Wet chemically produced nanomaterials for soft wearable biosensors
Ren
Wang
 ab,
Guangzhao
Mao
ab,
Guangzhao
Mao
 bc,
Dewei
Chu
bc,
Dewei
Chu
 d,
Noushin
Nasiri
d,
Noushin
Nasiri
 e,
Yuling
Wang
e,
Yuling
Wang
 f,
Marcela
Bilek
g,
Ken-Tye
Yong
f,
Marcela
Bilek
g,
Ken-Tye
Yong
 g,
Wallace
Wong
g,
Wallace
Wong
 h,
Stan
Skafidas
i,
Jefferson Zhe
Liu
h,
Stan
Skafidas
i,
Jefferson Zhe
Liu
 j,
Yuri
Kivshar
j,
Yuri
Kivshar
 k,
Madhu
Bhaskaran
l,
Yuerui
Lu
k,
Madhu
Bhaskaran
l,
Yuerui
Lu
 m,
Benjamin
Eggleton
n,
Arnold
Ju
m,
Benjamin
Eggleton
n,
Arnold
Ju
 g,
Qianqian
Shi
o,
Nam-Trung
Nguyen
g,
Qianqian
Shi
o,
Nam-Trung
Nguyen
 p,
Chwee Teck
Lim
p,
Chwee Teck
Lim
 qr and
Wenlong
Cheng
qr and
Wenlong
Cheng
 *ag
*ag
aDepartment of Chemical Engineering, Faculty of Engineering, Monash University, Clayton 3800, Victoria, Australia. E-mail: wenlong.cheng@sydney.edu.au
bSchool of Chemical Engineering University of New South Wales (UNSW Sydney), Sydney, New South Wales 2052, Australia
cSchool of Engineering, Institute for Materials and Processes, The University of Edinburgh, Robert Stevenson Road, Edinburgh, EH9 3FB, UK
dSchool of Materials Science and Engineering, University of New South Wales (UNSW Sydney), Sydney, New South Wales 2052, Australia
eSchool of Engineering, Faculty of Science and Engineering, Macquarie University, Sydney 2109, Australia
fSchool of Natural Sciences, Faculty of science and engineering, Macquarie University, Sydney, NSW 2109, Australia
gSchool of Biomedical Engineering, Faculty of Engineering, The University of Sydney, Sydney, NSW 2008, Australia
hSchool of Chemistry, Bio21 Institute, University of Melbourne, Parkville, Victoria 3010, Australia
iDepartment of Electrical and Electronic Engineering, Faculty of Engineering and Information Technology, The University of Melbourne, Parkville, VIC 3010, Australia
jDepartment of Mechanical Engineering, The University of Melbourne, Parkville, VIC 3010, Australia
kResearch School of Physics, Australian National University, Canberra, ACT 2601, Australia
lFunctional Materials and Microsystems Research Group, RMIT University, Melbourne, Victoria 3001, Australia
mSchool of Engineering, College of Engineering, Computing and Cybernetics, the Australian National University, Canberra, ACT, Australia
nSchool of Physics, The University of Sydney, Sydney, NSW 2006, Australia
oSchool of Environmental and Life Sciences, University of Newcastle, Callaghan 2308, New South Wales, Australia
pQueensland Micro- and Nanotechnology Centre, Griffith University, 170 Kessels Road, Nathan, Queensland 4111, Australia
qDepartment of Biomedical Engineering, National University of Singapore, Singapore 117576, Singapore
rInstitute for Health Innovation and Technology, National University of Singapore, Singapore 117599, Singapore
First published on 29th April 2025
Abstract
Wearable biosensors are gaining significant attention for their ability to monitor vital health signs remotely, continuously, and non-invasively. Nanomaterials offer transformative potential for next-generation soft wearable sensors, enabling seamless skin integration with enhanced comfort and data accuracy. Wet chemistry provides a scalable, cost-effective approach to producing nanomaterials, transforming rigid sensors into soft, flexible, and stretchable devices for broader wearable applications. This review highlights recent advances in soft wearable biosensors based on wet chemically produced nanomaterials, including metals, carbons, conducting polymers, conductive hydrogels, and liquid metals. It discusses fabrication techniques such as conductive ink formulation, ink delivery, electroless coating, and fiber integration, along with applications in physiological, physical, and biochemical monitoring. The review concludes by addressing challenges and opportunities, emphasizing the potential of these sensors in revolutionizing medical technology and personalized healthcare.
1. Introduction
Wearable biosensors are a rapidly evolving field, bridging the gap between biotic and abiotic systems and driving significant advancements in healthcare, including diagnostics, therapy, prosthetics, and a deeper understanding of physiological processes.1,2 The human body continuously performs physiological activities, generating unique bio-electrical, bio-mechanical, and bio-chemical signals that reflect health and activity status.3 Biosensors can transduce signals across the tissue-device interface, enabling them to accurately measure, precisely predict, and effectively regulate biological activities within healthcare systems. We have seen rapid advancements in biosensors spanning across chemistry, materials, engineering, biology, life sciences and digital technology.Wearable biosensors represent a rapidly growing sector in healthcare, with the potential to transform traditional hospital-centered diagnostics into patient-centered, connected healthcare systems of the future.4 The wearable technology market size was valued at USD 84.20 billion in 2024 and is projected to expand at a compound annual growth rate of 13.6% from 2025 to 2030 and will reach USD 186.14 billion in 2030, based on the report from Grand View Research. The worldwide COVID-19 pandemic also expanded the role of these wearable devices in the healthcare sector. The growing market needs drive the significant research efforts in academia to quest for solution to design and fabricate next-generation soft wearable biosensors.
The key components of next-generation wearable biosensors are soft electrodes, which interface directly with on-body environments such as the skin, eyes, and teeth.5 The rational design of soft electrodes is considered pivotal for next-generation healthcare biosensors, contributing to enhanced quality of life.6,7 In electrophysiological biosensors, soft electrodes must maintain high conductivity to collect electrical signals from the skin while exhibiting minimal resistance changes under strain to mimic natural skin movements. In physical biosensors, particularly stress–strain sensors, the focus shifts toward achieving significant changes in transduction signals (e.g. resistance, capacitance or piezoelectric etc.) under strain to ensure high sensitivity to mechanical deformation. For electrochemical biosensors, sensitivity depends on active sensing materials—such as enzymes, antibodies, and aptamers—designed to target specific biomarkers.8 One crucial aspect of all those applications is the rational design of soft electrodes. For example, in the case of electrophysiology biosensors, soft electrode must maintain low resistance changes when subjected to strain to ensure reliable performance.
The key challenge lies at mismatch in Young's moduli of dissimilar materials interface, as illustrated in Fig. 1 – particularly between soft biological tissues and rigid man-made electronics. The mechanical stiffness of conventional electronics can cause adverse effects on the skin, including discomfort, irritation, and allergic reactions.9 The ideal wearable biosensors should be soft, lightweight, flexible, and even stretchable for continuous, non-invasive, comfortable real-time monitoring. Our skin undergoes routine stretching in normal life (for example, 30% stretching for arm area and 80% stretching for elbow area).10 The key challenge lies in the maintenance of their biosensing functionalities in everyday activities such as walking or running.11–13 This challenge may be addressed by soft wearable biosensors, for which there have been excellent reviews covering this topic from different perspectives, such as epidermal electronics,14 prosthetic electronic skin,15 wearable electrochemical biosensors,16 nature-inspired flexible electronics,17 stretchable organic electronics,18 nanomaterial-based soft bioelectronics,19 disruptive soft wearable sensors,20 hydrogel bioelectronics,21 wearable sweat sensors22 and electronic tattoos.23 Despite significant advances in the field, a comprehensive review specifically addressing wet-chemically synthesized nanomaterials remains absent. Such nanomaterials can be readily integrated into soft substrates via wet chemical fabrication, a low-cost and scalable method typically conducted under mild conditions, thereby circumventing the need for harsh environments such as high temperatures, high pressures, or costly cleanroom facilities.24
This review highlights the use of nanomaterials synthesized through wet chemical approaches for designing next-generation soft wearable biosensors. It begins with the discussion of “stretchable electrodes” through the lens of novel nanomaterials utilized, including metals, carbons, conductive polymers (CPs), conductive hydrogels (CHs) and Liquid metals (LMs). Subsequently, we provide a detailed examination of wet chemical fabrication methods including ink formulation (chemical reduction, hydrothermal and sol–gel synthesis), ink delivery such as drop-casting, pen writing, Langmuir–Blodgett (LB) and printing technologies. This is followed by an description of electroless coating, electrodeposition, and fiber integration techniques, including twisting, dry-spinning, and electrospinning. Afterwards, we cover the state-of-the-art progress of wearable biosensors in healthcare-related applications. The review concludes with a summary of viewpoints into the current challenges and future prospects in the field.
Fig. 2 illustrates the overall wet chemical enabled wearable biosensors in terms of active materials (inner ring), wet manufacturing methods (middle ring) and various applications (outer ring). Nanosized active materials are usually formulated into conductive electronic inks, which can be deposited onto or into elastomeric substrates by various fabrication methods including printing,25 drawing26 and mixing.27 Alternatively, some direct electroless coating approaches may enable direct conductive coating of nanomaterials onto elastomeric surfaces.28 Those nanomaterials-incorporated elastomers have been used to demonstrate various wearable applications. Wearable electrocardiography (ECG), electroencephalography (EEG) and electromyography (EMG) can detect heart conditions,29 brain and muscle activities30 or human–machine interface.31 Wearable physical biosensors can detect physical signals such as heart rate,32 skin motion,33 and blood pressure.34 Wearable electrochemical biosensors, integrated with bio- or chemo-receptors such as enzymes, antibodies, ion exchange membranes, and aptamers, can detect signal changes associated with various biomarkers in body sweat.35,36
 | ||
| Fig. 2 A schematic illustrating wet chemically produced nanomaterials for wearable biosensors, highlighting key aspects such as active materials, fabrication methods, and applications. | ||
2. Wet chemically produced nanomaterials for stretchable conductors
Stretchable conductors are vital for wearable biosensors such as electrophysiological, physical, and chemical/biochemical sensors. These soft conductors provide the flexibility and durability needed to maintain consistent performance while conforming to the dynamic movements of the human body, such as skin deformation. They enable reliable signal transmission and data acquisition, which are critical for accurately monitoring biometrics such as heart rate, blood pressure, electrocardiograms, and sweat composition. Their inherent stretchability ensures seamless integration into daily activities, enabling continuous health monitoring without compromising user comfort or device functionality.To fabricate stretchable conductors, conductive nanomaterials are utilized to sustain conductivity through a percolation network embedded within a soft substrate. This approach imparts intrinsic stretchability to soft electrodes, which can be finely tuned by adjusting the concentration of conductive nanofillers incorporated into or onto the substrate matrix.
According to the percolation theory,37–39 conductivity is achieved by introducing conductive fillers into a polymer matrix. When the fillers are randomly distributed, a conductive network forms at a critical filler concentration, known as the percolation threshold. At this threshold, the composite material exhibits a sharp increase in conductivity as the filler loading surpasses the critical point. Using graphene filler in a polymer matrix as an example40 (illustrated in Fig. 3), three distinct regions are highlighted: three regions are observed: the insulating region (below the threshold), the percolating region (at the threshold), and the conductive region (beyond the threshold as graphene filler content increases).
 | ||
| Fig. 3 A schematic illustrating the conduction percolation theory, showing the effect of increasing graphene content in a polymer matrix. Adapted with permission.40 Copyright 2018 IOP publishing. | ||
However, there is an inherent trade-off between mechanical stretchability and conductivity. In the insulating region, where the concentration of conductive nanofillers is low, the material retains excellent stretchability, reflecting the inherent flexibility of the soft substrate. As the filler concentration increases, conductivity improves, but stretchability diminishes until reaching the conduction region. The primary challenge in designing stretchable electrodes lies in balancing mechanical and electrical properties—achieving high stretchability without compromising conductivity, particularly within the percolation region.
2.1. Metallic nanomaterials
Bulk metals have long been used as electrodes in biosensors and healthcare devices, but their inherent lack of stretchability has been a limitation. Recent advancements in nano-engineering and wet chemical fabrication techniques have significantly improved the development of metallic nanomaterials, enabling the design of inherently stretchable, electrochemically active, and biocompatible conductors.24,41A notable example of 0D AuNPs for fabricating stretchable conductors involved the use of polyurethane (PU) and the layer-by-layer (LBL) assembly technique to obtain nanocomposites, as shown in Fig. 4a–d.43 0D AuNPs were synthesized via a citrate-based redox process in an aqueous solution. After many LBL deposition cycles, the AuNPs/PU electrode achieved a remarkably high conductivity of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S cm−1. Impressively, it can maintain a conductivity of 2400 S cm−1 at 110% stretching due to the ability of the AuNPs to reorganize into a conductive pathway aligned with the direction of strain. This re-organization could lead to 1.7-fold increase in conductivity after 5000 stretching cycles.
000 S cm−1. Impressively, it can maintain a conductivity of 2400 S cm−1 at 110% stretching due to the ability of the AuNPs to reorganize into a conductive pathway aligned with the direction of strain. This re-organization could lead to 1.7-fold increase in conductivity after 5000 stretching cycles.
 | ||
| Fig. 4 Wet chemically produced gold nanomaterials for stretchable electrodes. (a)–(d) The scheme of 0D AuNPs based electrode at different stretching level. (e)–(h) SEM and optical images of 1D v-AuNWs on soft elastomer substrate when stretching-releasing cycle up to 300% ((e)–(h) adapted with permission.28 Copyright 2018 American Chemical Society). (i) The SEM images of v-AuNWs encapsulated in PDMS ((i) adapted with permission.44 Copyright 2019 John Wiley and Sons). (j)–(m) SEM and optical images of 2D AuNSs based electrode when stretching-releasing cycle up to 30% ((e)–(h) adapted with permission.45 Copyright 2013 John Wiley and Sons). | ||
1D gold nanowires (AuNWs) are highly favored in flexible bioelectronics due to their high aspect ratio, which enables percolation conductivity with minimal material usage.46 This approach achieves the desired conductivity without significantly compromising stretchability. Notably, ultrathin AuNWs, with diameters around 2 nm and aspect ratios exceeding 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, have been successfully developed by mixing an agent solution with a gold precursor in hexane solution. These AuNWs are employed in the creation of intrinsically stretchable electrodes for applications such as soft pressure sensors and wearable strain sensors.32,47
000, have been successfully developed by mixing an agent solution with a gold precursor in hexane solution. These AuNWs are employed in the creation of intrinsically stretchable electrodes for applications such as soft pressure sensors and wearable strain sensors.32,47
Another example of AuNWs in soft electronics is vertically aligned AuNWs (v-AuNWs) (Fig. 4e), which can directly grow on a wide variety of elastomeric substrates by using the seed-mediated method.28,48 It requires surface chemical modification and seed attachment, followed by the spontaneous growth of v-AuNWs in a solution containing a gold precursor with reducing agent. This approach could offer ultrahigh stretchability before electrical failure. Compared to bulk gold films, the superior stretchability of v-AuNWs films is attributed to the formation of minute cracks rather than catastrophic large cracks. As shown in Fig. 4f–h, the surface texture did not change much before, during and after 300% strain. Such wet chemically produced nanowire film was compatible with photolithography patterning,49 enabling the embedding of the v-AuNWs into polydimethylsiloxane (PDMS) with the tail side exposed (Fig. 4i). This configuration substantially enhanced durability of v-AuNW films leading to strain-insensitive conductors,44 enabling the fabrication of various transferable tattoo-like devices for wearable applications on human skin.
2D gold nanosheets (AuNSs) are a promising alternative for developing stretchable electrodes due to their unique assembly and structural properties. These 2D nanosheets can be efficiently self-assembled using a hydrothermal method at 95 °C in the air–water interface, resulting in structures with lateral dimensions in the micrometer range and a nanoscale thickness of approximately 20 nm (Fig. 4j).45 As shown in Fig. 4k–m, multilayered 2D AuNS electrodes can be fabricated through a repeated transfer process. These electrodes achieve a low sheet resistance of 2–3 Ω sq−1 after eight cycles and exhibit excellent electrical stability under 100% strain. This resilience is attributed to the reversible sliding and deformability of the AuNSs when subjected to stretching forces. It exhibited minimal changes in resistivity, even after 1000 strain-release cycles, due to the flexible nature of the AuNSs.
 | ||
| Fig. 5 Wet chemically produced silver nanomaterials for stretchable electrodes. (a) The top view SEM images of a stretchable electrode fabricated using 1D AgNWs with PDMS ((a) adapted with permission.50 Copyright 2012 John Wiley and Sons). (b) The schematic of a stretchable electrode fabricated by in situ formation of silver nanoparticles from silver flakes. (c) The Ag–Au core–sheath NW composite electrode with the Au–Ag core–shell structure (insert). | ||
Another example features a highly conductive electrode fabricated using a percolation network of 2D silver flakes and 0D AgNPs formed in situ. This was achieved by blending micrometer-sized silver flakes with fluorine rubbers and a surfactant. The 0D AgNPs are formed in situ from silver flakes through surfactant-assisted reduction of Ag+ ions diffused from the flake surfaces during a controlled heating and annealing process, as illustrated in Fig. 5b.51 The high-density formation of 2–10 nm AgNPs within the elastomer matrix, confirmed via TEM imaging, ensuring efficient tunnelling conduction for enhanced conductivity. Its high conductivity is attributed to the dense dispersion of AgNPs, which not only enhanced the percolation pathways between the particles but also minimized crack formation under mechanical strain, demonstrating an impressive conductivity of 6168 S cm−1 without strain, maintaining 935 S cm−1 even under 400% strain. The conductor demonstrates outstanding cyclic durability, maintaining stable resistance over 547 cycles at 50% strain, attributed to the surfactant and PU encapsulation layer.
An innovative approach has been developed to create Ag–Au core–sheath structured NWs through a galvanic-free deposition process using a gold–sulfite complex to selectively deposit a uniform Au shell. This method involves coating AgNWs with a layer of gold to prevent the leaching of cytotoxic silver ions, which could pose health risks. The scheme and backscattered electron image (insert) are shown in Fig. 5c.52 While local cracks may form in Ag–Au nanowire-rich regions under stretching, the overall percolation network remains intact due to the interconnected nature of the NWs. Additional electrical pathways are formed through adjacent nanowire-rich regions, ensuring the maintenance of electrical conductivity. The resulting Ag/Au- poly(styrene-butadiene-styrene) (SBS) nanocomposite electrode demonstrated remarkable stretchability of up to 266% (optimized) and up to 840% (maximum with heat rolling-press treatment) and outstanding conductivity of approximately 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S cm−1.
000 S cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Ω when bending at an angle to 180 degrees. The bending test exhibits a stable current of approximately 0.5 mA after 5000 cycles at a 50° bending angle.
000 Ω when bending at an angle to 180 degrees. The bending test exhibits a stable current of approximately 0.5 mA after 5000 cycles at a 50° bending angle.
 | ||
| Fig. 6 Wet chemically produced copper nanomaterials for stretchable electrodes. (a)–(c) The photo, TEM and SEM image of draw-on electrode inks made from CuNWs ((a)–(c) adapted with permission.53 Copyright 2015 American Chemical Society). | ||
2.2. Carbon-based nanomaterials
Various carbon nanomaterials have been extensively explored for applications in soft sensors.54 1D carbon nanotubes (CNTs) are appealing building blocks but are prone to agglomeration in solutions, a property paradoxically exploited to enhance the stretchability of electrodes. This is achieved by forming buckled CNT bundles that adapt to stretching forces. For instance, stretchable electrodes incorporating buckled CNTs on a PDMS substrate have been successfully fabricated by ultrasonication of arc-discharge CNTs in N-methylpyrrolidone (NMP). After sonication, the solution is centrifuged to remove large bundles and contaminants, producing a stable and homogeneous CNT dispersion. The stretchable electrodes are then doped with tetrafluorotetracyanoquinodimethane (F4TCNQ) to enhance their electrical conductivity, resulting in a sheet resistance of 328 Ω sq−1. The orientation of the CNTs can be readily adjusted through the stretching and releasing of the soft substrate, as illustrated in Fig. 7a and b.55 These spring-like CNT bundles achieve stretchability of up to 150% while maintaining a high conductivity of approximately 2200 S cm−1. It also showed only a 22% increase in resistance after 1500 stretching cycles at 25% strain, followed by a gradual increase up to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cycles. In another example, a stretchable CNT-based thin-film transistor achieved a high unit density of 347 transistors per cm2 by laminating a flexible styrene–ethylene–butylene–styrene (SEBS) substrate onto the CNT electrodes, followed by a water-soaking process to transfer the device from a rigid silicon substrate onto human skin.56 The CNT dispersion is prepared using bath sonication and centrifugation to ensure uniformity. By employing techniques such as spray coating, high-density CNT networks were successfully achieved. The transistor arrays maintained high charge-carrier mobility even under significant mechanical strain (Fig. 7c), demonstrating excellent electrical stability and mechanical robustness when stretched up to 100% and after 1000 stretching cycles. The array was highly stretchable up to 600% strain and maintained highly stable electrical performance, even when subjected to pressure, twisting, and stretching.
500 cycles. In another example, a stretchable CNT-based thin-film transistor achieved a high unit density of 347 transistors per cm2 by laminating a flexible styrene–ethylene–butylene–styrene (SEBS) substrate onto the CNT electrodes, followed by a water-soaking process to transfer the device from a rigid silicon substrate onto human skin.56 The CNT dispersion is prepared using bath sonication and centrifugation to ensure uniformity. By employing techniques such as spray coating, high-density CNT networks were successfully achieved. The transistor arrays maintained high charge-carrier mobility even under significant mechanical strain (Fig. 7c), demonstrating excellent electrical stability and mechanical robustness when stretched up to 100% and after 1000 stretching cycles. The array was highly stretchable up to 600% strain and maintained highly stable electrical performance, even when subjected to pressure, twisting, and stretching.
2D graphene sheets could form crumpled structures through wet-chemical processes, such as etching graphene films from nickel substrates with FeCl3 and transferring them onto biaxially pre-stretched polymer substrates and subsequently introducing controlled sequential relaxation of the pre-strains. Graphene films can self-organize into hierarchical structures after unfolding back from biaxially stretched, as shown in Fig. 7d.57 The as-prepared crumpled graphene electrodes exhibit minimal resistance increases even at high stretching levels up to 450% with excellent durability across 50 crumpling-unfolding cycles.
2.3. Conducting polymers
CPs provide a foundational framework for stretchable electrodes through their robust molecular-level organic polymer networks, which enable the mixed transport of ions and electrons. This dual transport capability is particularly advantageous for establishing seamless interfaces with soft substrates and biological tissues, which is essential for minimizing interfacial impedance-a critical parameter in many biosensing applications.58–60 Typical CPs materials include polyaniline (PANI), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) etc.CPs are organic in nature but consist of rigid, non-stretchable aromatic rings. To overcome these limitations, aqueous dispersions of PEDOT:PSS have been combined with ionic stretchability and electrical conductivity (STEC) enhancers. These additives attenuate the interaction between PSS and PEDOT, effectively softening the PSS domains and significantly enhancing conductivity, as demonstrated in Fig. 8a.61 By incorporating these enhancers, PEDOT:PSS/SEBS electrodes have achieved prominent conductivity levels exceeding 3100 S cm−1 without strain. Interestingly, the conductivity even increased to 4100 S cm−1 under 100% strain due to the alignment of polymer chains. Remarkably, the conductivity remains above 100 S cm−1 even under 600% strain. These enhanced properties make CPs well-suited for use as interconnects in electronic devices, where they can maintain stable conductivity under extreme deformation and even upon mechanical puncture. The conductor retains 92% of its original conductivity after 1000 cycles at 50% strain.
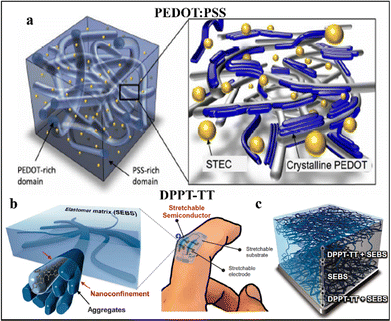 | ||
| Fig. 8 Conducting polymer-based nanomaterials for stretchable electrodes. (a) The scheme diagram representing the morphology of stretchable PEDOT:PSS conductor with enhancers ((a) adapted with permission.61 Copyright 2017 American Association for the Advancement of Science). (b) and (c) A 3D schematic illustrating the targeted morphology features embedded nanoscale networks of conductive polymer semiconductors for constructing highly stretchable and wearable thin-film transistors ((b) and (c) adapted with permission.62 Copyright 2017 American Association for the Advancement of Science). | ||
In another example, a poly(2,5-bis(2-octyldodecyl)-3,6-di(thiophen-2-yl)diketopyrrolo[3,4-c]pyrrole-1,4-dione-alt-thieno[3,2-b]thiophene) (DPPT-TT), a widely studied conjugated polymer, is fabricated using a wet chemical phase separation approach known as the conjugated-polymer/elastomer phase separation-induced elasticity method. In this process, DPPT-TT and the elastomer SEBS are dissolved in a common chlorobenzene solvent, ensuring homogeneous mixing before phase separation occurs.62 During the phase separation, nanoconfined fibrillar structures of DPPT-TT are embedded within the SEBS elastomer matrix, resulting in a highly stretchable and conductive film (Fig. 8b). The nanoconfinement process enhances polymer chain dynamics, reducing the modulus of DPPT-TT and significantly delaying crack formation under strain (up to 100%) while preserving its structural integrity without visible cracks and electrical performance (Fig. 8c). The stretchable transistors demonstrate exceptional biaxial stretchability, retaining stable on-current performance with 1.32 cm2 V−1 s−1 at 100% strain as a finger-wearable driver even under same mechanical stretchability. Additionally, the elastomer SEBS is compatible with other conjugated polymers discussed in the study, offering a generalised strategy for transforming conventional rigid conjugated polymers into stretchable semiconducting materials. This significantly broadens their potential applications in flexible and wearable electronics. The conductor also exhibits excellent long-term stability, maintaining its charge mobility after 100 cycles at 100% strain, and remaining stable for over a year in storage without noticeable degradation.
2.4. Conductive hydrogels
Hydrogels, which are water-swollen, porous 3D network polymers formed through physical or chemical crosslinking, are distinguished by their low Young's moduli. This makes them great candidates for bridging the gap between conventional rigid, dry, high-modulus electronics and soft, wet, low-modulus biological tissues. For biosensing applications, one important property of CHs is often their conductivity, a fundamental requirement for efficient electrical performance.63–65Hydrogels, being fully infiltrated with water, serve not only as highly stretchable soft substrates but also as intrinsically ionic conductive materials. Remarkable progress has been made in the field of hydrogel iontronics, where hydrogels are utilized as ionic conductors.66 An innovative soft ionic actuator employs two pieces of polyacrylamide (PAAM) hydrogel ionic conductors that were simply crosslinked by UV light for monomer, crosslinker and photo-initiator in water,67 with a dielectric layer sandwiched in between, connected to metal electrodes, as illustrated in Fig. 9a.67 The ionic conductivity of hydrogels can be tuned by modifying the concentration of salt dissolved within the hydrogel matrix. When a voltage is applied, the ionic conductors generate oppositely charged ions that attract each other. This attraction compresses the ionic conductors and the central dielectric elastomer, leading to a contraction in thickness and an expansion in surface area. Additionally, the ionic conductivity of hydrogels can be tuned by modifying the concentration of salt dissolved within the hydrogel matrix as an ionic conductor. Thanks to the inherent soft properties of hydrogels, the stretchability of such devices can reach up to 5.9 times their original length, accompanied by linear capacitance changes. The capacitance response remains stable for over 4000 stretching cycles at 2% strain.
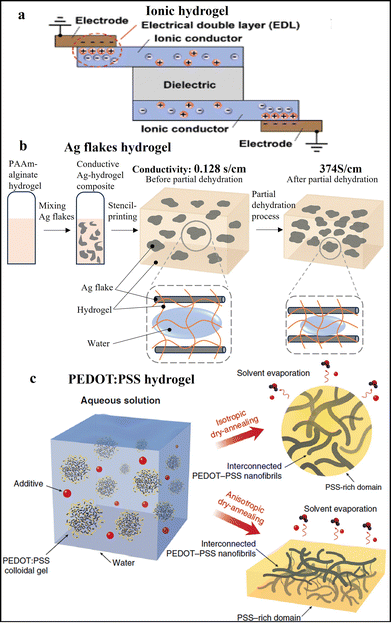 | ||
| Fig. 9 Hydrogels for stretchable electrodes. (a) The scheme of a stretchable hydrogel-based ionic conductor worked as a soft actuator ((a) adapted with permission.67 Copyright 2014 John Wiley and Sons). (b) The scheme of a silver/PAAM/Alginate composite hydrogel at initial and partial dehydrated states. (c) The scheme of a pure PEDOT:PSS hydrogel fabricated by dry-annealing ((c) adapted with permission.69 Copyright 2019 Springer Nature). | ||
To enhance conductivity, metallic conductive nanofillers have been incorporated into hydrogel matrices, leading to the development of novel composite materials with high conductivity. For instance, silver flakes were introduced into a pre-gel solution and subsequently subjected to in-situ polymerization, resulting in an Ag/PAAM/alginate composite double networks conductive CH.68 A partial dehydration process was utilized to compact this CH, enabling the formation of more dense percolation networks. This modification significantly enhanced conductivity, increasing from 0.128 S cm−1 to an impressive 374 S cm−1, as illustrated in Fig. 9b. Furthermore, the composite hydrogel demonstrated minimal resistance hysteresis during stretch-release cycles of up to 250%. The resistance increases slightly from 2.5 Ω to 4.2 Ω after 1000 stretching cycles at 100%.
CPs can also be formulated into hydrogels, offering a promising electrical interface with polymer networks. A PANI hydrogel was synthesized using phytic acid to reduce the π–π stacking distance, thereby facilitating improved electron transport. This approach results in a mesh-like hydrogel network with a high surface area and porosity.70 The 3D porous structure, featuring coral-like dendritic nanofibers, provides significant conductivity of 0.11 S cm−1 and stretchability under up to 100% strain. In another example, a pure PEDOT:PSS hydrogel was synthesized by incorporating the volatile additive dimethyl sulfoxide (DMSO) into aqueous PEDOT:PSS solutions, followed by controlled dry-annealing and rehydration processes, as shown in Fig. 9c.69 This pure PEDOT:PSS hydrogel exhibited high conductivity, achieving 20 S cm−1 in phosphate-buffered saline (PBS) and 40 S cm−1 in water after rehydration. Additionally, the hydrogel can be patterned with high resolution on polyethylene terephthalate (PET) substrates and displays tunable isotropic or anisotropic swelling behavior in wet physiological environments.
2.5. Liquid metals
LMs have been extensively utilized in soft wearable biosensors due to their unique properties. At room temperature, LMs retain a liquid-like state while offering excellent metallic conductivity and biocompatibility.71–73 For instance, LMs have been used to fabricate stretchable and permeable fiber mats through dip-coating with eutectic gallium–indium alloy (EGaIn) into electrospun SBS mats, forming a continuous conductive layer. As illustrated in Fig. 10a,74 this innovative structure self-organizes into a mesh-like, laterally and vertically buckled configuration, providing exceptional permeability, biocompatibility, stretchability (up to 1800%), and conductivity (up to 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S m−1). The LM mat electrode retains conductivity after 10
000 S m−1). The LM mat electrode retains conductivity after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 stretching cycles at 100%.
000 stretching cycles at 100%.
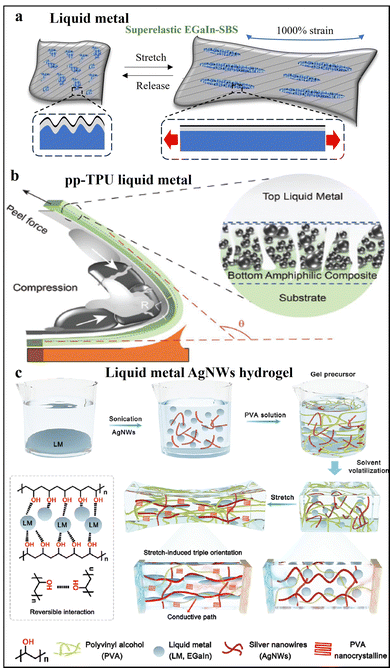 | ||
| Fig. 10 Wet chemically treated liquid metal particles for stretchable electrodes. (a) The schematic of a superplastic and permeable LM fiber mat at initial and 1000% stretched states. (b) The schematic of peeling activation of bilayer liquid–solid conductor ((b) adapted with permission.75 Copyright 2023 John Wiley and Sons). (c) The schematic of fabrication process of a LM/AgNWs composite hydrogel and photos at initial and 5000% stretching state ((c) adapted with permission.76 Copyright 2024 John Wiley and Sons. | ||
To realize an ultrahigh and strain-insensitive conductor, the bilayer liquid–solid conductor is fabricated using a wet chemical approach that involves preparing LM particles through sonication of eutectic gallium-indium alloy in cyclohexanone, followed by mixing with a polyester polyol-rich thermoplastic polyurethane (pp-TPU) binder to form a conductive ink.75 This ink is stencil-printed onto substrates, and the printed traces are activated via a peeling process, creating a bilayer structure with a liquid metal top layer and a composite bottom layer. The LM forms a continuous, self-healing conductive layer that accommodates strain without breaking, while the underlying composite layer of LM particles embedded in the TPU matrix provides mechanical support and strain redistribution, showing in Fig. 10b. This bilayer structure ensures that the conductive pathways remain intact even under extreme stretching, deformation, or damage, enabling self-healing capability stems from the fluidity and deformability of LM. The incorporation of liquid metal provides exceptional conductivity up to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 532 S cm−1 and ultrahigh stretchability up to 2260% strain, along with strain-insensitive performance, demonstrated as an excellent conductive and stretchable material for wearable devices. The bilayer liquid–solid conductor retains its conductivity with negligible resistance change over 10
532 S cm−1 and ultrahigh stretchability up to 2260% strain, along with strain-insensitive performance, demonstrated as an excellent conductive and stretchable material for wearable devices. The bilayer liquid–solid conductor retains its conductivity with negligible resistance change over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 stretching cycles at 100% strain.
000 stretching cycles at 100% strain.
By using these advanced conductive nanomaterials, as mentioned above, a novel highly conductive composite material has been developed by dispersing LMs particles and AgNWs into a pre-heated polyvinyl alcohol (PVA) solution. The mixture is then poured into silicone moulds and left to dry at room temperature, forming uniform hydrogels with well-distributed conductive components. Liquid metal is essential for the hydrogel's performance, providing electrical conductivity, preventing crack propagation under strain, and enabling strain-insensitive conductive pathways in synergy with AgNWs, as illustrated in Fig. 10c.76 This approach leverages the stretch-induced alignment of AgNWs, the deformability of LM, and the nanocrystalline structure of PVA to significantly enhance the hydrogel's mechanical properties, providing a stretchability of up to 5300%. The electrical conductivity of the hydrogels shows remarkable improvement from 4.05 × 10−3 to 24 S m−1 when stretched from 0% to 4200% strain. The composite material exploits the positive piezoconductivity effect, where conductivity increases with strain-contrasting the typical negative piezoconductivity observed in most materials. It also maintains conductivity after 1000 strain cycles at 100% strain with minimal resistance fluctuation.
In another example, a novel stimuli-responsive LM-elastomer architecture employs a wet chemical fabrication approach to achieve tunable conductivity and exceptional stretchability (up to 800%).77 Iron-incorporated gallium microparticles are dispersed within an elastomer matrix using HCl-assisted processing, enabling mechanically induced solidification, magnetically controlled phase switching, and Joule heating-based reconfiguration. This phase transition mechanism allows the material to shift from an insulating state (>200 MΩ) to a highly conductive state (<10 Ω). Its stimuli-responsive behaviour lies in the ability to self-adapt conductivity, enabling real-time thermal imaging and encrypted data storage via mechanical deformation, magnetic fields, or localized heating.
The stretchable conductors discussed above rely partially or entirely on wet chemical synthesis, which presents both advantages and limitations. As no single material meets all performance criteria, a comparative evaluation is necessary. Table 1 summarises key aspects of wet chemical processing methods, conductivity, stretchability, and biocompatibility. Although a wide range of stretchability values has been reported, our comparison is standardised to 100% strain—a threshold typically sufficient for most wearable applications.
| Type of nanomaterials | Wet chemical synthesis | Processing methods | Conductivity | >100% stretchability |
|---|---|---|---|---|
| 0D AuNPs | Citrate reduction43,78 | LBL and vacuum-assisted flocculation (VAF)43 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S cm−1 (ref. 43) 000 S cm−1 (ref. 43) |
Yes43 |
| 1D v-AuNWs | Aqueous reduction,28 oleyamine reduction in organic phase47 | Electroless deposition,28 dip-coating,47 drop-casting32 | ∼200 S cm−1,28 ∼2.5 MΩ sq−1,47 ∼2.03 MΩ sq−1 (ref. 32) | Yes28 |
| 2D AuNSs | L-Arginine-assisted reduction,45 ascorbic reduction79 | Water-assisted transfer,45 drop-casting79 | ∼2–3 Ω sq−1,45 1600 S cm−1 (ref. 79) | Yes45 |
| 1D AgNWs | Polyol reduction80 | Drop-casting,50 self-assembly at the water–oil interface81 | 8130 S cm−1,50 165![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 S cm−1 (ref. 81) 700 S cm−1 (ref. 81) |
Yes50 |
| 2D Ag flakes + 0D AgNPs51 | In situ reduction AgNPs | Screen-printing | 6168 S cm−1 | Yes |
| Ag–Au core–sheath NWs52 | AgNWs by polyol method and Au by galvanic-free deposition | Drop-casting | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 S cm−1 850 S cm−1 |
Yes |
| CuNWs53 | Glucose-based reduction,53 solution reduction82 | Pen-writing,53 freeze-drying and PDMS embedding82 | ∼850 Ω,53 8.1 S cm−1 (ref. 82) | Yes53 |
| 1D single-walled CNTs,55 CNT56 | N/A | Spray-coating and surface doping,55 spray-coating56 | 2200 S cm−1 (at 150% strain)55 | Yes55 |
| 2D graphene57 | FeCl3 etching | Stamp transfer | 200 Ω sq−1 before crumpling | Yes |
| PEDOT:PSS/STEC,61 DPPT-TT62 | Aqueous dispersions61 | Spin-coating,61 Phase separation62 | 4100 S cm−1 (ref. 61) | Yes61,62 |
| PAAM hydrogel,67 Ag flakes/PAAM/Alginate hydrogel,68 PEDOT:PSS hydrogel69 | Radical polymerization in water for hydrogel67 by mixing Ag flakes in pre-gel solution,68 Dry annealing and rehydration69 | N/A | Dependent on the concentration of dissolved salt,67 374 S cm−1 after dehydration,68 20 S cm−1 in PBS and 40 S cm−1 in water69 | Yes,67,68 No69 |
| Liquid metal fibre mat,74 pp-TPU liquid metal75 PVA/AgNWs liquid metal hydrogel,76 Fe/liquid metal77 | Forming composite with pp-TPU,75 mixed in solution and water evaporating,76 HCl-assisted processing77 | Electrospinning of fiber mat and screen printing,74 screen printing,75 phase transition77 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S m−1,74 22 000 S m−1,74 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 532 S cm−1,75 24 S m−1 at strain = 100%,76 from 200 MΩ to 10 Ω77 532 S cm−1,75 24 S m−1 at strain = 100%,76 from 200 MΩ to 10 Ω77 |
Yes74–77 |
3. Wet chemical fabrication methods
In general, increasing the amount of conductive nanomaterials deposited onto or embedded within elastomeric sheets improves electrical conductivity but often compromises mechanical compliance, and vice versa. To address this trade-off, various fabrication strategies have been developed to carefully balance the mechanical flexibility and electrical performance of materials used in stretchable electrodes.24 Among these approaches, wet chemical fabrication methods are not only cost-effective but also operate under mild processing conditions, making them particularly well-suited for scalable manufacturing with low cost.Common techniques for fabricating stretchable electrodes include a variety of ink delivery methods, such as drop-casting, spin-coating, pen-writing, screen printing, inkjet printing, and 3D printing (Fig. 1). The conductive inks used in these processes are typically prepared via chemical reduction, hydrothermal synthesis, or sol–gel methods. Additional deposition techniques, including Langmuir–Blodgett (LB) assembly and electrodeposition, are also employed.
To achieve high-resolution patterning on stretchable electrodes, wet chemical processes can be integrated with top-down fabrication techniques, enabling the development of wearable biosensors with micro- and nanoscale precision. Conductive fibers represent another versatile class of stretchable electrodes. These are fabricated using methods such as film twisting, dry-spinning, and electrospinning, which allow for the creation of highly flexible and stretchable network structures.
Each fabrication method offers distinct advantages and limitations depending on the specific requirements for conductivity, mechanical compliance, and the target application. A summary of various wet chemical approaches used in the fabrication of sensing devices is provided in Table 2.
| Chemical Method | Applications | Advantages | Disadvantages |
|---|---|---|---|
| Drop-casting | Strain sensor,32 ECG and strain sensor83 | Extremely simple, low-cost, no specialized equipment required | Poor uniformity and reproducibility |
| Dip-coating | Pressure sensor47 | Equipment-free, easy to perform | Weak adhesion to substrate |
| Spray-coating | Flexible circuit84 | Good uniformity, scalable over large areas | Significant material loss due to overspray |
| Spin-coating | Stretchable transistor array56 | Precise thickness control | Limited to flat surfaces; not suitable for complex geometries |
| Pen writing | Strain sensor,85 fuel cell86 | Low-cost, highly accessible, customizable patterns | Low throughput, limited resolution |
| Screen printing | Stretchable transistors,87 stretchable RFID tag88 | Scalable, compatible with various inks, cost-effective | Requires mesh tooling, less precise thickness control |
| Langmuir–Blodgett | Electroresistive heater89 | High film uniformity, good control over monolayer thickness | Poor adhesion, solvent-intensive process |
| Inkjet printing | Stretchable transistor,90 transistor arrays25 | Widely adopted, high resolution, adaptable to diverse nanomaterials | Requires expensive equipment, variable layer thickness |
| Roll-to-roll printing | Electrochemical sensor,91 stretchable circuit92 | Industrial scalability, low cost per unit, continuous production | Requires complex equipment, potential non-uniformity, limited resolution |
| 3D printing | ECG & nerve electrode,93 EMG & EOG94 | Enables complex 3D architectures, high material versatility | High equipment cost, relatively slower throughput |
| Electroless deposition | ECG,29 strain sensor,28 pressure sensor,34 electrochemical sensor95 | Inexpensive, no external power or equipment needed | Limited control over patterning structures |
| Electrochemical deposition | Fuel cell,96 electrochemical gas sensor97 | Excellent thickness control, suitable for patterned deposition | Requires conductive substrate and electrochemical setup |
| Dry spinning | Electrochemical sensor98 | Simple setup, low cost, continuous fiber production | Limited range of usable materials |
| Electrospinning | Strain sensor,33 strain & EMG sensor99 | High material versatility, controlled fiber diameter, moderate scalability | Requires high voltage setup, specialized equipment |
3.1. Conductive ink formulation
Seed-mediated chemical reduction synthesis is a powerful method for preparing conductive inks, offering precise control over nanoparticle size, shape, and surface functionality.42,100 This level of control is achieved by carefully selecting reducing agents, ligands, and dispersing agents to tailor key properties such as concentration, viscosity, and rheology—critical for the fabrication of soft electrodes. The seed-mediated approach enables robust regulation of nanoparticle nucleation and growth, wherein small, pre-formed “seed” particles act as nucleation centers for the controlled enlargement of nanoparticles. By tuning parameters such as the type of reductant, surfactant concentration, and growth duration, a wide range of nanostructures—from nanospheres and nanorods to nanowires—can be synthesized.101Conductive ink could be formulated without the need for seeds in some cases. For instance, ultrathin AuNWs with an aspect ratio exceeding 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 have been successfully synthesized using oleylamine as a capping agent in hexane solution.47 These AuNWs are soft and flexible, making them suitable for flexible wearable strain or pressure sensors.
000 have been successfully synthesized using oleylamine as a capping agent in hexane solution.47 These AuNWs are soft and flexible, making them suitable for flexible wearable strain or pressure sensors.
Another seedless approach is hydrothermal synthesis, which leverages high thermal energy to break the chemical bonds of precursors, leading to the formation of nanoparticles.102 This process involves chemical reactions in aqueous solutions at elevated temperatures, where nucleation and controlled growth yield nanoparticles with specific sizes and shapes. For example, a self-assembled graphene hydrogel can be produced via a one-step hydrothermal reduction of graphene oxide, driven by hydrophobic interactions and π–π stacking. This results in a robust, interconnected 3D porous network with physical cross-linking that retains water, forming a mechanically strong and conductive hydrogel ideal for electrochemical applications.103
Sol–gel processing is another widely used wet chemical technique, characterized by the transformation of a liquid “sol” (a colloidal suspension) into a solid “gel” phase. This method typically involves stages such as hydrolysis, condensation, aging, and calcination, starting from liquid-phase precursors.104 The resulting gel can be further processed into various material forms, including thin films and fibrous structures, offering remarkable versatility. For instance, ZnO nanocrystals with morphologies such as cones, hexagonal cones, and rods can be synthesized via non-hydrolytic ester elimination sol–gel reactions. This method allows for precise control over nanocrystal shape and uniformity, yielding high-purity structures well-suited for applications in optoelectronics, sensing, and catalysis.105
3.2. Conductive ink delivery
Once conductive inks are formulated, they must be deposited onto or into a soft substrate to fabricate stretchable electrodes. This can be accomplished using various techniques, including dip-coating,106 drop-casting,32 spin-coating,56 spray-coating,84 pen writing,85 LB89 and different printing technologies,27 such as screen printing,87 inkjet printing107 and 3D printing.93Drop-casting is a straightforward, equipment-free method for directly fabricating thin films from conductive ink solutions. For example, as illustrated in Fig. 11a,32 a stretchable thin film can be produced using drop-casting in combination with a polyimide (PI) mask. The resulting SEM image reveals polymer-like entangled and bundled morphologies of gold nanowires (AuNWs), which contribute to the film's impressive stretchability of up to 300% without cracking or delamination. This flexibility allows the film to conform to curved surfaces, such as a bending finger, as shown in Fig. 11b. In another example, drop-casting was used to fabricate a dual-layer percolation network comprising a 2D silver nanowire (AgNW) interfacial layer and a protruding 3D AgNW network embedded within an elastic polymer matrix. This architecture achieves both high conductivity (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 S cm−1) and outstanding stretchability of up to 660%.108 This approach ensures the uniform distribution and alignment of AgNWs, significantly enhancing electrode performance and stability under various conditions, including cyclic stretching, high temperatures, and physical damage.
500 S cm−1) and outstanding stretchability of up to 660%.108 This approach ensures the uniform distribution and alignment of AgNWs, significantly enhancing electrode performance and stability under various conditions, including cyclic stretching, high temperatures, and physical damage.
 | ||
| Fig. 11 Controlled conductive ink delivery of solution casting for stretchable electrodes. (a) and (b) The scheme and photo of an AuNWs film fabricate after dropping nano-ink onto substrate and attached onto skin ((a) and (b) adapted with permission.32 Copyright 2019 John Wiley and Sons). (c) A tattoo-like flower shape AuNWs/PANI electrode was prepared using direct writing by Chinese penbrush onto glove and stretching ((c) adapted with permission.85 Copyright 2015 American Chemical Society). (d) and (e) A typical LB assembly process at air–water interface and the close-packed AuNWs in mono- and multi-layer. | ||
Pen-writing offers a simple and versatile approach for creating draw-on electrodes with customizable designs on a variety of substrates. For instance, the pen-on-paper technique enables the direct writing of conductive features onto paper, facilitating the fabrication of flexible electronics. By using a rollerball pen filled with conductive silver ink, this method achieves a high conductivity of 4.34 × 10−6 Ω cm−1 following annealing under ambient conditions.26 Furthermore, it demonstrates excellent durability, with minimal resistance increases observed after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending cycles at a 1.6 mm bend radius. Another example involves a tattoo-like, flower-shaped AuNWs/PANI (well-mixed in ethanol/hexane solution as the conductive ink) electrode drawn directly onto a latex glove using a Chinese brush, as shown in Fig. 11c.85 This cost-effective approach not only produces soft, flexible electrodes but also showcases the potential for aesthetically versatile and customizable biosensors.
000 bending cycles at a 1.6 mm bend radius. Another example involves a tattoo-like, flower-shaped AuNWs/PANI (well-mixed in ethanol/hexane solution as the conductive ink) electrode drawn directly onto a latex glove using a Chinese brush, as shown in Fig. 11c.85 This cost-effective approach not only produces soft, flexible electrodes but also showcases the potential for aesthetically versatile and customizable biosensors.
The Langmuir–Blodgett (LB) technique is another effective method for fabricating stretchable electrodes. For example, LB assembly at the air–liquid interface can be employed to produce conductive films in monolayer or multilayer configurations on a variety of elastomeric substrates. As illustrated in Fig. 11d and e,89 The nanoparticle film supported at the air/water interface can be transferred onto an elastomeric substrate through simple immersion and lift-off steps. The film thickness, dictated by the number of layers, can be precisely controlled by adjusting the number of LB cycles.
Printing technology offers significant advantages for the fabrication of stretchable electrodes, including high precision, scalability, material versatility, and seamless integration with other components. These benefits span a wide range of methods—from traditional screen printing and widely adopted inkjet printing to scalable roll-to-roll processes,91 and advanced 3D printing techniques. While rooted in conventional techniques, screen printing has evolved into a critical platform for manufacturing soft, printable electronics. For instance, stretchable sensors integrated with passive radiofrequency identification (RFID) tags have been successfully fabricated using screen printing. In this approach, silver flakes blended with an elastomer-based ink are printed onto SEBS substrates.88 The resulting wearable sensor system features a skin-like modulus and enables continuous monitoring of physiological parameters—such as pulse, respiration, and movement—when conformally mounted on various areas of the body. Communication with a printed circuit board (PCB) is achieved via RFID, as shown in Fig. 12a. Remarkably, the sensors maintain full functionality under 50% strain and with varying RFID mounting geometries on the wrist, demonstrating excellent durability and practical suitability for wearable applications (Fig. 12b).
 | ||
| Fig. 12 Controlled printing technology for stretchable electrodes. (a) and (b) The schematic of wearable sensor with RFID for sensing pulse on human arm and a person wearing stretchable RFID sensor system on wrist. (c) The circuit schematic of inkjet printed circuit with sensors and images complementary ring oscillators. (d) The scheme of hybrid 3D printing including SLA 3D printing and DIW ((d) adapted with permission.94 Copyright 2024 John Wiley and Sons). | ||
Using a desktop inkjet printer, stretchable field-effect transistor (FET) arrays have been fabricated using novel materials, such as ionic PEDOT, ionic poly(vinylidene fluoride-co-hexafluoropropylene), and single-walled carbon nanotube (SWCNT) networks, achieving stretchability of up to 20%.25 In another study, inkjet printing was employed to deposit polymer-based organic semiconductors and AgNPs onto flexible substrates to fabricate electrodes. This technology enables the creation of complementary organic FETs and pressure-sensitive circuits, offering scalable and cost-effective production of flexible, high-performance sensors for wearable and prosthetic devices.90 The additive, maskless nature of inkjet printing facilitates scalable production and easy customization of device layouts in large scale, reducing processing complexity and enhancing device integration and interconnectivity, as shown in Fig. 12c.
3D printing enables the fabrication of intricate microscale architectures, including complex bio-inspired structures that are difficult to achieve using traditional techniques. For example, inspired by octopus suckers, stretchable electrode microstructures have been designed with micro-dome geometries embedded with serpentine microgrooves, providing superior adhesion, stretchability, and breathability compared to conventional patch electrodes.94 A hybrid 3D printing strategy—combining stereolithography (SLA) for structural formation with direct ink writing (DIW) for electrode patterning—allows precise control over the electrode architecture,94 as illustrated in Fig. 12d. An Ag/AgCl composite ink, synthesized via solution-based chemical reduction, ensures low resistivity and stable impedance, maintaining performance after repeated stretching up to 50%. These electrodes demonstrate excellent stretchability, strong adhesion, and consistent conductivity, making them ideal for long-term wearable monitoring applications, including ECG, EMG, and electrooculogram (EOG).
3.3. Electroless coating and electrodeposition
Electroless deposition offers a low-cost, scalable, and uniform method for coating conductive films onto elastomeric substrates without the need for external electric fields. A recent advancement has demonstrated the direct plating of gold nanowires (AuNWs) onto a variety of polymeric surfaces, including PET, Ecoflex, PDMS, and polyurethane sponges.109 The process begins with the anchoring of gold seed particles onto plasma-treated elastomer surfaces functionalised with amino groups. Vertical AuNWs (v-AuNWs) are then grown by immersing the substrate into a growth solution containing gold precursors, ligands, and reducing agents in a water/ethanol mixture, as illustrated in Fig. 13a.109 The use of strong-binding ligands passivates the exposed surfaces of the gold seeds, promoting vertically directed growth and resulting in unique Janus structural characteristics. Interestingly, as shown in the SEM and photographic images in Fig. 13b and c, the seed side (head) appears as a shiny gold surface, whereas the tail side (base of the grown AuNWs) appears dark black.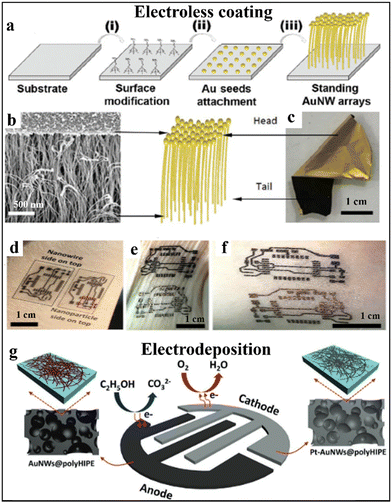 | ||
| Fig. 13 Electroless coating and electrodeposition approaches to fabricate stretchable electrodes. (a)–(c) The solution-based electroless coating of Janus v-AuNWs on elastomers ((a)–(c) adapted with permission.109 Copyright 2018 American Chemical Society). (d)–(f) The photos of complicated pattern of v-AuNWs conformal contact to human skin ((d)–(f) adapted with permission.44 Copyright 2019 John Wiley and Sons). (g) The scheme of electrodes for stretchable fell cell via electrodeposition of Pt on it ((g) adapted with permission.96 Copyright 2020 John Wiley and Sons). | ||
Such wet chemically electroless deposition could be combined with top-down fabrication methods such as photolithography, electron beam lithography (EBL), and shadow mask lithography for patterning micro and nanoscale structures for various applications.1,110 An example is the fabrication of patterned v-AuNWs stretchable tattoos as versatile sensors.44
To enhance the interfacial bonding, a polymethyl methacrylate (PMMA)-mediated approach can be used to embed v-AuNWs into elastomeric PDMS, offering super-strong soft/hard interfacial adhesion.44 Further introduction of photomask and photolithography enabled the generation of artificial PVA-based, transferable tattoos (Fig. 13d–f). This ultra-thin PDMS-supported gold tattoo could have an overall thickness of less than 10 μm, which could offer natural bonding forces with human skins for wearable applications of detecting physical and chemical biometric information, including pressure, strain, temperature, glucose, lactate and pH.
Once elastomeric electrodes are fabricated, electrochemical deposition could be used for fabricating functional stretchable electrodes, such as depositing catalysts. Adopting the electrochemical deposition method, Pt can provide a uniform coating on complex structures such as a porous polymerized high internal phase emulsions (polyHIPEs) surface for a skin-like fuel cell, as shown in Fig. 13g.96 Such electrodes can be intimately mounted on human skin with strain applied, retaining 88.7% power density under 40% strain.
3.4. Fiber integration
Fiber-type stretchable electrodes hold great promise for next-generation wearable biosensors, as textiles have been used by humans for millennia, offering inherent softness, flexibility, breathability, durability, and washability. These qualities make fibers a great platform for integrating wearable electronic devices.111–113 Nanomaterials can be incorporated into fibers to create stretchable sensors114 and energy devices.115 For example, a CNT fiber was fabricated from aligned CNT array sheets using a twisting process. The resulting free-standing fiber exhibits a unique spring-like structure, allowing it to withstand large strains of up to 300% without any degradation in performance.116In addition, it has been reported stretchable conductive gold fiber electrodes could be fabricated via a dry spinning process as shown in Fig. 14a.98 Initially, ultrathin AuNWs were mixed with SEBS in a THF solution, enabling the fabrication of AuNW-impregnated SEBS fibers through simple extrusion via a microneedle using a syringe pump. While these as-spun fibers exhibited low conductivity, their surfaces could be enhanced with a conductive gold layer through electroless plating. To improve stretchability and maintain conductivity, a pre-strain release strategy was employed, causing the surface-bonded gold film to form a naturally wrinkled structure upon strain release, as shown in the SEM image in Fig. 14b. This wrinkled gold configuration, combined with the fiber's intrinsic elasticity, resulted in minimal conductivity loss even under 100% strain.
 | ||
| Fig. 14 Dry and electrospinning for fiber-type stretchable electrodes. (a) and (b) The scheme and SEM image of a highly stretchable gold fiber with wrinkled design ((a) and (b) adapted with permission.98 Copyright 2020 Royal Society of Chemistry). (c) A schematic of an electrospun Au nanomesh conductors fabricated by dissolving electrospinning PVA fiber. | ||
Electrospinning has long been demonstrated to be a highly effective technique for fabricating fibers. In one example, it has been demonstrated the dry-spun fibers could be used to create flexible, gas-permeable, lightweight, and stretchable sensors using PVA Fig. 14c.99 The PVA nanomesh serves a dual purpose: it acts as a mask for gold plating and as a directly laminated layer onto the skin. After application, the PVA is dissolved, leaving behind a gold-plated nanomesh network. This interconnected network ensures efficient electron transport within the percolating pathways while maintaining excellent biocompatibility. The design is optimized for extended use to human skin without causing inflammation, making it ideal for wearable applications.
4. Wearable sensing applications
The previous sections have outlined the key attributes of wet chemically produced nanomaterials and their versatility in fabricating soft electronic devices. This section will further explore their typical applications in wearable biosensing, focusing on three major categories: wearable electrophysiological biosensors, wearable physical biosensors, and wearable electrochemical biosensors.4.1. Electrophysiological biosensors
Wearable electrophysiological biosensors, such as those used for ECG, EEG, and EMG, are specifically designed to monitor cardiovascular activity, brain function, and muscle movements, respectively. These sensors work by detecting biopotential signals and dynamic fluctuations at the skin surface. A key challenge in their design lies in achieving intimate and conformal skin contact to establish a seamless interface. This is essential for minimizing skin–electrode contact impedance and maintaining stable conductivity in soft bioelectrodes during normal body movement, thereby enabling prolonged and continuous monitoring in real-world environments.117,118Currently, commercial wet gel electrodes are considered the benchmark for electrophysiological testing, particularly in ECG applications. However, their long-term usability is limited due to rapid dehydration in open environments. In contrast, dry electrodes provide a more stable configuration without the risk of drying out, making them well-suited for extended use. For instance, a deformable electrode was developed using an AuNW-impregnated sponge fabricated via an electroless coating method. This durable electrode can function effectively as an ECG sensor and is capable of generating wireless signals when integrated with a flexible PCB, as illustrated in Fig. 15a.29 It maintains a dynamic signal quality index (dSQI) above 0.85 for over 3 hours, with minimal impedance changes under stretching (40%), compression (80%), and twisting (1080°). Importantly, high-quality ECG signals were obtained during real-world dynamic conditions, including driving, sleeping, eating, and office work. The dry AuNW foam electrode offers a wearable, comfortable solution for continuous cardiac monitoring, with promising applications in healthcare and sports medicine.
 | ||
| Fig. 15 Wearable electrophysiological biosensors. (a) The scheme of gold sponge-based wearable ECG sensor system ((a) adapted with permission.29 Copyright 2022 Elsevier B.V.). (b)–(d) The scheme of the design of ultrathin nanomesh hydrogel sensor for electrophysiological testing and the image of this hydrogel sensor mounting and peeling-off from skin ((b)–(d) adapted with permission.119 Copyright 2024 American Association for the Advancement of Science). | ||
Additionally, printed soft electrodes have shown great potential for EEG and EMG applications. Their skin-like properties help minimize irritation, enabling prolonged wear without discomfort or adverse reactions. In one example, printed biostickers made from a biphasic Ag/liquid metal (LM) composite demonstrated significantly lower skin–electrode impedance (6.99 × 104 Ω) compared to conventional Ag/AgCl electrodes, highlighting their promise for high-performance, long-term electrophysiological monitoring.120 The system captures high power spectral density in the 1–100 Hz range, delivering robust signal acquisition even under ambulatory conditions. For EMG monitoring, it maintains a high signal-to-noise ratio (SNR), enabling clear differentiation of muscle activity and hand gestures. The use of digital printing allows for rapid, customizable fabrication of the biostickers, which conform seamlessly to the skin and remain securely attached for several days—even during physical activity and bathing.
To enhance skin adhesion and sensing performance, a 10 μm thick nanomesh hydrogel sensor was fabricated by dip-coating electrospun PU nanomeshes into a pre-gel solution with thermally controlled phase transition properties (Fig. 15b).119 The ECG sensors achieve a SNR of 32.8 dB, surpassing that of commercial gel electrodes (29.7 dB), and maintain a low skin–electrode impedance of 31.3 kΩ, enabling stable cardiac monitoring for over eight days. Similarly, EEG signals remain stable for 24 hours, effectively capturing alpha rhythms with high power spectral density, demonstrating reliable neural activity tracking. The EMG monitoring device achieves an SNR of 38.2 dB, comparable to that of commercial electrodes, supporting accurate and consistent muscle signal detection. This ultrathin hydrogel demonstrates high stretchability, up to 696%, along with significant gas permeability and anti-drying capabilities when mounted on human skin, showing in Fig. 15c and d. It exhibited high-fidelity electrophysiological sensing performance comparable to commercial gels, able to measure motor conduction velocity (MCV), EOG, EEG, auditory brainstem response (ABR), and visual evoked potential (VEP) signals, besides ECG and EMG. These hydrogel sensors provide long-term wearability, high signal fidelity, and flexibility, reducing motion artifacts and skin irritation. While environmental factors like humidity and mechanical stress may affect performance, nanomesh hydrogels remain a promising alternative to traditional wet electrodes for precise, non-invasive, and durable electrophysiological monitoring in biomedical and healthcare settings.
4.2. Physical biosensors
Wearable physical biosensors are adept at detecting a wide array of physical signals from humans,29,121 including heart rate, blood pressure, respiratory rate, and acoustics etc. Besides comfortably conforming to the skin without impeding normal movement, these sensors are often required to have high sensitivity, commonly referred to as the gauge factor (GF). A wide range of nanomaterial and wet chemical fabrication methods enable GF adjustability, which allows the sensors to be fine-tuned according to specific monitoring needs, whether for subtle physiological changes or more significant health biometrics.Utilizing these principles, a skin movement detection strain sensor was fabricated by thermally evaporating gold onto a PU-PDMS core-sheath nanomesh. These were electrospun from a solution of N,N-dimethylformamide and methyl ethyl ketone in PU, and subsequently dip-coated with PDMS. This sensor is characterized by its thin thickness and ultra-conformability to human skin, providing minimal mechanical interference on natural skin deformations, thereby enabling continuous monitoring of motion activities.33 When these nanomesh strain mapping sensors are mounted on the human face, facial movements can be efficiently detected without any hysteresis or discrepancy compared to control markers. In another study, a float assembly method was demonstrated for fabricating highly stretchable electrodes, where Ag NWs or Ag–Au NWs are assembled at a water–toluene interface.81 This process leverages Marangoni flow, induced by solvent evaporation, to produce tightly packed, aligned nanowires embedded within a thin elastomer matrix, such as SEBS or thermoplastic PU, as shown in Fig. 16a. The method enables the creation of exceptionally conductive nanomembranes (exceeding 165![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S cm−1) that are directly integrated into flexible substrates and can withstand strains of up to 540%.81 After patterning these nanomembranes, a multifunctional skin-mounted wearable sensor array could be fabricated to detect various biometrics including strains.
000 S cm−1) that are directly integrated into flexible substrates and can withstand strains of up to 540%.81 After patterning these nanomembranes, a multifunctional skin-mounted wearable sensor array could be fabricated to detect various biometrics including strains.
 | ||
| Fig. 16 Wearable physical biosensors. (a) The scheme of the solution injection process to fabricate the monolayer assembly of close-packing NWs through marangoni flow under water–oil interface. (b) Schematic of the wearable hierarchically resistive skin as a single biosensor to report multiple biometrics information ((b) adapted with permission.123 Copyright 2023 Springer Nature). (c) and (d) The scheme of fabrication process of v-AuNWs based hierarchical pressure biosensor for pulse wave monitoring ((c) and (d) adapted with permission.34 Copyright 2019 John Wiley and Sons). (e) and (f) The scheme of the copper electrodes that are laminated with a PU thin film to form the parallel-plate pressure sensor structure and the gait tracking during rehabilitation and athletics ((e) and (f) adapted with permission.124 Copyright 2024 American Association for the Advancement of Science). | ||
While resistive signal transduction approach has been demonstrated facility of detecting strains,125 a hierarchically resistive wearable skin sensor has recently reported as shown in Fig. 16b.123 In this work, three kinds of nanomaterials are laminated including ultrathin gold nanowire inks and electrolessly plated v-AuNWs. The top layer features high conductivity and sensitivity achieved using sputter-coated platinum. The middle layer consists of v-AuNWs on PDMS, produced via the electroless coating method, offering medium conductivity and sensitivity. The bottom layer, providing low conductivity and sensitivity, was made from ultrathin AuNWs which are chemically reduced from gold precursors in hexane. In this design, different layers could detect strain signals with different sensitivity and GF, specific to physical activities in the throat related to heart rates, respiration, tactile touch, neck movement and acoustic vibration.
In one example, PDMS micro-pyramid arrays were integrated with electrolessly grown v-AuNWs, as illustrated in Fig. 16c and d.34 This configuration provides high sensitivity, particularly in the low-pressure range (below 600 Pa), owing to its hierarchical gold-film structure. With this sensor setup, high-quality arterial pulses can be recorded, where the systolic peak, diastolic peak, and reflective peak are clearly distinguishable. These results highlight the outstanding performance of this wet chemically produced wearable pressure biosensor. In another instance, as depicted in Fig. 16e,124 an advanced additive manufacturing technique known as continuous liquid interface production was utilized to fabricate stretchable capacitive pressure sensors featuring various lattice designs, such as Kagome and tetrahedral structures. This method involves designing and printing a 3D lattice dielectric layer, curing, and assembly by sandwiching it between two flexible electrodes with carbon grease added as the conductive medium to enhance sensing capabilities. These sensors exhibit high responsiveness to normal pressure, shear forces, and torsion, demonstrating excellent durability over more than 850 loading cycles. Their versatile applications include gait analysis, sports performance monitoring (Fig. 16f), integration into helmet linings for cranial impact detection, and force sensing in robotic grasping.
4.3. Electrochemical biosensors
Wearable electrochemical biosensors have recently emerged as innovative tools for detecting and analyzing biomarkers in biofluids such as sweat, interstitial fluid, and saliva, with the overarching goal of enabling disease prevention, early diagnosis, and personalized treatment.128–130 To ensure optimal performance, it is essential to rigorously evaluate and optimize key parameters such as sensitivity, selectivity, detection limit, and response time. These metrics are critical for the accurate and reliable analysis of a broad spectrum of biomarkers, including metabolites (such as glucose,131 lactate,10 uric acid132), electrolytes (including pH,98 sodium,95 chloride,133 potassium,133 calcium134 ions), as well as hormones (like cortisol135), and nutrients (e.g., amino acids136).Beyond single-analyte detection, integrated sensing systems have been developed to advance wearable biosensors toward real-world applications, positioning them as a key component in modern medical technology. These devices not only enhance patient comfort and compliance by reducing the need for frequent invasive testing but also enable continuous multi-parameter health monitoring and early disease detection.
 | ||
| Fig. 17 Wearable electrochemical biosensors for signal analytes analysis. (a) and (b) The scheme of sweat collection from the fingertip, through a PVA gel to sensor and the enzymatic reaction of glucose biosensor ((a) and (b) adapted with permission.138 Copyright 2021 American Chemical Society). (c) and (d) The scheme and Na+ ion testing performance of wearable ISE biosensors ((c) and (d) adapted with permission.95 Copyright 2019 American Chemical Society). (e) The reagentless in situ quantification of oestradiol using a AuNPs-MXene sensor coupled with a target-induced strand displacement aptamer switch ((e) adapted with permission.139 Copyright 2023 Springer Nature). (f) The mechanism of wearable skin-interfaced system direct detection for Tyr. | ||
Similarly, the levels of ions such as Na+, K+, and Ca2+ in sweat are essential for normal metabolism and are associated with various diseases. For example, pH levels representing hydrogen ion concentration play a significant role in physiological cycles, influencing overall health status and the wound-healing process.141,142 To monitor these electrolyte concentrations effectively, wearable ion-selective electrodes (ISEs) can be developed by modifying the working electrode with an ion-selective membrane, such as an ionophore for Na+ detection. The adoption of innovative electrode nanomaterials that conform well to human skin can substantially minimize signal drift and enhance stretchability of these sensors. As shown in Fig. 17c,95 v-AuNWs arrays, which are electrolessly coated on PDMS, function as stretchable electrodes. After electrodeposition with PANI and drop-casting ion-selective membranes, these electrodes have been utilized for monitoring pH, Na+, and K+, exhibiting good sensitivity and selectivity. These sensors can be comfortably mounted onto a human forehead with flexible PCB, allowing for efficient and unobtrusive sweat analysis. Notably, these sensors maintain relatively stable performance under stretching up to 30%, showing in Fig. 17d.
Proteins, including hormones found in human biofluids, are closely linked to various diseases and mental health conditions. As such, biosensors capable of detecting these proteins play a vital role in advancing personalized healthcare diagnostics. One example is a bandage-type biosensor developed for the detection of tyrosinase—an important enzyme biomarker for melanoma. This sensor is fabricated by screen-printing mechanically robust inks, composed of Ag/AgCl and carbon ink blended with Ecoflex, onto a medical bandage to ensure flexibility under mechanical deformation. It enables rapid, electrochemical detection of tyrosinase through the enzymatic conversion of catechol to benzoquinone, representing the first wearable enzyme-based platform for real-time protein sensing.136 In another example, the hormone cortisol, an important biomarker for chronic mental health conditions can significantly disrupt the homeostasis of the cardiovascular, renal, skeletal, immune, and endocrine systems if not properly monitored.143 Oestradiol, a pivotal female hormone oestrogen, is critical during the reproductive years and menstrual cycles.144 A novel wearable aptamer-based biosensor enables automatic and non-invasive monitoring of oestradiol from sweat, as depicted in Fig. 17e.139 This sensor integrates AuNP–MXene nanomaterials with a target-induced strand displacement aptamer switch, achieving an ultra-low detection limit of 0.14 pM. The square wave voltammetry (SWV) response of the aptamer-based estradiol sensor in artificial sweat exhibits a clear linear correlation with physiologically relevant estradiol concentrations. When combined with signal processing and wireless communication technologies, the sensor effectively captures cyclical fluctuations in estradiol levels from human participants, enabling real-time, non-invasive hormone monitoring.
Amino acids (AAs) are crucial biomarkers for various health conditions, deriving primarily from dietary intake and gut microbiota synthesis. For example, a wearable electrochemical biosensor has been developed to monitor the trace levels of all nine essential AAs, along with vitamins, metabolites, and lipids.140 This biosensor utilizes electrochemically redox-active reporters and molecularly imprinted polymer (MIP) strategies for sensitive detection of electroactive molecules directly, as shown in Fig. 17f. In this device, electroactive molecules such as tyrosine and tryptophan, which are chemically bonded to a MIP electrode, can be directly measured using differential pulse voltammetry (DPV), providing a direct, quantitative assessment during vigorous exercise.
Moreover, there exists a vast array of underexplored biomarkers that hold potential as targets for detection using wearable technologies.145–147 The transition of biomarkers from traditional laboratory-based methodologies to innovative wearable strategies represents a significant advancement in the field of healthcare monitoring.
The first fully integrated wearable sweat analysis platform is capable of monitoring different electrolytes (sodium and potassium ions) and metabolites (glucose and lactate) from human skin.148 This device demonstrated high sensitivity to diverse chemicals, enabling the real-time, wireless analysis of the sweat's physiological state in human subjects during physical activities. This platform opened a new route in real-time personalized diagnostics and physiological monitoring for multiple analytes. Subsequent developments have expanded the multiplexed sensing system to include smart textiles, thereby enhancing the user experience with breathable, comfortable, and cloth-like attributes.151 For example, a patch based on silk fabric-derived carbon textiles has been introduced for the simultaneous detection of metabolites and ions, including glucose, lactate, ascorbic acid, uric acid, Na+, and K+. Its hierarchical woven and porous structure makes it an outstanding platform for a comfortable user experience.
Further integration has resulted in the development of a wearable sensing system combined with a transdermal drug delivery platform, specifically tailored for diabetes management. This comprehensive system continuously monitors glucose, pH, temperature, and humidity, even under 20% tensile strain.152 By coupling with drug delivery (the right image of Fig. 18a), this innovative approach realizes a closed-loop system for point-of-care diabetes treatment for chronic conditions.
 | ||
| Fig. 18 Wearable integrated electrochemical sensing system. (a) The scheme and mechanism of in situ microfluidic sweat CRP analysis system that involves fully automatic sweat sampling, reagent routing and detection ((a) adapted with permission.153 Copyright 2023 Springer Nature). (b) The scheme of the fingertip-wearable microgrid system, which includes lactate-based biofuel cells, AgCl–Zn batteries, flexible PCB and wearable electrochemical sensors with an osmotic sweat extraction assisted paper fluidic system. (i) main schematic, (ii) ventral side and (iii) dorsal side. ((b) adapted with permission.154 Copyright 2024 Springer Nature). (c) The schematic and mechanism of FTENG charge distribution. (d) The schematic of a wearable sweat sensor system that integrates human motion energy harvesting, signal processing, microfluidic technology, and wireless data transmission for real-time health monitoring ((c) and (d) adapted with permission.155 Copyright 2024 American Association for the Advancement of Science). | ||
Wearable biosensors have demonstrated their applications in monitoring individuals with chronic conditions such as obstructive pulmonary disease, past infections, or heart failure, where elevated concentrations of C-reactive protein (CRP) serve as a critical biomarker, as shown in Fig. 18a.153 To address this need, a wearable patch has been developed that shows a strong correlation between C-reactive protein (CRP) levels measured by the device and those found in serum. This multifunctional platform integrates several key components essential for comprehensive health monitoring: iontophoretic sweat induction, microfluidic channels for sweat collection and reagent delivery, and real-time electrochemical detection of the inflammatory biomarker CRP. The device is fabricated using laser-engraved graphene on a polyimide (PI) substrate, with electrodeposited gold nanoparticles (AuNPs) functionalized with anti-CRP capture antibodies. Electrodeposition enables precise control over the size and distribution of AuNPs, resulting in a uniform, densely packed nanoparticle layer that enhances the surface area for antibody immobilization. The microfluidic module, constructed from PET and medical-grade adhesives, channels sweat to the sensing region, while the iontophoresis module uses carbachol-loaded hydrogels to stimulate sweat production. In addition to CRP, the sensor quantitatively measures ionic strength, pH, and temperature, allowing for real-time calibration and improved accuracy. By enabling sensitive, non-invasive analysis of inflammatory proteins directly from sweat, this wearable biosensor holds significant potential for the continuous management of chronic diseases.
As described from Fig. 18b, a revolutionary fingertip-wearable microgrid system that integrates energy harvesting and metabolic monitoring into a single platform.154 By combining a sweat-powered biofuel cell and a rechargeable AgCl–Zn battery, the system achieves autonomous and sustainable energy management, enabling extended use without reliance on external power sources. The device features stretchable electrodes fabricated using screen printing on SEBS substrates, coupled with hydrogels to passively extract sweat from the fingertip, which can hold for 20% stretching and twisting. It facilitates simultaneous monitoring of multiple biomarkers, including glucose, lactate and vitamin C, levodopa, through enzyme-functionalized electrodes and a charge-transfer complex designed electrochemical sensing interface. The osmotic sweat extraction method ensures non-invasive and continuous biomarker collection, eliminating the need for physical activity. Additionally, the microgrid system supports uninterrupted operation for over 16 hours, maintaining robust performance under mechanical deformation such as bending and stretching.
Eliminating the need for external power sources, a freestanding triboelectric nanogenerator (FTENG), as shown in Fig. 18c, harvests mechanical energy from human motion to power biosensors, enabling self-sustained, long-term operation and real-time, on-the-go diagnostics.155 The electrodes, fabricated via electroless nickel/immersion gold plating on a flexible PCB and laminated with polytetrafluoroethylene (PTFE) to enhance triboelectric performance, are shown in Fig. 18d. Unlike conventional power sources, the FTENG operates through contact electrification and in-plane charge transfer between copper and PTFE tribo-pairs to efficiently generate electricity. Its design incorporates an interdigital stator and a grating-patterned slider, which together maximize charge transfer and achieve a peak power output of approximately 416 mW m−2. The harvested energy is regulated by a power management circuit and stored in capacitors, enabling continuous, wireless operation of the biosensor. Lightweight, durable, and optimized for on-body use, the system supports real-time monitoring of sweat biomarkers such as Na+ and pH during physical activity.
4.4. Integrated smart biosensing system
With the rise of the Internet of Things (IoT) and big data, artificial intelligence (AI) and machine learning (ML) have greatly advanced signal processing in wearable biosensing systems. They enable automated data analysis, noise reduction, and real-time accuracy, supporting multimodal signal decoupling, pattern recognition, and adaptive filtering. Integrated with biosensors, these technologies drive personalized health monitoring, autonomous diagnostics, and seamless human–machine interaction in next-generation wearables.156–159For the example shown in Fig. 16b, powered by an AI algorithm (Fig. 19a), the hierarchical resistive sensor successfully disentangles a single resistive signal into five distinct physical signals, achieving an impressive accuracy of 92.73 ± 0.82%. This AI-enabled wearable sensor offers the potential to detect multiple biometric signals with minimal electrical wiring, paving the way for automated classification and intelligent decision-making in real-time health monitoring.
 | ||
| Fig. 19 Wearable integrated smart biosensing system. (a) The diagram of the data processing associated deep learning architecture for the wearable hierarchically resistive skin ((a) adapted with permission123. Copyright 2023 Springer Nature). (b) The scheme and working principle of wearable imager that fabricated by multi-layer stretchable electrodes and components. (c) The schematic workflow illustrates that pre-processed images are utilized for training the FCN-32 model. Once trained, the model processes raw ultrasound images to predict left ventricular (LV) volume, which is then used to compute stroke volume, cardiac output, and ejection fraction ((b) and (c) adapted with permission.160 Copyright 2023 Springer Nature). (d) The scheme of the GO–MS structure for moist-electric potential fluctuations in reaction to various external stimuli (left). The diagram of ML model to simultaneously detect changes in light intensity, temperature, humidity, and pressure (right) ((d) adapted with permission.161 Copyright 2022 John Wiley and Sons). | ||
In another smart integrated system (as illustrated in Fig. 19b), the electrodes utilize a LM composite, which is screen-printed onto a SEBS polymer matrix.160 This process is followed by solvent welding using a toluene–ethanol solution, which softens the polymer and enhances interfacial bonding. This wet chemical fabrication approach not only achieves high conductivity (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 S m−1) but also provides biaxial mechanical stretchability of up to 110% strain. These properties make the wearable imager well-suited for long-term use, significantly improving signal fidelity in ultrasound imaging. A key innovation of this work is the integration of a CNN-supported deep learning framework (Fig. 19c) that enables multimodal signal decoupling. In wearable ultrasound systems, signals often include overlapping mechanical deformations and physiological variations, complicating real-time interpretation. The fully convolutional network (FCN-32) processes continuous ultrasound data, extracting left ventricular volume waveforms while separating motion artifacts from true cardiac signals. Through automatic feature extraction, spatial filtering, and temporal alignment, the model enhances the accuracy of stroke volume, cardiac output, and ejection fraction estimations. This AI-driven, multimodal approach enables real-time, operator-independent cardiac monitoring, outperforming traditional manual echocardiography under dynamic and ambulatory conditions. The integration of wet-chemically fabricated stretchable electrodes with advanced AI analysis establishes a transformative platform for continuous, high-fidelity cardiovascular monitoring.
800 S m−1) but also provides biaxial mechanical stretchability of up to 110% strain. These properties make the wearable imager well-suited for long-term use, significantly improving signal fidelity in ultrasound imaging. A key innovation of this work is the integration of a CNN-supported deep learning framework (Fig. 19c) that enables multimodal signal decoupling. In wearable ultrasound systems, signals often include overlapping mechanical deformations and physiological variations, complicating real-time interpretation. The fully convolutional network (FCN-32) processes continuous ultrasound data, extracting left ventricular volume waveforms while separating motion artifacts from true cardiac signals. Through automatic feature extraction, spatial filtering, and temporal alignment, the model enhances the accuracy of stroke volume, cardiac output, and ejection fraction estimations. This AI-driven, multimodal approach enables real-time, operator-independent cardiac monitoring, outperforming traditional manual echocardiography under dynamic and ambulatory conditions. The integration of wet-chemically fabricated stretchable electrodes with advanced AI analysis establishes a transformative platform for continuous, high-fidelity cardiovascular monitoring.
Leveraging AI-driven signal processing, a self-powered multimodal sensor system based on a moisture-electric graphene oxide single-component (GO-MS) has been developed and reported.161 The sensor consists of a GO and sodium alginate composite, fabricated by dispersing GO with sodium alginate in ethanol and freeze-drying the mixture to form a porous aerogel structure. This self-powered system harnesses ambient moisture to generate a stable electrical output, eliminating the need for external energy sources. As shown in Fig. 19d, the sensor integrates a porous aerogel framework with embedded gold mesh electrodes, achieving high sensitivity and operational stability. A key innovation of this work is the application of machine learning (ML) for enhanced signal decoupling, addressing the persistent challenge of mixed signal interference in multimodal sensing. By employing a long short-term memory (LSTM) neural network, the system effectively separates overlapping signals, significantly improving the accuracy of real-time physiological monitoring. This advancement enables a range of high-impact applications, including gesture recognition, pulse tracking, and continuous monitoring of vital signs.
5. Challenges and opportunities
This review presents recent progress in the development of wet chemically synthesized nanomaterials for soft wearable bioelectronic sensing applications. These materials offer distinct advantages, including precise control over particle size, morphology, and surface functionality—key factors in optimizing biosensor performance. Additionally, the tunable solubility, dispersion stability, and rheological properties of wet-processed inks make them highly compatible with scalable, low-cost fabrication techniques such as drop-casting, printing, coating, and self-assembly. Unlike physical or vapor-phase deposition methods, wet chemical synthesis provides a cost-effective, solution-based approach that supports large-scale production. It also allows for seamless integration with soft, flexible substrates, enabling the fabrication of stretchable, skin-conformal devices ideal for continuous, real-time physiological monitoring. These attributes make wet chemically produced nanomaterials a promising platform for next-generation biosensors, flexible electronics, and wearable energy systems.In spite of the promises of wet chemically produced nanomaterials, challenges such as stability under physiological conditions, potential cytotoxicity, and long-term durability must be addressed to enable widespread adoption. The use of protective coatings, and hybrid material systems can enhance mechanical resilience and signal reliability. Additionally, the batch-to-batch variability, reagent costs, and complex purification steps can increase production costs and limit industrial scalability. Developing automated, continuous-flow synthesis processes and integrating green chemistry principles can enhance cost efficiency and environmental sustainability,162 making wet chemically synthesized nanomaterials more attractive for commercial applications. Also, in situ characterization techniques are necessary to monitor nanomaterial behavior over time, ensuring reproducibility and robust performance in real-world applications. Overcoming these challenges is essential to fully realize the potential of wet chemically synthesized nanomaterials in next-generation wearable and biomedical devices.
From a real-world application perspective, the long-term stability and reliability of wet chemically synthesized nanomaterials directly impact the usability and commercial viability of wearable biosensors. Nanomaterials are sometimes prone to degradation due to prolonged exposure to sunlight, moisture, sweat, temperature fluctuations, and mechanical stress. Key factors affecting stability include oxidation, ion diffusion, polymer matrix degradation, and nanoparticle aggregation, leading to signal drift, reduced sensitivity, and compromised biocompatibility. Addressing these issues requires continuous research efforts, driven by the rising demand for wearable devices. Innovations in material science, surface functionalization, and encapsulation techniques can enhance mechanical durability and environmental resistance. For example, chemically synthesized gold nanowire sponges have demonstrated superior ambient stability,163 while embedding nanomaterials into elastomeric substrates significantly improves adhesion and prevents delamination.49 Cyclic stability testing under real-world conditions is essential to assess electrical, mechanical, and biochemical resilience, ensuring consistent sensor performance for potential commercialization.
Looking ahead, the future landscape of wearable biosensors presents exciting opportunities for innovation. The integration of AI and machine learning into soft wearable biosensors is still in its early stages, with vast potential for more precise health assessments and personalized recommendations through closed-loop devices. Additionally, the establishment of standards for device interoperability is imperative. Developing global manufacturing standards will facilitate seamless data sharing, integration across platforms, and large-scale adoption, paving the way for widespread commercialization and impact in healthcare, sports, and beyond.
Author contributions
Ren Wang led the manuscript development. Wenlong Cheng and Guangzhao Mao supervised the research and provided guidance throughout the process. The rest of the authors are members of soft electronics alliance (https://soft-electronics-eng.sydney.edu.au/) and contributed to discussion on the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from Australian Research Council via its discovery project scheme (DP200100624 and DP230101377), as well as USYD-NUS Ignite grant. We also acknowledge the USYD Soft Electronics Alliance for facilitating this collaborative work.References
- S. Gong, Y. Lu, J. Yin, A. Levin and W. Cheng, Chem. Rev., 2024, 124, 455–553 CrossRef CAS PubMed.
- Y. Luo, M. R. Abidian, J. H. Ahn, D. Akinwande, A. M. Andrews, M. Antonietti, Z. Bao, M. Berggren, C. A. Berkey, C. J. Bettinger, J. Chen, P. Chen, W. Cheng, X. Cheng, S. J. Choi, A. Chortos, C. Dagdeviren, R. H. Dauskardt, C. A. Di, M. D. Dickey, X. Duan, A. Facchetti, Z. Fan, Y. Fang, J. Feng, X. Feng, H. Gao, W. Gao, X. Gong, C. F. Guo, X. Guo, M. C. Hartel, Z. He, J. S. Ho, Y. Hu, Q. Huang, Y. Huang, F. Huo, M. M. Hussain, A. Javey, U. Jeong, C. Jiang, X. Jiang, J. Kang, D. Karnaushenko, A. Khademhosseini, D. H. Kim, I. D. Kim, D. Kireev, L. Kong, C. Lee, N. E. Lee, P. S. Lee, T. W. Lee, F. Li, J. Li, C. Liang, C. T. Lim, Y. Lin, D. J. Lipomi, J. Liu, K. Liu, N. Liu, R. Liu, Y. Liu, Y. Liu, Z. Liu, Z. Liu, X. J. Loh, N. Lu, Z. Lv, S. Magdassi, G. G. Malliaras, N. Matsuhisa, A. Nathan, S. Niu, J. Pan, C. Pang, Q. Pei, H. Peng, D. Qi, H. Ren, J. A. Rogers, A. Rowe, O. G. Schmidt, T. Sekitani, D. G. Seo, G. Shen, X. Sheng, Q. Shi, T. Someya, Y. Song, E. Stavrinidou, M. Su, X. Sun, K. Takei, X. M. Tao, B. C. K. Tee, A. V. Thean, T. Q. Trung, C. Wan, H. Wang, J. Wang, M. Wang, S. Wang, T. Wang, Z. L. Wang, P. S. Weiss, H. Wen, S. Xu, T. Xu, H. Yan, X. Yan, H. Yang, L. Yang, S. Yang, L. Yin, C. Yu, G. Yu, J. Yu, S. H. Yu, X. Yu, E. Zamburg, H. Zhang, X. Zhang, X. Zhang, X. Zhang, Y. Zhang, Y. Zhang, S. Zhao, X. Zhao, Y. Zheng, Y. Q. Zheng, Z. Zheng, T. Zhou, B. Zhu, M. Zhu, R. Zhu, Y. Zhu, Y. Zhu, G. Zou and X. Chen, ACS Nano, 2023, 17, 5211–5295 CrossRef CAS PubMed.
- D. Gao, K. Parida and P. S. Lee, Adv. Funct. Mater., 2020, 30, 1907184 CrossRef CAS.
- T. R. Ray, J. Choi, A. J. Bandodkar, S. Krishnan, P. Gutruf, L. Tian, R. Ghaffari and J. A. Rogers, Chem. Rev., 2019, 119, 5461–5533 CrossRef CAS PubMed.
- Y. Fang, L. Meng, A. Prominski, E. N. Schaumann, M. Seebald and B. Tian, Chem. Soc. Rev., 2020, 49, 7978–8035 RSC.
- Q. Lyu, S. Gong, J. Yin, J. M. Dyson and W. Cheng, Adv. Healthcare Mater., 2021, 10, 2100577 CrossRef CAS.
- D. Mukasa, M. Wang, J. Min, Y. Yang, S. A. Solomon, H. Han, C. Ye and W. Gao, Adv. Mater., 2023, 35, 2212161 CrossRef CAS.
- Y. Yu, D. Chen, Y. Yang and Q. Yuan, Nano Biomed. Eng., 2024, 16, 309–330 CrossRef.
- S. H. Sunwoo, K. H. Ha, S. Lee, N. Lu and D. H. Kim, Annu. Rev. Chem. Biomol. Eng., 2021, 12, 359–391 CrossRef CAS.
- R. Wang, Q. Zhai, T. An, S. Gong and W. Cheng, Talanta, 2021, 222, 121484 CrossRef CAS.
- N. Matsuhisa, X. Chen, Z. Bao and T. Someya, Chem. Soc. Rev., 2019, 48, 2946–2966 RSC.
- D. C. Kim, H. J. Shim, W. Lee, J. H. Koo and D. H. Kim, Adv. Mater., 2019, 32, 1902743 CrossRef PubMed.
- Z. Hui, L. Zhang, G. Ren, G. Sun, H. D. Yu and W. Huang, Adv. Mater., 2023, 35, 2211202 CrossRef CAS PubMed.
- D. H. Kim, N. Lu, R. Ma, Y. S. Kim, R. H. Kim, S. Wang, J. Wu, S. M. Won, H. Tao, A. Islam, K. J. Yu, T. I. Kim, R. Chowdhury, M. Ying, L. Xu, M. Li, H. J. Chung, H. Keum, M. McCormick, P. Liu, Y. W. Zhang, F. G. Omenetto, Y. Huang, T. Coleman and J. A. Rogers, Science, 2011, 333, 838–843 CrossRef CAS PubMed.
- A. Chortos, J. Liu and Z. Bao, Nat. Mater., 2016, 15, 937–950 CrossRef CAS.
- J. Kim, A. S. Campbell, B. E. de Avila and J. Wang, Nat. Biotechnol., 2019, 37, 389–406 CrossRef CAS PubMed.
- Y. Liu, K. He, G. Chen, W. R. Leow and X. Chen, Chem. Rev., 2017, 117, 12893–12941 CrossRef CAS PubMed.
- T. Sekitani and T. Someya, Adv. Mater., 2010, 22, 2228–2246 CrossRef CAS.
- K. W. Cho, S. H. Sunwoo, Y. J. Hong, J. H. Koo, J. H. Kim, S. Baik, T. Hyeon and D. H. Kim, Chem. Rev., 2022, 122, 5068–5143 CrossRef CAS PubMed.
- Y. Ling, T. An, L. W. Yap, B. Zhu, S. Gong and W. Cheng, Adv. Mater., 2019, 32, 1904664 CrossRef.
- H. Yuk, B. Lu and X. Zhao, Chem. Soc. Rev., 2019, 48, 1642–1667 RSC.
- J. Min, J. Tu, C. Xu, H. Lukas, S. Shin, Y. Yang, S. A. Solomon, D. Mukasa and W. Gao, Chem. Rev., 2023, 123, 5049–5138 CrossRef CAS.
- H. Li, P. Tan, Y. Rao, S. Bhattacharya, Z. Wang, S. Kim, S. Gangopadhyay, H. Shi, M. Jankovic, H. Huh, Z. Li, P. Maharjan, J. Wells, H. Jeong, Y. Jia and N. Lu, Chem. Rev., 2024, 6, 3220 CrossRef.
- B. Zhu, S. Gong and W. Cheng, Chem. Soc. Rev., 2019, 48, 1668–1711 RSC.
- F. Molina-Lopez, T. Z. Gao, U. Kraft, C. Zhu, T. Ohlund, R. Pfattner, V. R. Feig, Y. Kim, S. Wang, Y. Yun and Z. Bao, Nat. Commun., 2019, 10, 2676 Search PubMed.
- A. Russo, B. Y. Ahn, J. J. Adams, E. B. Duoss, J. T. Bernhard and J. A. Lewis, Adv. Mater., 2011, 23, 3426–3430 CrossRef CAS PubMed.
- R. Y. Tay, Y. Song, D. R. Yao and W. Gao, Mater. Today, 2023, 71, 135 CrossRef CAS PubMed.
- Y. Wang, S. Gong, S. J. Wang, X. Yang, Y. Ling, L. W. Yap, D. Dong, G. P. Simon and W. Cheng, ACS Nano, 2018, 12, 9742–9749 CrossRef CAS PubMed.
- S. Gong, L. W. Yap, Y. Zhang, J. He, J. Yin, F. Marzbanrad, D. M. Kaye and W. Cheng, Biosens. Bioelectron., 2022, 205, 114072 CrossRef CAS PubMed.
- R. J. Sugden, V. L. Pham-Kim-Nghiem-Phu, I. Campbell, A. Leon and P. Diamandis, Bioelectron. Med., 2023, 9, 12 CrossRef PubMed.
- H. Kim, J. Lee, U. Heo, D. K. Jayashankar, K. C. Agno, Y. Kim, C. Y. Kim, Y. Oh, S. H. Byun, B. Choi, H. Jeong, W. H. Yeo, Z. Li, S. Park, J. Xiao, J. Kim and J. W. Jeong, Sci. Adv., 2024, 10, eadk5260 CrossRef PubMed.
- S. Gong, D. T. H. Lai, B. Su, K. J. Si, Z. Ma, L. W. Yap, P. Guo and W. Cheng, Adv. Electron. Mater., 2015, 1, 1400063 CrossRef.
- Y. Wang, S. Lee, T. Yokota, H. Wang, Z. Jiang, J. Wang, M. Koizumi and T. Someya, Sci. Adv., 2020, 6, eabb7043 CrossRef CAS.
- B. Zhu, Y. Ling, L. W. Yap, M. Yang, F. Lin, S. Gong, Y. Wang, T. An, Y. Zhao and W. Cheng, ACS Appl. Mater. Interfaces, 2019, 11, 29014–29021 CrossRef CAS PubMed.
- Q. Zhai and W. Cheng, Mater. Today Nano, 2019, 7, 100041 CrossRef.
- T. He, F. Wen, Y. Yang, X. Le, W. Liu and C. Lee, Anal. Chem., 2023, 95, 490–514 CrossRef CAS PubMed.
- J. Li, P. C. Ma, W. S. Chow, C. K. To, B. Z. Tang and J. K. Kim, Adv. Funct. Mater., 2007, 17, 3207–3215 CrossRef CAS.
- J. Li and J.-K. Kim, Compos. Sci. Technol., 2007, 67, 2114–2120 CrossRef CAS.
- G. E. Pike and C. H. Seager, Phys. Rev. B, 1974, 10, 1421–1434 CrossRef.
- A. J. Marsden, D. G. Papageorgiou, C. Vallés, A. Liscio, V. Palermo, M. A. Bissett, R. J. Young and I. A. Kinloch, 2D Mater., 2018, 5, 032003 CrossRef.
- D. Dong, S. S. Dhanabalan, P. F. M. Elango, M. Yang, S. Walia, S. Sriram and M. Bhaskaran, Appl. Phys. Rev., 2023, 10, 031314 CAS.
- S. J. Tan, M. J. Campolongo, D. Luo and W. Cheng, Nat. Nanotechnol., 2011, 6, 268–276 CrossRef CAS.
- Y. Kim, J. Zhu, B. Yeom, M. Di Prima, X. Su, J. G. Kim, S. J. Yoo, C. Uher and N. A. Kotov, Nature, 2013, 500, 59–63 CrossRef CAS PubMed.
- S. Gong, L. W. Yap, B. Zhu, Q. Zhai, Y. Liu, Q. Lyu, K. Wang, M. Yang, Y. Ling, D. T. H. Lai, F. Marzbanrad and W. Cheng, Adv. Mater., 2019, 31, 1903789 CrossRef CAS.
- G. D. Moon, G. H. Lim, J. H. Song, M. Shin, T. Yu, B. Lim and U. Jeong, Adv. Mater., 2013, 25, 2707–2712 CrossRef CAS PubMed.
- D. Huo, M. J. Kim, Z. Lyu, Y. Shi, B. J. Wiley and Y. Xia, Chem. Rev., 2019, 15, 8972 CrossRef.
- S. Gong, W. Schwalb, Y. Wang, Y. Chen, Y. Tang, J. Si, B. Shirinzadeh and W. Cheng, Nat. Commun., 2014, 5, 3132 CrossRef PubMed.
- T. An, B. Zhu, Y. Ling, S. Gong and W. Cheng, J. Mater. Chem. A, 2019, 7, 14233–14238 RSC.
- B. Zhu, S. Gong, F. Lin, Y. Wang, Y. Ling, T. An and W. Cheng, Adv. Electron. Mater., 2019, 5, 1800509 CrossRef.
- F. Xu and Y. Zhu, Adv. Mater., 2012, 24, 5117–5122 CrossRef CAS PubMed.
- N. Matsuhisa, D. Inoue, P. Zalar, H. Jin, Y. Matsuba, A. Itoh, T. Yokota, D. Hashizume and T. Someya, Nat. Mater., 2017, 16, 834–840 CrossRef CAS.
- S. Choi, S. I. Han, D. Jung, H. J. Hwang, C. Lim, S. Bae, O. K. Park, C. M. Tschabrunn, M. Lee, S. Y. Bae, J. W. Yu, J. H. Ryu, S. W. Lee, K. Park, P. M. Kang, W. B. Lee, R. Nezafat, T. Hyeon and D. H. Kim, Nat. Nanotechnol., 2018, 13, 1048–1056 CrossRef CAS.
- N. N. Jason, W. Shen and W. Cheng, ACS Appl. Mater. Interfaces, 2015, 7, 16760–16766 CrossRef CAS.
- V. Schroeder, S. Savagatrup, M. He, S. Lin and T. M. Swager, Chem. Rev., 2019, 119, 599–663 CrossRef CAS.
- D. J. Lipomi, M. Vosgueritchian, B. C. Tee, S. L. Hellstrom, J. A. Lee, C. H. Fox and Z. Bao, Nat. Nanotechnol., 2011, 6, 788–792 CrossRef CAS PubMed.
- S. Wang, J. Xu, W. Wang, G. N. Wang, R. Rastak, F. Molina-Lopez, J. W. Chung, S. Niu, V. R. Feig, J. Lopez, T. Lei, S. K. Kwon, Y. Kim, A. M. Foudeh, A. Ehrlich, A. Gasperini, Y. Yun, B. Murmann, J. B. Tok and Z. Bao, Nature, 2018, 555, 83–88 CrossRef CAS.
- J. Zang, S. Ryu, N. Pugno, Q. Wang, Q. Tu, M. J. Buehler and X. Zhao, Nat. Mater., 2013, 12, 321–325 CrossRef CAS.
- L. Zhang, J. Li, S. Yue, H. He and J. Ouyang, Adv. Funct. Mater., 2021, 31, 2102745 Search PubMed.
- F. Zhao, Y. Shi, L. Pan and G. Yu, Acc. Chem. Res., 2017, 50, 1734–1743 CrossRef CAS PubMed.
- T. Nezakati, A. Seifalian, A. Tan and A. M. Seifalian, Chem. Rev., 2018, 118, 6766–6843 Search PubMed.
- Y. Wang, C. Zhu, R. Pfattner, H. Yan, L. Jin, S. Chen, F. Molina-Lopez, F. Lissel, J. Liu, N. I. Rabiah, Z. Chen, J. W. Chung, C. Linder, M. F. Toney, B. Murmann and Z. Bao, Sci. Adv., 2017, 3, e1602076 CrossRef PubMed.
- J. Xu, S. Wang, G. N. Wang, C. Zhu, S. Luo, L. Jin, X. Gu, S. Chen, V. R. Feig, J. W. To, S. Rondeau-Gagne, J. Park, B. C. Schroeder, C. Lu, J. Y. Oh, Y. Wang, Y. H. Kim, H. Yan, R. Sinclair, D. Zhou, G. Xue, B. Murmann, C. Linder, W. Cai, J. B. Tok, J. W. Chung and Z. Bao, Science, 2017, 355, 59–64 CrossRef PubMed.
- X. Liu, J. Liu, S. Lin and X. Zhao, Mater. Today, 2020, 36, 102 CrossRef.
- T. Zhou, H. Yuk, F. Hu, J. Wu, F. Tian, H. Roh, Z. Shen, G. Gu, J. Xu, B. Lu and X. Zhao, Nat. Mater., 2023, 22, 895–902 CrossRef PubMed.
- D. Liu, C. Huyan, Z. Wang, Z. Guo, X. Zhang, H. Torun, D. Mulvihill, B. B. Xu and F. Chen, Mater. Horiz., 2023, 10, 2800–2823 RSC.
- C. Yang and Z. Suo, Nat. Rev. Mater., 2018, 3, 125–142 CrossRef.
- J. Y. Sun, C. Keplinger, G. M. Whitesides and Z. Suo, Adv. Mater., 2014, 26, 7608–7614 CrossRef PubMed.
- Y. Ohm, C. Pan, M. J. Ford, X. Huang, J. Liao and C. Majidi, Nat. Electron., 2021, 4, 185–192 CrossRef.
- B. Lu, H. Yuk, S. Lin, N. Jian, K. Qu, J. Xu and X. Zhao, Nat. Commun., 2019, 10, 1043 CrossRef PubMed.
- L. Pan, G. Yu, D. Zhai, H. R. Lee, W. Zhao, N. Liu, H. Wang, B. C. Tee, Y. Shi, Y. Cui and Z. Bao, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9287–9292 CrossRef.
- J. Yang, W. Cheng and K. Kalantar-zadeh, Proc. IEEE, 2019, 107, 2168–2184 Search PubMed.
- T. Shay, O. D. Velev and M. D. Dickey, Soft Matter, 2018, 14, 3296–3303 RSC.
- S. Chen, S. Fan, H. Chan, Z. Qiao, J. Qi, Z. Wu, J. C. Yeo and C. T. Lim, Adv. Funct. Mater., 2024, 34, 2309989 CrossRef CAS.
- Z. Ma, Q. Huang, Q. Xu, Q. Zhuang, X. Zhao, Y. Yang, H. Qiu, Z. Yang, C. Wang, Y. Chai and Z. Zheng, Nat. Mater., 2021, 20, 859 CrossRef CAS.
- S. Chen, S. Fan, J. Qi, Z. Xiong, Z. Qiao, Z. Wu, J. C. Yeo and C. T. Lim, Adv. Mater., 2023, 35, 2208569 CrossRef CAS.
- X. Wang, S. Zheng, J. Xiong, Z. Liu, Q. Li, W. Li and F. Yan, Adv. Mater., 2024, 36, 2313845 CrossRef CAS.
- H. Wang, B. Yuan, X. Zhu, X. Shan, S. Chen, W. Ding, Y. Cao, K. Dong, X. Zhang, R. Guo, Y. Yao, B. Wang, J. Tang and J. Liu, Sci. Adv., 2024, 10, eadp5215 CrossRef CAS.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55 RSC.
- C. Lim, C. Park, S. H. Sunwoo, Y. G. Kim, S. Lee, S. I. Han, D. Kim, J. H. Kim, D. H. Kim and T. Hyeon, ACS Nano, 2022, 16, 10431–10442 CrossRef PubMed.
- S. Coskun, B. Aksoy and H. E. Unalan, Cryst. Growth Des., 2011, 11, 4963–4969 CrossRef.
- D. Jung, C. Lim, H. J. Shim, Y. Kim, C. Park, J. Jung, S. I. Han, S. H. Sunwoo, K. W. Cho, G. D. Cha, D. C. Kim, J. H. Koo, J. H. Kim, T. Hyeon and D. H. Kim, Science, 2021, 373, 1022–1026 CrossRef PubMed.
- Y. Tang, S. Gong, Y. Chen, L. W. Yap and W. Cheng, ACS Nano, 2014, 8, 5707–5714 CrossRef CAS PubMed.
- K.-H. Teng and M.-H. Lee, J. Environ. Chem. Eng., 2025, 13, 115716 CrossRef CAS.
- J. E. Mates, I. S. Bayer, J. M. Palumbo, P. J. Carroll and C. M. Megaridis, Nat. Commun., 2015, 6, 8874 CrossRef CAS.
- S. Gong, D. T. Lai, Y. Wang, L. W. Yap, K. J. Si, Q. Shi, N. N. Jason, T. Sridhar, H. Uddin and W. Cheng, ACS Appl. Mater. Interfaces, 2015, 7, 19700–19708 CrossRef CAS.
- Y. Lu, Z. Yong, S. Gong, Q. Shi, F. Lin, Q. Zhai, R. Wang and W. Cheng, Biosens. Bioelectron., 2023, 221, 114924 CrossRef CAS.
- N. Matsuhisa, M. Kaltenbrunner, T. Yokota, H. Jinno, K. Kuribara, T. Sekitani and T. Someya, Nat. Commun., 2015, 6, 7461 CrossRef CAS PubMed.
- S. Niu, N. Matsuhisa, L. Beker, J. Li, S. Wang, J. Wang, Y. Jiang, X. Yan, Y. Yun, W. Burnett, A. S. Y. Poon, J. B. H. Tok, X. Chen and Z. Bao, Nat. Electron., 2019, 2, 361–368 CrossRef.
- D. Son, J. Lee, S. Qiao, R. Ghaffari, J. Kim, J. E. Lee, C. Song, S. J. Kim, D. J. Lee, S. W. Jun, S. Yang, M. Park, J. Shin, K. Do, M. Lee, K. Kang, C. S. Hwang, N. Lu, T. Hyeon and D. H. Kim, Nat. Nanotechnol., 2014, 9, 397–404 Search PubMed.
- B. C. Tee, A. Chortos, A. Berndt, A. K. Nguyen, A. Tom, A. McGuire, Z. C. Lin, K. Tien, W. G. Bae, H. Wang, P. Mei, H. H. Chou, B. Cui, K. Deisseroth, T. N. Ng and Z. Bao, Science, 2015, 350, 313–316 Search PubMed.
- M. Bariya, Z. Shahpar, H. Park, J. Sun, Y. Jung, W. Gao, H. Y. Y. Nyein, T. S. Liaw, L. C. Tai, Q. P. Ngo, M. Chao, Y. Zhao, M. Hettick, G. Cho and A. Javey, ACS Nano, 2018, 12, 6978–6987 Search PubMed.
- O.-H. Huttunen, T. Happonen, J. Hiitola-Keinänen, P. Korhonen, J. Ollila and J. Hiltunen, Ind. Eng. Chem. Res., 2019, 58, 19909–19916 CrossRef CAS.
- Y. Hui, Y. Yao, Q. Qian, J. Luo, H. Chen, Z. Qiao, Y. Yu, L. Tao and N. Zhou, Nat. Electron., 2022, 5, 893–903 CrossRef.
- A. A. Alsharif, A. M. Syed, X. Li, N. A. Alsharif, G. Lubineau and N. El-Atab, Adv. Funct. Mater., 2024, 34, 2406341 CrossRef.
- Q. Zhai, L. W. Yap, R. Wang, S. Gong, Z. Guo, Y. Liu, Q. Lyu, J. Wang, G. P. Simon and W. Cheng, Anal. Chem., 2020, 92, 4647–4655 Search PubMed.
- S. Gong, S. Du, J. Kong, Q. Zhai, F. Lin, S. Liu, N. R. Cameron and W. Cheng, Small, 2020, 16, 2003269 Search PubMed.
- R. Wang, M. Kilani, J. Lin, R. Chandrawati and G. Mao, Adv. Sens. Res., 2025, 4, 2400167 CrossRef.
- R. Wang, Q. Zhai, Y. Zhao, T. An, S. Gong, Z. Guo, Q. Shi, Z. Yong and W. Cheng, J. Mater. Chem. B, 2020, 8, 3655–3660 Search PubMed.
- A. Miyamoto, S. Lee, N. F. Cooray, S. Lee, M. Mori, N. Matsuhisa, H. Jin, L. Yoda, T. Yokota, A. Itoh, M. Sekino, H. Kawasaki, T. Ebihara, M. Amagai and T. Someya, Nat. Nanotechnol., 2017, 12, 907–913 CrossRef.
- J. Lin, M. Kilani, M. Baharfar, R. Wang and G. Mao, Nanoscale, 2024, 19564 RSC.
- C. J. Murphy and N. R. Jana, Adv. Mater., 2002, 14, 80–82 CrossRef.
- Y. X. Gan, A. H. Jayatissa, Z. Yu, X. Chen and M. Li, J. Nanomater., 2020, 2020, 1–3 Search PubMed.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef.
- D. Bokov, A. Turki Jalil, S. Chupradit, W. Suksatan, M. Javed Ansari, I. H. Shewael, G. H. Valiev, E. Kianfar and Z. Wang, Adv. Mater. Sci. Eng., 2021, 1–21 Search PubMed.
- J. Joo, S. G. Kwon, J. H. Yu and T. Hyeon, Adv. Mater., 2005, 17, 1873–1877 CrossRef.
- J. Ge, H. B. Yao, X. Wang, Y. D. Ye, J. L. Wang, Z. Y. Wu, J. W. Liu, F. J. Fan, H. L. Gao, C. L. Zhang and S. H. Yu, Angew. Chem., Int. Ed., 2013, 52, 1654–1659 Search PubMed.
- K.-H. Choi, J. Yoo, C. K. Lee and S.-Y. Lee, Energy Environ. Sci., 2016, 9, 2812–2821 RSC.
- H. Zhang, Y. Shao, R. Xia, G. Chen, X. Xiang and Y. Yu, Adv. Mater., 2024, 36, 2401550 CrossRef.
- Y. Wang, S. Gong, D. Gomez, Y. Ling, L. W. Yap, G. P. Simon and W. Cheng, ACS Nano, 2018, 12, 8717–8722 CrossRef.
- D. Qi, Z. Liu, M. Yu, Y. Liu, Y. Tang, J. Lv, Y. Li, J. Wei, B. Liedberg, Z. Yu and X. Chen, Adv. Mater., 2015, 27, 3145–3151 Search PubMed.
- W. Zeng, L. Shu, Q. Li, S. Chen, F. Wang and X. M. Tao, Adv. Mater., 2014, 26, 5310–5336 Search PubMed.
- J. Lee, B. Llerena Zambrano, J. Woo, K. Yoon and T. Lee, Adv. Mater., 2020, 32, 1902532 CrossRef PubMed.
- L. Wang, X. Fu, J. He, X. Shi, T. Chen, P. Chen, B. Wang and H. Peng, Adv. Mater., 2020, 32, 1901971 CrossRef.
- Y. M. Zhao, D. S. Dong, S. Gong, L. Brassart, Y. Wang, T. An and W. L. Cheng, Adv. Electron. Mater., 2019, 5, 1800462 CrossRef.
- Y. Zhao, D. Dong, Y. Wang, S. Gong, T. An, L. W. Yap and W. Cheng, ACS Appl. Mater. Interfaces, 2018, 10, 42612–42620 CrossRef PubMed.
- Y. Zhang, W. Bai, X. Cheng, J. Ren, W. Weng, P. Chen, X. Fang, Z. Zhang and H. Peng, Angew. Chem., Int. Ed., 2014, 53, 14564–14568 CrossRef PubMed.
- X. Pan, Q. Wang, P. He, K. Liu, Y. Ni, X. Ouyang, L. Chen, L. Huang, H. Wang and Y. Tan, ACS Sustainable Chem. Eng., 2019, 7, 7918–7925 CrossRef.
- F. Stauffer, M. Thielen, C. Sauter, S. Chardonnens, S. Bachmann, K. Tybrandt, C. Peters, C. Hierold and J. Voros, Adv. Healthcare Mater., 2018, 7, 1700994 CrossRef PubMed.
- Z. Zhang, J. Yang, H. Wang, C. Wang, Y. Gu, Y. Xu, S. Lee, T. Yokota, H. Haick, T. Someya and Y. Wang, Sci. Adv., 2024, 10, eadj5389 CrossRef CAS PubMed.
- M. Reis Carneiro, C. Majidi and M. Tavakoli, Adv. Funct. Mater., 2022, 32, 2205956 CrossRef CAS.
- S. Gong, L. W. Yap, Y. Zhu, B. Zhu, Y. Wang, Y. Ling, Y. Zhao, T. An, Y. Lu and W. Cheng, Adv. Funct. Mater., 2020, 30, 1910717 CrossRef CAS.
- J. C. Yang, J. Mun, S. Y. Kwon, S. Park, Z. Bao and S. Park, Adv. Mater., 2019, 31, 1904765 CrossRef CAS PubMed.
- S. Gong, X. Zhang, X. A. Nguyen, Q. Shi, F. Lin, S. Chauhan, Z. Ge and W. Cheng, Nat. Nanotechnol., 2023, 18, 889–897 CrossRef CAS.
- A. Berman, K. Hsiao, S. E. Root, H. Choi, D. Ilyn, C. Xu, E. Stein, M. Cutkosky, J. M. DeSimone and Z. Bao, Sci. Adv., 2024, 10, eadq8866 Search PubMed.
- N. N. Jason, M. D. Ho and W. Cheng, J. Mater. Chem. C, 2017, 5, 5845–5866 Search PubMed.
- X. Sun, F. Yao and J. Li, J. Mater. Chem. A, 2020, 18605 RSC.
- J. He, Y. Zhang, R. Zhou, L. Meng, T. Chen, W. Mai and C. Pan, J. Materiomics, 2020, 6, 86–101 CrossRef.
- J. R. Sempionatto, J. A. Lasalde-Ramirez, K. Mahato, J. Wang and W. Gao, Nat. Rev. Chem., 2022, 6, 899–915 CrossRef PubMed.
- Y. Yang and W. Gao, Chem. Soc. Rev., 2019, 48, 1465–1491 RSC.
- T. Someya and M. Amagai, Nat. Biotechnol., 2019, 37, 382–388 CrossRef PubMed.
- T. Saha, R. Del Cano, K. Mahato, E. De la Paz, C. Chen, S. Ding, L. Yin and J. Wang, Chem. Rev., 2023, 123, 7854–7889 Search PubMed.
- J. Kim, S. Imani, W. R. de Araujo, J. Warchall, G. Valdes-Ramirez, T. R. Paixao, P. P. Mercier and J. Wang, Biosens. Bioelectron., 2015, 74, 1061–1068 Search PubMed.
- G. Xu, C. Cheng, W. Yuan, Z. Liu, L. Zhu, X. Li, Y. Lu, Z. Chen, J. Liu, Z. Cui, J. Liu, H. Men and Q. Liu, Sens. Actuators, B, 2019, 297, 2104347 Search PubMed.
- H. Y. Nyein, W. Gao, Z. Shahpar, S. Emaminejad, S. Challa, K. Chen, H. M. Fahad, L. C. Tai, H. Ota, R. W. Davis and A. Javey, ACS Nano, 2016, 10, 7216–7224 CrossRef PubMed.
- F. Mohammadi, H. Zahraee, M. Izadpanah Kazemi, Z. S. Habibi, S. M. Taghdisi, K. Abnous, Z. Khoshbin and C. H. Chen, Talanta, 2024, 266, 125010 Search PubMed.
- B. Ciui, A. Martin, R. K. Mishra, B. Brunetti, T. Nakagawa, T. J. Dawkins, M. Lyu, C. Cristea, R. Sandulescu and J. Wang, Adv. Healthcare Mater., 2018, 7, 1701264 CrossRef PubMed.
- F. Alam, S. RoyChoudhury, A. H. Jalal, Y. Umasankar, S. Forouzanfar, N. Akter, S. Bhansali and N. Pala, Biosens. Bioelectron., 2018, 117, 818–829 CrossRef.
- J. R. Sempionatto, J. M. Moon and J. Wang, ACS Sens., 2021, 6, 1875–1883 CrossRef PubMed.
- C. Ye, M. Wang, J. Min, R. Y. Tay, H. Lukas, J. R. Sempionatto, J. Li, C. Xu and W. Gao, Nat. Nanotechnol., 2023, 330 Search PubMed.
- M. Wang, Y. Yang, J. Min, Y. Song, J. Tu, D. Mukasa, C. Ye, C. Xu, N. Heflin, J. S. McCune, T. K. Hsiai, Z. Li and W. Gao, Nat. Biomed. Eng., 2022, 6, 1225–1235 CrossRef CAS PubMed.
- M. T. Ghoneim, A. Nguyen, N. Dereje, J. Huang, G. C. Moore, P. J. Murzynowski and C. Dagdeviren, Chem. Rev., 2019, 119, 5248–5297 CrossRef PubMed.
- T. Guinovart, G. Valdés-Ramírez, J. R. Windmiller, F. J. Andrade and J. Wang, Electroanalysis, 2014, 26, 1345–1353 CrossRef.
- A. Kaushik, A. Vasudev, S. K. Arya, S. K. Pasha and S. Bhansali, Biosens. Bioelectron., 2014, 53, 499–512 CrossRef PubMed.
- V. N. Luine, Horm. Behav., 2014, 66, 602–618 CrossRef PubMed.
- M. Bariya, H. Y. Y. Nyein and A. Javey, Nat. Electron., 2018, 1, 160–171 CrossRef.
- J. Wu, H. Liu, W. Chen, B. Ma and H. Ju, Nat. Rev. Bioeng., 2023, 1, 346–360 CrossRef PubMed.
- K. Mahato and J. Wang, Sens. Actuators, B, 2021, 344, 130178 CrossRef CAS.
- W. Gao, S. Emaminejad, H. Y. Y. Nyein, S. Challa, K. Chen, A. Peck, H. M. Fahad, H. Ota, H. Shiraki, D. Kiriya, D. H. Lien, G. A. Brooks, R. W. Davis and A. Javey, Nature, 2016, 529, 509–514 CrossRef CAS.
- L. Yin, M. Cao, K. N. Kim, M. Lin, J.-M. Moon, J. R. Sempionatto, J. Yu, R. Liu, C. Wicker, A. Trifonov, F. Zhang, H. Hu, J. R. Moreto, J. Go, S. Xu and J. Wang, Nat. Electron., 2022, 5, 694–705 CrossRef.
- E. De la Paz, A. Barfidokht, S. Rios, C. Brown, E. Chao and J. Wang, Anal. Chem., 2021, 93, 12767–12775 CrossRef.
- W. He, C. Wang, H. Wang, M. Jian, W. Lu, X. Liang, X. Zhang, F. Yang and Y. Zhang, Sci. Adv., 2019, 5, eaax0649 CrossRef PubMed.
- H. Lee, T. K. Choi, Y. B. Lee, H. R. Cho, R. Ghaffari, L. Wang, H. J. Choi, T. D. Chung, N. Lu, T. Hyeon, S. H. Choi and D. H. Kim, Nat. Nanotechnol., 2016, 11, 566–572 CrossRef.
- J. Tu, J. Min, Y. Song, C. Xu, J. Li, J. Moore, J. Hanson, E. Hu, T. Parimon, T. Y. Wang, E. Davoodi, T. F. Chou, P. Chen, J. J. Hsu, H. B. Rossiter and W. Gao, Nat. Biomed. Eng., 2023, 7, 1293–1306 CrossRef PubMed.
- S. Ding, T. Saha, L. Yin, R. Liu, M. I. Khan, A.-Y. Chang, H. Lee, H. Zhao, Y. Liu, A. S. Nazemi, J. Zhou, C. Chen, Z. Li, C. Zhang, S. Earney, S. Tang, O. Djassemi, X. Chen, M. Lin, S. S. Sandhu, J.-M. Moon, C. Moonla, P. Nandhakumar, Y. Park, K. Mahato, S. Xu and J. Wang, Nat. Electron., 2024, 7, 788–799 CrossRef.
- Y. Song, J. Min, Y. Yu, H. Wang, Y. Yang, H. Zhang and W. Gao, Sci. Adv., 2020, 6, eaay9842 CrossRef.
- Z. Ballard, C. Brown, A. M. Madni and A. Ozcan, Nat. Mach. Intell., 2021, 3, 556–565 CrossRef.
- K. Zhang, J. Wang, T. Liu, Y. Luo, X. J. Loh and X. Chen, Adv. Healthcare Mater., 2021, 10, 2100734 CrossRef PubMed.
- C. Xu, S. A. Solomon and W. Gao, Nat. Mach. Intell., 2023, 5, 1344–1355 CrossRef.
- Y. Zhang, Y. Hu, N. Jiang and A. K. Yetisen, Biosens. Bioelectron., 2023, 219, 114825 CrossRef.
- H. Hu, H. Huang, M. Li, X. Gao, L. Yin, R. Qi, R. S. Wu, X. Chen, Y. Ma, K. Shi, C. Li, T. M. Maus, B. Huang, C. Lu, M. Lin, S. Zhou, Z. Lou, Y. Gu, Y. Chen, Y. Lei, X. Wang, R. Wang, W. Yue, X. Yang, Y. Bian, J. Mu, G. Park, S. Xiang, S. Cai, P. W. Corey, J. Wang and S. Xu, Nature, 2023, 613, 667–675 CrossRef PubMed.
- C. Yang, H. Wang, J. Yang, H. Yao, T. He, J. Bai, T. Guang, H. Cheng, J. Yan and L. Qu, Adv. Mater., 2022, 34, 2205249 CrossRef PubMed.
- X. Z. Lin, A. D. Terepka and H. Yang, Nano Lett., 2004, 4, 2227–2232 CrossRef.
- F. Lin, D. Vera Anaya, S. Gong, L. W. Yap, Y. Lu, Z. Yong and W. Cheng, ACS Sens., 2024, 9, 5414–5424 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |


