DOI:
10.1039/D5DT01100K
(Paper)
Dalton Trans., 2025, Advance Article
Flux crystal growth, structure, and optical properties of non-centrosymmetric oxysulfides Ln3Ga3Ge2S3O10 (Ln = La, Ce, Pr, Nd)†
Received
9th May 2025
, Accepted 4th June 2025
First published on 12th June 2025
Abstract
Single crystals of the non-centrosymmetric oxysulfides Ln3Ga3Ge2S3O10 (Ln = La, Ce, Pr, Nd) in a hexagonal space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c were grown by a flux crystal growth method using a eutectic BaCl2–NaCl molten salt. Single-crystal X-ray diffraction analysis of them revealed that the Ga and Ge atoms were located on the 6g and 4f sites, which were tetrahedrally coordinated with two O and two S atoms, and four O atoms, respectively. In the structure, the GaS2O2 and GeO4 tetrahedra form 1∞[Ga3S3O3] triangular tubes and Ge2O7 tetrahedral dimers aligned along the c axis, which are surrounded by LaS2O6 square prisms in the ab plane. Neutron powder diffraction studies on polycrystalline samples of La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 which were prepared by high-temperature solid state reactions supported the Ga/Ge cation order determined by the single-crystal structure analysis. UV-Vis-NIR absorption spectra revealed band gaps larger than 4.60 eV for La3Ga3Ge2S3O10, Pr3Ga3Ge2S3O10, and Nd3Ga3Ge2S3O10, while Ce3Ga3Ge2S3O10 was found to have a small band gap value of 3.51 eV because of the Ce-4f1 electronic configuration.
2c were grown by a flux crystal growth method using a eutectic BaCl2–NaCl molten salt. Single-crystal X-ray diffraction analysis of them revealed that the Ga and Ge atoms were located on the 6g and 4f sites, which were tetrahedrally coordinated with two O and two S atoms, and four O atoms, respectively. In the structure, the GaS2O2 and GeO4 tetrahedra form 1∞[Ga3S3O3] triangular tubes and Ge2O7 tetrahedral dimers aligned along the c axis, which are surrounded by LaS2O6 square prisms in the ab plane. Neutron powder diffraction studies on polycrystalline samples of La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 which were prepared by high-temperature solid state reactions supported the Ga/Ge cation order determined by the single-crystal structure analysis. UV-Vis-NIR absorption spectra revealed band gaps larger than 4.60 eV for La3Ga3Ge2S3O10, Pr3Ga3Ge2S3O10, and Nd3Ga3Ge2S3O10, while Ce3Ga3Ge2S3O10 was found to have a small band gap value of 3.51 eV because of the Ce-4f1 electronic configuration.
Introduction
Metal oxychalcogenides, in which oxide and chalcogenide ions coexist in one structure, have provided a playground for solid-state scientists to explore fascinating properties because of the large differences in their ionic radii, electronegativities, oxidation states, and polarizabilities.1,2 One recent significant advantage of oxychalcogenide compounds is the design of nonlinear optical (NLO) materials.3,4 Stabilizing metal-centered heteroleptic coordination environments containing both oxide and chalcogenide ligands offers a chance to significantly increase the optical band gaps and second harmonic generation (SHG) responses compared with chalcogenides, because of the presence of O 2p orbitals in the valence band maximum and acentric polyhedral units with polar atomic displacement. Since the discovery of the first phase-matchable oxyselenide BaGeOSe2 and oxysulfide SrZn2S2O with strong SHG intensities,5,6 a number of new oxychalcogenides have been reported to show good balanced properties with high SHG responses, high laser damage thresholds, and wide optical band gaps.3,7 While most of the reported NLO oxychalcogenide compounds were focused on applications in the infrared region because of their relatively small band gap values (<4 eV),7 a new oxysulfide La3Ga3Ge2S3O10 consisting of (Ga/Ge)O4 and (Ga/Ge)S2O2 tetrahedra exhibited promising properties for applications in the UV regions (Fig. 1): the exceptionally large band gap value (4.70 eV), short cutoff edge (250 nm), and strong SHG response twice that of the KH2PO4 benchmark compound.8 The fundamental origin of the large bandgap and strong SHG response can be attributed to the lack of an all-sulfide coordination environment and the presence of a heteroleptic coordination environment,9,10 as in [Ba2F2][Ge2O3S2] (1.4 × AgGaS2),11 Nd3Ga3Ge2S3O10 (1.7 × KDP, 0.8 × AgGaS2),12 and La3Ga3Si2S3O10 (1.7 × KDP, 0.3 × AgGaS2).13
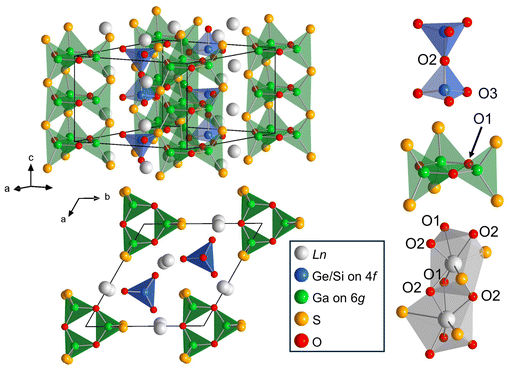 |
| | Fig. 1 Side and top views of the crystal structure of Ln3Ga3Ge2S3O10 (Ln = La, Ce, Pr, Nd), which possesses one-dimensional chains of LnS2O6 square antiprisms, and isolated dimers and triangular tubes composed of M1O4 tetrahedra and M2S2O2 tetrahedra, respectively. | |
Single-crystal X-ray diffraction (SCXRD) analyses of La3Ga3Ge2S3O10, La3Ga3Si2S3O10, and Nd3Ga3Ge2S3O10 have demonstrated that they are isostructural and adopt a hexagonal unit cell in the space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c.8,12,13 However, they exhibited different atomic distribution patterns, as described below. In the latter two phases, the Ga and Ge/Si atoms were located on Wyckoff positions 6g and 4f, respectively, to take the GaS2O4 and (Ge/Si)O4 tetrahedral geometries (Fig. 1). These tetrahedra formed 1∞[Ga3S3O3] triangular tubes and (Ge/Si)2O7 tetrahedral dimers, which were separated by (La/Nd)S2O6 square antiprisms in the ab plane. In contrast, La3Ga3Ge2S3O10 exhibited a random distribution of Ga and Ge over the 6g and 4f sites at an atomic ratio of 3
2c.8,12,13 However, they exhibited different atomic distribution patterns, as described below. In the latter two phases, the Ga and Ge/Si atoms were located on Wyckoff positions 6g and 4f, respectively, to take the GaS2O4 and (Ge/Si)O4 tetrahedral geometries (Fig. 1). These tetrahedra formed 1∞[Ga3S3O3] triangular tubes and (Ge/Si)2O7 tetrahedral dimers, which were separated by (La/Nd)S2O6 square antiprisms in the ab plane. In contrast, La3Ga3Ge2S3O10 exhibited a random distribution of Ga and Ge over the 6g and 4f sites at an atomic ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. It is reasonable for Si ions to adopt an all-oxygen homoleptic coordination geometry because they rarely form heteroleptic coordination polyhedra, while the difference in cation ordering patterns in La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 is non-trivial. In previous studies, the bond-valence-sum (BVS) values for Ga/Ge on 6g and 4f were estimated to be 3.04 and 3.92, respectively. This implied a Ga/Ge ordered state as in the Nd analog, although structural refinements based on the cation-ordered model were unstable. In oxides containing both Ga and Ge atoms, the identification of the distribution of these cations by XRD analysis is of major concern because of their similar X-ray scattering factors.14–16
2. It is reasonable for Si ions to adopt an all-oxygen homoleptic coordination geometry because they rarely form heteroleptic coordination polyhedra, while the difference in cation ordering patterns in La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 is non-trivial. In previous studies, the bond-valence-sum (BVS) values for Ga/Ge on 6g and 4f were estimated to be 3.04 and 3.92, respectively. This implied a Ga/Ge ordered state as in the Nd analog, although structural refinements based on the cation-ordered model were unstable. In oxides containing both Ga and Ge atoms, the identification of the distribution of these cations by XRD analysis is of major concern because of their similar X-ray scattering factors.14–16
In this study, we reexamined the Ga/Ge cation disordered state of La3Ga3Ge2S3O10 and newly synthesized a series of Ln3Ga3Ge2S3O10 (Ln = La, Ce, Pr, Nd) by both molten chloride flux methods and conventional solid-state reactions. Neutron powder diffraction (NPD), in addition to SCXRD, was employed to characterize the crystal structure, especially the Ga/Ge cation ordered pattern.
Experimental section
Reagents
La2S3 (Kojundo Chemical Laboratory, 3 N), La2O3 (Rare Metallic, 4 N), CeO2 (Rare Metallic, 4 N), Pr6O11 (Rare Metallic, 3 N), Nd2O3 (Rare Metallic, 3 N), Ga2O3 (Rare Metallic, 3 N), GeO2 (Rare Metallic, 4 N), Ge (Kojundo Chemical Laboratory, 4 N), S (Kojundo Chemical Laboratory, 4 N), BaCl2 (Rare Metallic, 3 N), and NaCl (Rare Metallic, 4 N) powders were used as received. BaCl2 and NaCl were heated overnight at 370 °C prior to use. La2O3 was preheated at 1000 °C in air prior to use. All raw materials were stored in an argon-filled glovebox (moisture and oxygen levels less than 0.1 ppm), and all manipulations before starting the reaction were carried out in a glovebox or under vacuum.
Crystal growth and elemental analysis
Single crystals of Ln3Ga3Ge2S3O10 (Ln = La, Ce, Pr, Nd) were obtained by the flux growth method using a BaCl2–NaCl eutectic molten salt. For Ln = La, La2S3 (0.5 mmol), Ga2O3 (0.5 mmol), and GeO2 (0.5 mmol) were combined according to the literature.8 For Ln = Ce and Pr, CeO2 (0.6 mmol)/Pr6O11 (0.1 mmol), Ga2O3 (0.3 mmol), Ge (0.4 mmol), and S (0.6 mmol) were combined. For Ln = Nd, Nd2O3 (0.3 mmol), Ga2O3 (0.3 mmol), Ge (0.3 mmol), S (0.6 mmol) and GeO2 (0.1 mmol) were combined. Each set of starting materials was loaded in an alumina crucible with BaCl2 (2.7 mmol) and NaCl (4.0 mmol). The crucibles were flame-sealed in fused silica tubes under a vacuum of 1 Pa, heated in a muffle furnace to 850 °C at 5 °C min−1, held at this temperature for 24 h, cooled to 550 °C at 0.08 °C min−1, and finally cooled naturally to room temperature. The products were then washed with sonicated water and extracted from the flux. Transparent rod-shaped crystals of the title compounds were collected by vacuum filtration with approximately yields of 65% for La, 10% for Ce, 30% for Pr, and 60% for Nd based on lanthanide (Fig. 2). Elemental analysis of single crystals of Ln3Ga3Ge2S3O10 was performed using a scanning electron microscope (SEM, HITACHI, S-43000) equipped with an energy dispersive X-ray (EDX) spectrometer. The accelerating voltage was set to 15 keV. EDX analysis indicated a Ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga
Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ge
Ge![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S atomic ratio of approximately 3
S atomic ratio of approximately 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, which was in good agreement with the chemical composition determined by single-crystal structure analysis.
3, which was in good agreement with the chemical composition determined by single-crystal structure analysis.
 |
| | Fig. 2 Photographs of single crystals of Ln3Ga3Ge2S3O10 (Ln = La, Ce, Pr, Nd) on a 1 mm-grid glass plate. | |
Solid state reaction
Polycrystalline powder samples of Ln3Ga3Ge2S3O10 (Ln = La–Nd) were synthesized by conventional solid-state reactions. The Ln = La, Pr, and Nd phases were synthesized from a stoichiometric mixture of La2O3 (0.3 mmol), Ga2O3 (0.3 mmol), GeO2 (0.1 mmol), Ge (0.3 mmol), and S (0.6 mmol) for Ln = La, Pr6O11 (0.1 mmol), Ga2O3 (0.3 mmol), Ge (0.4 mmol) and S (0.6 mmol) for Ln = Pr, Nd2O3 (0.3 mmol), Ga2O3 (0.3 mmol), GeO2 (0.1 mmol), Ge (0.3 mmol), and S (0.6 mmol) for Ln = Nd. The Ln = Ce phase was synthesized using a mixture of CeO2 (0.6 mmol), Ga2O3 (0.3 mmol), Ge (0.4 mmol), and S (0.6 mmol), which contains 0.1 mmol excess of oxygen compared with the stoichiometry of Ce3Ga3Ge2S3O10. Each set of starting materials was ground with an agate mortar and pestle, pressed into a pellet, sealed in a silica tube under a vacuum of 1 Pa, and heated in a muffle furnace at 1000 °C for 24 h. For neutron powder diffraction studies, scaled-up syntheses (approximately 10 times the amount) of polycrystalline powder samples of La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 were performed using a similar synthesis procedure.8,12
Single crystal structure determination
X-ray intensity data of single crystals of Ln3Ga3Ge2S3O10 (Ln = La–Nd) were collected using a Rigaku XtaLAB mini II diffractometer (Mo Kα radiation). Data collection covered more than 96% of the reciprocal space to 2θmax ∼ 60° with Rint = 4.17% for La, 3.42% for Ce, 3.19% for Pr, and 3.31% for Nd after absorption correction. The crystal structure was solved using a dual-space algorithm method (SHELXT)17 and refined using a full-matrix least-squares method with SHELXL18 using an Olex219 graphical user interface.
Powder XRD and UV-Vis-NIR
Powder XRD patterns were collected on a Rigaku MiniFlex-600 diffractometer (Cu Kα radiation) from the 2θ range of 5–70° with a step of 0.02° at room temperature. The UV-vis–NIR reflectance spectra were collected using a Shimadzu UV- 2600 UV-Vis-NIR spectrometer (used in the diffuse reflectance mode) equipped with an integrating sphere in the range of 220–1200 nm. Deuterium and halogen lamps were used as sources of UV and visible-NIR light, respectively. The recorded reflectance spectra were converted into the absorption data via the Kubelka–Munk function.
Powder neutron diffraction
Time-of-flight neutron powder diffraction (NPD) measurements were conducted at room temperature using an iMATERIA20 installed at J-PARC MLF BL20 in Japan. La3Ga3Ge2S3O10 (2.44 g) and Nd3Ga3Ge2S3O10 (2.57 g) were independently loaded into a vanadium can with 5.8 mm inner diameter, and the diffraction data were collected using a backscattering detector bank. The NPD data were analyzed by Rietveld refinement using the Z-code program.21
Results and discussion
Structure determination using single-crystal X-ray diffraction data
Typical dimensions of single crystals of Ln3Ga3Ge2S3O10 (Ln = La–Nd) were ranged from 0.1 × 0.1 × 0.2 to 0.5 × 0.5 × 0.8 mm3, indicating that these crystals grew preferentially along the c-axis. Single crystals of La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 were colorless and pale purple, respectively, as previously reported (Fig. 2). Ce3Ga3Ge2S3O10 and Pr3Ga3Ge2S3O10 were colorless and lime-green, respectively. The colors of Pr3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 are characteristic of f–f transitions in Pr3+ and Nd3+. These single crystals are stable in air and water insoluble. Single-crystal structure analysis of Ln3Ga3Ge2S3O10 (Ln = La–Nd) was performed at room temperature. As reported previously, the Ln = La phase adopts the hexagonal space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c (no. 190) with lattice parameters of a = 10.1701(4) Å and c = 7.5198(3) Å.8 Additionally, the structure refinement based on the cation ordered model that Ga and Ge atom occupied 6g and 4f sites, respectively, smoothly converged in contrast to our previous report. The Ln = Ce–Nd analogs were also found to adopt the same space group with lattice parameters, which were proportional to the ionic radius of Ln3+ ions (Fig. 3). The structure refinements indicated the Ga/Ge ordering, as reported previously for Nd3Ga3Ge2S3O10.12 The details of the final refined structure for all the phases are listed in Table 1. The atomic coordinates and isotropic thermal displacement parameters are listed in Table 2 and the anisotropic displacement parameters are listed in Table S1.† Selected interatomic distances and angles are listed in Table S2.† The metal–ligand bond distances in GeO4 and GaS2O2 tetrahedra in all phases are very close to the sum of their ionic radii (rGe4+ = 0.39 Å, rGa3+ = 0.47 Å, rO2− = 1.4 Å, rS2− = 1.84 Å).22 In contrast, in the LnS2O6 tetrahedron, the Ln–O/Ln–S bond distances are broad compared to the sum of their ionic radii, but their average bond distances are in good agreement with the sum of their ionic radii. Bond valence sum (BVS) calculations23 were carried out for all the atoms, as shown in Table 3. The BVS values of Ln (= La, Ce, Pr, Nd), Ga, and Ge atoms were consistent with the nominal oxidation number expected from the chemical composition.
2c (no. 190) with lattice parameters of a = 10.1701(4) Å and c = 7.5198(3) Å.8 Additionally, the structure refinement based on the cation ordered model that Ga and Ge atom occupied 6g and 4f sites, respectively, smoothly converged in contrast to our previous report. The Ln = Ce–Nd analogs were also found to adopt the same space group with lattice parameters, which were proportional to the ionic radius of Ln3+ ions (Fig. 3). The structure refinements indicated the Ga/Ge ordering, as reported previously for Nd3Ga3Ge2S3O10.12 The details of the final refined structure for all the phases are listed in Table 1. The atomic coordinates and isotropic thermal displacement parameters are listed in Table 2 and the anisotropic displacement parameters are listed in Table S1.† Selected interatomic distances and angles are listed in Table S2.† The metal–ligand bond distances in GeO4 and GaS2O2 tetrahedra in all phases are very close to the sum of their ionic radii (rGe4+ = 0.39 Å, rGa3+ = 0.47 Å, rO2− = 1.4 Å, rS2− = 1.84 Å).22 In contrast, in the LnS2O6 tetrahedron, the Ln–O/Ln–S bond distances are broad compared to the sum of their ionic radii, but their average bond distances are in good agreement with the sum of their ionic radii. Bond valence sum (BVS) calculations23 were carried out for all the atoms, as shown in Table 3. The BVS values of Ln (= La, Ce, Pr, Nd), Ga, and Ge atoms were consistent with the nominal oxidation number expected from the chemical composition.
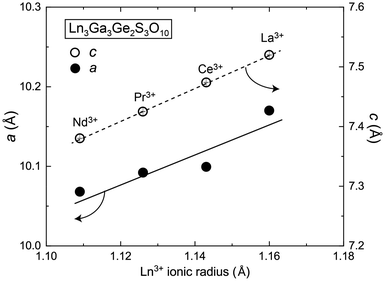 |
| | Fig. 3 The a-axis and c-axis lengths plotted as a function of the ionic radius of Ln3+. | |
Table 1 Results of structure refinement of Ln3Ga3Ge2S3O10 (Ln = La, Ce, Pr, Nd) using single-crystal XRD data
| Formula |
La3Ga3Ge2S3O10 |
Ce3Ga3Ge2S3O10 |
Pr3Ga3Ge2S3O10 |
Nd3Ga3Ge2S3O10 |
| Formula weight |
1027.25 |
1030.88 |
1033.25 |
1043.24 |
| T (K) |
297 |
297 |
293 |
294 |
| Crystal system |
Hexagonal |
Hexagonal |
Hexagonal |
Hexagonal |
| Space group |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c 2c |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c 2c |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c 2c |
P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c 2c |
| a (Å) |
10.1701(4) |
10.0993(4) |
10.0921(7) |
10.0682(5) |
| c (Å) |
7.5198(3) |
7.4738(4) |
7.4251(5) |
7.3802(5) |
| α (°) |
90 |
90 |
90 |
90 |
| β (°) |
90 |
90 |
90 |
90 |
| γ (°) |
120 |
120 |
120 |
120 |
| V (Å3) |
673.58(6) |
660.17(6) |
654.93(9) |
647.89(8) |
| Z |
2 |
2 |
2 |
2 |
| ρcalc (g cm−3) |
5.065 |
5.186 |
5.240 |
5.348 |
| μ (mm−1) |
20.096 |
21.201 |
22.042 |
23.023 |
| F000 |
912 |
918 |
924 |
930 |
| θ (°) |
3.534–30.53 |
2.329–30.64 |
3.5840–30.38 |
2.336–30.607 |
| Rint (%) |
4.17 |
3.42 |
3.19 |
3.31 |
| No. of reflections (collected/unique) |
9330/734 |
9529/723 |
2246/467 |
2524/658 |
| Goodness of fit on F2 |
1.061 |
1.083 |
0.977 |
1.041 |
| R1, wR2 [I > 2σ(I)] |
0.0109, 0.0251 |
0.0109/0.0208 |
0.0128/0.0234 |
0.0192/0.0378 |
| R1, wR2 (all data) |
0.0114, 0.0252 |
0.0126/0.0210 |
0.0136/0.0236 |
0.0216/0.0386 |
| Diff peak, hole (e Å−3) |
0.433, −0.387 |
0.454, −0.494 |
0.373, −0.330 |
0.712, −0.644 |
| Flack parameter |
−0.002(13) |
0.001(12) |
−0.03(3) |
−0.05(3) |
Table 2 Crystallographic and refinement data obtained from single-crystal structure analysis of Ln3Ga3Ge2S3O10 (Ln = La, Ce, Pr, Nd)
| Atom |
Site |
x |
y |
z |
Occupancy |
Uiso/ Å2 |
| La3Ga3Ge2S3O10 |
| La |
6h |
0.35751(3) |
0.38034(3) |
1/4 |
1 |
0.00771(8) |
| Ge |
4f |
2/3 |
1/3 |
0.51367(7) |
1 |
0.00446(13)a |
| Ga |
6g |
0 |
0.18935(5) |
0 |
1 |
0.00652(12)a |
| S |
6h |
0.01248(14) |
0.32093(14) |
1/4 |
1 |
0.0106(2) |
| O1 |
6g |
0.1757(3) |
0.1757(2) |
0 |
1 |
0.0089(7) |
| O2 |
12i |
0.5052(3) |
0.3439(3) |
0.4667(3) |
1 |
0.0111(5) |
| O3 |
2c |
2/3 |
1/3 |
3/4 |
1 |
0.033(2) |
| |
| Ce3Ga3Ge2S3O10 |
| Ce |
6h |
0.35730(3) |
0.38047(3) |
1/4 |
1 |
0.00888(7) |
| Ge |
4f |
2/3 |
1/3 |
0.51309(7) |
1 |
0.00542(13) |
| Ga |
6g |
0 |
0.19034(6) |
0 |
1 |
0.00761(11) |
| S |
6h |
0.01396(15) |
0.32373(15) |
1/4 |
1 |
0.0120(3) |
| O1 |
6g |
0.1770(3) |
0.1770(3) |
0 |
1 |
0.0111(7) |
| O2 |
12i |
0.5038(3) |
0.3439(3) |
0.4663(3) |
1 |
0.0115(5) |
| O3 |
2c |
2/3 |
1/3 |
3/4 |
1 |
0.032(2) |
| |
| Pr3Ga3Ge2S3O10 |
| Pr |
6h |
0.35754(5) |
0.38139(6) |
1/4 |
1 |
0.00797(13) |
| Ge |
4f |
2/3 |
1/3 |
0.51145(11) |
1 |
0.0039(2) |
| Ga |
6g |
0 |
0.19057(10) |
0 |
1 |
0.0070(2) |
| S |
6h |
0.01632(2) |
0.3260(2) |
1/4 |
1 |
0.0118(5) |
| O1 |
6g |
0.1764(6) |
0.1764(6) |
0 |
1 |
0.0135(15) |
| O2 |
12i |
0.5033(4) |
0.3433(4) |
0.4658(5) |
1 |
0.0106(9) |
| O3 |
2c |
2/3 |
1/3 |
1/4 |
1 |
0.036(4) |
| |
| Nd3Ga3Ge2S3O10 |
| Nd |
6h |
0.35688(5) |
0.38212(6) |
1/4 |
1 |
0.00923(14) |
| Ge |
4f |
2/3 |
1/3 |
0.51014(13) |
1 |
0.0056(2) |
| Ga |
6g |
0 |
0.19121(11) |
0 |
1 |
0.0076(2) |
| S |
6h |
0.0183(3) |
0.3287(3) |
1/4 |
1 |
0.0121(5) |
| O1 |
6g |
0.1778(6) |
0.1778(6) |
0 |
1 |
0.0123(14) |
| O2 |
12i |
0.5029(5) |
0.3435(5) |
0.4643(6) |
1 |
0.0114(9) |
| O3 |
2c |
2/3 |
1/3 |
1/4 |
1 |
0.036(4) |
Table 3 The values of bond-valence-sum calculations for Ln, Ge, and Ga sites obtained from the single crystal structure analysis
| Formula |
Ln at 6h |
Ga at 6g |
Ge at 4f |
| La3Ga3Ge2S3O10 |
2.81 |
3.03 |
3.91 |
| Ce3Ga3Ge2S3O10 |
2.83 |
2.95 |
4.03 |
| Pr3Ga3Ge2S3O10 |
2.92 |
2.98 |
4.05 |
| Nd3Ga3Ge2S3O10 |
2.81 |
2.98 |
4.04 |
X-ray powder diffraction
Fig. 4 shows the room-temperature X-ray powder diffraction patterns of polycrystalline Ln3Ga3Ge2S3O10 (Ln = La–Nd) samples synthesized by conventional high-temperature solid-state reactions. These compounds were obtained in nearly a single phase. All of these XRD patterns could be readily indexed to the hexagonal cell in the space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c, except for a few minor peaks that could be assigned to GeO2. Similar to the single-crystal structure analysis, the lattice constants of the polycrystalline samples also varied with the ionic radius of Ln3+ (Fig. S1†).
2c, except for a few minor peaks that could be assigned to GeO2. Similar to the single-crystal structure analysis, the lattice constants of the polycrystalline samples also varied with the ionic radius of Ln3+ (Fig. S1†).
 |
| | Fig. 4 Room-temperature X-ray powder diffraction patterns of Ln3Ga3Ge2S3O10 (Ln = La, Ce, Pr, Nd) polycrystalline samples obtained via high-temperature solid-state reactions. All phases can be assigned to a hexagonal cell in the space group P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c. 2c. | |
Structure determination using neutron powder diffraction data
To investigate the Ga and Ge atom distribution in La3Ga3Ge2S3O10, neutron powder diffraction experiments were performed on La3Ga3Ge2S3O10 at room temperature, together with Nd3Ga3Ge2S3O10, for comparison. Fig. 5 shows the results of Rietveld refinements of these two oxysulfides. For both La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10, the profile fitting to the Ga/Ge ordered model smoothly converged well with reliable factors, Rwp = 5.40, Rp = 4.24, RB = 2.58 for La and Rwp = 8.24, Rp = 5.79, RB = 5.97 for Nd (Fig. 5). To examine the possibility of Ga/Ge atoms being disordered, structure refinement was performed under the assumption that Ga and Ge atoms are disordered at the 6g and 4f sites. The results showed that 6g and 4f sites were more than 99% occupied by Ga and Ge atoms, respectively, within statistical errors. Thus, we conclude that the Ga and Ge atoms in the polycrystalline samples are ordered like the single crystals. Tables S3 and Table 3 summarize the final obtained crystallographic data including isotropic displacement parameters for all atoms. The occupancy factors of Ga on 6g and Ge on 4f were fixed at unity in the final refinement.
 |
| | Fig. 5 Neutron powder diffraction patterns collected from (a) La3Ga3Ge2S3O10 and (b) Nd3Ga3Ge2S3O10 at room temperature. Rietveld refinements were performed on the basis of the structural models determined by single crystal structure analysis. | |
Ga/Ge cation ordering and size effect of Ln3+ on the lattices
The present structure refinements against the SCXRD and NPD data of La3Ga3Ge2S3O10 revealed the Ga/Ge cation order, which differed from the Ga/Ge disorder characterized in the previous study.8 One possible reason for the cation disordering can be ascribed to a technical problem in the structure refinements. We reviewed the previous SCXRD data from the beginning and found that the cation ordered state was also stabilized. Perhaps, due to similar X-ray scattering factors of Ga and Ge atoms, the previous refinements fell into a local minimum that stabilizes the cation disordered state.
As shown in Fig. 3, the lattice parameters change linearly with the ionic radius of the Ln3+. On the other hand, the distortion index24 (D) around the metal centers, which was calculated using the metal–anion bond distances determined from the SCXRD data, exhibits unusual behaviors against the size of Ln3+ (Table S4†). The D values for the Ge-centered tetrahedra in Ln = La are 0.00911, which are remarkably larger than those for the corresponding tetrahedra in the other Ln ions by 15–31%, while the D values for the Ln- and Ga-centered polyhedra remained similar regardless of the Ln species. This can be rationalized by considering the volume of LnO6S2 square antiprisms, which form three-member rings via common S atoms in the ab plane and surround the Ga/Ge-centered tetrahedra. The GeO4 tetrahedron may be too small to fit into a framework consisting of LaS2O6, which has the largest volume compared to other Ln-centered polyhedra. We attempted to synthesize other members with Ln3+ smaller than Nd3+, but it was not successful.
Optical properties and bandgap
Fig. 6a shows the UV-Vis–NIR diffuse reflectance of Ln3Ga3Ge2S3O10 (Ln = La–Nd). The spectra of Ln = Pr and Nd exhibited a UV cutoff edge close to 250 nm, as seen for La3Ga3Ge2S3O10, which can be attributed to an optical transition from the valence band maximum composed of Ln-5d and Ga-4s, 4p orbitals to the conduction band minimum composed of O-2p and S-3p orbitals.8,12 A series of complex optical bands in a broad range of wavelengths result from the f–f transitions characteristic of the localized 4f orbitals of Ln ions. In contrast, the Ln = Ce phase exhibited stepwise absorption below 430 nm, followed by a cutoff edge at 340 nm. This characteristic absorption is due to the presence of the Ce-4f ground state between the VBM and CBM: optical transitions from the Ce-4f ground state to the lowest and second lowest Ce-4d states occur. Fig. 6b shows the absorption spectra converted from the diffuse reflectance spectra using the Kubelka–Munk function. The optical band gaps of Ln = La, Ce, Pr, and Nd estimated by the extrapolation method were 4.70, 3.51, 4.60, and 4.64 eV, respectively. Similar band gap values for Ln = La, Pr, and Nd are consistent with the similar Ln 5d energy levels.25
 |
| | Fig. 6 (a) UV-Vis–NIR diffuse reflectance and (b) absorption spectra of Ln3Ga3Ge2S3O10 (Ln = La, Ce, Pr, Nd). The bandgaps were estimated to be 4.70, 3.51, 4.60, and 4.64 eV, respectively. | |
SHG measurements
The powder SHG intensities of Ln3Ga3Ge2S3O10 (Ln = Ce, Pr) were measured using the Kurtz–Perry method at λ = 1064 nm, with polycrystalline KDP as a reference compound. The SHG signals are plotted as a function of particle size in Fig. 7. The SHG intensities of both phases increased with increasing particle size in the size region smaller than 150 μm, and were almost saturated in the larger particle size region. These behaviors indicate that they are type-I phase-matchable like La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10.8,12 The SHG intensities of Ln = Ce and Pr are comparable to that of KDP at the largest particle sizes measured, but somewhat smaller than those of the La and Nd analogs (∼2 × KDP). These high SHG intensities are consistent with a previous theoretical conclusion that the Ga/Ge-centered tetrahedra forming non-centrosymmetric sublattices are mainly responsible for the SHG response of La3Ga3Ge2S3O10.8,26,27
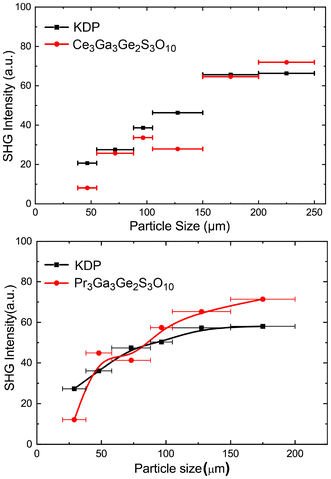 |
| | Fig. 7 Plots of SHG intensity vs. particle size at 1064 nm for Ln3Ga3Ge2S3O10 (Ln = Ce, Pr) and the reference compound KH2PO4 (KDP). Solid lines are drawn as guides to the eyes. | |
Conclusions
Although a Ga/Ge disordered state was previously suggested for single crystals of La3Ga3Ge2S3O10, re-investigation by single-crystal XRD and neutron diffraction experiments performed in this study showed that La3Ga3Ge2S3O10 has a Ga/Ge ordered state. Similar cation-ordered arrangements were observed for other lanthanide analogues. UV-Vis-NIR absorption spectra revealed band gaps larger than 4.60 eV for La3Ga3Ge2S3O10, Pr3Ga3Ge2S3O10, and Nd3Ga3Ge2S3O10, except for Ce3Ga3Ge2S3O10 with a small band gap value of 3.51 eV because of Ce-4f1 electronic configuration.
Conflicts of interest
The authors declare no competing financial interests.
Data availability
The data supporting this article have been included as part of ESI. X-ray crystallographic files for the structure have been deposited in Cambridge Crystallographic Data Centre (CCDC) with no. of 2449159, 2449160, 2449238, and 2449239.†
Acknowledgements
This study was supported by World Premier International Research Center Initiative (WPI), the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant no. 25K01507, 25K01657, 25H01652), Bilateral Program (No. JPJSBP120237714), and Core-to-Core Program (JPJSCCA20200004). Y. T. acknowledges the grant from the Murata Science Foundation. We also acknowledge Dr Ishigaki for his assistance in the NPD experiments with iMATERIA with the approval of J-PARC (Proposal No. 2023PM2001, 2024PM2001, 2025PM2002) and Prof. Z. Yang and Prof. S. Pan from Xinjiang Technical Institute of Physics and Chemistry, CAS for the SHG measurements.
References
- H. Kageyama, K. Hayashi, K. Maeda, J. P. Attfield, Z. Hiroi, J. M. Rondinelli and K. R. Poeppelmeier, Nat. Commun., 2018, 9, 772 CrossRef PubMed.
- M. Orr, G. R. Hebberd, E. E. McCabe and R. T. Macaluso, ACS Omega, 2022, 7, 8209–8218 CrossRef CAS PubMed.
- J. Huang, S. Shu and G.-M. Cai, Cryst. Growth Des., 2022, 22, 1500–1514 CrossRef CAS.
- Y. Zhang, H. Wu, Z. Hu and H. Yu, Chem. – Eur. J., 2023, 29, e202203597 CrossRef CAS PubMed.
- B. W. Liu, X. M. Jiang, G. E. Wang, H. Y. Zeng, M. J. Zhang, S. F. Li, W. H. Guo and G. C. Guo, Chem. Mater., 2015, 27, 8189–8192 CrossRef CAS.
- Y. Tsujimoto, C. Juillerat, W. Zhang, K. Fujii, M. Yashima, P. S. Halasyamani and H.-C. zur Loye, Chem. Mater., 2018, 30, 6486–6493 CrossRef CAS.
- J. jing Xu and K. Wu, Coord. Chem. Rev., 2023, 486, 215139 CrossRef CAS.
- H. Yan, Y. Matsushita, K. Yamaura and Y. Tsujimoto, Angew. Chem., Int. Ed., 2021, 60, 26561–26565 CrossRef CAS PubMed.
- B. Zhang, G. Shi, Z. Yang, F. Zhang and S. Pan, Angew. Chem., Int. Ed., 2017, 56, 3916–3919 CrossRef CAS PubMed.
- G. Shi, Y. Wang, F. Zhang, B. Zhang, Z. Yang, X. Hou, S. Pan and K. R. Poeppelmeier, J. Am. Chem. Soc., 2017, 139, 10645–10648 CrossRef CAS PubMed.
- H. Liu, Z. Song, H. Wu, Z. Hu, J. Wang, Y. Wu and H. Yu, ACS Mater. Lett., 2022, 4, 1593–1598 CrossRef CAS.
- M. Y. Ran, S. H. Zhou, W. B. Wei, B. X. Li, X. T. Wu, H. Lin and Q. L. Zhu, Small, 2023, 19, 2300248 CrossRef CAS PubMed.
- M. S. Zhang, S. M. Pei, X. M. Jiang, B. W. Liu and G. C. Guo, ACS Mater. Lett., 2024, 3, 312–318 Search PubMed.
- A. P. Dudka and B. V. Mill’, Crystallogr. Rep., 2013, 58, 594–603 CrossRef CAS.
- L. A. Gorelova, V. A. Yukhno, M. G. Krzhizhanovskaya and O. S. Vereshchagin, J. Solid State Chem., 2024, 329, 124429 CrossRef CAS.
- H. Bazzaoui, C. Genevois, D. Massiot, V. Sarou-Kanian, E. Veron, S. Chenu, P. Beran, M. J. Pitcher and M. Allix, Inorg. Chem., 2022, 61, 9339–9351 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A:Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C:Struct. Chem., 2015, 71, 3–8 Search PubMed.
- O. V. Dolomanov, A. J. Blake, N. R. Champness and M. Schröder, J. Appl. Crystallogr., 2003, 36, 1283–1284 CrossRef CAS.
- T. Ishigaki, A. Hoshikawa, M. Yonemura, T. Morishima, T. Kamiyama, R. Oishi, K. Aizawa, T. Sakuma, Y. Tomota, M. Arai, M. Hayashi, K. Ebata, Y. Takano, K. Komatsuzaki, H. Asano, Y. Takano and T. Kasao, Nucl. Instrum. Methods Phys. Res., Sect. A, 2009, 600, 189–191 CrossRef CAS.
- R. Oishi, M. Yonemura, Y. Nishimaki, S. Torii, A. Hoshikawa, T. Ishigaki, T. Morishima, K. Mori and T. Kamiyama, Methods Phys. Res., Sect. A, 2009, 600, 94–96 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A, 1976, 32, 751–767 CrossRef.
- N. E. Brese and M. O'Keeffe, Acta Crystallogr., Sect. B:Struct. Sci., 1991, 47, 192–197 CrossRef.
- W. H. Baur, Acta Crystallogr., Sect. B, 1974, 30, 1195–1215 CrossRef CAS.
- Y. Shimizu and K. Ueda, J. Lumin., 2015, 168, 14–19 CrossRef CAS.
- B.-H. Lei, S. Pan, Z. Yang, C. Cao and D. J. Singh, Phys. Rev. Lett., 2020, 125, 187402 Search PubMed.
- Z. Yang and S. Pan, Chin. Sci. Bull., 2024, 69, 4197–4208 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article e,
Masatomo Yashima
e,
Masatomo Yashima c,
Kazunari Yamaura
c,
Kazunari Yamaura ab and
Yoshihiro Tsujimoto
ab and
Yoshihiro Tsujimoto *ab
*ab
![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c were grown by a flux crystal growth method using a eutectic BaCl2–NaCl molten salt. Single-crystal X-ray diffraction analysis of them revealed that the Ga and Ge atoms were located on the 6g and 4f sites, which were tetrahedrally coordinated with two O and two S atoms, and four O atoms, respectively. In the structure, the GaS2O2 and GeO4 tetrahedra form 1∞[Ga3S3O3] triangular tubes and Ge2O7 tetrahedral dimers aligned along the c axis, which are surrounded by LaS2O6 square prisms in the ab plane. Neutron powder diffraction studies on polycrystalline samples of La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 which were prepared by high-temperature solid state reactions supported the Ga/Ge cation order determined by the single-crystal structure analysis. UV-Vis-NIR absorption spectra revealed band gaps larger than 4.60 eV for La3Ga3Ge2S3O10, Pr3Ga3Ge2S3O10, and Nd3Ga3Ge2S3O10, while Ce3Ga3Ge2S3O10 was found to have a small band gap value of 3.51 eV because of the Ce-4f1 electronic configuration.
2c were grown by a flux crystal growth method using a eutectic BaCl2–NaCl molten salt. Single-crystal X-ray diffraction analysis of them revealed that the Ga and Ge atoms were located on the 6g and 4f sites, which were tetrahedrally coordinated with two O and two S atoms, and four O atoms, respectively. In the structure, the GaS2O2 and GeO4 tetrahedra form 1∞[Ga3S3O3] triangular tubes and Ge2O7 tetrahedral dimers aligned along the c axis, which are surrounded by LaS2O6 square prisms in the ab plane. Neutron powder diffraction studies on polycrystalline samples of La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 which were prepared by high-temperature solid state reactions supported the Ga/Ge cation order determined by the single-crystal structure analysis. UV-Vis-NIR absorption spectra revealed band gaps larger than 4.60 eV for La3Ga3Ge2S3O10, Pr3Ga3Ge2S3O10, and Nd3Ga3Ge2S3O10, while Ce3Ga3Ge2S3O10 was found to have a small band gap value of 3.51 eV because of the Ce-4f1 electronic configuration.![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c.8,12,13 However, they exhibited different atomic distribution patterns, as described below. In the latter two phases, the Ga and Ge/Si atoms were located on Wyckoff positions 6g and 4f, respectively, to take the GaS2O4 and (Ge/Si)O4 tetrahedral geometries (Fig. 1). These tetrahedra formed 1∞[Ga3S3O3] triangular tubes and (Ge/Si)2O7 tetrahedral dimers, which were separated by (La/Nd)S2O6 square antiprisms in the ab plane. In contrast, La3Ga3Ge2S3O10 exhibited a random distribution of Ga and Ge over the 6g and 4f sites at an atomic ratio of 3
2c.8,12,13 However, they exhibited different atomic distribution patterns, as described below. In the latter two phases, the Ga and Ge/Si atoms were located on Wyckoff positions 6g and 4f, respectively, to take the GaS2O4 and (Ge/Si)O4 tetrahedral geometries (Fig. 1). These tetrahedra formed 1∞[Ga3S3O3] triangular tubes and (Ge/Si)2O7 tetrahedral dimers, which were separated by (La/Nd)S2O6 square antiprisms in the ab plane. In contrast, La3Ga3Ge2S3O10 exhibited a random distribution of Ga and Ge over the 6g and 4f sites at an atomic ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. It is reasonable for Si ions to adopt an all-oxygen homoleptic coordination geometry because they rarely form heteroleptic coordination polyhedra, while the difference in cation ordering patterns in La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 is non-trivial. In previous studies, the bond-valence-sum (BVS) values for Ga/Ge on 6g and 4f were estimated to be 3.04 and 3.92, respectively. This implied a Ga/Ge ordered state as in the Nd analog, although structural refinements based on the cation-ordered model were unstable. In oxides containing both Ga and Ge atoms, the identification of the distribution of these cations by XRD analysis is of major concern because of their similar X-ray scattering factors.14–16
2. It is reasonable for Si ions to adopt an all-oxygen homoleptic coordination geometry because they rarely form heteroleptic coordination polyhedra, while the difference in cation ordering patterns in La3Ga3Ge2S3O10 and Nd3Ga3Ge2S3O10 is non-trivial. In previous studies, the bond-valence-sum (BVS) values for Ga/Ge on 6g and 4f were estimated to be 3.04 and 3.92, respectively. This implied a Ga/Ge ordered state as in the Nd analog, although structural refinements based on the cation-ordered model were unstable. In oxides containing both Ga and Ge atoms, the identification of the distribution of these cations by XRD analysis is of major concern because of their similar X-ray scattering factors.14–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga
Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ge
Ge![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S atomic ratio of approximately 3
S atomic ratio of approximately 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, which was in good agreement with the chemical composition determined by single-crystal structure analysis.
3, which was in good agreement with the chemical composition determined by single-crystal structure analysis.

![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c (no. 190) with lattice parameters of a = 10.1701(4) Å and c = 7.5198(3) Å.8 Additionally, the structure refinement based on the cation ordered model that Ga and Ge atom occupied 6g and 4f sites, respectively, smoothly converged in contrast to our previous report. The Ln = Ce–Nd analogs were also found to adopt the same space group with lattice parameters, which were proportional to the ionic radius of Ln3+ ions (Fig. 3). The structure refinements indicated the Ga/Ge ordering, as reported previously for Nd3Ga3Ge2S3O10.12 The details of the final refined structure for all the phases are listed in Table 1. The atomic coordinates and isotropic thermal displacement parameters are listed in Table 2 and the anisotropic displacement parameters are listed in Table S1.† Selected interatomic distances and angles are listed in Table S2.† The metal–ligand bond distances in GeO4 and GaS2O2 tetrahedra in all phases are very close to the sum of their ionic radii (rGe4+ = 0.39 Å, rGa3+ = 0.47 Å, rO2− = 1.4 Å, rS2− = 1.84 Å).22 In contrast, in the LnS2O6 tetrahedron, the Ln–O/Ln–S bond distances are broad compared to the sum of their ionic radii, but their average bond distances are in good agreement with the sum of their ionic radii. Bond valence sum (BVS) calculations23 were carried out for all the atoms, as shown in Table 3. The BVS values of Ln (= La, Ce, Pr, Nd), Ga, and Ge atoms were consistent with the nominal oxidation number expected from the chemical composition.
2c (no. 190) with lattice parameters of a = 10.1701(4) Å and c = 7.5198(3) Å.8 Additionally, the structure refinement based on the cation ordered model that Ga and Ge atom occupied 6g and 4f sites, respectively, smoothly converged in contrast to our previous report. The Ln = Ce–Nd analogs were also found to adopt the same space group with lattice parameters, which were proportional to the ionic radius of Ln3+ ions (Fig. 3). The structure refinements indicated the Ga/Ge ordering, as reported previously for Nd3Ga3Ge2S3O10.12 The details of the final refined structure for all the phases are listed in Table 1. The atomic coordinates and isotropic thermal displacement parameters are listed in Table 2 and the anisotropic displacement parameters are listed in Table S1.† Selected interatomic distances and angles are listed in Table S2.† The metal–ligand bond distances in GeO4 and GaS2O2 tetrahedra in all phases are very close to the sum of their ionic radii (rGe4+ = 0.39 Å, rGa3+ = 0.47 Å, rO2− = 1.4 Å, rS2− = 1.84 Å).22 In contrast, in the LnS2O6 tetrahedron, the Ln–O/Ln–S bond distances are broad compared to the sum of their ionic radii, but their average bond distances are in good agreement with the sum of their ionic radii. Bond valence sum (BVS) calculations23 were carried out for all the atoms, as shown in Table 3. The BVS values of Ln (= La, Ce, Pr, Nd), Ga, and Ge atoms were consistent with the nominal oxidation number expected from the chemical composition.
![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c
2c![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c
2c![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c
2c![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c
2c![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c, except for a few minor peaks that could be assigned to GeO2. Similar to the single-crystal structure analysis, the lattice constants of the polycrystalline samples also varied with the ionic radius of Ln3+ (Fig. S1†).
2c, except for a few minor peaks that could be assigned to GeO2. Similar to the single-crystal structure analysis, the lattice constants of the polycrystalline samples also varied with the ionic radius of Ln3+ (Fig. S1†).






