Impact of catalyst support on water-assisted CO oxidation over PdO/MO2 (M = Sn, Ti, and Si) catalysts: experimental and theoretical investigation†
Kun
Liu
*a,
Luliang
Liao
b and
Guangfu
Liao
 *c
*c
aSchool of Resources and Environment, Nanchang University, 999 Xuefu Road, Nanchang, Jiangxi 330031, China. E-mail: liukun@ncu.edu.cn
bJiangxi Science Technology Normal University, Nanchang, Jiangxi, China
cCollege of Materials Engineering, Fujian Agriculture and Forestry University, Fuzhou 350002, China. E-mail: liaogf@mail2.sysu.edu.cn
First published on 26th October 2024
Abstract
This study examines the impact of different supports, SnO2, TiO2, and SiO2, on the catalytic performance and water resistance of PdO-based catalysts for CO oxidation. By merging experimental data with DFT calculations, we reveal the distinct characteristics exhibited by each catalyst. Specifically, PdO/TiO2 stands out with exceptional CO oxidation activity, attributed to its minute Pd grain size, robust CO adsorption capacity, and optimal Pd dispersion on the TiO2 surface. In stark contrast, PdO/SnO2 demonstrates heightened activity in the presence of water vapor, whereas PdO/SiO2 experiences minimal effects, as evidenced by quantitative H2O-TPD analysis and DFT simulations of surface interactions. Water vapor exerts differential impacts on the catalytic performance of these catalysts by modulating the energy barriers associated with the CO oxidation mechanisms. On PdO/TiO2, the presence of H2O or H–OH elevates the energy barrier for CO to abstract surface oxygen, thereby diminishing catalyst activity under humid conditions and gradually leading to deactivation due to accumulated surface H2O and OH species. Conversely, on PdO/SnO2, when H2O is present in the form of OH, the energy barrier diminishes, augmenting CO oxidation activity owing to the beneficial effects of surface OH groups.
Introduction
CO oxidation is extensively studied due to its scientific and industrial importance, being crucial for removing CO from exhaust gases and purifying hydrogen in fuel cells.1–8 As one of the three harmful substances targeted by catalytic converters, CO is typically converted to CO2 through oxidation.5,9–12 While basic research on gas-phase reactions often uses dry gases, real-world conditions inevitably involve H2O. For applications, under harsher conditions, like vehicle exhaust treatment during startup and catalytic CO removal from flue gas, catalysts must remain active at low temperatures and in the presence of water vapor.13–20In 1977, Fuller et al.21 investigated CO oxidation on PdO/SnO2 catalysts under humid conditions and discovered that water vapor not only does not poison the catalyst but it significantly enhances its activity. This synergistic effect is due to the accelerated migration of activated CO from the PdO surface to the SnO2 surface in the presence of water. Conversely, the catalytic efficiency of SnO2 and PdO/SiO2 diminishes under humid conditions. One plausible explanation is that H2O generates hydroxylated sites on the SnO2 surface, which act as chemical bridges facilitating CO spillover activated by PdO. Adsorbed proton acceptors, such as water and alcohols, can aid the transfer of activated hydrogen from the noble metal surface to the oxide surface. However, the precise mechanism by which PdO activates CO prior to spillover remains unclear. Wang et al.22 explored a SnO2-modified PdO/Al2O3 catalyst for CO oxidation, revealing that its activity increases in the presence of H2O and remains stable under prolonged steam conditions. Similarly, Xu et al.23 observed that H2O enhances CO oxidation over PdO/SnO2 catalysts supported by Sn–Al solid solutions. Sun et al.24 demonstrated that Sn-modified Co3O4 catalysts are effective for CO oxidation, with Sn addition significantly inhibiting H2O adsorption and enhancing water resistance. Similarly, Liu et al.25 found that water deactivates Hopcalite catalysts, but SnO2 addition improves their water resistance. Choi et al.26 used theoretical calculations to show that H2O on the PdO (101) surface promotes CO oxidation by stabilizing the carboxyl-like transition state through hydrogen bonding, thus lowering the CO oxidation energy barrier. Despite these findings, the impact of water on PdO-based catalysts and the mechanisms involved require further systematic study. Additionally, the effect of supports on the water resistance of PdO catalysts is rarely reported. Rutile TiO2 and SnO2, with similar crystal structures and cell parameters, are common catalyst supports in heterogeneous catalysis. While PdO/TiO2 shows good catalytic oxidation performance, studies on the effect of water on PdO/TiO2 catalysts for CO oxidation are yet to be reported.27
The current study investigates the influence of SnO2, TiO2 and SiO2 supports on the catalytic performance and water resistance of PdO-based catalysts for CO oxidation through a combination of experimental results and density functional theory (DFT) calculations. PdO/SnO2 and PdO/TiO2 catalysts were synthesized using SnO2 and rutile-type TiO2 supports, both sharing the same crystal structure, while PdO/SiO2 was prepared as a diluent with a similar specific surface area to characterize the intrinsic properties of PdO. Different corresponding catalyst models were constructed while employing a combined theoretical and experimental approach to examine the effect of these supports on CO oxidation activity and water resistance. Our findings reveal that PdO/TiO2 exhibits superior CO oxidation activity due to optimal Pd dispersion, PdO/SnO2 shows enhanced activity in the presence of water vapor, and PdO/SiO2 remains largely unaffected. These results underscore the critical role of catalyst supports in determining CO oxidation efficiency and water tolerance.
Experiment
Methodology and modeling
The quantum chemical calculations in this study were conducted using VASP software with the DFT-D3 method, incorporating van der Waals corrections and spin polarization. PAW–PBE pseudopotentials were used to describe electronic and ionic interactions with a cutoff energy of 400 eV. The k-point grid size (4 × 2 × 1 or 2 × 2 × 1) was selected based on the cell size and generated using the Monkhorst–Pack method. The convergence criteria were set at 0.01 meV for energy and 0.01 eV Å−1 for forces, and a 15 Å vacuum layer with dipole corrections along the z-direction was included. To correct the interactions between Ti 3d electrons, we applied a Hubbard U parameter (U – J = 4). The CI-NEB method was used to locate transition states, verified by the single imaginary frequency criterion.The PdO space group is P42/mmc, while SnO2 and rutile-type TiO2 belong to the P42/mnm space group, characterized by lattice parameters a = b ≠ c and α = β = γ = 90°. The lattice parameters, as listed in Table S1,† align with the experimental values. Sun et al.28 studied H2 adsorption and dissociation on n ML PdO supported on TiO2, finding that the properties of 2–4 ML PdO resemble pure PdO, whereas 1 ML PdO/TiO2 exhibits unique characteristics. Given the low PdO loading in this study, we used a 1 ML PdO model on TiO2 and SnO2 surfaces to form PdO/TiO2 and PdO/SnO2 catalyst models. The respective crystallographic planes are as follows: PdO (101) R90 with dimensions 3.058 Å × 6.223 Å, TiO2 (110) with dimensions 2.990 Å × 6.462 Å, and SnO2 (110) with dimensions 3.220 Å × 6.740 Å. The PdO (101) R90 and TiO2 (110) parameters are well matched, whereas PdO (101) R90 and SnO2 (110) have different parameters; thus, the average cell parameters are used for alignment. A 15 Å vacuum layer was added in the z-direction to prevent interlayer interactions. The specific model is depicted in Fig. 1, where the bottom 9 atomic layers are fixed in the bulk phase, and the others are fully relaxed during calculations.
 | ||
| Fig. 1 Model diagrams of the PdO/TiO2 and PdO/SnO2 catalysts that were constructed utilizing VASP software by application of the DFT-D3 method. | ||
The adsorption energy is defined as Eads = Eadsorbate/substrate − (Eadsorbate + Esubstrate), where Eadsorbate/substrate is the energy of the entire system after adsorption, Eadsorbate is the energy of the adsorbate in its free state, and Esubstrate represents the energy of the substrate. When the adsorption energy is negative, it indicates an exothermic process.
Results and discussion
The specific surface areas of the catalysts and their supports were measured using the N2-BET method, as detailed in Table S2.† The supports (SnO2, TiO2, SiO2) demonstrated similar surface areas of 34, 27, and 26 m2 g−1, respectively, thereby minimizing the influence of the support surface area in the experiments. After loading with PdO, the PdO/SnO2, PdO/TiO2, and PdO/SiO2 catalysts exhibited surface areas of 30, 25 and 25 m2 g−1, respectively. The X-ray diffraction (XRD) results of these catalysts are presented in Fig. 2. For PdO/SiO2, the broad peak at 21.6° corresponds to SiO2, indicating low crystallinity. The peak at 33.96° observed in both PdO/SiO2 and PdO/TiO2 samples corresponds to the (101) plane of PdO. In PdO/TiO2, peaks corresponding to rutile-type TiO2 were also detected alongside PdO peaks. For PdO/SnO2, peaks at 26.61°, 33.89°, and 51.78° are characteristic of SnO2, with the 33.89° peak coinciding with the PdO (101) peak at 33.96° seen in the PdO/SiO2 and PdO/TiO2 samples, indicating the presence of PdO features in PdO/SnO2. The crystallite sizes of PdO were calculated as 24 nm on SiO2 and 13 nm on TiO2, suggesting better dispersion of PdO on TiO2 surfaces.Catalyst activity testing for CO oxidation and the influence of H2O on CO oxidation performance
The catalyst activity for CO oxidation and the influence of H2O on CO oxidation performance are shown in Fig. 3. Under dry conditions, PdO/TiO2 exhibited the highest catalytic activity, achieving 10% CO conversion at 60 °C and 100% at 130 °C. In contrast, PdO/SiO2 showed the lowest activity, with a T10 (temperature at 10% conversion) of 185 °C and a T100 (temperature for complete conversion) of 210 °C. The catalytic activity for CO oxidation followed the sequence: PdO/TiO2 > PdO/SnO2 > PdO/SiO2.In the presence of water vapor, the CO oxidation activity of PdO/TiO2 decreased significantly, with T10 increasing to 90 °C and achieving 100% conversion at 140 °C. Conversely, under dry conditions, the CO oxidation activity of PdO/SnO2 increased, with T10 decreasing from 115 °C to 100 °C, and the complete conversion temperature reducing from 140 °C to 130 °C. For PdO/SiO2, the CO oxidation activity slightly decreased at lower temperatures (T10 increased from 185 °C to 195 °C) under wet conditions, but showed no significant change at higher temperatures. Table S3† presents a quantitative analysis of the surface CO oxidation reactivity of various catalysts, focusing on their apparent activation energy (Ea) and reaction rates at 100 °C, derived using Arrhenius plots. Under anhydrous conditions, the Ea values for surface CO oxidation increased in the order: PdO/TiO2 > PdO/SnO2 > PdO/SiO2, consistent with their reaction activity. In the presence of water, the Ea value for PdO/TiO2 increased by 8.77 kJ mol−1, while for PdO/SnO2 it decreased by 8.31 kJ mol−1, correlating with the changes in catalytic activity. Additionally, normalized reaction rates (Rw) for low conversions (<20%) by catalyst mass showed Rw values in the sequence: PdO/TiO2 > PdO/SnO2 > PdO/SiO2, consistent with their activity levels. Fig. 4a shows the stability test results of the PdO/SnO2 catalyst for CO oxidation at 110 °C under humid conditions. At 110 °C without water, the CO conversion rate is around 10%, which increases to about 90% stable for 30 hours in the presence of water. Furthermore, the catalyst activity remains stable after water removal, showcasing the PdO/SnO2 catalyst's enhanced CO oxidation activity and its potential for water-resistant applications.
Fig. 4b shows that at a reaction temperature of 110 °C for the PdO/TiO2 catalyst, the CO oxidation conversion rate is around 95%, which gradually decreases to about 20% over 20 hours and then stabilizes in the presence of water. Meanwhile, upon removing the water, the activity immediately returns to the dry condition level of a stable high conversion rate (90%), illustrating the poor water resistance behavior of the PdO/TiO2 catalyst, where water can reversibly affect the CO oxidation process. For the PdO/SiO2 catalyst shown in Fig. 4c, the CO conversion rate can be noticed at around 95% at 190 °C, while fluctuating between 88% and 95% in the presence of water, with no significant decline in activity over 30 hours. This indicates that water has no impact on the catalyst's activity, and the catalyst exhibits water resistance during CO oxidation. Further investigations were carried out using XPS, TPR and TPD characterization studies to elucidate the different CO oxidation activity trends and stability characteristics of these catalysts under wet conditions.
Analysis of the surface composition and H2O and CO adsorption properties on catalyst surfaces by different characterization approaches
The surface properties of the catalysts were investigated by XPS, with the results shown in Fig. 5 and Table S4.† The Pd3d spectra revealed peaks at 336.6 and 342.0 eV corresponding to Pd2+, indicating that Pd species on the surface of all three catalysts exist as Pd2+. The quantitative results listed in Table S4† illustrate that the PdO/TiO2 catalyst boasts the highest surface Pd content of 4.76 wt%, followed by PdO/SnO2 with 2.31 wt% and PdO/SiO2 with 1.08 wt%. However, the ICP results showed that the bulk Pd content was similar across the three catalysts, with PdO/TiO2, PdO/SnO2, and PdO/SiO2 containing 1.69, 1.64 and 1.75 wt% Pd, respectively. These observations suggest that TiO2 enhances Pd dispersion more effectively, a conclusion supported by XRD grain size measurements. The CO-TPD results (Table S4†) also indicated that surface Pd dispersion follows the order PdO/TiO2 > PdO/SnO2 > PdO/SiO2, underscoring the critical role of Pd dispersion in the performance of PdO-based catalysts. Meanwhile for the deconvolution of the O 1s peaks in PdO/TiO2 samples, the binding energy at 529.3 eV can be attributed to surface lattice oxygen (Olatt), and the peaks at 530.1 eV and 531.5 eV can be assigned to surface adsorbed oxygen (Oads).29 In the PdO/SnO2 samples, the binding energies are shifted to 530.5 eV and 531.8 eV, corresponding to Olatt and Oads, respectively, which further deviate to 531.9 eV (Olatt) and 532.8 eV (Oads) for the PdO/SiO2 catalyst.30 The quantitative analysis of surface oxygen species (listed in Table S4†) showing the Oads/(Oads + Olatt) ratio of PdO/SiO2 samples follows the sequence of PdO/TiO2 > PdO/SnO2 > PdO/SiO2. This sequence matching the catalytic activity for CO oxidation demonstrates that the amount of surface adsorbed oxygen is another factor influencing catalytic activity. | ||
| Fig. 5 The XPS spectra of all freshly prepared catalysts are presented as follows: (a) O 1s and (b) Pd 3d XPS spectra of PdO/TiO2, PdO/SnO2, and PdO/SiO2. | ||
To study the effects of various supports on PdO, H2-TPR tests were conducted. The redox properties of the three samples were analyzed by H2-TPR techniques, with the results shown in Fig. 6a. All three samples exhibit an H2 desorption peak below 100 °C, which is attributed to hydrogen adsorption on the reduced Pd species. Additionally, the hydrogen spillover effect causes the H2 consumption peak temperature of SnO2 to shift to a lower position, while the TiO2 and SiO2 supports do not show any reduction peaks. This indicates that PdO maintains strong metal–support interactions with SnO2, TiO2, and SiO2 supports. Three catalysts were tested for water adsorption through H2O-TPD. The results in Fig. 6b show that PdO/SiO2 has minimal water adsorption, whereas PdO/TiO2 and PdO/SnO2 exhibit significant adsorption. The distinct desorption peaks below and above ∼170 °C correspond to water and OH desorption behaviors, respectively.25 Table S5† provides a quantitative analysis of the desorbed water peak areas across different temperature ranges. The comparative analysis of different peaks highlights that PdO/SnO2 and PdO/TiO2 have significant H2O desorption peaks, unlike PdO/SiO2, which shows only a minimal peak, illustrating the unchanged catalytic activity of PdO/SiO2 under both hydrated and dehydrated conditions during CO oxidation. Conversely, PdO/SnO2 and PdO/TiO2 exhibit similar large water adsorption capacities with both H2O and OH surface adsorption, yet display different trends in CO oxidation activity, suggesting the need for further investigation to understand the specific reasons. The CO-TPD spectra (Fig. 6c) and quantitative results (Table S5,† based on integrated desorption peak areas) suggest that PdO/TiO2 favors the highest CO adsorption behavior with the following order PdO/TiO2 > PdO/SnO2 > PdO/SiO2 correlating with a CO oxidation activity sequence. However, a different behavior observed in the presence of water, where PdO/TiO2 activity decreases, PdO/SnO2 increases, and PdO/SiO2 remains consistent, highlights a different scenario. The ambiguous underlying reasons for support and water effects on catalyst activity prompt the construction of a catalyst model for quantum chemical calculations to elucidate the support effects on CO oxidation in the presence of H2O.
Discussion of DFT calculation results
Fig. 8a indicates that CO initially adsorbs at the Pd–O bridge site while releasing 2.08 eV of energy on the clean PdO/TiO2 catalyst surface. The C–O bond lengthens from the free molecule length of 1.144 Å to 1.227 Å, activating the CO molecule. The activated CO forms an Ocus–C–O species with surface coordinatively unsaturated O (Ocus), ultimately extracting the surface Ocus to generate the CO2* species, as illustrated in Fig. 7c. This process requires overcoming an energy barrier of 0.83 eV. In the transition state structure (Fig. 8a), the Ocus–C–O species initially breaks the Pd–Ocus bond to form the adsorbed CO2* species. The bond length of Ocus–C shortens from its initial adsorption length of 1.308 Å to 1.219 Å, indicating enhanced interaction between C and Ocus. The generated CO2* species exhibits C–O bond lengths of 1.168 Å and 1.119 Å, with an O–C–O angle of 175°. In contrast, the C–O bond length in a free CO2 molecule is 1.177 Å, with an O–C–O angle of 180°, highlighting the weaker adsorption of CO2 on the catalyst surface compared to its free form. On the surface of the PdO/TiO2 catalyst in the presence of H2O, as depicted in Fig. 8b, CO initially adsorbs at Pd–O bridge sites, releasing 2.34 eV of energy. The C–O bond lengthens from its molecular length of 1.144 Å to 1.241 Å, activating the CO molecule. Activated CO then reacts with surface Ocus to form Ocus–C–O species, eventually departing from the surface as CO2* species. This process requires a barrier of 1.25 eV to be overcome (Fig. 7c).
In the transition state structure, CO is oxidized with surface Ocus to form a bent Ocus–C–O species, where the Ocus–O bond length shortens from the initial 1.308 Å upon adsorption to 1.169 Å, approaching the C–O bond length in CO2 molecules. The generated CO2* species exhibit C–O bond lengths of 1.170 Å and 1.183 Å, with an O–C–O angle of 179°. These results indicate that the presence of H2O impedes the process of CO oxidation with surface Ocus. In the presence of H–OH on the PdO/TiO2 surface in dissociative form, as shown in Fig. 8c, CO adsorption at Pd–O bridge sites releases 2.04 eV of energy, which is similar to the energy released during CO adsorption on a clean PdO/TiO2 surface. The process of CO oxidation with surface O requires an energy barrier of 1.37 eV to be overcome. In the transition state structure (Fig. 8a), the Ocus–C–O species initially breaks the Pdcus–C bond to form the adsorbed CO2* species. The bond length of Ocus–C changes from 1.313 Å upon adsorption to 1.203 Å, and the resulting CO2* species has C–O bond lengths of 1.172 and 1.180 Å, with an ∠O–C–O angle of 179°. When H2O is present in the dissociated form H–OH on the PdO/TiO2 surface, as shown in Fig. 8c, CO adsorption on Pd–O bridge sites releases 2.04 eV of energy, which is similar to the energy released when CO adsorbs on a clean PdO/TiO2 surface. The process of CO oxidation with surface O involves overcoming a barrier of 1.37 eV. In the transition state structure (Fig. 8a), the Ocus–C–O species initially breaks the Pdcus–C bond to form the adsorbed CO2* species, with the bond length of Ocus–C changing from 1.313 Å upon adsorption to 1.203 Å. The resulting CO2* species has C–O bond lengths of 1.172 and 1.180 Å, and an O–C–O angle of 179°. The presence of H–OH alters the bond-breaking process during CO oxidation with surface O, leading to an increased reaction barrier. This indicates that the presence of H–OH further impedes the process of CO oxidation with surface oxygen, which aligns with experimental observations where the presence of H2O reduces CO oxidation activity.
On the PdO/SnO2 catalyst surface, the process of CO oxidation with surface oxygen is illustrated in Fig. 9a–c, with corresponding changes in potential energy shown in Fig. 7d and Table S8.† The initial and final states of CO during oxygen extraction exhibit structures nearly identical to those on the PdO/TiO2 surface. However, on the PdO/SnO2 surface, during the transition state formation of the CO2* species where CO is oxidized with surface oxygen, the Ocus–C–O species breaks the Pdcus–C bond rather than the Pd–Ocus bond. The breaking of the Pdcus–C bond is a dynamically more challenging process, resulting in a higher energy barrier for CO oxidation compared to the PdO/TiO2 surface. When surface H2O exists in molecular form, the energy barrier for CO oxidation with oxygen is 1.48 eV with an adsorption energy of 1.28 eV. However, when H2O is in the dissociated form H–OH, the energy barrier decreases to 1.25 eV with an adsorption energy of 0.91 eV. This lower barrier and reduced adsorption energy compared to the clean surface and molecular H2O conditions indicate that on the PdO/SnO2 catalyst surface, the enhanced activity in CO oxidation with H2O primarily stems from the presence of OH species. Moreover, this process is more favorable both kinetically and thermodynamically.
Conclusion
This work combines experimental and density functional theory (DFT) calculations to investigate the support effects of PdO/MO2 (M = Sn, Ti, and Si) catalysts on CO oxidation and the impact of the presence of water on their catalytic performance. As the active component, PdO shows different catalytic activities for CO oxidation when supported on TiO2, SnO2 and SiO2 supports, with the activity order: PdO/TiO2 > PdO/SnO2 > PdO/SiO2. PdO/TiO2 exhibits the smallest Pd grain size, the highest CO adsorption capacity, the maximum surface oxygen content, and the highest Pd dispersion on TiO2, resulting in superior CO oxidation activity. DFT calculations show that the energy barrier for CO oxidation with surface oxygen is the lowest for PdO/TiO2, consistent with experimental observations. Upon introducing H2O into the reaction system, the activity of PdO/TiO2 decreases, while that of PdO/SnO2 increases, and PdO/SiO2 activity remains nearly unchanged. Quantitative results from H2O-TPD indicate minimal H2O adsorption on PdO/SiO2 and significant adsorption on PdO/TiO2 and PdO/SnO2, with varying strengths for H2O and OH adsorption. This explains why H2O has a minimal impact on PdO/SiO2 but significant effects on PdO/TiO2 and PdO/SnO2 activities. DFT calculations reveal that the presence of H2O or H–OH species on the surface of PdO/TiO2 increases the energy barrier for CO oxidation with surface oxygen, leading to reduced activity under wet conditions and eventual deactivation under a humid atmosphere, attributed to the combined effect of surface H2O and OH. Conversely, on PdO/SnO2 surfaces, when H2O exists in the OH state, the energy barrier for CO oxidation with surface oxygen decreases, indicating enhanced CO oxidation activity due to the role of surface OH species.Author contributions
Kun Liu: investigation, formal analysis, and writing – review & editing. Luliang Liao: investigation. Guangfu Liao: conceptualization and supervision.Data availability
All the data for this article are available in the main text and the ESI† or upon reasonable request from the corresponding author.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful for financial support from the Jiangxi Provincial Natural Science Foundation (20242BAB20121).References
- G. Liao, J. Fang, Q. Li, S. Li, Z. Xu and B. Fang, Nanoscale, 2019, 11, 7062–7096 RSC.
- S. Lu, S. Zhang, Q. Liu, W. Wang, N. Hao, Y. Wang, Z. Li and D. Luo, Carbon Neutralization, 2024, 3, 142–168 CrossRef.
- A. Naitabdi, A. Boucly, F. Rochet, R. Fagiewicz, G. Olivieri, F. Bournel, R. Benbalagh, F. Sirotti and J.-J. Gallet, Nanoscale, 2018, 10, 6566–6580 RSC.
- G. Liao, Y. He, H. Wang, B. Fang, N. Tsubaki and C. Li, Device, 2023, 1, 100173 CrossRef.
- L. Huang, X. Song, Y. Lin, C. Liu, W. He, S. Wang, Z. Long and Z. Sun, Nanoscale, 2020, 12, 3273–3283 RSC.
- G. Liao, G. Ding, B. Yang and C. Li, Precis. Chem., 2024, 2, 49–56 CrossRef PubMed.
- G. Ding, C. Li, L. Chen and G. Liao, Energy Environ. Sci., 2024, 17, 5311–5335 RSC.
- G. Ding, C. Li, Y. Ni, L. Chen, L. Shuai and G. Liao, EES Catal., 2023, 1, 369–391 RSC.
- M. P. Salinas-Quezada, J. K. Pedersen, P. Sebastián-Pascual, I. Chorkendorff, K. Biswas, J. Rossmeisl and M. Escudero-Escribano, EES Catal., 2024, 2, 941–952 RSC.
- M. Chatterjee, N. Hiyoshi, T. Fukuda and N. Mimura, Sustainable Energy Fuels, 2023, 7, 1878–1892 RSC.
- H. Zhu, W. Qiu, R. Wu, K. Li and H. He, Catal. Sci. Technol., 2024, 14, 3050–3063 RSC.
- Y. Zhao, G. Chen, N. Zheng and G. Fu, Faraday Discuss., 2014, 176, 381–392 RSC.
- J. Cai, Z. Liu, K. Cao, Y. Lang, S. Chu, B. Shan and R. Chen, J. Mater. Chem. A, 2020, 8, 10180–10187 RSC.
- Y. Li and W. Shen, Chem. Soc. Rev., 2014, 43, 1543–1574 RSC.
- X. Liu, Y. Tang, M. Shen, W. Li, S. Chu, B. Shan and R. Chen, Chem. Sci., 2018, 9, 2469–2473 RSC.
- Y. Liao, K. Wang, G. Liao, M. A. Nawaz and K. Liu, Catal. Sci. Technol., 2024 10.1039/D4CY00679H.
- H. Zhu, L. Gou, C. Li, X. Fu, Y. Weng, L. Chen, B. Fang, L. Shuai and G. Liao, Device, 2024, 2, 100283 CrossRef.
- F. Tian, X. Wu, J. Chen, X. Sun, X. Yan and G. Liao, Dalton Trans., 2023, 52, 11934–11940 RSC.
- J. P. N. Kembo, J. Wang, N. Luo, F. Gao, H. Yi, S. Zhao, Y. Zhou and X. Tang, New J. Chem., 2023, 47, 20222–20247 RSC.
- G. Liao, C. Li, S.-Y. Liu, B. Fang and H. Yang, Phys. Rep., 2022, 983, 1–41 CrossRef.
- G. Croft and M. J. Fuller, Nature, 1977, 269, 585–586 CrossRef.
- X. Wang, J. S. Tian, Y. H. Zheng, X. L. Xu, W. M. Liu and X. Z. Fang, ChemCatChem, 2014, 6, 1604–1611 CrossRef.
- X. Xu, X. Wang, Y. Li, J. Tian, W. Liu and Z. Gao, Z. Phys. Chem., 2014, 228, 27–48 CrossRef.
- X. Xu, X. Sun, H. Han, H. Peng, W. Liu, X. Peng, X. Wang and X. Yang, Appl. Surf. Sci., 2015, 355, 1254–1260 CrossRef.
- Y. Liu, Y. Guo, H. Peng, X. Xu, Y. Wu, C. Peng, N. Zhang and X. Wang, Appl. Catal., A, 2016, 525, 204–214 CrossRef.
- J. Choi, L. Pan, V. Mehar, F. Zhang, A. Asthagiri and J. F. Weaver, Surf. Sci., 2016, 650, 203–209 CrossRef.
- C. Zhang, Y. Li, Y. Wang and H. He, Environ. Sci. Technol., 2014, 48, 5816–5822 CrossRef CAS.
- X. Sun, X. Peng, X. Xu, H. Jin, H. Wang and X. Wang, J. Mol. Model., 2016, 22, 204 CrossRef.
- R. Ruus, A. Saar, J. Aark, A. Aidla, T. Uustare and A. Kikas, J. Electron Spectrosc. Relat. Phenom., 1998, 93, 193–199 CrossRef CAS.
- S. Salimian and M. Delfino, J. Appl. Phys., 1991, 70, 3970–3972 CrossRef.
- X. Li, X. Sun, X. Xu, W. Liu, H. Peng, X. Fang, H. Wang and X. Wang, Appl. Surf. Sci., 2017, 401, 49–56 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03963g |
| This journal is © The Royal Society of Chemistry 2024 |



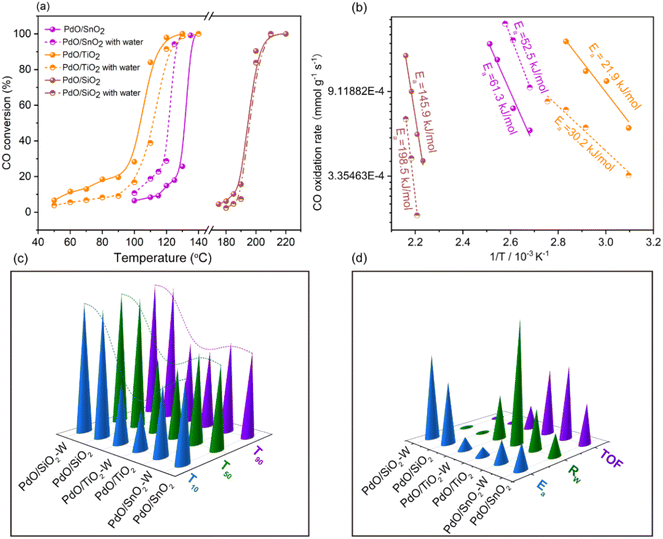
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. (c) The corresponding reaction temperatures for all catalysts at T10, T50, and T90 (Note: T10 represents the reaction temperature corresponding to a 10% CO conversion rate for the catalyst). (d) The intrinsic reaction performance parameters for all catalysts (
000 mL g−1 h−1. (c) The corresponding reaction temperatures for all catalysts at T10, T50, and T90 (Note: T10 represents the reaction temperature corresponding to a 10% CO conversion rate for the catalyst). (d) The intrinsic reaction performance parameters for all catalysts (



