Unexpected effect of an axial ligand mutation in the type 1 copper center in small laccase: structure-based analyses and engineering to increase reduction potential and activity†
Abstract
Type 1 copper (T1Cu) centers are crucial in biological electron transfer (ET) processes, exhibiting a wide range of reduction potentials 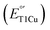 to match their redox partners and optimize ET rates. While tuning
to match their redox partners and optimize ET rates. While tuning 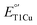 in mononuclear T1Cu proteins like azurin has been successful, it is more difficult for multicopper oxidases. Specifically, while replacing axial methionine to leucine in azurin increased its
in mononuclear T1Cu proteins like azurin has been successful, it is more difficult for multicopper oxidases. Specifically, while replacing axial methionine to leucine in azurin increased its 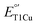 by ∼100 mV, the corresponding M298L mutation in small laccase from Streptomyces coelicolor (SLAC) unexpectedly decreased its
by ∼100 mV, the corresponding M298L mutation in small laccase from Streptomyces coelicolor (SLAC) unexpectedly decreased its 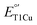 by 12 mV. X-ray crystallography revealed two axial water molecules in M298L-SLAC, leading to the decrease of
by 12 mV. X-ray crystallography revealed two axial water molecules in M298L-SLAC, leading to the decrease of 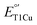 due to decreased hydrophobicity. Structural alignment and molecular dynamics simulation indicated a key difference in T1Cu axial loop position, leading to the different outcome upon methionine to leucine mutation. Based on structural analyses, we introduced additional F195L and I200F mutations, leading to partial removal of axial waters, a 122-mV increase in
due to decreased hydrophobicity. Structural alignment and molecular dynamics simulation indicated a key difference in T1Cu axial loop position, leading to the different outcome upon methionine to leucine mutation. Based on structural analyses, we introduced additional F195L and I200F mutations, leading to partial removal of axial waters, a 122-mV increase in 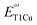 , and a 7-fold increase in kcat/KM from M298L-SLAC. These findings highlight the complexity of tuning
, and a 7-fold increase in kcat/KM from M298L-SLAC. These findings highlight the complexity of tuning  in multicopper oxidases and provide valuable insights into how structure-based protein engineering can contribute to the broader understanding of T1Cu center,
in multicopper oxidases and provide valuable insights into how structure-based protein engineering can contribute to the broader understanding of T1Cu center,  and reactivity tuning for applications, such as in solar energy transfer, fuel cells, and bioremediation.
and reactivity tuning for applications, such as in solar energy transfer, fuel cells, and bioremediation.

- This article is part of the themed collection: Spotlight Collection: Bioinorganic Chemistry


 Please wait while we load your content...
Please wait while we load your content...