some limitations, however, mainly related to the need to spectroscopically resolve the bands involved which is not always simple as there may be band overlap. In addition, a good thermometric material should be as thermally and chemically stable as possible while its preparation process should be robust enough to reproduce the results of luminescence evaluation.27,28,31,38,49,50
Hereby, we present the inorganic solid-state mixed-anion phosphor terbium(III) oxide carbodiimide (Tb2O2NCN) as a candidate multifunctional material, coupling magnetism and photosensitivity in the same compound. The temperature-dependent photoluminescence exhibits typical thermal-quenching behavior at low temperatures but reveals, at moderate temperatures, an antithermal-quenching mechanism, so as to establish ratiometric thermometry with only one luminescent center. This peculiar characteristic, together with the fact that Tb2O2NCN presents high chemical, thermal and mechanical stability and a facile solid-state synthesis process, make it a promising material for thermal sensing at cryogenic temperatures. In addition, we present an approach to thermometry, which is receiving increasing attention, based on dimensional reduction through principal component analysis (PCA): not only the emission of two bands but the complete data of the emission spectrum (high dimensional space) are considered for the treatment of the information, thus eliminating problems related to spectral overlap or changes in the shape of the emission spectrum.51–55
Tb2O2NCN also exhibits a temperature-dependent magnetic behavior demonstrating a giant magnetocaloric effect (MCE) such that it is also a promising candidate for a low-temperature refrigerant. The large ground-state spin of Tb3+ ions and the high magnetic density in Tb2O2NCN lead to a large magnetic entropy change (−ΔSM) manifesting its role for magnetic refrigeration.56,57 Finally, this compound exhibits slow magnetic relaxation as a consequence of the inherent anisotropy in the ions’ magnetic ordering, thus demonstrating a strong structure–property relation; the geometry of the crystal structure determines the processes related to the energy migration of the photoluminescence as well as the magnetic interaction between the Tb3+ ions.
Results and discussion
The fully inorganic 2D Tb2O2NCN was synthesized via solid-state metathesis by heating a solid, homogenized mixture of Li2NCN and TbOCl to 600 °C under argon flow, as described recently.58 The powder X-ray diffraction pattern reveals high phase purity (Fig. 2a) with hexagonal lattice parameters a = 3.75 Å and c = 8.20 Å. Details of the Rietveld refinement, crystallographic information and atomic positions are available in the ESI (Fig. S1 and Tables S1, S2†). The structure of Tb2O2NCN (P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1, no. 164) is composed of a double-layer of Tb3+ ions with a bipyramidal-type coordination environment, i.e., three NCN2− units on one side and three oxygen atoms on the opposite side, plus an additional oxygen atom forming a Tb–O bond along the c-stacking axis (Fig. 2b). Each of the TbN3O4 polyhedra shares an edge with six other polyhedra in the same layer and with three others in the next layer. The terbium cations within the double-layer are bridged to each other by O2− anions, while the connection between terbium atoms in different layers relies on NCN2− anions. The arrangement of the Tb3+ magnetic ions in each sheet of the double-layer forms an equilateral triangular lattice with distances dintrasheet ≈ 3.75 Å and dintralayer ≈ 3.65 Å (Fig. 2b). An important characteristic is that these triangular Tb motifs are displaced from each other in such a way that the Tb3+ ion in one layer is above/below the center of the triangle formed by the terbium ions in the next layer of the double-layer, as shown in Fig. 2c. Thus, the triangular layers alternate with the NCN2− anion in a stacking with an AB-NCN-AB-NCN-AB arrangement and an interlayer distance dinterlayer ≈ 5.69 Å, so that we can expect a behavior characteristic for a two-dimensional system.
m1, no. 164) is composed of a double-layer of Tb3+ ions with a bipyramidal-type coordination environment, i.e., three NCN2− units on one side and three oxygen atoms on the opposite side, plus an additional oxygen atom forming a Tb–O bond along the c-stacking axis (Fig. 2b). Each of the TbN3O4 polyhedra shares an edge with six other polyhedra in the same layer and with three others in the next layer. The terbium cations within the double-layer are bridged to each other by O2− anions, while the connection between terbium atoms in different layers relies on NCN2− anions. The arrangement of the Tb3+ magnetic ions in each sheet of the double-layer forms an equilateral triangular lattice with distances dintrasheet ≈ 3.75 Å and dintralayer ≈ 3.65 Å (Fig. 2b). An important characteristic is that these triangular Tb motifs are displaced from each other in such a way that the Tb3+ ion in one layer is above/below the center of the triangle formed by the terbium ions in the next layer of the double-layer, as shown in Fig. 2c. Thus, the triangular layers alternate with the NCN2− anion in a stacking with an AB-NCN-AB-NCN-AB arrangement and an interlayer distance dinterlayer ≈ 5.69 Å, so that we can expect a behavior characteristic for a two-dimensional system.
To investigate the optical properties of Tb2O2NCN, photoluminescence emission spectra were recorded in the temperature range of 3–320 K (Fig. 3a). Upon excitation at 270 nm the oxide carbodiimide exhibits typical Tb3+ luminescence with narrow and well-resolved emission bands. The spectrum consists of multiple bands along five regions centered around 487, 543, 583, 621, and 658 nm corresponding to the [Xe]4f8–[Xe]4f8 intraconfigurational transitions of the Tb3+ ions from the 5D4 level towards the 7FJ levels with J = 6, 5, 4, 3, and 2, respectively. The temperature dependence of the emission spectra of Tb2O2NCN is provided in Fig. 3b and reveals two types of behavior. First, as the temperature increases from 3 to 140 K, the intensity of the main emission (at 543 nm) decreases to about 55% of the intensity at 3 K, which typically is due to the thermal activation of non-radiative decay mechanisms, an observation well-known as thermal quenching. Quite surprisingly, however, starting at 140 K the intensity increases progressively with temperature up to 320 K, an increase of about 200% compared to the intensity at 140 K, thus demonstrating an anti-thermal quenching behavior.
 | ||
| Fig. 3 (a) Emission spectra of Tb2O2NCN under λexc = 270 nm in the temperature range between 3 and 320 K, resulting in the transitions 5D4 → 7FJ. (b) Temperature-dependent emission spectra showing the anti-thermal quenching effect for the main emission at 543 nm. (c) Temperature-dependence of integrated intensities for the total emission and 5D4 → 7FJ transitions, R1 (J = 6), R2 (J = 5), R3 (J = 4), R4 (J = 3) and R5 (J = 2). (d) Schematic energy diagram of the cross-relaxation process between 5D3 → 7F0 and 7F6 → 5D4. (e) Suggested configurational coordinate diagram for the anti-thermal quenching process in Tb2O2NCN. (f) Decay curves for the transition 5D4 → 7F5 at 100, 300, and 500 K. Inset: temperature-dependence of the experimental radiative lifetime (τ0). For the excitation spectrum of Tb2O2NCN, please see ref. 58. | ||
To facilitate the analysis of the thermal and anti-thermal quenching of the 5D4 → 7FJ transitions, we segmented the emission spectrum into five different regions: R1 (J = 6, 480–500 nm), R2 (J = 5, 535–555 nm), R3 (J = 4, 577–602 nm), R4 (J = 3, 613–629 nm), and R5 (J = 2, 656–660 nm). The behavior of the intensities (expressed as integrated area) of the five Ri regions with temperature, as well as the total emission, are shown in Fig. 3c. Clearly, the intensity of the regions R1, R2 (related to the main emission at 543 nm) and R3 mirror the thermal and antithermal quenching behavior, although the effect is not as pronounced in the R1 case. In contrast, the intensity of the R4 region is not strongly affected by temperature until 200 K at which point the intensity starts to increase up to 150% at T = 320 K compared to the value at 3 K. The R5 region looks practically unaffected. Thus, the anti-thermal quenching effect becomes dominant in four out of five regions from T = 200 K onwards.
This temperature behavior can be explained by two different processes. A first mechanism takes place at low temperatures, where under excitation at 270 nm the electrons pass directly from the ground state to the 5d levels (Fig. 3d), where by non-radiative processes they populate the 5DJ levels and emit light mainly by 5D4 → 7FJ transitions. Thus, as the temperature increases, the non-radiative transfer process becomes more dominant and the intensity of the emission decreases, which constitutes the thermal-quenching effect. This is observed in the intensity behavior of regions R2 and R3 where the intensity clearly decreases as the temperature increases. Nonetheless, the fact that the intensity in the R1 region (transition 5D4 → 7F6) is not so strongly affected by temperature can be explained by a compensation effect resulting from Tb–Tb energy transfer through a cross-relaxation mechanism between the 5D3 → 7F0 and 7F6 → 5D4 transitions. At moderate temperatures, as the temperature increases multiple vibration states above the 5d levels are formed. If they are energetically close enough to the Tb-NCN charge-transfer state, the 5d electrons relax directly towards the 5D4 levels, thereby increasing the intensity of all radiative emissions towards the 7FJ levels and explaining the anti-thermal quenching effect, as shown in Fig. 3e (violet line). Therefore, in this temperature regime, this second mechanism compensates and exceeds the intensity reduction originating from the intrinsic thermal-quenching effect, so all the intensities of the Ri regions increase. If the temperature continues to increase, i.e., the compound is over-heated, electrons could be thermally induced from the Tb-NCN state to the ground state without light emission, thus increasing the non-radiative energy loss (Fig. 3e yellow line).
To obtain information about the energy transfer process between Tb3+ ions, time-dependent luminescence measurements (λexc = 270 nm) were carried out for the 5D4 → 7F5 transition in the 100–500 K range, as depicted in Fig. 3f. In all cases, the decay curves do not show mono-exponential behavior but the long-time part follows the mono-exponential decay model: I = I0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ0). By fitting the experimental data with this equation, an experimental radiative lifetime of τ0 = 13.6 μs at T = 100 K is obtained, which increases to 19.4 μs at 500 K, revealing that the energy migration process slows with temperature and is consistent with the thermal intensity behavior in the emission spectrum. Now, it is known that the probability (or rate) of energy transfer (P) can be expressed as P = C
exp(−t/τ0). By fitting the experimental data with this equation, an experimental radiative lifetime of τ0 = 13.6 μs at T = 100 K is obtained, which increases to 19.4 μs at 500 K, revealing that the energy migration process slows with temperature and is consistent with the thermal intensity behavior in the emission spectrum. Now, it is known that the probability (or rate) of energy transfer (P) can be expressed as P = C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−2R/L), where R is the distance between the luminescent centers, C is an interaction constant between them, and L is the effective average Bohr radius (≈0.30 Å for Tb3+ ions). Since in the Tb2O2NCN structure the intrasheet distance (≈3.75 Å) is considerably smaller than the interlayer distance (≈5.69 Å) the energy-migration rate is dominant along the Tb3+ ion double-layer (Pintrasheet/Pinterlayer ≈ 8 × 105). To investigate the nature of these short-distance interactions, the value of the critical transfer distance (RC) compared to the Tb–Tb distance in the triangular network (dintrasheet ≈ 3.75 Å) has been evaluated. This very critical distance can be calculated according to
exp(−2R/L), where R is the distance between the luminescent centers, C is an interaction constant between them, and L is the effective average Bohr radius (≈0.30 Å for Tb3+ ions). Since in the Tb2O2NCN structure the intrasheet distance (≈3.75 Å) is considerably smaller than the interlayer distance (≈5.69 Å) the energy-migration rate is dominant along the Tb3+ ion double-layer (Pintrasheet/Pinterlayer ≈ 8 × 105). To investigate the nature of these short-distance interactions, the value of the critical transfer distance (RC) compared to the Tb–Tb distance in the triangular network (dintrasheet ≈ 3.75 Å) has been evaluated. This very critical distance can be calculated according to  where V is the unit-cell volume, n is the number of activators per unit cell, and xC is the activator concentration (1.0 for Tb2O2NCN, a stoichiometric compound fully occupied by Tb3+ ions). Thus, one obtains RC ≈ 4.57 Å > dintrasheet, indicating that the energy-migration process is favored through an exchange interaction mechanism.
where V is the unit-cell volume, n is the number of activators per unit cell, and xC is the activator concentration (1.0 for Tb2O2NCN, a stoichiometric compound fully occupied by Tb3+ ions). Thus, one obtains RC ≈ 4.57 Å > dintrasheet, indicating that the energy-migration process is favored through an exchange interaction mechanism.
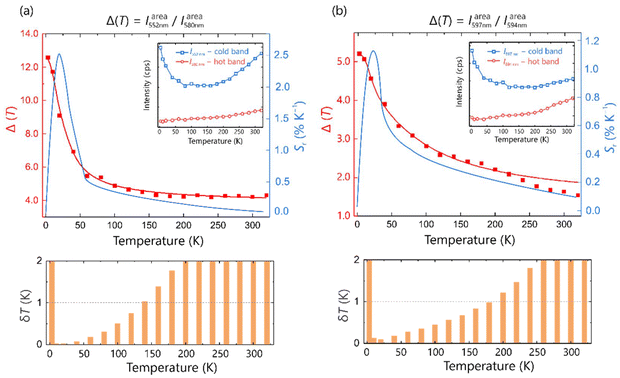
In an attempt to take advantage of the particular thermal behavior of the emission spectrum, Tb2O2NCN was tested for ratiometric thermometry. We found that the thermal variation of the intensities (expressed as integrated area, Iareaλ) of the emission peaks in regions R2 and R3 provide good combinations suitable for thermometric parameters,  . The choice of λ1 and λ2 is made so that one of the intensities is strengthened at low temperatures (cold band) while the other is weakened (hot band). In this way, we have explored the temperature behavior of two thermometric parameters Δ552/580 = Iarea552 nm/Iarea580 nm and Δ597/594 = Iarea597 nm/Iarea594 nm as shown in Fig. 4. The experimental data can be appropriately described according to the Mott–Seitz equation59,60 according to which ΔT = Δ0/[1 + α1
. The choice of λ1 and λ2 is made so that one of the intensities is strengthened at low temperatures (cold band) while the other is weakened (hot band). In this way, we have explored the temperature behavior of two thermometric parameters Δ552/580 = Iarea552 nm/Iarea580 nm and Δ597/594 = Iarea597 nm/Iarea594 nm as shown in Fig. 4. The experimental data can be appropriately described according to the Mott–Seitz equation59,60 according to which ΔT = Δ0/[1 + α1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔE1/kB·T) + α2
exp(−ΔE1/kB·T) + α2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔE2/kB·T)], where Δ0, α1 and α2 are fitting parameters and ΔE1 and ΔE2 are the activation energies for the emissions. Fitting the model yields that for thermometry based on Δ552/580 only one channel is required (i.e., ΔE2 ≈ 0) so that ΔE1 ≈ 37 K while for the thermometric parameter Δ597/594 values of ΔE1 ≈ 225 K and ΔE2 ≈ 38 K (see ESI† for details) are obtained. Small values in the activation energy could be related to a multiphonon quenching mechanism while the higher value would be associated with the thermal crossover mechanism described in Fig. 3. Moreover, as shown in Fig. 4, the values of Δ552/580 decrease exponentially with temperature, which explains the presence of only one non-radiative decay channel, while Δ597/594 shows an “S-shaped” decrease, making the presence of two channels ΔE1 and ΔE2 with a considerable energy difference understandable.
exp(−ΔE2/kB·T)], where Δ0, α1 and α2 are fitting parameters and ΔE1 and ΔE2 are the activation energies for the emissions. Fitting the model yields that for thermometry based on Δ552/580 only one channel is required (i.e., ΔE2 ≈ 0) so that ΔE1 ≈ 37 K while for the thermometric parameter Δ597/594 values of ΔE1 ≈ 225 K and ΔE2 ≈ 38 K (see ESI† for details) are obtained. Small values in the activation energy could be related to a multiphonon quenching mechanism while the higher value would be associated with the thermal crossover mechanism described in Fig. 3. Moreover, as shown in Fig. 4, the values of Δ552/580 decrease exponentially with temperature, which explains the presence of only one non-radiative decay channel, while Δ597/594 shows an “S-shaped” decrease, making the presence of two channels ΔE1 and ΔE2 with a considerable energy difference understandable.
The performance analysis of both thermometric curves was carried out by inspecting the relative thermal sensitivity, Sr = |∂ΔT/∂T|/Δ, and temperature uncertainty, δT = |δΔ/Δ|/Sr.27,28,38 This evaluation reveals that the wavelengths chosen provide good values of Sr and δT (i.e., Sr > 1% K−1 and δT < 1 K) at relatively low temperatures. The thermometric parameter Δ552/580 offers an operating range between 10 and 140 K with a maximum sensitivity Sr = 2.42% K−1 at 20 K while Δ597/594 provides a maximum sensitivity Sr = 1.13% K−1 also at 20 K but with a wider temperature reading range between 10 and 180 K. Similar results can also be obtained with the intensities associated with the maximum emission (Fig. S2†). Furthermore, it is possible to establish a direct linear correlation between temperature and the thermometric parameters Δ594/597 and Δ580/597, involving wavelengths of the transition 5D4 → 7F4, in addition to Δ622/597 related to the transitions 5D4 → 7F3 and 5D4 → 7F4, respectively. Hence, the thermometry appears in three different temperature regimes: cryogenic (10–120 K), low (80–200 K) and medium (180–320 K) as shown in Fig. 5. This type of thermometry is simpler, more robust and reliable, and it does not require any additional calibration. In addition, large values in the slopes demonstrate a high sensitivity indicating that Tb2O2NCN is an excellent self-referencing luminescent thermometer.
One of the biggest challenges related to ratiometric thermometry is to extract intensities reliably and easily. In the case of Tb2O2NCN, the wavelengths offering the best results in sensitivity (Sr) and precision (δT) involve the 5D4 → 7FJ transitions (J = 4 and 3), which involve a high degree of complexity due to the splitting of the emissions by the crystal field, and this could hinder a future real application. For this reason, we have explored the option of a thermometry involving, during the analysis, information from the entire emission spectrum and not just part of it, thereby eliminating any error upon intensity extraction. To do so, we propose a multivariant thermometry based on principal component analysis (PCA), a statistical dimensionality-reduction method used to find combinations of variables describing the behavior of large datasets. In this way, the collected data were arranged in a 18 × 251 matrix X where the number of rows corresponds to the number of observations (i.e., the different temperatures in the range 3–320 K) while the number of columns represents the number of variables, in our case the intensities at the wavelengths of the emission spectrum between 450–700 nm. Thus, the PCA was performed on this matrix X, producing a set of new variables, or principal components (PCi), which are related to the original intensities I(λj) at a given temperature, by means of PCi = ∑ci(λj)I(λj) where ci(λj) represents the coefficients describing the behavior of each principal component.54,55
After applying PCA to our experimental datasets, we found that only three principal components are sufficient to describe more than 90% of the variance of the original data. In addition, the coefficients associated with each of the three principal components reproduce the emission spectrum in considerable detail (Fig. 6a), showing a shape and variability very close to the original information in the regions of interest Ri (that is, where the transitions 5D4 → 7FJ occur) while in the areas outside their contribution is basically null, so that our principal components are not only statistically relevant but also have physical meaning. As a result, temperature was related to these new variables by multivariate linear regression, obtaining a good correlation as shown in Fig. 6b. In order to evaluate the performance parameters, the linear regression equation was used as a basis Tcalc = β0 + ∑βiPCi obtaining sensitivity values (Sr) well above 1.0% as shown in Fig. 6b and a precision (δT) of ≈1.0 K (see ESI† for details). It is rather obvious that PCA-thermometry offers better performance results since it uses the entire experimental dataset to identify the parameters that correlate with temperature, without the need to pre-select regions or specific thermometric parameters.
The magnetic behavior of μ-crystalline Tb2O2NCN was investigated by susceptibility measurements using a SQUID magnetometer. The value of the magnetic susceptibility χMT at room temperature is 22.4 cm3 K mol−1 in good agreement with the theoretical value of 23.6 cm3 K mol−1 expected for two isolated Tb3+ ions (J = 6, gL = 3/2).37,61–63 Upon cooling, χMT remains practically constant up to about 150 K, from where, due to the depopulation of the excited levels of the 7F6 ground multiplet, it starts to decrease to a value of 2.1 cm3 K mol−1 at 3 K as shown in Fig. 7a. In the temperature range between 50–300 K the susceptibility χM follows the Curie–Weiss law  quite well, so that by fitting a 1/χMvs. T curve the parameters θCW = −16.6 K and C = 23.75 cm3 K mol−1 are obtained. The negative value of θCW indicates predominantly antiferromagnetic interactions between Tb3+ ions in the paramagnetic state, while the effective magnetic moment calculated from C yields 13.98μB (i.e., 9.70μB per Tb3+ ion), in agreement with the expected theoretical value
quite well, so that by fitting a 1/χMvs. T curve the parameters θCW = −16.6 K and C = 23.75 cm3 K mol−1 are obtained. The negative value of θCW indicates predominantly antiferromagnetic interactions between Tb3+ ions in the paramagnetic state, while the effective magnetic moment calculated from C yields 13.98μB (i.e., 9.70μB per Tb3+ ion), in agreement with the expected theoretical value  .37,64 Under 50 K, the curve χ(T) clearly shows the characteristic peak for a paramagnetic transition to long-range antiferromagnetic order at TN ≈ 6.5 K (Fig. 7b). This low ordering temperature reflects the Tb–Tb interactions: as already mentioned, the arrangement of the Tb3+ in Tb2O2NCN corresponds to three Tb–Tb distances: dintrasheet ≈ 3.75 Å, dintralayer ≈ 3.65 Å, and dinterlayer ≈ 5.69 Å. For our analysis we may ignore the intralayer interactions in the same double-layer because (Fig. 7c) this interaction (J2) should be small since a positional shift in the triangular network frustrates the intralayer magnetic coupling. Therefore, we will limit ourselves to comparing the dintrasheet (J1) and dinterlayer (J3) interactions. Given that the NCN2− anion is between the Tb3+ ions in different layers, these are relatively far away from each other, so a small value of J3 can be expected compared to J1. In fact, this can be seen if comparing the scale of the dipolar interactions65–68
.37,64 Under 50 K, the curve χ(T) clearly shows the characteristic peak for a paramagnetic transition to long-range antiferromagnetic order at TN ≈ 6.5 K (Fig. 7b). This low ordering temperature reflects the Tb–Tb interactions: as already mentioned, the arrangement of the Tb3+ in Tb2O2NCN corresponds to three Tb–Tb distances: dintrasheet ≈ 3.75 Å, dintralayer ≈ 3.65 Å, and dinterlayer ≈ 5.69 Å. For our analysis we may ignore the intralayer interactions in the same double-layer because (Fig. 7c) this interaction (J2) should be small since a positional shift in the triangular network frustrates the intralayer magnetic coupling. Therefore, we will limit ourselves to comparing the dintrasheet (J1) and dinterlayer (J3) interactions. Given that the NCN2− anion is between the Tb3+ ions in different layers, these are relatively far away from each other, so a small value of J3 can be expected compared to J1. In fact, this can be seen if comparing the scale of the dipolar interactions65–68 (here R is the Tb–Tb distance) from which D1/D3 = 6.01 K/1.72 K = 3.49 is obtained, reinforcing the idea of a two-dimensional system with magnetic moments located on the triangular lattice. Furthermore, the frustration index f = |θCW/TN| = 2.55 of Tb2O2NCN reflects a geometrically frustrated system according to the criteria established by Ramirez.69,70 Given that magnetic ordering is observed at low temperature, however, we can conclude that such geometrical frustration does not completely hinder the emergence of magnetic ordering in Tb2O2NCN.
(here R is the Tb–Tb distance) from which D1/D3 = 6.01 K/1.72 K = 3.49 is obtained, reinforcing the idea of a two-dimensional system with magnetic moments located on the triangular lattice. Furthermore, the frustration index f = |θCW/TN| = 2.55 of Tb2O2NCN reflects a geometrically frustrated system according to the criteria established by Ramirez.69,70 Given that magnetic ordering is observed at low temperature, however, we can conclude that such geometrical frustration does not completely hinder the emergence of magnetic ordering in Tb2O2NCN.
Isothermal magnetization measurements (Fig. 8a) in the range of μ0H = 0–9 T at various temperatures show a lack of saturation, indicative of magnetocrystalline anisotropy. The slow relaxation dynamics of Tb2O2NCN was studied by AC susceptibility measurements at 2 K. In a zero DC field with an AC field of 5 Oe in the frequency range of 10–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz, a clear dependence of the out-of-phase component of the susceptibility (χ′′) on the frequency is revealed, although without the presence of any maximum in the measurement range. Fitting simultaneously χ′ and χ′′ to a generalized Debye model71–74 (see ESI† for more details) allows us to extract (Fig. 8b and c) the characteristic magnetic relaxation time of τ0 ≈ 5.7 μs and a parameter α = 0.01, indicating that the relaxation of the magnetic configurations takes place simultaneously. Additionally, the curves M(H) at temperatures between 2.5–8 K show a slope change at ≈1.8 T that becomes evident when comparing the behavior at low and high magnetic fields, clearly observed in the ∂M/∂H plot of Fig. 8a, which typically suggests a metamagnetic transition. To confirm and determine the nature of the magnetic transition, the M–H data were converted into M2–μ0H/M curves (Arrott plot) depicted in Fig. 8d. According to the Banerjee criterion,75 a negative slope in the Arrott plot would indicate a first-order magnetic transition.
000 Hz, a clear dependence of the out-of-phase component of the susceptibility (χ′′) on the frequency is revealed, although without the presence of any maximum in the measurement range. Fitting simultaneously χ′ and χ′′ to a generalized Debye model71–74 (see ESI† for more details) allows us to extract (Fig. 8b and c) the characteristic magnetic relaxation time of τ0 ≈ 5.7 μs and a parameter α = 0.01, indicating that the relaxation of the magnetic configurations takes place simultaneously. Additionally, the curves M(H) at temperatures between 2.5–8 K show a slope change at ≈1.8 T that becomes evident when comparing the behavior at low and high magnetic fields, clearly observed in the ∂M/∂H plot of Fig. 8a, which typically suggests a metamagnetic transition. To confirm and determine the nature of the magnetic transition, the M–H data were converted into M2–μ0H/M curves (Arrott plot) depicted in Fig. 8d. According to the Banerjee criterion,75 a negative slope in the Arrott plot would indicate a first-order magnetic transition.
These characteristics in the behavior of the M(H) curves, together with the fact that Tb2O2NCN has a high magnetic density (2 × MTb/Mw = 0.82) and is also magnetically reversible (no magnetic hysteresis, see Fig. S3†), make it particularly attractive for magnetic refrigeration applications. The magnetocaloric performance was evaluated by means of the magnetic entropy change (−ΔSM), calculated from the isothermal magnetization curves using the Maxwell equation  .76–80Fig. 9a depicts the magnetic entropy change curves as a function of temperature in the 1–7 T range. The maximum −ΔSM at low field (3 T) reaches a value of ≈3.6 J kg−1 K−1 while in high field (7 T) a maximum of ≈21.1 J kg−1 K−1 is observed, but still far from the theoretical maximum of −ΔSM = nR
.76–80Fig. 9a depicts the magnetic entropy change curves as a function of temperature in the 1–7 T range. The maximum −ΔSM at low field (3 T) reaches a value of ≈3.6 J kg−1 K−1 while in high field (7 T) a maximum of ≈21.1 J kg−1 K−1 is observed, but still far from the theoretical maximum of −ΔSM = nR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2J + 1)/MW ≈ 109.0 J kg−1 K−1 (here, n is the number of magnetic centers, R is the gas constant and MW represents the formula weight).57 A comparison of the magnetocaloric performance of Tb2O2NCN with other Tb-based compounds is summarized in Table 1, together with sketches of the magnetic sublattices in Fig. 9b. These compounds can be grouped according to the dimensionality of the connected terbium ions. In the group of compounds with 3D ordering, TbCrO4 stands out: the TbO8 polyhedra are arranged in the tetragonal zircon (ZrSiO4) structure, with Tb–Tb ≈ 3.9 Å and a maximum magnetic entropy change of 16.2 J kg−1 K−1 at 23 K.81 In turn, in TbBO3, 2D triangular nets of Tb3+ are observed forming monolayers with intra- and inter-layer distances of 3.8 Å and 4.4 Å, respectively, with a value of −ΔSM of 5.97 J kg−1 K−1 at 2 K.82 In LiTbP4O12, terbium ions form 1D channels with intra- and inter-channel distances of 5.6 Å and 6.4 Å, the maximum value of −ΔSM being 15.9 J kg−1 K−1 at 2 K.83 Tb2O2NCN, however, with a double layer arrangement and intra- and inter-layer distances of 3.75 Å and 5.69 Å, far surpasses all their performances under the same applied field and temperature. Apparently, the effect of magnetic frustration compensation on Tb–Tb interactions in this type of structure plays an important role. We conclude that Tb2O2NCN would be an excellent candidate for practical application as a cryogenic refrigerant.
ln(2J + 1)/MW ≈ 109.0 J kg−1 K−1 (here, n is the number of magnetic centers, R is the gas constant and MW represents the formula weight).57 A comparison of the magnetocaloric performance of Tb2O2NCN with other Tb-based compounds is summarized in Table 1, together with sketches of the magnetic sublattices in Fig. 9b. These compounds can be grouped according to the dimensionality of the connected terbium ions. In the group of compounds with 3D ordering, TbCrO4 stands out: the TbO8 polyhedra are arranged in the tetragonal zircon (ZrSiO4) structure, with Tb–Tb ≈ 3.9 Å and a maximum magnetic entropy change of 16.2 J kg−1 K−1 at 23 K.81 In turn, in TbBO3, 2D triangular nets of Tb3+ are observed forming monolayers with intra- and inter-layer distances of 3.8 Å and 4.4 Å, respectively, with a value of −ΔSM of 5.97 J kg−1 K−1 at 2 K.82 In LiTbP4O12, terbium ions form 1D channels with intra- and inter-channel distances of 5.6 Å and 6.4 Å, the maximum value of −ΔSM being 15.9 J kg−1 K−1 at 2 K.83 Tb2O2NCN, however, with a double layer arrangement and intra- and inter-layer distances of 3.75 Å and 5.69 Å, far surpasses all their performances under the same applied field and temperature. Apparently, the effect of magnetic frustration compensation on Tb–Tb interactions in this type of structure plays an important role. We conclude that Tb2O2NCN would be an excellent candidate for practical application as a cryogenic refrigerant.
| Material | θ CW (K) | T N (K) | −ΔSM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (J kg−1 K−1) at 5 T a (J kg−1 K−1) at 5 T |
Tb-arrangement |
|---|---|---|---|---|
| a At 2 T for LiTb6O6(BO3)3. b This work. | ||||
TbCrO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81 81 |
— | 22.1 | 16.2 (T = 23 K) | 3D framework |
TbBO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82 82 |
−11.0 | <2 | 5.97 (T = 2 K) | 2D layers |
LiTbP4O12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83 83 |
0.86 | <2 | 15.9 (T = 2 K) | 1D channels |
LiTb6O6(BO3)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 84 84 |
−12.4 | <2 | 6.58 (T = 4 K) | 3D framework |
TbMnO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 85 |
— | 7.0 | 6.75 (T = 16 K) | 3D framework |
| Tb2O2NCNb | −16.6 | 6.5 | 11.7 (T = 9 K) | 2D double layers |
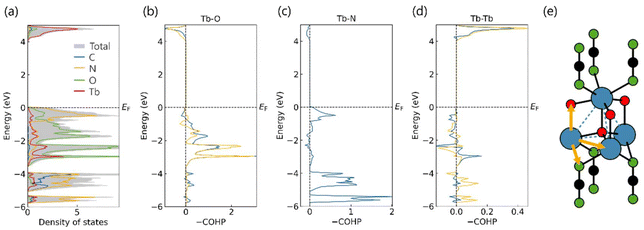
At this point, it is clear that the arrangement of Tb3+ ions in the Tb2O2NCN structure plays a key role in determining the properties even though it is not only the arrangement: the interatomic communication may perhaps be even more decisive. Hence, the density of states (DOS) and crystal orbital Hamilton population (COHP) for the interactions involving Tb were calculated to analyze the electronic structure of Tb2O2NCN (Fig. 10). From the local DOS, the expected, dominant contribution of the N 2p and O 2p levels is seen at the valence band maximum (VBM) between −2.5 and 0 eV, while the conduction band minimum (CBM), between 3.75 and 5 eV, is Tb-centered. The Crystal Orbital Hamilton Populations (COHP) for Tb–X (X = Tb, O, N) interactions shows that Tb–O bonds are stronger than Tb–N bonds (a higher value in the integrated COHP), while, curiously, Tb–Tb interactions show a bonding character in the empty conduction band, unlike Tb–O and Tb–N interactions. This suggests that under excitation at 270 nm (≈4.45 eV) Tb–Tb communication (i.e., the energy migration process in luminescence) would take place directly (attractively in terms of Tb–Tb but not necessarily reducing terbium) and not through O2− or NCN2−. In contrast, the strong bonding character of the Tb–N bond near the Fermi level (EF) indicates that Tb–Tb magnetic interactions will occur through the NCN2− unit, thus explaining the communication of the triangular nets of different layers when long-range magnetic order is established.
Conclusions
Both spectroscopic and magnetic investigations on solid-state Tb2O2NCN have allowed us to explore its potential for luminescent thermometry and magnetic refrigeration applications at low temperatures. The crystal structure can be understood as a two-dimensional system, formed by alternating layers of the extended NCN2− anion and [Tb2O2]2+ which, in turn, consist of a double-layer of Tb triangular lattice, all stacked along the [001] direction. Thanks to its anti-thermal quenching behavior, Tb2O2NCN is suitable for optical thermometry using appropriate thermometric parameters offering good sensitivity (Sr > 1% K−1) and precision (δT > 1 K). In addition, the option of an instantaneous, linear and simple thermometry has been explored that allows covering a wider temperature range thanks to dimensionality reduction (PCA) offering better performance. In turn, magnetic investigations show that Tb2O2NCN contains magnetic frustration between Tb3+ ions. This frustration is topological, a consequence of the arrangement geometry, since the Tb-triangular lattices are shifted from each other in the double-layer, separated by the large NCN2− anion. Given the comparatively large Tb3+ magnetic moment, however, the frustration is insufficient to suppress the magnetic order observed from TN = 6.5 K. Furthermore, magnetocalorics of Tb2O2NCN was investigated, thanks to first-order metamagnetic transitions taking place at around 1.8 T. The maximum for the magnetic entropy change achieves −ΔSM ≈ 21.1 J kg−1 K−1 at 7 T. Furthermore, −ΔSM ≈ 11.7 J kg−1 K−1 at 5 T is considerably larger than those reported for other materials incorporating trivalent terbium under the same magnetic field change and temperature. These excellent results establish that Tb2O2NCN is an extremely promising material for applications in thermometry and magnetic refrigeration.Experimental section
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio under argon protective atmosphere. The mixture was homogenized using a mortar and pestle agate, transferred into an open dry glass capillary and loaded into a glass ampoule. The sample was heated in a horizontal tubular furnace at 600 °C under flowing argon for 18 h, with heating and cooling rates of 2 °C min−1. The product is an air-stable powder which was washed several times with water and acetone to remove the LiCl metathesis salt, finally dried in air at 100 °C.
1 molar ratio under argon protective atmosphere. The mixture was homogenized using a mortar and pestle agate, transferred into an open dry glass capillary and loaded into a glass ampoule. The sample was heated in a horizontal tubular furnace at 600 °C under flowing argon for 18 h, with heating and cooling rates of 2 °C min−1. The product is an air-stable powder which was washed several times with water and acetone to remove the LiCl metathesis salt, finally dried in air at 100 °C.
Powder X-ray diffraction analysis
Powder X-ray diffraction data of washed Tb2O2NCN were collected employing a STOE STADI-P powder diffractometer with a flat sample holder (Cu–Kα1, linear PSD, 2θ = 4–120° with individual steps of 0.015°). Rietveld refinement was performed using GSAS II.86 In the final cycles of least-squares refinement, lattice parameters, fractional coordinates, site-occupancy factors and isotropic thermal displacement parameters (Uiso) were refined for all atoms with a constrained Uiso for chemically reasonable sorts of atoms, in particular all C and N atoms.Luminescence spectroscopy
Excitation and emission spectra of Tb2O2NCN sample were recorded using a fluorescence spectrometer FLS920 (Edinburgh Instruments) equipped with a 450 W xenon discharge lamp (OSRAM) as the radiation source. For the data collection, a R2658P single-photon-counting photomultiplier tube from Hamamatsu was used. For temperature adjustment a cryostat “MicrostatN” from Oxford Instruments had been mounted on the spectrometer. Liquid nitrogen was used as a cooling agent. The photoluminescence decay curves were also recorded on the FLS920 spectrometer, while a 270 nm laser diode from Edinburgh Instruments was used as an excitation source.Magnetic measurements
Magnetic susceptibility measurements of powderous Tb2O2NCN were performed using a Quantum Design SQUID MPMS-XL magnetometer. Samples were placed in PTFE containers and data were recorded in the temperature range 4–300 K in a field of 200 Oe. Magnetization data were collected at several temperatures in the field range 0 ≤ μ0H(T) ≤ 9. The AC magnetic susceptibility measurements were performed in a PPMS-DynaCool system using the vibrating sample magnetometry option in an oscillating AC field of 5 Oe with frequencies from 10 Hz to 10 kHz in a zero-DC field. The magnetic susceptibilities were corrected for the sample holder and the intrinsic diamagnetic contribution of the compound (χM,dia = −1.95 × 10−4 cm3 mol−1).Dimensionality reduction
Before performing the PCA, the data in the X matrix (18 observations × 251 wavelengths) were pre-processed. First, each observation (i.e., each row) was normalized using the intensity maximum, and subsequently each column was adjusted to the normal distribution by the transformation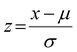 . The resulting matrix Z served as input for PCA. In overall, PCA is a technique that transforms a set of correlated variables p in a new set of independent variables q, denominated as principal components. The first stage involves the calculation of the covariance matrix and then, the eigenvalues and eigenvectors are determined, sorted in descending order according to their eigenvalues. The number of principal components was determined by using the scree plot as criterion (Fig. S4†).
. The resulting matrix Z served as input for PCA. In overall, PCA is a technique that transforms a set of correlated variables p in a new set of independent variables q, denominated as principal components. The first stage involves the calculation of the covariance matrix and then, the eigenvalues and eigenvectors are determined, sorted in descending order according to their eigenvalues. The number of principal components was determined by using the scree plot as criterion (Fig. S4†).
Computational details
Density-functional theory calculations were performed using the Vienna ab initio simulation package (VASP, version 6.1.1).87,88 Projector augmented wave pseudopotentials with Perdew–Burke–Ernzerhof (PBE)89 parameterization were used. The generalized gradient approximation (GGA)90 scheme was chosen for treating both electron exchange and correlation. 800 eV was set as the cutoff for the plane-wave basis set. A Γ-centered Monkhorst–Pack k point mesh was used throughout for sampling the first Brillouin zone, the distance between two adjacent k points being always below 2π × 0.02 Å−1. The experimentally obtained structure was first optimized on this level of theory until energy and force differences between consecutive iterations fell below thresholds of 10−8 eV and 10−6 eV Å−1, respectively. Gaussian smearing with 0.02 eV as the smearing width was set to treat partial occupancies for each orbital. It is worth noting that, due to the well-known self-interaction errors, the 4f orbitals of Tb were downfolded in the core, and a special “trivalent” GGA potential (designated Tb_3) was used. Following the structure optimization, self-consistent field (SCF) calculations were carried out. Only time-reversal symmetry was considered during the SCF calculations and tetrahedron method with Blöchl corrections91 was utilized for Brillouin zone integration. Wavefunctions were written out by the end of SCF calculations. Subsequently, a reciprocal space unitary transformation92 was performed by using the Local-Orbital Basis Suite Towards Electronic Structure Reconstruction (LOBSTER, version 5.1.1)93,94 suite to extract both density of states (DOS) and chemical-bonding information. The outputs from LOBSTER were automatically postprocessed by LOBSTER's postprocessor (LOPOSTER).95Author contributions
All authors have accepted responsibility for the entire content of this submitted manuscript and approved the submission.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflicts of interest regarding this article.Acknowledgements
We thank Mr T. Storp for his assistance with PXRD measurements and Ms C. Houben for her assistance with magnetic measurements. J. Medina-Jurado is grateful for the financial support from Deutscher Akademischer Austauschdienst (DAAD). A. Corkett is indebted to the Deutsche Forschungsgemeinschaft (DFG) for funding (project number 441856704).References
- A. J. Corkett, O. Reckeweg, R. Pöttgen and R. Dronskowski, Chem. Mater., 2024, 36, 9107–9125 CrossRef CAS.
- M. Becker and M. Jansen, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2001, 57, 347–348 CrossRef CAS PubMed.
- X. Liu, M. Krott, P. Müller, C. Hu, H. Lueken and R. Dronskowski, Inorg. Chem., 2005, 44, 3001–3003 CrossRef CAS PubMed.
- X. Liu, R. Dronskowski, R. K. Kremer, M. Ahrens, C. Lee and M.-H. Whangbo, J. Phys. Chem. C, 2008, 112, 11013–11017 CrossRef CAS.
- X. Liu, L. Stork, M. Speldrich, H. Lueken and R. Dronskowski, Chem. – Eur. J., 2009, 15, 1558–1561 CrossRef CAS PubMed.
- M. Krott, X. Liu, B. P. T. Fokwa, M. Speldrich, H. Lueken and R. Dronskowski, Inorg. Chem., 2007, 46, 2204–2207 CrossRef CAS PubMed.
- S. K. Deb and A. D. Yoffe, Trans. Faraday Soc., 1959, 55, 106–113 Search PubMed.
- M. Becker, J. Nuss and M. Jansen, Z. Naturforsch. B, 2000, 55, 383–385 Search PubMed.
- E. Leysour de Rohello, Y. Suffren, O. Merdrignac-Conanec, O. Guillou, C. Calers and F. Chevire, J. Solid State Chem., 2021, 300, 122240 CrossRef CAS.
- M. Kubus, C. Castro, D. Enseling and T. Jüstel, Opt. Mater., 2016, 59, 126–129 CrossRef CAS.
- M. Krings, G. Montana, R. Dronskowski and C. Wickleder, Chem. Mater., 2011, 23, 1694–1699 CrossRef CAS.
- Y. Masubuchi, S. Nishitani, A. Hosono, Y. Kitagawa, J. Ueda, S. Tanabe, H. Yamane, M. Higuchi and S. Kikkawa, J. Mater. Chem. C, 2018, 6, 6370–6377 Search PubMed.
- D. Dutczak, M. Ströbele, D. Enseling, T. Jüstel and H.-J. Meyer, Eur. J. Inorg. Chem., 2016, 25, 4011–4016 CrossRef.
- J. Glaser, L. Unverfehrt, H. Bettentrup, G. Heymann, H. Huppertz, T. Jüstel and H.-J. Meyer, Inorg. Chem., 2008, 47, 10455–10460 CrossRef CAS PubMed.
- D. Dutczak, A. Siai, M. Ströbele, D. Enseling, T. Jüstel and H.-J. Meyer, Eur. J. Inorg. Chem., 2020, 41, 3954–3958 CrossRef.
- E. Säilynoja, M. Lastusaari, J. Hölsä and P. Porcher, J. Lumin., 1997, 72, 201–203 CrossRef.
- J. Hölsä, R.-J. Lamminmäki, M. Lastusaari, P. Porcher and E. Säilynoja, J. Alloys Compd., 1998, 275, 402–406 CrossRef.
- J. Hölsä, R.-J. Lamminmäki, M. Lastusaari, E. Säilynoja, P. Porcher, P. Dereń and W. Strȩk, Spectrochim. Acta, Part A, 1998, 13, 2065–2069 CrossRef.
- M. Takahashi, Y. Hashimoto, S. I. Kikkawa and H. Kobayashi, J. Soc. Mater. Sci., 2000, 11, 1230–1234 CrossRef.
- T. Takeda, N. Hatta and S. Kikkawa, Chem. Lett., 2006, 9, 988–989 CrossRef.
- M. Kubus, R. Heinicke, M. Ströbele, D. Enseling, T. Jüstel and H.-J. Meyer, Mater. Res. Bull., 2015, 62, 37–41 CrossRef CAS.
- J. Medina-Jurado, H. Bourakhouadar, Y. Wang, A. J. Corkett, D. Enseling, T. Jüstel and R. Dronskowski, Chem. Mater., 2025, 37, 2506–2515 CrossRef CAS.
- Y. Hashimoto, M. Takahashi, S. Kikkawa and F. Kanamaru, J. Solid State Chem., 1996, 125, 37 CrossRef CAS.
- M. Li, W. Yuan, J. Wang and C. Gu, Powder Diffr., 2007, 22, 59 CrossRef CAS.
- J. Sindlinger, J. Glaser, H. Bettentrup, T. Jüstel and H.-J. Meyer, Z. Anorg. Allg. Chem., 2007, 633, 1686–1690 CrossRef CAS.
- L. Wang, S. Yuan, Y. Yang, F. Chevire, F. Tessier and G. Chen, Opt. Mater. Express, 2015, 5, 2616–2624 CrossRef CAS.
- A. Ansari, A. Parchur, M. K. Nazeeruddin and M. Tavakoli, Coord. Chem. Rev., 2021, 444, 214040 CrossRef CAS.
- M. D. Dramićanin, J. Appl. Phys., 2020, 128, 040902 CrossRef.
- Y. Shen, X. Han, S. Wang, H. Yu and H. Zhang, Inorg. Chem., 2025, 64, 133–141 Search PubMed.
- R. M. Diaz-Rodriguez, D. A. Gálico, D. Chartrand, E. A. Suturina and M. Murugesu, J. Am. Chem. Soc., 2022, 144, 912–921 CrossRef CAS PubMed.
- D. K. Amarasinghe and F. A. Rabuffetti, Chem. Mater., 2019, 31, 10197–10204 Search PubMed.
- M. Suta, Ž. Antić, V. Ðorđević, S. Kuzman, M. D. Dramićanin and A. Meijerink, Nanomaterials, 2020, 10, 543 CrossRef CAS PubMed.
- X. Liu, A. Skripka and Y. Lai, et al. , Nat. Commun., 2021, 12, 6401 CrossRef CAS PubMed.
- S. Wang, Y. Xu, T. Chen, W. Jiang, J. Liu, X. Zhang, W. Jiang and L. Wang, Chem. Eng. J., 2021, 404, 125912 Search PubMed.
- P. Dang, W. Wang, H. Lian, G. Li and J. Lin, Adv. Opt. Mater., 2022, 10, 2102287 Search PubMed.
- K. Karachousos-Spiliotakopoulos, V. Tangoulis, N. Panagiotou, A. Tasiopoulos, V. Nastopoulos, E. Moreno-Pineda, W. Wernsdorfer, M. Schulze, A. M. P. Botas and L. D. Carlos, Inorg. Chem., 2022, 61, 18629–18639 Search PubMed.
- J. Wang, J. J. Zakrzewski, M. Zychowicz, Y. Xin, H. Tokoro, S. Chorazy and S.-I. Ohkoshi, Angew. Chem., Int. Ed., 2023, 62, e202306372 CrossRef CAS PubMed.
- C. D. S. Brites, R. Marin, M. Suta, A. N. Carneiro-Neto, E. Ximendes, D. Jaque and L. D. Carlos, Adv. Mater., 2023, 35, 2302749 CrossRef CAS PubMed.
- Y. Ma, A. Aierken, Y. Wang and A. Meijerink, J. Colloid Interface Sci., 2023, 638, 640–649 CrossRef CAS PubMed.
- Y. Wang, L. Lei, R. Ye, G. Jia, Y. Hua, D. Deng and S. Xu, ACS Appl. Mater. Interfaces, 2021, 13, 23951–23959 Search PubMed.
- K. Elzbieciak-Piecka and L. Marciniak, Sci. Rep., 2022, 12, 16364 CrossRef CAS PubMed.
- G. Brunet, R. Marin, M.-J. Monk, U. Resch-Genger, D. A. Gálico, F. A. Sigoli, E. A. Suturina, E. Hemmer and M. Murugesu, Chem. Sci., 2019, 10, 6799–6808 RSC.
- Y. Cui, H. Xu, Y. Yue, Z. Guo, J. Yu, Z. Chen, J. Gao, Y. Yang, G. Qian and B. Chen, J. Am. Chem. Soc., 2012, 134, 3979–3982 CrossRef CAS PubMed.
- X. Shi, Y. Xue, Q. Mao, L. Pei, X. Li, M. Liu, Q. Zhang and J. Zhong, Inorg. Chem., 2023, 62, 893–903 CrossRef CAS PubMed.
- M. Back, J. Ueda, H. Nambu, M. Fujita, A. Yamamoto, H. Yoshida, H. Tanaka, M. Brik and S. Tanabe, Adv. Opt. Mater., 2021, 9, 2100033 Search PubMed.
- J. Stefańska, A. Bednarkiewicz and L. Marciniak, J. Mater. Chem. C, 2022, 10, 5744–5782 Search PubMed.
- Y. H. Wang, S. Yang, Q. F. Xu, C. L. Yang, S. F. Zhang, S. Y. J. Yu and M. M. Jiao, J. Phys. Chem. C, 2022, 126, 15517–15527 Search PubMed.
- P. Du, X. Huang and J. Yu, Inorg. Chem. Front., 2017, 4, 1987–1995 RSC.
- K. M. N. de Souza, R. N. Silva, J. A. B. Silva, C. D. S. Brites, B. Francis, R. A. S. Ferreira, L. D. Carlos and R. L. Longo, Adv. Opt. Mater., 2022, 10, 2200770 CrossRef CAS.
- Z. Liang, J. Wu and Y. Cui, Light: Sci. Appl., 2023, 12, 36 Search PubMed.
- K. Trejgis, K. Ledwa and K. Maciejewska, et al. , Sci. Rep., 2022, 12, 5847 CrossRef CAS.
- I. E. Kolesnikov, D. V. Mamonova, M. A. Kurochkin, M. A. Khodasevich, V. A. Medvedev, E. Y. Kolesnikov and A. A. Manshina, J. Mater. Chem. C, 2023, 11, 14814–14825 RSC.
- Š. Dragutin, et al. , Tehnika, 2020, 75, 279–283 CrossRef.
- E. Ximendes, R. Marin and L. D. Carlos, et al. , Light: Sci. Appl., 2022, 11, 237 CrossRef CAS PubMed.
- A. Rajčić, Z. Ristić, Z. J. Periša, B. Milićević, S. Aldawood, A. N. Alodhayb, Ž. Antić and M. D. Dramićanin, Technologies, 2024, 12, 131 CrossRef.
- Z. Li, A. Arauzo, J. G. Planas and E. Bartolomé, Dalton Trans., 2024, 53, 8969–8979 Search PubMed.
- B. Wang, X. Liu, F. Hu, J. Wang, J. Xiang, P. Sun, J. Wang, J. Sun, T. Zhao, Z. Mo, J. Shen, Y. Chen, Q. Huang and B. Shen, J. Am. Chem. Soc., 2024, 146, 35016–35022 Search PubMed.
- J. Medina-Jurado, H. Bourakhouadar, A. J. Corkett, D. Enseling, T. Jüstel and R. Dronskowski, Z. Anorg. Allg. Chem., 2025, e202400212 CrossRef CAS.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, Nanoscale, 2012, 4, 4799–4829 Search PubMed.
- D. V. Lapaev, V. G. Nikiforov, V. S. Lobkov, A. A. Knyazev and Y. G. Galyametdinov, J. Mater. Chem. C, 2018, 6, 9475–9481 RSC.
- C. Ritchie, E. G. Moore, M. Speldrich, P. Kögerler and C. Boskovic, Angew. Chem., Int. Ed., 2010, 49, 7702 Search PubMed.
- K. Katoh, S. Yamashita, N. Yasuda, Y. Kitagawa, B. K. Breedlove, Y. Nakazawa and M. Yamashita, Angew. Chem., Int. Ed., 2018, 57, 9262 Search PubMed.
- H. Wang, Z. Zhu, L. La Droitte, W. Liao, O. Cador, B. Le Guennic and J. Tang, Chem. Sci., 2023, 14, 7208 RSC.
- S. Mugiraneza and A. M. Hallas, Commun. Phys., 2022, 5, 1 CrossRef.
- N. P. Raju, M. Dion, M. J. P. Gingras, T. E. Mason and J. E. Greedan, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 14489 CrossRef CAS.
- S. E. Palmer and J. T. Chalker, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 488 CrossRef CAS.
- M. J. P. Gingras, C. Lacroix, P. Mendels and F. Mila, Introduction to Frustrated Magnetism, Springer, Berlin, 2011 Search PubMed.
- O. A. Petrenko and D. McK Paul, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 63, 024409 CrossRef.
- V. Biondo, P. C. W. Sarvezuk, F. F. Ivashita, K. L. Silva, A. Paesano Jr. and O. Isnard, Mater. Res. Bull., 2014, 54, 41–47 CrossRef CAS.
- A. P. Ramirez, Annu. Rev. Mater. Sci., 1994, 24, 453 CrossRef CAS.
- P. V. Madduri, S. Sen, B. Giri, D. Chakrabartty, S. Manna, S. Parkin and A. Nayak, Phys. Rev. B, 2020, 102, 174402 CrossRef CAS.
- O. Pastukh, P. Konieczny, M. Laskowska and Ł. Laskowski, Magnetochemistry, 2021, 7, 122 CrossRef CAS.
- M. Balanda, Acta Phys. Pol. A, 2013, 124, 964 CrossRef.
- C. V. Topping and S. J. Blundell, J. Phys.: Condens. Matter, 2019, 31, 013001 Search PubMed.
- B. K. Banerjee, Phys. Lett., 1964, 12, 16 CrossRef.
- K. P. Shinde, S. H. Jang, J. W. Kim, M. Ranot and K. C. Chung, Dalton Trans., 2015, 44, 20386–20391 RSC.
- M. Kumar, L. Li, J. K. Zareba, L. Tashi, S. C. Sahoo, M. Nyk, S. Liu and H. Sheikh, Cryst. Growth Des., 2020, 20, 6430–6452 Search PubMed.
- S.-J. Liu, S.-D. Han, J.-P. Zhao, J. Xu and X.-H. Bu, Coord. Chem. Rev., 2019, 394, 39–52 CrossRef CAS.
- S.-J. Liu, C. Cao, S.-L. Yao, T.-F. Zheng, Z.-X. Wang, C. Liu, J.-S. Liao, J.-L. Chen, Y.-W. Li and H. R. Wen, Dalton Trans., 2017, 46, 64–70 RSC.
- Y. Wang, H. Zhang, M.-L. Wu, K. Tao, Y.-W. Li, T. Yan, K.-W. Long, T. Long, Z. Pang and Y. Long, Chin. Phys. B, 2016, 25, 127104 CrossRef.
- E. Palacios, M. Castro, J. Romero de Paz, J. M. Gallardo-Amores and R. Sáez-Puche, J. Solid State Chem., 2022, 314, 123356 CrossRef CAS.
- P. Mukherjee, Y. Wu, G. I. Lampronti and S. E. Dutton, Mater. Res. Bull., 2018, 98, 173–179 CrossRef CAS.
- D. N. Petrov, P. T. Long, Y. S. Koshkid'Ko, J. Ćwik and K. Nenkov, J. Phys. D: Appl. Phys., 2020, 53, 495005 CrossRef CAS.
- Y. Chen, W. Liu, J. Feng, R. Guo, F. Fan, J. Shen, G. Zhang and H. Tu, Cryogenics, 2022, 124, 103476 CrossRef CAS.
- M. Staruch, K. Luna, A. McDannald and M. Jain, J. Magn. Magn. Mater., 2015, 377, 117–120 CrossRef CAS.
- B. Toby, J. Appl. Crystallogr., 2001, 34, 210 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh and C. Fiolhais, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 6671–6687 CrossRef CAS PubMed.
- P. E. Blöchl, O. Jepsen and O. K. Andersen, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 16223–16233 CrossRef PubMed.
- S. Maintz, V. L. Deringer, A. L. Tchougréeff and R. Dronskowski, J. Comput. Chem., 2013, 34, 2557–2567 CrossRef CAS PubMed.
- S. Maintz, V. L. Deringer, A. L. Tchougréeff and R. Dronskowski, J. Comput. Chem., 2016, 37, 1030–1035 CrossRef CAS PubMed.
- R. Nelson, C. Ertural, J. George, V. L. Deringer, G. Hautier and R. Dronskowski, J. Comput. Chem., 2020, 41, 1931–1940 CrossRef CAS PubMed.
- Y. Wang, P. C. Müller, D. Schnieders and R. Dronskowski, J. Comput. Chem. Search PubMed, under review.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5dt01003a |
| This journal is © The Royal Society of Chemistry 2025 |