Recent advances in tungsten oxide-based chromogenic materials: photochromism, electrochromism, and gasochromism
Yaqi
Zhang
ab,
Yilin
Ding
a,
Fan
Lan
a,
Wenjing
Zhang
a,
Jingfa
Li
 *b and
Rufan
Zhang
*b and
Rufan
Zhang
 *a
*a
aBeijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China. E-mail: zhangrufan@tsinghua.edu.cn
bJiangsu Key Laboratory of New Energy Devices & Interface Science, School of Chemistry and Materials Science, Nanjing University of Information Science and Technology, Nanjing 210044, China. E-mail: aplijf@nuist.edu.cn
First published on 18th October 2024
Abstract
As n-type and wide-bandgap semiconductor materials which are widely found in nature, tungsten oxides (WOx) have attracted extensive attention because of their rich phase structures and unique sub-stoichiometric properties. Tungsten oxides have a good chromogenic response to optical, electrical, and gaseous stimuli, in which their phase changes with the change of temperature and ionic embeddedness, accompanied by significant changes in their optical properties. In addition, due to the presence of oxygen defects, the conductivity and adsorption capacity of tungsten oxides for surface substances are enhanced. These properties endow tungsten oxides with promising application potential in the optical and electronic device areas. This paper reviews the structural and optoelectrical properties of tungsten oxide-based chromogenic materials. Then we focus on the working mechanisms, performance indexes, and preparation methods of tungsten oxides in the field of intelligent chromogenic technology, including photochromism, electrochromism, and gasochromism of tungsten oxide-based chromogenic materials. Finally, a conclusion and outlook are provided, which may help to further advance the application of tungsten oxides in the field of smart chromogenic changes.
1. Introduction
In recent years, the fast-growing global energy consumption and carbon dioxide emission have posed serious challenges to the sustainable development of human society. Therefore, it is of significant importance to develop green, clean, and renewable energy technologies. Tremendous efforts have been made in recent years on solar cells,9,10 fuel cells,14 photocatalytic materials,19 and photothermal management materials.20,21 Among the most commonly used photothermal management materials, intelligent chromogenic materials have attracted wide attention because of their advantages in smart windows, building envelope materials and infrared camouflage.22–25 The optical properties of intelligent chromogenic materials can be largely changed under external stimuli such as light, electricity, heat, pressure, etc. As abundant semiconductor materials found in nature, tungsten oxides (WOx) have a chromogenic response to a wide range of external stimuli such as light, electricity, and gas, which are therefore considered as among the most popular inorganic intelligent chromogenic materials.Tungsten oxides have various phase structures, which can be converted between each other with changes in temperature and the extent of ionic embedding, accompanied by significant changes in their optical properties, such as visible and infrared light transmittance, absorbance, reflectance, etc. Therefore, tungsten oxide materials have photochromic, electrochromic, gasochromic and thermochromic properties. A perfect WO3 crystal is a cubic crystal composed of [WO6] octahedra in a co-vertex mode and is the same as the ReO3-type chalcogenide structure, which endows WO3 with an excellent charge storage and transfer capability. In addition, WOxx has unique sub-stoichiometric properties. Due to the presence of different degrees of oxygen defects, its electrical conductivity, adsorption capacity for surface substances, etc. are enhanced to different extents. In recent years, the complex multivalent properties and multi-defect structure of WOx have shown great potential for applications in lithium batteries, supercapacitors, gas sensors, and smart windows (Fig. 1).29–33
Herein, we briefly review the structural and sub-stoichiometric properties of WOx materials and introduce the influence of these properties on their optoelectrical performance. Then we discuss the working mechanism of WOx materials in intelligent chromogenic technology, including photochromism, electrochromism and gasochromism, evaluation indicators and the methods of performance enhancement.
2. Fundamental properties of WOx
Materials are intricately linked between their structures and properties. The WOx family is a series of materials with diverse structures and complex chemical states, a feature that makes the variation of its optical and electrical properties of great interest.2.1 Crystallography and sub-chemometrics
As a common member of the WOx family, the WO3 crystal is a cubic crystal composed of [WO6] octahedra in a co-vertex manner, which is shown in Fig. 2a. In other WO3 crystal forms, it can be seen as an assembly of structural units obtained by rotating or twisting on the basis of an ideal WO3 crystal structure. As the temperature increases, WO3 undergoes a phase transition, which varies sequentially from ε-WO3 (monoclinic phase, below −43 °C) to δ-WO3 (triclinic phase, −43–17 °C), γ-WO3 (monoclinic phase, 17–330 °C), β-WO3 (orthorhombic phase, 330–740 °C), and α-WO3 (tetragonal phase, above 740 °C) (Fig. 2b–f).4,34 Among various types of WO3, γ-WO3 is the most common stable phase at room temperature. Besides the above-mentioned crystal types, h-WO3 (hexagonal phase) is also a relatively stable crystal type of WO3 (Fig. 2g).4 However, it cannot be obtained from other crystalline structures by heat treatment but can only be prepared by dehydration of WO3 with water of crystallization. Such specific preparation conditions result in the presence of many hexagonal and triangular pores in h-WO3, which facilitates the transport of ions inside the structure and also provides active space for redox reactions. Compared with crystalline tungsten oxides, amorphous tungsten oxides have disordered bond lengths and bond angles, and there are a large number of dangling bonds and various structural distortions and defects in their distorted structures with a more active electronic structure.35 Higher atomic disorder and interstitial spacing of amorphous phase tungsten oxide can serve as a fast channel for ion diffusion, exhibiting excellent properties and performance of tungsten oxides-based electrochromic devices.36 In general, amorphous tungsten oxides have enhanced optical modulation and coloring efficiency over bulk crystalline films due to their relatively loose structure, yet their stability is limited. | ||
| Fig. 2 (a) Ideal WO3 crystal. (b) Monoclinic WO3. (c) Triclinic WO3. (d) Monoclinic WO3 (17–330 °C). (e) Orthorhombic WO3. (f) Tetragonal WO3. (g) Hexagonal WO3. Reproduced with permission.4 Copyright 2019, John Wiley and Sons. (h) Sub-stoichiometric WO3−x. Reproduced with permission.12 Copyright 2021, Springer Nature. | ||
Based on the content of oxygen vacancies in the lattice, WOx is classified as stoichiometric WOx and sub-stoichiometric WOx. In 1989, Glember and Saurr first found the sub-stoichiometric properties of WOx.37,92 Since then, a large number of WOx materials with sub-stoichiometric ratios have been continuously reported, in which the value of x is between 2 and 3, which represents the concentration of oxygen vacancies. Thus, a series of sub-stoichiometric WOx materials (including W32O84, W3O8, W18O49, W17O47, W5O14, W20O58, etc.) exist between the two stable tungsten oxides, WO3 and WO2 (Fig. 2h).12 The crystal structure of sub-stoichiometric WOx is similar to that of stoichiometric WO3, also composed of co-rimmed [WO6] octahedra, with the difference that the ordered oxygen defect surface acts as its crystal shear (CS) surface to split it.38 WO2.87 and higher concentrations of oxygen vacancies in WOx result in the formation of pentagonal columns (PCs), leading to the appearance of new crystalline phases.12 Taking the advantage of the structural features of the [WO6] octahedral edges, the WOx lattice can withstand a considerable degree of oxygen deficiency, and part of W6+ atoms are reduced to W5+. The absence of lattice oxygen atoms affects the energy gap and free electron density of WOx. With the increase of oxygen vacancy concentration, the metallic properties of WOx are revealed. Therefore, diverse and interesting physicochemical properties can be tapped by simply modulating the stoichiometric ratio of WOx.
2.2 Optical and electrical properties
WOx is an n-type and wide-bandgap semiconductor material with an indirect bandgap between the valence and conduction bands, where the valence band consists mainly of O 2p orbitals and the conduction band consists mainly of W 5d orbitals.37 W 5d orbital occupancy is strongly correlated with lattice distortion, thus the change in crystal phase has a significant effect on the band gap of WOx. The bandgap of bulk phase γ-WO3 is usually in the range of 2.6–2.8 eV. The bandgap varies with the degree of distortion of the crystal structure, therefore amorphous WOx with large lattice distortion has a wide bandgap of 3.4 eV. Apart from the crystalline phase structure, the size of the WOx material also affects its band gap. Due to the quantum confinement effect, when the size of WOx is close to or smaller than its exciton Bohr radius (3 nm), its bandgap becomes wider as the particle size decreases. In contrast, when the crystal size is larger than the exciton Bohr radius, the effect of size on the bandgap diminishes.39Standard stoichiometric WO3 powder is yellow, while sub-stoichiometric WOx is variable in color. For instance, WO2.9 is bluish-purple, WO2.72 is purplish-red, and WO2 is brown. The light absorption range of WOx covers the entire solar spectrum, i.e., the ultraviolet, visible, and near-infrared bands, and the whole process involves electron interbond leaps, polariton leaps, and plasmon resonance (LSPR).40–42 Absorption in the visible region may be related to the level of oxygen defects, a process that can be elucidated in terms of the polariton jump mechanism. The injected electrons are first trapped by the W 5d orbitals, and the lattice around them undergoes polarization to produce polaritons. Incident photons can induce mutual leaps of polaritons from two neighboring W sites, which is thought to be the reason for the blue color of WOx materials when stimulated by light, gases, and electric fields.37
In recent years, near-infrared absorption of sub-stoichiometric WOx has also received extensive attention. Sub-stoichiometric WOx has oxygen vacancies that increase its carrier density. At higher concentrations of oxygen vacancies, the absorption of WOx in the near-infrared (NIR) band is greatly increased, which is usually attributed to the generation of the LSPR effect.43,44 LSPR is usually found in noble metal nanoparticles, which absorb photon energy strongly when the incident photon frequency matches the overall vibrational frequency of the carriers, which results in a strong resonance absorption peak in the spectrum. Sub-stoichiometric WOx is distinguished from other semiconductor oxides due to its rather high carrier density, which is also necessary for the strong LSPR effect.8,45–48 The tunable plasmon resonance of sub-stoichiometric WOx gives it a strong absorption in the near-infrared region, and therefore has a great potential for application in the field of building energy efficiency.
3. Photothermal management applications
Owing to its unique physicochemical properties, WOx offers great potential for its application in optical and electrical fields. This section focuses on the working mechanism, performance indexes, and preparation methods of WOx in intelligent chromogenic technologies, including photochromism, electrochromism, and gasochromism.3.1 Photochromism
Photochromism is one of important branch of photochemical and photophysical applications which is a reversible process triggered by light absorption and other external stimuli (e.g., light avoidance, thermal stimuli, etc.). As shown in Fig. 3a, a chemical reaction occurs when A is irradiated with light to produce product B. B is then able to be reconverted to A after overcoming an energy barrier or a photothermal stimulus, with a distinct difference in its absorption spectrum and a change in color.1 Photostimulation has the advantages of being clean and contactless, and can be adjusted remotely and precisely (wavelength, intensity, coverage area, duration, etc.). Therefore, photochromic materials have a promising application in the fields of data storage,49,50 information displays,11 sensors,26 erasable rewritable paper,5 and smart windows.51–53 | ||
| Fig. 3 (a) Photochromic process. Reproduced with permission.1 Copyright 2011, Royal Society of Chemistry. (b) Illustration of the photochemical reaction mechanism in the WO3/PVA/PAA hybrid system. (c) Time evolution and color holographic reconstruction of the first-order diffraction efficiency in the recorded configurations at 457 nm, 473 nm and 532 nm for WO3 and WO3/PVA/PAA films. Reproduced with permission.13 Copyright 2022, Royal Society of Chemistry. (d) Transient photovoltaic and photocurrent responses of pristine WO3, pristine Bi2WO6 and WO3/Bi2WO6. (e) Schematic insertion of Bi atoms on the [WO6] octahedral framework, WO3/Bi2WO6 photogenerated carrier separation and the photocatalytic color change mechanism. Reproduced with permission.26 Copyright 2023, Elsevier. | ||
WOx, as the most representative inorganic photochromic material, has received much attention. Although the photochromism of WOx has been studied for several decades, the mechanism of photochromism is still not well defined. Researchers have proposed many different models to explain the photochromic phenomenon on the basis of their respective experiments. Here, we give a brief overview of the photochromic mechanism of WO3. Under UV irradiation, WO3 produces photogenerated electrons and holes (eqn (1)). The photogenerated holes decompose the adsorbed water of the material, thereby generating protons, which participate in water decomposition together with the photogenerated electrons (eqn (2)). The photogenerated electrons and protons then reduce some of the W6+ ions in the WO3 to W5+, forming a blue tungsten bronze at the same time (eqn (3)). The tungsten bronze is oxidized by oxygen in the air (eqn (4)), a process known as photochromic bleaching.
 | (1) |
| H2O + 2h+ → 2H+ + O | (2) |
| WO3 + xH+ + xe− → HxW1−x6+Wx5+O3 | (3) |
 | (4) |
Excitation wavelength, coloring time, bleaching conditions, bleaching time, and the degree of color changes are key parameters for evaluating photochromic performance. The excitation wavelength is the maximum absorption wavelength that can trigger the photochromic behavior, and a larger excitation wavelength not only improves the solar photoelectricity utilization, but also generates more photogenerated electrons. The relationship between the band gap (Eg) and the excitation wavelength (λ) of semiconductor materials is shown as follows:
 | (5) |
Although the band gap can be modified to enhance light absorption, the complexation of photogenerated electrons and holes needs to be taken into account to obtain a better photochemical energy conversion efficiency. Suppressing photogenerated electron–hole pair composites, improving the carrier lifetime, increasing the number of photogenerated electrons in the system, and utilizing these carriers to initiate photochemical reactions lead to higher chemical reaction rates and photochemical energy–chemical energy conversion efficiencies. The construction of heterojunctions is one of the commonly used strategies to inhibit photogenerated electron–hole pair complexation. Dong et al. synthesized WO3/Bi2WO6 heterostructure nanoparticles with efficient sunlight-responsive photochromism by introducing Bi atoms into the [WO6] framework to form a [Bi2O2] component (Fig. 3d).26 The WO3/Bi2WO6-type II heterostructure ensured efficient photoexcited electron transfer from Bi2WO6 to WO3, and the abundant [WO6] units served as fast sites for trapping and consuming photogenerated electrons, effectively promoting the separation of photogenerated electrons and holes (Fig. 3e). In addition, compounding with organics or noble metals (Au, Ag, etc.) is also a common solution. Precious metals are deposited on the surface of semiconductors, forming a Schottky barrier at the interface between the two and facilitating the separation of photogenerated carriers.56,57 As organics are electron donors and act as hole sacrificers to eliminate photogenerated holes in semiconductors, the compounding of photogenerated electrons and holes in the semiconductors themselves is prevented.58,59 Si et al. introduced fluor-silane and designed a WO3-based photochromic material with superamphiphobicity, low adhesion, heat and chemical resistance, as well as UV and abrasion resistance (Fig. 4a).5 Fluor-silane acted as an electron donor to promote rapid electron transfer and proton insertion, which significantly improved the photochromic properties of WO3. Subsequently, they verified the photochromic performance of the material and its surface antifouling and self-cleaning ability by UV repetitive writing erasure, as illustrated in Fig. 4b.
 | ||
| Fig. 4 (a) Schematic illustration of the F-WO3/ZnO superamphiphobic powder surface adapting to harsh environmental conditions. (b) Process flow diagram for ultraviolet (UV) mask writing and thermal erasure. Reproduced with permission.5 Copyright 2024, Elsevier. (c) Schematic demonstration of the photochromic process in WO3·0.33H2O hybrid nanostructures. (d) Schematic photochromic mechanism diagrams of different WO3·0.33H2O hybrid nanostructures. Reproduced with permission.16 Copyright 2022, Elsevier. (e) HR-TEM pattern of the WO3 nanofiber. (f) UV-Diffused reflectance spectra of the WO3 nanofiber. (g) Number of photochromic reruns of the WO3 nanofiber. Reproduced with permission.27 Copyright 2022, Springer Nature. | ||
The photochromic behavior depends on the electronic energy band structure and charge transfer process, which are affected by the physical and chemical properties of the photochromic materials. G. Deonikar et al. introduced plasma ions (Cu and Al) and non-plasma ions (Zn and Sm) to modulate tungsten oxide hydride (WO3·0.33H2O) nanostructures in order to enhance their reversible photochromic ability (Fig. 4c).16 Based on the double insertion-extraction model of ions and electrons, they explained the effect of different metal ions on the photochromic mechanism of WO3 (Fig. 4d). They found that the insertion of hot electrons generated by plasma metals (Cu and Al) into the WO3·0.33H2O nanostructures inhibited the complexation of photogenerated electrons and holes, enhanced light absorption, and improved the photochromic ability of the hybrid materials.
In addition to the structural design of WOx photochromic materials, the modulation of morphology and size is also an important part to be considered in order to improve their photochromic properties, especially the light modulation ability and bleaching efficiency. Ejeromedoghene et al. reported a WO3 nanofiber synthesized by non-electrostatic spinning under moderate conditions with particle diameters in the range of 200 nm (Fig. 4e).27 This WO3 fiber rapidly changed its visual color from white to blue with excellent reflectance (72%), reproducibility and stability after exposure to UV light for up to 7 s, as shown in Fig. 4f and g. Zhu et al. prepared extremely small-sized WO3 quantum dots (WQDs) and WQDs-polyvinyl alcohol (PVA) transparent films by the casting method (Fig. 5a and b).6 The WQDs are monodisperse near-spherical crystals with an average diameter of 1.2 nm.60 Owing to the size effect of WQDs and the provision of protons by polyol, the composite films exhibited a fast light response (<60 s) and a large optical modulation amplitude (>90%) before and after coloration (Fig. 5c and d). However, their bleaching time was more than 8 h. Recently, Meng et al. reported a method for the in situ growth of WO3 nanoparticles in PMMA matrix and obtained highly dispersed small-sized WO3 nanoparticles in composite films simply and efficiently by utilizing the spatial confinement effect of PMMA chains (Fig. 5e).18 This method allowed the preparation of photochromic films with high luminescence transparency (transmittance Tlum = 91%) and scalability (30 × 350 cm2) at low cost. The high modulation of visible light (ΔTlum = 73%) and solar heat (modulated solar transmittance ΔTsol = 73% and modulated solar heat gain coefficient ΔSHGC = 0.5) by the films improved indoor daylight comfort and energy efficiency (Fig. 5f and g). Furthermore, they introduced Cu ions into this film to accelerate its bleaching process (20 min). This photochromic film, with its excellent light modulation capability, short bleaching time and ease of large-area preparation, offered an attractive strategy for achieving more energy-efficient buildings and carbon neutrality.
 | ||
| Fig. 5 (a) SEM image of the PVA WQD/PVA film. (b) Digital photographs of colored and bleached WQD-PVA films (25 cm × 30 cm). (c) Variation of the transmittance of the WQD-PVA film with UV irradiation time and its transmittance change at different times in the dark. (d) Real-time data of the outdoor experiment of the WQD-PVA film. Reproduced with permission.6 Copyright 2022, Elsevier. (e) Schematic diagram of the preparation process of Cu-W-PC films. (f) Transmission spectra of Cu-W-PC films to standardized AM1.5 solar spectra (green shading) under coloring and bleaching conditions (6 h of outdoor sunlight). (g) Photographs of 30 × 40 cm2 Cu-W-PC films prepared by the blade coating method in the bleached (left) and colored (right) states. Reproduced with permission.18 Copyright 2023, John Wiley and Sons. | ||
3.2 Electrochromism
Electrochromism is the phenomenon in which a material reversibly changes its color or optical properties (absorbance, transmittance, reflectance, etc.) through redox reactions in the presence of an applied voltage or current.61–63 In 1961, the concept of “electrochromism” was first proposed by Platt.64 Subsequently, Deb discovered the electrochromic phenomenon of WO3 for the first time,65 and proposed the electrochromic principle of the color-centered theory on the basis of this research, which became an important milestone in the history of the development of electrochromism.66 As the most classic inorganic electrochromic material, WOx has been widely used in the fields of infrared stealth,67–69 flexible electronic devices,70 and smart windows.2,21,71,72Regarding the electrochromic mechanism of WO3, the F-color core model and the dual ion implantation model are mainly discussed and accepted at present. The F-color center model was first proposed by Deb in 1973,73 aiming to explain the electrochromic mechanism of amorphous WO3 at that time. He suggested that amorphous WO3 has an ionic crystal structure with oxygen vacancies in localized states, and that when a negative voltage is applied, electrons enter the oxygen vacancies and are trapped by them to form the F-color center, and the electrochromic film changes from colorless to blue. When a positive voltage is applied, the electrons are removed from the oxygen vacancies and the F-color center disappears, and the electrochromic film changes from blue to colorless. The dual ion implantation model, also known as the Faughnan model, is currently the most accepted mechanism model for electrochromism in the field since it explains not only the phenomenon of WO3 electrochromism, but also other common electrochromic materials.74 When a negative voltage is applied, the electrons enter into the interior of the material, and the cations enter at the same time and undergo valence transitions to produce the color change. When the opposite voltage is applied, the electrons and ions are simultaneously withdrawn, causing the color to return to its initial state.
There are mainly six performance parameters for evaluating electrochromic materials and devices. The first is the contrast ratio (CR), which reflects the degree of color changes of a material or device during the coloring or bleaching process, and is the basic index for evaluating the effect of color change. The second is optical density (OD), which describes the transmittance of the material or device at a constant wavelength, and is defined as:
 | (6) |
 | (7) |
Researchers have proposed various methods to enhance the performance of WOx electrochromic materials, for instance, control of the structure and morphology, doping and compounding with other inorganic materials, and compounding with organics. Different synthesis approaches can yield WOx with different nanostructures. Huang et al. found that the concentration of precursors plays an essential role in morphology control.8 The morphology of WO3−x (0 < x < 1) nanostructures gradually changed from nanowires (NWs), nanoclusters (NCs), to nanoflowers (NFs) with the increase of precursor concentration (Fig. 6a and b) and the color of the as-prepared WO3−x nanostructure solutions gradually changed from light blue to dark blue, which indicated the increase of light absorption. It was also found that the lattice-stripe spacing of the WO3−x nanostructures widened with increasing solution concentration. The weaker long-range ordering and lower crystallinity of WO3−x NFs compared to WO3−x NWs and WO3−x NCs suggest that WO3−x NFs are more conducive to the insertion/extraction of electrolyte ions in the electrochromic process. As shown in Fig. 6c, the WO3−x NFs have the best optical modulation ability of 62.98% over WO3−x NWs (52.06%) and WO3−x NCs (61.01%) in the VIS and NIR regions, showing the importance of nanostructures. Fig. 6d shows the bright, cold and dark modulation modes of the WO3−x NF electrochromic film, which demonstrates its potential application electrochromic smart windows. Du et al. prepared novel WO3−x hydrate nanosheets by the template method, and the fabricated electrochromic films exhibited excellent electrochemical and electrochromic properties (Fig. 6e and g).11
 | ||
| Fig. 6 (a) Synthesis process of WO3−x with different structures and morphologies. (b) SEM images of WO3−x NWs, WO3−x NCs, and WO3−x NFs. (c) Optical transmittance spectra of WO3−x NWs, WO3−x NCs and WO3−x NFs in 1.0 M LiClO4/PC electrolyte at −1.5 and 1.0 V. (d) Illustration of dual-band electrochromic smart windows in bright, cool, and dark modes. Reproduced with permission.8 Copyright 2023, Springer Nature. (e) TEM images of the WO3−x hydrate nanosheets. (f) Transmittance spectra in the bleached and colored states of the H-WO3−x film. (g) In situ transmittance change curve between the colored state and the bleached state of the H-WO3−x film in a cycle. Reproduced with permission.11 Copyright 2022, Elsevier. | ||
The crystalline phase structure of WOx thin films has a significant effect on their electrochemical properties. Crystalline films prepared by conventional evaporation and magnetron sputtering have better cycling stability, yet they tend to be dense, which is not conducive to the diffusion of ions and will affect their coloring efficiency and response time.75,76 It is generally recognized that amorphous films have a looser structure and more holes, which facilitates the diffusion of ions in them, and provides greater color contrast and speed of color change. Zhang et al. developed a fast-switching dual-band electrochromic smart window based on a single-component amorphous porous WO3 (AP-WO3) cathode (Fig. 7a and b).3 The amorphous and porous structure of WO3 not only significantly improves the ion transport, but also provides a large surface area for Li+ adsorption, resulting in tunable surface plasmon resonance in the near-infrared range (Fig. 7c and d). As a result, the single-component AP-WO3 films can independently and effectively modulate the near-infrared and visible transmittance through three different modes with high optical modulation and a fast switching speed, but their stability is slightly poor (Fig. 7e), which is also a common problem of amorphous WO3 electrochromic films.35,77,78 Pham et al. prepared WO3 porous films on indium tin oxide (ITO) substrates using a simple three-pulse electrodeposition method.79 These porous films outperformed the corresponding dense films formed by continuous electrodeposition in terms of long-term stability, with insignificant changes after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. This research result will inspire more researchers to improve the preparation of electrodeposited films, reduce the preparation cost of electrochromic films, and promote the industrialization of electrochromic devices.
000 cycles. This research result will inspire more researchers to improve the preparation of electrodeposited films, reduce the preparation cost of electrochromic films, and promote the industrialization of electrochromic devices.
 | ||
| Fig. 7 (a) Surface and cross-section SEM images of AP-WO3 thin films. (b) Transmittance spectra of AP-WO3 thin films before and after coloring. (c) Cyclic stability test of AP-WO3 thin films. (d) Transmittance spectra of the AP-WO3 electrochromic device in bright, cold, and dark modes and (e) corresponding digital photographs. Reproduced with permission.3 Copyright 2022, John Wiley and Sons. (f) SEM images of Nb18W16O93 thin films. (g) Transmission spectra and (h) coloring efficiencies of WO3, Nb18W16O93, and Nb2O5 films in initial, colored, and bleached states. (i) Cycling stability test of the Nb18W16O93 film. (j) Schematic diagram of the electrochromic energy storage device assembled with the Nb18W16O93 film (green) as an electrochromic layer. Reproduced with permission.15 Copyright 2022, John Wiley and Sons. | ||
Compounding and doping of WOx with other inorganic materials or elements is one of the most commonly used means to enhance its electrochromic properties. Most of the doping and compounding processes of WOx were based on transition metals, especially focusing on transition metal oxides with electrochromic properties, such as Mo,80 Ti,81,82etc. Cai et al. synthesized a Nb18W16O93 nanomaterial with superstructural motifs and prepared homogeneous self-supported electrochromic thin films on transparent conductive substrates (Fig. 7f–i),15 which showed large optical modulation (93% at 633 nm and 89% at 1200 nm), high color rendering efficiency (105.6 cm2 C−1), excellent multiplication capability and long-term electrochemical stability (6000 cycles). Based on this high-performance electrochromic film, they further assembled a multifunctional electrochromic energy storage device to realize the combination of dynamic dimming and energy storage applications (Fig. 7j). Such devices can not only manage solar thermal radiation entering a building and protect personal privacy, but also deposit electrical energy into it. The electrochromic films prepared in this work are low-cost and scalable, providing a new material option for electrochromic smart windows. Zhao et al. have constructed one-dimensional WO3@PB arrays with porous core–shell structures.7 As shown in Fig. 8a and b, the modification of WO3 enables the film to have dual-band modulation capability at low voltage. Moreover, the porous one-dimensional structure of PB@WO3 provides sufficient space for the lattice expansion of PB during the EC process, which improves the stability of PB (Fig. 8c). Owing to the formation of a heterostructure between PB and WO3, the electron transfer is accelerated and the electronic interactions are enhanced, which further improves its electrochemical activity and stability.
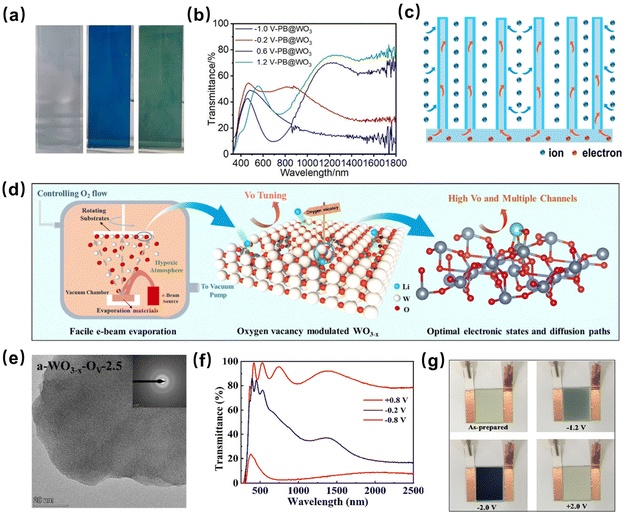 | ||
| Fig. 8 (a) Digital photos of the PB@WO3 film electrode at −0.2, 0.6, and 1.2 V. (b) UV–vis–NIR transmittance spectra of PB@WO3 under different voltages of −1.0, −0.2, 0.6, and 1.2 V. (c) Schematic illustration of the EC mechanism in PB@WO3. Reproduced with permission.7 Copyright 2023, American Chemical Society. (d) Schematic illustration of the preparation and structure of oxygen vacancy-modulated a-WO3−x–Ov thin films. (e) HRTEM image of the a-WO3−x–Ov film. (f) Transmission spectrum of the a-WO3−x–Ov thin film. (g) Corresponding digital photographs of the a-WO3−x–Ov electrochromic device at different applied voltages. Reproduced with permission.2 Copyright 2023, Royal Society of Chemistry. | ||
Moreover, as a new doping means, oxygen vacancy doping significantly improves the electrochromic properties of WOx, especially the modulation ability in the near-infrared band. Chen et al. reported a novel oxygen vacancy-modulated amorphous tungsten oxide (a-WO3−x–Ov, 0 < x < 1), as shown in Fig. 8d and e.2 It was found that the introduction of oxygen vacancies not only enabled this film to modulate the near-infrared transmittance independently through the LSPR effect, but also provided more sites for ion diffusion and binding, which resulted in the optimum conductivity performance (Fig. 8f). They assembled these films into devices with effective energy storage performance and significant energy savings, which can greatly reduce the energy consumption of air conditioning and lighting in buildings, as shown in Fig. 8g. Additionally, as demonstrated in Fig. 9a, it can selectively control visible and NIR transmittance in three different modes: bright mode (+0.8 V, NIR and visible transparency), cold mode (0.2 V, visible transparency, mostly NIR opacity), and dark mode (0.8 V, NIR and visible opacity). Among the non-metallic element doping N83 and phosphorus84 doped WO3 films have also been reported. It is worth mentioning that due to the superior electrochromic properties of WOx itself, the doping of W atoms into various electrochromic oxides also leads to a better enhancement in their properties.85 Wang et al. fabricated single tungsten atom (W)-modified hydrangea-like porous V2O5 nanoflowers (HLP-W/V2O5) with a high loading content of atomic W (13.7 wt%) using a low-temperature solvothermal method (Fig. 9b and c).17 The ion diffusion and electronic conductivity of HLP-W/V2O5 were greatly improved with the assistance of W atom modification. Below 1.2 V, HLP-W/V2O5 exhibited three reversible color transformations (yellow, blue, and brown) and excellent cycling stability (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles without significant decay), as demonstrated in Fig. 9d–f.
000 cycles without significant decay), as demonstrated in Fig. 9d–f.
 | ||
| Fig. 9 (a) Application scenarios of the a-WO3−x–Ov electrochromic device in bright, cool, and dark operation modes. Reproduced with permission.2 Copyright 2023, Royal Society of Chemistry. (b) SEM images of HLP-W/V2O5. (c) AC HAADF-STEM image of HLP-W/V2O5. (d) Prototype of the panda fabricated based on HLP-W/V2O5 and photographs at different colored states. (e) Structural schematic diagram of the solid EC device and photographs of the solid EC device at different color states. (f) Cycling stability of HLP-V2O5 and HLP-W/V2O5 with multi-potential steps between −0.6 and 1 V for 79.4 h. Reproduced with permission.17 Copyright 2023, Elsevier. (g) Transmittance of PANI-WO3 thin films at different applied voltages. Reproduced with permission.28 Copyright 2021, Elsevier. | ||
As a typical representative of inorganic electrochromic materials, WOx possesses excellent chemical stability, but it also has the drawbacks of singular color change and a slow response speed, thus compositing with organic materials is a reliable method to realize its fast response and multiple color change. Nguyen et al. reported an electrochromic hybrid film based on polyaniline and WO3, which achieved multiple color changes (green, blue, violet and dark blue) in a single film and has ultra-short switching times (1.5 s for each of the coloring and bleaching processes) and cycle lifetimes (Fig. 9g).28 Xue et al. constructed an electrolyte-free, integrated electrochromic device using WO3 sols doped with carbon nanodots (CDs).86 Unlike conventional WO3 that exhibit color transitions between transparent and blue, the device enabled reversible color changes between yellow and green due to the intrinsic color of the carbon dots. Their study provided a new idea for the composite of WOx and organic materials.
3.3 Gasochromism
The gasochromism effect refers to the interaction of a material with a target gas, which causes changes in the optical properties of the material, such as transparency, reflectivity, and color changes. Typically, the material returns to its initial state after isolation of the target gas, and the process is reversible.87 The target gas may be different for different materials. As for WO3, the target gas is H2. Therefore, the gasochromism of WO3 can also be called hydrochromic response.Since the gasochromism of WO3 was studied later than its electrochromism, there are relatively limited studies on its gasochromic mechanism. In this review, we choose the commonly used double-injection model to explain the H2 gasochromic mechanism of WO3. Compared with electrochromism, the coloration process of WO3 under an H2 atmosphere is much more complicated. During the coloration process, H2 molecules are adsorbed on the catalyst surface and dissociate into H atoms, which further diffuse along the surface and finally inject into the WO3 in the form of H+ and electrons, which is known as the so-called double injection. There is only one charge transfer reaction in the double injection process, where an electron from the H atom is transferred into WO3, reducing the W6+ ion to W5+. In the bleaching process, O2 adsorbed on the surface dissociates and transfers to form chemically active O atoms or O2−, which attracts and combines with the H atoms to form an H2O molecule, and then the H2O is desorbed, thus causing discoloration of the film.88 The above coloring and bleaching processes can be described by eqn (8) and (9):
 | (8) |
 | (9) |
It can be analyzed from the WO3 hydrochromic mechanism that the discoloration rate of WO3 is mainly related to the adsorption of H2, the diffusion of H atoms in WO3, and the chemical reaction between H atoms and WO3. On the other hand, the adsorption and diffusion of H2 are closely related to the distribution of the catalyst as well as the composition and structure of the material. Except for the effect of temperature, it is difficult to accelerate the chemical reaction between H atoms and WO3 by other methods. Hence, it can be designed from the morphological structure and composition in order to improve the hydrochromic performance of WO3.
Foroushan et al. prepared three WO3 nanofiber meshes by electrostatic spinning.89 WO3 nanofiber meshes had good sensitivity to hydrogen gas (in argon) down to 2% at room temperature. Upon exposure to hydrogen, the almost colorless nanofibers turned blue in less than 2 min. Liu et al. synthesized sub-stabilized hexagonal WO3 with three typical morphologies using a hydrothermal method, which exhibited excellent hydrochromic properties.90 Moreover, they pointed out that the correlation between the morphology and the hydrochromic properties was mainly attributed to the growth difference of the preferred crystalline surface of WO3. The evaluation of the morphology of WO3 nanowires, microspheres and nanorods exhibited a decrease in the aspect ratio, which corresponds to the microscopically preferred growth orientation changing from (002) to (100). Increasing the exposure ratio of oxygen ions on the c-axis by morphological modulation can improve the injection process of WO3 and promote the coloring process. This is helpful to deepen the understanding of the morphology effect of WO3 in the H2 gas chromatography process and to provide theoretical guidance for the preparation of highly sensitive WO3 gas chromatography materials. A detailed study of the hydrochromic properties of WO3 at different operating temperatures, solvation conditions (low, medium, high) and doping concentrations has been carried out by Nisha et al. and the effect of precious metal (Pt, Pd) doping on the hydrochromic properties has been discussed.91 The results show that the hydrochromic response increases significantly with increasing working temperature, and the Pt-doped samples exhibit better hydrochromic response than the Pd-doped samples. A low detection limit of 0.01% was observed for the samples at temperatures between 50 °C and 100 °C, and they did not show any cross-sensitivity to other gases. It is a positive contribution to the development of hydrogen sensors based on WO3. More and further research is needed to be put into the gasochromism of WO3, which would be a significant advancement forward in gas sensing.
4. Summary and outlook
This review focuses on the functional mechanisms, performance indexes, properties, and applications of WOx in photochromism, electrochromism and gasochromism. WOx materials have been widely investigated in recent years due to their tunnel-like structure, sub-stoichiometric properties, and polycrystalline phase. However, there are still many challenges for WOx, such as the structural design, stability improvement, large-scale and low-cost preparation, etc. Much effort should be made for the development of WOx in intelligent chromogenic technology. It is crucial to improve the long-term stability of WOx materials to realize their practical applications. There is an urgent need to combine nanostructure design and multiple synthesis methods to construct a series of WOx materials with long-term stable recycling. Since WOx-based photothermal management devices typically have a multilayer structure and interactions between the layers, an appropriate device structural design is critical. In the case of electrochromic devices, the selection of different substrates, transparent conductive layers, and electrolytes has a significant impact on the performance and sustainability of the devices. In addition, large-scale preparation of high-performance WOx films at a reasonable cost is crucial. Currently, electrodeposition, electrostatic spinning, sol–gel, etc. are the main approaches for the preparation of WOx films. However, their cost and scale are not suitable for practical production. Hence, it is essential to improve the traditional preparation techniques and investigate new preparation techniques to promote its industrial development and application.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Tsinghua-Toyota Joint Research Fund, the National Key Research and Development Program of China (Grant No. 2020YFC2201103 and 2020YFA0210702), the Jiangsu Specially Appointed Professor program and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX24_1486).References
- R. Pardo, M. Zayat and D. Levy, Chem. Soc. Rev., 2011, 40, 672–687 RSC.
- M. Chen, X. Zhang, D. Yan, J. Deng, W. Sun, Z. Li, Y. Xiao, Z. Ding, J. Zhao and Y. Li, Mater. Horiz., 2023, 10, 2191–2203 RSC.
- S. Zhang, Y. Peng, J. Zhao, Z. Fan, B. Ding, J. Y. Lee, X. Zhang and Y. Xuan, Adv. Opt. Mater., 2023, 11, 2202115 CrossRef CAS.
- P. A. Shinde and S. C. Jun, ChemSusChem, 2020, 13, 11–38 CrossRef CAS PubMed.
- W. Si, S. Wang, Z. Yu, X. Dai, M. Guo and Z. Guo, Chem. Eng. J., 2024, 492, 152232 CrossRef CAS.
- Y. Zhu, Y. Yao, Z. Chen, Z. Zhang, P. Zhang, Z. Cheng and Y. Gao, Sol. Energy Mater. Sol. Cells, 2022, 239, 111664 CrossRef CAS.
- S. Zhao, B. Wang, Y. Huang, Y. Zhang, X. Wu, Q. Jiang, D. Gao, F. Wang, R. Li, Y. Li, Y. Zhao, J. Li and R. Zhang, ACS Appl. Nano Mater., 2023, 6, 15021–15028 CrossRef CAS.
- Y. Huang, B. Wang, P. Lyu, S. Zhao, X. Wu, S. Zhang, R. Li, Q. Jiang, F. Wang, Y. Zhao and R. Zhang, Nano Res., 2023, 16, 12165–12172 CrossRef CAS.
- K. O. Brinkmann, P. Wang, F. Lang, W. Li, X. Guo, F. Zimmermann, S. Olthof, D. Neher, Y. Hou, M. Stolterfoht, T. Wang, A. B. Djurišić and T. Riedl, Nat. Rev. Mater., 2024, 9, 202–217 CrossRef CAS.
- Y. Li, X. Ru, M. Yang, Y. Zheng, S. Yin, C. Hong, F. Peng, M. Qu, C. Xue, J. Lu, L. Fang, C. Su, D. Chen, J. Xu, C. Yan, Z. Li, X. Xu and Z. Shao, Nature, 2024, 626, 105–110 CrossRef CAS PubMed.
- J. Du, Z. Zhang, C. Yue, Z. Nie, H. Tan, Z. Tang, N. Li, L. Xu and J. Xu, Mater. Today Chem., 2022, 26, 101089 CrossRef CAS.
- S. Bandi and A. K. Srivastav, J. Mater. Sci., 2021, 56, 6615–6644 CrossRef CAS.
- M. Zhang, J. Miao, H. Liu, S. Fu, X. Li, Y. Tao, X. Qi and X. Zhang, J. Mater. Chem. C, 2022, 10, 7690–7698 RSC.
- X. Qiu, Y. Wang and M. Shao, Joule, 2024, 8, 881–882 CrossRef CAS.
- G. Cai, R. Zhu, S. Liu, J. Wang, C. Wei, K. J. Griffith, Y. Jia and P. S. Lee, Adv. Energy Mater., 2022, 12, 2103106 CrossRef CAS.
- V. G. Deonikar and H. Kim, Mater. Today Chem., 2022, 26, 101080 CrossRef CAS.
- B. Wang, Y. Huang, Y. Han, S. Zhao, W. Ding, W. Zhang, R. Li, X. Wu, Q. Jiang, Y. Li, D. Gao, Y. Zhao, F. Wang, H. Jiang and R. Zhang, Cell Rep. Phys. Sci., 2023, 4, 101408 CrossRef CAS.
- W. Meng, A. J. J. Kragt, Y. Gao, E. Brembilla, X. Hu, J. S. van der Burgt, A. P. H. J. Schenning, T. Klein, G. Zhou, E. R. van den Ham, L. Tan, L. Li, J. Wang and L. Jiang, Adv. Mater., 2024, 36, 2304910 CrossRef CAS PubMed.
- A. Dhakshinamoorthy, Z. Li, S. Yang and H. Garcia, Chem. Soc. Rev., 2024, 53, 3002–3035 RSC.
- S. Li, G. Du, M. Pan, X. Wang, X. Dong, T. Huang, D. Hu, T. Ren, X. Li, H. Chen and X. Mai, Adv. Compos. Hybrid Mater., 2024, 7, 15 CrossRef CAS.
- Z. Shao, A. Huang, C. Cao, X. Ji, W. Hu, H. Luo, J. Bell, P. Jin, R. Yang and X. Cao, Nat. Sustain., 2024, 7, 796–803 CrossRef.
- F.-X. Zhao, M.-H. Wang, Z.-Y. Huang, M.-H. Zhu, C. Chen, Q.-H. Pan, B. Yu, Y.-T. Wang, X. Guo, Y.-J. Qian, L.-W. Zhang, X.-J. Qiu, S.-Z. Sheng, Z. He, J.-L. Wang and S.-H. Yu, Adv. Mater., 2024, 36, 2408192 CrossRef CAS PubMed.
- J. Zhao, S. Zhang, S. Chang, C. Li, C. Fang, X. Xia, L. Shen, J. Yang Lee, C. Cao, X. Zhang and Y. Xuan, Chem. Eng. J., 2024, 480, 148010 CrossRef CAS.
- Y. Deng, Y. Yang, Y. Xiao, X. Zeng, H.-L. Xie, R. Lan, L. Zhang and H. Yang, Adv. Mater., 2024, 36, 2401869 CrossRef CAS PubMed.
- Y. Li, P. Sun, J. Chen, X. Zha, X. Tang, Z. Chen, Y. Zhang, S. Cong, F. Geng and Z. Zhao, Adv. Mater., 2023, 35, 2300116 CrossRef CAS PubMed.
- X. Dong, Y. Shao, X. Ren, Z. Li, K. Li, Y. Tong, X. Liu and Y. Lu, Appl. Surf. Sci., 2023, 636, 157783 CrossRef CAS.
- O. Ejeromedoghene, X. Zuo, S. O. Ogungbesan, O. Oderinde, F. Yao, S. Adewuyi and G. Fu, J. Mater. Sci.: Mater. Electron., 2022, 33, 7371–7379 CrossRef CAS.
- T. V. Nguyen, H. H. Do, T. Q. Trung, Q. V. Le, T. P. Nguyen, S. H. Hong, H. W. Jang, S. H. Ahn and S. Y. Kim, J. Alloys Compd., 2021, 882, 160718 CrossRef CAS.
- Y. Wang, H. Yin, X. Zhao, Y. Qu, A. Zheng, H. Zhou, W. Fang and J. Li, Appl. Catal., B, 2024, 341, 123266 CrossRef CAS.
- Y. Zhang, W. Zheng, H. Wu, R. Zhu, Y. Wang, M. Wang, T. Ma, C. Cheng, Z. Zeng and S. Li, SusMat, 2024, 4, 106–115 CrossRef.
- K. Wang, L. Luo, C. Wang and J. Tang, Chin. J. Catal., 2023, 46, 103–112 CrossRef CAS.
- Y.-G. Lee, G. Yoo, Y.-R. Jo, H.-R. An, B.-R. Koo and G.-H. An, Adv. Energy Mater., 2023, 13, 2300630 CrossRef CAS.
- M. Zhang, K. Liu, X. Zhang, B. Wang, X. Xu, X. Du, C. Yang and K. Zhang, J. Adv. Ceram., 2022, 11, 1860–1872 CrossRef CAS.
- P. Roussel, P. Labbe and D. Groult, Acta Crystallogr., Sect. B: Struct. Sci., 2000, 56, 377–391 CrossRef PubMed.
- P. Liu, B. Wang, C. Wang, L. Ma, W. Zhang, E. Hopmann, L. Liu, A. Y. Elezzabi and H. Li, Adv. Funct. Mater., 2024, 34, 2400760 CrossRef CAS.
- W. Cheng, J. He, K. E. Dettelbach, N. J. J. Johnson, R. S. Sherbo and C. P. Berlinguette, Chem, 2018, 4, 821–832 CAS.
- S. Cong, F. Geng and Z. Zhao, Adv. Mater., 2016, 28, 10518–10528 CrossRef CAS PubMed.
- A. Al Mohammad and M. Gillet, Thin Solid Films, 2002, 408, 302–309 CrossRef CAS.
- H. Watanabe, K. Fujikata, Y. Oaki and H. Imai, Chem. Commun., 2013, 49, 8477–8479 RSC.
- Z. Li, J. Zi, X. Luan, Y. Zhong, M. Qu, Y. Wang and Z. Lian, Adv. Funct. Mater., 2023, 33, 2303069 CrossRef CAS.
- C. Hu, J. Yin, S. Xun, L. Zhu, H. Li, M. He, P. Wu, H. Li and W. Zhu, Appl. Catal., B, 2024, 355, 124155 CrossRef CAS.
- J. A. Delgado-Notario, D. López-Díaz, D. McCloskey and J. M. Caridad, Adv. Funct. Mater., 2024, 34, 2401599 CrossRef CAS.
- X. Liu, L. Yang, M. Huang, Q. Li, L. Zhao, Y. Sang, X. Zhang, Z. Zhao, H. Liu and W. Zhou, Appl. Catal., B, 2022, 319, 121887 CrossRef CAS.
- Y. Huang, K. Dai, J. Zhang and G. Dawson, Chin. J. Catal., 2022, 43, 2539–2547 CrossRef CAS.
- H. Gu, Y. Zhao, M. Tan, S. Zhang, P. Wen, H. Cheng, Y. Yan and D. Hu, Mater. Lett., 2022, 321, 132407 CrossRef CAS.
- M. Zhang, C. Yang, Z. Zhang, W. Tian, B. Hui, J. Zhang and K. Zhang, Adv. Colloid Interface Sci., 2022, 300, 102596 CrossRef CAS PubMed.
- Z. Zhang, J. Liang, K. Liu, W. Tian, X. Liang, K. Zhao and K. Zhang, ACS Sens., 2024, 9, 4196–4206 CrossRef CAS PubMed.
- Z. Zou, Z. Zhao, Z. Zhang, W. Tian, C. Yang, X. Jin and K. Zhang, Anal. Chem., 2023, 95, 2110–2118 CrossRef CAS PubMed.
- X. Li, M. Liu, J. Shao, H. Sun, Q. Zhang, D. Peng and F. Liu, Adv. Funct. Mater., 2024, 34, 2402603 CrossRef CAS.
- P. Li, Y. Wang, X. He, Y. Cui, J. Ouyang, J. Ouyang, Z. He, J. Hu, X. Liu, H. Wei, Y. Wang, X. Lu, Q. Ji, X. Cai, L. Liu, C. Hou, N. Zhou, S. Pan, X. Wang, H. Zhou, C.-W. Qiu, Y.-Q. Lu and G. Tao, Light: Sci. Appl., 2024, 13, 48 CrossRef CAS PubMed.
- T. Ma, B. Li, S. Tian, J. Qian, L. Zhou, Q. Liu, B. Liu, X. Zhao and G. Sankar, Chem. Eng. J., 2023, 468, 143587 CrossRef CAS.
- X. Dong, Y. Lu, Z. Wu, X. Liu and Y. Tong, Chem. Eng. J., 2023, 477, 147064 CrossRef CAS.
- W. Meng, A. J. J. Kragt, X. Hu, J. S. van der Burgt, A. P. H. J. Schenning, Y. Yue, G. Zhou, L. Li, P. Wei, W. Zhao, Y. Li, J. Wang and L. Jiang, Adv. Funct. Mater., 2024, 34, 2402494 CrossRef CAS.
- Ş. Neaţu, V. I. Pârvulescu, G. Epure, E. Preda, V. Şomoghi, A. Damin, S. Bordiga and A. Zecchina, Phys. Chem. Chem. Phys., 2008, 10, 6562–6570 RSC.
- J. Akoth Okwako, S. H. Song, S. Park, H. V. Tran, B. O. Aduda, S. Waita, Y.-S. Hong, S. Hong and C.-H. Han, Electrochem. Commun., 2024, 165, 107762 CrossRef CAS.
- S. Yamazaki and K. Isoyama, J. Phys. Chem. B, 2022, 126, 6520–6528 CrossRef CAS PubMed.
- X. Yan, W. Zhong, S. Qu, Z. Li and L. Shang, J. Colloid Interface Sci., 2023, 646, 855–862 CrossRef CAS PubMed.
- M. S. Kim, H. K. Lee, J. H. Yoon, H. M. Kim, Y. S. Kim and J. P. Kim, Colloids Surf., A, 2024, 694, 134083 CrossRef CAS.
- Q. Zhang, R. Wang, Y. Lu, Y. Wu, J. Yuan and J. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 4220–4229 CrossRef CAS PubMed.
- Y. Yao, Q. Zhao, W. Wei, Z. Chen, Y. Zhu, P. Zhang, Z. Zhang and Y. Gao, Nano Energy, 2020, 68, 104350 CrossRef CAS.
- B. Deng, Y. Zhu, X. Wang, J. Zhu, M. Liu, M. Liu, Y. He, C. Zhu, C. Zhang and H. Meng, Adv. Mater., 2023, 35, 2302685 CrossRef CAS PubMed.
- S. Kandpal, T. Ghosh, C. Rani, A. Chaudhary, J. Park, P. S. Lee and R. Kumar, ACS Energy Lett., 2023, 8, 1870–1886 CrossRef CAS.
- P. Lei, J. Wang, Y. Gao, C. Hu, S. Zhang, X. Tong, Z. Wang, Y. Gao and G. Cai, Nano-Micro Lett., 2023, 15, 34 CrossRef CAS PubMed.
- J. R. Platt, J. Chem. Phys., 1961, 34, 862–863 CrossRef CAS.
- S. K. Deb, Appl. Opt., 1969, 8(Suppl 1), 192–195 CrossRef PubMed.
- S. K. Deb, Philos. Mag., 1973, 27, 801–822 CrossRef CAS.
- K. Chein Sheng Ly, H. Zhang, X. Liu, D. Tang, J. Xu, X. Fu, H. Zhou and T. Fan, J. Am. Ceram. Soc., 2021, 104, 2143–2157 CrossRef CAS.
- M. Li, W. Jiang, Y. Lin, C. Huang, P. Hao, W. Wang, L. Yang, Y. Wang and D. Wang, J. Mater. Chem. C, 2024, 12, 5420–5430 RSC.
- T. Ghosh, S. Kandpal, L. Bansal, B. Sahu, D. K. Rath, C. Rani and R. Kumar, Adv. Eng. Mater., 2024, 26, 2400013 CrossRef CAS.
- L. Brändler, L. Niklaus, M. Schott and P. Löbmann, Adv. Mater. Technol., 2023, 8, 2201903 CrossRef.
- M. Chen, X. Zhang, W. Sun, Y. Xiao, H. Zhang, J. Deng, Z. Li, D. Yan, J. Zhao and Y. Li, Nano Energy, 2024, 123, 109352 CrossRef CAS.
- X. Tong, J. Wang, P. Zhang, P. Lei, Y. Gao, R. Ren, S. Zhang, R. Zhu and G. Cai, Chem. Eng. J., 2023, 470, 144130 CrossRef CAS.
- S. K. Deb, Sol. Energy Mater. Sol. Cells, 2008, 92, 245–258 CrossRef CAS.
- Y. Huang, B. Wang, F. Chen, Y. Han, W. Zhang, X. Wu, R. Li, Q. Jiang, X. Jia and R. Zhang, Adv. Opt. Mater., 2022, 10, 2101783 CrossRef CAS.
- K. Pandurangarao, N. Purnachand and V. Ravi Kumar, Opt. Mater., 2020, 101, 109791 CrossRef CAS.
- T.-S. Yang, Z.-R. Lin and M.-S. Wong, Appl. Surf. Sci., 2005, 252, 2029–2037 CrossRef CAS.
- Z. Han, M. Tong, C. Zhang, X. Guo, Y. Chen, W. Chen, H. Zhong, J. Wan, S. Cai, Y. Ma, C. Wang, S. Cong and Z. Wang, Sol. Energy Mater. Sol. Cells, 2024, 273, 112939 CrossRef CAS.
- Z. Li, Z. Liu, L. Zhao, Y. Chen, J. Li and W. Yan, J. Alloys Compd., 2023, 930, 167405 CrossRef CAS.
- N. S. Pham, L. T. Nguyen, H. T. Nguyen, V. Q. Nguyen, T. B. T. Nguyen, C. D. Tran, B. N. Nguyen and A. Q. K. Nguyen, Ceram. Int., 2023, 49, 33413–33417 CrossRef CAS.
- P. J. Morankar, R. U. Amate, A. M. Teli, S. A. Beknalkar, G. T. Chavan, N. A. Ahir and C.-W. Jeon, J. Energy Storage, 2024, 84, 110978 CrossRef.
- Q. Meng, S. Cao, J. Guo, Q. Wang, K. Wang, T. Yang, R. Zeng, J. Zhao and B. Zou, J. Energy Chem., 2023, 77, 137–143 CrossRef CAS.
- M. Hassan, A. Ghaffar, G. Lou, Z. Miao, Z. Peng and K. Celebi, Adv. Funct. Mater., 2024, 34, 2310535 CrossRef CAS.
- G. Atak, İ. Bayrak Pehlivan, J. Montero, D. Primetzhofer, C. G. Granqvist and G. A. Niklasson, Mater. Today: Proc., 2020, 33, 2434–2439 CAS.
- C. O. Avellaneda and L. O. S. Bulhões, J. Solid State Electrochem., 2003, 7, 183–186 CrossRef CAS.
- G. Gao, X. Tao, Y. He, Z. Li, J. Zhuang, L. He, Y. Li, Y. Wang, D. Sun and A. Xie, Appl. Surf. Sci., 2023, 640, 158346 CrossRef CAS.
- Z. Xue, Q. Li, M. Yu, Y. Lv, H. Sun, Y. Zhang and D. Wang, Phys. Lett. A, 2020, 384, 126822 CrossRef CAS.
- J. Domaradzki, D. Kaczmarek, D. Wojcieszak and M. Mazur, Sens. Actuators, B, 2014, 201, 420–425 CrossRef CAS.
- S. Lee, H. Cheong, P. Liu, D. Smith, C. E. Tracy, A. Mascanrenhas, J. R. Pitts and S. K. Deb, J. Appl. Phys., 2000, 88, 3076–3078 CrossRef CAS.
- F. Tavakoli Foroushani, H. Tavanai, M. Ranjbar and H. Bahrami, Sens. Actuators, B, 2018, 268, 319–327 CrossRef CAS.
- D. Liu, Z. Geng, A. Han, P. Yu, K. Zhang, H. Liu and Y. Liu, Int. J. Hydrogen Energy, 2024, 60, 20–27 CrossRef CAS.
- Nisha and P. K. Basu, IEEE Sens. J., 2023, 23, 1854–1866 CAS.
- O. Glember and H. Saurr, Z. Anorg. Chem., 1943, 252(3–4), 144–159 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |





