Tattoo electrodes in bioelectronics: a pathway to next-generation wearable systems
Jinwoo Lee
a and
Seung Hwan Ko
 *abc
*abc
aWearable Soft Electronics Lab, Department of Mechanical Engineering, Seoul National University, 1, Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea. E-mail: maxko@snu.ac.kr
bInstitute of Engineering Research/Institute of Advanced Machinery and Design (SNU-IAMD), Seoul National University, 1, Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea
cInterdisciplinary Program in Bioengineering, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul, Korea
First published on 10th June 2025
Abstract
Tattoo-based electronics have emerged as a transformative platform for next-generation wearable bioelectronics. Unlike conventional wearable devices, which rely on substrates, tattoo electrodes are directly formed or transferred onto the skin or internal organs, ensuring superior comfort, breathability, and long-term usability. This intimate interface minimizes motion-induced artifacts and enables reliable biosignal acquisition across diverse physiological and anatomical regions. However, the absence of a supporting substrate imposes unique challenges in fabrication and material design. The fabrication processes must be tailored to accommodate direct skin application, and the selection of functional materials is more constrained. Materials must not only be biocompatible and flexible but also capable of maintaining performance under the dynamic conditions of the human body. This review presents a comprehensive overview of tattoo electrode technology, beginning with fabrication strategies, including direct and indirect patterning methods. We then discuss a range of materials, such as metallic networks, carbon-based materials, polymers, and materials recently being studied. Finally, we explore the diverse applications of tattoo electrodes in strain and electrophysiological sensing, temperature and humidity detection, biochemical monitoring, and energy harvesting and storage. Through this review, we aim to highlight the potential and future directions of tattoo-based electronic systems.
1. Introduction
Various types of soft and stretchable electrodes have been studied, attracting substantial attention in wearable bioelectronics. By utilizing flexible substrates and materials, researchers have progressed in acquiring biosignals from the human skin, heart, and other internal organs.1 These biosignals are subsequently processed using computational technologies, including machine learning and deep learning technologies, to extract valuable information.2–4 However, electrodes based on flexible substrates still face some limitations in terms of conformality, user convenience, permeability, and biocompatibility, which hinder long-term use of the electrodes.5,6As a promising alternative, tattoo electrodes offer several universal advantages due to their ultrathin, substrate-free configurations.7 Their intimate conformality minimizes motion artifacts and enhances signal fidelity. In addition, their low mechanical modulus, comparable to that of biological tissues, improves mechanical compatibility with the human body (Table 1).8 Tattoo electrodes also reduce the risk of irritation and inflammation, particularly when implemented using breathable, porous materials that preserve natural skin functions such as sweating and thermoregulation. These features make them suitable for long-term, unobstructive wearable electronics.9 However, extreme manufacturing processes, such as high temperature, high pressure, or chemically reactive methods that can be harmful, are generally avoided, since the electrodes are intended for direct contact with the human body.
This review addresses challenges that tattoo electrodes encounter and investigates fabrication methods and materials. It also outlines future directions for tattoo electrodes to expand their applications into a wider range of fields (Fig. 1).
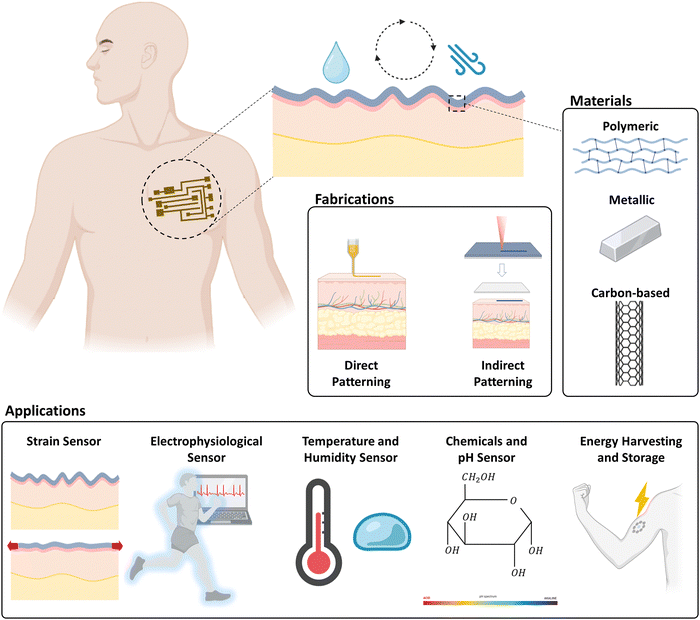 | ||
| Fig. 1 Schematic overview of tattoo-based electronic systems. This schematic was created with https://BioRender.com. | ||
2. Fabrication
Tattoo electrodes differ from many conventional electrodes not merely due to their substrate-free nature, but because they are designed for direct, conformal contact with the human body. This imposes unique constraints on fabrication, as harsh processing conditions such as high temperature, vacuum environment, or chemical treatments cannot be applied directly to the body without risking adverse physiological effects.10 To address these challenges, a variety of manufacturing techniques have been developed to enable the efficient formation of skin-conformal electrodes under biocompatible conditions. This section discusses these fabrication methods in detail.2.1. Direct patterning
Direct patterning refers to the process of creating electrode patterns directly on the human body without the use of an intermediate substrate. Due to the absence of a supporting substrate, there are stringent constraints on fabrication conditions, particularly regarding the tolerance to harsh processing environments. Additionally, inevitable movements such as breathing and pulse activity lead to distortion of the pattern, even when user motion is minimized. Nevertheless, direct patterning offers an intuitive and highly effective approach, particularly for rapid prototyping.Among the methods for direct patterning, spray-coating stands out as a simple fabrication technique that requires few processing steps. Typically, the areas where electrode deposition is unwanted are covered using a physical mask; thus, it is crucial to ensure the mask and body make close contact to prevent any gaps. Any gaps can lead to unwanted electrode deposition, therefore contaminating unintended areas. For example, Kim et al. developed a substrate-less nanomesh receptor for strain sensing, employing silver–gold core–shell nanowires as the electrode material to ensure biocompatibility (Fig. 2a).11 They created a lightweight and portable printing machine and used a stencil mask to cover the unwanted areas so that mass production could be available in a scalable and transportable manner. One of the key advantages of spray-coating is its ability to pattern electrodes over large areas, which makes it highly suitable for rapid prototyping.
 | ||
| Fig. 2 Diverse types of fabrication methods to generate tattoo electrodes. (a) (i) Photographs of the portable printing machine. (ii) Schematic illustration of a nanomesh receptor mimicking a cutaneous receptor, with wireless measurement of resistance changes. Adapted with permission from ref. 11 Copyright 2022, Springer Nature. (b) (i) Photographs of the in situ biogel applied and squeezed on skin. (ii) Photographs of the biogel undergoing rapid in situ gelation. (iii) Photographs demonstrating the biogel's skin adhesion and mechanical toughness. Adapted with permission from ref. 14 Copyright 2025, Springer Nature. (c) (i) Schematic illustration of nanomesh conductors and their deposition onto skin, along with an optical image on skin (scale bar: 1 mm) and an SEM image on a skin replica (scale bar: 5 μm). (ii) Photographs of nanomesh conductors attached to the finger when the hand is opened and closed. Adapted with permission from ref. 22 Copyright 2017, Springer Nature. (d) (i) Schematic illustration of reversible selective laser-induced redox enabling monolithic patterning of CuNW, Cu2ONW, and CuONW networks. (ii) Optical images of CuNW, Cu2ONW, and CuONW networks patterned via laser processing. (iii) Optical images showing laser-induced drawing, conversion, and erasing. Adapted with permission from ref. 26 Copyright 2022, Springer Nature. | ||
Another approach for direct patterning involves printing or drawing directly onto the target area. In printing-based methods, the three-dimensional geometry of the application site should be scanned while restraining user movement, allowing for precise electrode deposition that conforms to the body's contours.12,13 Drawing methods, on the other hand, utilize a brush or a similar tool to create customized electrode patterns manually (Fig. 2b).14 During the subsequent transition from the liquid to the solid phase, either through solvent evaporation or temperature-induced phase change, the adhesion between the electrode and the body is enhanced. Although these methods may result in lower pattern resolution than mask-assisted techniques, they offer significant advantages: electrodes can uniformly cover the entire target surface without shaded regions caused by mask misalignment, and desired patterns can be produced more rapidly and flexibly.
Additionally, to acquire biosignals from the deeper regions of the body, conductive materials can be injected beneath the dermis with a tattoo needle.15 In contrast to the electrodes deposited on the surface, which collect signals only from the exterior of the skin or organs, this technique enables capturing signals from deeper layers, allowing for more accurate and varied data collection. While most tattoo electrodes have been primarily used for electrical signal detection and a limited range of chemical sensing, this method extends their functionality to diverse chemical sensing. Utilizing specific reactive materials can collect biological data, enabling the tracking of glucose levels, proteins, inflammation markers, and other biochemical signals.16,17 Nevertheless, user discomfort and challenges in complete tattoo removal still pose significant obstacles.
In conclusion, improving the resolution without harsh manufacturing procedures is required to enable fine electrode patterning for tattoo electrodes made using the direct patterning method to be used efficiently. Additionally, the electrodes must also have a high degree of adhesion to the body surface to ensure long-term stability and prevent delamination. When these issues are properly addressed, the direct patterning method is highly beneficial for mass manufacturing, rapid prototyping, and a simplified fabrication process, which makes it a good choice for wearable electronics.
2.2. Indirect patterning
Unlike direct patterning, indirect patterning involves patterning the electrode on an external substrate before transferring it onto the body. Water-soluble substrates, which act as a temporary medium for depositing conductive materials, are the mainstay of such production procedures. The utilization of harsh fabrication techniques like laser processing, UV curing, and chemical reactions is made possible by the greatly decreased fabrication limitations resulting from the patterning process occurring on a substrate rather than directly on the human body.18 Furthermore, the accurate application of inkjet printing, which necessitates a steady and motionless environment, is made possible by the lack of movement during processing.12 This method is especially useful for rapid production while preserving the inherent properties of both the substrate and the electrode.One approach for indirect patterning employs water-soluble substrates.19 In this method, the conductive material only remains on the body after the substrate dissolves in water. It is important to set the temporary substrate to an appropriate thickness. If the substrate is too thick, forming precise electrode patterns on intricate surfaces like fingerprints or extremely curved and wrinkled areas may become difficult since conformal contact between the skin and substrates becomes challenging. On the other hand, an excessively thin substrate makes it difficult to work with; for best results, a balance in thickness is needed. Furthermore, the substrate material should also be readily detachable and biocompatible to guarantee safe application on the body. Among many choices, polyvinyl alcohol (PVA) is frequently utilized as a substrate material since it is highly water-soluble and biocompatible, making removal simple without causing any harm to humans.20,21
For example, Miyamoto et al. employed a PVA electrospun membrane as a substrate that dissolves in water (Fig. 2c).22 After applying a gold nanomesh solution, the membrane is placed on the skin. Then, the membrane is dissolved in water, and only the gold nanomesh is left on the skin, providing remarkable conformality even in highly dynamic regions like finger joints, where adhesion is difficult. Additionally, the gold nanomesh electrode demonstrated high stretchability due to its ability to repeatedly and reversibly split and repair, while its high permeability enabled seamless exchange of sweat and air through the electrode.
Moreover, indirect patterning reduces process constraints, allowing for the adoption of a wide range of fabrication techniques. Specifically, laser direct writing can achieve high resolution and induce photothermal chemical reactions with patterning materials.23 For instance, when polyimide undergoes a photothermal chemical reaction, it transforms into graphene, which can serve as electrodes due to its high conductivity.24,25 Similarly, copper can be reduced and subsequently oxidized via wet oxidation to form Cu2O, a semiconducting material suitable for optoelectronic applications (Fig. 2d).26 Additionally, photolithography can be employed, in which photoresist layers are patterned through light exposure and subsequently developed using chemicals to achieve fine and precise structures.27,28
Thus, in indirect patterning, patterns can be easily transferred by dissolving water-soluble substrates. Alternatively, laser direct patterning and photolithography can also be used to improve resolution, enabling the formation of accurate and fine patterns. These methods provide distinct approaches for achieving precise patterning in different contexts.
3. Materials
Tattoo electrodes rely significantly on the selection of electrode materials, which must satisfy several requirements: mechanical compliance to match soft biological tissues, high electrical conductivity for efficient signal transmission, biocompatibility to ensure safety during prolonged contact with the body, and, in many cases, permeability to preserve natural skin functions.29,30 Traditionally, metallic, carbon-based, and polymeric materials have been widely used as tattoo electrode materials. Recent developments have additionally brought liquid metals and MXene family as alternative materials with improved characteristics for specific applications. Table 2 summarizes the typical materials, electrode structure, electrical characteristics, and electrical failure strain of various tattoo electrodes. The components and construction of tattoo electrodes will be discussed in this part, highlighting both conventional and newly developed materials under ongoing research.| Material class | Material name | Electrode structure | Electrical characteristics | Electrical failure strain | Ref. |
|---|---|---|---|---|---|
| Metallic | Ag–Au | Nanomesh | ∼1.4 Ω sq−1 | >30% | 11 |
| Au | 5.3 × 10−7 Ω m | ∼50% | 22 | ||
| Serpentine | — | ∼100% | 91 | ||
| Wrinkled thin film | 66–88 Ω sq−1 | >200% | 34 | ||
| Cu | Serpentine | R/R0 < 1.01 | >300% | 90 | |
| Ag | Nanomesh | 7.62 Ω sq−1 | >80% | 38 | |
| Au/Ti | Hollow-carved structure | 4–12 kΩ cm | >100% | 39 | |
| Ni/EGaIn | Intrinsically stretchable | Na+ sensitivity ∼56.8 mV per decade | 10–30% | 40 | |
| Carbon-based | Graphene | Serpentine | Skin-electrode impedance ∼200–300 kΩ at 10 kHz | 10–30% | 50 |
| ∼2 kΩ sq−1 | >25% | 51 | |||
| Multi-stacking | ∼0.55 ± 0.25 kΩ sq−1 | >30% | 52 | ||
| Skin-electrode impedance ∼1–10 kΩ at 10 kHz | 10–30% | 53 | |||
| Polymeric | ETE-COONa/PVA | Intrinsically stretchable | ∼0.25 S cm−1 | >30% | 13 |
| Polyacrylamide/polypyrrole | R/R0 < 1.06 | >200% | 66 | ||
| PEDOT:PSS | Skin-electrode impedance −72 kΩ at 50 Hz | 10–30% | 85 | ||
| 100–200 Ω sq−1 | 10–30% | 94 | |||
| PVA/PEDOT:PSS | 0.6–1.8 S m−1 | >349% | 57 | ||
| 2.3 S m−1 | >100% | 59 | |||
| WPU/PEDOT:PSS | 11 S cm−1 | >400% | 61 | ||
| Poly(aniline) | Stencil pattern | — | 10–30% | 97 | |
| Hybrid | EGaIn/CNT | Intrinsically stretchable | <100 Ω sq−1 | >40% | 45 |
| CNT/PDMS | ∼10−2 S m−1 | ∼200% | 55 | ||
| Ag/TPU | 38% increase in sheet resistance over 4 days | ∼100% | 92 | ||
| Others | Mo2C | Ultrathin film | 60–150 Ω sq−1 | 10–30% | 89 |
| Ti3C2Tx/EGaIn | Intrinsically stretchable | 2.2 Ω sq−1 | >50% | 78 | |
3.1. Metallic materials
Metallic materials have been widely used in bioelectronics due to their high electrical conductivity and ease of patterning. Ensuring conformal contact is essential for tattoo electrodes to accurately collect signals from the highly textured and non-uniform surfaces of the human body. However, bulk metals are inherently brittle and have low stretchability, making it difficult for them to conform to the body. Therefore, structural modifications and solution-processed metals have been employed to overcome these limitations.Structurally engineered electrode patterns exhibit greater stretchability while maintaining significant conductivity under strain.31 Additionally, when metal films are patterned into serpentine or other porous structures by laser processing or etching, permeability is also enhanced, making them highly beneficial for strain sensing applications.32,33 Furthermore, research has demonstrated that depositing gold onto shape-memory polymers can further improve stretchability and mechanical resilience.34
On the other hand, metal nanowires in solution form have been extensively explored for tattoo electrode applications. Depending on their structural arrangement, nanowires can form either a dense nanomesh structure or a nano-percolation network. In the nanomesh structure, individual nanowires rearrange under strain while maintaining electrical connection without fracture.22 In contrast, nanowires create a porous structure that can be integrated with a polymer matrix when a percolation network is formed. By embedding metal nanowires into stretchable polymer matrices, a conductive composite can be formed that intrinsically combines mechanical deformability with electrical conductivity (Fig. 3a).35 This approach eliminates the need for post-deposition processing and enables conformal contact with dynamic surfaces. The polymer matrix provides elasticity and structural support, while the embedded nanowires establish percolated conductive pathways that remain stable under strain, offering excellent performance for stretchable tattoo electronics.36 Furthermore, chemical stability and biocompatibility are also critical factors for direct contact with the body. Therefore, gold nanowires, copper nanowires, and silver–gold core–shell nanowires are commonly used in tattoo electrodes.37–39
 | ||
| Fig. 3 Metallic and carbon-based materials that can be utilized for the formation of tattoo electrodes. (a) (i) Schematic illustrations depicting the manufacturing process of a nanowire-embedded polymer. (ii) Photograph of the nanowire-embedded polymer for EMG sensing. Adapted with permission from ref. 35 Copyright 2025, Springer Nature. (b) (i) Schematic illustration depicting the assembly of a Pt-decorated CNT on liquid metal particles (CMPs) on the skin. (ii) Photographs showing diverse applications of CMPs, including ECG sensing, electrical stimulation, biosensing, and photothermal heating. (iii) Schematic illustration of a CMP-based electrical stimulator applied to a rat. Adapted with permission from ref. 45 Copyright 2022, Wiley-VCH. (c) (i) Photographs of graphene electronic tattoo (GET) on the skin under 25% compression and stretching. (ii) Demonstration of the GET used for skin hydration and temperature sensing. Adapted with permission from ref. 51 Copyright 2017, American Chemical Society. (d) (i) Photographs of the CNT/PDMS composite sensor for EEG monitoring from top and bottom views. (ii) Sensor attached behind the ear. (iii) FFT spectrogram of EEG signals before and after eye closure. Adapted with permission from ref. 54 Copyright 2023, Springer Nature. | ||
On the other hand, liquid metal-based tattoo electrodes, such as eutectic gallium–indium (EGaIn), have recently gained great attention. EGaIn is the most well-known liquid metal, capable of being directly patterned in a liquid state, as it remains in a liquid phase until exposed to external stimuli.40 However, EGaIn particles, mechanically fractured from bulk EGaIn, are typically employed instead since bulk EGaIn lacks intrinsic stretchability.41 When EGaIn particles are deposited into a desired pattern, oxide layers rapidly form on their surface. These layers can be mechanically fractured through mechanical force or laser sintering, allowing the particles to connect and restore electrical conductivity.42 However, once these EGaIn particles reconnect, they behave similarly to bulk EGaIn, which easily fractures under strain. To address this limitation, elastomeric materials or metal flakes are often incorporated to enhance stretchability.43,44 Additionally, Lee et al. demonstrated the enhanced mechanical robustness of liquid metal-based tattoo electrodes by attaching Pt-decorated carbon nanotubes (CNTs) onto EGaIn, further expanding the potential of metallic tattoo electrode materials (Fig. 3b).45
As a result, metallic electrodes can be effectively utilized by engineering their structures or incorporating liquid-state metals, enabling them to be applied even on highly dynamic areas subjected to frequent movements.
3.2. Carbon-based materials
Carbon-based materials have unique bonding structures that enable their high electrical conductivity. In well-packed carbon materials, such as carbon nanotubes (CNTs) and graphene, the sp2 hybridized structure creates delocalized π-electron networks, facilitating electron transport.46,47 The carbon materials exhibit metallic behavior with an electron bandgap close to 0 eV, and their conductivity is further enhanced.48 Additionally, carbon-based tattoo electrodes have been developed to employ carbon black ink with conductive polymers, broadening the variety of carbon-based materials for tattoo applications.49 Overall, their high conductivity, mechanical flexibility, and chemical stability make them ideal for skin-conformal and stretchable electronic applications. For these reasons, carbon-based materials, such as graphene and carbon nanotubes (CNTs), are among the most commonly utilized materials for tattoo electrodes.Graphene, a one-atom-thick 2D material, comprises carbon atoms arranged in a honeycomb lattice. Graphene has been widely used in flexible displays and wearable sensors owing to its high flexibility and mechanical strength. Although the material is not inherently stretchable and is prone to break under tensile strain, strategies have been developed to overcome this limitation.50 Kabiri Ameri et al. developed filamentary serpentine graphene electrodes, improving the stretchability to over 40%, whereas straight-line graphene electrodes rupture at strains below 20% (Fig. 3c).51 Another strategy developed to enhance the resilience of graphene-based tattoo electrodes is multilayer stacking, in which graphene is stacked into multilayers to maintain electrical conductivity and function as a backup when a layer breaks under strain.52 Multilayer stacking enables greater stretchability and durability, making it suitable for generating stretchable and transparent electrodes for wearable applications.
CNTs, on the other hand, are composed of cylindrical carbon structures and exhibit great flexibility. However, the material also lacks stretchability, limiting its uses to low-strain regions.53 This limitation can be improved by blending the material with an elastomer, such as polydimethylsiloxane (PDMS), to form CNT–elastomer composites (Fig. 3d).54 These composites can withstand strains exceeding 100%, enabling their use in high-strain sensor applications. Furthermore, a high gauge factor is another characteristic that makes CNT-based electrodes ideal for ultra-sensitive strain sensors in tattoo electronics.55
Given their high electrical conductivity, biocompatibility, and outstanding mechanical strength, carbon-based materials remain one of the most promising candidates for tattoo electrodes. They also have immense potential for wearable bioelectronics and next-generation flexible devices.
3.3. Polymeric materials
The most prevalent polymer-based material for tattoo electrodes is poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS). This material exhibits high electrical conductivity, excellent biocompatibility, and strong processability. However, pristine PEDOT:PSS exhibits very low intrinsic stretchability (2% before mechanical failure), which makes it prone to delamination under body movements.56 Moreover, in humid environments, the hydrophilic nature of the PSS component leads to excessive swelling and redispersion of the film, limiting its applicability in wearable bioelectronics without further engineering.To address these challenges, network engineering strategies have been employed, incorporating PEDOT:PSS into polymer matrices such as poly(vinyl alcohol) (PVA), poly(acrylic acid) (PAA), and gelatin.57–59 These networks improve mechanical stretchability and suppress water-induced degradation (Fig. 4a).60 Alternatively, phase separation approaches have been explored. For instance, the addition of dimethyl sulfoxide induces the formation of reconfigured PEDOT:PSS.61 Other methods use additives like polyurethane to decouple mechanical and electrical phases, creating distinct PEDOT:PSS conductive pathways within mechanically robust matrices.60,62 Recently, Won et al. introduced a laser treatment technique that spatially separates PEDOT and PSS phases (Fig. 4b).63 Upon hydration, the PEDOT-rich domains form continuous electrical pathways, while the PSS-rich domains act as ionic conductors, collectively suppressing water-induced degradation. Moreover, the use of laser processing enables high-resolution, selective patterning on targeted regions.
 | ||
| Fig. 4 Polymeric and alternative materials that can be utilized to form tattoo electrodes. (a) (i) Schematic illustrations of composites integrating PEDOT:PSS and hydrophilic polyurethane. (ii) Photographs of the bioelectronic interface on a fingertip demonstrating its stretchability. (iii) Photograph of the bioelectronic interface on a rat sciatic nerve. Adapted with permission from ref. 60 Copyright 2023, Springer Nature. (b) (i) PEDOT:PSS pattern fabricated via laser processing, demonstrating strong adhesion to the substrate under wet conditions, and SEM image of the dried PEDOT:PSS hydrogel cross-section on a PET substrate. (ii) Comparison of wet adhesion to the substrate under shear force. Top: Only EG-treated. Bottom: Laser-treated. Adapted with permission from ref. 63 Copyright 2024, Springer Nature. (c) (i) Schematic illustrations of fabrication steps for a hydrogel-based electronic tattoo sensor. (ii) Photographs of different hydrogel electronic tattoo sensors. HSHS, HRTD, and HEPS represent hydrogel-based hydration sensors, hydrogel-based resistance temperature sensors, and hydrogel-based electrophysiological sensors, respectively (scale bar: 1 cm). (iii) SEM image showing its microstructural morphology. Adapted with permission from ref. 66 Copyright 2024, Springer Nature. (d) (i) Schematic illustrations of a sprayable liquid metal–MXene composite electronic tattoo sensor applied onto the skin. (ii) Top: Photographs of the nanocomposite along different strains (0–50%) (scale bar: 200 μm). Bottom: Schematic illustration of the self-healing mechanism of the cracked nanocomposite. (iii) Demonstrations of LEDS in a tattoo circuit maintaining stable operation on the skin during compression, stretching, and twisting. Adapted with permission from ref. 78 Copyright 2024, Elsevier. | ||
In addition, conductive pathways can be embedded within hydrogel matrices to fabricate tattoo-like sensors and interfaces.64,65 For example, Zhuo and colleagues developed a reusable hydrogel-based tattoo sensor, in which polypyrrole was incorporated to enable monitoring of skin temperature and hydration levels (Fig. 4c).66 Owing to their extremely low mechanical modulus, hydrogels offer exceptional mechanical conformity with biological tissue, making them ideal for bioelectronic interfaces and wearable sensors. Unlike conventional polymers, hydrogels exhibit high water retention within their internal matrix, which prevents dehydration-related failures and ensures stable performance in humid or sweaty environments that often degrade other tattoo electrode materials. Their inherently porous structure also enables the incorporation of ionic components, allowing internal ions to migrate freely under mechanical strain and maintain stable current flow even during deformation.67 Additionally, their high viscosity allows hydrogels to be processed into printable inks, thereby facilitating patterning via 3D printing and other additive manufacturing techniques.68,69
Overall, polymeric materials strike a crucial balance between flexibility, biocompatibility, and conductivity in tattoo electrodes. Hydrogel-based systems, in particular, offer superior ionic conductivity and mechanical softness, enabling advanced functionalities for sensing and human–machine interfaces in next-generation wearable biomedical devices.
3.4. Other materials
The previously discussed materials – metallic, carbon-based, and polymeric – have long been employed as electrode materials. To enhance their suitability for tattoo electrodes, researchers have explored combining them with elastomers or forming composites that leverage the advantages of each material.43,55,70,71Lately, the MXene family has been receiving significant interest as possibly useful materials for tattoo electrodes. MXenes are a family of 2D nanomaterials with remarkable electrical properties, high ionic conductivity, and great flexibility that make them ideal materials for skin-conformal bioelectronics.72 MXenes can be derived from MAX phases through chemical or mechanical exfoliation.73 Early production techniques, however, suffered from scalability, limiting their general deployment. However, large-scale synthesis techniques have made it feasible to generate consistent, high-quality MXenes, and hence, their use has expanded greatly in various research fields, including energy storage and wearable bioelectronics.74
MXene-based electrodes' capacity to be processed into ultrathin films, allowing their use in biosignal sensing and energy storage, is one of their main advantages.75,76 Their unique combination of conductivity, flexibility, large surface area and biocompatibility further expands the potential of tattoo electrodes in wearable and implantable bioelectronics, thus opening the path for next-generation soft electronic systems.77 However, MXenes suffer from their intrinsic structural brittleness under tension. To overcome this, Wang et al. developed a composite by incorporating a liquid metal to enhance its mechanical resilience and stretchability (Fig. 4d).78 Under strain, the oxide layer of the liquid metal nanocapsules mechanically breaks, allowing the liquid metal within to flow and re-establish electrical pathways, thereby effectively solving the brittleness issue. MXene films produced in this work demonstrated outstanding resistance to mechanical stress and water exposure while allowing high-fidelity biosignal acquisition.
In addition to MXenes, transition metal dichalcogenides (TMDs) such as MoS2 and WS2 are also gaining interest in the context of tattoo electrodes.79,80 TMDs are two-dimensional semiconductor materials with layered structures similar to graphene.81 They exhibit several attractive properties, including strong exciton binding energies, fast charge transport, and intense light–matter interactions.82 Furthermore, TMDs allow for tunable electronic and optical properties through phase engineering, doping, or strain modulation. These characteristics make TMDs promising candidates not only for conformable sensors, but also for next-generation optoelectronic devices, transistors, and catalysts.83 Their atomic thinness enables excellent skin conformality, while their semiconducting nature provides diverse functionalities in bio-integrated systems.
In summary, MXenes and TMDs represent promising emerging materials for tattoo electrodes, offering exceptional electrical, mechanical, and optical properties that enable multifunctionality in wearable bioelectronics. Their unique characteristics, such as skin conformability, conductivity, and tunable behavior, open new possibilities for next-generation bio-integrated systems beyond conventional materials.
4. Applications
Tattoo electrodes, leveraging their skin-conformal structure and material versatility, have found wide-ranging applications in wearable bioelectronics. Their intimate contact with the body enables highly accurate strain and electrophysiological signal acquisition, supporting diverse use cases such as motion tracking, facial expression monitoring, and real-time cardiac or neural assessments. In addition, tattoo electrodes are being employed for temperature and humidity sensing through responsive materials, providing valuable data for both virtual reality systems and clinical diagnostics. Their adaptability also extends to chemical and pH sensing, where electrochemical mechanisms enable non-invasive detection of biochemical markers. Beyond sensing, tattoo electrodes are emerging energy harvesting and storage platforms, particularly through sweat-powered biofuel cells, capacitive systems, and wireless power transfer, paving the way for self-sustaining, wire-free wearable systems. This section explores the expanding role of tattoo electrodes in health monitoring, human–machine interaction (HMI), and the development of fully integrated, next-generation wearable electronics.4.1. Strain sensors
The exceptional performance of tattoo electrodes in strain sensing is one of their significant advantages in biosignal monitoring. Conventional strain sensors fabricated on flexible substrates often struggle to maintain intimate contact with the body, especially over highly contoured, wrinkled, or dynamic surfaces such as joints or facial regions.84 This imperfect interface can compromise signal fidelity and accuracy. In contrast, tattoo electrodes are directly deposited onto the skin or tissue surface, achieving gap-free, conformal adhesion without the need for adhesives or an external support. This ultra-thin, substrate-free architecture provides a skin-like interface that ensures precise transduction of mechanical deformations into electrical signals, thereby significantly enhancing the accuracy and reliability of strain sensing.85Tattoo electrodes are frequently employed to monitor various forms of body movement, including finger motion, facial expressions, and micro-strains from skin deformation (Fig. 5a).11,86,87 Beyond cutaneous applications, they can also be integrated with internal organs such as the heart and lungs, owing to their soft mechanical properties.13 This mechanical compatibility minimizes inflammation and delamination, enabling stable long-term in vivo operation without triggering adverse immune responses.
 | ||
| Fig. 5 Various types of applications achieved with tattoo-based electronic systems. (a) Applications implemented by tattoo strain sensors for (i) finger motion, (ii) facial expressions, and (iii) micro-strains from skin deformation. Adapted with permission from ref. 11, 86 and 87 Copyright 2022, Springer Nature, 2024, The American Association for the Advancement of Science, and 2022, Springer Nature. (b) Implementation of polysomnographic techniques using tattoo electrophysiological sensors. Adapted with permission from ref. 90 Copyright 2023, The American Association for the Advancement of Science. (c) Optical and infrared images of temperature sensors integrated into the skin. Adapted with permission from ref. 91 Copyright 2013, Springer Nature. (d) (i) Schematic illustration of a tattoo-based humidity sensing system and (ii) current response measured before, during, and after exercise. Adapted with permission from ref. 96 Copyright 2024, Springer Nature. (e) Schematic illustrations of the manufacturing process of a pH-responsive tattoo sensor. Adapted with permission from ref. 98 Copyright 2021, Wiley-VCH. (f) Sensing mechanisms of tattoo-based electrodes for detecting (i) glucose and (ii) alcohol. Adapted with permission from ref. 16 and 17 Copyright 2014, American Chemical Society and 2016, American Chemical Society. (g) Schematic illustrations of thermoelectric generation using tattoo-like wearable devices. Adapted with permission from ref. 104 Copyright 2024, Wiley-VCH. (h) Photographs of the tattoo electrode system for energy harvesting and storage. Adapted with permission from ref. 106 Copyright 2017, Wiley-VCH. | ||
The strain signals collected by tattoo sensors are typically processed using machine learning algorithms, allowing for high-level interpretation and classification.3,4 Applications such as hand gesture recognition, facial expression detection, and non-verbal communication interfaces have been successfully demonstrated, paving the way for advanced HMI and neuroprosthetic control systems.
In summary, the ultra-conformal, substrate-free design of tattoo electrodes offers a distinct advantage in strain sensing by enabling seamless integration with both external skin and internal organs. This intimate interface ensures high-fidelity signal acquisition even on complex, dynamic surfaces, while minimizing mechanical mismatch and immune responses. Coupled with machine learning-based signal processing, tattoo strain sensors have demonstrated strong potential in diverse applications.
4.2. Electrophysiological sensors
Electrophysiological signals, including electromyography (EMG), electrocardiography (ECG), electroencephalography (EEG), and electrooculography (EOG), are essential for monitoring neuromuscular activities, cardiac rhythms, brain function, and ocular motion.88 However, traditional electrodes, often composed of rigid metal components or gel-based adhesives, face inherent limitations in long-term and dynamic monitoring. One major issue is their susceptibility to motion artifacts, which arises from poor mechanical conformity and unstable contact with the skin during body movement. Additionally, the imperfect interface between the electrode and skin often leads to high interfacial impedance, degrading the quality and fidelity of acquired signals.Tattoo electrodes address these limitations by forming a conformal, ultra-thin interface that adheres intimately to the skin's microtopography.89 Unlike conventional electrodes, they do not require bulky adhesives or conductive gels, minimizing the risk of delamination or contact variation. This configuration effectively reduces motion-induced signal distortion and ensures low-impedance coupling, even under continuous or dynamic conditions. As a result, tattoo electrodes enable reliable acquisition of high-fidelity electrophysiological signals suitable for both clinical and wearable applications. A representative example is polysomnography, where simultaneous monitoring of multiple physiological parameters during sleep is critical (Fig. 5b).90 Conventional setups often involve cumbersome electrodes and wiring that can interfere with natural sleep. Tattoo electrodes offer a lightweight and unobtrusive solution, enabling continuous, high-resolution sleep monitoring without compromising user comfort or signal integrity.
Thanks to their skin-like mechanical properties and stable electrical interface, tattoo electrodes are particularly well-suited for electrophysiological sensing in biomedical applications. Their ability to acquire high-fidelity EMG, ECG, EEG, and EOG signals over extended periods enables continuous monitoring of muscular, cardiac, neurological, and ocular functions in a non-invasive and patient-friendly manner. This makes them highly attractive for use in neurological disorder diagnosis, cardiac health tracking, sleep studies, and assistive technologies. By enabling accurate and long-term bioelectrical monitoring, tattoo sensors hold great potential to advance personalized medicine, adaptive neuroprosthetics, and real-time health feedback systems.
4.3. Temperature and humidity sensors
In addition to mechanical and electrophysiological sensing, tattoo electrodes can be engineered to detect temperature and humidity by incorporating materials that exhibit temperature-dependent or hygroscopic electrical properties. Their ultra-thin, conformal form allows for precise and continuous monitoring of skin temperature and moisture levels with minimal interference to the user. This is particularly valuable in scenarios where subtle thermal changes reflect underlying physiological conditions, such as inflammation, infection, fever onset, or wound healing dynamics.One notable work by Webb et al. demonstrated ultrathin, conformal temperature sensors capable of continuous thermal mapping of the skin with clinical-grade accuracy (Fig. 5c).91 Such systems can act as early indicators of localized physiological abnormalities, enabling timely intervention in medical settings. Furthermore, skin-integrated tattoo thermosensors can be seamlessly combined with other tattoo-based sensors to create multimodal diagnostic platforms that monitor biochemical and biophysical parameters in real-time. Beyond healthcare, temperature-sensitive tattoo electrodes also show strong potential in virtual and augmented reality environments, where realistic thermal feedback can enhance immersion and interaction.92–94 By providing location-specific temperature changes in sync with digital stimuli, tattoo sensors enable more lifelike tactile experiences, thereby expanding the possibilities for next-generation human–machine interfaces. In addition, humidity-sensing tattoo electrodes, often based on materials like graphene oxide or MXene-based composites, can track perspiration levels to infer the hydration status, stress response, or metabolic activity (Fig. 5d).95,96 When integrated into wearable systems, they support continuous sweat analysis, athletic performance monitoring, and thermal regulation studies.
Overall, tattoo-based temperature and humidity sensors offer a compelling combination of wearability, sensitivity, and application diversity, making them valuable tools in clinical diagnostics and interactive technology platforms.
4.4. Chemical and pH sensors
Tattoo electrodes can be functionalized to detect chemical biomarkers and pH levels in biofluids such as sweat, blood, and urine, providing a powerful platform for non-invasive, continuous health monitoring. These biofluids contain physiologically relevant analytes, including electrolytes, metabolites, and protons (pH), which can indicate the hydration status, metabolic conditions, and localized inflammation.One effective approach for tattoo-based chemical sensing involves the use of electrochemical mechanisms, in which chemical reactions at the sensor surface produce measurable electrical signals.97 A representative example is the temporary tattoo pH sensor developed using a pH-responsive hydrogel (Fig. 5e).98 In this system, the hydrogel swells or contracts in response to local pH, altering the electrical double layer at the electrode interface. This change is transduced into an electrochemical signal, enabling real-time tracking of skin pH. Such devices demonstrate excellent mechanical conformity, reversibility, and stability, all while maintaining user comfort.
Beyond pH, tattoo-based chemical sensors have been developed to monitor glucose, lactate, sodium, potassium, and cortisol levels using enzyme-functionalized electrodes or ion-selective membranes (Fig. 5f).16,17,99,100 These sensors typically utilize potentiometric or amperometric mechanisms to convert ion concentration or redox reactions into voltage or current signals.
Unlike rigid chemical sensors that may require bulky instrumentation or invasive sampling, tattoo sensors are ultra-thin, breathable, and skin-conformal, allowing for unobtrusive operation over extended periods. These features make tattoo chemical and pH sensors ideal for early disease detection, sports performance monitoring, and personalized health diagnostics, especially in decentralized healthcare settings.101 They also show strong potential for emerging applications such as pet health monitoring and ingestible or edible sensors with guaranteed biodegradability.102,103
4.5. Energy harvesting and storage
Tattoo electrodes are not only capable of transmitting bioelectrical and externally applied signals but also hold great potential for on-skin energy harvesting and storage. Their ultra-thin, breathable, and highly conformal architecture enables efficient energy interaction with the skin and surrounding environment, opening up new opportunities for developing self-powered wearable systems.Recent studies have demonstrated the feasibility of such systems using tattoo-based platforms. For instance, a thermoelectric tattoo system has been developed that harvests energy from the temperature gradient between the human body and ambient air, enabling continuous temperature mapping without an external power source (Fig. 5g).104 In another example, a fully untethered and battery-free electronic tattoo was realized using near-field communication-based wireless energy harvesting to support real-time biosignal monitoring.105 These examples highlight how tattoo electrodes can serve not only as sensing interfaces but also as integral components of autonomous energy systems, advancing the development of next-generation self-powered bioelectronics.
Moreover, tattoo electrodes can also function as energy storage components, particularly in the form of capacitors. A typical configuration consists of two conductive layers separated by a dielectric material, enabling the system to store and release electrical energy (Fig. 5h).106 In capacitive coupling systems, the stratum corneum can act as the dielectric medium, allowing tattoo electrodes to relay signals or charge through the skin without direct electrical contact.107 Furthermore, triboelectric nanogenerators have been demonstrated by sandwiching elastic dielectric layers, such as electrospun fibers, between conductive tattoo electrodes.108 This configuration enables energy harvesting through motion-induced contact and separation, creating self-powered systems suitable for dynamic body movements.
Collectively, these developments in energy harvesting and storage enable tattoo electronics to function as fully independent systems with sustained operation. Therefore, tattoo-integrated energy solutions can contribute significantly to the development of next-generation autonomous bioelectronic systems by providing compact, eco-friendly, and non-invasive power sources.
5. Conclusion and perspectives
This review has explored fabrication methods, materials, and diverse applications of tattoo-based electronics. While traditional substrate-based wearables often struggle with issues of conformality, user comfort, and mechanical compatibility, tattoo electronics offer a promising alternative through their seamless integration with the skin. Building on these advantages, tattoo-based electronics are now redefining the landscape of wearable technology by enabling ultrathin and conformal platforms for health monitoring, HMI, and energy systems. However, to fully realize their potential, several unresolved challenges must still be addressed, along with the exploration of new technological directions.First, enhancing long-term durability and mechanical robustness remains critical. Tattoo electrodes often suffer from delamination, mechanical failure, or degradation under repeated strain, especially under humid or dynamic skin conditions. Strategies involving self-healing materials, moisture-tolerant encapsulation, and stretchable interconnects are needed to ensure consistent long-term performance. Second, the development of biodegradable tattoo electronics is an emerging direction with high relevance for both medical and environmental applications. Biodegradable tattoo sensors can offer transient wound healing or drug delivery monitoring without requiring removal, minimizing environmental impact and improving user comfort. Third, incorporating a wider range of materials can allow tattoo sensors to detect multiple types of signals simultaneously, enabling more compact and lightweight designs. Incorporating 2D materials, bioresorbable composites, and biocompatible nanostructures can drive progress in this area. Finally, integrating data processing and wireless communication in ultrathin formats is essential to create fully autonomous systems. Advanced machine learning approaches and power-efficient circuitry can enable real-time analysis and feedback in decentralized healthcare or immersive HMI environments.
Therefore, addressing these frontiers will shape tattoo-based electronics into truly autonomous, versatile platforms for next-generation bioelectronic systems.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (grant number RS-2025-11092968, RS-2022-NR068144) and the Air Force Office of Scientific Research under award number FA2386-24-1-4089.References
- Y. Liu, H. Wang, W. Zhao, M. Zhang, H. Qin and Y. Xie, Sensors, 2018, 18, 645 CrossRef PubMed.
- M. Jafari, G. Marquez, H. Dechiraju, M. Gomez and M. Rolandi, Cell Rep. Phys. Sci., 2023, 4, 101535 CrossRef CAS.
- J. Xie, Y. Zhao, D. Zhu, J. Yan, J. Li, M. Qiao, G. He and S. Deng, ACS Appl. Mater. Interfaces, 2023, 15, 12551–12559 CrossRef CAS PubMed.
- Q. Yang, W. Jin, Q. Zhang, Y. Wei, Z. Guo, X. Li, Y. Yang, Q. Luo, H. Tian and T.-L. Ren, Nat. Mach. Intell., 2023, 5, 169–180 CrossRef.
- W. Heng, S. Solomon and W. Gao, Adv. Mater., 2022, 34, e2107902 CrossRef PubMed.
- Y. Yuan, B. Liu, H. Li, M. Li, Y. Song, R. Wang, T. Wang and H. Zhang, Biosensors, 2022, 12, 1069 CrossRef CAS PubMed.
- A. J. Bandodkar, W. Jia and J. Wang, Electroanalysis, 2015, 27, 562–572 CrossRef CAS.
- K. Lim, H. Seo, W. G. Chung, H. Song, M. Oh, S. Y. Ryu, Y. Kim and J.-U. Park, Commun. Mater., 2024, 5, 49 CrossRef CAS.
- A. Spanu, A. Mascia, G. Baldazzi, B. Fenech-Salerno, F. Torrisi, G. Viola, A. Bonfiglio, P. Cosseddu and D. Pani, Front. Bioeng. Biotechnol., 2022, 10, 820217 CrossRef PubMed.
- Y. Wang, L. Yin, Y. Bai, S. Liu, L. Wang, Y. Zhou, C. Hou, Z. Yang, H. Wu, J. Ma, Y. Shen, P. Deng, S. Zhang, T. Duan, Z. Li, J. Ren, L. Xiao, Z. Yin, N. Lu and Y. Huang, Sci. Adv., 2020, 6, eabd0996 CrossRef CAS PubMed.
- K. K. Kim, M. Kim, K. Pyun, J. Kim, J. Min, S. Koh, S. E. Root, J. Kim, B.-N. T. Nguyen, Y. Nishio, S. Han, J. Choi, C. Y. Kim, J. B. H. Tok, S. Jo, S. H. Ko and Z. Bao, Nat. Electron., 2022, 6, 64–75 Search PubMed.
- L. W. Lo, J. Zhao, H. Wan, Y. Wang, S. Chakrabartty and C. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 21693–21702 CrossRef CAS PubMed.
- X. Strakosas, H. Biesmans, T. Abrahamsson, K. Hellman, M. S. Ejneby, M. J. Donahue, P. Ekström, F. Ek, M. Savvakis, M. Hjort, D. Bliman, M. Linares, C. Lindholm, E. Stavrinidou, J. Y. Gerasimov, D. T. Simon, R. Olsson and M. Berggren, Science, 2023, 379, 795–802 CrossRef CAS PubMed.
- T. Li, H. Qi, C. Zhao, Z. Li, W. Zhou, G. Li, H. Zhuo and W. Zhai, Nat. Commun., 2025, 16, 88 CrossRef PubMed.
- B. Wei, Z. Wang, H. Guo, F. Xie, S. Cheng, Z. Lou, C. Zhou, H. Ji, M. Zhang, X. Wang, X. Jiao, S. Ma, H.-M. Cheng and X. Xu, Cell Rep. Phys. Sci., 2023, 4, 101335 CrossRef CAS.
- J. Kim, I. Jeerapan, S. Imani, T. N. Cho, A. Bandodkar, S. Cinti, P. P. Mercier and J. Wang, ACS Sens., 2016, 1, 1011–1019 CrossRef CAS.
- A. J. Bandodkar, W. Jia, C. Yardimci, X. Wang, J. Ramirez and J. Wang, Anal. Chem., 2015, 87, 394–398 CrossRef CAS PubMed.
- M. Kim, J. J. Park, C. Cho and S. H. Ko, Adv. Funct. Mater., 2023, 33, 2303286 CrossRef CAS.
- T. W. Kang, J. Lee, Y. Kwon, Y. J. Lee and W. H. Yeo, Adv. NanoBiomed Res., 2024, 4, 2300169 CrossRef CAS.
- L. M. Ferrari, U. Ismailov, J.-M. Badier, F. Greco and E. Ismailova, npj Flexible Electron., 2020, 4, 4 CrossRef CAS.
- X. Zhou, A. Rajeev, A. Subramanian, Y. Li, N. Rossetti, G. Natale, G. A. Lodygensky and F. Cicoira, Acta Biomater., 2022, 139, 296–306 CrossRef CAS PubMed.
- A. Miyamoto, S. Lee, N. F. Cooray, S. Lee, M. Mori, N. Matsuhisa, H. Jin, L. Yoda, T. Yokota, A. Itoh, M. Sekino, H. Kawasaki, T. Ebihara, M. Amagai and T. Someya, Nat. Nanotechnol., 2017, 12, 907–913 CrossRef CAS PubMed.
- C. Cho, W. Shin, M. Kim, J. Bang, P. Won, S. Hong and S. H. Ko, Small, 2022, 18, e2202841 CrossRef PubMed.
- J. Zhu, Y. Xiao, X. Zhang, Y. Tong, J. Li, K. Meng, Y. Zhang, J. Li, C. Xing, S. Zhang, B. Bao, H. Yang, M. Gao, T. Pan, S. Liu, F. Lorestani, H. Cheng and Y. Lin, Adv. Mater., 2024, 36, e2400236 CrossRef PubMed.
- F. M. Vivaldi, A. Dallinger, A. Bonini, N. Poma, L. Sembranti, D. Biagini, P. Salvo, F. Greco and F. Di Francesco, ACS Appl. Mater. Interfaces, 2021, 13, 30245–30260 CrossRef CAS PubMed.
- J. Bang, Y. Jung, H. Kim, D. Kim, M. Cho and S. H. Ko, Nano-Micro Lett., 2022, 14, 49 CrossRef CAS PubMed.
- I. Byun, A. W. Coleman and B. Kim, J. Micromech. Microeng., 2013, 23, 085016 CrossRef.
- K. J. Lee, K. A. Fosser and R. G. Nuzzo, Adv. Funct. Mater., 2005, 15, 557–566 CrossRef CAS.
- S. Wang, J. Y. Oh, J. Xu, H. Tran and Z. Bao, Acc. Chem. Res., 2018, 51, 1033–1045 CrossRef CAS PubMed.
- K. Zhao, Y. Zhao, R. Qian and C. Ye, Chem. Eng. J., 2023, 477, 147109 CrossRef CAS.
- L. Yin, R. Kumar, A. Karajic, L. Xie, J. M. You, D. Joshuia, C. S. Lopez, J. Miller and J. Wang, Adv. Mater. Technol., 2018, 3, 1800013 CrossRef.
- S. Yin and L. Dong, Adv. Mater. Technol., 2024, 9, 2302073 CrossRef CAS.
- T. Widlund, S. Yang, Y.-Y. Hsu and N. Lu, Int. J. Solids Struct., 2014, 51, 4026–4037 CrossRef CAS.
- J. Kim, S. J. Park, T. Nguyen, M. Chu, J. D. Pegan and M. Khine, Appl. Phys. Lett., 2016, 108, 061901 CrossRef PubMed.
- Y. Jung, K. R. Pyun, S. Yu, J. Ahn, J. Kim, J. J. Park, M. J. Lee, B. Lee, D. Won, J. Bang and S. H. Ko, Nano-Micro Lett., 2025, 17, 127 CrossRef CAS PubMed.
- Y. Chen, R. S. Carmichael and T. B. Carmichael, ACS Appl. Mater. Interfaces, 2019, 11, 31210–31219 CrossRef CAS PubMed.
- S. J. Joo, S. H. Park, C. J. Moon and H. S. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 5674–5684 CrossRef CAS PubMed.
- J. S. Oh, J. S. Oh and G. Y. Yeom, Nanomaterials, 2020, 10, 633 CrossRef CAS PubMed.
- H. Wang, J. Wang, D. Chen, S. Ge, Y. Liu, Z. Wang, X. Zhang, Q. Guo and J. Yang, IEEE Sens. J., 2022, 22, 3817–3827 CAS.
- W. Zhao, C. Liu, Y. Wang, K. Li, Z. He, S. Zhou, J. Zeng, O. O. Ibrahim, S. Zhang and Q. Wang, Sens. Actuators, B, 2025, 428, 137245 CrossRef CAS.
- G.-H. Lee, H. Kim, J. Lee, J.-Y. Bae, C. Yang, H. Kim, H. Kang, S. Q. Choi, S. Park, S.-K. Kang, J. Kang, Z. Bao, J.-W. Jeong and S. Park, Mater. Today, 2023, 67, 84–94 CrossRef CAS.
- W. Ge, R. Wang, X. Zhu, H. Zhang, L. Sun, F. Wang, H. Li, Z. Li, X. Du, H. Chen, F. Zhang, H. Shi, H. Hu, Y. Xi, J. He, L. Hu and H. Lan, J. Mater. Chem. A, 2024, 12, 657–689 RSC.
- M. Reis Carneiro, C. Majidi and M. Tavakoli, Adv. Eng. Mater., 2021, 24, 2100953 CrossRef.
- S. Kim, B. Yoo, M. Miller, D. Bowen, D. J. Pines and K. M. Daniels, Sens. Actuators, A, 2022, 342, 113659 CrossRef CAS.
- G. H. Lee, H. Woo, C. Yoon, C. Yang, J. Y. Bae, W. Kim, D. H. Lee, H. Kang, S. Han, S. K. Kang, S. Park, H. R. Kim, J. W. Jeong and S. Park, Adv. Mater., 2022, 34, e2204159 CrossRef PubMed.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132–145 CrossRef CAS PubMed.
- K. J. Hughes, K. A. Iyer, R. E. Bird, J. Ivanov, S. Banerjee, G. Georges and Q. A. Zhou, ACS Appl. Nano Mater., 2024, 7, 18695–18713 CrossRef CAS.
- Z. Zhai, L. Zhang, T. Du, B. Ren, Y. Xu, S. Wang, J. Miao and Z. Liu, Mater. Des., 2022, 221, 111017 CrossRef CAS.
- L. Bareket, L. Inzelberg, D. Rand, M. David-Pur, D. Rabinovich, B. Brandes and Y. Hanein, Sci. Rep., 2016, 6, 25727 CrossRef CAS PubMed.
- D. Kireev, S. K. Ameri, A. Nederveld, J. Kampfe, H. Jang, N. Lu and D. Akinwande, Nat. Protoc., 2021, 16, 2395–2417 CrossRef CAS PubMed.
- S. Kabiri Ameri, R. Ho, H. Jang, L. Tao, Y. Wang, L. Wang, D. M. Schnyer, D. Akinwande and N. Lu, ACS Nano, 2017, 11, 7634–7641 CrossRef CAS PubMed.
- D. Kireev, J. Kampfe, A. Hall and D. Akinwande, npj 2D Mater. Appl., 2022, 6, 46 CrossRef CAS.
- D. Kireev, K. Sel, B. Ibrahim, N. Kumar, A. Akbari, R. Jafari and D. Akinwande, Nat. Nanotechnol., 2022, 17, 864–870 CrossRef CAS PubMed.
- J. E. Jin, S. Kim, H. Yu, K. N. Lee, Y. R. Do and S. M. Lee, Biomed. Eng. Lett., 2023, 13, 495–504 CrossRef PubMed.
- L. Liu, X. Zhang, D. Xiang, Y. Wu, D. Sun, J. Shen, M. Wang, C. Zhao, H. Li, Z. Li, P. Wang and Y. Li, Prog. Nat. Sci.: Mater. Int., 2022, 32, 34–42 CrossRef CAS.
- H. Li, J. Cao, R. Wan, V. R. Feig, C. M. Tringides, J. Xu, H. Yuk and B. Lu, Adv. Mater., 2025, 37, e2415151 CrossRef PubMed.
- J. Yu, R. Wan, F. Tian, J. Cao, W. Wang, Q. Liu, H. Yang, J. Liu, X. Liu, T. Lin, J. Xu and B. Lu, Small, 2024, 20, e2308778 CrossRef PubMed.
- J. Jin, S. Wang, Z. Zhang, D. Mei and Y. Wang, Soft Sci., 2023, 3, 8 Search PubMed.
- Q. Gao, C. Li, M. Wang, J. Zhu, D. R. Munna, P. Wang, C. Zhu, J. Gao and C. Gao, J. Mater. Chem. C, 2022, 10, 6271–6280 RSC.
- T. Zhou, H. Yuk, F. Hu, J. Wu, F. Tian, H. Roh, Z. Shen, G. Gu, J. Xu, B. Lu and X. Zhao, Nat. Mater., 2023, 22, 895–902 CrossRef CAS PubMed.
- B. Lu, H. Yuk, S. Lin, N. Jian, K. Qu, J. Xu and X. Zhao, Nat. Commun., 2019, 10, 1043 CrossRef PubMed.
- N. Kim, S. Lienemann, I. Petsagkourakis, D. Alemu Mengistie, S. Kee, T. Ederth, V. Gueskine, P. Leclere, R. Lazzaroni, X. Crispin and K. Tybrandt, Nat. Commun., 2020, 11, 1424 CrossRef CAS PubMed.
- D. Won, H. Kim, J. Kim, H. Kim, M. W. Kim, J. Ahn, K. Min, Y. Lee, S. Hong, J. Choi, C. Y. Kim, T.-S. Kim and S. H. Ko, Nat. Electron., 2024, 7, 475–486 CrossRef CAS.
- H. Cao, L. Duan, Y. Zhang, J. Cao and K. Zhang, Signal Transduction Targeted Ther., 2021, 6, 426 CrossRef CAS PubMed.
- E. M. Ahmed, J. Adv. Res., 2015, 6, 105–121 CrossRef CAS PubMed.
- S. Zhuo, A. Tessier, M. Arefi, A. Zhang, C. Williams and S. K. Ameri, npj Flexible Electron., 2024, 8, 49 CrossRef.
- S. Correa, A. K. Grosskopf, H. Lopez Hernandez, D. Chan, A. C. Yu, L. M. Stapleton and E. A. Appel, Chem. Rev., 2021, 121, 11385–11457 CrossRef CAS PubMed.
- M. Song, C. Gong and X. Liu, J. Polym. Mater., 2025, 42, 111–123 CrossRef.
- J. Yu, F. Tian, W. Wang, R. Wan, J. Cao, C. Chen, D. Zhao, J. Liu, J. Zhong, F. Wang, Q. Liu, J. Xu and B. Lu, Chem. Mater., 2023, 35, 5936–5944 CrossRef CAS.
- J. H. Kim, J. Y. Hwang, H. R. Hwang, H. S. Kim, J. H. Lee, J. W. Seo, U. S. Shin and S. H. Lee, Sci. Rep., 2018, 8, 1375 CrossRef PubMed.
- Y. Ding, Y. Shi, D. Yu and W. Wang, Colloids Surf., A, 2023, 675, 132060 CrossRef CAS.
- M. Shekhirev, C. E. Shuck, A. Sarycheva and Y. Gogotsi, Prog. Mater. Sci., 2021, 120, 100757 CrossRef CAS.
- A. VahidMohammadi, J. Rosen and Y. Gogotsi, Science, 2021, 372, eabf1581 CrossRef CAS PubMed.
- N. A. Koshi, A. K. Mandia, B. Muralidharan, S. C. Lee and S. Bhattacharjee, Nanoscale, 2023, 15, 10254–10263 RSC.
- C. J. Zhang, B. Anasori, A. Seral-Ascaso, S. H. Park, N. McEvoy, A. Shmeliov, G. S. Duesberg, J. N. Coleman, Y. Gogotsi and V. Nicolosi, Adv. Mater., 2017, 29, 1702678 CrossRef.
- Y. Dong, S. S. K. Mallineni, K. Maleski, H. Behlow, V. N. Mochalin, A. M. Rao, Y. Gogotsi and R. Podila, Nano Energy, 2018, 44, 103–110 CrossRef CAS.
- M. Salauddin, S. M. S. Rana, M. Sharifuzzaman, M. T. Rahman, C. Park, H. Cho, P. Maharjan, T. Bhatta and J. Y. Park, Adv. Energy Mater., 2021, 11, 2002832 CrossRef CAS.
- L. Wang, Y. Lin, C. Yang, Q. Wang, T. Fang, C. Bai, J. Wang and D. Kong, Chem. Eng. J., 2024, 500, 157504 CrossRef CAS.
- J. Y. Oh, J. H. Lee, S. W. Han, S. S. Chae, E. J. Bae, Y. H. Kang, W. J. Choi, S. Y. Cho, J.-O. Lee, H. K. Baik and T. I. Lee, Energy Environ. Sci., 2016, 9, 1696–1705 RSC.
- Z. Xie, S. Yu, X. Ma, K. Li, L. Ding, W. Wang, D. A. Cullen, H. M. Meyer, H. Yu, J. Tong, Z. Wu and F.-Y. Zhang, Appl. Catal., B, 2022, 313, 121458 CrossRef CAS.
- S. Manzeli, D. Ovchinnikov, D. Pasquier, O. V. Yazyev and A. Kis, Nat. Rev. Mater., 2017, 2, 17033 CrossRef CAS.
- Q. Zhang, E. Linardy, X. Wang and G. Eda, ACS Nano, 2020, 14, 11482–11489 CrossRef CAS PubMed.
- S. Joseph, J. Mohan, S. Lakshmy, S. Thomas, B. Chakraborty, S. Thomas and N. Kalarikkal, Mater. Chem. Phys., 2023, 297, 127332 CrossRef CAS.
- T. Zhang, Y. Wang, X. Feng, Y. Zuo, H. Yu, H. Bao, F. Jiang and S. Jiang, iScience, 2024, 27, 110707 CrossRef CAS PubMed.
- R. Vikhe, S. Masure, M. K. Das, A. Mishra, E. Sreehari, U. Kulhari, B. D. Sahu, L. N. Sharma, S. Loganathan and S. Kumar, ACS Appl. Electron. Mater., 2025, 7, 1611–1621 CrossRef CAS.
- S. Liu, T. Fawden, R. Zhu, G. G. Malliaras and M. Bance, Sci. Adv., 2024, 10, eado9576 CrossRef PubMed.
- H. Jang, K. Sel, E. Kim, S. Kim, X. Yang, S. Kang, K. H. Ha, R. Wang, Y. Rao, R. Jafari and N. Lu, Nat. Commun., 2022, 13, 6604 CrossRef CAS PubMed.
- S. Shustak, L. Inzelberg, S. Steinberg, D. Rand, M. David Pur, I. Hillel, S. Katzav, F. Fahoum, M. De Vos, A. Mirelman and Y. Hanein, J. Neural Eng., 2019, 16, 026024 CrossRef PubMed.
- S. Wang, X. Wang, W. Zhang, X. Shi, D. Song, Y. Zhang, Y. Zhao, Z. Zhao and N. Liu, Nano Res., 2023, 16, 4100–4106 CrossRef CAS.
- S. Kwon, H. S. Kim, K. Kown, H. Kim, Y. S. Kim, S. H. Lee, Y. T. Kown, J. W. Jeong, L. M. Trotti, A. Duarte and W. H. Yeo, Sci. Adv., 2023, 9, eadg9671 CrossRef CAS PubMed.
- R. C. Webb, A. P. Bonifas, A. Behnaz, Y. Zhang, K. J. Yu, H. Cheng, M. Shi, Z. Bian, Z. Liu, Y. S. Kim, W. H. Yeo, J. S. Park, J. Song, Y. Li, Y. Huang, A. M. Gorbach and J. A. Rogers, Nat. Mater., 2013, 12, 938–944 CrossRef CAS PubMed.
- Y. Zheng, Y. Li, Y. Zhao, X. Lin, S. Luo, Y. Wang, L. Li, C. Teng, X. Wang, G. Xue and D. Zhou, Nano Energy, 2023, 107, 108092 CrossRef CAS.
- H. Yuan, Y. Li, J. Yang, H. Li, Q. Yang, C. Guo, S. Zhu and X. Shu, Micromachines, 2021, 12, 1521 CrossRef PubMed.
- M. Galliani, F. Greco, E. Ismailova and L. M. Ferrari, ACS Appl. Electron. Mater., 2025, 7, 1408–1414 CrossRef CAS PubMed.
- R. Park, H. Kim, S. Lone, S. Jeon, Y. W. Kwon, B. Shin and S. W. Hong, Sensors, 2018, 18, 1857 CrossRef PubMed.
- T. Li, T. Zhao, H. Zhang, L. Yuan, C. Cheng, J. Dai, L. Xue, J. Zhou, H. Liu, L. Yin and J. Zhang, npj Flexible Electron., 2024, 8, 3 CrossRef.
- A. J. Bandodkar, V. W. Hung, W. Jia, G. Valdes-Ramirez, J. R. Windmiller, A. G. Martinez, J. Ramirez, G. Chan, K. Kerman and J. Wang, Analyst, 2013, 138, 123–128 RSC.
- K. Unger, F. Greco and A. M. Coclite, Adv. Mater. Technol., 2021, 7, 2100717 CrossRef.
- A. J. Bandodkar, D. Molinnus, O. Mirza, T. Guinovart, J. R. Windmiller, G. Valdes-Ramirez, F. J. Andrade, M. J. Schoning and J. Wang, Biosens. Bioelectron., 2014, 54, 603–609 CrossRef CAS PubMed.
- N. K. Singh, S. Chung, A. Y. Chang, J. Wang and D. A. Hall, Biosens. Bioelectron., 2023, 227, 115097 CrossRef CAS PubMed.
- D. Kim, J. Lee, M. K. Park and S. H. Ko, Commun. Mater., 2024, 5, 41 CrossRef CAS.
- G. E. Bonacchini, C. Bossio, F. Greco, V. Mattoli, Y. H. Kim, G. Lanzani and M. Caironi, Adv. Mater., 2018, 30, e1706091 CrossRef PubMed.
- W. T. Fonseca, T. Parra Vello, G. C. Lelis, A. V. Ferreira Deleigo, R. K. Takahira, D. S. T. Martinez and R. F. de Oliveira, ACS Sens., 2025, 10, 3222–3238 CrossRef CAS PubMed.
- M. A. S. Almeida, A. L. Pires, J. L. Ramirez, S. B. Malik, S. de la Flor, E. Llobet, A. T. Pereira and A. M. Pereira, Adv. Sci., 2025, 12, e2403775 CrossRef CAS PubMed.
- J. Alberto, C. Leal, C. Fernandes, P. A. Lopes, H. Paisana, A. T. de Almeida and M. Tavakoli, Sci. Rep., 2020, 10, 5539 CrossRef CAS PubMed.
- S. Hong, J. Lee, K. Do, M. Lee, J. H. Kim, S. Lee and D. H. Kim, Adv. Funct. Mater., 2017, 27, 1704353 CrossRef.
- L. M. Ferrari, U. Ismailov, F. Greco and E. Ismailova, Adv. Mater. Interfaces, 2021, 8, 2100352 CrossRef CAS.
- N. Gogurla and S. Kim, Adv. Energy Mater., 2021, 11, 2100801 CrossRef CAS.
- A. Wahlsten, A. Stracuzzi, I. Luchtefeld, G. Restivo, N. Lindenblatt, C. Giampietro, A. E. Ehret and E. Mazza, Acta Biomater., 2023, 170, 155–168 CrossRef PubMed.
- S. Budday, R. Nay, R. de Rooij, P. Steinmann, T. Wyrobek, T. C. Ovaert and E. Kuhl, J. Mech. Behav. Biomed. Mater., 2015, 46, 318–330 CrossRef PubMed.
- R. Emig, C. M. Zgierski-Johnston, V. Timmermann, A. J. Taberner, M. P. Nash, P. Kohl and R. Peyronnet, Biophys. Rev., 2021, 13, 587–610 CrossRef PubMed.
- A. E. Samir, M. Dhyani, A. Vij, A. K. Bhan, E. F. Halpern, J. Méndez-Navarro, K. E. Corey and R. T. Chung, Radiology, 2015, 274, 888–896 CrossRef PubMed.
- S. R. Polio, A. N. Kundu, C. E. Dougan, N. P. Birch, D. E. Aurian-Blajeni, J. D. Schiffman, A. J. Crosby and S. R. Peyton, PLoS One, 2018, 13, e0204765 CrossRef PubMed.
- A. Karimi, A. Shojaei and P. Tehrani, J. Chem. Neuroanat., 2017, 86, 15–18 CrossRef PubMed.
- G. H. Borschel, K. F. Kia, W. M. Kuzon, Jr. and R. G. Dennis, J. Surg. Res., 2003, 114, 133–139 CrossRef PubMed.
- D. B. Camasao and D. Mantovani, Mater. Today Bio, 2021, 10, 100106 CrossRef CAS PubMed.
- G. Nakao, T. Kodesho, T. Kato, Y. Yokoyama, Y. Saito, Y. Ohsaki, K. Watanabe, M. Katayose and K. Taniguchi, J. Med. Ultrason., 2023, 50, 275–283 CrossRef PubMed.
- M. Ferrara, G. Lugano, M. T. Sandinha, V. R. Kearns, B. Geraghty and D. H. W. Steel, Eye, 2021, 35, 1818–1832 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |


