Flexible fibrous electrodes for implantable biosensing
Hanfei
Li
abcd,
Chenyang
Li
bc,
Hang
Zhao
bc,
Qingsong
Li
 bc,
Yang
Zhao
bc,
Jianhong
Gong
ad,
Guanglin
Li
bc,
Huan
Yu
*bc,
Qiong
Tian
bc,
Yang
Zhao
bc,
Jianhong
Gong
ad,
Guanglin
Li
bc,
Huan
Yu
*bc,
Qiong
Tian
 *bc,
Zhiyuan
Liu
*bc,
Zhiyuan
Liu
 *bce and
Fei
Han
*bce and
Fei
Han
 *bce
*bce
aSchool of Mechanical, Electrical & Information Engineering, Shandong University, 264209 Weihai, China
bGuangdong-Hong Kong-Macao Joint Laboratory of Human-Machine Intelligent Systems, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China. E-mail: h.yu2@siat.ac.cn; qiong.tian@siat.ac.cn; zy.liu1@siat.ac.cn; fei.han@siat.ac.cn
cResearch Center for Neural Engineering, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China
dWeiHai Research Institute of Industrial Technology of Shandong University, 264209 Weihai, China
eGuangdong Provincial Key Laboratory of Multimodality Non-Invasive Brain-Computer Interfaces, Shenzhen 518055, China
First published on 18th March 2025
Abstract
Flexible fibrous electrodes have emerged as a promising technology for implantable biosensing applications, offering significant advancements in the monitoring and manipulation of biological signals. This review systematically explores the key aspects of flexible fibrous electrodes, including the materials, structural designs, and fabrication methods. A detailed discussion of electrode performance metrics is provided, covering factors such as conductivity, stretchability, axial channel count, and implantation duration. The diverse applications of these electrodes in electrophysiological signal monitoring, electrochemical sensing, tissue strain monitoring, and in vivo electrical stimulation are reviewed, highlighting their potential in biomedical settings. Finally, the review discusses the eight major challenges currently faced by implantable fibrous electrodes and explores future development directions, providing critical technical analysis and potential solutions for the advancement of next-generation flexible implantable fiber-based biosensors.
1. Introduction
In recent years, implantable sensors have garnered widespread attention due to their immense potential in clinical diagnostics and long-term health monitoring.1–7 These sensors can continuously and effectively track critical physiological parameters within the body, such as neural activity,8–10 muscle contractions,11–13 tissue strain,14–17 and biochemical concentrations,18–20 which are essential for diagnosing and treating various diseases. In the context of personalized medicine, implantable sensors offer a real-time, customized approach to therapy and intervention.2,21,22 This aligns with the evolving trend of precision medicine, which not only focuses on specific diseases but also considers individual variations in genetics, environment, and lifestyle. The rising prevalence of chronic diseases and the increasing demand for real-time health monitoring solutions have significantly amplified the market potential for implantable biosensors and Invasive diagnostic catheter instruments. It is estimated that the global biosensor market will expand from USD 27.5 billion in 2021 to USD 49.6 billion by 2026, with a compound annual growth rate (CAGR) of 7.7%,23 while the invasive diagnostic catheter instruments market, valued at 4.1 billion in 2022, is projected to reach more than 7.7 billion by 2031, growing at a CAGR of 7.1%.24 Despite this growth, significant gaps persist in the current market, including the limited availability of high-density, multifunctional, and long-term stable biosensors that can seamlessly integrate with biological tissues. At the same time, advancements in sensor integration, wearable monitoring tools, and practical and cost-effective catheter sensors have been notable in recent years. These innovations, along with improvements in catheter placement, longer duration, and positioning precision, have driven strides in medical technology, leading to better patient care and treatment outcomes. Together, these developments highlight the growing intersection of biosensors and Invasive diagnostic catheter instruments in addressing the evolving needs of modern healthcare.Integrating implantable sensors, particularly for neural and muscular applications,7,25,26 presents a significant challenge. While traditional rigid electrode systems have been effective in short-term studies, their mechanical mismatch with soft tissues such as the brain, heart, and skeletal muscles often leads to complications. These tissues are constantly moving and deforming during normal physiological activities, and rigid electrodes may cause irritation, inflammation, or even long-term tissue damage, ultimately impairing device function and compromising patient health. This issue has driven the search for flexible, stretchable, and biocompatible alternatives that can seamlessly integrate with soft tissues while maintaining stable electrical performance over extended periods.27–29
In biosensing, flexible electrodes offer several advantages over traditional rigid systems. Firstly, their mechanical properties enable them to stretch and bend in concert with the natural movement of tissues, reducing the risk of inflammation and promoting long-term biocompatibility.28,30–34 This is particularly important in applications such as brain-machine interfaces or neuromuscular monitoring, where electrodes must remain functional in dynamic environments over long durations. Moreover, advancements in materials science have spurred the development of metallic nanowires,35–39 conductive polymers,40–44 liquid metal45–47 and novel conductive materials48,49 that not only exhibit high electrical conductivity but also possess flexibility and stretchability, further enhancing the performance of flexible electrodes in implantable systems. In addition, advanced biosensor technologies, such as fifth-generation sensors, nose-on-chip systems, and hospital-on-chip platforms, represent significant breakthroughs by offering unmatched precision, scalability, and multifunctionality.23 Sensors based on biogenic materials, MXene, and borophene further enhance biosensing capabilities by leveraging their exceptional electrical, mechanical, and biocompatibility properties.50–53 These advancements illustrate how next-generation biosensors can address existing gaps, bridging the transition from research innovations to practical healthcare solutions.
Compared to two-dimensional flexible thin-film electrodes54–57 and three-dimensional flexible electrodes,58,59 one-dimensional flexible fibrous electrodes29,60–62 offer significant advantages in terms of electrode scale, functional integration, implantation methods, tissue inflammatory response, and long-term stability. Typically made from materials that are both conductive and mechanically flexible, these fiber electrodes conform to the contours of biological tissues and deform with them, without causing damage or compromising signal quality. Their small volume and lightweight nature contribute to reduced wound trauma upon implantation, leading to decreased inflammatory and immune responses. This minimizes the impact on the subject, ensuring prolonged signal stability and enhancing patient comfort.29,63–65 Additionally, flexible fiber electrodes can be designed to integrate multiple sensing modalities, enabling simultaneous detection of electrical, mechanical, and chemical signals at the same site. This multifunctionality is especially useful for monitoring complex physiological processes, such as neural activity or muscle contractions, which are often influenced by a combination of electrical, chemical, and mechanical factors.
Flexible fiber electrodes have a wide range of applications, from monitoring electrophysiological signals in the brain65–67 and peripheral nervous system68–70 to detecting biochemical markers in body fluids.71–73 For example, in neural applications, fiber electrodes can record local field potentials and electrocorticography, providing valuable insights into brain function and facilitating the development of advanced neuroprosthetic devices. In the peripheral nervous system, these electrodes, with their ultrafine size and small electrode footprint, can monitor intramuscular electromyography (EMG) signals and even the electrophysiological signals of muscle fibers, which are critical for controlling prosthetics or restoring motor functions in paralyzed patients. Additionally, fiber electrodes can be employed in electrochemical sensing applications, detecting specific ions or biomolecules in body fluids such as sweat, blood, or interstitial fluid, providing real-time information about a patient's health status.
Beyond sensing, implantable flexible fiber electrodes also offer significant advantages in electrical stimulation applications in biomedicine.74–76 These electrodes can deliver precise electrical stimulation to target tissues such as nerves or muscles, which is crucial for therapies like neurostimulation, muscle rehabilitation, and cardiac pacing. The fibrous structure facilitates better integration with tissues and enables the possibility of multi-channel stimulation, improving spatial resolution and functional outcomes. Moreover, these electrodes maintain stable electrical performance under mechanical strain, ensuring reliable and efficient electrical stimulation even in dynamic environments, such as muscles or peripheral nerves.
The development of flexible fiber electrodes for implantable biosensing is a multidisciplinary effort involving expertise from materials science, biomedical engineering, electronics, and clinical medicine. While significant progress has been made in recent years in the field of flexible fiber electrodes, there remain substantial challenges in realizing the next generation of intelligent implantable fiber electrodes. To this end, this review will first explore the latest advancements in flexible fiber electrodes for implantable biosensing (Fig. 1), with a focus on their materials, structures, and fabrication methods. We will also discuss their current and potential applications in monitoring neural, muscular, biochemical signals, as well as in vivo strain sensing and electrical stimulation. Furthermore, we will examine the challenges these electrodes face in electrode performance and interface integration with biological tissues and propose important future directions in the field, such as the development of ultra-high-density flexible fiber electrodes and the pursuit of intelligence and controlled movement. The ultimate goal of this review is to provide a comprehensive overview of the latest technologies in flexible fiber electrodes for implantable biosensing and to demonstrate the transformative potential of these devices in healthcare through continuous real-time physiological signal monitoring.
 | ||
| Fig. 1 Overview of implantable flexible fibrous electrode, including materials and structure,77–81 methods,78,79,82,83 applications65,73,75,84 and prospects.85 This figure has been reproduced from ref. 65 with permission from the Springer Nature, copyright: 2024; ref. 73 with permission from the Springer Nature, copyright: 2020; ref. 75 with permission from the Springer Nature, copyright: 2024; ref. 77 with permission from the American Chemical Society, copyright: 2021; ref. 78 with permission from the American Association for the Advancement of Science, copyright: 2017; ref. 79 with permission from bioRxiv Cold Spring Harbor Laboratory, copyright: 2023; ref. 80 with permission from the American Chemical Society, copyright: 2020; ref. 81 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2015; ref. 82 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2018; ref. 83 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2021; ref. 84 with permission from bioRxiv Cold Spring Harbor Laboratory, copyright: 2023; ref. 85 with permission from the Springer Nature, copyright: 2015. | ||
2. Materials, methods and performance
With the advancement of technology, researchers have continuously innovated in conductive materials, structural design, and preparation processes, promoting the rapid development of fibrous electrodes, as shown in Fig. 2.60,73,75,80,86–91 | ||
| Fig. 2 Materials, structure, and fabrication methods of fibrous electrodes. (a) Schematic illustration of the fabrication processes of the flexible tubular microelectrode, mainly including: fabrication of parylene thin film microelectrode, wrapping and gluing on the polyimide capillary, and electrochemical deposition of conducting polymer.86 This figure has been reproduced from ref. 86 with permission from the Springer Nature, copyright: 2016. (b) Schematic illustration of the fabrication process of the stretchable fiber-based glucose sensor, schematic illustration of the fiber working electrode and the two main reactions on the interface of the electrode and the solution.88 This figure has been reproduced from ref. 88 with permission from the American Chemical Society, copyright: 2019. (c) Overview concept and material design of the bioabsorbable electrical stimulation suture for treating muscle gashes.92 This figure has been reproduced from ref. 100 with permission from the Springer Nature, copyright: 2024. (d) Schematic illustration of a micro-structured core–sheath fiber based interdigited electrodes-free gas sensor and its surface morphologies.87 This figure has been reproduced from ref. 87 with permission from the Elsevier, copyright: 2021. (e) Schematic diagram of flexible piezoelectric fiber generator structure and Schematic diagram of fabrication method.89 This figure has been reproduced from ref. 89 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2015. (f) Implantable fiber biosensors based on CNTs.60 This figure has been reproduced from ref. 60 with permission from the American Chemical Society, copyright: 2021. (g) Schematic illustration of biohybrid artificial muscle. Muscle fiber consists of a bundle of myofibril (left). Similar to the muscle fiber, biohybrid artificial muscle is composed of CNT fiber, PU fiber, and skeletal muscle fiber (right).91 This figure has been reproduced from ref. 91 with permission from the Springer Nature, copyright: 2021. (h) Fabrication process and surface morphology characterization of the PU@PMA@EGaIn fiber.80 This figure has been reproduced from ref. 80 with permission from the American Chemical Society, copyright: 2020. (i) The structure and SEM image of fiber-shaped all-in-one organic electrochemical transistors (OECTs).93 This figure has been reproduced from ref. 94 with permission from the Springer Nature, copyright: 2020. | ||
Conductive materials are the core components of flexible fiber electrodes, and their selection directly affects the conductivity and mechanical properties of the electrode. Common conductive materials mainly include metal materials (Fig. 2a–c).67,75,86,88,94 carbon-based materials (Fig. 2d–g),60,87,89,91,95 liquid metal (Fig. 2h)80 and conductive polymers93 (Fig. 2i) and MXene.96 Metal materials are also widely used in flexible fiber electrodes due to their excellent conductivity. Common metal materials include silver nanowires, copper fibers, gold nanowires, and gold thin films. Silver nanowires are highly favored due to their extremely high conductivity and excellent flexibility.81 Gold nanowires and gold thin films are one of the most stable and reliable conductive materials for flexible and stretchable electrodes due to their excellent ductility and oxidation resistance.79,88 Additionally, liquid metal has garnered widespread attention in recent years for fiber electrode fabrication due to its excellent conductivity and fluidity.83
Carbon based materials play an important role in flexible fiber electrodes, and their excellent conductivity and mechanical properties make them an ideal choice.97,98 Carbon based materials include graphene and carbon nanotubes (CNTs). Graphene is a two-dimensional material composed of a single layer of carbon atoms arranged in a hexagonal structure, with extremely high conductivity and strength.76,99 The preparation of graphene fibers usually adopts wet spinning or chemical vapor deposition technology, which is widely used in fields such as sensors and batteries. Carbon nanotubes are a type of carbon material with unique structure, high conductivity, and strength, suitable for preparing stretchable conductive fibers.60,100 Carbon nanotube fibers can be prepared by chemical vapor deposition or electrospinning methods and are widely used in energy storage and flexible electronic devices. Conductive polymers are widely used materials in flexible fiber electrodes, and are highly valued for their excellent mechanical flexibility and good conductivity. PEDOT/PSS conductive polymer is an ultra-thin conductive polymer coating with a conductivity of up to 1000 S cm−1.93
The structural design of flexible fiber electrodes is critical to their performance. These electrodes typically consist of conductive materials, flexible substrates, and functional layers.94 The conductive layer is the core component, primarily responsible for electrical signal conduction. Factors such as the thickness, morphology, and material uniformity of the conductive layer directly influence the electrode's conductivity.78 Ideally, the conductive layer should demonstrate continuity and sufficient thickness to resist breaking during stretching or bending. Flexible substrates serve as the supportive structure for the electrode, with commonly used materials including polyurethane,80 polyimide,65 polydimethylsiloxane,78 and SEBS.79,83 These substrates not only provide flexibility but also essential support and protection. Different flexible substrate materials affect the overall flexibility and stability of the electrode. The functional layer is employed to enhance specific properties, such as electrochemical activity, hydrophilicity, and biocompatibility, enabling diverse functional integration within the electrode through customization of the functional layer's characteristics.
In the design and application of flexible electronic devices, the adhesion performance between conductive materials and flexible substrates is one of the key factors affecting the stretchability, stability, and durability of the devices. A good interface bonding between conductive materials and substrates not only ensures the stability of electrical performance, but also effectively enhances the reliability of devices under complex mechanical deformations such as tension, bending, or twisting. Some researchers enhance the interfacial compatibility between conductive materials and flexible substrates through surface treatment techniques such as plasma treatment, chemical modification, coatings, etc., improving the physical and chemical bonding between materials.101 They can form chemical bonds between conductive materials and substrates by introducing functionalized molecules or modified materials, thereby improving the adhesion of the interface. In addition, interface layers (such as thin layers of polymers or metal film) can also be used as transition layers to effectively alleviate the thermal expansion differences between materials and enhance interface stability.72 Finally, for flexible fiber electrodes, a strong encapsulation layer can effectively enhance the stability and durability of the device.102
The fabrication methods of flexible fiber electrodes directly impact their performance and suitability for various applications. These methods primarily include 3D printing, coating, microfluidic injection, and dimensional transformation techniques.73,80,86–88 In recent years, 3D printing technology has been progressively applied to the fabrication of flexible fiber electrodeshod facilitates the creation of complex electrode shapes and multi-channel structures, allowing the geometric configuration of the electrode to be optimized for improved conductivity and flexibility.103 In coating processes, functional materials can be evenly applied to the electrode surface either through liquid immersion or by spraying conductive materials uniformly onto the substrate (Fig. 2e).78 This trelatively uniform coating thickness and is suitable for fabricating electrodes with intricate shapes. The microfluidic injection method involves injecting conductive material into flexible microchannels to form continuous fiber-like structures.80,83 By using precision-designed microchannel molds, conductive materials can form fiber electrodes with specific dimensions and shapes during injection, achieving highly precise and controlled electrode structures. This method enhances electrode flexibility and conductivity and is compatible with various conductive materials, such as liquid metals. Dimensional transformation refers to converting a two-dimensional film electrode into a one-dimensional fiber-like electrode through operations such as winding or rolling, facilitating multi-channfiber electrodes with ease.84,86,89 Our research group has utilized this method high-density, multi-channel flexible stretchable fiber electrodes, with stretchability up to 90% and the integration of 64 channels on a single fiber electrode.79
The development of flexible fiber electrodes in conductive materials, structural design, and preparation processes provides strong support for their promotion in various application fields. In the future, with the continuous emergence of new materials and technologies, the performance of flexible fiber electrodes will be further improved, bringing new opportunities for the development of flexible electronic technology. Through interdisciplinary research, it is expected to achieve wider applications in fields such as biomedicine, smart wearable devices, and wearable sensors.
Soft fiber shaped electrode devices with different functions can achieve different sensing functions. Due to their different flexible substrates, conductive materials, and preparation processes, their functions and performance parameters also vary. In Table 1, we listed various fibrous electrode devices and conducted statistical analysis on their materials, functions, sizes, flexibility and number of channels, etc. From the table, it can be seen that implementing multi-channel fiber like flexible electrodes is quite difficult, and it is also a huge challenge to ensure that the device has both normal functionality and stretchability in terms of flexibility.
| Conductive material | Support material | Size (diameter) | Softness | Channel number | Electrical property | Biocompatibility | Durability | Function | Merits | Demerits | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEDOT-TFB | Carbon fibers | 1–5 μm | Hard | 64 | 4.84 MΩ | Non toxic, non inflammatory. | Long-term monitoring | Record neuronal signals | Multiple channels, detailed and reliable data | Modulus is much larger than brain tissue, and there is an inevitable immune response | 104 |
| Ag ink | TPU fiber | 5.5 mm | Stretchable | 1 | 500 Ω with 200% strain | Non-interface detachment enhancing biocompatibility. | 1200 cyclic test with 30% strain | Strain sensor | Featuring dual-mode sensing performance and a wide detection range | Unable to prepare multi-channel array, unable to implant in vivo | 105 |
| Carbon nanotube | Calcium ion crosslinked sodium alginate | 20 μm | Flexible | 1 | 10 kΩ at 1 kHz | Negligible neuronal cell loss and glial response. | Soak in artificial cerebrospinal fluid for one week to maintain stability | Record neuronal signals | The modulus of the electrode can be adjusted, which greatly reduces the occurrence of immune reactions | Lack of high integration and insufficient signal resolution | 66 |
| Pt | Graphene-fiber | 50 μm | Flexible | 1 | 5 MΩ μm2 at 1 kHz | Graphene microfibers have natural biocompatibility. | Maintain 77.6% charge storage capacity after 200 times 360° folding | Record neuronal signals | Low impedance, high signal-to-noise ratio | It is difficult to achieve multi-channel electrical signal monitoring and electrical stimulation | 69 |
| Tungsten or copper | PC–PVDF–PMMA | 500 μm | Flexible | 4 | 22.71 MΩ cm2 at 1 kHz | The cell density of Iba1+ SC neurons was significantly lower at 2 weeks after implantation. | Long-term (at least 10 weeks) simultaneous optical stimulation and neural recording | Optical stimulation and neural recording | Long term dual-mode light stimulation and electrical signal recording function in the body, with a small signal-to-noise ratio | Lack of highly arrayed signal acquisition and feedback | 106 |
| PCL/Mo conductive composite | Polycaprolactone fiber | 300–500 μm | Flexible | 1 | ≈43.5 Ω cm−1 | The cell viability is observed to be over 90% after 7 days. | Durability for more than 4000 bending cycles | Suturable temperature sensor, electrical stimulator | Biodegradable, highly conductive, and mechanical robustness | Larger size, lower resolution | 107 |
| PEDOT:PSS/Au-coated nylon fiber | CNT fiber | 120 μm | Flexible | 1–5 | 1000 Ω at 1 kHz | Fiber-shaped OECTs implantation caused negligible immune activation. | Stable after 2000 bending cycles | Biochemical detection | Multiple biochemical tests with high sensitivity and strong anti-interference ability | The preparation is relatively complex and lacks electrical signal monitoring and stimulation functions | 93 |
| Carbon nanotube, Pt wire, Ag/AgCl electrode | Carbon nanotube | 150 μm | Flexible | 1 | 500 Ω at 1 kHz | The dopamine-sensing fiber owned high biocompatibility. | Impedance of DSF was well maintained during 200 bending cycles | Long-term monitoring of dopamine | Can simultaneously monitor dopamine and electrical signals in the brain | The backend devices are complex, with a single number of channels and limited information content | 108 |
| Carbon fiber | Carbon fiber | 8.4 μm | Hard | 16 | 118 kΩ ± 28 kΩ at 1 kHz | Poses no toxicity to neural tissue. | — | Neural unit recording | Small electrode size and spacing, capable of array based monitoring | The modulus is large, causing damage to the tissue and making long-term monitoring impossible | 109 |
| MXene | PU fiber | 200 μm | Stretchable | 1 | 6700 S cm−1 | — | — | Gas sensor | Having high sensitivity and high signal-to-noise ratio | Large size, single function | 87 |
| Gold film | SEBS with AuNWs | 80 μm | Stretchable | 1 | 90 S cm−1 | — | After 100 stretching cycles, the current retention was about 88%. | Monitoring chemical/biological markers | High stretchability and excellent sensitivity | Complex structure, difficult packaging, wearable but non degradable | 110 |
| Au or Pt | Flexible polymer | 500 μm | Flexible | 4 | 3000 Ω at 1 kHz | Good biocompatibility | No mention | Electrophysiological recording and drug delivery | Simultaneously recording electrophysiological signals and delivering drugs | Hollow pipes mean larger invasion of the wound | 86 |
| Pt/PEDOT:PSS/Gox/Pb2+ | CNT fiber | 50 μm | Flexible | 4 | ≈100 Ω at 1 kHz | Good biocompatibility | The impedance remained almost unchanged after bending and twisting for 100 cycles | Monitor multiple disease biomarkers | Injectable, low stress, wireless transmission, long-term implantation capability | Not biodegradable in the body, and the number of single fiber electrodes is relatively small | 73 |
| EGaIn | PU/PMA fiber | 200 μm | Stretchable | 1 | 3000 S cm−1 | Nontoxic to organisms | 40% strain for 8000 cycles | Monitor human activities | High conductivity and high thermal stability | Liquid metal cannot be implanted into the body | 80 |
| Carbon nanotube | Restorative gel sheath | 150 μm | Flexible | 1 | 1 × 104 S cm−1 | Non toxic, no inflammatory response | Impedance stabilizes after 1000 cycles of bending | Monitoring of biochemical dynamics in amniotic fluid | Low biological damage and high monitoring accuracy | Single application scenario and lack of universality | 90 |
| Pt | PI | 180 μm | Flexible | 1024 | 0.194 MΩ at 1 kHz | Non-toxic, high biocompatibility | Still stable after bending ± 15 degrees ten times | Neural unit recording | Realize in vivo monitoring with thousands of channels and over a hundred weeks | Complex preparation and high equipment cost | 65 |
| Carbon nanotube nanofibers | Hydrophilic polyurethane | 100 μm | Flexible | 1 | 3.55 (±0.41) S cm-1 electrical conductance | — | Up to 4% in the stretching and releasing cycle | Actuation of biohybrid artificial muscle | As a new paradigm of artificial muscles, it has the potential to be applied to future soft robot systems | Lack of multifunctional integration leads to application difficulties | 91 |
3. Implantable applications
Fiber electrodes have exhibited great potential as suitable implantable electrodes owing to their high structural flexibility, tailorable mechanical stiffness, and minimal size of recording sites.60,64 Here, we examine four key applications of fiber electrodes in implantable devices: electrophysiological recording, electrochemical sensing, strain sensing, and electrical stimulation. By reviewing these diverse applications, we aim to provide valuable insights into the functional development and performance optimization of implantable fiber electrodes.3.1 Electrophysiological recording
Implantable fiber electrodes are now a critical focus in biomedical engineering due to their capability of continuous and precise monitoring of neural and physiological activity.111–114 For neural electrodes, it is necessary to select materials with appropriate stiffness to stabilize the electrode-tissue interface and enable feasible operation. Additionally, the design must incorporate high conductivity and biocompatibility to achieve long-term and effective signal transmission.70,115,116 Tang et al. reported a multilayer microfiber electrode with an adjustable elastic modulus, consisting of a carbon nanotube core and a sodium alginate shell.117 Hydrating sodium alginate softens it after implantation, ensures dynamic stability of the interface, and reduces inflammatory reactions, thus enabling monitoring of stable electrical signals of cerebral cortex neurons within 4 weeks. Gong et al. demonstrated a one-step and catalyst-free chemical vapor deposition process for tailoring the Young's modulus and geometry of carbon nanotube fibers, which is beneficial for a stable electrode–neural interface (Fig. 3a).68 Peak-to-peak action potential of sciatic nerve is 310% higher than the commercial Pt electrode (Fig. 3b). Recording the signals of individual brain neurons during intense activity remains a long-term challenge due to the enormous deformities of the brain.118 Materials that are axially stretchable and possess high conductivity, along with a low intrinsic modulus, need to be designed to accommodate large brain deformations. A soft fiber neural device that can track individual neurons in the deep brain of running cats with stability is reported.119 The fiber neural device features a conductive gel fiber that possesses a network-in-liquid structure created with conjugated polymers and liquid matrices (Fig. 3c). The structure assists neurons in maintaining their connections and transmiting neural signals even under vigorous activities (Fig. 3d). Also, advances in wireless power and signal processing are driving the next generation of implantable fiber electrodes. Integrating optical devices, like optical fibers, into fiber electrodes is a breakthrough in monitoring biological signals.120 This combination enables the simultaneous collection of both electrical and optical signals from the body, resulting in high-precision, multi-modal monitoring of physiological functions.85,121 Tapered optical fibers integrated with microelectrodes offer a promising solution for localized nerve signal collection in the cerebral cortex while reducing interference.122 The fabrication process, which combines focused ion beam milling and non-planar two-photon polymerization, allows for the precise manipulation of microstructures on the surface of the optical fiber. Park et al. reported that the hydrogel hybrid device was also shown to be effective for electrophysiological, optogenetic, and behavioral studies of neural circuits, and it was able to track stable, isolated single-neuron potentials of the cerebral cortex in freely moving mice for up to six months after implantation (Fig. 3e–g).123 These research advancements provide novel ideas and approaches for applying implantable fiber electrodes to record electrophysiological signals, which are expected to bring more breakthroughs and innovations in the field of biomedical engineering in the future.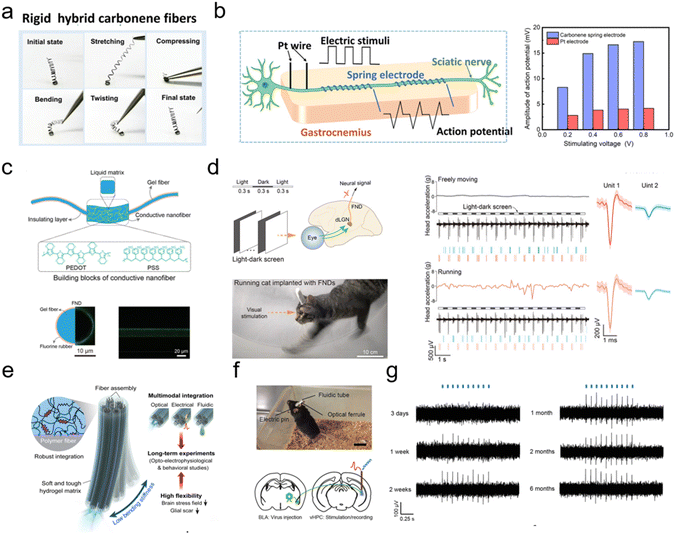 | ||
| Fig. 3 Implantable fibrous electrodes for electrophysiological recording. (a) Hybrid carbonene fiber with mechanical stability.68 This figure has been reproduced from ref. 68 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2021. (b) The difference in the amplitude of action potential of the sciatic nerve between the carbonene spring electrode and the Pt electrode.68 This figure has been reproduced from ref. 68 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2021. (c) Design and structure of the soft-fiber neural device.119 This figure has been reproduced from ref. 119 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2024. (d) Neural recordings obtained by the fiber neural device implanted in the cat brain as the animal performed vigorous activities.119 This figure has been reproduced from ref. 119 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2024. (e) Fabrication of the hydrogel hybrid probe.123 This figure has been reproduced from ref. 123 with permission from the Springer Nature, copyright: 2021. (f) A photograph of a mouse implanted with the hydrogel hybrid probe and an illustration of optogenetic modulation and electrophysiological recording in a specific projection circuit.123 This figure has been reproduced from ref. 123 with permission from the Springer Nature, copyright: 2021. (g) Electrophysiological recordings in the ventral hippocampus during optical stimulation with the probes within 6 months post-implantation.123 This figure has been reproduced from ref. 123 with permission from the Springer Nature, copyright: 2021. | ||
3.2 Electrochemical sensing
Implantable fiber electrodes have demonstrated significant potential in the field of electrochemical sensing, playing a crucial role in exploring physiological functions, understanding disease mechanisms, and developing treatments for various health conditions. For instance, neurotransmitters are closely related to neurological disorders such as Parkinson's disease and epilepsy,124,125 glucose levels indicate the presence of diabetes,126 and electrolyte concentrations are linked to serious conditions like cardiovascular diseases and kidney failure.127,128Unlike electrophysiological recording electrodes, which focus on capturing electrical signals, electrodes used for electrochemical sensing emphasize the selective detection of chemical substances, optimization of electrochemical reaction kinetics, and long-term stability of sensing performance.60,129,130 Enhancing the active surface area of the fiber electrodes can lead to a substantial improvement in the sensitivity of the chemical sensors.131 Carbon nanotubes with a multilevel helical structure endow the injectable fiber electrode with high specific surface and high flexibility.132 This structure allows it to efficiently bind different active substances and detect a variety of chemicals, such as calcium ions, glucose and proteins (Fig. 4a). Electrochemical deposition of platinum nanoparticles on the fiber surface can enhance the sensitivity of H2O2 detection in tumors (Fig. 4b and c). In addition, creating a stable interface between the electrode and biological tissue is vital for maintaining accurate and consistent measurements, especially for long-term implantations. Li et al. presented a modified multilayer fiber electrode coated with a restorative gel, which ensures seamless adhesion and uniform stress distribution across the interface (Fig. 4d).90 This novel design ensures stable and reliable electrochemical sensing, facilitating real-time non-invasive monitoring of the dynamic biochemical states of amniotic fluid during pregnancy (Fig. 4e and f). High selectivity is another critical factor in the design of electrochemical sensors.133 Zou et al. designed a carbon nanotube fiber modified with molecularly imprinted polymers, which resulted in a highly selective implantable electrochemical fiber sensor.72 It only allows molecules that precisely match the recognition binding sites to pass through, ensuring efficient electrochemical reactions and providing the sensor with in vivo selectivity for homovanillic acid (Fig. 4g and h). Another promising approach involves the use of organic electrochemical transistors for localized amplification of electrochemical signals.93,134
 | ||
| Fig. 4 Implantable fibrous electrodes for electrochemical sensing. (a) Schematic of the hierarchical structure of muscle. Representative transmission electron microscope image of a multi-walled CNT. Representative SEM images of a primary CNT fibre and a hierarchically helical CNT fibre assembled from primary CNT fibres.132 This figure has been reproduced from ref. 129 with permission from the Springer Nature, copyright: 2020. (b) Multiply sensing fibres were injected in a nude mouse with tumour tissue.132 This figure has been reproduced from ref. 129 with permission from the Springer Nature, copyright: 2020. (c) Mapping of the spatial and temporal distribution of H2O2 in a mature tumour in vivo.132 This figure has been reproduced from ref. 129 with permission from the Springer Nature, copyright: 2020. (d) Schematic illustration of the IEFS implanted in the uterus for real-time monitoring of biochemical signals during pregnancy. The signals monitored by the sensor were wirelessly transmitted through a flexible chip attached to the skin.90 This figure has been reproduced from ref. 90 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2024. (e) Photograph of the skin surface of a pregnant rat after implantation.90 This figure has been reproduced from ref. 90 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2024. (f) Accuracy of the electrochemical fiber sensor to lactate, glucose, NO, and pH after implantation for different days within a gestation period of the pregnant rat.90 This figure has been reproduced from ref. 90 with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, copyright: 2024. (g) Schematic illustration of a highly selective implantable electrochemical homovanillic acid fiber sensor.72 This figure has been reproduced from ref. 72 with permission from the American Chemical Society, copyright: 2024. (h) Changes of dopamine concentration in hippocampus and homovanillic acid concentration in blood using fiber sensor.72 This figure has been reproduced from ref. 72 with permission from the American Chemical Society, copyright: 2024. | ||
With the continuous advancement of biosensing technologies, the integration of hospital-on-chip, breathomics, nose-on-chip, 5th-generation biosensors, 6th-generation biosensors, and quantum biosensors has paved the way for new directions in medical diagnostics and real-time health monitoring.135–143 Nose-on-chip sensors enable early disease detection, such as the early diagnosis of lung cancer, by detecting volatile organic compounds (VOCs) in exhaled breath, while breathomics offers non-invasive health monitoring by analyzing biomarkers in breath.135,144,145 Additionally, 5th-generation biosensors combine artificial intelligence (AI), the Internet of Things (IOT), and big data analytics to provide portable, real-time detection capabilities,136,138 while 6th-generation biosensors further integrate quantum sensing, holography, and 6G communication, enhancing detection sensitivity and expanding remote medical applications.135,146 Quantum biosensors, leveraging quantum effects like quantum dots and quantum tunneling, significantly improve detection accuracy, particularly in detecting low-concentration biomarkers.140,147 Incorporating these advanced technologies into fiber electrodes, especially for applications in breath monitoring, sweat analysis, and body fluid analysis, not only enhances sensor sensitivity and selectivity but also allows for the precise identification of complex biological signals (such as early lung cancer screening) through AI algorithms and quantum material integration. Moreover, the use of 6G communication enables wireless data transmission, supporting remote healthcare and personalized health management.142,146
3.3 In vivo strain sensing
Implantable fiber-based strain sensing electrodes provide an innovative solution for real-time monitoring of mechanical strain in biological tissues, making them highly applicable in areas such as rehabilitation training and dynamic tissue monitoring.148–150 One of the major challenges in this field is developing strain sensors that can achieve both high sensitivity and ultra-stretchability across a broad working strain range.151 Combining highly elastic materials, such as silicone or thermoplastic elastomers, with conductive materials like carbon nanotubes or graphene has proven to be an effective solution.149,152–154 For example, Li et al. designed a highly stretchable strain sensor with a wide strain range (>1135%) and a fast response time (16 ms).153 They achieved this using a buckled core structure made from a multi-layer composite of carbon nanotubes and thermoplastic elastomers. By attaching this sensor to a rat's tendon, they could monitor muscle movement in real time and assess it quantitatively. This demonstrates the potential for precise and effective monitoring of mechanical deformations in biological tissues. In order to achieve long-term implantation and real-time monitoring, it is particularly important to develop a more stable, more flexible, and low-energy-consuming electrode system. Advancements in passive and wireless transmission and signal processing are driving the application of implantable fiber electrodes.111 Lee et al. developed a wireless, stretchable strain sensing system by integrating a capacitive optical fiber strain sensor with an inductive coil (Fig. 5a).155 Thanks to the resonant circuit formed by the integration of the capacitive fiber optic strain sensor and the inductive coil, passive operation is achieved through the energy of an external electromagnetic field. This system was capable of monitoring the stretching strain during the movement of a pig's leg, showing the potential of wireless, implantable sensors in monitoring muscle and joint activities without the need for invasive wired connections (Fig. 5b and c). The triboelectric nanogenerator, which converts mechanical energy into electricity through the combination of frictional charging and electrostatic induction, offers a versatile platform for self-powered sensors. Wang et al. desiged a spiral-shaped strain sensor based on a triboelectric nanogenerator using an organic gel/silicone fiber structure.156 This sensor was designed for passive, suture-compatible ligament strain monitoring, highlighting its potential use in orthopedic surgeries and injury recovery monitoring. Real-time monitoring of hemodynamic information is crucial for the prevention and management of cardiovascular diseases. Zhang et al. developed a flexible, fiber-based capacitive strain sensor using inkjet printing technology.105 The sensor features parallel spiral silver electrodes encased in high-dielectric materials, providing excellent dual-peak sensing capabilities. The energy supply is achieved by harnessing the energy from an external radio frequency electromagnetic field. This fiber sensor was able to wirelessly monitor blood pressure and heart rate within a simulated artificial blood vessel environment. The success of this design demonstrates the potential for using flexible fiber sensors in cardiovascular monitoring, offering a non-invasive, real-time approach to tracking vital signs and preventing cardiovascular conditions. With the development of emerging technologies such as artificial intelligence and machine learning, implantable fiber electrode strain sensors will bring more intelligent and personalized medical solutions. | ||
| Fig. 5 Implantable fibrous electrodes for in vivo strain sensing and electrical stimulation. (a) Schematic illustration of a passive wireless strain-sensing system based on a fibre strain sensor.155 This figure has been reproduced from ref. 155 with permission from the Springer Nature, copyright: 2021. (b) Photographs showing the wireless measurement of the fiber strain-sensing system implanted in the minipig according to bending and stretching of the leg.155 This figure has been reproduced from ref. 155 with permission from the Springer Nature, copyright: 2021. (c) The implanted wireless fiber strain-sensing system's resonant frequency after 3 weeks of repeated bending and stretching of the porcine leg.155 This figure has been reproduced from ref. 155 with permission from the Springer Nature, copyright: 2021. (d) The design of the bioadhesive pacing lead and the tools for minimally invasive implantation.157 This figure has been reproduced from ref. 157 with permission from the American Association for the Advancement of Science; copyright: 2024. (e) Bioadhesive pacing lead supported continuous and telemetric cardiac pacing in a porcine model.157 This figure has been reproduced from ref. 157 with permission from the American Association for the Advancement of Science; copyright: 2024. (f) Wound-healing mechanism between endogenous and applied electrical stimulation and representative optical images of wound areas after 10 days of recovery.75 This figure has been reproduced from ref. 75 with permission from the Springer Nature, copyright: 2024. | ||
3.4 Electrical stimulation
Electrical stimulation has become a key clinical approach for treating various neurological and psychiatric disorders.158–160 Implantable flexible fiber electrodes for electrical stimulation offer innovative solutions that enhance therapeutic outcomes by providing more targeted and minimally invasive treatments.76,96,161,162 Conductive biomaterials provide the potential for the combination of rehabilitation and regenerative medicine by promoting the regeneration of nerves and tissues and supporting the transmission of electrical signals.74,163–166 For example, Yao et al. have developed aligned conductive hydrogel fibers by introducing carbon nanotubes into methacrylated gelatin hydrogels through rotary bath electrospinning.74 The aligned structure of the hydrogel can induce nerve fiber regeneration and combine with electrical stimulation to enhance myelin sheath and axon regeneration. Incorporating 3D printing technology, Deng et al. reported a bio-adhesive cardiac pacemaker fiber device that demonstrated minimally invasive and stable cardiac pacing in rats and pigs for 10 to 14 days (Fig. 5d and e).157 This device can be removed on demand, offering flexibility and safety. Compared with commercially available temporary cardiac pacing leads, the bio-adhesive pacing lead exhibited a significantly higher charge injection capacity of up to 450 μC cm−2, as well as improved capture thresholds and sensing amplitudes. Electrical stimulation is also an effective non-drug wound care strategy due to the fact that it mimics the natural healing mechanism of endogenous electric fields.75,167,168 Sun et al. proposed a novel bioabsorbable electromechanical suture that promotes wound healing by converting the mechanical energy of motion into electrical energy and generating electrical stimulation (Fig. 5f).75 This suture is composed of multiple coaxial structures, including polylactic acid hydroxyacetic acid copolymer, polycaprolactone, and magnesium, which can be safely degraded in vivo. The combined device was placed underwater to generate electricity by inducing a potential difference of contact-separation between the nanofiber and the sheath in the suture during the stretch-recovery process.4. Challenges in implantable applications
Flexible fiber electrodes for implantable applications present a variety of challenges related to material selection, mechanical durability, signal transmission, and system integration. Addressing these challenges is essential to ensure long-term functionality, safety, and reliable performance within biological environments. In this section, we discuss key hurdles, including material biocompatibility, electrochemical stability, mechanical flexibility, microfabrication techniques, packaging, and power management, which must be overcome to enable the successful development of implantable flexible fiber electrodes (Fig. 6).4.1 Biocompatibility and long-term stability
Biocompatibility remains a fundamental requirement for implantable electrodes. Various materials, such as conductive polymers, metal and the oxide nanoparticles, and carbon-based materials, have demonstrated potential in maintaining compatibility with biological tissue while minimizing adverse immune responses.60,169,170 Some studies have shown that electrodes composed of aligned carbon nanotube (CNT) fibers exhibit biocompatibility for up to 30 days post-implantation,171 while surface coatings and modifications (e.g., platinum coatings on graphene fibers) improve the electrochemical properties and reduce tissue reactivity.99 Studies have found that certain carbon nanotubes can cause inflammatory reactions in the body, indicating that even nanomaterials with good conductivity require in-depth research on their biocompatibility.172 There is still a lack of long-term exposure experimental data for nanomaterials, and future research in this area needs to be strengthened to comprehensively evaluate their potential impacts on human health.Despite these advances, long-term biocompatibility and stability are still critical challenges. Materials degrade over time due to exposure to bodily fluids, leading to a reduction in electrode performance and potential failure. Additionally, fibrotic tissue formation and glial scarring can significantly increase impedance at the electrode-tissue interface, diminishing signal quality.173,174 Future research must focus on creating more robust, biocompatible materials that maintain functionality over extended periods and minimize the body's foreign body response. Furthermore, piezoelectric materials can generate electrical energy during human movement. Integrating them with flexible fiber electrodes is expected to provide part of the energy for the electrodes. Thermoelectric materials can generate electricity using human body heat, offering new possibilities for the energy supply of implantable devices. Future research is needed to further explore their application effects in flexible fiber electrodes.
4.2 Electrochemical stability and signal transmission
Current research has shown that flexible fiber electrodes can maintain relatively low impedance and a high signal-to-noise ratio through advanced material choices and surface modifications. For example, platinum-coated graphene fibers demonstrate improved signal transmission and low impedance.99 Optimizing electrochemical stability is crucial for reliable long-term implantation, as electrodes must continuously transmit electrical signals without interference from the surrounding biological environment.However, many issues remain unresolved in maintaining long-term electrochemical stability. Over time, electrodes face increasing impedance due to biofouling and tissue response, which can degrade signal quality.174 Additionally, motion artifacts and electromagnetic interference in dynamic environments, such as muscle or neural tissues, continue to compromise signal transmission. More work is needed to develop materials and surface treatments that reduce impedance and improve the consistency of signal transmission over time.
4.3 Mechanical mismatch
Flexible fiber electrodes have been designed to withstand the dynamic mechanical environment of the body, demonstrating improvements in tensile strength, fatigue resistance, and bending endurance. Multi-walled carbon nanotubes twisted into helical fiber bundles, for example, mimic the structure of muscle, providing low bending stiffness and high resilience under mechanical stress.73 These developments enhance the mechanical flexibility needed for long-term implantation in areas like muscles or joints.However, challenges in mechanical matching between the electrodes and biological tissues persist. Continuous movement and mechanical strain can cause fatigue and eventual fracture of the electrode material, leading to loss of function. Moreover, mechanical mismatches between the modulus of fiber electrodes and target tissues, especially during dynamic deformations such as cardiac contractions and muscle fiber movements, can lead to slippage at the device-tissue interface. This results in inaccurate monitoring and potential tissue damage. Developing fiber electrodes with mechanical properties that dynamically match those of the surrounding tissues remains a significant challenge. Addressing these mechanical challenges is critical for ensuring the reliability and longevity of implanted devices.
4.4 Biodegradability and functional lifespan
Biodegradable flexible fiber electrodes have emerged as a promising solution for temporary implants, providing functionality while reducing long-term health risks. Recent studies have developed biodegradable fibers that degrade within a specified timeframe without causing significant harm to surrounding tissues. For example, calcium ion sensors made from biodegradable materials can function in vivo for several weeks before degrading.175 Additionally, a significant breakthrough involved incorporating Mo microparticles into the outer layer of a polycaprolactone (PCL) fiber scaffold, allowing the fiber to serve as an interconnect, suturable temperature sensor, and in vivo electrical stimulator. This fiber electrode exhibited a notable decrease in weight, down to 42%, after 77 days of immersion in PBS solution at 37 °C, reflecting its controlled degradation profile.101 Such materials show great promise for applications requiring temporary monitoring or therapeutic interventions.However, challenges remain in ensuring controlled biodegradability and managing the precise functional lifespan of these electrodes. A key difficulty lies in achieving reliable performance during the device's operational period while guaranteeing predictable degradation afterward. Uncontrolled or premature degradation could lead to device failure before the completion of its intended use, while incomplete biodegradation might leave residual material in the body, potentially causing adverse effects. Current research efforts are focused on refining biodegradable polymers and composite materials to improve their degradation kinetics, mechanical properties, and biocompatibility. Further advancements are needed to enhance the predictability of degradation rates and to develop mechanisms that ensure safe and complete dissolution within biological environments, thereby minimizing the risk of long-term complications.
To address these challenges, future research is increasingly focusing on the development of “green” electrodes and sensors, which are not only biodegradable but also environmentally sustainable throughout their lifecycle.176 Green electrodes, made from renewable resources or biocompatible materials, offer a promising avenue for reducing the environmental footprint of temporary implants. For instance, green semiconductors derived from organic or bio-based materials, such as cellulose, chitosan, or other natural polymers, are being explored for their potential to create sensors that are both functional and eco-friendly.177–183 These green semiconductors can be engineered to exhibit tunable electrical properties, enabling their use in a variety of sensing and stimulation applications while maintaining compatibility with biological tissues. Moreover, the integration of green semiconductors into biodegradable fiber electrodes could further enhance their performance and sustainability. For example, sensors made from green semiconductors could be combined with biodegradable polymers like polycaprolactone (PCL) or polylactic acid (PLA) to create multifunctional devices that degrade harmlessly after use.178,179,181,183–185 Such devices could be designed to monitor physiological parameters, such as temperature, pH, or ion concentrations, while also providing therapeutic electrical stimulation. The use of green materials in these devices not only ensures their safe degradation but also aligns with global efforts to reduce the environmental impact of medical technologies. By leveraging advances in green materials and energy-harvesting technologies, researchers can create next-generation biodegradable implants that are both highly functional and environmentally responsible. In conclusion, the future of flexible implants lies in the convergence of green materials, biodegradable electronics, and sustainable design principles. By addressing the challenges of controlled biodegradability and functional lifespan, these innovations hold the potential to revolutionize medical devices, offering safer, more effective, and environmentally friendly solutions for temporary monitoring and therapeutic interventions.
4.5 Microfabrication and multi-channel resolution
The microfabrication of flexible fiber electrodes enables the creation of high-resolution, multi-channel devices critical for precise neural signal recording and stimulation. Techniques such as electrospinning, 3D printing, and nanomanufacturing have been explored to produce these devices, allowing for intricate electrode patterns with enhanced functional properties. However, ensuring consistent spatial resolution, particularly when scaling to multi-channel systems, remains challenging. Recent advances, such as the development of Neurotassel/PEG composite fibers with 1024 channels fabricated using an elastocapillary technique, have shown promise in reducing implantation footprints while increasing channel density.186Despite these advancements, achieving precise electrode placement remains difficult, especially in complex environments like the brain. Flexible multi-strand fiber devices can be inserted into a helical scaffold and implanted, where they spread after implantation, offering improved biocompatibility compared to previous rigid electrodes.187 However, this approach introduces new challenges in controlling the exact positioning of the fibers.188 Ensuring spatial accuracy while maintaining flexibility and biocompatibility is a critical area for further development in neural interface design.
4.6 Encapsulation and system integration
Encapsulation and system integration are essential for implantable flexible fiber electrodes to ensure long-term functionality. Packaging needs to protect the electrodes and their connection points from fluid infiltration, which can cause corrosion, electrical failure, and degradation of materials over time. Reliable connections between electrodes and external systems, such as signal processing units, are also crucial for maintaining performance without sacrificing flexibility. For example, one-dimensional line connectors have been demonstrated to work for multi-channel electrodes in small-scale systems,104 while two-dimensional array connectors have enabled monitoring of neural activity in larger, 8640-bundle metallic fiber arrays, connected to CMOS arrays for signal acquisition in the motor cortex or striatum.188However, challenges persist in ensuring consistent high spatial resolution for flexible fiber electrodes, especially when integrating multiple functionalities like sensing, stimulation, or even drug delivery. Three-dimensional structures in silicon probes189 and multi-functional electrodes compatible with magnetic resonance imaging (MRI), such as CNT fibers and polymers, have shown promise.190 Still, achieving optimal biocompatibility and system effectiveness requires further refinement. Techniques such as PDMS encapsulation combined with PHEMA gel coating have been explored to address these issues,191 but long-term stability and the prevention of signal degradation remain areas for further research.
4.7 Power consumption and management
Power consumption in implantable devices presents a critical challenge, as low-power operation is essential to ensure device longevity while minimizing adverse thermal effects on surrounding tissues. Traditional implantable systems rely on external power sources or batteries, which can limit their lifespan and create additional challenges such as the need for periodic replacements or recharging. Innovations in low-power electrode design and high-power implantable biofuel cell192 are crucial to enhancing energy efficiency. However, these designs must maintain signal quality and avoid trade-offs like reduced data accuracy or slower processing speeds.Huisheng Peng's pioneering work on energy-harvesting solutions provides a promising direction for addressing these challenges. Peng and his team developed fiber-shaped supercapacitors that can utilize physiological fluids as electrolytes, allowing for in vivo energy storage and power generation.100 These biocompatible fibers not only extend device lifespans but also minimize thermal effects by efficiently managing power consumption without relying on bulky external power sources. Such innovations can alleviate the limitations posed by traditional battery-dependent designs and offer a pathway towards self-powered implantable systems. However, challenges remain in optimizing the integration of these energy storage devices into multifunctional electrodes, ensuring that they provide consistent power without compromising mechanical flexibility or biocompatibility.
The use of such fiber-based energy solutions also introduces the challenge of balancing energy harvesting and consumption. While Peng's fiber-shaped supercapacitors, generator and batteries193–195 offer a solution to power supply limitations, maintaining stable and sufficient energy output for continuous operation in dynamic biological environments remains difficult. Additionally, ensuring that these systems do not generate excessive heat, which could lead to tissue damage, requires further refinement. Future research should focus on improving the efficiency of these integrated energy systems while minimizing their impact on surrounding biological tissues, enabling reliable and long-lasting implantable devices.
4.8 Regulatory and commercialization
Implantable fibrous biosensors face significant regulatory and commercialization challenges that must be addressed for clinical translation. Regulatory hurdles include ensuring long-term biocompatibility and safety, as required by standards like ISO 10993, and demonstrating device stability under physiological conditions. Extensive preclinical and clinical testing is necessary to validate their performance, including accurate signal acquisition, minimal drift, and reliability over time. On the commercialization side, scalable and cost-effective manufacturing remains a critical barrier, as advanced fabrication methods such as 3D printing and injection molding must meet both precision and affordability requirements. Furthermore, intellectual property protection, clear approval pathways, and collaborations with healthcare stakeholders are essential for market entry. Material scientists, medical professionals, industry, and regulatory agencies each have distinct and crucial roles. Material scientists are responsible for developing advanced materials that meet strict biocompatibility and performance requirements. Medical professionals can offer valuable insights from a clinical perspective, guiding the design and evaluation of these electrodes. The industry should focus on optimizing manufacturing processes for scalability and cost – effectiveness while maintaining high-quality standards. Regulatory agencies need to establish clear, science – based regulations and efficient approval pathways. Addressing these challenges will require interdisciplinary efforts and innovations in regulatory science, manufacturing processes, and stakeholder engagement to unlock the potential of flexible fibrous electrodes for transformative healthcare applications.The long-term sustainability of implantable fibrous biosensors hinges not only on overcoming regulatory and commercialization challenges but also on their potential to make a profound societal impact. By enabling continuous, real-time monitoring of physiological parameters, these devices can revolutionize personalized medicine, allowing for early detection of diseases, optimized treatment plans, and improved patient outcomes. Their minimally invasive nature and biodegradability further align with the growing demand for patient-centric and environmentally sustainable medical technologies. Moreover, the widespread adoption of implantable fibrous biosensors could contribute to the advancement of global health equity. By providing affordable and accessible diagnostic tools, these devices have the potential to bridge healthcare disparities, particularly in underserved or resource-limited regions. From an environmental perspective, the development of green and biodegradable biosensors aligns with the broader goals of reducing electronic waste and promoting sustainable practices in the medical device industry. By utilizing eco-friendly materials and ensuring complete degradation after use, these devices minimize their environmental footprint, contributing to a circular economy in healthcare. This approach not only addresses the growing concern over medical waste but also sets a precedent for the development of future medical technologies that prioritize both human health and planetary well-being.
5. Future directions and prospects
With the continuous advancement of implantable flexible electrode technologies, future research and application prospects are promising. This chapter explores three key developmental directions: multichannel multifunctional fiber electrodes, intelligent fiber electrodes, and motile fiber electrodes. These directions represent not only the cutting-edge trends in technology but also have the potential to drive major breakthroughs in the field of implantable biosensors.5.1 Multichannel multifunctional fiber electrodes
Multichannel and multifunctionally integrated flexible fiber electrodes can significantly enhance the precision and breadth of signal acquisition.65,73,79,196 One of the primary advantages of multichannel fiber electrodes is their structure, which allows multiple sensing channels to be uniformly distributed along the fiber axis. This design enables high-density signal acquisition in relatively small implantation areas, making it particularly suitable for recording complex physiological signals. For instance, in neural applications, multichannel fiber electrodes can simultaneously record neural activities from various locations, offering a comprehensive understanding of neural network dynamics far beyond the capabilities of single-channel or low-density electrodes. In muscle monitoring, multichannel designs capture the spatial distribution of muscle contractions, helping to analyze the activities of different muscle fiber groups. This high-density data acquisition holds significant value in developing more advanced brain-machine interfaces (BMIs), neural prostheses, and electromyographic monitoring devices.Another notable advantage of multichannel fiber electrodes is their ability to integrate multiple sensing functionalities. Traditional electrodes typically capture only a single type of electrical signal, whereas multifunctional fiber electrodes can simultaneously monitor various physiological signals, such as electrical, mechanical (e.g., pressure, strain), and chemical signals (e.g., pH, ion concentration). This multimodal monitoring is especially valuable in complex physiological environments. For example, during neuromuscular monitoring, changes in electrical signals are often accompanied by variations in mechanical stress, and multifunctional electrodes can capture these factors’ synergistic effects in real time, providing a more comprehensive picture of biological processes. In biochemical monitoring, multifunctional fiber electrodes can detect various chemical markers in body fluids, such as glucose, lactate, or potassium ion concentrations, offering real-time data support for metabolic monitoring and personalized medicine.
Despite the significant technical advantages of multichannel multifunctional fiber electrodes, several challenges remain in practical applications. For example, as the number of channels increases, electrical crosstalk between electrodes may affect signal accuracy. Researchers are addressing this issue by increasing the spacing between electrodes or incorporating shielding materials to reduce crosstalk. Additionally, data processing is a critical challenge, as the massive datasets generated by multichannel electrodes require more efficient signal processing algorithms and more robust hardware to ensure real-time, precise analysis.
Multichannel and multifunctional fiber electrodes open new possibilities in the field of implantable biosensing. They not only provide richer biological information for fundamental scientific research but also demonstrate great potential in clinical applications such as neural modulation, muscle function monitoring, personalized medicine, and real-time metabolic monitoring. As technology continues to advance, particularly in materials science, micro/nano fabrication techniques, and flexible electronics, multichannel multifunctional fiber electrodes are expected to play an even more significant role in future medical applications, revolutionizing disease monitoring and health management.
5.2 Intelligent fiber electrodes
Intelligent fiber electrodes represent one of the key developmental directions in implantable biosensing technologies, aiming to enhance the adaptability and responsiveness of flexible electrodes in complex physiological environments by integrating sensing, processing, and feedback functionalities. This technological breakthrough not only improves the precision of monitoring but also lays the foundation for more interactive and autonomous medical devices, with broad applications in neural modulation, precision medicine, and real-time health monitoring.197–201The core advantage of intelligent fiber electrodes lies in their ability to dynamically sense physiological signals and respond in real time. Traditional implantable electrodes are mostly passive devices used primarily for signal recording. However, with the introduction of intelligent technologies, fiber electrodes can be endowed with active regulation capabilities, enabling them to adjust their performance or therapeutic interventions based on the physiological signals they collect. For example, in neural modulation, intelligent fiber electrodes can automatically adjust the intensity and frequency of electrical stimulation based on real-time monitored neural activity, optimizing therapeutic outcomes. This adaptive regulation capability is particularly valuable for treating neurological diseases such as Parkinson's disease and epilepsy, reducing side effects and enhancing treatment precision.
Another important feature of intelligent fiber electrodes is their ability to integrate signal processing. By embedding microprocessors or other electronic components into the electrode structure, intelligent fiber electrodes can perform preliminary signal processing and data filtering after implantation. This capability not only alleviates the computational burden on external devices but also reduces potential interference and delays during signal transmission, ensuring the real-time accuracy of the signals. Moreover, intelligent electrodes can analyze trends in physiological signals, predicting potential health issues in advance, such as abnormal heart rates, respiratory arrest, or neurological disorders, thereby enabling preventive medical interventions.
In medical applications, intelligent fiber electrodes have the potential to transform the current operating modes of implantable devices. Traditional implantable devices often require continuous monitoring and control by external equipment, whereas intelligent fiber electrodes can achieve greater autonomy, reducing patient dependence on external devices. For example, in brain-machine interfaces, intelligent fiber electrodes can dynamically adjust signal acquisition parameters based on neural activity changes, helping patients control external devices, such as prosthetics or wheelchairs, more naturally. This autonomous control feature has the potential to significantly improve neurorehabilitation outcomes, allowing individuals with disabilities to adapt more quickly to prosthetic operation. Furthermore, the realization of intelligent fiber electrodes relies on advanced materials that can adjust their electrical properties according to environmental changes, ensuring stable operation over extended periods in the body. For instance, self-healing materials can automatically repair damage sustained during implantation, preventing signal interruption or quality degradation. Some intelligent materials can also respond to changes in the concentration of biomolecules or ions in bodily fluids, adjusting the electrode's conductivity in real time to enhance multimodal monitoring sensitivity.
However, the development of intelligent fiber electrodes faces several challenges. First, how to efficiently integrate electronic components and smart materials into the miniature structure of fiber electrodes remains a technical bottleneck. Although microelectronics technology has made significant strides, ensuring that these components function long-term in the body without compromising biocompatibility requires further investigation. Additionally, the energy supply for intelligent electrodes is a key challenge. Since replacing batteries after implantation is difficult, researchers are exploring ways to utilize physiological energy sources (e.g., blood flow, temperature gradients) or wireless power transfer technologies to provide long-term, stable energy for the electrodes.
Intelligent fiber electrodes represent a promising future direction in implantable biosensing technologies. By combining intelligent materials and electronic components with flexible electrodes, these devices can offer more precise signal monitoring and autonomous regulation in complex physiological environments, providing new solutions for personalized medicine and precision treatment. As advancements in materials science, flexible electronics, and micro/nano manufacturing continue, intelligent fiber electrodes are expected to play an increasingly important role in future medical applications, improving patient quality of life and driving the development of smarter, more autonomous medical devices.
5.3 Motile fiber electrodes
Motile fiber electrodes represent a frontier technology in implantable bioelectronics, incorporating motion and self-regulation functions into traditional flexible electrodes. This enables precise capture and feedback of signals in dynamic physiological environments. These electrodes not only possess flexibility and biocompatibility but also have the ability to perform controlled movements and displacements within the body, better adapting to tissue changes and maintaining stable performance in complex physiological conditions over the long term. Compared to traditional static implantable electrodes, motile fiber electrodes exhibit significant advantages in dynamic adaptability, signal quality, and lifespan.52,79,202–207Firstly, motile fiber electrodes are designed with adaptive structures that allow them to actively respond to dynamic changes in body tissues. Traditional implantable electrodes are prone to signal loss or failure due to mechanical mismatch when faced with tissue contraction, extension, or displacement. Motile electrodes, however, can adjust their shape in response to tissue movement, ensuring that the electrode remains in close contact with the target tissue. This is critical for long-term implants, particularly in the peripheral nervous system or muscle tissues, where frequent dynamic activities require the electrode to maintain high-quality signal acquisition without damaging the tissue. For example, in monitoring muscle electrical activity, motile fiber electrodes can adjust in response to muscle contraction and relaxation, preventing electrode slippage or detachment, thereby providing more stable electromyographic signals.
Secondly, the self-regulating capability of motile fiber electrodes enhances the accuracy and stability of signal capture. The physiological environment within the body is complex and variable, especially when monitoring neural or electromyographic signals, where electrodes must have strong dynamic adaptability. By incorporating motility mechanisms, these electrodes can not only adjust their position but also fine-tune within a certain range to optimize the electrode–tissue interface, reducing contact impedance and improving signal transmission efficiency. This ability is particularly valuable in neural interface applications, where small displacements can lead to significant signal changes. Through self-adjustment, motile electrodes dynamically optimize signal stability, enhancing the precision of electrophysiological monitoring.
Furthermore, motile fiber electrodes offer new pathways to reduce implantation trauma and improve patient comfort. Traditional electrodes often cause significant tissue trauma during implantation, and because their position is fixed, subsequent adjustments may require additional surgery. Motile electrodes, however, can be displaced and adjusted via external control without the need for additional surgical procedures, thus reducing trauma and increasing the adaptability of implanted electrodes. For instance, in brain-machine interfaces, the initial implantation position of the electrode may not be optimal, but with motility functions (controlled via magnetic, electrical, or optical means), the electrode can be fine-tuned post-surgery to optimize its position within the neural population, capturing clearer signals. This not only reduces surgical complexity but also significantly lowers post-operative discomfort and risks for the patient.
The realization of motile fiber electrodes relies on advances in materials and manufacturing technologies. To ensure the safe movement of electrodes within the body, the materials must possess high flexibility and extensibility while maintaining excellent conductivity. Emerging materials such as conductive polymers, metal nanowires, and carbon-based materials provide a solid foundation for the development of motile fiber electrodes. Additionally, the application of microelectromechanical systems (MEMS) enables precise control and feedback of the electrode's movement, ensuring that the electrodes can perform adjustments and movements on a microscale. These technologies collectively pave the way for the widespread application of motile fiber electrodes in medical fields.
Despite the theoretical advantages of motile fiber electrodes, several challenges remain for clinical applications. First, how to achieve controlled movement of the electrode in the body while ensuring its long-term biocompatibility and mechanical stability is a major research focus. Second, energy supply issues cannot be overlooked. Researchers are exploring miniaturized energy transmission systems or self-powered systems to provide sustained energy for motile electrodes. Additionally, the interaction between the electrode's motility mechanism and physiological tissues requires detailed study to avoid inflammation or immune responses caused by electrode movement.
Motile fiber electrodes, as a new generation of implantable electrodes, provide a powerful solution to the limitations of traditional electrodes in dynamic environments due to their adaptive capabilities and dynamic regulation functions. As material science, nanotechnology, and MEMS technology continue to advance, motile fiber electrodes are expected to play a more prominent role in neuroscience, muscle monitoring, and implantable therapies, driving medical devices toward greater intelligence and personalization. This technology not only offers new insights for biosensing and medical monitoring but also lays the foundation for future precision medicine and personalized treatment.
6. Conclusion
In conclusion, flexible fibrous electrodes for implantable biosensing represent a transformative advancement in real-time, long-term physiological monitoring and therapeutic interventions. These electrodes, with their adaptability to soft, dynamic tissue environments, overcome many limitations of traditional rigid electrode systems, promoting biocompatibility and reducing tissue irritation. Their multifunctionality—enabling simultaneous monitoring of electrical, mechanical, and chemical signals—provides a nuanced understanding of complex physiological processes, enhancing applications ranging from neural interfacing to electrochemical sensing.Despite significant progress, challenges remain in achieving ultra-high-density and multifunctional flexible fiber electrodes with robust integration and stable performance in vivo. Future developments should prioritize advanced materials and novel fabrication techniques that support both functional versatility and durability under physiological conditions. Moreover, the evolution towards intelligent, controlled electrode systems will open new avenues in precision medicine, allowing for personalized, responsive, and effective interventions. The long-term sustainability of implantable fibrous biosensors depends on overcoming regulatory and commercialization challenges while maximizing their societal impact through real-time health monitoring, personalized medicine, global health equity, and environmentally sustainable design, ultimately advancing both healthcare accessibility and eco-friendly medical innovation. As flexible fiber electrode technology advances, its potential for impacting clinical diagnostics, rehabilitation, and neuroprosthetics continues to expand, promising improved patient outcomes through minimally invasive, adaptive, and integrated biosensing solutions.
Author contributions
H. Li, C. Li, H. Yu, Q. Tian, and F. Han conducted the literature search and data collection, prepared the initial draft, and created the figures. H. Zhao, Q. Li, Y. Zhao, J. Gong, and G. Li contributed to the discussion of the manuscript. Q. Tian, Z. Liu, and F. Han provided in-depth revisions to the content and guidance on the overall framework and structure. Z. Liu and F. Han were responsible for the overall conceptualization, supervision, and final revisions of the review, ensuring its academic rigor and integrity. All authors reviewed and approved the final manuscript for publication.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Key R&D Program of China (2023YFC2414500, 2023YFC2414501), National Natural Science Foundation of China (62201558, 52473269, 62471462, 62101544, U81927804 and 62201559), Guangdong Provincial Key Laboratory of Multimodality Non-Invasive Brain-Computer Interfaces (grant no. 2024B1212010010), Shenzhen Science and Technology Program (KQTD20210811090217009 and KJZD20230923114108015), and Guangdong Basic and Applied Basic Research Foundation (2022A1515010879, 2023A1515011160).References
- M. Y. Lin, H. J. Hu, S. Zhou and S. Xu, Nat. Rev. Mater., 2022, 7, 850–869 CrossRef.
- G. H. Lee, H. Moon, H. Kim, G. H. Lee, W. Kwon, S. Yoo, D. Myung, S. H. Yun, Z. Bao and S. K. Hahn, Nat. Rev. Mater., 2020, 5, 149–165 CrossRef PubMed.
- N. Ashammakhi, A. L. Hernandez, B. D. Unluturk, S. A. Quintero, N. R. De Barros, E. H. Apu, A. Bin Shams, S. Ostrovidov, J. X. Li, C. Contag, A. S. Gomes and M. Holgado, Adv. Funct. Mater., 2021, 31, 2104149 CrossRef CAS.
- A. Yu, M. Zhu, C. Chen, Y. Li, H. Cui, S. Liu and Q. Zhao, Adv. Healthcare Mater., 2024, 13, e2302460 CrossRef PubMed.
- S.-H. Sunwoo, S. I. Han, C. S. Park, J. H. Kim, J. S. Georgiou, S.-P. Lee, D.-H. Kim and T. Hyeon, Nat. Rev. Bioengin., 2024, 2, 8–24 CrossRef CAS.
- J. Zhou, S. Zhou, P. Fan, X. Li, Y. Ying, J. Ping and Y. Pan, Nano-Micro Lett., 2023, 16, 49 CrossRef PubMed.
- T. Zhang, N. Liu, J. Xu, Z. Liu, Y. Zhou, Y. Yang, S. Li, Y. Huang and S. Jiang, Innovation, 2023, 4, 100485 Search PubMed.
- Q. Liang, X. Xia, X. Sun, D. Yu, X. Huang, G. Han, S. M. Mugo, W. Chen and Q. Zhang, Adv. Sci., 2022, 9, e2201059 CrossRef PubMed.
- A. Cutrone and S. Micera, Adv. Healthcare Mater., 2019, 8, e1801345 CrossRef PubMed.
- C. A. Chapman, N. Goshi and E. Seker, Adv. Funct. Mater., 2018, 28, 1703523 Search PubMed.
- B. Oh, Y. S. Lim, K. W. Ko, H. Seo, D. J. Kim, D. Kong, J. M. You, H. Kim, T. S. Kim, S. Park, D. S. Kwon, J. C. Na, W. K. Han, S. M. Park and S. Park, Biosens. Bioelectron., 2023, 225, 115060 CAS.
- X. Q. Huo, S. J. Luo, Z. Cao, Y. X. Zhou, Y. R. Hu, Z. L. Wang and Z. Y. Wu, Chem. Eng. J., 2024, 497, 154971 CAS.
- B. G. Molina, J. Sanz-Farnos, S. Sánchez and C. Alemán, Sens. Actuators, B, 2024, 416, 136005 CAS.
- B. Ryu, C. Y. Kim, S.-P. Park, K. Y. Lee and S. Lee, Adv. Funct. Mater., 2023, 33, 2305769 CAS.
- Q. Zhang, F. M. Bossuyt, N. C. Adam, B. L. Zambrano, F. Stauffer, P. Rennhard, R. Gubler, R. L. Küng, S. Abramovic, V. Useini, W. Herzog, T. Leonard, M. W. Scott, W. R. Taylor and C. R. Smith, Adv. Mater. Technol., 2023, 8, 2202041 CAS.
- C. Zhang, W. Ouyang, L. Zhang and D. Li, Microsyst. Nanoeng., 2023, 9, 158 CrossRef CAS PubMed.
- S. Deng, Y. Li, S. Li, S. Yuan, H. Zhu, J. Bai, J. Xu, L. Peng, T. Li and T. Zhang, Innovation, 2024, 5, 100596 CAS.
- D. Rodrigues, A. I. Barbosa, R. Rebelo, I. K. Kwon, R. L. Reis and V. M. Correlo, Biosensors, 2020, 10, 79 CrossRef PubMed.
- P. Zhang, B. Zhu, P. Du and J. Travas-Sejdic, Chem. Rev., 2024, 124, 722–767 CrossRef CAS PubMed.
- G. Liu, Z. Lv, S. Batool, M. Z. Li, P. Zhao, L. Guo, Y. Wang, Y. Zhou and S. T. Han, Small, 2023, 19, 2207879 CrossRef CAS PubMed.
- Y. Song, J. Min and W. Gao, ACS Nano, 2019, 13, 12280–12286 CrossRef CAS PubMed.
- J. Andreu-Perez, D. R. Leff, H. M. Ip and G.-Z. Yang, IEEE Trans. Biomed. Eng., 2015, 62, 2750–2762 Search PubMed.
- V. Chaudhary, V. Khanna, H. T. A. Awan, K. Singh, M. Khalid, Y. K. Mishra, S. Bhansali, C.-Z. Li and A. Kaushik, Biosens. Bioelectron., 2023, 220, 114847 CrossRef CAS PubMed.
- B. A. Taha, I. A. Al-Tahar, A. J. Addie, A. B. Mahdi, A. J. Haider, Y. A. Mashhadany, V. Chaudhary and N. Arsad, Appl. Mater. Today, 2024, 38, 102229 CrossRef.
- Q. Zeng and Z. L. Huang, Adv. Funct. Mater., 2023, 33, 2301223 CrossRef CAS.
- H. Li, H. Zhao, K. Song, F. Han, Z. Liu and Q. Tian, Nanoscale, 2024, 16, 6402–6428 RSC.
- M. Hassan, G. Abbas, N. Li, A. Afzal, Z. Haider, S. Ahmed, X. Xu, C. Pan and Z. Peng, Adv. Mater. Technol., 2022, 7, 2100773 CrossRef.
- M. Zhu, H. Wang, S. Li, X. Liang, M. Zhang, X. Dai and Y. Zhang, Adv. Healthcare Mater., 2021, 10, e2100646 CrossRef PubMed.
- J. J. Wang, L. Y. Wang, J. Y. Feng, C. Q. Tang, X. M. Sun and H. S. Peng, Adv. Fiber Mater., 2021, 3, 47–58 CrossRef CAS.
- X. Tang, H. Shen, S. Zhao, N. Li and J. Liu, Nat. Electron., 2023, 6, 109–118 CrossRef.
- X. K. Ju, J. Kong, G. H. Qi, S. P. Hou, B. Wang, X. K. Diao, S. J. Dong and Y. D. Jin, eScience, 2024, 4, 100223 CrossRef.
- Y. Liu, H. Luo, H. Xie, Z. Xiao, F. Wang, X. Jiang, X. Zhou and D. Zhang, Microstructures, 2023, 3, 2023008 CAS.
- Y. Hao, Innovation, 2024, 5, 100613 CAS.
- Z. Song, M. Chen, W. Li and L. Niu, Innov. Mater., 2023, 1, 100035 CrossRef.
- S.-H. Sunwoo, S. I. Han, D. Jung, M. Kim, S. Nam, H. Lee, S. Choi, H. Kang, Y. S. Cho and D.-H. Yeom, ACS Nano, 2023, 17, 7550–7561 CrossRef CAS PubMed.
- T. Y. Kim, S. Shin, H. Choi, S. H. Jeong, D. Myung and S. K. Hahn, ACS Appl. Bio Mater., 2021, 4, 4532–4541 CrossRef CAS PubMed.
- K. K. Kim, M. Kim, K. Pyun, J. Kim, J. Min, S. Koh, S. E. Root, J. Kim, B.-N. T. Nguyen and Y. Nishio, Nat. Electron., 2023, 6, 64–75 Search PubMed.
- J. Choi, J. Min, D. Kim, J. Kim, J. Kim, H. Yoon, J. Lee, Y. Jeong, C.-Y. Kim and S. H. Ko, ACS Nano, 2023, 17, 17966–17978 CrossRef CAS PubMed.
- H. Yoon, J. Choi, J. Kim, J. Kim, J. Min, D. Kim, S. Jeong, J. G. Lee, J. Bang and S. H. Choi, Adv. Funct. Mater., 2024, 2313504 CrossRef CAS.
- Z. Wang, H. Cui, S. Li, X. Feng, J. Aghassi-Hagmann, S. Azizian and P. A. Levkin, ACS Appl. Mater. Interfaces, 2021, 13, 21661–21668 CrossRef CAS PubMed.
- F. F. Lang, J. D. Pang and X. H. Bu, eScience, 2024, 4, 100231 CrossRef.
- D. Won, J. Kim, H. Kim, M. W. Kim, J. Ahn, K. Min, Y. Lee, S. Hong, J. Choi, C.-Y. Kim, T.-S. Kim and S. H. Ko, Nat. Electron., 2024, 7, 475–486 CrossRef CAS.
- J. Kim, D. Won, T. H. Kim, C.-Y. Kim and S. H. Ko, Biosens. Bioelectron., 2024, 258, 116327 CrossRef CAS PubMed.
- D. Won, J. Kim, J. Choi, H. Kim, S. Han, I. Ha, J. Bang, K. K. Kim, Y. Lee and T.-S. Kim, Sci. Adv., 2022, 8, eabo3209 CrossRef CAS PubMed.
- J. Oh, S. Kim, S. Lee, S. Jeong, S. H. Ko and J. Bae, Adv. Funct. Mater., 2021, 31, 2007772 CrossRef CAS.
- M. Kim, J. J. Park, C. Cho and S. H. Ko, Adv. Funct. Mater., 2023, 33, 2303286 CrossRef CAS.
- P. Won, S. Jeong, C. Majidi and S. H. Ko, iScience, 2021, 24, 102698 CrossRef CAS PubMed.
- L. J. Zhao, L. L. Wang, Y. Q. Zheng, S. F. Zhao, W. Wei, D. W. Zhang, X. Y. Fu, K. Jiang, G. Z. Shen and W. Han, Nano Energy, 2021, 84, 105921 CrossRef CAS.
- Y. Gao, X. Chen, X. Jin, C. Zhang, X. Zhang, X. Liu, Y. Li, Y. Li, J. Lin and H. Gao, eScience, 2024, 100292 CrossRef.
- V. Chaudhary, S. Rustagi and A. Kaushik, Curr. Opin. Green Sustainable Chem., 2023, 42, 100817 CrossRef.
- H. Sable, V. Kumar, V. Singh, S. Rustagi, V. Chaudhary and S. Pandit, J. Electrochem. Soc., 2024, 171, 037527 CrossRef CAS.
- B. A. Taha, I. A. Al-Tahar, A. J. Addie, A. B. Mahdi, A. J. Haider, Y. Al Mashhadany, V. Chaudhary and N. Arsad, Appl. Mater. Today, 2024, 38, 102229 CrossRef.
- B. A. Taha, A. J. Addie, E. M. Abbas, B. H. Aubaidan, N. M. Ahmed, A. J. Haider, V. Chaudhary and N. Arsad, J. Photochem. Photobiol., C, 2024, 100678 CAS.
- H. Li, F. Han, L. Wang, L. Huang, O. W. Samuel, H. Zhao, R. Xie, P. Wang, Q. Tian and Q. Li, Adv. Funct. Mater., 2023, 33, 2300859 CAS.
- Z. Liu, H. Wang, P. Huang, J. Huang, Y. Zhang, Y. Wang, M. Yu, S. Chen, D. Qi and T. Wang, Adv. Mater., 2019, 31, 1901360 CrossRef PubMed.
- J. X. Chen and W. T. Xu, eScience, 2023, 3, 100178 CrossRef.
- F. Han, H. Li, L. Huang, X. Zhou, R. Su, H. Yu, Q. Tian, H. Zhao, Q. Li and J. Sun, eScience, 2024, 100327 Search PubMed.
- G. Q. Li, M. Y. Zhang, S. H. Liu, M. Yuan, J. J. Wu, M. Yu, L. J. Teng, Z. W. Xu, J. H. Guo, G. L. Li, Z. Y. Liu and X. Ma, Nat. Electron., 2023, 6, 154–163 CrossRef.
- Z. Liu, X. Hu, R. Bo, Y. Yang, X. Cheng, W. Pang, Q. Liu, Y. Wang, S. Wang, S. Xu, Z. Shen and Y. Zhang, Science, 2024, 384, 987–994 CrossRef CAS PubMed.
- J. Y. Feng, C. R. Chen, X. M. Sun and H. S. Peng, Acc. Mater. Res., 2021, 2, 138–146 CrossRef CAS.
- C. Dang, Z. Wang, T. Hughes-Riley, T. Dias, S. Qian, Z. Wang, X. Wang, M. Liu, S. Yu, R. Liu, D. Xu, L. Wei, W. Yan and M. Zhu, Chem. Soc. Rev., 2024, 53, 8790–8846 CAS.
- Y. Zhang, Y. P. Li, Z. Z. Guo, J. B. Li, X. Y. Ge, Q. Z. Sun, Z. J. Yan, Z. Li and Y. H. Huang, eScience, 2024, 4, 100174 CrossRef.
- A. Canales, X. Jia, U. P. Froriep, R. A. Koppes, C. M. Tringides, J. Selvidge, C. Lu, C. Hou, L. Wei, Y. Fink and P. Anikeeva, Nat. Biotechnol., 2015, 33, 277–284 CrossRef CAS PubMed.
- B. Sadri and W. Gao, Appl. Phys. Rev., 2023, 10, 031303 CAS.
- Y. Liu, H. Jia, H. Sun, S. Jia, Z. Yang, A. Li, A. Jiang, Y. Naya, C. Yang, S. Xue, X. Li, B. Chen, J. Zhu, C. Zhou, M. Li and X. Duan, Nat. Neurosci., 2024, 27, 1620–1631 CrossRef CAS PubMed.
- C. Tang, S. Xie, M. Wang, J. Feng, Z. Han, X. Wu, L. Wang, C. Chen, J. Wang, L. Jiang, P. Chen, X. Sun and H. Peng, J. Mater. Chem. B, 2020, 8, 4387–4394 RSC.
- C. Won, U. J. Jeong, S. Lee, M. Lee, C. Kwon, S. Cho, K. Yoon, S. Lee, D. Chun, I. J. Cho and T. Lee, Adv. Funct. Mater., 2022, 32, 2205145 CrossRef CAS.
- Q. Gong, Y. Yu, L. Kang, M. Zhang, Y. Zhang, S. Wang, Y. Niu, Y. Zhang, J. Di, Q. Li and J. Zhang, Adv. Funct. Mater., 2021, 32, 2107360 CrossRef.
- K. Wang, C. L. Frewin, D. Esrafilzadeh, C. Yu, C. Wang, J. J. Pancrazio, M. Romero-Ortega, R. Jalili and G. Wallace, Adv. Mater., 2019, 31, e1805867 CrossRef PubMed.
- S. P. Lacour, G. Courtine and J. Guck, Nat. Rev. Mater., 2016, 1, 16063 CrossRef CAS.
- B. B. Jia, B. H. Zhang, Z. Cai, X. Y. Yang, L. D. Li and L. Guo, eScience, 2023, 3, 100112 CrossRef.
- K. Zou, Q. Li, D. Li, Y. Jiao, L. Wang, L. Li, J. Wang, Y. Li, R. Gao, F. Li, E. He, T. Ye, W. Tang, J. Song, J. Lu, X. Li, H. Zhang, X. Cao and Y. Zhang, ACS Nano, 2024, 18, 7485–7495 CrossRef CAS PubMed.
- L. Wang, S. Xie, Z. Wang, F. Liu, Y. Yang, C. Tang, X. Wu, P. Liu, Y. Li, H. Saiyin, S. Zheng, X. Sun, F. Xu, H. Yu and H. Peng, Nat. Biomed. Eng., 2020, 4, 159–171 CrossRef CAS PubMed.
- S. Yao, Y. Yang, C. Li, K. Yang, X. Song, C. Li, Z. Cao, H. Zhao, X. Yu, X. Wang and L. N. Wang, Bioact. Mater., 2024, 35, 534–548 CAS.
- Z. Sun, Y. Jin, J. Luo, L. Li, Y. Ding, Y. Luo, Y. Qi, Y. Li, Q. Zhang, K. Li, H. Shi, S. Yin, H. Wang, H. Wang and C. Hou, Nat. Commun., 2024, 15, 8462 CrossRef CAS PubMed.
- S. Zhao, G. Li, C. Tong, W. Chen, P. Wang, J. Dai, X. Fu, Z. Xu, X. Liu, L. Lu, Z. Liang and X. Duan, Nat. Commun., 2020, 11, 1788 CrossRef CAS PubMed.
- J. Y. Feng, C. R. Chen, X. M. Sun and H. S. Peng, Acc. Mater. Res., 2021, 2, 138–146 CrossRef CAS.
- C. Lu, S. Park, T. J. Richner, A. Derry, I. Brown, C. Hou, S. Rao, J. Kang, C. T. Mortiz, Y. Fink and P. Anikeeva, Sci. Adv., 2017, 3, e1600955 CrossRef PubMed.
- R. Xie, Q. Yu, D. Li, X. Han, X. Xu, J. Huang, W. Yan, X. Zhou, X. Deng, Q. Tian, Q. Li, H. Li, H. Zheng, G. Li, F. Han, T. Xu and Z. Liu, bioRxiv, 2023, preprint, DOI: 10.1101/2023.10.02.560392.
- G. Chen, H. Wang, R. Guo, M. Duan, Y. Zhang and J. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 6112–6118 CrossRef CAS PubMed.
- S. Lee, S. Shin, S. Lee, J. Seo, J. Lee, S. Son, H. J. Cho, H. Algadi, S. Al-Sayari, D. E. Kim and T. Lee, Adv. Funct. Mater., 2015, 25, 3114–3121 CrossRef CAS.
- C. Yang, Q. Cao, P. Puthongkham, S. T. Lee, M. Ganesana, N. V. Lavrik and B. J. Venton, Angew. Chem., Int. Ed., 2018, 57, 14255–14259 CrossRef CAS PubMed.
- Y. C. Lai, H. W. Lu, H. M. Wu, D. Zhang, J. Yang, J. Ma, M. Shamsi, V. Vallem and M. D. Dickey, Adv. Energy Mater., 2021, 11, 2170068 CrossRef CAS.
- M. Khatib, E. T. Zhao, S. Wei, A. Abramson, E. S. Bishop, C. H. Chen, A. L. Thomas, C. Xu, J. Park, Y. Lee, R. Hamnett, W. Yu, S. E. Root, L. Yuan, D. Chakhtoura, K. K. Kim, D. Zhong, Y. Nishio, C. Zhao, C. Wu, Y. Jiang, A. Zhang, J. Li, W. Wang, F. Salimi-Jazi, T. A. Rafeeqi, N. M. Hemed, J. B. Tok, X. Chen, J. A. Kaltschmidt, J. C. Y. Dunn and Z. Bao, bioRxiv, 2023, preprint, DOI: 10.1101/2023.10.02.560482.
- A. Canales, X. Jia, U. P. Froriep, R. A. Koppes, C. M. Tringides, J. Selvidge, C. Lu, C. Hou, L. Wei, Y. Fink and P. Anikeeva, Nat. Biotechnol., 2015, 33, 277–284 CrossRef CAS PubMed.
- H. C. Tian, J. Q. Liu, X. Y. Kang, L. J. Tang, M. H. Wang, B. W. Ji, B. Yang, X. L. Wang, X. Chen and C. S. Yang, Sci. Rep., 2016, 6, 26910 CrossRef CAS PubMed.
- Y. T. Tang, Y. L. Xu, J. Z. Yang, Y. Y. Song, F. X. Yin and W. J. Yuan, Sens. Actuators, B, 2021, 346, 130500 CrossRef CAS.
- Y. Zhao, Q. Zhai, D. Dong, T. An, S. Gong, Q. Shi and W. Cheng, Anal. Chem., 2019, 91, 6569–6576 CrossRef CAS PubMed.
- H. J. Sim, C. Choi, C. J. Lee, Y. T. Kim, G. M. Spinks, M. D. Lima, R. H. Baughman and S. J. Kim, Adv. Eng. Mater., 2015, 17, 1270–1275 CrossRef CAS.
- Q. Li, D. Li, J. Lu, K. Zou, L. Wang, Y. Jiao, M. Wang, R. Gao, J. Song, Y. Li, F. Li, J. Ji, J. Wang, L. Li, T. Ye, E. He, H. Chen, Y. Wang, J. Ren, C. Bai, S. Yang and Y. Zhang, Adv. Mater., 2024, 36, e2307726 CrossRef PubMed.
- Y. Jang, S. M. Kim, E. Kim, D. Y. Lee, T. M. Kang and S. J. Kim, Microsyst. Nanoeng., 2021, 7, 70 CrossRef CAS PubMed.
- Z. Sun, Y. Jin, J. Luo, L. Li, Y. Ding, Y. Luo, Y. Qi, Y. Li, Q. Zhang, K. Li, H. Shi, S. Yin, H. Wang, H. Wang and C. Hou, Nat. Commun., 2024, 15, 8462 Search PubMed.
- X. Wu, J. Feng, J. Deng, Z. Cui, L. Wang, S. Xie, C. Chen, C. Tang, Z. Han, H. Yu, X. Sun and H. Peng, Sci. China:Chem., 2020, 63, 1281–1288 CrossRef CAS.
- J. Wang, L. Wang, J. Feng, C. Tang, X. Sun and H. Peng, Adv. Fiber Mater., 2021, 3, 47–58 Search PubMed.
- Y. Teng, J. Li, J. Yao, L. Kang and Q. Li, Microstructures, 2023, 3, 2023019 CAS.
- L. Bi, R. Garg, N. Noriega, R. J. Wang, H. Kim, K. Vorotilo, J. C. Burrell, C. E. Shuck, F. Vitale, B. A. Patel and Y. Gogotsi, ACS Nano, 2024, 18, 23217–23231 CrossRef CAS.
- X. Fu, G. Li, Y. Niu, J. Xu, P. Wang, Z. Zhou, Z. Ye, X. Liu, Z. Xu, Z. Yang, Y. Zhang, T. Lei, B. Zhang, Q. Li, A. Cao, T. Jiang and X. Duan, Front. Neurosci., 2021, 15, 771980 CrossRef PubMed.
- Y. Lee, C. Kong, J. W. Chang and S. B. Jun, J. Korean Med. Sci., 2019, 34, e24 Search PubMed.
- K. Wang, C. L. Frewin, D. Esrafilzadeh, C. Yu, C. Wang, J. J. Pancrazio, M. Romero-Ortega, R. Jalili and G. Wallace, Adv. Mater., 2019, 31, e1805867 CrossRef PubMed.
- S. He, Y. Hu, J. Wan, Q. Gao, Y. Wang, S. Xie, L. Qiu, C. Wang, G. Zheng, B. Wang and H. Peng, Carbon, 2017, 122, 162–167 CrossRef CAS.
- J. Kim, C. Yang, T. Yun, S. Woo, H. Kim, M. Lee, M. Jeong, H. Ryu, N. Kim, S. Park and J. Lee, Adv. Sci., 2023, 10, e2206186 CrossRef PubMed.
- C. Zhang, W. Ouyang, L. Zhang and D. Li, Microsyst. Nanoeng., 2023, 9, 158 Search PubMed.
- M. Costantini, S. Testa, P. Mozetic, A. Barbetta, C. Fuoco, E. Fornetti, F. Tamiro, S. Bernardini, J. Jaroszewicz, W. Swieszkowski, M. Trombetta, L. Castagnoli, D. Seliktar, P. Garstecki, G. Cesareni, S. Cannata, A. Rainer and C. Gargioli, Biomaterials, 2017, 131, 98–110 CrossRef CAS PubMed.
- G. Guitchounts and D. Cox, Sci. Rep., 2020, 10, 3830 CrossRef CAS PubMed.
- C. Zhang, W. Ouyang, L. Zhang and D. Li, Microsyst. Nanoeng., 2023, 9, 158 Search PubMed.
- M. Du, L. Huang, J. Zheng, Y. Xi, Y. Dai, W. Zhang, W. Yan, G. Tao, J. Qiu, K. F. So, C. Ren and S. Zhou, Adv. Sci., 2020, 7, 2001410 CrossRef CAS PubMed.
- J. Kim, C. Yang, T. Yun, S. Woo, H. Kim, M. Lee, M. Jeong, H. Ryu, N. Kim, S. Park and J. Lee, Adv. Sci., 2023, 10, e2206186 CrossRef PubMed.
- L. Y. Wang, J. W. Chen, J. J. Wang, H. J. Li, C. R. Chen, J. Y. Feng, Y. Guo, H. B. Yu, X. M. Sun and H. S. Peng, Sci. China:Chem., 2021, 64, 1763–1769 CAS.
- P. R. Patel, K. Na, H. Zhang, T. D. Kozai, N. A. Kotov, E. Yoon and C. A. Chestek, J. Neural. Eng., 2015, 12, 046009 Search PubMed.
- R. Wang, Q. Zhai, T. An, S. Gong and W. Cheng, Talanta, 2021, 222, 121484 CAS.
- V. Kalidasan, X. Yang, Z. Xiong, R. R. Li, H. Yao, H. Godaba, S. Obuobi, P. Singh, X. Guan, X. Tian, S. A. Kurt, Z. Li, D. Mukherjee, R. Rajarethinam, C. S. Chong, J. W. Wang, P. L. R. Ee, W. Loke, B. C. K. Tee, J. Ouyang, C. J. Charles and J. S. Ho, Nat. Biomed. Eng., 2021, 5, 1217–1227 Search PubMed.
- C. Won, U. J. Jeong, S. Lee, M. Lee, C. Kwon, S. Cho, K. Yoon, S. Lee, D. Chun, I. J. Cho and T. Lee, Adv. Funct. Mater., 2022, 32, 2205145 CAS.
- X. Yang, T. Zhou, T. J. Zwang, G. Hong, Y. Zhao, R. D. Viveros, T. M. Fu, T. Gao and C. M. Lieber, Nat. Mater., 2019, 18, 510–517 CAS.
- S. F. Hu, J. Y. Song, Q. Tian, C. Zeng, Y. C. Jiang, Q. H. Li, J. Xu, W. Yan, J. Li, Z. Y. Liu, W. Q. Kong and M. F. Zhu, Adv. Fiber Mater., 2024, 6, 1980–1991 Search PubMed.
- L. Karumbaiah, T. Saxena, D. Carlson, K. Patil, R. Patkar, E. A. Gaupp, M. Betancur, G. B. Stanley, L. Carin and R. V. Bellamkonda, Biomaterials, 2013, 34, 8061–8074 CAS.
- X. Wu and H. Peng, Sci. Bull., 2019, 64, 634–640 CAS.
- C. Tang, S. Xie, M. Wang, J. Feng, Z. Han, X. Wu, L. Wang, C. Chen, J. Wang, L. Jiang, P. Chen, X. Sun and H. Peng, J. Mater. Chem. B, 2020, 8, 4387–4394 Search PubMed.
- Y. Cho, S. Park, J. Lee and K. J. Yu, Adv. Mater., 2021, 33, 2005786 Search PubMed.
- C. Tang, Z. Han, Z. Liu, W. Li, J. Shen, K. Zhang, S. Mai, J. Li, X. Sun, X. Chen, H. Li, L. Wang, J. Liang, M. Liao, J. Feng, C. Wang, J. Wang, L. Ye, Y. Yang, S. Xie, X. Shi, K. Zeng, X. Zhang, X. Cheng, K. Zhang, Y. Guo, H. Yang, Y. Xu, Q. Tong, H. Yu, P. Chen, H. Peng and X. Sun, Adv. Mater., 2024, 36, e2407874 CrossRef PubMed.
- X. Xu, S. Xie, Y. Zhang and H. Peng, Angew. Chem., Int. Ed., 2019, 58, 13643–13653 CrossRef CAS PubMed.
- C. Lu, U. P. Froriep, R. A. Koppes, A. Canales, V. Caggiano, J. Selvidge, E. Bizzi and P. Anikeeva, Adv. Funct. Mater., 2014, 24, 6594–6600 CrossRef CAS.
- B. Spagnolo, A. Balena, R. T. Peixoto, M. Pisanello, L. Sileo, M. Bianco, A. Rizzo, F. Pisano, A. Qualtieri, D. D. Lofrumento, F. De Nuccio, J. A. Assad, B. L. Sabatini, M. De Vittorio and F. Pisanello, Nat. Mater., 2022, 21, 826–835 CrossRef CAS PubMed.
- S. Park, H. Yuk, R. Zhao, Y. S. Yim, E. W. Woldeghebriel, J. Kang, A. Canales, Y. Fink, G. B. Choi, X. Zhao and P. Anikeeva, Nat. Commun., 2021, 12, 3435 CAS.
- A. F. Khan, Q. Adewale, S. J. Lin, T. R. Baumeister, Y. Zeighami, F. Carbonell, N. Palomero-Gallagher and Y. Iturria-Medina, Nat. Commun., 2023, 14, 6009 CAS.
- E. Akyuz, A. K. Polat, E. Eroglu, I. Kullu, E. Angelopoulou and Y. N. Paudel, Life Sci., 2021, 265, 118826 Search PubMed.
- B. Zhu, X. Li, L. Zhu, M. Qi, J. Cao, L. Zhou and B. Su, ACS Sens., 2023, 8, 4064–4070 CAS.
- L. Yung, T. T. Zhao and J. S. Yuan, Physiology, 2023, 38, 5733959 Search PubMed.
- E. H. J. Verschuren, C. Castenmiller, D. J. M. Peters, F. J. Arjona, R. J. M. Bindels and J. G. J. Hoenderop, Nat. Rev. Nephrol., 2020, 16, 337–351 CAS.
- C. D. Flynn, D. Chang, A. Mahmud, H. Yousefi, J. Das, K. T. Riordan, E. H. Sargent and S. O. Kelley, Nat. Rev. Bioeng., 2023, 1, 1–16 Search PubMed.
- Y. Zhao, K. Q. Jin, J. D. Li, K. K. Sheng, W. H. Huang and Y. L. Liu, Adv. Mater., 2023, e2305917 Search PubMed.
- N. Wongkaew, M. Simsek, C. Griesche and A. J. Baeumner, Chem. Rev., 2019, 119, 120–194 CAS.
- L. Wang, S. Xie, Z. Wang, F. Liu, Y. Yang, C. Tang, X. Wu, P. Liu, Y. Li, H. Saiyin, S. Zheng, X. Sun, F. Xu, H. Yu and H. Peng, Nat. Biomed. Eng., 2020, 4, 159–171 CAS.
- R. Gui, H. Jin, H. Guo and Z. Wang, Biosens. Bioelectron., 2018, 100, 56–70 CAS.
- A. Yang, Y. Li, C. Yang, Y. Fu, N. Wang, L. Li and F. Yan, Adv. Mater., 2018, 30, e1800051 Search PubMed.
- V. Chaudhary, B. A. Taha, Lucky, S. Rustagi, A. Khosla, P. Papakonstantinou and N. Bhalla, ACS Sens., 2024, 9, 4469–4494 CAS.
- V. Chaudhary, V. Khanna, H. T. Ahmed Awan, K. Singh, M. Khalid, Y. K. Mishra, S. Bhansali, C.-Z. Li and A. Kaushik, Biosens. Bioelectron., 2023, 220, 114847 CAS.
- L. P. Lin and M. T. T. Tan, Biosens. Bioelectron., 2023, 237, 115492 CAS.
- V. Chaudhary, A. Kaushik, H. Furukawa and A. Khosla, ECS Sens. Plus, 2022, 1, 013601 CAS.
- V. Chaudhary, P. Gaur and S. Rustagi, Sustainable Mater. Technol., 2024, 40, e00952 Search PubMed.
- S. Das, H. Mazumdar, K. R. Khondakar, Y. K. Mishra and A. Kaushik, ECS Sens. Plus, 2024, 3, 025001 CAS.
- H. Mazumdar, K. R. Khondakar, S. Das and A. Kaushik, Front. Nanotechnol., 2024, 6, 1434014 Search PubMed.
- N. S. Shrikrishna, R. Sharma, J. Sahoo, A. Kaushik and S. Gandhi, Chem. Eng. J., 2024, 490, 151661 Search PubMed.
- C. M. Leung, P. de Haan, K. Ronaldson-Bouchard, G.-A. Kim, J. Ko, H. S. Rho, Z. Chen, P. Habibovic, N. L. Jeon, S. Takayama, M. L. Shuler, G. Vunjak-Novakovic, O. Frey, E. Verpoorte and Y.-C. Toh, Nat. Rev. Methods Primers, 2022, 2, 33 Search PubMed.
- N. L. Lukman Hekiem, A. A. Md Ralib, M. A. B. Mat Hattar, F. B. Ahmad, A. N. Nordin, R. A. Rahim and N. F. Za'bah, Sens. Actuators, A, 2021, 329, 112792 CAS.
- S. Nag, L. Duarte, E. Bertrand, V. Celton, M. Castro, V. Choudhary, P. Guegan and J.-F. Feller, J. Mater. Chem. B, 2014, 2, 6571–6579 CAS.
- A. Ahad and M. Tahir, ECS Sens. Plus, 2023, 2, 011601 CrossRef.
- S. Chatterjee, M. Castro and J. F. Feller, J. Mater. Chem. B, 2013, 1, 4563–4575 RSC.
- M. Lee, Y. Lee, J. H. Choi, H. Kim, D. Jeong, K. Park, J. Kim, J. Park, W. Y. Jang, J. Seo and J. Lee, ACS Nano, 2024, 18, 12210–12224 CrossRef CAS PubMed.
- G. Q. Wu, Z. P. Yang, Z. Y. Zhang, B. Y. Ji, C. Y. Hou, Y. G. Li, W. Jia, Q. H. Zhang and H. Z. Wang, Electrochim. Acta, 2021, 395, 139141 Search PubMed.
- L. Liu, R. Li, F. Liu, L. Huang, W. Liu, J. Wang, Z. Wu, N. Reddy, W. Cui and Q. Jiang, ACS Nano, 2023, 17, 9600–9610 CAS.
- J. Z. Zhang, B. A. Xu, K. L. Chen, Y. Li, G. Li and Z. K. Liu, SusMat, 2024, 4, e207 CAS.
- D. Y. Khang, H. Jiang, Y. Huang and J. A. Rogers, Science, 2006, 311, 208–212 CAS.
- L. Li, H. Xiang, Y. Xiong, H. Zhao, Y. Bai, S. Wang, F. Sun, M. Hao, L. Liu, T. Li, Z. Peng, J. Xu and T. Zhang, Adv. Sci., 2018, 5, 1800558 Search PubMed.
- Y. Wu, T. Yan, K. Zhang and Z. Pan, Adv. Mater. Technol., 2022, 8, 2200777 Search PubMed.
- J. Lee, S. J. Ihle, G. S. Pellegrino, H. Kim, J. Yea, C. Y. Jeon, H. C. Son, C. Jin, D. Eberli, F. Schmid, B. L. Zambrano, A. F. Renz, C. Forró, H. Choi, K. I. Jang, R. Kung and J. Vörös, Nat. Electron., 2021, 4, 291–301 CAS.
- F. Sheng, B. Zhang, Y. Zhang, Y. Li, R. Cheng, C. Wei, C. Ning, K. Dong and Z. L. Wang, ACS Nano, 2022, 16, 10958–10967 Search PubMed.
- J. Deng, J. Wu, X. Chen, T. L. Sarrafian, C. E. Varela, W. Whyte, C. F. Guo, E. T. Roche, L. G. Griffiths, H. Yuk, C. S. Nabzdyk and X. Zhao, Sci. Transl. Med., 2024, 16, eado9003 CAS.
- E. Kim, S. Kim, Y. W. Kwon, H. Seo, M. Kim, W. G. Chung, W. Park, H. Song, D. H. Lee, J. Lee, S. Lee, I. Jeong, K. Lim and J. U. Park, Interdiscipl. Med., 2023, 1, e20230003 Search PubMed.
- R. Fabbri, A. Scida, E. Saracino, G. Conte, A. Kovtun, A. Candini, D. Kirdajova, D. Spennato, V. Marchetti, C. Lazzarini, A. Konstantoulaki, P. Dambruoso, M. Caprini, M. Muccini, M. Ursino, M. Anderova, E. Treossi, R. Zamboni, V. Palermo and V. Benfenati, Nat. Nanotechnol., 2024, 19, 1344–1353 CAS.
- J. C. Hsieh, H. Alawieh, Y. Li, F. Iwane, L. Zhao, R. Anderson, S. I. Abdullah, K. W. Kevin Tang, W. Wang, I. Pyatnitskiy, Y. Jia, J. D. R. Millan and H. Wang, Biosens. Bioelectron., 2022, 218, 114756 CAS.
- A. Sahasrabudhe, L. E. Rupprecht, S. Orguc, T. Khudiyev, T. Tanaka, J. Sands, W. Zhu, A. Tabet, M. Manthey, H. Allen, G. Loke, M. J. Antonini, D. Rosenfeld, J. Park, I. C. Garwood, W. Yan, F. Niroui, Y. Fink, A. Chandrakasan, D. V. Bohorquez and P. Anikeeva, Nat. Biotechnol., 2024, 42, 892–904 CAS.
- Y. Zhou, C. Gu, J. Liang, B. Zhang, H. Yang, Z. Zhou, M. Li, L. Sun, T. H. Tao and X. Wei, Microsyst. Nanoeng., 2022, 8, 118 CAS.
- Y. Li, L. Wei, L. Lan, Y. Gao, Q. Zhang, H. Dawit, J. Mao, L. Guo, L. Shen and L. Wang, Acta Biomater., 2022, 139, 157–178 CAS.
- E. A. Kiyotake, M. D. Martin and M. S. Detamore, Acta Biomater., 2022, 139, 43–64 CAS.
- R. Dong, P. X. Ma and B. Guo, Biomaterials, 2020, 229, 119584 CAS.
- G. X. Zhao, H. W. Zhou, G. R. Jin, B. R. Jin, S. M. Geng, Z. T. Luo, Z. G. Ge and F. Xu, Prog. Polym. Sci., 2022, 131, 101573 Search PubMed.
- H. Wu, Y. Q. Wang, H. Li, Y. Y. Hu, Y. D. Liu, X. R. Jiang, H. Sun, F. Liu, A. Xiao, T. R. Chang, L. Lin, K. Yang, Z. Y. Wang, Z. Z. Dong, Y. H. Li, S. T. Dong, S. Q. Wang, J. Chen, Y. L. Liu, D. D. Yin, H. D. Zhang, M. Liu, S. S. Kong, Z. Q. Yang, X. E. Yu, Y. Wang, Y. B. Fan, L. Wang, C. J. Yu and L. Q. Chang, Nat. Electron., 2024, 7, 299–312 CAS.
- R. Luo, J. Dai, J. Zhang and Z. Li, Adv. Healthcare Mater., 2021, 10, e2100557 Search PubMed.
- G. Liu, Z. Lv, S. Batool, M. Z. Li, P. Zhao, L. Guo, Y. Wang, Y. Zhou and S. T. Han, Small, 2023, 19, e2207879 Search PubMed.
- S. B. Kim, C. H. Kim, S. Y. Lee and S. J. Park, Nanoscale, 2024, 16, 16313–16328 RSC.
- Y. Zhao, C. R. Chen, Y. Y. Qiu, T. L. Mei, L. Ye, H. Feng, Y. Zhang, L. Y. Wang, Y. Guo, X. M. Sun, J. X. Wu and H. S. Peng, Adv. Fiber Mater., 2022, 4, 246–255 CrossRef CAS.
- R. K. Talreja, H. Sable, V. Chaudhary, S. Kadian, M. Singh, M. Kumar, J. Kishore, V. Chaudhary and A. Khosla, ECS Sens. Plus, 2024, 3, 041602 CrossRef CAS.
- Q. Yang, T. Wei, R. T. Yin, M. Wu, Y. Xu, J. Koo, Y. S. Choi, Z. Xie, S. W. Chen, I. Kandela, S. Yao, Y. Deng, R. Avila, T. L. Liu, W. Bai, Y. Yang, M. Han, Q. Zhang, C. R. Haney, B. Lee, K. Aras, T. Wang, M. H. Seo, H. Luan, S. M. Lee, A. Brikha, N. Ghoreishi-Haack, L. Tran, I. Stepien, F. Aird, E. A. Waters, X. Yu, A. Banks, G. D. Trachiotis, J. M. Torkelson, Y. Huang, Y. Kozorovitskiy, I. R. Efimov and J. A. Rogers, Nat. Mater., 2021, 20, 1559–1570 CrossRef CAS PubMed.
- H. Yuk, J. Wu and X. Zhao, Nat. Rev. Mater., 2022, 7, 935–952 CrossRef CAS.
- S. Yu, C. Tang, S. Yu, W. Li, J. Wang, Z. Liu, X. Yan, L. Wang, Y. Yang, J. Feng, J. Wu, K. Zhang, H. Guan, Y. Liu, S. Zhang, X. Sun and H. Peng, Adv. Healthcare Mater., 2024, 13, e2400675 CrossRef PubMed.
- V. Chaudhary, J. Electrochem. Soc., 2025, 172, 017501 Search PubMed.
- P. K. Kalambate, Z. Rao, Dhanjai, J. Wu, Y. Shen, R. Boddula and Y. Huang, Biosens. Bioelectron., 2020, 163, 112270 CrossRef CAS PubMed.
- B. Piro, H. V. Tran and V. T. Thu, Sensors, 2020, 20, 5898 CrossRef CAS PubMed.
- B. A. Prabowo and A. Purwidyantri, IEEE Nanotechnol. Mag., 2022, 16, 13–19 Search PubMed.
- H. Hamidi, J. Levieux, C. Larrigy, A. Russo, E. Vaughan, R. Murray, A. J. Quinn and D. Iacopino, Biosens. Bioelectron.:X, 2023, 15, 100403 CAS.
- J. Min, Y. Jung, J. Ahn, J. G. Lee, J. Lee and S. H. Ko, Adv. Mater., 2023, 35, 2211273 CrossRef CAS PubMed.
- S. C. Teixeira, N. O. Gomes, T. V. D. Oliveira, P. Fortes-Da-Silva, N. D. F. F. Soares and P. A. Raymundo-Pereira, Biosens. Bioelectron.:X, 2023, 14, 100371 CAS.
- F. Alam, M. Ashfaq Ahmed, A. Jalal, I. Siddiquee, R. Adury, G. Hossain and N. Pala, Micromachines, 2024, 15, 475 CrossRef PubMed.
- C. Pechyen, B. Tangnorawich, S. Toommee, R. Marks and Y. Parcharoen, Sens. Int., 2024, 5, 100287 CrossRef.
- R. K. Pal, A. A. Farghaly, C. Wang, M. M. Collinson, S. C. Kundu and V. K. Yadavalli, Biosens. Bioelectron., 2016, 81, 294–302 CrossRef CAS PubMed.
- S. Guan, J. Wang, X. Gu, Y. Zhao, R. Hou, H. Fan, L. Zou, L. Gao, M. Du, C. Li and Y. Fang, Sci. Adv., 2019, 5, eaav2842 CrossRef CAS PubMed.
- S. Jiang, D. C. Patel, J. Kim, S. Yang, W. A. Mills 3rd, Y. Zhang, K. Wang, Z. Feng, S. Vijayan, W. Cai, A. Wang, Y. Guo, I. F. Kimbrough, H. Sontheimer and X. Jia, Nat. Commun., 2020, 11, 6115 CrossRef CAS PubMed.
- A. Obaid, M. E. Hanna, Y. W. Wu, M. Kollo, R. Racz, M. R. Angle, J. Muller, N. Brackbill, W. Wray, F. Franke, E. J. Chichilnisky, A. Hierlemann, J. B. Ding, A. T. Schaefer and N. A. Melosh, Sci. Adv., 2020, 6, eaay2789 CrossRef CAS PubMed.
- J. J. Jun, N. A. Steinmetz, J. H. Siegle, D. J. Denman, M. Bauza, B. Barbarits, A. K. Lee, C. A. Anastassiou, A. Andrei, C. Aydin, M. Barbic, T. J. Blanche, V. Bonin, J. Couto, B. Dutta, S. L. Gratiy, D. A. Gutnisky, M. Hausser, B. Karsh, P. Ledochowitsch, C. M. Lopez, C. Mitelut, S. Musa, M. Okun, M. Pachitariu, J. Putzeys, P. D. Rich, C. Rossant, W. L. Sun, K. Svoboda, M. Carandini, K. D. Harris, C. Koch, J. O'Keefe and T. D. Harris, Nature, 2017, 551, 232–236 CrossRef CAS PubMed.
- L. Lu, X. Fu, Y. Liew, Y. Zhang, S. Zhao, Z. Xu, J. Zhao, D. Li, Q. Li, G. B. Stanley and X. Duan, Nano Lett., 2019, 19, 1577–1586 CrossRef CAS PubMed.
- J. Macron, A. P. Gerratt and S. P. Lacour, Adv. Mater. Technol., 2019, 4, 1900331 CrossRef CAS.
- L. Wang, E. He, R. Gao, X. Wu, A. Zhou, J. Lu, T. Zhao, J. Li, Y. Yun, L. Li, T. Ye, Y. Jiao, J. Wang, H. Chen, D. Li, X. Ning, D. Wu, H. Peng and Y. Zhang, Adv. Funct. Mater., 2021, 31, 2107160 Search PubMed.
- B. Yan, Y. Zhao and H. Peng, Small Methods, 2023, 7, e2300501 Search PubMed.
- Z. Qian, Y. Yang, L. Wang, J. Wang, Y. Guo, Z. Liu, J. Li, H. Zhang, X. Sun and H. Peng, Angew. Chem., Int. Ed., 2023, 62, e202303268 CrossRef CAS PubMed.
- S. S. He, A. N. Zhang, D. Z. Wang, H. Y. Song, H. W. Chu, F. L. Ni, Y. Y. Zhang, P. N. Chen, B. Zhang, L. B. Qiu and H. S. Peng, Chem. Eng. J., 2022, 441, 136106 CrossRef CAS.
- S. Jiang, T. Zhang, Y. Zhou, P. Lai and Y. Huang, Innovation, 2023, 4, 100447 Search PubMed.
- C. Chen, J. Feng, J. Li, Y. Guo, X. Shi and H. Peng, Chem. Rev., 2023, 123, 613–662 CrossRef CAS PubMed.
- S. C. Dhanabalan, B. Dhanabalan, X. Chen, J. S. Ponraj and H. Zhang, Nanoscale, 2019, 11, 3046–3101 RSC.
- S. Wang, Q. C. Xu and H. Sun, Adv. Fiber Mater., 2022, 4, 324–341 CrossRef CAS.
- G. Shan, X. Li and W. Huang, Innovation, 2020, 1, 100031 Search PubMed.
- F. Wu, P. Yu and L. Mao, Innov. Mater., 2023, 1, 100007 CrossRef.
- R. Jalili, A. Kanneganti, M. I. Romero-Ortega and G. G. Wallace, Curr. Opin. Electrochem., 2017, 3, 68–74 CrossRef CAS.
- J. Sun, R. Yang, Q. Li, R. Zhu, Y. Jiang, L. Zang, Z. Zhang, W. Tong, H. Zhao, T. Li, H. Li, D. Qi, G. Li, X. Chen, Z. Dai and Z. Liu, Adv. Mater., 2024, 36, e2400110 CrossRef PubMed.
- S. Yin, D. R. Yao, Y. Song, W. Heng, X. Ma, H. Han and W. Gao, Chem. Rev., 2024, 124, 11585–11636 CrossRef CAS PubMed.
- J. L. Jiang, L. D. Yang and L. Zhang, IEEE/ASME Trans. Mechatron., 2024 DOI:10.1109/Tmech.2024.3449393.
- Y. L. Zhang, J. C. Li, H. Zhou, Y. Q. Liu, D. D. Han and H. B. Sun, The Innovation, 2021, 2, 100168 CrossRef PubMed.
- X. Han, X. Lin, Y. Sun, L. Huang, F. Huo and R. Xie, ACS Appl. Mater. Interfaces, 2024, 16, 54976–55010 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |







