Open Access Article
Open Access ArticleNon-viral mRNA delivery to the lungs
Lauren
Healy†
 a,
Breanna Y.
Seto†
a,
Breanna Y.
Seto†
 a,
Haissi
Cui
a,
Haissi
Cui
 a and
Bowen
Li
a and
Bowen
Li
 *abcd
*abcd
aDepartment of Chemistry, University of Toronto, Toronto, Ontario M5S 3H6, Canada. E-mail: bw.li@utoronto.ca
bLeslie Dan Faculty of Pharmacy, University of Toronto, Toronto, Ontario M5S 3M2, Canada
cInstitute of Biomedical Engineering, University of Toronto, Toronto, Ontario M5S 3G9, Canada
dPrincess Margaret Cancer Centre, University Health Network, Toronto, Ontario M5G 2C1, Canada
First published on 23rd April 2025
Abstract
The rapid advancement of mRNA therapeutics, exemplified by COVID-19 vaccines, underscores the transformative potential of non-viral delivery systems. However, achieving efficient and targeted mRNA delivery to the lungs remains a critical challenge due to biological barriers such as pulmonary mucus, nanoparticle instability, and off-target accumulation particularly in the liver. Addressing these challenges is crucial for advancing treatments for respiratory diseases, including cystic fibrosis, primary ciliary dyskinesia, and lung cancers. This review highlights emerging strategies to enhance lung-targeted mRNA delivery, focusing on lipid nanoparticles, polymeric nanoparticles, lipid–polymer hybrids, and peptide/protein conjugates. By discussing advances in bioinspired design and nanoparticle reformulation, this review provides a roadmap for overcoming current delivery limitations and accelerating the clinical translation of lung-targeted mRNA therapies.
1. Introduction
Non-viral polymer and lipid-based delivery platforms are promising carriers for encapsulating and protecting large nucleic acid cargo like messenger RNA (mRNA), offering lower immunogenicity compared to viral vectors such as adeno-associated viruses (AAVs).1,2 Their transformative potential was exemplified by the clinical success of COVID-19 vaccines made by Pfizer-BioNTech and Moderna, which validated lipid nanoparticles (LNPs) as a safe and effective modality for mRNA delivery in humans.3–5 This breakthrough has catalyzed the development of non-viral mRNA delivery technologies for versatile applications including protein replacement therapy6 and gene editing.7–9 Despite this progress, realizing the full therapeutic potential of mRNA still requires the design of new delivery systems with high specificity to clinically relevant target tissues.Lung-targeted mRNA delivery holds significant therapeutic promise for treating pulmonary diseases including genetic disorders and cancers.10,11 Broadly, lung cells fall into three main cell types relevant for delivery: epithelial, endothelial, and immune cells.12 The optimal target cell type depends on the specific disease context. For example, selective delivery to the respiratory epithelium is essential for mRNA-based therapies for cystic fibrosis, primary ciliary dyskinesia, α-1 antitrypsin deficiency, and asthma.13,14 In contrast, targeting endothelial cells may facilitate treatment of vascular related conditions like lung cancers and pulmonary hypertension.15–17 Despite the potential of mRNA delivery using non-viral vectors, achieving safe, efficient, and precise delivery to different cell types within the lung remains a significant challenge.
Two primary routes of administration have been explored for mRNA delivery to the lungs: (1) local administration via inhalation, and (2) systemic administration via intravenous (IV) injection (Fig. 1).18 These routes differ substantially in their cellular tropism: systemic delivery predominantly targets endothelial cells, while inhalation primarily accesses epithelial cells.19–24 As such, selecting an appropriate route of administration requires alignment with the intended target cell type and an understanding of route-specific delivery barriers. Inhalation enables direct access to the airway epithelium but must overcome the pulmonary mucus barrier, which impedes nanoparticle diffusion and uptake. Mucociliary clearance further reduces nanoparticle residence time by continuously expelling foreign particles from the airways.25,26 Additionally, maintaining nanoparticle stability during aerosolization presents a significant formulation challenge.13,27,28 These are exacerbated in diseased lungs, such as in cystic fibrosis, where hyperviscous mucus severely hinders nanoparticle penetration.29,30 Conversely, systemic administration allows nanoparticles to bypass the lung's mucus barrier, but suffers from undesired off-target accumulation in the liver, which narrows the therapeutic window and raises safety concerns.31–34 Taken together, an effective mRNA delivery strategy to the lung requires careful consideration of the disease context, the target cell type, and the delivery route, alongside rational nanoparticle design to overcome route-specific and disease-specific biological barriers.
Multiple factors influence the organ-targeting ability of non-viral vectors. One key determinant is the formation of a protein corona during systemic circulation, which can alter nanoparticle biodistribution. For instance, cationic vectors tend to recruit vitronectin, promoting lung accumulation.35 In addition to modulating the protein corona, vector surfaces can be functionalized with ligands such as antibodies or peptides to achieve lung targeting through protein/receptor binding.36,37 Furthermore, the underlying chemistry and surface charge of lipid or polymer nanoparticles can be rationally engineered to modulate both organ and cellular tropism.38–40 Despite these insights, a comprehensive understanding of how these factors converge to dictate lung targeting remains incomplete, highlighting the need for further research to establish design guidelines for selective and efficient lung delivery.
Another challenge in the development of lung-targeted delivery vectors is the limited translatability between animal models and human physiology.41 Mouse models are commonly used for in vivo screening of top-performing nanoparticles, however, these models do not fully replicate human lung anatomy. For example, basal stem cells, critical targets for gene therapies in human intrapulmonary airways, are present only in the trachea of mice, not in their intrapulmonary airways.42 This anatomical discrepancy poses a barrier for therapies aiming to correct genetic diseases at the basal cell level. To address this hurdle, ferret and pig models have been increasingly used as alternative disease models for in vivo studies as their lung anatomy and cellular composition more closely resemble those of humans.43–46 These species differences highlight the importance of selecting appropriate animal models that properly mimic human lung biology to improve clinical translation.
This review discusses emerging strategies to enhance lung-targeted mRNA delivery, focusing on lipid nanoparticles (LNPs), polymeric nanoparticles (PNPs), lipid–polymer hybrids (LPHNPs), and peptide/protein conjugates (Table 1).
2. Lipid nanoparticles
Lipid nanoparticles (LNPs) are widely used as RNA delivery vectors because of their ability to effectively protect and deliver mRNA into target cells.47 Their clinical success in delivering small molecules,48 siRNA therapeutics,48 and mRNA5 has established them as a leading platform for RNA therapeutics. However, their inherent liver tropism remains a significant barrier to effective lung-targeted mRNA/LNP delivery. To highlight recent progress in this field, research efforts to develop lung-targeted mRNA/LNP systems can be categorized into two complementary approaches: modifying LNP composition and engineering ionizable lipids.2.1. Optimization of LNP formulation
The classical LNP formulation consists of four key components: ionizable lipids, which bind to negatively charged mRNA and assist in endosomal escape; helper phospholipids, which aid in cargo packing and provide structural integrity; cholesterol, which enhances nanoparticle stability and promotes membrane fusion; and PEGylated lipids, which reduce aggregation and improve circulation (Fig. 2).49,50 It is well established that varying the ratio and identity of these components can significantly influence the biodistribution and efficacy of mRNA/LNP systems.22,38 Given this tunability, researchers are actively exploring different formulation strategies to promote lung-specific delivery.A landmark study by Cheng et al. demonstrated that incorporating a fifth component, called SORT (selective organ-targeting) molecules, enabled precise organ-specific mRNA delivery to the liver, lungs, and spleen after IV administration.51,52 By increasing the molar percentage of the permanently cationic SORT lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) in LNPs, they successfully shifted mRNA expression from the liver to the lungs.51,52 The mechanism behind SORT LNP selectivity is hypothesized to be endogenous targeting, in which lung tropism is driven by the adsorption of distinct serum proteins, like vitronectin, to the LNP surface.53 This lung-optimized formulation efficiently transfected both endothelial and epithelial cells following systemic injection.51
While successful in redirecting delivery to the lungs, the addition of a fifth lipid component to LNPs further complicates what is already a complex formulation space.54 Motivated by this, researchers have determined that the direct replacement of traditional phospholipids with permanently cationic lipids in four-component LNP systems can achieve similar lung-selective delivery.23,54,55 In an effort to further simplify the classical four-component formulation, Su et al. challenge conventional LNP paradigms by demonstrating that three-component LNPs (ionizable lipid, permanently cationic lipid, PEG–lipid) can prevent typical liver tropism and instead promote pulmonary endothelial and epithelial transfection.22 Notably, their three-component LNPs outperform their cholesterol-containing five- or four-component counterparts in both efficacy and lung-selective delivery, while maintaining remarkable stability.22
Nebulization offers a non-invasive, localized route for mRNA delivery to the lungs, however, conventional LNP formulations often lack the stability to withstand this process, leading to aggregation and mRNA leakage, as seen in a recent cystic fibrosis gene therapy clinical trial.28,56,57 Recent research has tackled this challenge by optimizing LNP formulations to improve their stability for nebulized pulmonary delivery.2,28,58–60 Lokugamage et al. developed LNPs specifically designed for aerosolization, achieving exclusive and efficient mRNA delivery to the lungs following nebulized administration. Through their study, they established three key design principles for nebulized LNPs: (1) the inclusion of a PEG–lipid is essential for LNP stability, (2) nebulized mRNA lung delivery is enhanced by the presence of a cationic helper lipid and a higher molar fraction of PEG, and (3) LNPs formulated with a neutral phospholipid require less PEG than those with a cationic helper lipid. Their findings reinforce the growing consensus that cationic lipids are a key component for efficient lung delivery51 and highlight that LNP behavior is heavily formulation-dependent. In parallel, increasing PEG surface density has been shown to enhance nanoparticle diffusion through airway mucus, thus overcoming one of the primary biological barriers of local inhalation delivery.25,61,62 While pulmonary administered LNPs must remain stable throughout nebulization and mucus penetration, they must also be sufficiently labile to promote endosomal escape (Fig. 1).63 Kim et al. addressed this balance by similarly demonstrating that increasing PEG–lipid content is necessary for nebulized LNP stability.63 However, they uniquely introduced β-sitosterol as a cholesterol substitute, hypothesizing that its inclusion induces a polyhedral shape in LNPs that enhances endosomal escape.64 This modification, along with increased PEG–lipid content, successfully balanced LNP stability with efficacy, improving both mucosal diffusion and mRNA transfection following inhalation.
Alternatively, to overcome the challenges associated with nebulization, Gordon et al. introduced an inhalable dry powder formulation of mRNA-loaded LNPs using a spray-freeze-drying method with mannitol as a stabilizing excipient.65 This approach preserved mRNA integrity while producing powders with optimal aerodynamic properties for deep lung deposition. These dry powders offer greater stability and ease of administration with simple inhalers, making them a promising platform for delivering mRNA to treat lung diseases.
2.2. Engineering ionizable lipid structure
It is important to acknowledge that while incorporating permanently cationic lipids into LNP formulations enhances lung-selective mRNA expression,22,23,51,54 safety concerns severely limit their clinical translation. Positively charged LNPs, including those formulated with DOTAP, have been associated with thrombosis and pulmonary barrier disruption.66 The severe safety risks of these formulations outweigh their advantages in lung-selective delivery, highlighting the need for safer alternatives that achieve comparable results. As such, another important avenue for achieving lung-specific mRNA delivery is through the rational engineering of ionizable lipids. Among the four components of classical LNPs, ionizable lipids have garnered significant attention for their crucial role in stabilizing RNA molecules and facilitating endosomal escape, both of which directly impact transfection efficiency.67–69 Beyond these established roles, studies now highlight how the structure of ionizable lipids plays a significant role in governing the delivery of mRNA to specific organs and cell types.38,70–73Structurally, ionizable lipids typically consist of an ionizable headgroup, hydrophobic tails, and a linker connecting these components (Fig. 2).39,74 Although considerable progress has been made, the influence of ionizable lipid structure on the in vivo selectivity of LNPs remains poorly understood.38 To address this, recent work has leveraged combinatorial chemistry and high-throughput screening to systematically design and evaluate novel lipid structures optimized for lung-selective mRNA delivery.23,75,76 For example, Qiu et al. present a novel class of lung-targeting N-series LNPs, which incorporate amide bond-containing tails that enable systemic mRNA delivery to the lungs.38 By changing the ester group to an amide group in their tails, they showed that small structural changes can shift organ tropism from liver to lung targeting.
Beyond efficacy, safety remains a critical challenge in developing lung-targeted LNPs. Popoola et al. address this with their design of sulfonium lipid nanoparticles (sLNPs) as a novel alternative to DOTAP-containing LNPs, achieving highly specific lung mRNA delivery in vivo without toxicity or inflammation.24 Unlike amine-based ionizable lipids, sulfonium lipids carry a permanent positive charge, enhancing mRNA complexation and organ-specific accumulation. The best-performing sLNPs primarily transfected pulmonary endothelial cells, followed by epithelial and immune cells after systemic administration.
While many lipid engineering efforts have focused on headgroup and tail modifications,71,74–77 recent studies have uncovered the importance of linker chemistry in organ-selective mRNA delivery. Somu Naidu et al. demonstrated that modifying the linker region of ionizable lipids to contain amide and urea groups can significantly enhance chemical stability and lung-specific mRNA delivery.39 The authors proposed that this effect was driven by an increased LNP pKa, which improved intracellular trafficking and endosomal escape across multiple lung cell types. Despite progress in optimizing ionizable lipids for lung-specific mRNA delivery, discovery remains limited by narrow chemical diversity and labor-intensive synthesis and screening. AI-driven approaches, integrating deep learning with combinatorial chemistry, offer a powerful solution by accelerating lipid design and screening to rapidly uncover key structure–function relationships beyond traditional rational design. Witten et al. recently introduced LiON (lipid optimization using neural networks), an AI-driven approach that screened 1.6 million lipids in silico, identifying top candidates with potent lung-targeting properties.43 These LNPs efficiently delivered mRNA to murine lungs via nebulization and successfully transfected ferret lung epithelium, a model more relevant to human airway. Going beyond universal LNP design, Xu et al. introduced the AGILE (AI-Guided Ionizable Lipid Engineering) platform, which rapidly optimizes ionizable lipids for targeted mRNA delivery.77 Notably, AGILE revealed cell-specific preferences for ionizable lipids, reinforcing the need for tailored lipids rather than a one-size-fits-all approach. By reducing lipid development time from months or years to days, AGILE presents a promising strategy for refining mRNA delivery to specific cell types within the lung.
3. Polymeric nanoparticles
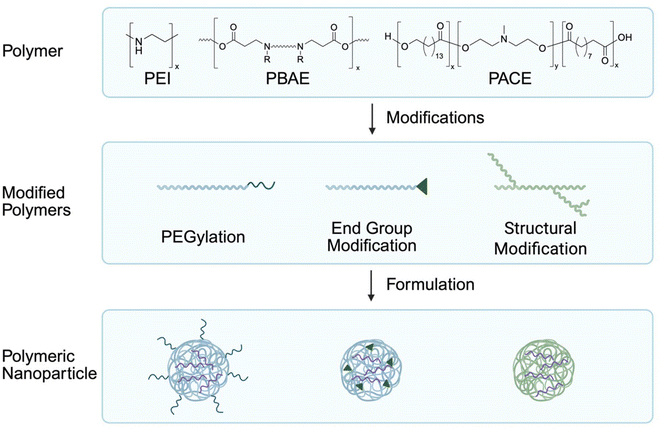
Polymeric nanoparticles (PNPs) are another type of nanoparticle that has been widely utilized for RNA delivery.78 Generally, cationic polymers are used to encapsulate negatively charged RNA through electrostatic interactions. Various kinds of polymers have been used for lung targeted mRNA delivery including polyethylenimine (PEI), poly(β-amino esters) (PBAE), and poly(amine-co-esters) (PACE).9,79–82 Traditionally, PNPs exhibit limitations due to their toxicity and low polymer stability.83,84 A few strategies can be used to increase the efficacy of PNPs such as incorporating polyethylene glycol (PEG)-grafted polymers, modifying the polymer structure, and changing end group structures (Fig. 3).82,85,86 PEGylation of PNPs can increase their stability and reduce toxicity by lowering surface charge, which minimizes interaction with blood plasma proteins during systemic administration.87 This in turn leads to longer systemic circulation time and decreased aggregation of PNPs.88 Particle stability can also be increased by incorporating branched polymer structures, which improves RNA delivery efficiency through elevating RNA release from nanoparticles.79 Additionally, end group structure modifications such as including amine end groups have notably been demonstrated to preferentially deliver PNPs to the lungs.89,90PEI is a widely used DNA transfection agent that has gained interest in RNA delivery. However, modifications to PEI are necessary to mitigate its inherent toxicity.81,84 Using PEG-grafted PEI and amine-containing terminal group PEI modifications, efficient transfection of pulmonary immune cells can be achieved by increasing the PNP stability and reducing the toxicity of PEI.9 PEI can also be functionalized with alkynoate tails, creating amino acrylate polymers that can be tuned for the delivery of mRNA in normoxic and hypoxic conditions through varying the PEG lipid molecular weight.91 Interestingly, by including adenosine triphosphate in their formulation, the authors observed higher mRNA expression in hypoxic conditions indicating the need to adjust formulations for hypoxic disease states.
PBAEs are tunable biodegradable polymers that can be structurally modified to aid in stabilizing nanoparticles for nebulized delivery.79,92 Despite the strong lung targeting ability of PBAEs and advances in improving particle stability, issues still arise during degradation due to the immunogenicity profile of these polymers.83 Hyperbranching of PBAEs (hPBAEs) has been shown to increase nanoparticle stability for nebulization and allow for lower toxicity. Nebulization of hPBAEs allowed for transfection of pulmonary epithelial cells and delivery of Cas13a mRNA for treatment of influenza and SARS-CoV-2 showing the clinical relevance of these polymers.79,93 Besides hyperbranching, poly-β-amino-thio-esters (PBTAEs) have also demonstrated efficient delivery of a Cas13a-encoding mRNA with low toxicity across animal models such as mice, hamsters, ferrets, and cows.94
PACE nanoparticles are another PNP that have been optimized for lung delivery. Using a PEG-grafting strategy and varying the PEG-conjugate percentage, Grun et al. found that the optimal PEG ratio depended on the method of administration with low PEGylation ratios showing better transfection of lung tissues after inhaled delivery.80 PACE polymers containing amine end group modifications and formulated with PEG-grafted-PACE polymers improved lung targeting and the resulting stabilization of PACE polyplexes allowed for delivery of a SARS-CoV-2 spike protein-encoding mRNA through a mucosal vaccine.95
Although various strategies have been adopted to increase PNP stability and mitigate their toxicity, no PNP–RNA therapy has undergone clinical trials or received FDA approval. This underlines the need to further optimize PNP structural modifications, change formulations to improve PNP safety profiles while maintaining potent and targeted mRNA delivery and use biodegradable polymers to mitigate toxicity seen with traditional PNPs.
4. Lipid polymer hybrid nanoparticles
Lipid polymer hybrid nanoparticles (LPHNPs) utilize helper polymers to formulate nanoparticles in a similar fashion to LNPs. Kaczmarek et al. observed increased serum stability and targeted lung transfection when utilizing a co-formulation of a PBAE terpolymer (containing alkylamine in the backbone) with PEG lipid following IV injection.96 Following this study, other PBAE formulations were tested containing a larger variety of lipids.PBAE terpolymers formulated with 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), cholesterol, and PEG lipids enabled targeted delivery of base editor and sgRNA encoded mRNA to pulmonary endothelial cells and immune cells following IV injection.19,97,98 Five-component LPHNPs have also been explored, incorporating DOTAP into the formulation with PBAE terpolymers, DOPE, cholesterol, and PEG lipid. This +DOTAP formulation promoted lung endothelium and immune cell targeting while preventing nanoparticle aggregation through repulsion.99 In addition to cationic polymers, the use of a zwitterionic polymer (ZIP) lipid hybrid nanoparticles has been reported.20 Replacing the PEG lipid with ZIP lipids and formulating LPHNPs using DOTAP, cholesterol, and a biodegradable ionizable lipid, the authors found that ZIP lipid particles retained transfection and encapsulation efficiency following nebulization and outperformed PEG formulations in delivering to pulmonary epithelial cells.
5. Peptide/protein conjugates
Cell-penetrating peptides have been utilized to enhance the targeted delivery of drugs to specific organs or cells.100 KL4, a cationic pulmonary surfactant protein B mimicking peptide has previously been reported to deliver siRNA in vitro to lung cells with minimal toxicity.101 Following this study, Qiu and colleagues created a KL4 peptide conjugated to a PEG lipid and observed efficient delivery of mRNA to deep tissues in the lungs following aerosolization of a spray-dried and spray-freeze-dried powder formulation.102 In addition to peptides, protein conjugates have also been explored to direct nanoparticles to the lungs. Using LNPs covalently conjugated with plasmalemma vesical associated protein (PV1) antibodies, lung targeting was achieved through interactions with caveolae expressing the PV1 protein in the endothelial membrane of lung blood capillaries.36 Both of these studies demonstrate that peptide/protein conjugates can facilitate the targeted delivery of mRNA to specific lung tissues.6. Outlook
Non-viral vectors have shown significant potential for delivering mRNA therapies, with LNPs achieving the greatest clinical momentum (Table 2). Several mRNA–LNP therapies targeting pulmonary diseases are now in early-phase clinical trials, including programs by Moderna/Vertex, Arcturus Therapeutics, and ReCode Therapeutics for cystic fibrosis and primary ciliary dyskinesia.103–106 These efforts reflect increasing confidence in the therapeutic potential of LNPs for lung delivery, yet also illustrate the complexity of translating promising preclinical results into durable clinical outcomes. A notable case is Translate Bio's MRT5005 program, which initially showed encouraging improvements in lung function (measured by ppFEV1) following a single dose in cystic fibrosis patients.11 However, subsequent results from the phase 1/2 trial revealed that neither escalating the single dose nor administering repeated lower doses yielded meaningful improvements in lung function. These findings underscore the importance of optimizing LNPs in diseased lung environments, particularly under conditions of chronic inflammation and thickened mucus barriers. Beyond LNPs, alternative nonviral systems such as PNPs, LPHNPs, or peptide conjugates have demonstrated preclinical promise. Yet, none have progressed to the clinical trial stage probably due to the potential toxicity, manufacturing complexity, and suboptimal delivery efficiency. The resulting translational bottleneck highlights the need for delivery platforms that are not only potent and biocompatible but also scalable and clinically robust.| Delivery vehicle | mRNA payload | Formulation (mol% if not specified) | Administration route | Organ/lung cell trophism | Ref. |
|---|---|---|---|---|---|
| LNP | AncNanoLuc, NLD1 mRNA | 7C1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTAP DOTAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C14–PEG2000 C14–PEG2000 |
Inhalation via nebulization | Pulmonary epithelial cells | 28 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
|||||
| LNP | Luc mRNA | 6Ac1–C12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTAP DOTAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMG–PEG2000 DMG–PEG2000 |
I.V. | Endothelial cells (71%) | 22 |
49.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49.5 49.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Epithelial cells (34%) | ||||
| Immune cells (15%) | |||||
| LNP | Luc, Cre, SpCas9 mRNA sgRNA | RCB-4–8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTAP DOTAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C14–PEG2000 C14–PEG2000 |
I.T. | Ciliated and club epithelial cells | 23 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|||||
| LNP | Luc, Cre mRNA | Sulfonium lipid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOPC DOPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMG–PEG2000 DMG–PEG2000 |
I.V. | Endothelial cells (67.0%) | 24 |
69.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.7 8.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.4 17.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.3 4.3 |
Epithelial cells (11.3%) | ||||
| Immune cells (3.5%) | |||||
| LNP | Luc, Cre, Cas9 hEPO, IL-10, mKL mRNA sgRNA | 5A2–SC8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOPE DOPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMG–PEG DMG–PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTAP DOTAP |
I.V. | Endothelial cells (<65%) | 51 |
11.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.9 11.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23.8 23.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
Epithelial cells (<40%) | ||||
| Immune cells (<21%) | |||||
| LNP | NLuc, Cre, CTFR mRNA | DLin–MC3–DMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DSPC DSPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β-sitosterol β-sitosterol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMG–PEG2000 DMG–PEG2000 |
Inhalation via nebulization | Epithelial cells | 63 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38.5 38.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
|||||
| LNP | Luc mRNA | cKK–E12/C12–200/DLin–MC3–DMA/SM102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTAP DOTAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholesterol cholesterol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C14–PEG2000 C14–PEG2000 |
Inhalation via dry powder | Pulmonary epithelial cells | 65 |
29.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.4 29.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38.2 38.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
|||||
| LNP | Luc, Cre, Tsc2 mRNA | 306–N16B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOPC DOPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMG–PEG2000 DMG–PEG2000 |
I.V. | Endothelial cells (69.6%) | 38 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38.5 38.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Macrophages (18.9%) | ||||
| Epithelium cells (7.3%) | |||||
| LNP | Luc, Cre, mmPE mRNA | Lipid 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DSPC DSPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG–DMG PEG–DMG |
I.V. | Endothelial cells (>60%) | 39 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.5 48.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Epithelial cells (∼40%) | ||||
| LNP | Luc, Cre mRNA | FO-32/FO-35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTAP DOTAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C14–PEG2000 C14–PEG2000 |
Nebulized I.N. | Pulmonary epithelial cells | 43 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|||||
| PNP | Luc, Cre mRNA | PEG-grafted-PEI, 0.5% PEG | I.V. | Immune cells (7.2%) | 9 |
| PNP | FLuc mRNA | Amino acrylate polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG PEG |
I.V. | Lung (>80%) | 91 |
99.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
|||||
| PNP | FLuc, Cre mRNA | hDD90–118 | Inhalation via nebulization | Epithelial cells (24.6%) | 79 |
| Endothelial cells (4.4%) | |||||
| Immune cells (0.4%) | |||||
| PNP | Cas13a, NLuc mRNA | hDD90–118 | Inhalation via nebulization | Lung | 93 |
| PNP | Cas13a, NLuc mRNA | P76 | Inhalation via nebulization | Lung | 94 |
| PNP | Cre mRNA | PBAE–E63 | I.N., I.V. | IN: Epithelial cells (8%) | 110 |
| Endothelial cells (<1%) | |||||
| Bronchial cells (6%) | |||||
| Alveolar cells (8%) | |||||
| IV: Epithelial cells (9–28%) | |||||
| Endothelial cells (4–17%) | |||||
| Bronchial cells (8–22%) | |||||
| Alveolar cells (2–19%) | |||||
| PNP | FLuc mRNA | PACE-grafted-PEG (5%) | I.T., I.V. | Lung | 80 |
| PNP | FLuc, Cre, SARS-CoV-2 spike protein mRNA | PACE–E14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG–PACE PEG–PACE |
I.N., I.T. | Epithelial cells (21.5%) | 95 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Lung leukocytes (19.6%) | ||||
| LPHNP | FLuc, Cre mRNA | DD90–C12–103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C14–PEG2000 C14–PEG2000 |
I.V. | Endothelial cells | 19 |
93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (w/w) 7 (w/w) |
|||||
| LPHNP | FLuc mRNA | DD90–C12–122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C14–PEG2000 C14–PEG2000 |
I.V. | Lung | 96 |
93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
|||||
| LPHNP | FLuc, Cre mRNA | A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOPE DOPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C14–PEG2000 C14–PEG2000 |
I.V. | Endothelial cells (75%) | 97 |
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Immune cells (2%) | ||||
| LPHNP | FLuc, Cre mRNA | (DOTAP + PBAE)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOPE DOPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMG–PEG DMG–PEG |
I.V. | Pulmonary epithelial cells (>3%) | 99 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38.5 38.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Lung endothelial cells (>20%) | ||||
| Immune cells (>5%) | |||||
| LPHNP | FLuc mRNA | C14–C14 ZIP–lipid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IR-117-17 IR-117-17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOTAP DOTAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Chol Chol |
Inhalation via nebulization | Endothelial cells | 20 |
2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45.6 45.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42.6 42.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.2 9.2 |
|||||
| Peptide conjugate | Fluc mRNA | mRNA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG12KL4 peptide/mRNA PEG12KL4 peptide/mRNA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mannitol mannitol |
I.T. | Epithelial cells | 102 |
0.1/0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/5 1/5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98.9/94.5 98.9/94.5 |
|||||
| LNP | FLuc mRNA | Dlin–MC3–DMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DSPC, Chol DSPC, Chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMG–PEG DMG–PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DSPE–PEG DSPE–PEG |
I.V. | Endothelial cells | 36 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.75 1.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
Looking ahead, the rational design of next-generation delivery systems will be critical to overcoming the distinct biophysical barriers of the lung. This includes fine-tuning lipid and polymer chemistry to enhance mucus penetration, minimize immunogenicity, improve intracellular trafficking, and achieve cell-type-specific targeting. Advances in formulation chemistry, lipid engineering, and nanoparticle surface modification are beginning to address these challenges. Integration of AI-guided lipid discovery77,107,108 and human-relevant models such as patient-derived organoids may further accelerate the development of non-viral vectors by providing predictive insights into delivery efficiency and tissue specificity.109
7. Conclusion
Lung-targeted mRNA delivery has the potential to revolutionize the treatment of lung diseases. Among non-viral platforms, LNPs have demonstrated encouraging results in both preclinical studies and early-stage clinical trials. However, key challenges persist, including limited delivery efficiency and formulation instability during aerosolization. Unlocking the full therapeutic potential of these systems will require more understanding of nanoparticle interactions within the lung microenvironment and the capacity to tailor delivery strategies to specific cellular and physiological contexts. Achieving this will require interdisciplinary efforts across biology, chemistry, and engineering to develop safe effective and scalable non-viral vectors for pulmonary mRNA therapeutics.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the funding support from GSK Chair Professorship, the Leslie Dan Faculty of Pharmacy startup fund, the Princess Margaret Cancer Center operating fund, Natural Sciences and Engineering Research Council of Canada (No. RGPIN-2023-05124), the Canada Research Chairs Program (No. CRC-2022-00575), National Institute of Health (1R01HL174773), Canadian Institutes of Health Research (No. PJH-185722, PJT-190109, PJT-192011, PJT-195669), Cystic Fibrosis Foundation (LI23G0, LI23I0), Cystic Fibrosis Canada (1188219), New Frontiers in Research Fund – Exploration (NFRFE-2023-00203), and the Canada Foundation for Innovation—John R. Evans Leaders Fund (No. 43711).References
- H. Zhang, T. F. Bahamondez-Canas, Y. Zhang, J. Leal and H. D. C. Smyth, Mol. Pharm., 2018, 15, 4814–4826 CrossRef CAS PubMed.
- H. Zhang, J. Leal, M. R. Soto, H. D. C. Smyth and D. Ghosh, Pharmaceutics, 2020, 12, 1042 CrossRef CAS PubMed.
- A. J. Barbier, A. Y. Jiang, P. Zhang, R. Wooster and D. G. Anderson, Nat. Biotechnol., 2022, 40, 840–854 CrossRef CAS PubMed.
- M. Papi, D. Pozzi, V. Palmieri and G. Caracciolo, Nano Today, 2022, 43, 101403 CrossRef CAS PubMed.
- L. R. Baden, H. M. El Sahly, B. Essink, K. Kotloff, S. Frey, R. Novak, D. Diemert, S. A. Spector, N. Rouphael, C. B. Creech, J. McGettigan, S. Khetan, N. Segall, J. Solis, A. Brosz, C. Fierro, H. Schwartz, K. Neuzil, L. Corey, P. Gilbert, H. Janes, D. Follmann, M. Marovich, J. Mascola, L. Polakowski, J. Ledgerwood, B. S. Graham, H. Bennett, R. Pajon, C. Knightly, B. Leav, W. Deng, H. Zhou, S. Han, M. Ivarsson, J. Miller, T. Zaks and COVE Study Group, N. Engl. J. Med., 2021, 384, 403–416 CrossRef CAS PubMed.
- M. L. Cacicedo, C. Weinl-Tenbruck, D. Frank, M. J. Limeres, S. Wirsching, K. Hilbert, M. A. Pasha Famian, N. Horscroft, J. B. Hennermann, F. Zepp, F. Chevessier-Tünnesen and S. Gehring, Front. Bioeng. Biotechnol., 2022, 10, 993298 CrossRef PubMed.
- A. Mohammadian Farsani, N. Mokhtari, S. Nooraei, H. Bahrulolum, A. Akbari, Z. M. Farsani, S. Khatami, M. sadat Ebadi and G. Ahmadian, Heliyon, 2024, 10, e24606 CrossRef CAS PubMed.
- A. Solomon, N. Engl. J. Med., 2021, 385, 1721–1722 CrossRef CAS PubMed.
- X. Ke, L. Shelton, Y. Hu, Y. Zhu, E. Chow, H. Tang, J. L. Santos and H.-Q. Mao, ACS Appl. Mater. Interfaces, 2020, 12, 35835–35844 CrossRef CAS PubMed.
- J. Spicer, N. Blais, S. Owen, A. G. Robinson, Q. Chu, C. Labbe, B. Shieh, P. Brown-Walker, J. Sederias, K. Jensen, A. F. Farago, M.-S. Tsao, T. R. Cottrell, B. Kidane, S. Laurie, R. Juergens, P. A. Bradbury, W. Tu and P.-O. Gaudreau, Clin. Lung Cancer, 2025, 26, 146–151 CrossRef CAS PubMed.
- S. M. Rowe, J. B. Zuckerman, D. Dorgan, J. Lascano, K. McCoy, M. Jain, M. S. Schechter, S. Lommatzsch, V. Indihar, N. Lechtzin, K. McBennett, C. Callison, C. Brown, T. G. Liou, K. D. MacDonald, S. Z. Nasr, S. Bodie, M. Vaughn, E. B. Meltzer and A. J. Barbier, J. Cystic Fibrosis, 2023, 22, 656–664 CrossRef CAS PubMed.
- N. Turuvekere Vittala Murthy, K. Vlasova, J. Renner, A. Jozic and G. Sahay, Adv. Drug Delivery Rev., 2024, 209, 115305 CrossRef CAS PubMed.
- A. Y. Jiang, J. Witten, I. O. Raji, F. Eweje, C. MacIsaac, S. Meng, F. A. Oladimeji, Y. Hu, R. S. Manan, R. Langer and D. G. Anderson, Nat. Nanotechnol., 2024, 19, 364–375 CrossRef CAS PubMed.
- T. Wan and Y. Ping, Adv. Drug Delivery Rev., 2021, 168, 196–216 CrossRef CAS PubMed.
- J. E. Dahlman, C. Barnes, O. Khan, A. Thiriot, S. Jhunjunwala, T. E. Shaw, Y. Xing, H. B. Sager, G. Sahay, L. Speciner, A. Bader, R. L. Bogorad, H. Yin, T. Racie, Y. Dong, S. Jiang, D. Seedorf, A. Dave, K. S. Sandu, M. J. Webber, T. Novobrantseva, V. M. Ruda, A. K. R. Lytton-Jean, C. G. Levins, B. Kalish, D. K. Mudge, M. Perez, L. Abezgauz, P. Dutta, L. Smith, K. Charisse, M. W. Kieran, K. Fitzgerald, M. Nahrendorf, D. Danino, R. M. Tuder, U. H. von Andrian, A. Akinc, A. Schroeder, D. Panigrahy, V. Kotelianski, R. Langer and D. G. Anderson, Nat. Nanotechnol., 2014, 9, 648–655 CrossRef CAS PubMed.
- L. Chi, M.-H. Na, H.-K. Jung, S. M. P. Vadevoo, C.-W. Kim, G. Padmanaban, T.-I. Park, J.-Y. Park, I. Hwang, K. U. Park, F. Liang, M. Lu, J. Park, I.-S. Kim and B.-H. Lee, J. Controlled Release, 2015, 209, 327–336 CrossRef CAS PubMed.
- C. E. Evans, N. D. Cober, Z. Dai, D. J. Stewart and Y.-Y. Zhao, Eur. Respir. J., 2021, 58, 2003957 CrossRef CAS PubMed.
- M. Zadory, E. Lopez, S. Babity, S.-P. Gravel and D. Brambilla, Biomater. Sci., 2022, 10, 6077–6115 RSC.
- J. C. Kaczmarek, A. K. Patel, L. H. Rhym, U. C. Palmiero, B. Bhat, M. W. Heartlein, F. DeRosa and D. G. Anderson, Biomaterials, 2021, 275, 120966 CrossRef CAS PubMed.
- A. Y. Jiang, S. Lathwal, S. Meng, J. Witten, E. Beyer, P. McMullen, Y. Hu, R. S. Manan, I. Raji, R. Langer and D. G. Anderson, J. Am. Chem. Soc., 2024, 146, 32567–32574 CrossRef CAS PubMed.
- M. Yuan, Z. Han, Y. Liang, Y. Sun, B. He, W. Chen and F. Li, Biomater. Res., 2023, 27, 90 CrossRef CAS PubMed.
- K. Su, L. Shi, T. Sheng, X. Yan, L. Lin, C. Meng, S. Wu, Y. Chen, Y. Zhang, C. Wang, Z. Wang, J. Qiu, J. Zhao, T. Xu, Y. Ping, Z. Gu and S. Liu, Nat. Commun., 2024, 15, 5659 CrossRef CAS PubMed.
- B. Li, R. S. Manan, S.-Q. Liang, A. Gordon, A. Jiang, A. Varley, G. Gao, R. Langer, W. Xue and D. Anderson, Nat. Biotechnol., 2023, 41, 1410–1415 CrossRef CAS PubMed.
- D. O. Popoola, Z. Cao, Y. Men, X. Li, M. Viapiano, S. Wilkens, J. Luo, Y. Teng, Q. Meng and Y. Li, Nano Lett., 2024, 24, 8080–8088 CrossRef CAS PubMed.
- J. T. Huckaby and S. K. Lai, Adv. Drug Delivery Rev., 2018, 124, 125–139 CrossRef CAS PubMed.
- S. P. Newman, Ther. Delivery, 2017, 8, 647–661 CrossRef CAS PubMed.
- A. Gordon, B. Li, J. Witten, H. Nguyen and D. G. Anderson, Adv. Healthcare Mater., 2024, 13, 2400509 CrossRef CAS PubMed.
- M. P. Lokugamage, D. Vanover, J. Beyersdorf, M. Z. C. Hatit, L. Rotolo, E. S. Echeverri, H. E. Peck, H. Ni, J.-K. Yoon, Y. Kim, P. J. Santangelo and J. E. Dahlman, Nat. Biomed. Eng., 2021, 5, 1059–1068 CrossRef CAS PubMed.
- N. Turuvekere Vittala Murthy, K. Vlasova, J. Renner, A. Jozic and G. Sahay, Adv. Drug Delivery Rev., 2024, 209, 115305 CrossRef CAS PubMed.
- B. Garcia and P. A. Flume, Semin. Respir. Crit. Care Med., 2019, 40, 804–809 CrossRef PubMed.
- K. A. Hajj, J. R. Melamed, N. Chaudhary, N. G. Lamson, R. L. Ball, S. S. Yerneni and K. A. Whitehead, Nano Lett., 2020, 20, 5167–5175 CrossRef CAS PubMed.
- D. M. S. Petersen, R. M. Weiss, K. A. Hajj, S. S. Yerneni, N. Chaudhary, A. N. Newby, M. L. Arral and K. A. Whitehead, Adv. Healthcare Mater., 2024, 13, e2400225 CrossRef PubMed.
- Z. Deng, G. T. Kalin, D. Shi and V. V. Kalinichenko, Am. J. Respir. Cell Mol. Biol., 2021, 64, 292–307 CrossRef CAS PubMed.
- R. N. Kularatne, R. M. Crist and S. T. Stern, Pharmaceuticals, 2022, 15, 897 CrossRef CAS PubMed.
- D. Chen, N. Parayath, S. Ganesh, W. Wang and M. Amiji, Nanoscale, 2019, 11, 18806–18824 RSC.
- Q. Li, C. Chan, N. Peterson, R. N. Hanna, A. Alfaro, K. L. Allen, H. Wu, W. F. Dall'Acqua, M. J. Borrok and J. L. Santos, ACS Chem. Biol., 2020, 15, 830–836 CrossRef CAS PubMed.
- M. Herrera-Barrera, R. C. Ryals, M. Gautam, A. Jozic, M. Landry, T. Korzun, M. Gupta, C. Acosta, J. Stoddard, R. Reynaga, W. Tschetter, N. Jacomino, O. Taratula, C. Sun, A. K. Lauer, M. Neuringer and G. Sahay, Sci. Adv., 2023, 9, eadd4623 CrossRef PubMed.
- M. Qiu, Y. Tang, J. Chen, R. Muriph, Z. Ye, C. Huang, J. Evans, E. P. Henske and Q. Xu, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2116271119 CrossRef CAS PubMed.
- G. Somu Naidu, R. Rampado, P. Sharma, A. Ezra, G. R. Kundoor, D. Breier and D. Peer, ACS Nano, 2025, 19, 6571–6587 CrossRef CAS PubMed.
- A. C. Kauffman, A. S. Piotrowski-Daspit, K. H. Nakazawa, Y. Jiang, A. Datye and W. M. Saltzman, Biomacromolecules, 2018, 19, 3861–3873 CrossRef CAS PubMed.
- S. Tenzer, D. Docter, J. Kuharev, A. Musyanovych, V. Fetz, R. Hecht, F. Schlenk, D. Fischer, K. Kiouptsi, C. Reinhardt, K. Landfester, H. Schild, M. Maskos, S. K. Knauer and R. H. Stauber, Nat. Nanotechnol., 2013, 8, 772–781 CrossRef CAS PubMed.
- M. C. Basil, J. Katzen, A. E. Engler, M. Guo, M. J. Herriges, J. J. Kathiriya, R. Windmueller, A. B. Ysasi, W. J. Zacharias, H. A. Chapman, D. N. Kotton, J. R. Rock, H.-W. Snoeck, G. Vunjak-Novakovic, J. A. Whitsett and E. E. Morrisey, Cell Stem Cell, 2020, 26, 482–502 CrossRef CAS PubMed.
- J. Witten, I. Raji, R. S. Manan, E. Beyer, S. Bartlett, Y. Tang, M. Ebadi, J. Lei, D. Nguyen, F. Oladimeji, A. Y. Jiang, E. MacDonald, Y. Hu, H. Mughal, A. Self, E. Collins, Z. Yan, J. F. Engelhardt, R. Langer and D. G. Anderson, Nat. Biotechnol., 2024, 1–10 Search PubMed.
- C. S. Rogers, D. A. Stoltz, D. K. Meyerholz, L. S. Ostedgaard, T. Rokhlina, P. J. Taft, M. P. Rogan, A. A. Pezzulo, P. H. Karp, O. A. Itani, A. C. Kabel, C. L. Wohlford-Lenane, G. J. Davis, R. A. Hanfland, T. L. Smith, M. Samuel, D. Wax, C. N. Murphy, A. Rieke, K. Whitworth, A. Uc, T. D. Starner, K. A. Brogden, J. Shilyansky, P. B. McCray, J. Zabner, R. S. Prather and M. J. Welsh, Science, 2008, 321, 1837–1841 CrossRef CAS PubMed.
- B. H. Rosen, M. Chanson, L. R. Gawenis, J. Liu, A. Sofoluwe, A. Zoso and J. F. Engelhardt, J. Cystic Fibrosis, 2018, 17, S28–S34 CrossRef CAS PubMed.
- K. A. Ryan, K. R. Bewley, S. A. Fotheringham, G. S. Slack, P. Brown, Y. Hall, N. I. Wand, A. C. Marriott, B. E. Cavell, J. A. Tree, L. Allen, M. J. Aram, T. J. Bean, E. Brunt, K. R. Buttigieg, D. P. Carter, R. Cobb, N. S. Coombes, S. J. Findlay-Wilson, K. J. Godwin, K. E. Gooch, J. Gouriet, R. Halkerston, D. J. Harris, T. H. Hender, H. E. Humphries, L. Hunter, C. M. K. Ho, C. L. Kennard, S. Leung, S. Longet, D. Ngabo, K. L. Osman, J. Paterson, E. J. Penn, S. T. Pullan, E. Rayner, O. Skinner, K. Steeds, I. Taylor, T. Tipton, S. Thomas, C. Turner, R. J. Watson, N. R. Wiblin, S. Charlton, B. Hallis, J. A. Hiscox, S. Funnell, M. J. Dennis, C. J. Whittaker, M. G. Catton, J. Druce, F. J. Salguero and M. W. Carroll, Nat. Commun., 2021, 12, 81 CrossRef CAS PubMed.
- X. Hou, T. Zaks, R. Langer and Y. Dong, Nat. Rev. Mater., 2021, 6, 1078–1094 CrossRef CAS PubMed.
- A. Akinc, M. A. Maier, M. Manoharan, K. Fitzgerald, M. Jayaraman, S. Barros, S. Ansell, X. Du, M. J. Hope, T. D. Madden, B. L. Mui, S. C. Semple, Y. K. Tam, M. Ciufolini, D. Witzigmann, J. A. Kulkarni, R. van der Meel and P. R. Cullis, Nat. Nanotechnol., 2019, 14, 1084–1087 CrossRef CAS PubMed.
- C. Hald Albertsen, J. A. Kulkarni, D. Witzigmann, M. Lind, K. Petersson and J. B. Simonsen, Adv. Drug Delivery Rev., 2022, 188, 114416 CrossRef CAS PubMed.
- J. Wang, Y. Ding, K. Chong, M. Cui, Z. Cao, C. Tang, Z. Tian, Y. Hu, Y. Zhao and S. Jiang, Vaccines, 2024, 12, 1148 CrossRef CAS PubMed.
- Q. Cheng, T. Wei, L. Farbiak, L. T. Johnson, S. A. Dilliard and D. J. Siegwart, Nat. Nanotechnol., 2020, 15, 313–320 CrossRef CAS PubMed.
- X. Wang, S. Liu, Y. Sun, X. Yu, S. M. Lee, Q. Cheng, T. Wei, J. Gong, J. Robinson, D. Zhang, X. Lian, P. Basak and D. J. Siegwart, Nat. Protoc., 2023, 18, 265–291 CrossRef CAS PubMed.
- S. A. Dilliard, Q. Cheng and D. J. Siegwart, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2109256118 CrossRef CAS PubMed.
- S. T. LoPresti, M. L. Arral, N. Chaudhary and K. A. Whitehead, J. Controlled Release, 2022, 345, 819–831 CrossRef CAS PubMed.
- K. J. Kauffman, M. A. Oberli, J. R. Dorkin, J. E. Hurtado, J. C. Kaczmarek, S. Bhadani, J. Wyckoff, R. Langer, A. Jaklenec and D. G. Anderson, Mol. Ther.–Nucleic Acids, 2018, 10, 55–63 CrossRef CAS PubMed.
- S. Liu, Y. Wen, X. Shan, X. Ma, C. Yang, X. Cheng, Y. Zhao, J. Li, S. Mi, H. Huo, W. Li, Z. Jiang, Y. Li, J. Lin, L. Miao and X. Lu, Nat. Commun., 2024, 15, 9471 CrossRef CAS PubMed.
- E. W. F. W. Alton, D. K. Armstrong, D. Ashby, K. J. Bayfield, D. Bilton, E. V. Bloomfield, A. C. Boyd, J. Brand, R. Buchan, R. Calcedo, P. Carvelli, M. Chan, S. H. Cheng, D. D. S. Collie, S. Cunningham, H. E. Davidson, G. Davies, J. C. Davies, L. A. Davies, M. H. Dewar, A. Doherty, J. Donovan, N. S. Dwyer, H. I. Elgmati, R. F. Featherstone, J. Gavino, S. Gea-Sorli, D. M. Geddes, J. S. R. Gibson, D. R. Gill, A. P. Greening, U. Griesenbach, D. M. Hansell, K. Harman, T. E. Higgins, S. L. Hodges, S. C. Hyde, L. Hyndman, J. A. Innes, J. Jacob, N. Jones, B. F. Keogh, M. P. Limberis, P. Lloyd-Evans, A. W. Maclean, M. C. Manvell, D. McCormick, M. McGovern, G. McLachlan, C. Meng, M. A. Montero, H. Milligan, L. J. Moyce, G. D. Murray, A. G. Nicholson, T. Osadolor, J. Parra-Leiton, D. J. Porteous, I. A. Pringle, E. K. Punch, K. M. Pytel, A. L. Quittner, G. Rivellini, C. J. Saunders, R. K. Scheule, S. Sheard, N. J. Simmonds, K. Smith, S. N. Smith, N. Soussi, S. Soussi, E. J. Spearing, B. J. Stevenson, S. G. Sumner-Jones, M. Turkkila, R. P. Ureta, M. D. Waller, M. Y. Wasowicz, J. M. Wilson and P. Wolstenholme-Hogg, Lancet Respir. Med., 2015, 3, 684–691 CrossRef CAS PubMed.
- J. Kim, A. Jozic and G. Sahay, Cell. Mol. Bioeng., 2020, 13, 463–474 CrossRef CAS PubMed.
- J. Kim, A. Jozic, Y. Lin, Y. Eygeris, E. Bloom, X. Tan, C. Acosta, K. D. MacDonald, K. D. Welsher and G. Sahay, ACS Nano, 2022, 16, 14792–14806 CrossRef CAS PubMed.
- A. Y. Jiang, J. Witten, I. O. Raji, F. Eweje, C. MacIsaac, S. Meng, F. A. Oladimeji, Y. Hu, R. S. Manan, R. Langer and D. G. Anderson, Nat. Nanotechnol., 2024, 19, 364–375 CrossRef CAS PubMed.
- B. Tafech, M.-R. Rokhforouz, J. Leung, M. M. Sung, P. J. Lin, D. D. Sin, D. Lauster, S. Block, B. S. Quon, Y. Tam, P. Cullis, J. J. Feng and S. Hedtrich, Adv. Healthcare Mater., 2024, 13, 2304525 CrossRef CAS PubMed.
- B. S. Schuster, J. S. Suk, G. F. Woodworth and J. Hanes, Biomaterials, 2013, 34, 3439–3446 CrossRef CAS PubMed.
- J. Kim, A. Jozic, Y. Lin, Y. Eygeris, E. Bloom, X. Tan, C. Acosta, K. D. MacDonald, K. D. Welsher and G. Sahay, ACS Nano, 2022, 16, 14792–14806 CrossRef CAS PubMed.
- Y. Eygeris, S. Patel, A. Jozic and G. Sahay, Nano Lett., 2020, 20, 4543–4549 CrossRef CAS PubMed.
- A. Gordon, B. Li, J. Witten, H. Nguyen and D. G. Anderson, Adv. Healthcare Mater., 2024, 13, 2400509 CrossRef CAS PubMed.
- S. Omo-Lamai, M. E. Zamora, M. N. Patel, J. Wu, J. Nong, Z. Wang, A. Peshkova, A. Majumder, J. R. Melamed, L. S. Chase, E.-O. Essien, D. Weissman, V. R. Muzykantov, O. A. Marcos-Contreras, J. W. Myerson and J. S. Brenner, Adv. Mater., 2024, 36, 2312026 CrossRef CAS PubMed.
- Y. Zhang, C. Sun, C. Wang, K. E. Jankovic and Y. Dong, Chem. Rev., 2021, 121, 12181–12277 CrossRef CAS PubMed.
- Y. Xu, F. Gong, A. Golubovic, A. Strilchuk, J. Chen, M. Zhou, S. Dong, B. Seto and B. Li, Proc. Natl. Acad. Sci. U. S. A., 2025, 122, e2409572122 CrossRef CAS PubMed.
- E. Samaridou, J. Heyes and P. Lutwyche, Adv. Drug Delivery Rev., 2020, 154–155, 37–63 CrossRef CAS PubMed.
- H. Ni, M. Z. C. Hatit, K. Zhao, D. Loughrey, M. P. Lokugamage, H. E. Peck, A. D. Cid, A. Muralidharan, Y. Kim, P. J. Santangelo and J. E. Dahlman, Nat. Commun., 2022, 13, 4766 CrossRef CAS PubMed.
- G. S. Naidu, S.-B. Yong, S. Ramishetti, R. Rampado, P. Sharma, A. Ezra, M. Goldsmith, I. Hazan-Halevy, S. Chatterjee, A. Aitha and D. Peer, Adv. Sci., 2023, 10, e2301929 CrossRef.
- H. Liao, J. Liao, L. Zeng, X. Cao, H. Fan and J. Chen, Wiley Interdiscip. Rev.:Nanomed. Nanobiotechnol., 2024, 16, e2004 CAS.
- K. Hashiba, M. Taguchi, S. Sakamoto, A. Otsu, Y. Maeda, Y. Suzuki, H. Ebe, A. Okazaki, H. Harashima and Y. Sato, Nano Lett., 2024, 24, 12758–12767 CAS.
- Y. Xu, A. Golubovic, S. Xu, A. Pan and B. Li, J. Mater. Chem. B, 2023, 11, 6527–6539 RSC.
- L. Xue, A. G. Hamilton, G. Zhao, Z. Xiao, R. El-Mayta, X. Han, N. Gong, X. Xiong, J. Xu, C. G. Figueroa-Espada, S. J. Shepherd, A. J. Mukalel, M.-G. Alameh, J. Cui, K. Wang, A. E. Vaughan, D. Weissman and M. J. Mitchell, Nat. Commun., 2024, 15, 1884 CrossRef CAS PubMed.
- J. Li, J. Hu, D. Jin, H. Huo, N. Chen, J. Lin and X. Lu, J. Nanobiotechnol., 2024, 22, 672 CrossRef CAS PubMed.
- Y. Xu, S. Ma, H. Cui, J. Chen, S. Xu, F. Gong, A. Golubovic, M. Zhou, K. C. Wang, A. Varley, R. X. Z. Lu, B. Wang and B. Li, Nat. Commun., 2024, 15, 6305 CrossRef CAS PubMed.
- M. A. Beach, U. Nayanathara, Y. Gao, C. Zhang, Y. Xiong, Y. Wang and G. K. Such, Chem. Rev., 2024, 124, 5505–5616 CrossRef CAS PubMed.
- A. K. Patel, J. C. Kaczmarek, S. Bose, K. J. Kauffman, F. Mir, M. W. Heartlein, F. DeRosa, R. Langer and D. G. Anderson, Adv. Mater., 2019, 31, 1805116 CrossRef PubMed.
- M. K. Grun, A. Suberi, K. Shin, T. Lee, V. Gomerdinger, Z. M. Moscato, A. S. Piotrowski-Daspit and W. M. Saltzman, Biomaterials, 2021, 272, 120780 CrossRef CAS PubMed.
- O. Boussif, F. Lezoualc'h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix and J. P. Behr, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 7297–7301 CrossRef CAS PubMed.
- D. G. Anderson, A. Akinc, N. Hossain and R. Langer, Mol. Ther., 2005, 11, 426–434 CrossRef CAS PubMed.
- J. I. Andorko, K. L. Hess, K. G. Pineault and C. M. Jewell, Acta Biomater., 2016, 32, 24–34 CrossRef CAS PubMed.
- J. Casper, S. H. Schenk, E. Parhizkar, P. Detampel, A. Dehshahri and J. Huwyler, J. Controlled Release, 2023, 362, 667–691 CrossRef CAS PubMed.
- A. Vila, H. Gill, O. McCallion and M. J. Alonso, J. Controlled Release, 2004, 98, 231–244 CrossRef CAS PubMed.
- Y. Jiang, Q. Lu, Y. Wang, E. Xu, A. Ho, P. Singh, Y. Wang, Z. Jiang, F. Yang, G. T. Tietjen, P. Cresswell and W. M. Saltzman, Nano Lett., 2020, 20, 1117–1123 CrossRef CAS PubMed.
- M. Ogris, S. Brunner, S. Schüller, R. Kircheis and E. Wagner, Gene Ther., 1999, 6, 595–605 CrossRef CAS PubMed.
- J. S. Suk, Q. Xu, N. Kim, J. Hanes and L. M. Ensign, Adv. Drug Delivery Rev., 2016, 99, 28–51 CrossRef CAS PubMed.
- P. S. Kowalski, U. Capasso Palmiero, Y. Huang, A. Rudra, R. Langer and D. G. Anderson, Adv. Mater., 2018, 30, 1801151 CrossRef PubMed.
- G. T. Zugates, W. Peng, A. Zumbuehl, S. Jhunjhunwala, Y.-H. Huang, R. Langer, J. A. Sawicki and D. G. Anderson, Mol. Ther., 2007, 15, 1306–1312 CrossRef CAS PubMed.
- P. B. Tiwade, Y. Ma, R. VanKeulen-Miller and O. S. Fenton, J. Am. Chem. Soc., 2024, 146, 17365–17376 CrossRef CAS PubMed.
- A. A. Eltoukhy, D. Chen, C. A. Alabi, R. Langer and D. G. Anderson, Adv. Mater., 2013, 25, 1487–1493 CrossRef CAS PubMed.
- E. L. Blanchard, D. Vanover, S. S. Bawage, P. M. Tiwari, L. Rotolo, J. Beyersdorf, H. E. Peck, N. C. Bruno, R. Hincapie, F. Michel, J. Murray, H. Sadhwani, B. Vanderheyden, M. G. Finn, M. A. Brinton, E. R. Lafontaine, R. J. Hogan, C. Zurla and P. J. Santangelo, Nat. Biotechnol., 2021, 39, 717–726 CrossRef CAS PubMed.
- L. Rotolo, D. Vanover, N. C. Bruno, H. E. Peck, C. Zurla, J. Murray, R. K. Noel, L. O'Farrell, M. Araínga, N. Orr-Burks, J. Y. Joo, L. C. S. Chaves, Y. Jung, J. Beyersdorf, S. Gumber, R. Guerrero-Ferreira, S. Cornejo, M. Thoresen, A. K. Olivier, K. M. Kuo, J. C. Gumbart, A. R. Woolums, F. Villinger, E. R. Lafontaine, R. J. Hogan, M. G. Finn and P. J. Santangelo, Nat. Mater., 2022, 22, 369–379 CrossRef PubMed.
- A. Suberi, M. K. Grun, T. Mao, B. Israelow, M. Reschke, J. Grundler, L. Akhtar, T. Lee, K. Shin, A. S. Piotrowski-Daspit, R. J. Homer, A. Iwasaki, H.-W. Suh and W. M. Saltzman, Sci. Transl. Med., 2023, 15, eabq0603 CrossRef CAS PubMed.
- J. C. Kaczmarek, A. K. Patel, K. J. Kauffman, O. S. Fenton, M. J. Webber, M. W. Heartlein, F. DeRosa and D. G. Anderson, Angew. Chem., Int. Ed., 2016, 55, 13808–13812 CrossRef CAS PubMed.
- J. C. Kaczmarek, K. J. Kauffman, O. S. Fenton, K. Sadtler, A. K. Patel, M. W. Heartlein, F. DeRosa and D. G. Anderson, Nano Lett., 2018, 18, 6449–6454 CrossRef CAS PubMed.
- Q. Chen, Y. Chang, X. He, Y. Ding, R. Wang, R. Luo, J. Yuan, J. Chen, G. Zhong, H. Yang, J. Chen and J. Li, ACS Nano, 2025, 19, 7835–7850 CrossRef CAS PubMed.
- Y. Cao, Z. He, Q. Chen, X. He, L. Su, W. Yu, M. Zhang, H. Yang, X. Huang and J. Li, Nano Lett., 2022, 22, 6580–6589 CrossRef CAS PubMed.
- P. Boisguérin, S. Deshayes, M. J. Gait, L. O'Donovan, C. Godfrey, C. A. Betts, M. J. A. Wood and B. Lebleu, Adv. Drug Delivery Rev., 2015, 87, 52–67 CrossRef PubMed.
- Y. Qiu, M. Y. T. Chow, W. Liang, W. W. Y. Chung, J. C. W. Mak and J. K. W. Lam, Mol. Pharm., 2017, 14, 4606–4617 CrossRef CAS PubMed.
- Y. Qiu, R. C. H. Man, Q. Liao, K. L. K. Kung, M. Y. T. Chow and J. K. W. Lam, J. Controlled Release, 2019, 314, 102–115 CrossRef CAS PubMed.
- Vertex Announces Investigational New Drug (IND) Application for VX-522, mRNA Therapy for People With Cystic Fibrosis, Cleared by FDA | Vertex Pharmaceuticals Newsroom, https://news.vrtx.com/news-releases/news-release-details/vertex-announces-investigational-new-drug-ind-application-vx-522, (accessed 31 March 2025).
- D. E. Geller, C. Crowley, J. Froehlich, C. Schwabe and M. O'Carroll, J. Cystic Fibrosis, 2024, 23, S19 CrossRef.
- D. Ishimaru, R. Bhattacharjee, J. Casillas, J. Cefalu, J. Chin, M. Hennig, S. Katikaneni, A. Lee, N. Mahmoudi, S. Mohapatra, T. Molla, S. Fakhari, D. Bartels, J. Engelhardt, H. Clark, V. Kharitonov, B. Wustman, S. Suliman and D. Lockhart, J. Cystic Fibrosis, 2024, 23, S9 CrossRef.
- ReCode Therapeutics Announces First Participants Dosed in a Phase 1 Healthy Volunteer Clinical Trial of Novel Disease-Modifying Genetic Medicine, RCT1100 for the Treatment of Primary Ciliary Dyskinesia, https://recodetx.com/recode-therapeutics-announces-first-participants-dosed-in-a-phase-1-healthy-volunteer-clinical-trial-of-novel-disease-modifying-genetic-medicine-rct1100-for-the-treatment-of-primary-ciliary-dyskinesi/, (accessed 2 April 2025).
- Y. Yuan, Y. Wu, J. Cheng, K. Yang, Y. Xia, H. Wu and X. Pan, Particuology, 2024, 90, 88–97 CrossRef CAS.
- J. Witten, I. Raji, R. S. Manan, E. Beyer, S. Bartlett, Y. Tang, M. Ebadi, J. Lei, D. Nguyen, F. Oladimeji, A. Y. Jiang, E. MacDonald, Y. Hu, H. Mughal, A. Self, E. Collins, Z. Yan, J. F. Engelhardt, R. Langer and D. G. Anderson, Nat. Biotechnol., 2024, 1–10 Search PubMed.
- X.-Y. Tang, S. Wu, D. Wang, C. Chu, Y. Hong, M. Tao, H. Hu, M. Xu, X. Guo and Y. Liu, Signal Transduction Targeted Ther., 2022, 7, 1–17 CrossRef PubMed.
- E. W. Kavanagh, S. Y. Tzeng, N. Sharma, G. R. Cutting and J. J. Green, Biomaterials, 2025, 313, 122753 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |