 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pushing the limit of triplet–triplet annihilation photon upconversion towards the UVC range†
Till J. B.
Zähringer
 a,
Corinna
Heusel
b,
Matthias
Schmitz
a,
Corinna
Heusel
b,
Matthias
Schmitz
 a,
Frank
Glorius
a,
Frank
Glorius
 b and
Christoph
Kerzig
b and
Christoph
Kerzig
 *a
*a
aDepartment of Chemistry, Johannes Gutenberg University Mainz, Duesbergweg 10–14, 55128, Mainz, Germany. E-mail: ckerzig@uni-mainz.de
bOrganisch-Chemisches Institut, University of Münster, Corrensstraße 36, 48149, Münster, Germany
First published on 22nd May 2025
Abstract
Triplet–triplet annihilation photon upconversion (TTA-UC) provides a milder alternative to traditional UVB/UVC photochemistry. However, suitable sensitizer-annihilator pairs are scarce, in particular in the high-energy UV regime. Herein, we present a benzene-based annihilator that, paired with a suitable sensitizer, generates upconverted emission approaching the UVC region for the first time.
Combining the energy of two photons into a single photon of higher energy offers significant advantages over traditional direct excitation. Using longer-wavelength incident light enhances penetration depth,1,2 which is relevant for biomedical applications, and enables the utilization of sub-bandgap photons in photovoltaics3,4 or photoelectrochemical cells5 thereby improving solar efficiency.6 Among the mechanisms for photon energy fusion,7,8 triplet–triplet annihilation upconversion (TTA-UC)9,10 stands out as the most promising, because it operates effectively under non-coherent and low-intensity excitation as that provided by commercial LEDs11 or even sunlight.12,13 The first reported TTA-UC system employed anthracene as an annihilator, producing violet emission.14 Since then, the field has advanced considerably in converting lower-energy near-infrared (NIR)12,15–17 or red light into higher energy visible light. In contrast, progress in visible-to-UV TTA-UC10,13,18–20—particularly below the UVA range (<315 nm)10,21–23—has been much slower. This lag is notable because it does not align with the practical needs of light sources for photochemical reactions:24,25 modern visible/UVA LEDs (>370 nm)26 are already highly efficient, and many photochemical reactions27 can readily utilize sunlight, which contains a non-negligible proportion of UVA.28 However, in the UVB (280–315 nm) and UVC (<280 nm) range, both are most inadequate. To this day, highly inefficient and hazardous UVB sources, such as mercury29 and excimer lamps,30 remain the commonly used options, underscoring the urgent need for TTA-UC as an alternative for generating light in this spectral region. The slow progress in UVB TTA-UC can be attributed to several challenges. A key obstacle is the limited availability of UV annihilator structures. To date only benzene,11 naphthalene,23,31 and biphenyl21,22 derivatives have been identified as being capable of approaching the UVB range while no structures are reported for the UVC range (Fig. 1). This absence is likely due to the inherently large singlet–triplet energy gap required for Vis-to-UVC TTA-UC and the limited understanding of how to precisely modulate these energy gaps in such molecules.32 The best results for UV annihilators have been achieved by modifying the annihilator scaffold with so-called TIPS-ethynyl ((triisopropylsilyl)ethynyl) groups that greatly reduced the triplet energy compared to the parent molecule.11,13,21,33 Recently, we reported a novel benzene-based annihilator substituted with two TIPS-ethynyl groups in para-position. That annihilator was capable of blue-to-UVB TTA-UC, achieving the highest UC emission energy recorded to date (Fig. 1).11 In contrast, the monosubstituted benzene derivative (TIPS-Bz) could not be utilized in such a blue-light-driven UC system, as its triplet state energy (ET) was estimated at 3.06 eV by DFT calculations and it is thus too high for sensitization. However, the high-energy emission of TIPS-Bz (Fig. 1) suggested its potential as a candidate for UVB/UVC photon upconversion when paired with an appropriate sensitizer. Yet, discovering suitable sensitizers for TIPS-Bz presents another significant challenge. This is because to enable sensitization of TIPS-Bz (ET > 3.0 eV) by violet/UVA light (<400 nm, >3.1 eV) energy losses must be minimized. In general sensitizers employed for TTA-UC exhibit small triplet–singlet energy splitting, such as B,N-heteroarene based34,35 and cyanoarene11,18 based donor–acceptor chromophores or metal complexes capable of direct triplet-state excitation.36 The triplet state energy of these sensitizers typically does not exceed ∼2.8 eV,37,38 which is insufficient to effectively sensitize potential UVB/C annihilators. In contrast, the metal complex [Ir(CF3-pmb)3] (see Fig. 2A for its structure) demonstrates an exceptional triplet state energy of 3.18 eV.39,40 The high triplet state energy was exploited by one of our groups in a challenging De Mayo-type ring expansion reaction that is not feasible with commercial high triplet state Ir sensitizers.39 We now report a novel sensitizer-annihilator pair based on TIPS-Bz as annihilator and the high triplet energy sensitizer [Ir(CF3-pmb)3] (Ir). A record-breaking upconverted UVB emission approaching the UVC range was achieved, with an unprecedented excited singlet state energy of 4.33 eV (Fig. 1). Mechanistic investigations are provided that highlight the advantages and challenges of this UC system. This study thus represents another breakthrough in the field of UV photon upconversion, advancing the practical limits of TTA-UC and paving the way for future developments.
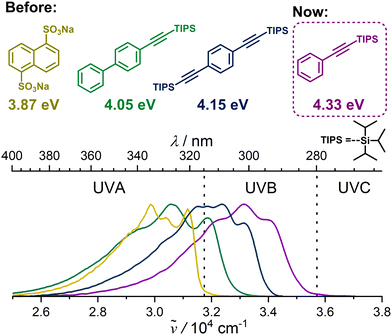 | ||
| Fig. 1 Structures and emission spectra of high-energy UV annihilators in comparison to the novel UV annihilator TIPS-Bz. | ||
The synthesis of the annihilator triisopropyl(ethynyl)-benzene and (TIPS-Bz) and fac-[Ir(CF3-pmb)3] (Ir) followed literature reports.11,39 The room-temperature absorption and emission spectra of TIPS-Bz and Ir are shown in Fig. 2B. TIPS-Bz has an excited singlet state energy of 4.33 eV, a low extinction coefficient of the long-wavelength absorption band with a maximum at 280 nm (ε280 < 1000 M−1 cm−1), a fluorescence quantum yield of 0.19 ± 0.02 and a fluorescence lifetime of 19.9 ns, in line with the Strickler–Berg relationship.41 A direct comparison of TIPS-Bz with benzene and other benzene-derived annihilators is provided in Chapter S4 (ESI†). Generally, the substitution of benzene with TIPS-ethynyl increases the fluorescence quantum yield, reduces the excited-state singlet lifetime, and induces a red shift in both the absorption and emission spectra, a trend that has been observed for naphthalene as well.33 We estimate a decreased triplet state energy of 3.06 eV from DFT calculations compared to the parent benzene molecule (3.9 eV).42Ir readily absorbs UVA light and can be selectively excited with 355 nm laser pulses. The triplet–triplet absorption spectrum is presented in Fig. 2C, and time-resolved measurements indicate a triplet state lifetime of 4 μs (Fig. 2D), which is sufficient to enable efficient bimolecular quenching. The high triplet state energy of 3.18 eV39 makes triplet–triplet energy transfer (TTET) to TIPS-Bz (3.06 eV) thermodynamically feasible, qualifying Ir as a potential sensitizer for the upconversion system under study. Indeed, the phosphorescence of Ir in Ar-saturated benzene is quenched upon addition of increasing TIPS-Bz concentrations (Fig. 2E). A Stern−Volmer analysis yielded a quenching rate constant of 1.14 × 109 M−1 s−1 (Fig. 2E, inset). To investigate the TTET process, a transient absorption (TA) spectrum of a solution containing TIPS-Bz and Ir was recorded after the initial deactivation of the sensitizer triplet. The relatively low sensitizer concentration minimizes filter effects at wavelengths λ < 320 nm. After quenching a TA band with a maximum at 304 nm is observed, which is attributed to the triplet–triplet absorption of 3TIPS-Bz (Fig. 3A). This assignment is supported by TD-DFT calculations, which predict a maximum oscillator strength at 291 nm. Time-resolved measurements show the simultaneous formation of 3TIPS-Bz as the luminescence of Ir is quenched (Fig. 3B). Under the selected conditions a triplet lifetime of 34 μs is measured for TIPS-Bz (Fig. S1, ESI†). After complete deactivation of 3TIPS-Bz only baseline level is detected (Fig. S3, ESI†). This gives us unequivocal proof of triplet–triplet energy transfer between the sensitizer and TIPS-Bz. With the TTET step established, we investigated the triplet–triplet annihilation (TTA) process of TIPS-Bz. For this study, a solution containing 25 μM Ir and 2 mM TIPS-Bz was selected, ensuring 95% deactivation of the excited sensitizer, a critical factor for efficient triplet–triplet annihilation.9 Reabsorption by TIPS-Bz does not play a significant factor since the lowest electronic transition (e.g. in the range of 280–320 nm) exhibits weak absorption presumably as it is symmetry forbidden.43 This is advantageous for upconversion since reabsorption typically leads to a reduced upconversion quantum yield.18 Time-gated emission spectra were recorded throughout the complete deactivation of 3TIPS-Bz (integration window: 4–204 μs after laser pulse excitation, Fig. 4A, blue spectrum). A signal in the UVB range is detected, which resembles the fluorescence spectrum of TIPS-Bz in diluted solution (Fig. 2B). Differences between these two spectra likely stems from inner filter effects of Ir owing to the large molar absorption coefficient ε of Ir in that range. Directly exciting the solution containing the UC system at 280 nm yields a practically identical emission spectrum in the UV (Fig. 4A, green spectrum). Further control experiments do not suggest the formation of excimers (Fig. S2, ESI†).33 Additionally, we detect post-quenching emission of Ir (compare to direct excitation at 280 nm, red spectrum). We assume that this emission stems from back-TTET and singlet energy transfer from 1TIPS-Bz* to Ir.11 Time-resolved transient absorption (TA) measurements of 3TIPS-Bz and the emission of 1TIPS-Bz* are shown in Fig. 4B. While strong artifacts are observed immediately after laser pulse excitation likely due to remaining stray light, the signals of both species concurrently reach a maximum, while the 1TIPS-Bz* emission decays faster than 3TIPS-Bz.9 Time-gated emission spectra were recorded with increasing laser pulse excitation intensities (Fig. 4C). Integration over the UC emission spectrum revealed a nonlinear dependence on the laser power, consistent with the bimolecular nature of the TTA process. Altogether, these findings provide clear evidence that the observed UVB emission originates from TTA-UC. Unfortunately, steady-state UC emission could not be detected with a 370 nm LED as light source. This absence is presumably due to the absorption characteristics of Ir, whose ε is two orders of magnitude lower at 370 nm than in the UVB region. Consequently, achieving sufficient concentrations of 3TIPS-Bz for TTA under mild 370 nm LED excitation necessitates a high concentration of Ir, which in turn enhances reabsorption losses. Ideally, the sensitizer should have an absorption minimum in the spectral region of UC emission.13,44 This highlights one of the major challenges of upconversion in the UVB/C range, where most photoactive compounds have large extinction coefficients (εUVB/C > 103 M−1 cm−1), which makes this a practically unavoidable loss channel in diffusion based UC in solution. Hence, under our UC conditions, considering the excitation and excited singlet state energy, the anti-Stokes shift (0.83 eV) remains below typical values of related systems.10 To address this challenge, TIPS-Bz was further modified by introducing a tert-butyl group at the para-position (4tBu-TIPS-Bz). This modification was intended to lower the triplet energy, potentially allowing sensitization by a broader range of sensitizers. However, the calculated triplet energy of 3.02 eV remains too high to provide significant benefits. While the UC system 4tBu-TIPS-Bz-Ir remains functional under 355 nm laser pulse excitation, its energy transfer constant and photostability are notably lower than those of TIPS-Bz, making it a less favorable system overall (see Chapter S5 for details, ESI†).
This study demonstrates that a benzene annihilator functionalized with a TIPS-ethynyl group can be integrated into a TTA-UC system, achieving emission near the UVC range. This was realized using the high-triplet-energy sensitizer fac-[Ir(CF3-pmb)3], which efficiently sensitizes the high-lying triplet state of TIPS-Bz. A thorough investigation of the TTA-UC mechanism provided unequivocal evidence of the process, pushing the feasibility of TTA-UC into higher-energy regions. However, these findings also highlight critical challenges for UVC upconversion. Future efforts towards Vis-to-UVC photon upconversion should prioritize increasing the singlet–triplet gap of the annihilator by appropriate functionalization, bringing it closer to the theoretical thermodynamic limit for TTA (i.e. 2 × ET1 ∼ ES1).32,45 This will effectively enable sensitization with sensitizers having lower triplet energies. Additionally, the development of an optimally matched sensitizer remains crucial—one that balances high absorption in the UVA/purple spectral range with minimal absorptivity in the UVB/C region to reduce losses.46
This work was supported by the German Research Foundation (DFG, grant number KE 2313/3-1 and GL 349/7-2 within priority program SPP 2102) and the German Federal Environmental Foundation (DBU, grant number 20022/028).
Data availability
All experimental data have been provided in the main text and the ESI.† The data sets shown in the main paper can be found under https://doi.org/10.25358/openscience-12308.Conflicts of interest
The authors declare no conflict of interest.Notes and references
- M. R. Hamblin, Dalton Trans., 2018, 47, 8571–8580 RSC.
- Q. Zhou, B. M. Wirtz, T. H. Schloemer, M. C. Burroughs, M. Hu, P. Narayanan, J. Lyu, A. O. Gallegos, C. Layton, D. J. Mai and D. N. Congreve, Adv. Mater., 2023, 35, 2301563 CrossRef CAS PubMed.
- Y. Shang, S. Hao, C. Yang and G. Chen, Nanomaterials, 2015, 5, 1782–1809 CrossRef CAS PubMed.
- D. Beery, T. W. Schmidt and K. Hanson, ACS Appl. Mater. Interfaces, 2021, 13, 32601–32605 CrossRef CAS PubMed.
- M. Barawi, F. Fresno, R. Pérez-Ruiz and V. A. De La Peña O’Shea, ACS Appl. Energy Mater., 2019, 2, 207–211 CrossRef CAS.
- T. Trupke, M. A. Green and P. Würfel, J. Appl. Phys., 2002, 92, 4117–4122 CAS.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808–5829 CAS.
- M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Angew. Chem., Int. Ed., 2009, 48, 3244–3266 CrossRef CAS PubMed.
- Y. Murakami and K. Kamada, Phys. Chem. Chem. Phys., 2021, 23, 18268–18282 RSC.
- M. Uji, T. J. B. Zähringer, C. Kerzig and N. Yanai, Angew. Chem., Int. Ed., 2023, 62, e202301506 CrossRef CAS PubMed.
- T. J. B. Zähringer, J. A. Moghtader, M. Bertrams, B. Roy, M. Uji, N. Yanai and C. Kerzig, Angew. Chem., Int. Ed., 2023, 62, e202215340 CrossRef PubMed.
- W. Liang, C. Nie, J. Du, Y. Han, G. Zhao, F. Yang, G. Liang and K. Wu, Nat. Photonics, 2023, 17, 346–353 CrossRef CAS.
- N. Harada, Y. Sasaki, M. Hosoyamada, N. Kimizuka and N. Yanai, Angew. Chem., Int. Ed., 2021, 60, 142–147 CrossRef CAS PubMed.
- C. A. Parker and C. G. Hatchard, Proc. R. Soc. Lond. Ser. Math. Phys. Sci., 1962, 269, 574–584 Search PubMed.
- Z. Huang, D. E. Simpson, M. Mahboub, X. Li and M. L. Tang, Chem. Sci., 2016, 7, 4101–4104 RSC.
- T. N. Singh-Rachford, A. Nayak, M. L. Muro-Small, S. Goeb, M. J. Therien and F. N. Castellano, J. Am. Chem. Soc., 2010, 132, 14203–14211 CrossRef CAS PubMed.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS.
- A. Olesund, J. Johnsson, F. Edhborg, S. Ghasemi, K. Moth-Poulsen and B. Albinsson, J. Am. Chem. Soc., 2022, 144, 3706–3716 CrossRef CAS PubMed.
- H.-L. Lee, M.-S. Lee, H. Park, W.-S. Han and J.-H. Kim, Korean J. Chem. Eng., 2019, 36, 1791–1798 CrossRef CAS.
- Y. Murakami, A. Motooka, R. Enomoto, K. Niimi, A. Kaiho and N. Kiyoyanagi, Phys. Chem. Chem. Phys., 2020, 22, 27134–27143 RSC.
- T. J. B. Zähringer, M.-S. Bertrams and C. Kerzig, J. Mater. Chem. C, 2022, 10, 4568–4573 RSC.
- L. Schmid, F. Glaser, R. Schaer and O. S. Wenger, J. Am. Chem. Soc., 2022, 144, 963–976 CrossRef CAS PubMed.
- X. Lin, Z. Chen, Y. Han, C. Nie, P. Xia, S. He, J. Li and K. Wu, ACS Energy Lett., 2022, 7, 914–919 CrossRef CAS.
- B. Lehmann, Photochem. Photobiol., 2005, 81, 1246–1251 CrossRef CAS PubMed.
- N. Zhang, G. Liu, H. Liu, Y. Wang, Z. He and G. Wang, J. Hazard. Mater., 2011, 192, 411–418 CAS.
- H. Hirayama, S. Fujikawa and N. Kamata, Electron. Commun. Jpn., 2015, 98, 1–8 CrossRef.
- T. J. B. Zähringer, M. Wienhold, R. Gilmour and C. Kerzig, J. Am. Chem. Soc., 2023, 145, 21576–21586 CrossRef PubMed.
- Reference Air Mass 1.5 Spectra, https://www.nrel.gov/grid/solar-resource/spectra.html, (accessed March 24, 2025).
- T. Chang, S. You, B. Yu and H. Kong, J. Hazard. Mater., 2007, 141, 784–792 CrossRef CAS PubMed.
- A. G. Griesbeck, N. Maptue, S. Bondock and M. Oelgemöller, Photochem. Photobiol. Sci., 2003, 2, 450–451 CrossRef CAS.
- B. Pfund, D. M. Steffen, M. R. Schreier, M.-S. Bertrams, C. Ye, K. Börjesson, O. S. Wenger and C. Kerzig, J. Am. Chem. Soc., 2020, 142, 10468–10476 CrossRef CAS PubMed.
- W. Zeng, C. Zhong, H. Bronstein and F. Plasser, Angew. Chem., Int. Ed., 2025, e202502485 CAS.
- A. Olesund, S. Ghasemi, K. Moth-Poulsen and B. Albinsson, J. Am. Chem. Soc., 2023, 145, 22168–22175 CrossRef CAS PubMed.
- J. Li, M. Zhang, L. Zeng, L. Huang and X. Wang, Angew. Chem., Int. Ed., 2023, 62, e202303093 CrossRef CAS PubMed.
- X. Cao, K. Pan, J. Miao, X. Lv, Z. Huang, F. Ni, X. Yin, Y. Wei and C. Yang, J. Am. Chem. Soc., 2022, 144, 22976–22984 CrossRef CAS PubMed.
- Y. Sasaki, S. Amemori, H. Kouno, N. Yanai and N. Kimizuka, J. Mater. Chem. C, 2017, 5, 5063–5067 RSC.
- F. Strieth-Kalthoff, M. J. James, M. Teders, L. Pitzer and F. Glorius, Chem. Soc. Rev., 2018, 47, 7190–7202 RSC.
- S. Dutta, J. E. Erchinger, F. Strieth-Kalthoff, R. Kleinmans and F. Glorius, Chem. Soc. Rev., 2024, 53, 1068–1089 RSC.
- T. O. Paulisch, L. A. Mai, F. Strieth-Kalthoff, M. J. James, C. Henkel, D. M. Guldi and F. Glorius, Angew. Chem., Int. Ed., 2022, 61, e202112695 CrossRef CAS PubMed.
- K. Tsuchiya, S. Yagai, A. Kitamura, T. Karatsu, K. Endo, J. Mizukami, S. Akiyama and M. Yabe, Eur. J. Inorg. Chem., 2010, 926–933 CrossRef CAS.
- M.-S. Bertrams and C. Kerzig, Chem. Commun., 2021, 57, 6752–6755 RSC.
- J. P. Doering, J. Chem. Phys., 1969, 51, 2866–2870 CrossRef CAS.
- J. N. Murrell and J. A. Pople, Proc. Phys. Soc. Sect. A, 1956, 69, 245–252 Search PubMed.
- A. Monguzzi, J. Mezyk, F. Scotognella, R. Tubino and F. Meinardi, Phys. Rev. B, 2008, 78, 195112 CrossRef.
- L. Naimovičius and A. B. Pun, Trends Chem., 2025, S2589597425000425 Search PubMed.
- Y. Zhou, F. N. Castellano, T. W. Schmidt and K. Hanson, ACS Energy Lett., 2020, 5, 2322–2326 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc01834j |
| This journal is © The Royal Society of Chemistry 2025 |



