Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Expanded polystyrene is not chemically degraded by mealworms†
Zahra Mohammadizadeh
Tahroudi
a,
Gavin
Flematti
 a,
Jitendra
Joshi
b,
Georg
Fritz
*a and
Rob
Atkin
a,
Jitendra
Joshi
b,
Georg
Fritz
*a and
Rob
Atkin
 *a
*a
aSchool of Molecular Sciences, The University of Western Australia, Perth, WA 6009, Australia. E-mail: zahra.mohammadizadehtahroudi@research.uwa.edu.au; gavin.flematti@uwa.edu.au; georg.fritz@uwa.edu.au; rob.atkin@uwa.edu.au
bWoodside Energy, Perth, WA 6000, Australia. E-mail: jitendra.joshi@woodside.com
First published on 19th November 2024
Abstract
Expanded polystyrene (EPS) is a widely used plastic material that poses significant environmental challenges due to its resistance to degradation. While mealworms have been reported to degrade EPS, several critical questions remain unanswered: (1) Do mealworms actually chemically degrade the polystyrene backbone in EPS? (2) Can mealworms effectively derive nutrition from EPS consumption? and (3) What mechanisms, if any, enable EPS degradation by mealworms? This study addresses these questions by feeding mealworms two types of EPS diets: pure EPS without additives and commercial EPS containing additives. Mealworms were individually housed (to prevent cannibalism) and categorized into age-specific groups, and their growth and survival were monitored on diets of pure EPS, commercial EPS, or under starvation conditions. Our results demonstrated that, compared to starvation, both pure and commercial EPS diets failed to sustain mealworm growth, and survival rates decreased, indicating that EPS consumption is toxic to mealworms. Gel permeation chromatography and attenuated total reflectance-Fourier transform infrared spectroscopy analyses of the frass revealed partial degradation of commercial EPS, characterized by a reduction in higher molecular weight fractions and increased carbonyl group formation. Additives likely caused EPS degradation. In contrast, pure EPS was essentially unaffected by passage through the mealworm digestive tract, providing clear chemical evidence that neither mealworms nor their gut microbiota possess enzymes capable of breaking down EPS for energy. These findings reveal that previous studies overstated the ability of mealworms to digest and derive energy from EPS, while providing new insights into the chemical processes involved in limited EPS degradation. Our results emphasize the need for further research into alternative organisms, pretreatment methods, and integrated waste management strategies that can more effectively address the challenge of EPS degradation.
Sustainability spotlightExpanded polystyrene (EPS) pollution poses significant environmental challenges due to its chemical stability. This study critically investigates the potential of mealworms for EPS biodegradation, revealing their inability to break down the carbon–carbon backbone of the polymer. Our findings show that additives in commercial EPS facilitate minimal oxidative degradation, evidenced by carbonyl group formation and reduced molecular weight, but at the cost of mealworm lifespan. This work advances sustainability by elucidating the chemical limitations of mealworm-mediated EPS degradation and emphasizing the need for more effective strategies. It aligns with UN Sustainable Development Goals 12 (Responsible Consumption and Production) and 14 (Life Below Water) by addressing plastic waste reduction and tackling a major source of marine pollution. This research highlights the importance of rigorous chemical analysis in developing sustainable solutions for plastic waste management. |
1. Introduction
Plastic pollution, particularly from synthetic polymers like expanded polystyrene (EPS), presents a significant challenge to global chemical sustainability efforts.1 EPS, a widely used thermoplastic material composed of styrene monomers polymerized into long hydrocarbon chains, exhibits remarkable chemical stability due to its molecular structure. This stability, while beneficial for applications in packaging and insulation, poses substantial obstacles for sustainable waste management and recycling processes, often leading to its disposal in landfills.2,3 The chemical inertness of EPS, attributed to its hydrophobic nature and strong carbon–carbon bonds, results in its persistence in various ecosystems. Furthermore, the incorporation of chemical additives in EPS formulations, such as antioxidants and flame retardants, introduces additional complexities to its environmental impact and potential degradation pathways. These additives, often halogenated organic compounds or phosphorus-based substances, can migrate from the polymer matrix and bioaccumulate in living organisms, raising concerns about their long-term effects on environmental and human health.4,5 While chemical degradation mechanisms for EPS exist, their limited scalability has led to exploration of alternative approaches, including biological degradation processes, which may offer insights into overcoming the chemical stability of EPS and its additives.To address the chemical stability of EPS, researchers have explored various degradation methods, primarily categorized into oxidative and thermal approaches.6,7 Oxidative degradation typically involves C–C bond scission in the polymer backbone through thermal, photo-, or catalytic oxidation. The process generally begins with the formation of free radicals via heat, light, or a catalyst, followed by reactions with oxygen to generate peroxy radicals, resulting in carbonyl group formation and chain scission.8 For instance, thermal oxidation at 200–400 °C produces a mixture of aromatic compounds including styrene monomers, benzaldehyde, and benzoic acid.9 However, this method faces challenges such as high energy consumption and the production of complex, potentially harmful byproducts.8 Thermal degradation, occurring at temperatures up to 420 °C for about 2 hours, involves random chain scission through radical formation, yielding mainly styrene monomer (60–80%) along with other aromatics.10 To reduce energy demands, researchers have investigated thermo-catalytic processes using solid catalysts such as zeolites,11 fluid catalytic cracking (FCC) catalysts,12 and metal oxides.13 These catalysts aim to lower the activation energy for C–C bond cleavage, often through electron transfer or acid–base interactions. While catalytic methods can operate at lower temperatures (up to 240 °C for metal oxide catalysts), they face challenges such as coke formation on catalysts and the potential for excessive cracking to light hydrocarbons.11,12 The high temperatures, complex product mixtures, and potential for harmful byproduct formation in these methods have limited their large-scale application.8 These limitations have led researchers to explore alternative approaches, including the use of catalysts and solvents,14 as well as biological degradation methods, which offer potentially more environmentally friendly solutions to the global EPS waste problem.
One promising solvent class for polymer degradation is the ionic liquids (ILs). ILs can function as both solvents and catalysts, enabling efficient depolymerization under mild conditions. They typically achieve high conversion rates and yields, often exceeding 90%, for various polymers including polyethylene terephthalate (PET), polycarbonate (PC), polyamide (PA), polylactic acid (PLA), poly(3-hydroxybutyrate) (PHB), polyethylene (PE), and epoxy resins.14 The mechanism generally involves IL cations and anions interacting with polymer chains and reactants, activating the polymer and facilitating nucleophilic attacks to break chemical bonds.14 ILs exhibit high thermal stability and can be easily separated and reused multiple times, enhancing their economic and environmental appeal.14 However, despite their promise for other polymers, ionic liquids have not yet demonstrated efficacy in PS degradation.
Despite the potential of chemical methods for EPS degradation, all these approaches face challenges in scaling up due to high costs, energy use, and potential environmental impacts. These limitations have led researchers to explore more sustainable options, such as biological breakdown methods utilizing various microorganisms and enzymes, which offer potentially more environmentally friendly and cost-effective approaches to addressing the global PS waste challenge.15
Among these, insect-mediated degradation has gained significant attention. Several insect species, including mealworms (Tenebrio molitor), superworms (Zophobas morio), waxworms (Galleria mellonella), and black soldier fly larvae (Hermetia illucens), have been reported to break down and potentially degrade various plastics, including EPS.16–18
The proposed mechanism for insect-mediated EPS degradation involves both mechanical breakdown and biochemical processes. Insects first mechanically fragment the EPS using their mandibles, increasing the surface area for subsequent degradation. The chemical breakdown is hypothesized to occur in the insect gut, where a combination of digestive enzymes and gut microbiota may act on the polymer chains.15 Some studies have suggested that these processes can lead to depolymerization of EPS, potentially yielding smaller molecular weight fragments or even monomeric units.19 However, the efficacy of this process, particularly for EPS, remains a subject of debate in the scientific community. The literature presents conflicting results regarding the ability of insects, especially mealworms, to survive and thrive on an EPS diet. Some studies report that mealworms can survive and even gain weight when fed EPS,20–24 while others observe significant weight loss and decreased survival rates.25–29
Despite the widespread use of additives to enhance the properties of plastics,30 their effect on insect-mediated EPS degradation remains largely unexplored. Commercial EPS products often contain trace amounts of additives that are difficult to detect due to their low concentrations and material heterogeneity.31 Only one study has investigated the fate of a common EPS additive, hexabromocyclododecane (HBCD), in mealworms,32 but it did not provide essential data on mealworm growth. Another study reported weight loss in mealworms fed with commercial EPS, but did not compare this to additive-free EPS.24 The potential role of these additives in facilitating or hindering EPS degradation by insects represents a significant gap in our understanding of the chemistry of this process.
The present study addresses three critical questions in EPS biodegradation research: (1) Can mealworms chemically break down the polystyrene backbone of EPS? (2) Is EPS a viable nutrient source for mealworm survival and growth? and (3) What role do additives play in EPS degradation by mealworms? To answer these questions, we employ a comprehensive experimental approach that addresses key limitations in previous studies. By individually housing mealworms, we eliminate cannibalism as a confounding factor that has complicated interpretation of past survival and growth data. Our comparison of pure, laboratory-synthesized EPS33 with commercial EPS provides the first controlled investigation of how additives influence both mealworm health and EPS degradation. Through careful monitoring of mealworm growth at different developmental stages and detailed chemical analysis using gel permeation chromatography (GPC) and attenuated total reflectance-Fourier transform infrared (ATR-FTIR), we generate definitive evidence regarding mealworms' capability to derive nutrition from and chemically modify EPS. This rigorous approach allows us to resolve conflicting claims in the literature and provide clear direction for future research into biological solutions for EPS waste management.
2. Materials and methods
2.1. Producing pure EPS
Polystyrene (PS) beads (Fig. S1a†) with an average molecular weight of 280 kDa (which is close to most commercial EPS),34 ethanol, pentane, and benzene were purchased from Sigma-Aldrich. These beads were expanded by a modified Stroe's method.33 This reaction was conducted in a 1 liter polymerization vessel equipped with a reflux condenser, mechanical stirrer, thermometer, and nitrogen and pentane access ports. At first, nitrogen bubbling was carried out to remove oxygen from the system. Then the beads were dissolved in benzene at a temperature of 90 °C for 2 hours. After 2 hours, 4 mL of pentane was added to the reaction mixture as a foaming agent to create expanded PS. The process resumed for 4 more hours at a temperature of 120 °C. After the solution cooled to room temperature, 500 mL of ethanol was added. Polystyrene's limited solubility in ethanol resulted in phase separation of the polymer in the solution. The polymer fragments were then extracted from the flask using forceps. They were then dried at room temperature overnight.The density of pure EPS was determined using a 30 mL pycnometer. To begin, the pycnometer was calibrated to ascertain its exact volume. Pure EPS pieces were subsequently placed inside and weighed. Water was then added slowly until the pycnometer was filled, and its mass was recorded. All measurements were conducted at 23 °C. Given that water has a known density of 0.99753 g cm−3 at this temperature, the volume of the added water was deduced. By subtracting this water volume from the pycnometer's calibrated volume, the volume of the pure EPS was obtained. The density of pure EPS was calculated from the mass and volume of the material.
2.2. Commercial EPS analysis
Commercial expanded polystyrene (EPS) sourced from Amazon packaging was subjected to comprehensive analysis to characterize its additives and molecular properties. The study employed Proton Nuclear Magnetic Resonance (1H NMR) spectroscopy and Gel Permeation Chromatography (GPC) to elucidate the molecular weight and composition of the commercial EPS samples.The sample extracts were dissolved in deuterated chloroform (CDCl3) and analyzed using a 400 MHz NMR spectrometer (Bruker Avance). To ensure reproducibility, samples were analyzed in duplicate, with the average result reported. The error was calculated based on the standard deviation. Quantification of analytes was achieved using the Electronic Reference To Access In vivo Concentrations (ERETIC) method, which provides a synthesized reference signal for determining absolute concentrations. The quality of this reference peak was validated using a solution containing Ph3PO, yielding a recovery of 100%.
The analysis was performed using an Agilent Technologies 1260 infinity II GPC system. Samples were prepared by dissolving commercial EPS in tetrahydrofuran (THF) at a concentration of 1 mg mL−1. Aliquots of 20 μL were injected into the GPC system, which employed THF as the mobile phase. The analysis was performed under isothermal conditions at 40 °C, with a flow rate of 1.0 mL min−1 over a 30 minute runtime. The chromatographic setup comprised a Polargel-M guard column (50 × 7.5 mm) and a Polargel-M main column (300 × 7.5 mm), specifically selected for their ability to separate polymers within the range of 1 to 500 kDa. To ensure statistical significance and reproducibility, the experiment was repeated in triplicate.
2.3. Mealworms cultivation, survival rate, EPS consumption
Mealworms (larvae of T. molitor, Petbarn, Perth, Australia) were continuously reared over three generations from the same batch of adults, as previously described.35 Briefly, the purchased larvae were reared on wheat bran, and the resulting pupae were separated and placed in new containers. After the adults emerged, they were allowed to mate and lay eggs, and the newly hatched larvae were transferred to another container and reared on bran. This cycle was repeated for three generations to ensure a consistent and healthy population of mealworms for the study.A comprehensive multi-group experiment was designed to evaluate the long-term effects of pure and commercial expanded polystyrene (EPS) on mealworm growth and development. The experimental protocol included a control group of 18 individually housed mealworms (initial weight = 24.4 ± 2.5 mg) fed with bran, with weights recorded at 5-day intervals until pupation (as illustrated in Fig. S2†). The experimental design for EPS feeding underwent methodological refinement during the study. Initially, individually housed mealworms (initial weight = 23 ± 2.5 mg) were to be fed both bran and EPS. However, this approach was modified to enhance the assessment of EPS effects at various growth stages. In the revised protocol, all mealworms were initially housed separately and fed bran. Subsequently, at 10 days intervals, nine mealworms of similar weight were selected and divided into three subgroups: commercial EPS (n = 3), pure EPS (n = 3), and starvation (n = 3). This selection process was repeated every 10 days, resulting in the formation of six distinct weight-based groups: Group 1 (T0) at 22.03 ± 0.09 mg, Group 2 (T10) at 38.1 ± 2.1 mg, Group 3 (T20) at 47.3 ± 3.6 mg, Group 4 (T30) at 62.2 ± 1.7 mg, Group 5 (T40) at 71.7 ± 1.6 mg, and Group 6 (T50) at 87.9 ± 1.3 mg. This stratified approach allowed for the evaluation of EPS effects on mealworms at different developmental stages, providing insights into potential age-dependent responses to EPS consumption. Furthermore, by feeding mealworms only EPS during the experimental phase, we were able to isolate and study the specific effects of EPS on mealworms, leading to more meaningful and unambiguous results.
The growth and health of mealworms were assessed by weighing them individually every five days. The average weight of each diet group was calculated. In case of mortality (usually of a weak mealworm), the average weight of the mealworms in that diet group was computed using the last recorded weight of the deceased mealworm. This approach prevented the artificial inflation of the average weight in the remaining group. The survival of mealworms was recorded along with their growth. The Kaplan–Meier survival analysis36 was used to estimate the survival rate. Kaplan–Meier survival curves depict the probability of survival over a given time interval. They are defined by36
| S(t)(%) = (S(t − 1) × (1 − d/N)) × 100, | (1) |
The EPS consumption of both pure and commercial types was determined by weighing the residual EPS cube after each mealworm's death. The average EPS consumption per individual mealworm per day was then calculated by
 | (2) |
2.4. Evaluation of additives and plasticizers on EPS degradation
In a 30-day experiment, 150 mealworms were divided into three equal groups: pure EPS-fed, commercial EPS-fed, and starved. Before EPS exposure, mealworms in the EPS-fed groups underwent a 48-hour starvation period to ensure gut clearance. Mealworms were cleaned of EPS debris every two days with an airstream before being transferred to a clean container for frass collection. After 48 hours, mealworms were returned to EPS containers. For the starvation group, mealworms remained in the same container throughout the experiment, with frass collected at 48-hour intervals. Frass was stored at −80 °C for subsequent analysis37,38 (Fig. S3†).Each collected sample of frass (50 mg) was placed into a 30 mL glass vial, and 10 mL of tetrahydrofuran (THF) was added. After incubation at room temperature for 2 hours the extract was filtered with a 0.22 μm PTFE sterile syringe filter (Sigma-Aldrich) into a clean 30 mL glass vial. THF was completely evaporated using a nitrogen evaporator, yielding a residue (20 mg). The polymer residue was then re-suspended in THF to a final concentration of 1 mg mL−1. Gel permeation chromatography (GPC) was performed to determine the Number-average molecular weight (Mn), Weight-average molecular weight (Mw), and Z-average molecular weight (Mz) of the samples. GPC analysis was performed as described above.
Functional group modifications were analyzed using attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy on an Agilent Cary 630 FTIR instrument. To prepare the samples, residual polymers were dissolved in THF and spread on the window, allowing the solvent to evaporate completely. The spectra were then recorded in the range of 650–4000 cm−1. Each experimental condition was replicated twice.35
3. Results and discussion
3.1. Experimental approach: EPS stability and mealworm growth and survival
This study directly addresses fundamental questions about EPS biodegradation through a systematic experimental approach designed to overcome limitations of previous research. Our investigation was motivated by conflicting reports in the literature regarding mealworms' ability to survive on and degrade EPS, with some studies reporting successful degradation and growth while others observed toxicity and mortality. To resolve these contradictions, we implemented three key methodological innovations: (1) individual housing of mealworms to eliminate cannibalism-related survival artifacts, (2) comparison of pure and commercial EPS to isolate the effects of additives, and (3) controlled introduction of EPS diets at specific developmental stages. This approach, combined with comprehensive chemical analysis using GPC and ATR-FTIR, allows us to definitively evaluate both the biological impact of EPS consumption on mealworms and their capability to chemically modify the polymer. By systematically controlling for confounding factors while gathering detailed growth, survival, and chemical data, we can directly connect our experimental observations to our research questions about EPS biodegradation mechanisms and feasibility.3.2. Properties of pure and commercial EPS
 | ||
| Fig. 1 1H NMR spectrum of extracted materials from commercial expanded polystyrene, compared to the spectrum of pure polystyrene as a reference, showing evidence of additives. | ||
| Component | Concentration (ppm) |
|---|---|
| Monoglycerides | 2909 ± 393 |
| Alkyl amine N-oxides | 116 ± 10 |
| Siloxanes | 1478 ± 949 |
| Aromatics (could include UV additives and some fire retardants) | 386 ± 14 |
3.3. Effect of EPS consumption on growth and survival of mealworms
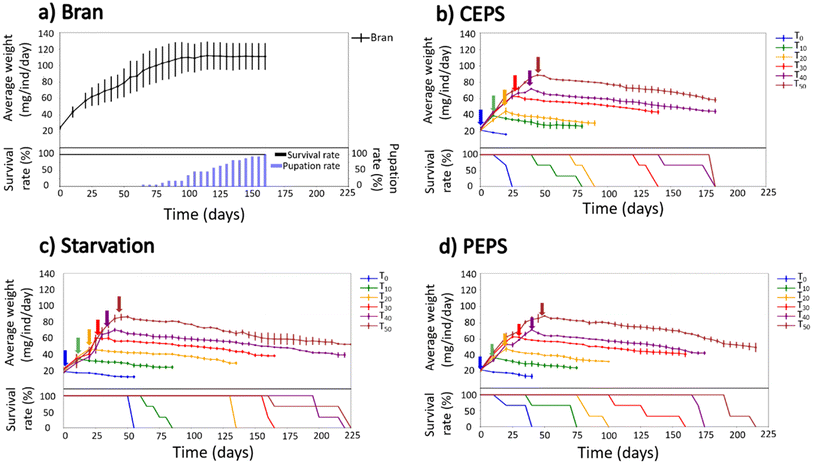
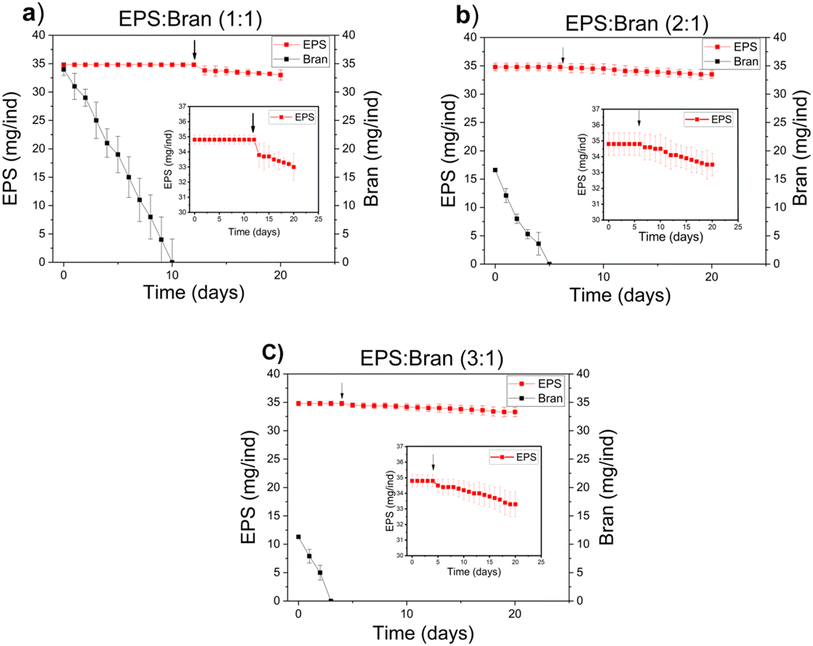
To establish a baseline for mealworm development, we first monitored the growth of individual mealworms on a standard bran diet. Specimens were isolated at approximately 2 months of age, weighing 20 mg, to eliminate potential confounding factors such as cannibalism—a common behavior in coleopteran insects that can skew survival data.39–42 This individual housing approach represents a methodological improvement over previous studies, allowing for a more precise assessment of nutritional impacts on mealworm physiology. The growth curve of bran-fed mealworms exhibited a sigmoidal pattern characteristic of insect development: an initial phase of rapid biomass accumulation lasting about 100 days, followed by a plateau at 110 ± 16 mg (Fig. 2a). This plateau coincided with the onset of pupation at day 65 (corresponding to a mealworm age of ∼125 days), marking the transition from larval to adult stages. Notably, all isolated mealworms successfully completed metamorphosis within 160 days, contrasting with group-reared populations where pupation failures often lead to late-stage weight loss and mortality.43 This improved developmental success rate in isolated conditions suggests that our experimental setup effectively mitigates stress factors associated with group housing, providing a robust framework for subsequent nutrient assimilation studies with EPS.Previous studies have used a mixture of bran and EPS as the food source for mealworms.44 Our investigation into mealworm feeding behavior with mixed diets revealed a clear preference for bran over EPS (Fig. 3). When presented with various ratios of bran to commercial EPS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), mealworms consistently consumed the bran component before engaging with the EPS. This selective feeding pattern complicates the assessment of EPS's nutritional value, as the observed growth could be primarily attributed to bran consumption. To address this confounding factor, we modified our experimental design to isolate the effects of EPS on mealworm development. Mealworms were initially reared on a bran diet, then at predetermined intervals (T0–T50, at 10 day increments), subsets were transitioned to one of three treatments: pure EPS, commercial EPS, or complete food deprivation. This approach allowed us to evaluate the impact of EPS consumption at different developmental stages while controlling for the nutritional history of the specimens. By eliminating the option of alternative nutrient sources, we could more accurately assess the capacity of mealworms to derive sustenance from EPS, a polymer not naturally encountered in their evolutionary history.
1), mealworms consistently consumed the bran component before engaging with the EPS. This selective feeding pattern complicates the assessment of EPS's nutritional value, as the observed growth could be primarily attributed to bran consumption. To address this confounding factor, we modified our experimental design to isolate the effects of EPS on mealworm development. Mealworms were initially reared on a bran diet, then at predetermined intervals (T0–T50, at 10 day increments), subsets were transitioned to one of three treatments: pure EPS, commercial EPS, or complete food deprivation. This approach allowed us to evaluate the impact of EPS consumption at different developmental stages while controlling for the nutritional history of the specimens. By eliminating the option of alternative nutrient sources, we could more accurately assess the capacity of mealworms to derive sustenance from EPS, a polymer not naturally encountered in their evolutionary history.
The results of our EPS feeding experiments revealed a stark contrast to the growth patterns observed in bran-fed mealworms. Fig. 2b–d illustrates the growth curves and survival rates for mealworms switched from bran to pure EPS, commercial EPS, or subjected to starvation at different developmental time points (T0–T50). Remarkably, regardless of the switch time or EPS type, all mealworms exhibited weight loss at rates indistinguishable from those under complete starvation. This consistent pattern of biomass reduction suggests that neither the presence of additives in commercial EPS nor the mealworm's developmental stage significantly influenced their ability to metabolize the polymer. Furthermore, no pupation was observed in any of the EPS-fed or starved groups, indicating a failure to complete the developmental cycle. These findings starkly contrast with previous reports of mealworm survival and even weight gain on EPS diets.20–24 The discrepancy between our results and earlier studies can be attributed to several factors we controlled for in our experimental design. For instance, one study reported that mealworms fed on EPS initially exhibited reduced growth, followed by an increase, and hypothesized that this pattern might be due to cannibalism, but did not provide suitable control experiments.43 Our study, by eliminating the possibility of cannibalism through individual housing, provides a clearer picture of EPS's true impact on mealworm growth and survival. The observed weight loss in our experiments likely reflects the catabolism of the mealworms' own tissues to maintain essential life functions, a process typically observed during starvation.45 These results suggest that EPS, regardless of its composition, cannot serve as a viable carbon or energy source for mealworm metabolism, challenging previous assumptions about the potential use of these insects in EPS biodegradation.
Analysis of survival rates revealed further insights into the effects of EPS consumption on mealworm viability. Interestingly, mealworms in the starvation control groups generally exhibited longer lifespans compared to those fed EPS diets, particularly evident in the T0, T20, and T40 groups (Fig. 2b–d). This unexpected result suggests that EPS consumption may be actively harmful to mealworms, potentially due to the induction of early cell death.45 The impact of commercial and pure EPS diets on mealworm survival varied across developmental groups, with some groups showing earlier mortality on pure EPS (e.g., T30) while others exhibited the opposite trend (e.g., T50). However, these inconsistencies did not reveal a clear pattern that would suggest a systematic influence of additives on mealworm survival.
These results collectively demonstrate that mealworms cannot survive when EPS is the sole carbon source, regardless of the presence of additives or the developmental stage at which the diet is introduced. Our findings challenge the hypothesis that essential elements, such as nitrogen, can be effectively assimilated through proposed nitrogen fixation by gut microbiota in EPS-fed mealworms.46 The inability of mealworms to complete their developmental cycle on EPS diets, coupled with their reduced survival compared to starvation conditions, strongly suggests that EPS cannot serve as a viable nutrient source for these insects. These observations have significant implications for the potential use of mealworms in EPS biodegradation strategies and highlight the need for a re-evaluation of previous claims regarding mealworm-mediated EPS degradation.
3.4. Effect of larval age on commercial and pure EPS consumption
To investigate the influence of developmental stage on EPS consumption, we quantified the intake of both commercial and pure EPS by mealworms at various time points throughout their larval development. Mealworms were fed either commercial or pure EPS starting at different developmental stages, denoted as T0 through T50, corresponding to 0 to 50 days after the initiation of the experiment. This approach allowed us to assess how EPS consumption patterns might change as the mealworms progress through their larval stages.Fig. 4a illustrates the average daily consumption of commercial EPS per mealworm across the different developmental time points. Mealworms at earlier developmental stages, from T0 to T30, exhibited a relatively consistent consumption rate of approximately 0.11 mg of commercial EPS per individual per day. However, a notable decrease in consumption was observed for mealworms at later developmental stages (T40 and T50), with the rate dropping to around 0.04 mg per individual per day. This reduction in EPS intake at later stages aligns with previous reports of lower feeding rates (0.04 mg per individual per day) observed in more mature mealworms weighing around 80 mg.47
 | ||
| Fig. 4 Average EPS consumption by mealworms on (a) commercial EPS and (b) pure EPS diets, quantified at different developmental time points T0–T50. | ||
A similar trend was observed for pure EPS consumption, as shown in Fig. 4b. Heavier mealworms demonstrated lower consumption rates compared to their lighter counterparts when fed a pure EPS diet. However, the consumption pattern for pure EPS showed slight variations across developmental stages. Mealworms at earlier stages (T0–T40) consumed more pure EPS than those at the final stage (T50), but the overall trend of decreased consumption with increased body mass remained consistent.
These results suggest mealworms have a preference for commercial EPS over pure EPS across all developmental stages. This may be attributed to the lower density of commercial EPS, which could make it easier for mealworms to mechanically process and ingest. The presence of additives in commercial EPS might also contribute to this preference, potentially altering the material's texture to make it more palatable to the mealworms.
These findings indicate that mealworms with greater body mass have a reduced propensity for consuming both commercial and pure EPS. This behaviour could be attributed to their enhanced ability to withstand periods of nutrient scarcity, a characteristic often observed in more mature insect larvae. This reduced consumption in older larvae might result in less exposure to stress induced by EPS ingestion.
The influence of mealworm age on their ability to consume and potentially degrade EPS has been a point of contention in previous research. Previous articles have examined this by feeding micro-polystyrene (MPS) to mealworms at different ages (1, 2, and 3 months) and showed that all groups exhibited the same trend of weight loss followed by gain.43 However, their study lacked a suitable control for cannibalism on the observed weight changes. Our study, by systematically examining mealworms at different developmental stages while controlling for cannibalism, provides a more robust assessment of how larval age affects EPS consumption and its impact on mealworm growth.
3.5. Effect of additives on EPS degradation
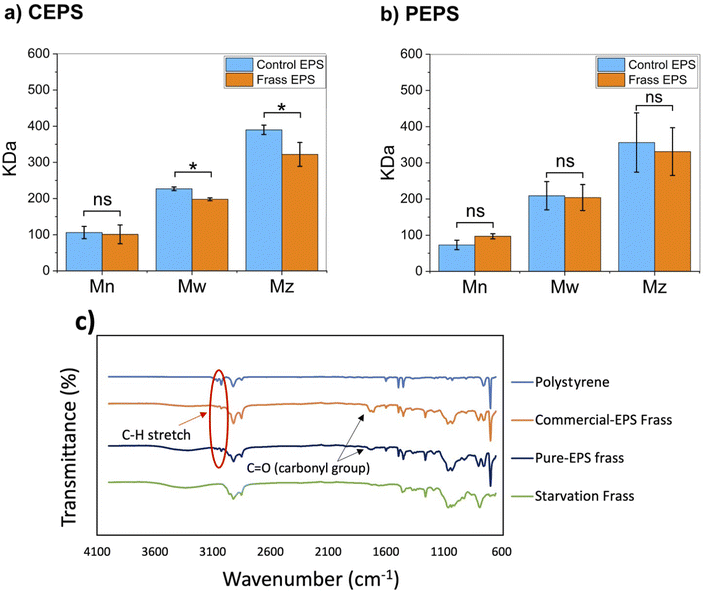
GPC and ATR-FTIR analyses were employed to elucidate the impact of additives on EPS degradation by examining polymer extracted from mealworm frass. GPC data revealed that for commercial EPS-fed mealworms, the Mw and Mz of PS in frass decreased by 12.7% and 17.4%, respectively, while Mn remained constant. In contrast, all molecular weight parameters for pure EPS were unchanged (Fig. 5a and b). This selective reduction in higher molecular weight fractions indicates partial degradation of commercial EPS, likely due to scission of longer PS chains.27,48 The observed partial degradation can be attributed to synergistic oxidative and mechanical processes during ingestion. Additives in commercial EPS, such as monoglyceride, alkyl amine N-oxide, and siloxane, introduce oxygen and nitrogen functionalities that may catalyze oxidative cleavage of PS chains, a known mechanism for C–C bond scission in polymer backbones.49 Moreover, the enhanced consumption rate of softer commercial EPS (Fig. 4a and b) suggests its increased susceptibility to mechanical breakdown by mealworm mandibles, potentially contributing to the observed molecular weight reduction. Conversely, the unaltered molar mass distribution of PS from pure EPS-fed mealworms indicates its greater resistance to both oxidative and mechanical degradation, likely due to the absence of additives and its more robust structural integrity.ATR-FTIR analysis provided further evidence for PS oxidation in the frass of mealworms fed both commercial and pure EPS diets (Fig. 5c). Spectral changes were observed in both datasets compared to pristine polystyrene, primarily in regions corresponding to R–OH (2500–3500 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1700 cm−1), and C–O (1050–1150 cm−1) stretches.20,21,50 However, most of these peaks (except the C
O (1700 cm−1), and C–O (1050–1150 cm−1) stretches.20,21,50 However, most of these peaks (except the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak) also appeared in the FTIR spectra of extracted materials from the frass of the starvation group. Notably, the C–H stretch peak at 3000 cm−1 decreased markedly in both pure and commercial EPS samples, indicating the oxidation of C–H bonded carbons, leading to the formation of C
O peak) also appeared in the FTIR spectra of extracted materials from the frass of the starvation group. Notably, the C–H stretch peak at 3000 cm−1 decreased markedly in both pure and commercial EPS samples, indicating the oxidation of C–H bonded carbons, leading to the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. The C
O bonds. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at 1735 cm−1 was more pronounced in samples from mealworms fed commercial EPS compared to those on a pure EPS diet. Given that absorbance is directly proportional to the concentration of absorbing species,51 the higher intensity of this functional group in commercial EPS—likely due to additives—may promote PS degradation through oxidation. The additives in commercial EPS, containing oxygen and nitrogen, could make it more susceptible to oxidative degradation by reactive oxygen species.52 These findings suggest that the presence of additives in commercial EPS facilitates minimal EPS degradation by mealworms, primarily through oxidation, at the cost of killing the insect. In the absence of plasticizers, the chemical structure of pure EPS is unaffected after passing through the mealworm.
O peak at 1735 cm−1 was more pronounced in samples from mealworms fed commercial EPS compared to those on a pure EPS diet. Given that absorbance is directly proportional to the concentration of absorbing species,51 the higher intensity of this functional group in commercial EPS—likely due to additives—may promote PS degradation through oxidation. The additives in commercial EPS, containing oxygen and nitrogen, could make it more susceptible to oxidative degradation by reactive oxygen species.52 These findings suggest that the presence of additives in commercial EPS facilitates minimal EPS degradation by mealworms, primarily through oxidation, at the cost of killing the insect. In the absence of plasticizers, the chemical structure of pure EPS is unaffected after passing through the mealworm.
The impact of additives on EPS biodegradation has been largely overlooked in previous studies, despite their ubiquity in commercial EPS products. While some researchers claimed to use additive-free EPS16,20,53 the heterogeneity and low concentrations of additives in commercial EPS often make their detection challenging.54 Our comparative analysis of pure synthesized EPS and commercial EPS provides crucial insights into the role of these additives in both mealworm physiology and EPS degradation kinetics. The observed differences in molecular weight distribution and oxidation patterns between pure and commercial EPS frass samples underscore the significant influence of additives on the degradation process. These findings highlight the importance of considering the effects of additives in future biodegradation studies and suggest that the chemical complexity of commercial EPS may lead to overestimation of mealworms' inherent ability to degrade the PS polymer backbone.
Our comprehensive analysis demonstrates three key findings regarding EPS degradation by mealworms. First, mealworms cannot effectively degrade the polystyrene backbone of pure EPS, as evidenced by the unchanged molecular weight distribution and minimal chemical modifications in frass samples. Second, while additives in commercial EPS enable limited oxidative degradation, resulting in a 12.7% decrease in Mw and increased carbonyl group formation, this degradation appears to be toxic to the mealworms rather than nutritionally beneficial. Third, the similar mortality rates between EPS-fed and starved mealworms, coupled with their inability to complete metamorphosis, definitively show that mealworms cannot derive sufficient nutrition from EPS regardless of its composition. These findings resolve contradictions in previous literature by revealing how experimental artifacts like cannibalism may have led to overestimation of mealworms' EPS degradation capabilities. The observed partial degradation of commercial EPS, while insufficient for waste management purposes, provides insight into how polymer additives might be leveraged to enhance degradation in future applications.
4. Conclusions
This study critically examined the ability of mealworms to consume and degrade EPS while controlling for confounding factors such as cannibalism and developmental stage. Our results demonstrated that mealworms cannot survive on EPS as their sole nutrient source – mortality rates matched or exceeded those of starved controls, and no mealworms completed metamorphosis when fed only EPS. Chemical analyses revealed that pure EPS passed through the digestive system unchanged, while commercial EPS showed limited degradation (12.7% reduction in molecular weight) through oxidative processes facilitated by additives. However, these same additives increased toxicity to mealworms, as shown by higher mortality rates compared to pure EPS or starvation conditions.Our findings indicate that neither mealworms nor their gut microbiota possess the enzymatic capability to break down the polystyrene backbone for nutritional purposes. The observed partial depolymerization of commercial EPS results from additive-mediated oxidation rather than biological degradation. These results challenge previous optimism about using mealworms for EPS biodegradation and emphasize the need to explore alternative approaches, including organisms with specialized enzymatic systems capable of cleaving carbon–carbon bonds in polystyrene, pretreatment methods, and integrated waste management strategies.
The observed partial depolymerization of commercial EPS, characterized by reduced molecular weight and increased carbonyl group formation, contrasts sharply with the minimal chemical changes in pure EPS. This highlights the interplay between polymer chemistry and the effects of additives in biological degradation processes. Importantly, the lack of chemical changes in pure EPS after passage through the mealworm digestive system provides strong evidence that neither mealworms nor their gut microbiota possess the enzymatic capability to break down the polystyrene backbone for nutritional purposes. This finding explains the observed inability of mealworms to derive energy from EPS, regardless of the presence of additives.
To address the growing problem of EPS waste, future studies should focus on exploring organisms with a higher tolerance for EPS consumption and more efficient degradation capabilities, potentially those with specialized enzymatic systems capable of cleaving the carbon–carbon bonds in polystyrene. Investigating the potential of physical or chemical pretreatment methods to enhance digestibility and evaluating the feasibility of integrating EPS-degrading organisms with other waste management strategies could lead to the development of more effective solutions.
Data availability
Data for this article, including figures and photographs are available at Zenodo at https://10.5281/zenodo.13880667.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the University of Western Australia and Woodside Energy Ltd.References
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan and K. L. Law, Marine pollution. Plastic waste inputs from land into the ocean, Science, 2015, 347(6223), 768–771, DOI:10.1126/science.1260352.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), e1700782 CrossRef PubMed.
- A. L. Andrady, Common plastics materials, Plast. Environ., 2003, 77–121 Search PubMed.
- B. T. Ho, T. K. Roberts and S. Lucas, An overview on biodegradation of polystyrene and modified polystyrene: the microbial approach, Crit. Rev. Biotechnol., 2018, 38(2), 308–320, DOI:10.1080/07388551.2017.1355293.
- B. Li, Z. Lan, L. Wang, H. Sun, Y. Yao, K. Zhang and L. Zhu, The release and earthworm bioaccumulation of endogenous hexabromocyclododecanes (HBCDDs) from expanded polystyrene foam microparticles, Environ. Pollut., 2019, 255, 113163, DOI:10.1016/j.envpol.2019.113163.
- A. Ong, J. Y. Teo, Z. Feng, T. T. Tan and J. Y. Lim, Organocatalytic aerobic oxidative degradation of polystyrene to aromatic acids, ACS Sustain. Chem. Eng., 2023, 11(34), 12514–12522 CrossRef CAS.
- A. M. Gonzalez-Aguilar, V. Pérez-García and J. M. Riesco-Ávila, A thermo-catalytic pyrolysis of polystyrene waste review: A systematic, statistical, and bibliometric approach, Polymers, 2023, 15(6), 1582 CrossRef CAS PubMed.
- S. Oh and E. E. Stache, Mechanistic insights enable divergent product selectivity in catalyst-controlled photooxidative degradation of polystyrene, ACS Catal., 2023, 13(16), 10968–10975 CrossRef CAS.
- J. D. Peterson, S. Vyazovkin and C. A. Wight, Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene and poly (propylene), Macromol. Chem. Phys., 2001, 202(6), 775–784 CrossRef CAS.
- C. Lu, H. Xiao and X. Chen, Simple pyrolysis of polystyrene into valuable chemicals, E-Polymers, 2021, 21(1), 428–432 CrossRef CAS.
- S. Lee, J. Yoon, J. Kim and D. Park, Catalytic degradation of polystyrene over natural clinoptilolite zeolite, Polym. Degrad. Stabil., 2001, 74(2), 297–305 CrossRef CAS.
- Y.-H. Lin, C.-C. Tseng, T.-T. Wei and C.-T. Hsu, Recycling of dual hazardous wastes in a catalytic fluidizing process, Catal. Today, 2011, 174(1), 37–45 CrossRef CAS.
- P. Tiwary and C. Guria, Effect of metal oxide catalysts on degradation of waste polystyrene in hydrogen at elevated temperature and pressure in benzene solution, J. Polym. Environ., 2010, 18, 298–307 CrossRef CAS.
- M. Zunita, H. P. Winoto, M. F. K. Fauzan and R. Haikal, Recent advances in plastics waste degradation using ionic liquid-based process, Polym. Degrad. Stab., 2023, 211, 110320 CrossRef CAS.
- L. Xu, Z. Li, L. Wang, Z. Xu, S. Zhang and Q. Zhang, Progress in polystyrene biodegradation by insect gut microbiota, World J. Microbiol. Biotechnol., 2024, 40(5), 143 CrossRef CAS PubMed.
- Y. Yang, J. Wang and M. Xia, Biodegradation and mineralization of polystyrene by plastic-eating superworms Zophobas atratus, Sci. Total Environ., 2020, 708, 135233, DOI:10.1016/j.scitotenv.2019.135233.
- S. S. Ali, T. Elsamahy, E. Koutra, M. Kornaros, M. El-Sheekh, E. A. Abdelkarim, D. Zhu and J. Sun, Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal, Sci. Total Environ., 2021, 771, 144719, DOI:10.1016/j.scitotenv.2020.144719.
- J. Wang, C. Liu, Q. Cao, Y. Li, L. Chen, Y. Qin, T. Wang and C. Wang, Enhanced biodegradation of microplastic and phthalic acid ester plasticizer: The role of gut microorganisms in black soldier fly larvae, Sci. Total Environ., 2024, 924, 171674 CrossRef CAS PubMed.
- E. Tsochatzis, J. A. Lopes, H. Gika and G. Theodoridis, Polystyrene biodegradation by Tenebrio molitor larvae: identification of generated substances using a GC-MS untargeted screening method, Polymers, 2020, 13(1), 17 CrossRef PubMed.
- L. Yang, J. Gao, Y. Liu, G. Zhuang, X. Peng, W. M. Wu and X. Zhuang, Biodegradation of expanded polystyrene and low-density polyethylene foams in larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae): Broad versus limited extent depolymerization and microbe-dependence versus independence, Chemosphere, 2021, 262, 127818, DOI:10.1016/j.chemosphere.2020.127818.
- B. Y. Peng, Y. Li, R. Fan, Z. Chen, J. Chen, A. M. Brandon, C. S. Criddle, Y. Zhang and W. M. Wu, Biodegradation of low-density polyethylene and polystyrene in superworms, larvae of Zophobas atratus (Coleoptera: Tenebrionidae): Broad and limited extent depolymerization, Environ. Pollut., 2020, 266, 115206, DOI:10.1016/j.envpol.2020.115206.
- A. M. Brandon, S. H. Gao, R. Tian, D. Ning, S. S. Yang, J. Zhou, W. M. Wu and C. S. Criddle, Biodegradation of Polyethylene and Plastic Mixtures in Mealworms (Larvae of Tenebrio molitor) and Effects on the Gut Microbiome, Environ. Sci. Technol., 2018, 52(11), 6526–6533, DOI:10.1021/acs.est.8b02301.
- J. Wang, Y. Wang, X. Li, Y. Weng, Y. Wang, X. Han, M. Peng, A. Zhou and X. Zhao, Different performances in polyethylene or polystyrene plastics long-term feeding and biodegradation by Zophobas atratus and Tenebrio molitor larvae, and core gut bacterial-and fungal-microbiome responses, J. Environ. Chem. Eng., 2022, 10(6), 108957 CrossRef CAS.
- X. Wang and T. Tang, Effects of Polystyrene Diet on the Growth and Development of Tenebrio molitor, Toxics, 2022, 10(10), 608, DOI:10.3390/toxics10100608.
- A. K. Urbanek, J. Rybak, M. Wrobel, K. Leluk and A. M. Mironczuk, A comprehensive assessment of microbiome diversity in Tenebrio molitor fed with polystyrene waste, Environ. Pollut., 2020, 262, 114281, DOI:10.1016/j.envpol.2020.114281.
- B. Y. Peng, Y. Su, Z. Chen, J. Chen, X. Zhou, M. E. Benbow, C. S. Criddle, W. M. Wu and Y. Zhang, Biodegradation of Polystyrene by Dark (Tenebrio obscurus) and Yellow (Tenebrio molitor) Mealworms (Coleoptera: Tenebrionidae), Environ. Sci. Technol., 2019, 53(9), 5256–5265, DOI:10.1021/acs.est.8b06963.
- B. Y. Peng, Y. Sun, P. Li, S. Yu, Y. Xu, J. Chen, X. Zhou, W. M. Wu and Y. Zhang, Biodegradation of polyvinyl chloride, polystyrene, and polylactic acid microplastics in Tenebrio molitor larvae: Physiological responses, J. Environ. Manage., 2023, 345, 118818, DOI:10.1016/j.jenvman.2023.118818.
- T. Q. Pham, S. Longing and M. G. Siebecker, Consumption and degradation of different consumer plastics by mealworms (Tenebrio molitor): Effects of plastic type, time, and mealworm origin, J. Clean. Prod., 2023, 403, 136842 CrossRef CAS.
- B. Y. Peng, Y. Sun, S. Xiao, J. Chen, X. Zhou, W. M. Wu and Y. Zhang, Influence of Polymer Size on Polystyrene Biodegradation in Mealworms (Tenebrio molitor): Responses of Depolymerization Pattern, Gut Microbiome, and Metabolome to Polymers with Low to Ultrahigh Molecular Weight, Environ. Sci. Technol., 2022, 56(23), 17310–17320, DOI:10.1021/acs.est.2c06260.
- A. L. Andrady, Plastics and the Environment, John Wiley & Sons, 2003 Search PubMed.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling, J. Hazard Mater., 2018, 344, 179–199 CrossRef CAS PubMed.
- A. M. Brandon, S. H. El Abbadi, U. A. Ibekwe, Y.-M. Cho, W.-M. Wu and C. S. Criddle, Fate of hexabromocyclododecane (HBCD), a common flame retardant, in polystyrene-degrading mealworms: elevated HBCD levels in egested polymer but no bioaccumulation, Environ. Sci. Technol., 2019, 54(1), 364–371 CrossRef PubMed.
- M. Stroe, M. Cristea, E. Matei, A. Galatanu, L. C. Cotet, L. C. Pop, M. Baia, V. Danciu, I. Anghel and L. Baia, et al., Optical Properties of Composites Based on Graphene Oxide and Polystyrene, Molecules, 2020, 25(10), 2419, DOI:10.3390/molecules25102419.
- C. Gutiérrez, M. T. García, I. Gracia, A. de Lucas and J. F. Rodríguez, The selective dissolution technique as initial step for polystyrene recycling, Waste Biomass Valorization, 2013, 4, 29–36 CrossRef.
- Z. Zhong, X. Zhou, Y. Xie and L. M. Chu, The interplay of larval age and particle size regulates micro-polystyrene biodegradation and development of Tenebrio molitor L, Sci. Total Environ., 2023, 857, 159335, DOI:10.1016/j.scitotenv.2022.159335.
- M. K. Goel, P. Khanna and J. Kishore, Understanding survival analysis: Kaplan-Meier estimate, Int. J. Ayurveda Res., 2010, 1(4), 274–278, DOI:10.4103/0974-7788.76794.
- Y. Lou, P. Ekaterina, S. S. Yang, B. Lu, B. Liu, N. Ren, P. F. Corvini and D. Xing, Biodegradation of Polyethylene and Polystyrene by Greater Wax Moth Larvae (Galleria mellonella L.) and the Effect of Co-diet Supplementation on the Core Gut Microbiome, Environ. Sci. Technol., 2020, 54(5), 2821–2831, DOI:10.1021/acs.est.9b07044.
- Y. Yang, J. Yang, W. M. Wu, J. Zhao, Y. Song, L. Gao, R. Yang and L. Jiang, Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 1. Chemical and Physical Characterization and Isotopic Tests, Environ. Sci. Technol., 2015, 49(20), 12080–12086, DOI:10.1021/acs.est.5b02661.
- T. C. R. White, Why Does the World Stay Green?: Nutrition and Survival of Plant-Eaters, Csiro Publishing, 2005 Search PubMed.
- L. R. Fox, Cannibalism in natural populations, Annu. Rev. Ecol. Systemat., 1975, 6(1), 87–106 CrossRef.
- M. L. Richardson, R. F. Mitchell, P. F. Reagel and L. M. Hanks, Causes and consequences of cannibalism in noncarnivorous insects, Annu. Rev. Entomol., 2010, 55, 39–53, DOI:10.1146/annurev-ento-112408-085314.
- J. L. C. Capinera, Encyclopedia of Entomology, 2008, pp. 710–714 Search PubMed.
- Z. Zhong, X. Zhou, Y. Xie and L. Chu, The interplay of larval age and particle size regulates micro-polystyrene biodegradation and development of Tenebrio molitor L, Sci. Total Environ., 2023, 857, 159335 CrossRef CAS PubMed.
- S. H. Smith and L. T. Taylor, Extraction of various additives from polystyrene and their subsequent analysis, Chromatographia, 2002, 56, 165–169 CrossRef CAS.
- E. D. Tsochatzis, I. E. Berggreen, N. P. Vidal, L. Roman, H. Gika and M. Corredig, Cellular lipids and protein alteration during biodegradation of expanded polystyrene by mealworm larvae under different feeding conditions, Chemosphere, 2022, 300, 134420, DOI:10.1016/j.chemosphere.2022.134420.
- Y. Yang, L. Hu, X. Li, J. Wang and G. Jin, Nitrogen fixation and diazotrophic community in plastic-eating mealworms Tenebrio molitor L, Microb. Ecol., 2023, 85(1), 264–276 CrossRef CAS PubMed.
- Z. Zhong, W. Nong, Y. Xie, J. H. L. Hui and L. M. Chu, Long-term effect of plastic feeding on growth and transcriptomic response of mealworms (Tenebrio molitor L.), Chemosphere, 2022, 287, 132063, DOI:10.1016/j.chemosphere.2021.132063.
- B. Y. Peng, S. Xiao, Y. Sun, Y. Liu, J. Chen, X. Zhou, W. M. Wu and Y. Zhang, Unveiling Fragmentation of Plastic Particles during Biodegradation of Polystyrene and Polyethylene Foams in Mealworms: Highly Sensitive Detection and Digestive Modeling Prediction, Environ. Sci. Technol., 2023, 57(40), 15099–15111, DOI:10.1021/acs.est.3c04406.
- J. F. Rabek, Oxidative degradation of polymers, in Comprehensive Chemical Kinetics, Elsevier, 1975, vol. 14, pp. 425–538 Search PubMed.
- S.-S. Yang, A. M. Brandon, J. C. A. Flanagan, J. Yang, D. Ning, S.-Y. Cai, H.-Q. Fan, Z.-Y. Wang, J. Ren and E. Benbow, Biodegradation of polystyrene wastes in yellow mealworms (larvae of Tenebrio molitor Linnaeus): factors affecting biodegradation rates and the ability of polystyrene-fed larvae to complete their life cycle, Chemosphere, 2018, 191, 979–989 CrossRef CAS PubMed.
- D. A. Skoog, F. J. Holler and S. R. Crouch, Principles of Instrumental Analysis, 2019 Search PubMed.
- Z. Chen, Y. Zhang, R. Xing, C. Rensing, J. Lu, M. Chen, S. Zhong and S. Zhou, Reactive Oxygen Species Triggered Oxidative Degradation of Polystyrene in the Gut of Superworms (Zophobas atratus Larvae), Environ. Sci. Technol., 2023, 57(20), 7867–7874, DOI:10.1021/acs.est.3c00591.
- B. Y. Peng, Y. Sun, X. Zhang, J. Sun, Y. Xu, S. Xiao, J. Chen, X. Zhou and Y. Zhang, Unveiling the residual plastics and produced toxicity during biodegradation of polyethylene (PE), polystyrene (PS), and polyvinyl chloride (PVC) microplastics by mealworms (Larvae of Tenebrio molitor), J. Hazard. Mater., 2023, 452, 131326, DOI:10.1016/j.jhazmat.2023.131326.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling, J. Hazard Mater., 2018, 344, 179–199 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00618f |
| This journal is © The Royal Society of Chemistry 2025 |