Flexible bidentate aluminum Lewis acids for host–guest complex formation†
Tz-Ching
Tsui
a,
Hao-Yuan
Lan
a,
Han-Jung
Li
 a,
Ting-Shen
Kuo
b and
Hsueh-Ju
Liu
a,
Ting-Shen
Kuo
b and
Hsueh-Ju
Liu
 *ac
*ac
aDepartment of Applied Chemistry, National Yang Ming Chiao Tung University, 1001 Daxue Rd, East District, Hsinchu City, Taiwan 300093. E-mail: hsuehjuliu@nycu.edu.tw
bDepartment of Chemistry, National Taiwan Normal University, Taipei 11677, Taiwan
cCenter for Emergent Functional Matter Science, National Yang Ming Chiao Tung University, 1001 Daxue Rd, East District, Hsinchu City, Taiwan 300093
First published on 13th February 2025
Abstract
This study investigates the design, synthesis, and reactivity of bidentate aluminum Lewis acids supported by amido and cyclopentadienyl donor sets within a flexible 1,4-disubstituted phenylene framework. The reactivity of these complexes with nitrogen-based ditopic donors was systematically explored, revealing their capacity to form host–guest assemblies. Reactions with equimolar pyrazine or quinoxaline yielded 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 colored adducts, highlighting the potential of these two systems as colorimetric sensors for ditopic nitrogen donors. The reaction with 4,4′-bipyridine, which features a longer N⋯N distance, resulted in a dimeric 2
1 colored adducts, highlighting the potential of these two systems as colorimetric sensors for ditopic nitrogen donors. The reaction with 4,4′-bipyridine, which features a longer N⋯N distance, resulted in a dimeric 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct. In contrast, sterically demanding nitrogen donors such as 2,3,5,6-tetramethylpyrazine or phenazine showed no reactivity with these bidentate Lewis acids. The results reveal not only the robust Lewis acidic nature of the aluminum centers but also the pivotal role of ligand flexibility in facilitating diverse molecular interactions.
2 adduct. In contrast, sterically demanding nitrogen donors such as 2,3,5,6-tetramethylpyrazine or phenazine showed no reactivity with these bidentate Lewis acids. The results reveal not only the robust Lewis acidic nature of the aluminum centers but also the pivotal role of ligand flexibility in facilitating diverse molecular interactions.
Introduction
Aluminum has long been recognized as a valuable Lewis acid in catalysis, owing to its low cost, high abundance, and versatile reactivity. Simple aluminum complexes including aluminum trihalides have been widely employed as catalysts or reagents in key organic reactions, including Friedel–Crafts alkylation and acylation, and the Meerwein–Ponndorf–Verley reduction.1 These applications leverage the Lewis acidic nature of Al(III) ions and have laid a strong foundation for advancements in aluminum coordination chemistry. Building on these early studies, modern approaches focus on creating more sophisticated aluminum-based systems that enhance catalytic performance and broaden the scope of their applications.One such advancement involves the strategic positioning of multiple Lewis acidic centers in close proximity, creating multi-dentate Lewis acidic sites within a single molecule. This concept parallels the use of multi-dentate ligands in coordination chemistry, where multiple donor groups provide enhanced binding capabilities to metal complexes. These multi-dentate Lewis acids show great promise in applications such as anion and molecular sensing and recognition2 and catalysis for small molecule activation.3 These studies underscore the potential of dual-site Lewis acids in cooperative binding and catalysis.
Our research builds on these concepts, focusing on multi-metallic clusters that incorporate Lewis acidic centers. Recently, we reported a di-zinc framework supported by bisphenoxymethanone ligands, where synergistic interactions between the two zinc centers significantly enhanced reactivity in ε-caprolactone ring-opening polymerization compared to monomeric zinc complexes with similar coordination environments.4 Additionally, we synthesized a unique anionic di-iron trihydride complex supported by a bis-cyclopentadienyl ligand, opening new avenues for heterobimetallic complex formation with coinage metals.5 Inspired by these findings, we have turned our attention to aluminum-based bidentate Lewis acids, supported by amido and cyclopentadienyl donor sets within a di-substituted-1,4-C6H4 scaffold. Herein, we report the synthesis, structural characterization, and reactivity of these flexible di-aluminum systems, with a particular focus on their ability to form host–guest complexes.
Results and discussion
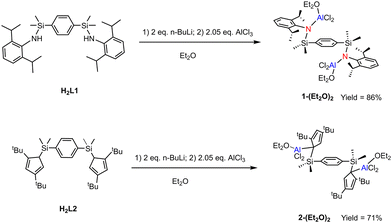
The synthesis of complexes 1-(Et2O)2 and 2-(Et2O)2 is outlined in Scheme 1, where 1 represents the L1-(AlCl2)2 moiety and 2 represents the L2-(AlCl2)2 moiety. Treatment of in situ prepared Li2L1 from H2L1 with 2.05 equiv. of AlCl3 in diethyl ether at ambient temperature yielded 1-(Et2O)2 as a white solid in 86% yield. Under similar conditions, using H2L2 afforded 2-(Et2O)2 in 71% yield.The 1H NMR spectra of both 1-(Et2O)2 and 2-(Et2O)2 confirmed the presence of coordinated Et2O molecules (δ = 3.59 and 0.67 ppm for 1-(Et2O)2; 3.53, 3.27, and 0.65 ppm for 2-(Et2O)2), indicating the Lewis acidic nature of both complexes. Notably, the α-protons of the coordinated Et2O in 2-(Et2O)2 are diastereotopic due to the asymmetric environment of aluminum centers in the L2-(AlCl2)2 moiety. Single-crystal X-ray diffraction analyses (see Fig. 1) provided the molecular structures of 1-(Et2O)2 and 2-(Et2O)2, revealing that the two AlCl2(OEt2) moieties in each complex adopt a trans-conformation relative to the central p-phenylene spacer. Both aluminum centers in 1-(Et2O)2 and 2-(Et2O)2 exhibit tetrahedral geometry. The Al–N bond length of 1.798(7) Å in 1-(Et2O)2 and the Al–C (η1-Cp coordination) bond length of 1.981(4) Å in 2-(Et2O)2 are consistent with those reported for four-coordinated aluminum amido6 and Al(η1-Cp)7 complexes in the literature.
To evaluate the Lewis acidity of 1-(Et2O)2 and 2-(Et2O)2, triethylphosphine oxide (TEPO) was employed as a probe molecule using the Gutmann–Beckett method.8 Complexation reactions of 1-(Et2O)2 and 2-(Et2O)2 with 2 equiv. of TEPO resulted in similar changes in the 31P chemical shift (Δ(δ31P) = +30.3 and +30.0 ppm for 1-(Et2O)2 and 2-(Et2O)2, respectively, relative to the δ31P of TEPO) in C6D6, indicating comparable Lewis acidity of 1 and 2 toward TEPO. The close Al–O bond lengths observed in the X-ray structures of 1-(Et2O)2 (1.884(7) Å) and 2-(Et2O)2 (1.872(3) Å) further confirm the similar Lewis acidity of the aluminum centers.
Given the Lewis acidic nature of 1-(Et2O)2 and 2-(Et2O)2, various donor molecules were used to assess their coordination capabilities. Replacing the Et2O ligand in 1-(Et2O)2 with the stronger donor 4-dimethylaminopyridine (DMAP) yielded colorless 1-(DMAP)2 in 63% yield, demonstrating the potential of 1-(Et2O)2 as a useful precursor for host–guest chemistry. The crystal structure of 1-(DMAP)2 is shown in Fig. 2a, featuring two AlCl2(DMAP) moieties arranged in an anti-configuration relative to the central p-phenylene spacer, similar to the structure of 1-(Et2O)2. Inspired by the work of Katz9 and Mitzel,10 which demonstrated that rigid bidentate boron Lewis acids serve as effective receptors for ditopic pyrimidine coordination, we envisioned that the bimetallic 1-(Et2O)2 and 2-(Et2O)2 might function as selective receptors for neutral Lewis bases. To explore this, we used pyrazine, which has a similar size to the central p-phenylene spacer in our bidentate Lewis acids, as a coordinating ligand in our study.
The reaction of 1-(Et2O)2 with 1 equiv. of pyrazine (pyz) in toluene rapidly generated an orange-red solution. The resulting product's 1H NMR spectrum indicated clean formation of a new species as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 self-assembled adduct. Red crystals of 1-κ2-pyz, obtained by diffusing pentane into a saturated toluene solution, were characterized by single-crystal X-ray diffraction. The solid-state structure of 1-κ2-pyz reveals that two AlCl2 fragments act as a bidentate Lewis acidic receptor for pyrazine binding, with the pyrazine molecule residing in the “binding pocket” in a κ2-binding mode (see Fig. 2b). This supramolecule features Al–Npyz bond lengths of 2.000(2) and 2.022(2) Å, and an interplanar distance of 3.440 Å (centroidpyz to centroidPh) between the pyrazine and central p-phenylene ring. The short distance between the two aromatic planes suggests intramolecular π–π interactions. Similarly, 2-κ2-pyz was obtained from the reaction of 2-(Et2O)2 with 1 equiv. of pyrazine, affording a dark green solid in 95% yield. Structural analysis by X-ray diffraction (Fig. 2e) confirms the formation of a 1
1 self-assembled adduct. Red crystals of 1-κ2-pyz, obtained by diffusing pentane into a saturated toluene solution, were characterized by single-crystal X-ray diffraction. The solid-state structure of 1-κ2-pyz reveals that two AlCl2 fragments act as a bidentate Lewis acidic receptor for pyrazine binding, with the pyrazine molecule residing in the “binding pocket” in a κ2-binding mode (see Fig. 2b). This supramolecule features Al–Npyz bond lengths of 2.000(2) and 2.022(2) Å, and an interplanar distance of 3.440 Å (centroidpyz to centroidPh) between the pyrazine and central p-phenylene ring. The short distance between the two aromatic planes suggests intramolecular π–π interactions. Similarly, 2-κ2-pyz was obtained from the reaction of 2-(Et2O)2 with 1 equiv. of pyrazine, affording a dark green solid in 95% yield. Structural analysis by X-ray diffraction (Fig. 2e) confirms the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct, with Al–N bond lengths of 2.024(2) and 2.029(2) Å, and a longer interplanar distance of 3.617 Å between the pyrazine and central p-phenylene spacer.
1 adduct, with Al–N bond lengths of 2.024(2) and 2.029(2) Å, and a longer interplanar distance of 3.617 Å between the pyrazine and central p-phenylene spacer.
The intriguing color change observed when equimolar amounts of pyrazine react with the colorless precursors 1-(Et2O)2 and 2-(Et2O)2 to form 1-κ2-pyz and 2-κ2-pyz, respectively, highlights the potential utility of these complexes as sensors for detecting ditopic aromatic N-donors. This is particularly notable since Al3+ complexes are typically colorless, even when coordinated with pyrazine, as recently demonstrated by Müller-Buschbaum and coworkers, who reported the formation of predominantly colorless polymeric or dimeric complexes of AlCl3 or InCl3 with pyrazine as a bridging ligand.11 These contrasting observations sparked our interest in understanding the origin of the color in our Al-pyrazine systems.
To investigate the host–guest interactions and electronic structures, titration experiments were performed using UV-vis spectroscopy. The colorless precursors 1-(Et2O)2 and 2-(Et2O)2 exhibit no noticeable absorption beyond 300 nm. For 1-(Et2O)2 (5 × 10−4 M), increasing the amount of pyrazine resulted in the appearance of a broad absorption band at 365 nm, which reached maximum intensity at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio (Fig. 3a), consistent with the clean formation of 1-κ2-pyz as confirmed by 1H NMR spectroscopy. In the case of 2-(Et2O)2, increasing the pyrazine concentration led to the appearance of a distinct absorption band at 570 nm, which also reached a maximum when 1 equiv. of pyrazine was added.
1 stoichiometric ratio (Fig. 3a), consistent with the clean formation of 1-κ2-pyz as confirmed by 1H NMR spectroscopy. In the case of 2-(Et2O)2, increasing the pyrazine concentration led to the appearance of a distinct absorption band at 570 nm, which also reached a maximum when 1 equiv. of pyrazine was added.
 | ||
| Fig. 3 UV-vis spectra and results showing the effects of varying amounts of pyrazine on (a) 1-(Et2O)2 and (b) 2-(Et2O)2. | ||
To gain further insights, the electronic structures of 1-κ2-pyz and 2-κ2-pyz were examined using DFT calculations. The results revealed that the HOMO and HOMO−1 in both systems primarily consist of the donor π-bonding orbitals of the 2,6-diisopropylphenyl groups (in 1-κ2-pyz) or cyclopentadienyl groups (in 2-κ2-pyz). Additionally, the p-phenylene π bonding orbitals appear as HOMO−6 and HOMO−6 in 1-κ2-pyz and HOMO−4 and HOMO−5 in 2-κ2-pyz (see Fig. S21 and S22†). The LUMO in both cases is dominated by the π* orbitals of pyrazine. The transitions from these occupied molecular orbitals to the LUMO likely account for the observed orange color in 1-κ2-pyz and green color in 2-κ2-pyz (see Fig. S21 and S22†).
Further reactivity studies revealed distinct behaviors for 1-κ2-pyz and 2-κ2-pyz when exposed to excess pyrazine. The reaction of 2-κ2-pyz with additional equivalents of pyrazine in toluene resulted in a rapid color change from dark green to pale yellow, with 1H NMR spectroscopy confirming the clean conversion to 2-(pyz)2. Single-crystal X-ray diffraction (Fig. 2f) revealed a trans-arrangement of two CptBu2AlCl2(κ1-pyz) fragments relative to the central p-phenylene spacer, with a slightly shorter Al–N(pyrazine) bond length of 1.979(4) Å compared to 2.024(2) and 2.029(2) Å in 2-κ2-pyz. UV-vis titration experiments further supported this observation: upon addition of >1 equiv. of pyrazine, the 570 nm band decreased significantly and ultimately vanished when >2 equiv. of pyrazine was present (see Fig. 3b).
In contrast, the addition of 1 equiv. of pyrazine to 1-κ2-pyz resulted in only ∼62% conversion to 1-(pyz)2, as determined by 1H NMR spectroscopy, indicating a dynamic equilibrium between 1-κ2-pyz and pyrazine. Upon further addition of excess pyrazine (>3 equiv.), a yellow precipitate formed, which was identified as 1-(pyz)2via X-ray crystallography (Fig. 2c). This structure features a unique triple-decker stacking arrangement of pyrazine-p-phenylene-pyrazine with an interplanar distance of 3.614 Å (centroidpyrazine to centroidphenylene). UV-vis titration experiments corroborated these findings, showing only ∼30% reduction of the 365 nm band with a three-fold excess of pyrazine to 1-κ2-pyz, consistent with the dynamic equilibrium observed in NMR. In contrast, an immediate reduction of the absorption band of 2-κ2-pyz was observed upon the addition of a slight excess of pyrazine (Fig. 3a).
The observed differences between systems 1 and 2 can likely be attributed to variations in intramolecular π interactions between pyrazine and the central p-phenylene ring. Despite comparable Lewis acidity for 1 and 2, as determined by the Gutmann–Beckett method, the p-phenylene-pyrazine π interaction appears to play a stabilizing role in 1-κ2-pyz, resulting in its distinct reactivity profile. The distinct color changes observed when 1-(Et2O)2 and 2-(Et2O)2 react with pyrazine, along with their differing reactivities toward excess pyrazine, demonstrate the ability of these bidentate aluminum Lewis acids to interact selectively with ditopic nitrogen donors. Moreover, the resistance of 1-κ2-pyz to complete conversion to 1-(pyz)2 in the presence of excess pyrazine suggests that 1-(Et2O)2 could serve as an ideal colorimetric sensor for pyrazine within a specific concentration range.
Examining the crystal structure of 1-κ2-pyz, we observe that while the pyrazine plane is not perfectly parallel to the central p-phenylene plane (with an interplanar angle of 14.7°), the pyrazine guest molecule resides well within the coordination pocket, attributed to its similar size to the central p-phenylene ring. Consequently, other ditopic nitrogen donors with comparable N⋯N distances to pyrazine should also serve as suitable guests for 1-(Et2O)2 and 2-(Et2O)2. Due to the poor solubility of N-donor adducts with 2-(Et2O)2, further studies with various N donors were conducted exclusively with 1-(Et2O)2.
To explore the ability of 1-(Et2O)2 as a bidentate chelator for ditopic Lewis bases, several aromatic and aliphatic N donors were tested, including quinoxaline (qul), phenazine or 2,3,5,6-tetramethylpyrazine. Treatment of 1-(Et2O)2 with 1 equiv. of quinoxaline in toluene produced an orange-brown solid in 56% yield (Scheme 2a). The molecular structure of 1-κ2-qul was determined by X-ray crystallography, revealing Al–Nqul bond lengths of 2.031(7) and 2.033(8) Å, comparable to those in 1-κ2-pyz (Fig. 2d). Interestingly, the more sterically demanding quinoxaline, which contains an additional fused aromatic ring, resulted in a larger interplanar angle of 31.4° between the quinoxaline and central p-phenylene planes—significantly larger than the 14.7° angle observed in 1-κ2-pyz. To accommodate quinoxaline within the binding pocket of 1-(Et2O)2, the two 2,6-diisopropylphenyl (dipp) groups were repositioned to one side, creating sufficient space for the inclined quinoxaline plane, as shown in Fig. S20.† This steric adjustment suggests the spatial constraints posed by bulkier guest molecules. Consequently, no reaction was observed between 1-(Et2O)2 and 1 equiv. of either phenazine or 2,3,5,6-tetramethylpyrazine, likely due to steric hindrance posed by these guest molecules.
In Mitzel's work,10 the reaction of a rigid diborane system with 3,3′-bipyridine resulted in an insoluble product, likely a polymeric structure. Similarly, Wagner demonstrated that reactions of ferrocene-containing diborane with ditopic Lewis bases, such as pyrazine or 4,4′-bipyridine, resulted in the formation of coordination polymers.12 In our previous research, a bidentate Lewis acidic di-zinc framework also reacted with 4,4′-bipyridine to produce a polymeric, ladder-type structure.4 By contrast, the introduction of flexible SiMe2 linkers in dinuclear organotin systems, as reported by Höpfl, Jurkschat, and coworkers, allows the formation of discrete 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 assemblies with substrates containing di-carboxylate or di-dithiocarbamate groups, suggesting the influence of structural flexibility on product formation.2g
1 assemblies with substrates containing di-carboxylate or di-dithiocarbamate groups, suggesting the influence of structural flexibility on product formation.2g
In our current system, it is evident that 4,4′-bipyridine, with its extended N⋯N distance, does not fit within the defined binding pocket of 1-(Et2O)2. This raises the intriguing possibility of an alternative binding mode, potentially involving the formation of intermolecular superstructures. To explore this, the reaction between 1-(Et2O)2 and 4,4′-bipyridine was carried out. Upon addition of 4,4′-bipyridine to a toluene solution of 1-(Et2O)2, light yellow precipitates formed immediately. By carefully layering a toluene solution of 4,4′-bipyridine over a solution of 1-(Et2O)2, we allowed for slow diffusion between the layers, which effectively yielded crystals suitable for single-crystal X-ray diffraction analysis (see Fig. 2g). The resulting molecular structure revealed the formation of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct, 12-μ-(bipy)2, where two equiv. of 1-(Et2O)2 and 4,4′-bipyridine assemble into a dimer. This unexpected dimerization highlights the flexibility of the SiMe2 linkages in the ligand system, enabling the formation of an intermolecular complex. The Al–N distances in 12-μ-(bipy)2 are approximately 1.96 Å, which is notably shorter than the Al–N distance observed in 1-κ2-pyz (2.02 Å), further underscoring the adaptability of the framework in accommodating various guest molecules.
2 adduct, 12-μ-(bipy)2, where two equiv. of 1-(Et2O)2 and 4,4′-bipyridine assemble into a dimer. This unexpected dimerization highlights the flexibility of the SiMe2 linkages in the ligand system, enabling the formation of an intermolecular complex. The Al–N distances in 12-μ-(bipy)2 are approximately 1.96 Å, which is notably shorter than the Al–N distance observed in 1-κ2-pyz (2.02 Å), further underscoring the adaptability of the framework in accommodating various guest molecules.
Conclusions
The design of a ditopic ligand within the 1,4-(SiMe2)2C6H4 disubstituted framework creates the flexibility and adaptability of the bidentate aluminum Lewis acid framework, exemplified by 1-(Et2O)2 and 2-(Et2O)2. These two systems demonstrate the capacity to accommodate a variety of nitrogen-based ditopic donors, each with distinct structural demands. These findings underscore the potential of 1-(Et2O)2 and similar frameworks in promoting tailored host–guest interactions. By accommodating guest molecules with varying steric and spatial requirements, this system expands the possibilities for designing flexible and cooperative Lewis acid frameworks that could be leveraged in supramolecular chemistry, sensing, and small-molecule capture applications. This study opens pathways for further exploration of ditopic Lewis acids with adjustable binding sites, potentially enhancing reactivity and selectivity in targeted applications.Experimental
General considerations
All manipulations with oxygen and moisture-sensitive materials were performed in a nitrogen-filled glovebox. Solvents were dried and deaerated using a solvent system (AsiaWong Enterprise Co., Ltd) prior to use. Benzene-d6 was dried over sodium and benzophenone, degassed via three freeze–pump–thaw cycles, and stored under nitrogen over 3 Å molecular sieves. CDCl3 was dried over 3 Å molecular sieves, degassed via three freeze–pump–thaw cycles, and stored under nitrogen. The NMR spectra were recorded using Bruker 300, Varian 400 or Varian 600 MHz spectrometers. The NMR spectra were referenced to residual protonated solvent for 1H NMR (7.16 ppm for the compound in benzene-d6; 7.26 ppm for the compound in CDCl3) and to deuterated solvent for 13C NMR (128.06 for the compound in benzene-d6; 77.16 ppm for the compound in CDCl3). All spectra were recorded at 25 °C. Complex multiplets are denoted as “m” and broad resonances as “br”. Elemental analyses were performed using an Elementar vario EL CUBE (CHN-OS Rapid, Germany). Mass spectrometry was performed using a JMS-T200GC AccuTOF GCx (source mode: FD (field desorption)). Di-tert-butylcyclopentadiene (CptBu2H), its deprotonated form CptBu2Li13 and p-Ph(SiMe2Cl)214 were synthesized according to the reported procedures.A suspension of AlCl3 (0.144 g, 1.11 mmol) in 10 mL of Et2O was added to the Li2L1 (0.300 g, 0.54 mmol) solution in Et2O (10 mL) at −35 °C. The mixture was then allowed to warm to room temperature and stirred for 18 h. Afterward, the volatile components were removed under vacuum, and the product was extracted with 20 mL of toluene. The mixture was filtered, and the volatiles were removed in vacuo to afford white powder L1[AlCl2(Et2O)]2 (1-(Et2O)2). Yield: 0.422 g, 88%. 1H NMR (400 MHz, benzene-d6, 298 K): δ 7.86 (s, 4H, ![[P with combining low line]](https://www.rsc.org/images/entities/i_char_0050_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif) ), 7.15–7.11 (m, 6H,
), 7.15–7.11 (m, 6H, ![[D with combining low line]](https://www.rsc.org/images/entities/i_char_0044_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif) ), 3.93 (m, 4H, C
), 3.93 (m, 4H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) Me2), 3.59 (q, J = 7.04 Hz, 8H, O(C
Me2), 3.59 (q, J = 7.04 Hz, 8H, O(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH3)2), 1.39–1.30 (m, 24H, CH
2CH3)2), 1.39–1.30 (m, 24H, CH![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), 0.67 (t, J = 6.98 Hz, 12H, O(CH2C
2), 0.67 (t, J = 6.98 Hz, 12H, O(CH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.34 (s, 12H, SiMe2) ppm. 13C{1H} NMR (100 MHz, benzene-d6, 298 K): δ 146.9, 143.1, 142.1, 134.3, 124.7, 124.4, 69.7, 28.1, 25.9, 25.2, 12.9, 1.0 ppm. Anal. calcd for C42H70Al2Cl4N2O2Si2: C 56.87, H 7.96, N 3.16. Found: C 57.33, H 8.21, N 3.40.
3)2), 0.34 (s, 12H, SiMe2) ppm. 13C{1H} NMR (100 MHz, benzene-d6, 298 K): δ 146.9, 143.1, 142.1, 134.3, 124.7, 124.4, 69.7, 28.1, 25.9, 25.2, 12.9, 1.0 ppm. Anal. calcd for C42H70Al2Cl4N2O2Si2: C 56.87, H 7.96, N 3.16. Found: C 57.33, H 8.21, N 3.40.
A suspension of AlCl3 (0.387 g, 2.90 mmol) in 10 mL of Et2O was added to the Li2L2 (1.00 g, 1.41 mmol) solution in Et2O (10 mL) at −35 °C. The mixture was then allowed to warm to room temperature and stirred for 18 hours. After this period, the mixture was filtered, and the filtrate was concentrated to about 1–2 mL under vacuum. The concentrated ether solution was carefully layered with 10 mL of pentane and cooled to −35 °C, yielding a white solid L2[AlCl2(Et2O)]2 (2-(Et2O)2), after drying. Yield: 0.893 g, 71%. 1H NMR (400 MHz, benzene-d6, 298 K): δ = 7.55–7.52 (br, 4H, ![[P with combining low line]](https://www.rsc.org/images/entities/i_char_0050_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif) ), 6.80 (s, 2H, Cp
), 6.80 (s, 2H, Cp![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), 6.36 (s, 2H, Cp
), 6.36 (s, 2H, Cp![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), 3.53 (br, 2H, O(C
), 3.53 (br, 2H, O(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH3)2), 3.27 (br, 2H, O(C
2CH3)2), 3.27 (br, 2H, O(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH3)2), 1.47 (s, 18H, tBu), 1.29 (s, 18H, tBu), 0.88 (s, 6H, Si
2CH3)2), 1.47 (s, 18H, tBu), 1.29 (s, 18H, tBu), 0.88 (s, 6H, Si![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), 0.65 (br, 12H, O(CH2C
2), 0.65 (br, 12H, O(CH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.50 (s, 6H, Si
3)2), 0.50 (s, 6H, Si![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2) ppm. 13C{1H} NMR (100 MHz, CDCl3, 298 K): δ = 161.6, 153.1, 141.3, 141.2, 133.8, 128.1, 126.6, 123.5, 123.4, 68.7, 68.6, 34.7, 33.9, 32.6, 31.3, 12.8, 12.7, 3.2, 3.1, −1.3 ppm. Elemental analysis calcd (%) for C44H76Al2Cl4O2Si2: C 59.45, H 8.62. Found: C 59.51, H 8.16.
2) ppm. 13C{1H} NMR (100 MHz, CDCl3, 298 K): δ = 161.6, 153.1, 141.3, 141.2, 133.8, 128.1, 126.6, 123.5, 123.4, 68.7, 68.6, 34.7, 33.9, 32.6, 31.3, 12.8, 12.7, 3.2, 3.1, −1.3 ppm. Elemental analysis calcd (%) for C44H76Al2Cl4O2Si2: C 59.45, H 8.62. Found: C 59.51, H 8.16.
![[D with combining low line]](https://www.rsc.org/images/entities/i_char_0044_0332.gif)
![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/i_char_0041_0332.gif)
![[P with combining low line]](https://www.rsc.org/images/entities/i_char_0050_0332.gif) ) 7.52 (s, 4H,
) 7.52 (s, 4H, ![[P with combining low line]](https://www.rsc.org/images/entities/i_char_0050_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif) ), 7.09–7.04 (m, 6H,
), 7.09–7.04 (m, 6H, ![[D with combining low line]](https://www.rsc.org/images/entities/i_char_0044_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif) ), 6.29 (d, 4H, 3JH,H = 6.90 Hz,
), 6.29 (d, 4H, 3JH,H = 6.90 Hz, ![[D with combining low line]](https://www.rsc.org/images/entities/i_char_0044_0332.gif)
![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/i_char_0041_0332.gif)
![[P with combining low line]](https://www.rsc.org/images/entities/i_char_0050_0332.gif) ), 3.74 (m, 4H, C
), 3.74 (m, 4H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) Me2), 3.06 (s, 12H, N
Me2), 3.06 (s, 12H, N![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), 1.25 (d, 12H, 3J = 6.84 Hz, CH
2), 1.25 (d, 12H, 3J = 6.84 Hz, CH![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), δ 1.12 (d, 12H, 3J = 6.84 Hz, CH
2), δ 1.12 (d, 12H, 3J = 6.84 Hz, CH![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), 0.27 (s, 12H, SiMe2) ppm. Satisfactory 13C NMR data were not obtained due to the poor solubility of 1-(DMAP)2 in CDCl3. Anal. calcd for C48H70Al2Cl4N6Si2: C 58.65, N 8.55, H 7.18. Found: C 59.18, N 8.33, H 7.34.
2), 0.27 (s, 12H, SiMe2) ppm. Satisfactory 13C NMR data were not obtained due to the poor solubility of 1-(DMAP)2 in CDCl3. Anal. calcd for C48H70Al2Cl4N6Si2: C 58.65, N 8.55, H 7.18. Found: C 59.18, N 8.33, H 7.34.
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/i_char_0079_0332.gif)
![[z with combining low line]](https://www.rsc.org/images/entities/i_char_007a_0332.gif) ), 7.21–7.17 (m, 6H,
), 7.21–7.17 (m, 6H, ![[D with combining low line]](https://www.rsc.org/images/entities/i_char_0044_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif) ), 7.14–7.05 (br, 4H,
), 7.14–7.05 (br, 4H, ![[P with combining low line]](https://www.rsc.org/images/entities/i_char_0050_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif) ), 4.95 (br, 4H, C
), 4.95 (br, 4H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) Me2), 1.50 (d, 12H, J = 6.40 Hz, CH
Me2), 1.50 (d, 12H, J = 6.40 Hz, CH![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), 1.42 (d, 12H, J = 6.77 Hz, CH
2), 1.42 (d, 12H, J = 6.77 Hz, CH![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), 0.42 (br, 12H, Si
2), 0.42 (br, 12H, Si![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2) ppm. 13C{1H} NMR (100 MHz, benzene-d6, 298 K): δ 146.5, 144.5, 142.4, 142.3, 133.6, 125.2, 124.6, 28.4, 25.8, 24.8, −0.43 ppm. Elemental analysis calcd (%) for C38H54Al2Cl4N4Si2: C 55.74, N 6.84, H 6.65. Found: C 55.51, N 6.60, H 6.69. UV-vis: 365 (ε = 1600) in toluene.
2) ppm. 13C{1H} NMR (100 MHz, benzene-d6, 298 K): δ 146.5, 144.5, 142.4, 142.3, 133.6, 125.2, 124.6, 28.4, 25.8, 24.8, −0.43 ppm. Elemental analysis calcd (%) for C38H54Al2Cl4N4Si2: C 55.74, N 6.84, H 6.65. Found: C 55.51, N 6.60, H 6.69. UV-vis: 365 (ε = 1600) in toluene.
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/i_char_0079_0332.gif)
![[z with combining low line]](https://www.rsc.org/images/entities/i_char_007a_0332.gif) ), 8.08 (br, 2H,
), 8.08 (br, 2H, ![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/i_char_0079_0332.gif)
![[z with combining low line]](https://www.rsc.org/images/entities/i_char_007a_0332.gif) ), 7.21–7.17 (m, 6H,
), 7.21–7.17 (m, 6H, ![[D with combining low line]](https://www.rsc.org/images/entities/i_char_0044_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif) ), 7.10 (s, 4H,
), 7.10 (s, 4H, ![[P with combining low line]](https://www.rsc.org/images/entities/i_char_0050_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif) ), 6.94 (s, 2H,
), 6.94 (s, 2H, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/char_0070_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.17 (s, 2H,
), 5.17 (s, 2H, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/char_0070_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 5.17 (s, 2H, He), 1.66 (s, 18H,
), δ 5.17 (s, 2H, He), 1.66 (s, 18H, ![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[B with combining low line]](https://www.rsc.org/images/entities/i_char_0042_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), 1.34 (s, 18H,
), 1.34 (s, 18H, ![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[B with combining low line]](https://www.rsc.org/images/entities/i_char_0042_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), δ 0.98 (s, 6H, Si
), δ 0.98 (s, 6H, Si![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), δ 0.24 (s, 6H, Si
2), δ 0.24 (s, 6H, Si![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2). Satisfactory 13C NMR data were not obtained due to the poor solubility of 2-κ2-pyz in C6D6. Repeated attempts at combustion elemental analysis did not produce satisfactory results. UV-vis: 570 (ε = 250) in toluene.
2). Satisfactory 13C NMR data were not obtained due to the poor solubility of 2-κ2-pyz in C6D6. Repeated attempts at combustion elemental analysis did not produce satisfactory results. UV-vis: 570 (ε = 250) in toluene.
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/i_char_0079_0332.gif)
![[z with combining low line]](https://www.rsc.org/images/entities/i_char_007a_0332.gif) ), 7.73 (br, 4H,
), 7.73 (br, 4H, ![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/i_char_0079_0332.gif)
![[z with combining low line]](https://www.rsc.org/images/entities/i_char_007a_0332.gif) ), 7.25 (s, 4H,
), 7.25 (s, 4H, ![[P with combining low line]](https://www.rsc.org/images/entities/i_char_0050_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif) ), 6.57 (d, 2H, 4J = 1.95 Hz,
), 6.57 (d, 2H, 4J = 1.95 Hz, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/char_0070_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 6.22 (d, 2H, 4J = 1.95 Hz,
), 6.22 (d, 2H, 4J = 1.95 Hz, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/char_0070_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 1.39 (s, 18H,
), 1.39 (s, 18H, ![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[B with combining low line]](https://www.rsc.org/images/entities/i_char_0042_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), 0.99 (s, 18H,
), 0.99 (s, 18H, ![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif)
![[B with combining low line]](https://www.rsc.org/images/entities/i_char_0042_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), δ 0.88 (s, 6H, Si
), δ 0.88 (s, 6H, Si![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), δ 0.73 (s, 6H, Si
2), δ 0.73 (s, 6H, Si![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2) ppm. 13C{1H} NMR (100.6 MHz, C6D6): δ 161.6, 153.7, 147.3, 141.9, 139.9, 133.4, 126.4, 122.2, 34.7, 33.9, 32.2, 30.9, 3.8, −0.6 ppm. Elemental analysis calcd (%) for C44H64Al2Cl4N4Si2: C 58.66, N 6.22, H 7.16. Found: C 58.61, N 5.86, H 7.03.
2) ppm. 13C{1H} NMR (100.6 MHz, C6D6): δ 161.6, 153.7, 147.3, 141.9, 139.9, 133.4, 126.4, 122.2, 34.7, 33.9, 32.2, 30.9, 3.8, −0.6 ppm. Elemental analysis calcd (%) for C44H64Al2Cl4N4Si2: C 58.66, N 6.22, H 7.16. Found: C 58.61, N 5.86, H 7.03.
![[q with combining low line]](https://www.rsc.org/images/entities/i_char_0071_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/i_char_006c_0332.gif) ), 8.82 (s, 2H,
), 8.82 (s, 2H, ![[q with combining low line]](https://www.rsc.org/images/entities/i_char_0071_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/i_char_006c_0332.gif) ), 7.43 (s, 4H,
), 7.43 (s, 4H, ![[P with combining low line]](https://www.rsc.org/images/entities/i_char_0050_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif) ), 7.25–7.17 (m, 6H,
), 7.25–7.17 (m, 6H, ![[D with combining low line]](https://www.rsc.org/images/entities/i_char_0044_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif) ), 7.06–7.00 (m, 2H,
), 7.06–7.00 (m, 2H, ![[q with combining low line]](https://www.rsc.org/images/entities/i_char_0071_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/i_char_006c_0332.gif) ), 3.98 (br, 4H, 3J = 6.75 Hz, C
), 3.98 (br, 4H, 3J = 6.75 Hz, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) Me2), 1.47 (d, 12H, 3J = 6.73 Hz, CH
Me2), 1.47 (d, 12H, 3J = 6.73 Hz, CH![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), δ 1.40 (br, 12H, CH
2), δ 1.40 (br, 12H, CH![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), δ 0.51 (s, 12H, Si
2), δ 0.51 (s, 12H, Si![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2) ppm. 13C{1H} NMR (100 MHz, benzene-d6, 298 K): δ 143.8, 143.4, 143.3, 138.8, 134.3, 134.0, 127.5, 125.2, 29.1, 25.7, 0.6 ppm. Elemental analysis calcd (%) for C42H56Al2Cl4N4Si2: C 58.06, N 6.45, H 6.50. Found: C 58.16, N 6.38, H 6.37.
2) ppm. 13C{1H} NMR (100 MHz, benzene-d6, 298 K): δ 143.8, 143.4, 143.3, 138.8, 134.3, 134.0, 127.5, 125.2, 29.1, 25.7, 0.6 ppm. Elemental analysis calcd (%) for C42H56Al2Cl4N4Si2: C 58.06, N 6.45, H 6.50. Found: C 58.16, N 6.38, H 6.37.
![[b with combining low line]](https://www.rsc.org/images/entities/i_char_0062_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/i_char_0079_0332.gif) ), 7.81 (s, 8H,
), 7.81 (s, 8H, ![[P with combining low line]](https://www.rsc.org/images/entities/i_char_0050_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/i_char_0068_0332.gif) ), 7.80–7.78 (br, 8H,
), 7.80–7.78 (br, 8H, ![[b with combining low line]](https://www.rsc.org/images/entities/i_char_0062_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/i_char_0079_0332.gif) ), 7.12–7.00 (m, 12H,
), 7.12–7.00 (m, 12H, ![[D with combining low line]](https://www.rsc.org/images/entities/i_char_0044_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/i_char_0069_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif)
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif) ), 3.69 (m, 8H, C
), 3.69 (m, 8H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) Me2), 1.22 (m, 24H, CH
Me2), 1.22 (m, 24H, CH![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), 0.89 (m, 24H, CH
2), 0.89 (m, 24H, CH![[M with combining low line]](https://www.rsc.org/images/entities/i_char_004d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/i_char_0065_0332.gif) 2), 0.32 (s, 24H, SiMe2) ppm. Satisfactory 13C NMR data were not obtained due to the poor solubility of 12-μ-(bipy)2 in CDCl3. Anal. calcd for C95H124Al4Cl8N8Si4 (12-μ-(bipy)2·toluene): C 60.63, N 5.95, H 6.64. Found: C 60.83, N 5.67, H 6.70.
2), 0.32 (s, 24H, SiMe2) ppm. Satisfactory 13C NMR data were not obtained due to the poor solubility of 12-μ-(bipy)2 in CDCl3. Anal. calcd for C95H124Al4Cl8N8Si4 (12-μ-(bipy)2·toluene): C 60.63, N 5.95, H 6.64. Found: C 60.83, N 5.67, H 6.70.
UV titration experiments
A 0.5 mL aliquot of a stock solution of 1-(Et2O)2 or 2-(Et2O)2 (5.0 × 10−3 M in toluene) was measured and mixed with varying amounts of pyrazine (2.5 × 10−3 M, 0–4 equivalents relative to 1-(Et2O)2 or 2-(Et2O)2) in a 5 mL volumetric flask. Toluene was then added to bring the total volume to 5 mL. The UV-vis spectra were recorded using a quartz cuvette (5.0 mL capacity and 1 cm path length).Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr. Li-Ching Shen (Center for Advanced Instrumentation at NYCU) for assistance with NMR experiments. This work is also supported by the Center for Emergent Functional Matter Science of National Yang Ming Chiao Tung University through The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.References
- H. Meerwein, G. Hinz, H. Majert and H. Sönke, J. Prakt. Chem., 1936, 147, 226–250 CrossRef CAS.
- (a) S. Solé and F. P. Gabbaï, Chem. Commun., 2004, 1284–1285 RSC; (b) V. Amendola, M. Bonizzoni, D. Esteban-Gómez, L. Fabbrizzi, M. Licchelli, F. Sancenón and A. Taglietti, Coord. Chem. Rev., 2006, 250, 1451–1470 CrossRef CAS; (c) C. R. Wade, A. E. J. Broomsgrove, S. Aldridge and F. P. Gabbaï, Chem. Rev., 2010, 110, 3958–3984 CrossRef CAS PubMed; (d) L. Gai, J. Mack, H. Lu, T. Nyokong, Z. Li, N. Kobayashi and Z. Shen, Coord. Chem. Rev., 2015, 285, 24–51 CrossRef CAS; (e) E. Galbraith and T. D. James, Chem. Soc. Rev., 2010, 39, 3831–3842 RSC; (f) D. Dakternieks, K. Jurkschat, H. Zhu and E. R. T. Tiekink, Organometallics, 1995, 14, 2512–2521 CrossRef CAS; (g) I. Rojas-León, H. Alnasr, K. Jurkschat, M. G. Vasquez-Ríos, G. Gómez-Jaimes, H. Höpfl, I. F. Hernández-Ahuactzí and R. Santillan, Organometallics, 2019, 38, 2443–2460 CrossRef; (h) V. Sharma, M. Simard and J. D. Wuest, J. Am. Chem. Soc., 1992, 114, 7931–7933 CrossRef CAS; (i) P. L. Lückert, J. Gilmer, A. Virovets, H.-W. Lerner and M. Wagner, Chem. Sci., 2025, 16, 147–155 RSC.
- (a) S. N. Kessler, M. Neuburger and H. A. Wegner, Eur. J. Org. Chem., 2011, 3238–3245 CrossRef CAS; (b) L. Hong, S. Ahles, A. H. Heindl, G. Tiétcha, A. Petrov, Z. Lu, C. Logemann and H. A. Wegner, Beilstein J. Org. Chem., 2018, 14, 618–625 CrossRef CAS PubMed; (c) A. Lorbach, M. Bolte, H.-W. Lerner and M. Wagner, Chem. Commun., 2010, 46, 3592–3594 RSC; (d) T. Ooi, M. Takahashi and K. Maruoka, J. Am. Chem. Soc., 1996, 118, 11307–11308 CrossRef CAS.
- D.-H. Wu, X.-J. Lin, W. Benchaphanthawee, Y.-H. Lee, Z.-C. Lin, H.-J. Li, P.-L. Chen, T.-S. Kuo, C.-L. Wang, Y.-K. Wu, C.-H. Peng and H.-J. Liu, ChemPlusChem, 2024, e202400382 Search PubMed.
- C.-C. Tseng, Y.-W. Ding, Z.-Y. Chen, H.-Y. Lan, H.-J. Li, Y.-S. Cheng, T.-S. Kuo, P.-L. Chen, W.-C. Wu, F.-K. Shi, T. Yang and H.-J. Liu, Inorg. Chem., 2024, 63, 11361–11368 CrossRef CAS PubMed.
- (a) M. Schiefer, H. Hatop, H. W. Roesky, H.-G. Schmidt and M. Noltemeyer, Organometallics, 2002, 21, 1300–1303 CrossRef CAS; (b) M. Schiefer, N. D. Reddy, H.-J. Ahn, A. Stasch, H. W. Roesky, A. C. Schlicker, H.-G. Schmidt, M. Noltemeyer and D. Vidovic, Inorg. Chem., 2003, 42, 4970–4976 Search PubMed.
- P. E. Romero, W. E. Piers, S. A. Decker, D. Chau, T. K. Woo and M. Parvez, Organometallics, 2003, 22, 1266–1274 CrossRef CAS.
- (a) U. Mayer, V. Gutmann and W. Gerger, Chem. Mon., 1975, 106, 1235–1257 CrossRef CAS; (b) V. Gutmann, Coord. Chem. Rev., 1976, 18, 225–255 CrossRef CAS; (c) M. A. Beckett, G. C. Strickland, J. R. Holland and K. Sukumar Varma, Polymer, 1996, 37, 4629–4631 CrossRef CAS.
- H. E. Katz, J. Org. Chem., 1989, 54, 2179–2183 CrossRef CAS.
- P. Niermeier, S. Blomeyer, Y. K. J. Bejaoui, J. L. Beckmann, B. Neumann, H.-G. Stammler and N. W. Mitzel, Angew. Chem., Int. Ed., 2019, 58, 1965–1969 CrossRef CAS PubMed.
- T. C. Schäfer, J. Becker, D. Heuler, M. T. Seuffert, A. E. Sedykh and K. Müller-Buschbaum, Aust. J. Chem., 2022, 75, 676–683 CrossRef.
- (a) M. Fontani, F. Peters, W. Scherer, W. Wachter, M. Wagner and P. Zanello, Eur. J. Inorg. Chem., 1998, 1998, 1453–1465 CrossRef; (b) M. Grosche, E. Herdtweck, F. Peters and M. Wagner, Organometallics, 1999, 18, 4669–4672 CrossRef CAS.
- C. E. Zachmanoglou, J. G. Melnick, B. M. Bridgewater, D. G. Churchill and G. Parkin, Organometallics, 2005, 24, 603–611 CrossRef CAS.
- K. Reuter, R. G. M. Maas, A. Reuter, F. Kilgenstein, Y. Asfaha and C. von Hänisch, Dalton Trans., 2017, 46, 4530–4541 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2390325, 2390330, 2390331, 2390335 and 2390401–2390405. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt03288h |
| This journal is © The Royal Society of Chemistry 2025 |