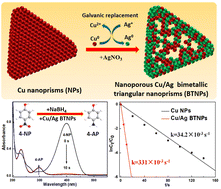
We report the synthesis of nanoporous Cu/Ag bimetallic triangular nanoprisms (BTNPs) using a galvanic replacement method. Based on ultraviolet–visible spectroscopy (UV–vis), scanning electron microscopy (SEM), transmission electron microscopy (TEM), high-resolution TEM (HRTEM), X-ray diffraction (XRD), energy dispersive spectrometry (EDS), selected area electron diffraction (SAED) and X-ray photoelectron spectroscopy (XPS) analyses, the structure of Cu/Ag BTNPs was characterized. The prepared Cu/Ag BTNPs exhibited excellent catalytic activity and good cycling stability for the reduction of 4-nitrophenol (4-NP) due to the synergistic effect between Cu and Ag elements. The kinetic rate constant (k) and turnover frequency (TOF) values reached 331 × 10−3 s−1 and 500 × 10−3 s−1, respectively, which were higher than those of previously reported Cu, Ag, Au, Cu/Ag or Cu/Au-based catalysts. We hope that the development of promising routes for high-quality BTNPs can broaden their applications in catalysis and environmental sustainability.