 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Field-enhanced chemical vapor deposition: new perspectives for thin film growth
Bhupendra Singh
 a,
Thomas Fischer
a,
Thomas Fischer
 a and
Sanjay Mathur
a and
Sanjay Mathur
 *ab
*ab
aInstitute of Inorganic and Materials Chemistry, University of Cologne, Greinstraße 6, 50939 Cologne, Germany. E-mail: sanjay.mathur@uni-koeln.de
bDepartment of Metals and Materials Engineering, Indian Institute of Technology Madras, 600036 Chennai, India
First published on 15th May 2025
Abstract
Chemical vapor deposition (CVD) is a versatile technique for producing thin films and coatings of functional materials with diverse mechanical, electrochemical, electrical, tribological, and optical properties. The CVD process is governed by various experimental parameters including precursor chemistry, feed rate, growth temperature, pressure, and carrier or reactive gases. The growth kinetics depends on precursor decomposition that can be influenced by plasma-chemical or photo-dissociation processes to supplement thermal energy. More recently, the application of electric or magnetic fields during the CVD process has impacted the film growth beyond the conventional parametric space. This review highlights the influence of external field effects (plasma, photo-radiation, electric field, and magnetic field) on key steps of thin film processing, such as nucleation, grain growth, texture, density, phase formation, anisotropy, and kinetic stabilization. The emphasis is on recent technical, material, and phenomenological innovations in the CVD technique, with applied fields as extrinsic processing parameters offering new insights into future directions in the research and development of high-fidelity functional films and coatings.
1 Introduction
Chemical vapor deposition (CVD) and its variants represent a platform technology to deposit a diverse range of materials as thin films and coatings that find large-scale applications from the food industry (e.g., antibacterial coatings) to precision-engineered semiconductor devices (e.g., light emitting diodes).1–6 By definition, CVD involves the deposition of a solid material by vapor phase decomposition of chemical species (precursors) on a substrate maintained at a specific temperature. When compared with physical vapor deposition techniques, such as evaporative deposition, sputtering, and pulsed laser deposition involving the generation of a plume of atomic or molecular species from a source material followed by their deposition (condensation) on a substrate, the CVD process relies upon chemical reactions of gaseous or aerosol precursors, occurring in the gas phase and/or on the substrate surface (Fig. 1(a)).2,7The fundamental parameters controlling the CVD process are the temperature of the substrate, pressure in the CVD reactor, precursor feed rate (flux), and the chemical nature of used precursors, as well as carrier gas that can be reactive or non-reactive. The film growth rates are governed by chemical kinetics and energy input, which influence the characteristics of the material, such as adhesion, morphology, microstructure, and crystallinity.8–13
The CVD technique encompasses numerous phenomenologically related processes (Fig. 1(a))2,14 that differ in energy input provided to activate the chemical precursors thermally, optically, or photochemically, resulting in different categories of CVD processes (Fig. 1(b)). In conventional CVD reactors, thin film deposition proceeds by thermal energy provided by resistive heating of the entire reaction zone (hot-wall CVD) or through a susceptor that selectively heats the substrate (cold-wall CVD). Alternatively, utilization of photons or plasma to decompose precursor species has led to the development of photo-assisted CVD (PACVD) and plasma-enhanced CVD (PECVD). The modification of CVD reactors and processes is largely driven by the necessity of achieving conformal coverage and chemically homogeneous deposition, which relies on the vapor pressure and chemical reactivity of the used precursor gases and chemicals. In addition, the necessity of applying functional coatings on temperature-sensitive substrates (e.g., aluminum, glass, polymers) has triggered the use of non-thermal plasmas (so-called cold plasma) in the CVD processes. The benefits of plasma-enhanced processes are two-fold: they can activate the substrate surface before deposition, remove any adsorbate layer that is inevitably present, and facilitate the growth kinetics. The attempts to diversify the range of precursors and to have greater control over the precursor chemistry, overcoming the barriers of non-volatility of precursors, have led to the development of aerosol-assisted CVD (AACVD) and metalorganic CVD (MOCVD). Similarly, the requirements for substrate cleaning in the semiconductor industry and the non-thermal initiation step in the polymerization of thermally sensitive materials have led to the development of low energy and/or pulsed plasma CVD.15–17
In thermal CVD, the role of temperature has been to fulfill the energy requirements for precursor decomposition, although it has further influenced the nucleation and growth kinetics.18–20 Temperature and other precursor-specific intrinsic parameters (composition, flow rate, precursor chemistry, etc.) are thermodynamics-dependent and often need macro-level control to execute micro-level variations in the properties of the resulting deposition. On the other hand, the characteristics of precursor-independent extrinsic parameters (photons, plasma, electric field, magnetic field) are controlled, independent of thermodynamics (Fig. 1(c) and (d)). Therefore, while using photon or plasma as an agent fulfilling the energy requirements, manipulations in their characteristics have provided additional means to manipulate and control the CVD process at the microscopic level. For example, besides providing energy for precursor decomposition, plasma also provides energetic species – electrons, photons, ions, excited atoms, and molecules – to the substrate/growth surface, causing surface reconstruction, stoichiometric change, and alteration in reaction rate and growth kinetics. Moreover, the interaction of the precursor with plasma species initiates an alternative decomposition pathway, enabling deposition without temperature assistance or at a significantly lowered temperature.15 Similarly, the use of laser radiation for precursor dissociation localizes the thermal energy and generates free carriers that further influence the thermodynamics and kinetics of the CVD steps.21 Additionally, photolysis and photosensitization reduce the energy of activation (Ea) of gaseous or surface-adsorbed species, accelerating the reaction kinetics.22
The energy associated with an electric field (EF) or magnetic field (MF) generated in a normal laboratory environment may not be sufficient to overcome the Ea for precursor decomposition in CVD. However, when applied in a CVD environment, the field associated with them interacts with various components (precursor, decomposition products, substrate, plasma, etc.), influencing the growth rate or modifying the film properties.23 For example, an external EF-assisted bias-enhanced nucleation has been key in the ‘‘seed-free’’ deposition of diamond on smooth surfaces.24,25 Similarly, the application of MF in the course of precursor flow or around the substrate, inside the reactor, has provided directional control over the movement of polar/charged species, altering the deposition and growth parameters.26 Moreover, coupling of an external MF with other CVD modulators, such as EF,27,28 plasma,29,30 or both,26,31 has generated a synergistic effect, imparting a unique microstructure and morphology to the deposited films.
The development of novel functional materials for targeted applications drives the need for new strategies to modulate the CVD process beyond conventional control parameters. In this context, the use of plasma and photo-irradiation, as well as the integration of external electric (EF) and magnetic fields (MF) into CVD setups, has shown significant potential to control intrinsic materials characteristics. These external fields can influence reaction kinetics and overcome the constraints of conventional CVD methods, enabling low-temperature deposition, alternative nucleation and growth mechanisms, selected area deposition, directional grain growth, molecular-scale engineering, and substrate versatility. This review is motivated by recent advances in CVD methodology that leverage such field effects – both contact-based (plasma and photo-irradiation) and non-contact (electric and magnetic fields)—to exert additional control at the microscale. These approaches help transcend the restrictions imposed by macroscopic parameters on growth rate, composition, phase, morphology, and microstructure.
This account highlights the key achievements made in field-assisted CVD, emphasizing how these techniques enable the fabrication of thin films with enhanced material properties. The review is organized into focused subsections; each begins with an introduction to the field-matter interaction during CVD, followed by a discussion of field as an external parameter to control the CVD process, either independently or in combination with conventional parameters. Each section is concluded by outlining the influence of fields on precursor decomposition, nucleation, film growth, and resulting characteristics.
2 Plasma-assisted/enhanced chemical vapor deposition
2.1 Plasma-matter interaction during chemical vapor deposition
The PECVD process primarily utilizes the high energy electrons present in the non-equilibrium plasma of partially ionized gases (reactive and/or carrier), having electron temperature Te ≤ 10 eV (1 eV = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 K) and gas temperatures ranging from room temperature to 104 K, to excite atomic and molecular precursor species to higher electronic and vibrational states.32 This initiates or/and promotes the dissociation of chemical bonds, consequently facilitating thin film deposition at significantly lower temperatures compared to conventional thermal CVD processes. Plasma electrons, owing to their low mass and high mobility, lead in receiving energy from the ionization source (electric field, heat, light, magnetic field, etc.) and transmit it to other species via inelastic collisions, leading to numerous gas-phase plasma-chemical processes, such as ionization/recombination, excitation/relaxation, dissociation/association, and charge-transfer reactions.33 The electron energy distribution function (EEDF; f(ε)), denotes the probability density for an electron to possess energy ε and is described by eqn (1)
600 K) and gas temperatures ranging from room temperature to 104 K, to excite atomic and molecular precursor species to higher electronic and vibrational states.32 This initiates or/and promotes the dissociation of chemical bonds, consequently facilitating thin film deposition at significantly lower temperatures compared to conventional thermal CVD processes. Plasma electrons, owing to their low mass and high mobility, lead in receiving energy from the ionization source (electric field, heat, light, magnetic field, etc.) and transmit it to other species via inelastic collisions, leading to numerous gas-phase plasma-chemical processes, such as ionization/recombination, excitation/relaxation, dissociation/association, and charge-transfer reactions.33 The electron energy distribution function (EEDF; f(ε)), denotes the probability density for an electron to possess energy ε and is described by eqn (1)
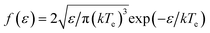 | (1) |
During the PECVD process, the quasi-neutrality of plasma bulk breaks due to the buildup of charge near the substrate surface, and an electrically non-neutral region known as the plasma sheath is formed. The sheath thickness, which is of the orders of Debye length, depends on electron energy and electron density. As the potential attained by the substrate (floating potential) is less than plasma potential (VP), positive ions diffuse through the plasma sheath, acquiring some additional energy en route to the substrate surface, which can be regulated by substrate biasing (VB) leading to the maximum kinetic energy given by eqn (2)
| Ei,max = e|VP − VB| + (ΔE/2) | (2) |
Santhosh et al.46 presented an interesting overview of different modes of generation and confinement in plasma systems used in PECVD (Fig. 2). The PECVD process relies on a complex interplay of plasma characteristics, precursor chemistry, and reactor design.48,49 Notwithstanding, high precursor decomposition efficiency, even at room temperature, is ensured by energy transfers in the form of kinetic and potential energies through plasma electrons and charged particles to the precursors, leading to the formation of free radicals and charged ions that are critical in influencing the deposition and growth.50,51 Additionally, the plasma-surface interaction under appropriate plasma conditions, apart from initiating/promoting precursor decomposition, also promotes adsorption, desorption, nucleation, adatom migration, implantation, sputtering and displacement of atoms, thus tuning the composition, phase, microstructure, and morphology of the deposited material.37,52,53
 | ||
| Fig. 2 A brief overview of different plasma systems used in PECVD.46,47 | ||
2.2 Overview of plasma-enhanced chemical vapor deposition
The plasma-assisted modulations in CVD induce gas-phase chemistry mediated by energetic plasma species, enabling the decomposition of precursors, even of low reactivity, leading to deposition on temperature-sensitive and morphologically intricate substrates.54,55 A recent review by Snyders et al.48 provides an impressive historical background of the stages of development and conceptual foundations of PECVD, tracing the origin of PECVD to 1911. Several other reviews discussing the basic chemical and physical phenomena associated with the PECVD process have been reported.17,46–48,50,56–60The PECVD process has been successfully used for depositing metals and ceramics,50,61–66 carbon-based materials,58,59,67–71 and organic polymers.17,48,72 In recent years, plasma-assisted modulations have focused on enhancing deposition and growth rates,73 achieving directional growth and crystallographic orientation of nanostructures,59,74–76 surface modification,77–80 doping and vacancy generation,81–85 and manipulating phase composition and crystallinity.86–89 Table 1 presents selected recent reports on PECVD, displaying its utility in thin film fabrication for a range of materials.
| Material/substrate | Plasma-type | Key features | Application | Ref. |
|---|---|---|---|---|
| a CCP – capacitively coupled plasma; ICP – inductively coupled plasma; DLC – diamond-like carbon; DPAEMA – 2-diisopropyl aminoethyl methacrylate; Eg – optical band gap; h-BN – hexagonal boron nitride; HMDS – hexamethyldisilazane; n-diamond – new-diamond (face centered cubic-carbon); TMDS – 1,1,3,3-tetramethyldisilazane; WVTR – water vapor transmission rate; ρ – resistivity; μH = hall mobility. | ||||
| Carbon-based materials | ||||
| Diamond films on 3D Si spheres | MW, (CH4, H2), 10 kPa | Uniform EF and plasma distribution by a Faraday cage | Uniform diamond coating on complex 3D geometries | 73 |
| Diamond films/Si | MW, 600–900 W, (H2, Ar, CH4), 12.6 kPa, 750 °C | Bias-enhanced nucleation led azimuthal texturing; Raman peak ∼1332 cm−1 | Quantum devices; coatings with aligned N vacancy defect centres, ∼70% azimuthal orientation | 90 |
| DLC/tungsten carbide | Cathode arc discharge (120 A DC), (N2, Ar, C2H2), 0.9 Pa, 450 °C | Role of particle energy on structure and properties | Cutting tool protection film; coefficient of friction = 0.112 | 91 |
| (n-Diamond)-carbon nanowalls hybrid/Si(100) | CCP-RF, 300 W, (CO, CH4, Ar, H2), ∼33 Pa, 450 °C | Large area (4 inch) simultaneous deposition | — | 92 |
| Fluorescent nanodiamond with Si impurity/Si | MW, (H2, CH4), 2.5 kPa, 700 °C | A vertically aligned Si source in the plasma path gives highly fluorescent (7–10 times enhanced) diamond film | Photoluminescent films | 85 |
| Vertical graphene (VG)/quartz | RF, 500 W, (C2H2, H2), ∼1 × 105 Pa, 900 °C | Position-induced control over morphology; superhydrophobicity and ultrahigh emissivity (0.999) | Black body and infrared thermometer | 93 |
| Graphene/sapphire | ECR, 500 W, (C2H2, H2), ∼0.54–5.4 kPa, 600–700 °C | Direct growth of uniform and continuous graphene on insulators at reduced temperature | Graphene film for optoelectronic device; transmittance ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) > >![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90%, sheet resistance 90%, sheet resistance ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) < <![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 kΩ) 1 kΩ) |
94 |
| Vertical graphene/SiO2 | RF (remote), 300 W, (CH4, Ar), 0.1 Pa, 300–1000 °C | Interfacial EF between plasma and substrate-governed vertical growth | — | 95 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Metalloids | ||||
| SiNx/SiOF/SiNx flexible moisture barrier film/polymer substrate | MW, 1500 W, (Ar, SiF4, N2O), 9 Pa, 45 °C | Films with high WVTR (3.94 × 10−4 g ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m−2 m−2 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) day−1) and visible light transmittance (93.32%) day−1) and visible light transmittance (93.32%) |
Roll-to-roll processing of optical devices | 96 |
| Amorphous hydrogenated silicon carbonitride/Si | MW (remote), 120 W, (TMDS/HMDS, H2, N2), 43–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pa, 30–400 °C Pa, 30–400 °C |
Precursors with Si–H bonds are necessary for initiation of remote plasma-CVD with a single source | Tribological coatings | 97 |
| Si film on different substrates | CCP-RF, 15 W, (SiF4, H2, Ar), 306 Pa, 150 °C | Area-selective Si deposition; surface-dependent nucleation delay due to a fluorinated Si precursor | — | 98 |
| Hydrogenated silicene/polycrystalline Ag | — | First report of silicone synthesis by PECVD | — | 99 |
| Phosphorus-doped nanocrystalline SiOx/glass | RF (13.56, 27, 40 MHz), (318, 333, 256 W), (SiH4, H2/CO2/PH3), 200 °C | Industrial-scale deposition using a very high-frequency PECVD system | Si heterojunction solar cells | 100 |
| 2D hexagonal-boron nitride (h-BN)/SiO2/Si | RF, 30 W (BH3-NH3, Ar/H2), ∼115 kPa, 300 °C | First report of catalyst-free directly-on-substrate growth | Dielectric interface for WSe2 field-effect transistor | 101 |
| Multilayered h-BN/Si, SiO2/Si, and quartz | ICP-RF, 40–180 W, (borazine, Ar, N2, H2) 10–100 Pa, 300–900 °C | Catalyst-free low-temperature growth of multilayered (>50 nm) h-BN | Optoelectronics; comparable/better optical characteristics than commercial h-BN, Eg = 5.8 eV | 40 |
| h-BN/Si | MW, 1000 W, (H2, NH3, B2H6), 2 kPa | Regardless of B2H6 flow rate (0.6–3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sccm), h-BN with B/N ratio = 1 is formed sccm), h-BN with B/N ratio = 1 is formed |
High-hardness coatings | 102 |
| 2D boron carbonitride (BCxN)/SiO2/Si or SiO2 | 30 W, (BH3·NH3, CH4, Ar, H2), 80 Pa, 450–620 °C | First report of catalyst-free temperature-dependent BCxN growth; Eg = 2.3 eV | Field effect transistors and sensor; H2O2 detection limit = 0.1 μM | 103 |
| Boron film on (100) Si | RF, 1000 W, (Ar, B2H6), 133–239 Pa | Comparison of PECVD films with other related deposition techniques | Mask layer/structural material in micro-machining | 104 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Metals, oxides, chalcogenides, nitrides | ||||
| Fe, Co, and Ni metallic films on different substrates | DC hallow cathode, 50–150 W, 50 Pa, 70–100 °C | Plasma electrons as reducing agents; electrical conductivity of the substrate plays a major role | Catalysts and electronic devices | 105 |
| Cu2O on different substrates | CCP-RF, 10 W, (Ar), 26 Pa, 200 °C | Large area (2.88 × 3.13 m2) uniform deposition | Thin-film semiconductors; ρ = 105 Ω cm; Eg = 2.53–2.59 eV, μH = 1.48 cm2 (V s)−1 | 106 |
| MoS2/sapphire or SiO2/Si | RF direct/remote plasma, 300 W, (MoO3, H2S, Ar), 400 °C | Photoluminescence (direct plasma deposited only at 2.0 eV; remote plasma dep. at 1.8 and 2.0 eV) | Hydrogen evolution reaction (HER) | 107 |
| N-alloyed Ga2O3 films (Ga2O3, GaON, and GaN)/c-Al2O3 | RF, 200 W, (Ga, Ar, N2, O2), 850 °C | Bandgap tuning and O vacancy density (Vo) reduction by adjusting N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 gas ratio O2 gas ratio |
Photodetectors; Eg = 4.64–3.25 eV, Vo = 32.89–19.87% | 108 |
| MoS2 | ICP-RF, 300 W, (S8, Ar), 29–53 Pa, 500 °C | Single-step deposition using elemental S and MoCl5 as precursors | — | 109 |
| Mo2C/MoS2 or Mo2C/MoSe2 on Cu foil | RF, 150 W, (S/Se, H2, N2), 750–350 °C | Phase-engineering of Mo2C by plasma-assisted selenization and sulfurization | HER; (Tafel slope/mV dec−1: Mo2C/MoS2 = 83, Mo2C/MoSe2 = 66); (overpotential/mV: Mo2C/MoS2 = 2263, Mo2C/MoSe2 = 257) | 110 |
| Amorphous carbon-tin film/glass | Glow discharge (40 kHz), 500 W, 4.2 Pa | Coupling capacitance of the reactor controls the semiconductor/insulator characteristics | HER or CO2 reduction; transport gap = 5.2 eV, Eg = 3.1 eV, electron affinity = 2.1 eV, ionization potential = 7.3 eV | 111 |
| Cr–N coating on Cr layer | MW, 5000 W, (H2 and N2), ∼22.6 kPa, 750–950 °C | MW plasma-assisted in situ reactions on a chromium pre-coated substrate | Tribological coatings; coefficient of friction <0.55 (in 750–950 °C range), wear resistance = 0.085 and resistance to plastic deformation = 0.142 at 800 °C | 112 |
| GaS on different substrates | RF (40.68 MHz) discharge, 50 W, 13.3 Pa, 250 °C | Influence of substrate on stoichiometry, structure, and surface morphology investigated | — | 113 |
| TiN, TiBN or TiB2 hard coating/steel | Pulsed DC, (BBr3, TiCl4, N2, H2, Ar), ∼933–1200 Pa, 500 °C | Use of BBr3 as a B precursor; hardness of TiN, TiBN and TiB2 films was 15.74, 19.44 and 23.47 GPa, respectively | Tribological coatings; for TiB2 coating, elastic modulus = 298 GPa and coefficient of friction = 0.23 | 75 |
| GeSn film on Si | RF, 30 W, (GeCl4, SnCl4, H2), 53 Pa, 180 °C | Low-temperature GeSn film without any buffer layer or post-annealing (growth rate ≈15 nm min−1 at 180 °C) | Short-wave infrared Si photonics; high Sn (>9%) and low threading dislocation density (1 × 107 cm−2) | 114 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Others | ||||
| Methyl ammonium iodide (MAI) | RF, 60 W, (MAI, H2O, Ar), ∼13 Pa | First report of MAI deposition using PECVD for MAPbI3 films; Eg = 1.57 eV, refractive index = 3.07, extinction coefficient = 0.13 | perovskite/Si photodiode; rectifying ratio = 1.58 × 104 at 1.0 V, photo-sensitivity = 7.46 × 102 | 115 |
| Poly(DPAEMA) film | RF, 20–80 W, (diisopropyl aminoethyl methacrylate, N2), ∼13–67 Pa, 10–40 °C | Initiator-free polymerization; large area (>16 cm diameter) deposition rate = 18.6 nm min−1 at 40 °C | pH-responsive polymers | 116 |
In PECVD, the excitation frequency of the plasma source provides a key tool to modulate the deposition process.100,117–122 Generally, an RF plasma source uses industrial 13.56 MHz frequency for plasma excitation, and for increasing the plasma density to accelerate the precursor decomposition and subsequent film growth rate, the plasma power is increased. However, an increase in power also causes increased ion bombardment, which often proves detrimental to the film quality. To overcome this, very high frequency (VHF; 30–300 MHz)123–125 or dual frequency126–129 plasma configurations are employed. With VHF plasma, increased frequency imparts higher energy to electrons via so-called stochastic heating but reduces the sheath thickness and lowers the electrical field intensity, thereby reducing the ion-bombardment energy.120 A dual frequency configuration involves two different frequencies on the same electrode or on separate electrodes, where high frequency imparts a stable discharge generating the reactive species and the low frequency controls the ion bombardment.119 The dual frequency configuration has proven its utility in improving the deposition quality in terms of film stress, step coverage, and stability.129
In catalyst-mediated PECVD processes, plasma-catalyst interactions play a key role during the pretreatment step and deposition. Such plasma-assisted restructuring or activation has proven to be important for controlling the mechanism, growth rate, composition, morphology, and microstructure.130–135 Restructuring of metal catalysts used for the growth of carbon nanostructures,130–134 silicon nanowires135–139 and other metal/metal oxide nanowires140,141 by plasma pre-treatment or/and during plasma-assisted catalytic vapor–liquid–solid142,143 and solid–liquid–solid141,144 growth mechanisms is well documented. For example, Zheng et al.141 demonstrated that during GeSn nanowire growth using Sn nanoparticle catalysts H2 plasma-assisted annealing inhibited the wetting of Sn during and at the end of nanowire growth, preventing the pinning of Sn NPs around the nanowires. Similarly, during in situ fabrication of graphitic nanosheet supported N-doped carbon-coated LiFePO4 (LFP@NC/GNS), plasma assisted the reduction of Fe2+ ions to Fe0, which catalyzed in situ formation of graphitic nanosheets on LFP@NC, and simultaneously removed  antisites, a commonly observed detrimental defect in LFP for its performance in Li-ion batteries.134
antisites, a commonly observed detrimental defect in LFP for its performance in Li-ion batteries.134
Over the years, there has been a growing trend to integrate PECVD with other deposition techniques for the deposition of a diverse range of materials. In PECVD of rare-earth element-doped Si-based materials, metal-organic precursors are used for in situ metal doping. However, the use of metal-organics causes significant hydrogen contamination. To overcome this limitation, a hybrid deposition technique was developed by in situ coupling PECVD with a magnetron sputtering source having the rare-earth target.145–150 Kulczyk-Malecka et al.150 proposed a high-power impulse magnetron sputtering-PECVD system where the magnetron-generated plasma also acted as an electron source to drive the precursor decomposition in the PECVD process, thus requiring only a single power supply. Moreover, the application of a variable magnetic field strength magnetron resulted in precise control over dopant content without adjusting the power supply. Lee et al.151 used a similar PECVD-sputtering hybrid system to fabricate Ti-doped diamond-like carbon (DLC) coatings, where metal doping helped overcome the residual stress in the deposits. Moreover, a multi-step PECVD or its integration with other deposition techniques resulted in a specific nanostructured assembly.152–156 For example, Huang et al.153 reported an anode-assisted reactive magnetron sputtering coupled with a DC PECVD system to fabricate amorphous hydrogenated DLC coatings with CrC interlayers, ameliorating the adhesion properties of DLC coatings by reducing the residual stress. Dias et al.157 reported large-volume deposition (∼19 mg min−1) of metal oxide/sulfide anchored N-graphene for supercapacitor applications by spraying a controlled metal-oxide(sulfide) microparticle jet into the plasma afterglow region, leading to plasma-induced size reduction and subsequent binding to the N-graphene sheets. Furthermore, Su et al.158 reported an integrated process encompassing plasma-assisted roll-to-roll deposition of vertical graphene (VG, which is graphene-containing carbon nanosheets grown vertically onto the substrate) and simultaneous syngas production from greenhouse gas mixtures. Such hybrid approaches have effectively expanded the application spectrum of PECVD, overcoming the technological barriers of limited precursor availability.
Over the years, manipulation of plasma characteristics and plasma delivery has enhanced the CVD process for tailoring film properties for desired applications. In this regard, PECVD using pulsed plasma flow with dynamic pulse parameters such as pulse frequency, duty ratio, and peak power has displayed remarkable effects on the deposition qualities.51,159–164 Pulse modulation, in combination with other plasma parameters, has been successful in significantly decreasing deposition temperature while maintaining or even enhancing characteristics like growth rate and composition.159,165,166 Interestingly, significant film growth also occurred during the ‘‘off’’ plasma duration167 and the pulsed mode resulted in additional morphological variations.168,169 For example, during PECVD growth of 2D copper sulphide superstructures, Taplick et al.169 observed that site-dependent charging–discharging dynamics during plasma pulsing played a crucial role in the directed assembly of nanoplatelets in the gas phase. The plasma pulsing promoted side-by-side growth, whereas continuous plasma additionally caused pronounced spike-like growth perpendicular to the basal plane. Similarly, pulsed PECVD displayed great usefulness in PECVD of insulating thin films, where substrates need to be under an alternating signal.170 In this regard, it is worthwhile to mention the hybrid plasma-immersion ion implantation and deposition systems for DLC coatings from hydrocarbon precursors, where ion-implantation renders improved tribological and mechanical properties.171–173 Recently, Tran et al.160 used a similar hybrid system to fabricate high surface area activated carbon coatings for biomedical applications, where the growing coatings were subjected to implantation of ion-aggregates formed in the plasma in a high voltage dielectric barrier discharge PECVD.
Moreover, efforts have been made to further modulate pulsed PECVD by coupling it with externally-controlled MF for plasma confinement.162,163,174 To fabricate pinhole-free amorphous silicon carbo-nitride, SiCN, at a high deposition rate, e.g., Matsutani and coworkers162 designed an MF coupled pulsed PECVD system, where a permanent magnet kept below the plasma electrode (which also holds the substrate) in a parallel electrode setup led to an increase in plasma current and deposition rate, as compared to that without MF. In a subsequent study, they generated pulsed plasma alternatively on both electrodes, while keeping the permanent magnet below the substrate electrode, to overcome the etching-induced film thinning due to the charging effect.163 The MF confinement of plasma during the plasma-immersion ion implantation and deposition of DLC coating resulted in improved diamond-like character with defect-free morphology and reduced roughness in as-grown films.174
Plasma-assisted processes have been demonstrated for large-scale fabrication of various carbon nanostructures for electronics, optoelectronics, energy and environmental applications.59,67,175–179 In addition, PECVD processing has enabled a controllable preparation strategy for the growth of desirable carbon nanoarchitectures at significantly lower temperatures112 and even without the need of catalysts,180 ensuring high purity by eliminating metal contamination. In the catalyst-free growth of VG,181 high-energy ion bombardment activated the surface for nucleation by inducing defects and facilitated growth mainly from the surface of the substrate.176,180 PECVD has enabled substrate-independent growth of graphene, facilitating its growth at 700 °C on Si and 3C–SiC/Si surfaces, important in electronic/optoelectronic heterojunction devices, which could not be achieved by thermal CVD due to the extremely low diffusion of carbon over these surfaces, as indicated by the theoretical studies.182 For deposition of amorphous carbon/DLC films, the control over plasma-ion energy by adjusting the bias power and bias voltage has conferred control over the H-content and ID/IG and sp2/sp3 ratios, regulating their amorphous or DLC characteristics and resulting in mechanical, electrical, optical, or tribological properties. For example, high ion density plasma, such as microwave plasma and magnetically enhanced plasma, promoted low H-content whereas low-density RF plasma promoted high H-content growth.68,69 A high ion density helped in the removal of H atoms from C–H bonds and raised the sp2/sp3 ratio in the deposited films.69
An important aspect of catalyst-aided or catalyst-free PECVD of various carbon nanomaterials is its ability to aid in vertically aligned growth, as the electric field associated with the plasma sheath helps the nanofibers grow vertically from the substrate.59,95,183–185 Various studies demonstrate, however, that the mechanistic implications of the role of plasma and other process parameters on the vertical growth mechanism are not well understood.95,186,187 Theoretical studies suggest that during VG growth, the coulombic repulsion due to the accumulated electrons on the flakes eventually push the growth to change from 2D to 3D mode.186 Sun et al.186 studied the role of plasma sheath EF in the mechanism for the growth of vertically grown graphene nanostructures. Using GaN nanowires grown onto a silicon substrate (GaN/Si) (Fig. 3(a)), much denser and longer growth of VG flakes was observed at the nanowire tips than on the nanowire bodies (Fig. 3(b)), indicating a local field-enhancement effect due to the higher field strengths and charge accumulation at the wire tips (Fig. 3(c)). Furthermore, when EF is selectively screened by employing metal meshes of different sizes, growth occurred only along the holes (Fig. 3(d)–(f)), due to partial blocking of the electric field formed in the plasma sheath. Moreover, using SiO2 nanoparticles as substrate, out of two possible modes of growth (Fig. 3(g)), the VG exclusively grew perpendicular to the particles' surface (Fig. 3(h)–(k)).186 These findings set the foundations for further mechanistic studies on the vertical growth of 2D materials.
 | ||
| Fig. 3 Schematic diagram of plasma-assisted vertical growth of carbon nanostructures (a), adopted with permission from MDPI;46 acanning electron microscopy (SEM) images of GaN nanowires (b) before and (c) after the VG growth in remote PECVD; SEM images of growth on the SiO2/Si substrate surface, nearly complete growth inhibition of VG growth when EF is completely screened (d), growth along the mesh holes when EF is partly blocked (e), normal VG growth without screening (f); schematic illustration of possible growth modes of VG on nanoparticles – (left) perpendicular to the substrate and (right) alongside the normal directions of the nanoparticles (g); SEM images confirming the latter growth mode with increasing flow rates of C2H2 and growth times (h)–(k), reused with permission from American Chemical Society (ACS).186 SEM images of VG deposited at 600 °C with different plasma powers, 100 (l), 200 (m), and 300 (n); and schematic showing the difference in growth behavior of VG under different plasma densities (o), reused with permission from ACS.95 | ||
Zhang and coworkers188–191 demonstrated the use of auxiliary vapors (chloroform, fluorobenzene, water, etc.) along with the chemical precursor to generate a special collection of plasma and reactive species to enhance the growth rate of VG in an electric field-assisted PECVD. For example, the introduction of fluorobenzene and water in the deposition chamber promoted the decomposition of the carbon source and etching of amorphous carbon, respectively, to enable higher growth rates (∼14.4 μm h−1).189 Zhang et al.95 observed that milder plasma and temperature conditions are helpful for vertical growth, as the higher growth temperatures, precursor and carrier flow rates, and applied plasma power also promoted branching (Fig. 3(p)), apart from increasing the growth rate (Fig. 3(i)–(n)).
In vacuum or atmospheric PECVD, plasma-assisted effects have demonstrated synergistic regulation of nanostructures and defect engineering in carbon nanostructures.46,93,192–197 For example, Ma et al.93 demonstrated a continuous variation of morphology, from “porous” to “tree-like” and then to “wall-like” structure, by adjusting the growth positions during PECVD of VG. Similarly, Dias et al.198 reported a PECVD approach capable of varying the density and energy of basic building blocks within the high energy-density plasma environment in the assembly zone, allowing precise control over the energy and material fluxes directed towards the evolving nanostructures for sustained gram-scale (30 mg min−1) production of graphene and allied derivatives from a low-cost feedstock of ethanol and acetonitrile. Moreover, plasma-assisted vertically-aligned growth is not limited to carbon-based nanostructures and it has been successfully used in the growth of vertically aligned metal oxides, nitrides, and chalcogenides168,199–206 as well as polymeric and heterostructures.207,208
Plasma-assisted CVD of functional inorganic materials with specific composition, phase, morphology and microstructure has led to promising applications in integrated electronics, energy storage and conversion, catalysis, and sensors.106,168,209–211 Among chalcogenides, transition metal dichalcogenides (TMDs) present unique properties depending on their polymorphic phases, such as 1T (metallic), 1T′ (semimetal-like), and 2H (semiconductor).212 In recent years, the fabrication of phase-pure and polymorphic heterostructures of these compounds has been realized by temperature-controlled plasma-assisted sulfurization/selenization of metal.84,89,110,200,210,213–218 Kim et al.201,210 reported an optimized PECVD system with Ar + H2S plasma for 1T-WS2 and 1T-MoS2 synthesis, which is otherwise challenging due to their metastable nature. The pre-deposited metal was sulfurized by accelerating the H2S+, ionized through the penning effect, in the plasma (positive) to the substrate (relatively negative) by the electric field in the sheath region. The ion bombardment caused the metal layer to break into nanocrystals and the H2S+ simultaneously penetrated it for efficient sulfurization. Unlike the microcrystalline nature of the deposits obtained from other chemical methods, the nanocrystalline structure of 1T-TMDs obtained from PECVD transformed it from metastable to a highly stable 1T phase structure with a lower surface energy than the 2H phase. However, in plasma-assisted sulfurization/selenization at higher temperatures, the 2H phase is predominantly formed.89,219
PECVD has been proven to be a convenient method for the fabrication of few-layer TMD–TMD heterostructure interfaces that exhibit interesting properties for diverse applications, due to their uniform band edges and tunable band gap through numerous layers.200,220 Kim et al.221 executed Ar plasma-induced phase transformation of SnS2 to SnS for the fabrication of a vertical SnS–SnS2 p–n junction heterostructure. The bombardment of Ar+ radicals on the SnS2 surface preferentially cleaved Sn–S bonds leading to selective etching of more volatile sulfur, thereby inducing the formation of the SnS phase. Similarly, Li et al.222 reported fabrication of a vertical SnSe/SnSe2 p–n heterojunction by NH3 plasma-induced phase transformation. Seok et al.200 reported wafer-scale MoS2–WS2 vertical heterostructures via a single-step plasma sulfurization of sequentially deposited seed layers of W and Mo (1 nm each) onto the SiO2/Si substrate. By optimizing the plasma and deposition temperature, Seok et al.220 reported wafer-scale growth of phase-selective 1T-MoS2/1T-WS2 or 2H-MoS2/2H-WS2 vertical heterostructures. Likewise, Sino et al.213 demonstrated a similar top-down approach for high-yield MoSSe asymmetric structures from MoS2 flakes where plasma-assisted selenization resulted in a Janus MoSSe layered structure at low temperature (200 °C) and a polymorphic alloy MoSSe at high temperatures (400–600 °C). Moreover, plasma-assisted sulfurization/selenization has also been extended for the fabrication of heterostructures of TMDs with metal oxides and carbides.110,218,223,224
For the fabrication of photovoltaic devices, PECVD is extensively employed in the deposition of antireflection coatings, passivation layers, dielectric layers, and so on.60,225–228 To ensure high throughput, avoiding the standing wave effect in a large-area uniform deposition process, it is essential to control the spatial distribution of plasma parameters and the complex interplay of plasma physics and chemistry with the deposition-chamber dimensions. Accordingly, numerous theoretical and experimental studies have been performed to address the uniformity of plasma over a large area.122,229 Zhang et al.229 investigated the plasma density distribution under different voltage and pressure conditions in a H2 capacitively coupled plasma (CCP) discharge sustained by multiple consecutive harmonics. Using a 2D fluid model, Kim et al.230 analyzed the nature of the substrate and reactor sidewall material on plasma density distribution in a CCP reactor. They also investigated the role of dilution gas in the spatial distribution of the plasma parameters in a CCP reactor.231 Similarly, in deposition of hydrogenated nanocrystalline silicon oxide (nc-SiOx:H), Yu et al.100 designed an industrial-scale VHF PECVD system with a minimized standing wave effect, by using a curved electrode and a glass plate over it, thus reducing the degree of non-uniformity for VHF1 (27 MHz) and VHF2 (40 MHz) to ±4.6% and ±10%, respectively, as against to ±27% and ±70%, for the same frequencies using a flat electrode configuration and without a glass plate. As a result, the thickness uniformity was improved in nc-SiOx:H layers. When employed in a metric 6 mm screw thread (M6)-size bifacial silicon heterojunction solar cells with silver electrodes, high power conversion efficiency with a narrower distribution range (25.7–25.9%) under VHF1 was observed as compared to the VHF2 (25.3–25.9%). On replacing the Ag with Cu electrodes, the device displayed a power conversion efficiency of 26.41%. Besides enhancing the growth rate, dual-frequency PECVD operations have effectively improved film stress, chemical composition, step coverage, and stability.37,126,232,233
As plasma characteristics are highly dependent upon plasma sources or/and coupling mechanisms, some desirable effects for specific applications may be attained only by a particular plasma source.32 For example, during the growth of carbon nanostructures using an RF plasma source, the ICP led to vertical graphene, whereas the CCP led to carbon nanotubes (CNT) due to the coupling mechanism affecting the plasma density.234 From the standpoint of innovation in plasma processing and confinement for PECVD, magnetized plasma sources such as helicon wave plasma (HWP) and electron cyclotron resonance plasma, in which the excited electrons are launched as spiral beams along the MF lines as a function of the MF and excitation frequency, enable a resonant-like increase of plasma density useful in various applications.235–239 The HWP source, with its electrodeless and magnetized plasma features, has provided an important means to regulate the energetic ion-bombardment flux on the stress level, structure, and resulting properties of the deposited films. Along with the flow rate, parameters – RF power and MF strength – can be used to regulate the high-density HWP.240 Accordingly, HWP-CVD is employed for the deposition of carbonaceous,42,239,241–243 metal carbide,244,245 and metal oxide246 coatings. During PECVD of DLC coatings, high plasma density of HWP enabled a high growth rate.247,248 Qian et al.249 reported room temperature PECVD of DLC in HWP with different magnetic field (B0 = 1200–2400 G). The optical emission spectroscopy analyses indicated that the precursor decomposition followed the pathway (CH4 + e− → CH3 + CH2 + CH + C + H), along with the formation of C2 species by the recombination of CH4 and other C-containing radicals. An increased MF provided high plasma density for achieving a higher growth rate. The C2 and CH emission intensities increased to a maximum value at B0 = 2100 G, leading to an enhanced deposition rate (800 nm min−1) and sp3/sp2 ratio, as numerous energized H atoms generated by HWP helped in graphitic carbon removal and hydrogen incorporation.
An electron cyclotron resonance plasma source is another example where MF-assisted electron confinement plays a key role in plasma density enhancement, influencing the PECVD growth rate.236,238,250,251 For example, during epitaxial growth of silicon thin film, the MF variation shifted the ECR plasma zone close to the SiH4 outlet, causing increased concentration of free radicals responsible for deposition and resulting in an increased growth rate.236 Nevertheless, the MF-assisted plasma confinement for enhanced growth and/or microstructural evolution has not been limited to a magnetized plasma sources alone, as demonstrated by Miller et al.237 with an RF-powered hollow cathode PECVD system for DLC films. The MF applied via an electromagnetic coil around the hollow cathode tube influenced the magnitude of cathode DC self-bias, with an MF of ∼95 G inducing maximum DC self-bias, indicating an optimal gas ionization that was independent of the applied RF power. Similarly, in a CCP system, the application of an MF (typically order of ∼102 G) parallel to the electrodes normally enhanced the performance of CCP, although stronger MF may cause plasma non-uniformity.252 Moreover, combined plasma, magnetic and electric field (substrate bias voltage) modulation to control the number of electrons and their movement path, influencing the ability of electrons to collide and ionize the gas for the deposition of DLC films is reported.91 Nevertheless, recently, some efforts focusing on plasma diagnostics and measurement of flux and energy of plasma and reactive species in magnetically confined plasma have been reported,252–256 which could be valuable for a better understanding of plasma chemistry to design suitable deposition conditions for film growth and microstructure.
In recent years, the requirements of fast processing with minimal crystalline defects in many device fabrication processes with enhanced plasma density at controlled ion-bombardment have prompted the testing of a variety of plasma excitation and delivery methods, such as gas-jet electron beam plasma,257–260 hallow-anode plasma,45,261,262 and radical injection plasma.45,263–266 There have been reports on the usage of highly ionized plasma, designed using continuous or pulsed high power schemes and often coupled with substrate bias to impart a high degree of energetic bombardment, influencing the deposition conditions and resultant phase, microstructure and quality.267–270 High-power plasma conditions involving excited ions with high power densities and ion energy have been important in the 3D growth of carbon nanostructures (thickness, branching, alignment height, etc.).271 For example, the nucleation of carbon nanowalls in the initial growth period occurred only under optimal balance between ion and radical fluxes, with ion bombardment modifying the surface adsorption mechanism from physical to chemical.266,272 It is important to mention here, however, that although it is generally accepted that high ion energies (∼200–250 eV) and ion fluxes (∼3.3–3.8 μA cm−2) are necessary to induce nanowall deposition, there are many reports of PECVD growth of nanowalls in low-energy ions, following a different growth mechanism.59,273,274
Plasma-enhanced chemical vapor deposition is also extended to the deposition of organic polymer films as an initiated-PECVD (i-PECVD) process where usually a plasma of low power density is used for quasi-selective breaking of the weak peroxide bond (O–O) in the initiator molecules, facilitating subsequent polymerization of monomers.275–280 Alternatively, i-PECVD is also performed directly with monomers only, without using any initiator.281,282 However, limiting the purview of this review, the reader is referred to the existing literature283,284 for detailed information. Similarly, as an alternative to plasma-assisted precursor decomposition, localized substrate-heating by a high-energy (often in the keV range) focused ion or electron beam is used, enabling nanoscale-level control over the material fabrication in CVD. For detailed information about such focused ion/electron beam-assisted CVD, relevant ref. 285–290 can be referred to. Here, the recent reports of plasma electron-assisted CVD of metallic films105,291–293 are notable, which rely upon electron-induced redox chemistry, unlike the thermal effects in focused ion/electron beam-assisted CVD.
2.3 Perspective on plasma-enhanced chemical vapor deposition
Modulations based on nonconventional energy vectors (plasma and photons) and fields (electric field and magnetic field) have significantly enriched the landscape of CVD, enabling the facile deposition of unique nanoarchitectures with desired properties at significantly low temperatures and faster rates compared to the conventional thermal CVD. The use of auxiliary gases along with the key precursor to generate a special collection of plasma and reactive species in the reactor has constantly been popular, enabling lowered deposition temperature and/or increased deposition rate.74,189,190,294 Future opportunities lie in designing novel configurations of plasma power, precursor and reactor design, controlling the plasma parameters (energy density and the composition and density of species such as photons, ions, excited fragments, etc.) for better control over the kinetics and imparting added functionalities (vacancies, dopants, etc.) in the deposited materials. With a judicious selection of plasma chemistry and substrate attributes, for example, PECVD can perform area selective deposition.98,293,295–298 PECVD has been more focused in utilizing the energy of plasma electrons, often ignoring the role of ionic species, which can be partially due to the emergence of related technology of plasma-immersion ion implantation and deposition. Increased attention towards tuning the plasma ionic species can provide an additional degree of freedom for influencing the properties of the thin films grown by PECVD.37,299,300Given the fact that the plasma-assisted processes are quite complex and involve numerous physical and chemical processes, the study of the deposition and growth mechanism has always been challenging. Additionally, most of the reported data on PECVD are largely based on the in-house plasma source and reactor, making the comparison of data somewhat challenging. Therefore, generalized relations between film characteristics and growth parameters such as temperature, dosage of precursors and carrier gases, and plasma power are still less established to date, hindering its utility in large-scale production of advanced materials and devices for specific applications. To overcome this, greater attention on establishing a generalized and direct correspondence between the plasma state, thermodynamic conditions and characteristics of deposited structures is required.
3 Photo-assisted chemical vapor deposition
3.1 Photoirradiation-matter interaction during chemical vapor deposition
Photoirradiation-based processing serves various applications, including deposition, doping, chemical etching and ablation.301–303 Photo-assisted or photo-initiated CVD utilizes photons generated from sources such as gas discharge lamps, inert gas-ion lasers, solid-state lasers, or excimer lasers to drive precursor decomposition. Typically, photons in the visible or ultraviolet (UV) spectrum (wavelength ∼100–700 nm) are used, though infrared (IR) lasers are occasionally employed for precursor decomposition.304,305 Precursor decomposition may occur through thermal effects (pyrolytic decomposition), non-thermal effects (photolytic/photochemical decomposition), or by a combination of both photophysical processes.306,307 In addition to temperature, factors such as pressure, nature of the reactant, and thermal conductivities of the substrate, gas, and resulting deposit also influence the deposition rate during the pyrolytic process.308In the pyrolytic process, a suitable laser is typically used to heat the substrate locally, creating a Gaussian distribution of irradiance on the focused region. This ensures that only the central part of the focused spot reaches the desired temperature for precursor decomposition and deposition. Conversely, in the photolytic process, directed radiation activates precursor molecules (e.g., AB) on the substrate or in the gas phase (eqn (3)), leading to dissociation (eqn (4)) or reactive dissociation (eqn (5)), resulting in decomposition and deposition.
| Absorption: AB + hν → AB* | (3) |
| Dissociation: AB* → A + B | (4) |
| Reactive decomposition: AB* + D → E + F | (5) |
Additionally, if the energy supplied by the UV lamp or laser is high enough, ionization (eqn (6)–(8)) and radical formation (eqn (9)) are concomitant reactions, leading to the formation of reactive species.309,310
| Ionisation: AB + hν → [AB]+ + e− | (6) |
| AB + hν → A+ + B + e− | (7) |
| AB + hν → A+ + B− | (8) |
| Radical formation: AB + hν → A˙ + B˙ | (9) |
In the photolytic process, precursor decomposition can occur at temperatures significantly lower than the pyrolysis temperature. This is achieved through energy transfer from the excited electronic states to the vibrational states of the precursor molecules.311 The fraction of excited molecules undergoing bond dissociation and the corresponding reaction rate (Ra) can be represented using a modified Beer's relationship, eqn (10)
Ra = (I0/hν)F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−σλpl/kT) exp(−σλpl/kT)
| (10) |
Photolytic PACVD typically employs vacuum ultraviolet (VUV, wavelength <200 nm) lamps or lasers, emitting high-energy (5–15 eV) photons capable of cleaving most chemical bonds, as the initiation source. However, the limited light transmission at these wavelengths necessitates specialized reactor windows, such as MgF2 and LiF, or advanced reactor designs. To overcome these limitations, short-wave UV light (200–280 nm) is often used, which can be transmitted through cost-effective quartz windows. This approach requires careful selection of precursors with reactive chemical bonds that can be activated at these wavelengths. To broaden the range of applicable precursors, especially for the deposition of polymers, photosensitizers and/or photo-initiators are used along with the monomers.315
3.2 Overview of photo-assisted chemical vapor deposition
The PACVD technique was initially developed to meet the demands of the semiconductor and microelectronics fabrication industry. Early reports of pyrolytic laser-assisted CVD (LACVD) include the deposition of silicon using a 50 W infrared CO2 laser316 and the deposition of carbon using a visible Ar+-ion laser.317 Important studies by Liu et al.,318,319 Ruzin and Nemirovsky,320 Irvine et al.,321 and Santos et al.322 explored the theoretical and mechanistic aspects of LACVD. Comprehensive discussions of the key principles underlying PACVD can be found in earlier reference works.312,323,324 Similarly, reviews by Sankur,325 Zhao et al.,22 and Hwang et al.326 provide valuable insights into the advancements and applications of PACVD. Table 2 presents selected reports on PACVD, displaying its importance in thin film fabrication for a range of materials.| Material/substrate | Photon source/reaction | Key features | Application | Ref. |
|---|---|---|---|---|
| a DLE – deep level emission; HEMA – 2-hydroxyethyl methacrylate; NBE – near-band-edge emission; PTFE – polytetrafluoroethylene; PO2 – oxygen partial pressure; PL – photoluminescence; TDMAD – tetrakis(dimethylamino)diboron; YAP – yttrium aluminum perovskite; YAG – yttrium–aluminum garnet. | ||||
| Carbon-based materials | ||||
| Diamond/Si (100) | Laser (wavelength: 532 nm)/photolytic | Coupling of laser with PECVD for increased crystal growth | Coatings; hardness = 91 GPa, Young's modulus = 721 GPa | 327 |
| Diamond/WC or cobalt | UV laser (wavelength 193 and 248 nm)/photolytic | Nondiamond carbon accumulation was minimised | — | 328 |
| Diamond/SS 316 | CO2 laser | 19 μm-thick diamond coating with a quality factor of 96% | Coatings; enhanced adherence with metallic substrate due to stress relief and improved mechanical bonding | 329 |
| Diamond/WC | Wavelength-tunable CO2 laser | Enhanced growth rate by resonant vibrational excitation of the CH2-wagging mode in C2H4 molecules | Synthetic diamond; grain size without laser (2.6 μm) increased to 10.494–14.3 μm with laser | 330 |
| Carbon-based materials/Si or Cu | Nd:YAG laser | Pulsed laser CVD; Cu foil was a more suitable substrate to grow pyrolytic carbon than SiO2 | Pyrolytic carbon with graphene as its fundamental building block | 331 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Metalloids | ||||
| SiOC–SiC/graphite | CO2 laser/pyrolytic | Temperature-dependent auxiliary formation of amorphous SiO2, β-SiC nanocrystals, and/or Si crystals | — | 332 |
| 3C–SiC/Si (001) epitaxial films | YAG diode laser | Density of microtwins was controlled by dilution gas (H2) flow | — | 333 |
| SiC–TaCN/Si3N4 | Nd:YAG laser | For 1000 °C deposited film, hardness and Young's modulus ≈30.4 and ≈232 GPa, respectively | Tribological coating; (extreme penetration = 59.0 μm and surface roughness = 3.5 μm) | 334 |
| Silicon nitride | InGaAlAs diode laser/photolytic | Lowest deposition temperature (1100 °C) so far for crystalline film; max. rate 972 μm h−1 at 1300 °C | Coatings; Vickers microhardness and nano-hardness 25.1 GPa and 34.8 GPa at 1300 °C, respectively | 308 |
| SiN encapsulation film | ArF excimer laser | Two-step fabrication of SiN film using LACVD and LAPECVD | Thin-film encapsulation for organic light emitting diodes (encapsulation increases lifetime by 3.59 times) | 335 |
| Boron carbon oxynitride/Si | Nd:YAG laser, 1050 °C; TDMAD precursor | Composition BwCxNyOz was varied by regulating the flow rate of the oxidation gas (i.e., air) | Phosphor; PL emission bands at 386–570 nm; intense emission bands at 534 and 570 nm for C- and O-rich films | 336 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Metals, oxides, chalcogenides, nitrides | ||||
| Ru on thiolated self-assembled monolayers (SAMs) | Hg arc lamp | Ru and RuOx deposit on –CH3- and –OH-terminated SAMs; no deposition on –COOH-terminations | — | 337 |
| N-doped α-Fe2O3/Si | Ar+ laser/pyrolytic; pulsed Nd:YAG laser | Ar+ laser for deposition and pulsed Nd:YAG laser for crystallization and minimize the surface irregularities | Magnetic devices and photoelectrodes | 338 |
| Li-doped NiZnO/c-axis oriented sapphire | Tungsten–halogen lamp (cutoff wavelength ∼200 nm) | Compared to CVD, PACVD results in decreased defect density and lattice stress | Optoelectronics; NBE/DLE ratio = 15.6 for PACVD compared to that of 3.2 for CVD | 339 |
| SmBa2Cu3O7−δ/LaAlO3 | Semiconductor laser, (wavelength: 808 nm) | PO2 dependence of deposition rate, critical temperature (Tc) and critical current density (Jc) | Superconductive films; highest Tc (89.2 K) and Jc (2.04 MA cm−2) for film deposited with PO2 = 200 Pa | 340 |
| Eu3+-, Dy3+-, or Tb3+-doped HfTiO4/quartz | Diode laser (continuous-wave mode; 1470 nm; 75 W) | Fast deposition rate (36 μm h−1) | Phosphor; Eu3+, Dy3+, and Tb3+ activated films displayed red, yellow, and green photoluminescence | 341 |
| HfO2/Si(100) | InGaAlAs diode laser/pyrolytic | Max. deposition rate (362 μm h−1) was 102–104 times higher than that obtained using existing methods | High-dielectric constant (k) material; k = 16–22 | 342 |
| Arsenic doped p-type ZnO/(GaAs/Al2O3) | Tungsten–halogen lamps (∼200 nm cutoff; total input power 600 W) | The GaAs layer acted as a doping source and its thickness controlled the doping level | Photoelectric device; hole concentration 3.1 × 1016 to 2.4 × 1017 cm−3 | 343 |
| Tb3+-,Eu3+-doped YAP/(100) SrTiO3 | CO2 laser (10.6 μm; 60 W), ZnSe window | Deposition rate (53 μm h−1) 50–90 times faster than for thermal CVD at 900–1003 °C | X-ray scintillation screen; fluorescence decay constants 1.89 ms (Tb3+:YAP) and 1.96 ms (Eu3+:YAP) | 344 |
| Ce3+-,Eu3+-doped YAG-Al2O3 ordered eutectic system | CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) laser (10.6 μm; 60 W), ZnSe window, 791–1030 °C laser (10.6 μm; 60 W), ZnSe window, 791–1030 °C |
First report on the formation of an α-Al2O3 rod-like structure in the YAG-Al2O3 composite | X-ray scintillation screen | 345 |
| Al-doped β-Ga2O3/c-axis oriented sapphire | CO2 laser (10.6 μm; 60 W), 750–900 °C | Fast epitaxial deposition (15 μm h−1) | X-ray scintillation screen; scintillation light yield = 5400 photons per 5.5 MeV, decay response = 5.1 ns | 346 |
| Gallium nitride/(0001) GaN-on-sapphire | CO2 laser (9.219 μm; 250 W)/photolytic | ∼60% reduction in carbon impurity incorporation (5.5 × 1015 cm−3); (max. growth rate 4.5 μm h−1) | Electron mobilities were 600–750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) and 750–1275 cm2 V−1 s−1, respectively, for normal and laser-assisted growth and 750–1275 cm2 V−1 s−1, respectively, for normal and laser-assisted growth |
311 |
| GaN/GaN | CO2 laser (9.219 μm; 200 W)/photolytic | High laser power suppressed the incorporation of carbon impurity | Trap concentrations were higher in laser-assisted GaN (up to 2 × 1015 cm−3) | 347 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Others | ||||
| Poly(HEMA-co-4VP)/electrospun cPCL | UV lamp, tert-butyl peroxide as initiator | piCVD-assisted crosslinking | Polymer degradation and drug delivery | 348 |
| Oligomeric film on CNTs | Short-wave UV lamp/photolytic, H2O2 initiator | piCVD; vertical chemical gradient formation to control hydrophilicity/hydrophobicity | Surface modification (wettability control) | 349 |
| Janus HEMA-PTFE membrane | UV (254 nm)/photolytic | piCVD | Membrane distillation (antifouling effect) | 350 |
Photo-assisted CVD has been widely used for the deposition of metals,351 oxides,352 carbides,334 nitrides,311,347 carbonitrides,334 and organic polymers,353 targeting applications such as coatings and functional materials. PACVD has demonstrated significant versatility in tailoring the characteristics of deposited materials by independently or synergistically modulating the characteristics of photo-radiation such as intensity or source type, or integrating it with substrate heating or PECVD processes. Akazawa et al.354,355 reported increased reactivity of the GeH4 precursor in PACVD of germanium on an SiO2 substrate using a VUV beam as the heat source, due to its gas phase photolytic decomposition. Lari et al.356 reported a continuous aerosol photopolymerization technique for coating nanoparticles, where photosensitive nanoparticles initiated surface photopolymerization of monomers onto their surfaces. Kasparek et al.357 studied the influence of different VUV sources on thiol-terminated films deposited from C2H2 + H2S gas mixtures, indicating that the absorption coefficients of gases significantly affected the photolytic reaction kinetics and resulting film composition.
Photo-assisted MOCVD has resulted in the deposition of oxide films (TiO2, NiO, ZnO, and NiZnO) with improved crystallinity.339,343,358,359 It also provided a convenient method for depositing metals at a reasonable growth rate on thermally delicate structures, such as self-assembled monolayers.337 McElwee-White and coworkers extensively investigated the photochemistry of suitable precursors,337,351,360–362 indicating that the secondary photo-processes often strongly influenced the overall efficacy of precursor decomposition.337
In laser-assisted CVD, depending upon the laser fluence and mode of its application (direct or parallel to substrate, scan speed, etc.), the laser-matter interaction can be expressed via laser pyrolysis,332,363,364 laser photolysis328,365–367 or laser resonance sensitization.368–370 LACVD displayed its utility in the fabrication of thin films of metals,371–373 oxides,338,344 nitrides,152,308,311,347,374 carbides,375 sulphides,376–378 carbon-based materials,329,331,379 and composites.334,380,381 Several general or material-specific reviews highlight the versatility of LACVD.307,370,379,382–388
Odusanya et al.331 investigated the role of deposition parameters (gas flow rate, temperature, laser power, time, substrate) in a pulsed laser-assisted CVD of carbon nanomaterials. Um et al.379 reported laser-assisted direct writing of >10 nm thick highly-ordered graphite film on a Ni substrate. Wu et al.329,389 utilized femtosecond laser texturing of metal substrates to improve the adhesion of diamond coatings in laser-assisted combustion flame CVD. Moreover, laser-assisted photolysis of hydrocarbon precursors has been shown to effectively suppress nondiamond carbon formation and promote preferential crystallographic orientation in CVD diamond.327,328,390
Tu et al.342 reported LACVD of HfO2 films via a single growth process using an InGaAlAs diode laser that created a lateral temperature gradient (100 K mm−1) over the Si(100) substrate and resulted in four regions with different deposition temperatures (Fig. 4). The four regions followed different deposition kinetics, resulting in differential phase, microstructure, and morphological developments in these regions. The maximum deposition rate was 102 to 104 times higher than that in previously reported deposition methods. On the other hand, Zhang et al.380 achieved a vertical temperature gradient between the top and bottom of the coating during the growth of graphene/SiC composites over Si nanowires. The light-trapping structure of nanowires enabled full utilization of the photothermal effect of the laser at the top of nanowires, resulting in a larger surface area in the deposited film.
 | ||
| Fig. 4 SEM images of the cross-sections and surfaces of HfO2 films in the four regions, obtained using the high-throughput growth process. (a), (e), (i), (m) region I at T = 1300 K, (b), (f), (j), (n) region II at T = 1400 K, (c), (g), (k), (o) region III at T = 1500 K, (d), (h), (l), (p) region IV at T = 1600 K, respectively. T represents deposition temperature, reused with permission from Royal Society of Chemistry.342 | ||
Zhang and coworkers380,391–393 utilized LACVD to fabricate graphene/cubic silicon carbide (3C–SiC) composite films using hexamethyldisilane as a single precursor. In these composites, the formation of graphene relied on the multi-step decomposition of hexamethyldisilane, depending on the deposition temperature and pressure:391
At lower deposition temperature and high pressure (i.e. at lower phonon energy per hexamethyldisilane molecule), hexamethyldisilane decomposition mostly proceeded to step (2), resulting in a graphene-rich graphene/3C–SiC composite. However, at high deposition temperature and low pressure (i.e. at sufficient phonon energy per hexamethyldisilane molecule), complete decomposition of hexamethyldisilane ensured sufficient supply of [(CH3)n–Si] clusters, resulting in pure epitaxial SiC films. A high distribution of graphene at the bottom of the film in transmission electron microscopy (TEM) analysis further supports this mechanism, as the formation of graphene predominantly occurred in the early stages of the heating ramp.
Laser-assisted CVD has emerged as a preferred method for the maskless fabrication of microdevices.307,364,379,394 Laser-assisted vapor phase epitaxy, a variation of LACVD, resulted in selected area epitaxial growth of high structural and optical quality layers of III–V compound semiconductors, without masking or surface structuring.395 LACVD has achieved a growth rate of ∼84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μm h−1 compared to ∼2–3 μm h−1 in normal CVD for the deposition of gallium nitride (GaN) transistors and diodes.347,396 In CVD of GaN using ammonia as a nitrogen source, poor decomposition efficiency of ammonia leads to abundant carbon impurities. Conventional approaches to overcoming carbon impurities, such as increased carrier gas flow and high NH3-to-Ga precursor ratio, have negatively affected the growth rate. LACVD has helped solve this issue without compositing with the growth rate. For example, by using a high-power CO2 laser in LACVD of GaN film from a trimethylgallium precursor, Zhao and coworkers311,397 achieved >60% reduction in carbon content with a high growth rate (4.5 μm h−1). A strong interaction of the CO2 laser with NH3 molecules at a wavelength (9.219 μm) matching with the rotational–vibrational transition of N–H wagging mode (1084.63 cm−1) of NH3 facilitated the NH3 decomposition.311
μm h−1 compared to ∼2–3 μm h−1 in normal CVD for the deposition of gallium nitride (GaN) transistors and diodes.347,396 In CVD of GaN using ammonia as a nitrogen source, poor decomposition efficiency of ammonia leads to abundant carbon impurities. Conventional approaches to overcoming carbon impurities, such as increased carrier gas flow and high NH3-to-Ga precursor ratio, have negatively affected the growth rate. LACVD has helped solve this issue without compositing with the growth rate. For example, by using a high-power CO2 laser in LACVD of GaN film from a trimethylgallium precursor, Zhao and coworkers311,397 achieved >60% reduction in carbon content with a high growth rate (4.5 μm h−1). A strong interaction of the CO2 laser with NH3 molecules at a wavelength (9.219 μm) matching with the rotational–vibrational transition of N–H wagging mode (1084.63 cm−1) of NH3 facilitated the NH3 decomposition.311
Laser-assisted PECVD (LAPECVD) processes are developed for electronic and optoelectronic applications to avoid key drawbacks of PECVD, such as ion bombardment-induced substrate damage, nonstoichiometric composition, and residual stress.152,335 For example, Kim et al.152 used an ArF laser along with the NH3/SiH4 plasma (Fig. 5(a)) for the dissociation of reactive gases during the deposition of silicon nitride films. The LAPECVD-grown film displayed increased deposition rate (Fig. 5(b) and (c)) and lower residual stress compared to the normal PECVD-grown film (Fig. 5(d)), due to the shift in composition towards the stoichiometric Si3N4. The LAPECVD-grown film displayed higher etching resistance due to the high film density resulting from its higher nitridation. Moreover, the organic light-emitting device employing the LAPECVD-grown passivation layer displayed improved performance without any electrical damage.
 | ||
Fig. 5 (a) Schematic of the LAPECVD system; deposition rates for PECVD and LAPECVD, measured as functions of NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiH4 ratio (b) and RF plasma power (c); and (d) residual stress of silicon nitride films with plasma RF power, reused with permission from Elsevier.152 SiH4 ratio (b) and RF plasma power (c); and (d) residual stress of silicon nitride films with plasma RF power, reused with permission from Elsevier.152 | ||
The initiated chemical vapor deposition (iCVD) is widely used for organic and hybrid polymer films, where thermal activation of a volatile initiator is employed to commence the polymerization of monomers.353 In iCVD, thermal activation generally needs an active cooling strategy to keep the substrate temperature conducive for adsorption, which can be arduous for complex or poorly thermal-conducting substrates. To address this, initiation-polymerization can be achieved by gas phase or surface photoactivation of initiator/monomer(s) in photo-initiated CVD (piCVD), thus eliminating the requirement of high-temperature sources.279,315 Typically, piCVD needs a judicious selection of a primary monomer reaction mixture, often without any initiator and sensitizer, based on its capacity to undergo photo-induced polymerization in the gas phase or onto the substrate. In recent years, piCVD has been widely used for the deposition of polymeric films and the surface functionalization of nanomaterials.22,348,349,353,398 The utilization of photon energy to initiate and propagate the polymerization process in functional films for biochemical/biological applications has eliminated the requirement of potentially toxic initiators and crosslinkers.348,399,400 The piCVD has been preferred for surface modification.22,315,348,349,398,401 In this regard, to overcome the constraints of a limited range of photochemically active precursors, initiators and sensitizers, a syngas piCVD is proposed, where syngas (H2 + CO) is photoexcited by short-wave UV radiation to produce radicals and reactive species.315,398,402,403 Tavares and coworkers studied the kinetics of syngas piCVD,315,402,404 and employed it for the deposition of thin films and surface treatment of polymers and nanoparticles.398,405–407 The piCVD achieved large-scale (5 g) encapsulation of magnetic iron oxide nanoparticles in a jet-assisted fluidized bed configuration.406 The piCVD is used to regulate the surface wetting behavior.349,407,408
Photo-assisted manipulations employing the ablation and annealing effects of laser are extensively used complementary to the main CVD process, influencing the composition, crystallographic orientation, and morphology of the substrate and ‘preparing’ it for the deposition of a desired material in the subsequent CVD step.326,375,409,410
3.3 Perspective on photo-assisted chemical vapor deposition
Photo-assisted manipulations in CVD enable the deposition of films and arrays with impressive substrate adhesion, high quality, and high deposition rate. Using PACVD, low-temperature deposition of selected area (using laser) and relatively large area (using UV lamps) film on thermally labile substrates is easily achieved. Unlike the PECVD where bombardment of aggressive plasma species may cause some damage to the film, PACVD-grown films are free from such artifacts. However, for using a VUV source, window composition has been one of the major factors in reactor design. The range of photolytic PACVD has been limited by the availability of photosensitive reactants. Photosensitizers and photoinitiators are employed to extend this range; however, their high cost is still a major constraint. The paucity of a universal and readily activated photoinitiator, such as the thermally activated peroxides in iCVD, is still a concern.Syngas piCVD has been a convenient and economical method for organic film deposition and surface functionalization. However, it is limited to carbonaceous materials and requires further understanding of the role of syngas composition and iron species on deposition chemistry and kinetics. Moreover, organic substrates and deposited films can often be susceptible to photo-induced degradation, limiting the quality of the films.
PACVD and its variants provide a convenient method for the deposition over thermally delicate structures, such as self-assembled monolayers. However, to achieve this, the development of new precursors with a greater understanding of their photochemistry is desired. PACVD is not suitable for deposition on transparent substrates. Similarly, the importance of LACVD rests in ensuring selective area deposition; however, the area of deposition is limited by the laser spot size. The Gaussian distribution of irradiance around the focused spot even renders it less suitable for nanoscale direct writing over a large area. In this regard, plasmon-assisted CVD, suggested by Boyd et al.,411 where localized heating induced by the surface plasmon of nanostructured metals is used for nanoscale heating and subsequent deposition, can be an alternative in some cases, especially where contamination by nanostructured metal layer is not an issue. However, a potential use of this Gaussian distribution of laser irradiance can be in creating a nanoscale temperature gradient during the molten metal-catalyzed growth of nanostructures. This, under some conditions, can manifest in interesting physicochemical effects on deposition and growth kinetics, e.g., the Marangoni effect in SiO2 during field-assisted CVD growth of CNTs412 and in differential phase, microstructure, and morphological developments in HfO2.342 Moreover, the idea of using a laser for selective alteration of specific chemical bonds or excitation of vibration modes in reacting species and thus imparting chemical selectivity413 is less explored in CVD and needs greater attention.
4 Electric field-assisted chemical vapor deposition
4.1 Electric field-matter interaction during chemical vapor deposition
The interaction of the external electric fields with matter (atoms or molecules) has a range of effects, with the Stark effect displaying the splitting of energy levels being the most prominent manifestation. Theoretical calculations suggest that an EF of 1–10 V nm−1 can alter the Ea of gas phase reactions by several kJ mol−1. The application of such an intense EF on reaction volume is challenging due to the dielectric breakdown of the medium.414 However, an externally controlled moderate EF provides efficient ways to alter the reaction chemistry via its direct interaction with the participating species (atoms, molecules, or other complex matter), influencing the electron transfer/charge migration, geometry and/or bond strength, and interfacial behavior.415–417 When an external EF is coupled with the CVD process, the precursor molecules having a permanent or induced dipole interact with the field and gain kinetic energy, increasing their probability of reaching the substrate surface.418 The gaseous precursor may assume a preferred orientation towards the substrate and, if EF is sufficiently strong, some of the bonds in precursors get activated along the direction of the dipole, reducing the energy of nucleation and executing a preferred growth orientation.23During the CVD process, externally controlled electrical biasing of the substrate-containing electrode plays an important role in bias-enhanced nucleation (BEN)90,419,420 and bias-enhanced growth.421 In processes involving the gas phase formation of charged nanoclusters, the application of electrical bias directs these clusters toward the substrate, where they act as nucleation centers for deposition.419 For example, during the deposition of diamond and carbon nanotubes, the gas-phase flux contains, respectively, the positively and negatively charged carbon fragments. Therefore, the substrates, which are negatively-biased (in diamond deposition) and positively-biased (in carbon nanotube deposition), promote nucleation and growth (Fig. 6(a)).424–428 Additionally, the nature of external electrical bias (i.e., DC/AC) has been an additional control parameter, with voltage and AC bias frequency significantly affecting the microstructure evolution and growth rate.23,422,423,429
 | ||
| Fig. 6 (a) Schematics of the bias effect on the evolution process for the granular structure of diamond films with (w-E) and without (w/o-E) EF. The bias condition displayed an increased and instantaneous nucleation (i), moderate grain-growth (ii), and coexistence of some graphitic phase (iii), reused with permission from ACS;421 (b) schematic showing a single-walled carbon nanotube in an EF (i) and its vibration in an EF (ii);422 (c) SEM images showing the effect of EF on the growth of vertical graphene arrays and graphene nanowalls, reused with permission from Wiley;188 and (d) effect of electrical bias on plasma appearance in PECVD of diamond films.423 | ||
From a non-classical nucleation point of view, the charged nanoclusters originating at the initial stages of precursor decomposition in CVD act as building blocks for 1D nanostructures such as nanowires and nanorods. For CVD growth of such nanostructures, the applied external electrical bias, apart from influencing their velocity and population at the substrate via a repelling or attracting influence, also provides directional control over the growing nanostructures.430 For example, during EF-assisted growth of 1D materials such as CNTs, the dipole-induced torque directed the growing 1D nanostructure to align along the EF, enabling a sustained oriented 1D growth (Fig. 6(b)).188,431 Similarly, external EF-induced in situ manipulation helped the formation of doped nanostructures, e.g., by inducing electromigration of dopant species,432 and homojunctions, e.g., by sequential growth of the Sb-doped p-type ZnO microwires (with EF) along the undoped n-type ZnO microwires (without EF).433
In PECVD, external EF has been used to influence the distribution of plasma around the substrate. The negatively charged plasma wall, due to the higher mobility of electrons compared to the ions, is attracted or repelled toward the substrate, depending upon the substrate bias (Fig. 6(d)). The external EF also led to the decoupling of ion flux and ion energy251,263,434 and exerted control over the electric field concomitant with the plasma.183,435,436 Such external bias-controlled ion energy and plasma flow characteristics have influenced various phenomena such as ad-atom migration, desorption, etching, and displacement of lattice atoms in the growing film, affecting its characteristics-growth rate, crystallinity, density, and stoichiometry.437,438
4.2 Overview of electric field-assisted chemical vapor deposition
The use of externally applied electrical bias in CVD can be traced back to the work of Sawabe and Inuzuka24 on the hot filament CVD (HFCVD) of diamond film, displaying an increased deposition rate due to a 150 V bias-induced enhanced electron bombardment on the substrate surface. Yugo et al.25,419 used an external EF-induced pre-deposition process to generate diamond nuclei under a minimum bias voltage of −70 V during the PECVD of diamond. Yoshimura et al.439 reported the fabrication of molecular-aligned polymer film by employing a strong (0.78 MV cm−1) external EF. The external EF-assisted CVD process gained momentum in the 1990s,418,439–441 and was well-received during the 2010s.23,433,442–454 It has been employed in CVD of metal455 and 1D/2D materials (hexagonal boron nitride,456 carbon microcoils and nanotubes,28,457 and graphene183), AACVD of metal oxides,449,453,454,458–461 and PECVD of microcrystalline silicon.462 The EF assistance in CVD has been focused on manipulation of deposition and growth,463 growth direction and crystallographic orientation in nanostructures,183,433,454,464 defects,435 and selective growth (phase composition and crystallinity).431,433,465 Table 3 presents selected examples of EFCVD used for the deposition of functional coatings.| Material/substrate | Bias | Key effect of EF | Application | Ref. |
|---|---|---|---|---|
| a Ca – specific areal capacitance; C– specific capacitance; j – photocurrent density (in μA cm−2); TRC – thermal contact resistance. | ||||
| Metal oxides, carbides, nitrides, etc. | ||||
| ZnO/Si | 0–700 V | No deposition in the absence of EF; shape and size changes with increasing bias | — | 466 |
| β-Ga2O3/sapphire | 0–80 V | Improved growth rate & crystal quality; a blue shift in absorption with increasing V | Direct wide band gap semiconductors; Eg = 4.59–4.94 eV | 465 |
| ZnO, CdO and PbO/fluorine-doped tin oxide (FTO) | ∼625 V cm−1 | EFCVD films displayed enhanced photocurrent in 0.1 M Na2SO4 compared to those deposited without EF | Photoelectrochemical; j values for ZnO, CdO and PbO in light and darkness were 207, 263, 237 and 1.55, 215, 1.67, respectively | 463 |
| VO2/FTO | 1–10 V cm−1 | Reduction in crystallite size and thermochromic transition temperature | Glazing material | 452 |
| TiO2/glass | 0–30 V | EF induced changes in particle size and shapes, agglomeration, and composition | Photocatalytic dye degradation | 453 |
| TiO2/FTO | 0–30 V | EF induced changes in particle size and shapes, and preferential crystal orientations | Antimicrobial and photocatalytic application | 454 |
| Silicon | ±1000 V | −ve bias is more favorable than the +ve bias | — | 467 |
| SiC | ±50 V | −ve bias promotes epitaxial growth | — | 468 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Carbon-based materials | ||||
| Vertical graphene (VG)/Si | 0–60 V | EF assistance in PECVD; height of VG arrays increased with the bias (18.7 μm with an EF of 30 V cm−1) | Thermal interface material; high vertical thermal conductivity (53.5 W m−1 K−1) and a low TRC (11.8 K mm2 W−1) | 188 |
| Vertical graphene (VG)/Si | −60–250 V | EF assistance in PECVD; high growth rate (11.5 μm h−1) | Thermal interface material; vertical thermal conductivity (34.2 W m−1 K−1) and a low TRC (18.2 K mm2 W−1) | 190 |
| Vertical graphene/Cu | −400–400 V | Deposition rate decreased with increasing −ve bias but opposite effect with +ve bias | — | 431 |
| Vertical graphene | 30 V cm−1 | Straight pore structure & enhanced electrochemically active surface area for efficient ion/electron transport pathways | Electrochemical capacitors; Ca = 1.72 mF cm−2 at Φ120 = 80.6° after 500k cycles, energy density = 0.33 μW h ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−2 cm−2 |
191 |
| Quenched-produced diamond/Ti | −40 V | A spontaneously formed TiC interfacial layer caused a 3-fold increase in adhesion strength | Coatings for biomedical devices; hardness = 96 GPa | 426 |
| Diamond/Si(100) | 150–250 V | Bias voltage plays a key role in the formation of azimuthally (70%) textured diamond film | Quantum devices; diamond films with N vacancy defect centres | 90 |
| Vertical graphene | 100 V | EF-assisted PECVD; max. growth rate = 15.9 μm h−1; VG height ∼144 μm | Light shielding; ultra-low reflectance of 0.25% | 189 |
| Carbon nanotubes | 0–30 V | Growth rate increased and diameter decreased with higher +ve voltage | EF-assisted mass production of CNTs | 469 |
| Carbon nanotubes | 0–700 V | “Herringbone structure” (diameter 25 nm) in no field, uniform parallel to the tube axis (diameter 12 nm) in EF | Supercapacitor; C = 237 F g−1 (3-fold increase compared to no field) | 470 |
| “Cow-nipple-like” submicro-nano carbon | 0–300 V | A vertically grown carbon nanotube (dia. 10–40 nm) upon a carbon ball (dia. 30–120 nm) | — | 471 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Others | ||||
| Perfluoropolyether on diamond-like carbon | 0–300 V | An EF-modulated photoelectron-assisted CVD | Ultrathin perfluoropolyether/DLC hybrid coatings for magnetic disks | 472 |
External EF assistance has shown a remarkable impact in aerosol-assisted CVD (AACVD) of metal oxides.449,453,454,458–461 Beyond the magnitude and polarity of the applied bias, factors such as the type of current (AC vs. DC), frequency,458 aerosol delivery setup and substrate orientation461 significantly influence particle size and shapes, agglomeration, and phase composition and preferential crystal orientations.453,454 The trajectory and flight time of aerosol droplets, charged either due to polarized molecules or static surface charges are influenced by the EF parameters. For example, in the deposition of WO3 using the [W(OPh)6] aerosol in toluene, applying AC voltage rather than DC voltage resulted in aligned and evenly distributed fibrous growth. This is attributed to the higher impedance of AC fields, which prevents the rapid dissipation of induced dipoles in the growing material, thus maintaining the alignment of the fibers with the field.458 In the CVD of β-Ga2O3 on a patterned sapphire substrate, external EF (0–80 V) was found to significantly affect the growth rate, morphology, and crystallinity.450,465 The growth rate increased with increasing EF, forming uniform and regularly arranged microstructures. X-ray diffraction (XRD) analysis indicated increased preferential growth along the (−201) plane of the monoclinic crystalline phase of β-Ga2O3 with increasing external voltage.465
The EF-assisted CVD processes have played an important role in understanding the non-classical charged nanocluster-based nucleation and growth. Hwang and coworkers429,466–468,473–475 have studied the formation of charged nanoclusters and their deposition behavior under different bias conditions (substrate bias, filament bias in HFCVD, AC vs. DC bias) in CVD. In HFCVD of SiC, a negative bias of hot filament led to the formation of smaller charged nanoclusters compared to that in positive or zero bias, which displayed liquid-like properties and promoted epitaxial growth through epitaxial recrystallization.468 In CVD of diamonds, the electrical bias accelerated the charged nanoclusters formed in the gas phase towards the substrate to act as nucleation centers and execute a bias-enhanced growth.476,477 In the CVD process, diamond deposition is also accompanied by an undesirable graphitic phase and EF-assisted +ve substrate biasing suppresses this graphitic phase.476 Wang et al.427 studied the evolution of nucleation and growth of a heteroepitaxial single-crystal diamond film with substrate bias on an Ir substrate, displaying a close relation between bias voltage and bias time. The bias-enhanced nucleation experienced incubation and domain-nucleation periods where domains can enlarge and diminish with bias time, resulting in the nucleation pathways changing from “isolated-crystal nucleation” to “typical domain nucleation” and again back to “isolated-crystal nucleation”.
In HFCVD of anisotropic carbon-based nanomaterials, the external electrical bias influences the deposition rate, composition, and orientation of growing nanostructures.478,479 For example, during catalytic deposition, the axis polarizability of 1D-carbon nanostructures in EF has caused them to be lifted upward from the horizontal direction, enabling them to grow more readily from the catalyst surface.443,470,480 Zhang and Pan481 demonstrated a bias-dependent diameter variation of carbon nanofibers using Ni nanocatalysts. An external bias of 0, 25 and 50 kV m−1, respectively, influenced the size of nanocatalysts, which become liquid at the deposition temperature, resulting in nanofibers with 19.2 ± 8.6, 13.8 ± 4.7, and 8.0 ± 2.4 nm diameter. Similarly, Peng et al.443 exploited the greater polarizability of metallic-CNTs compared to the semiconducting-CNTs to raise the percentage of metallic-CNTs to 80% with EF-bias of 200 V cm−1 compared to that of 47% with zero field bias. Luo et al.470 observed that the CNTs grown under EF displayed a smaller and uniform diameter, improved crystallinity and graphitic nature, and fewer graphite layers parallel to the tube axis. Issman et al.464 used external EF for continuous alignment of CNT bundles (Fig. 7). The theoretical model simulating the alignment process indicated that a CNT stiffening effect (introduced by a z-pinch mechanism induced by the Lorentz force of the AC field, rather than DC fields) enabled a means to control CNT bundle diameters. The EF-assisted samples displayed a 75–90% increase in specific electrical conductivity and 260–320% increase in specific tensile stress to failure, compared to the zero-field sample.
 | ||
| Fig. 7 (a) An illustration of an EFCVD reactor with an RF electrode inserted at its front, while the forming CNT aerogel acts as a counter electrode. (b) A magnified schematic shows the occurrence in the interelectrode gap: (i) AC field induced “Lorentz pinch” stiffens the ultralong CNT, (ii) Stiff, ultralong CNT is under the influence of a field-induced aligning torque, (iii) CNT is aligned according to the field lines. (c) Finite element method numerical results of field distribution inside the reactor tube portraying equipotential lines (blue) and orthogonal field lines (red). The packing density of the equipotential lines indicates the local field intensity. The model shows the presence of alignment-inducing field lines bridging the two electrodes within the interelectrode gap (50 mm wide). (d–f) Z-pinch mechanism, (d) illustration of electromagnetic fields in a CNT relevant for the z-pinch stiffening effect. Axial current (orange) is confined to the CNT walls and induces a circumferential magnetic field (blue). (e and f) The cross-section free-body diagram of the continuum CNT model for the z-pinch. Internal forces on both faces along the contour are shown in red. Pressure acting on CNT wall (e) and equivalent restoring force (f) are shown in blue, adopted with permission from ACS.464 | ||
Linnik et al.482 reported the RF (13.56 MHz) bias-enhanced nucleation of diamond on dielectric substrates where the nucleation densities obtained on sapphire and Si substrates were 1010 and 1011 cm−2, respectively. In PECVD of carbon-based nanomaterials, internal EF associated with the plasma plays an important role in oriented growth.183,186,483 However, additional electrical biasing has also been enforced, ensuring vertical growth and/or enhancing desired materials attributes.188 For large-area deposition of high electrical conducting graphite-like carbon film in PECVD, high and uniform plasma density and effective ion bombardment (i.e., high plasma energy) are needed to increase graphitic character. To this, Bae et al.434 used a high negative substrate bias (up to 2 kV) to decouple the ion flux with ion energy, enhancing the ion bombardment energy (≥1.3 keV) of the Ar/C6H6 surface-wave plasma (plasma density ≥1017 m−3) for the deposition of sp2 carbon-rich films. With increasing bias, the deposition rate decreased, but the graphitic character increased, resulting in a highly conducting large area (16 cm) film. In PECVD, diffused/remote plasma configurations overcome the issues of defect creation and etching, which are observed in the direct plasma configuration. However, in vertical growth of carbon nanomaterials, diffused/remote plasma configurations may also lead to horizontal growth.484–487 The application of an additional external substrate bias with respect to the electrically grounded chamber wall minimized the horizontal growth.59,184,186 Butcher et al.488 used a biased grid placed between the plasma and the substrate, shielding the substrate from the strong EFs generated by the RF plasma during PECVD of GaN. PECVD with a grounded grid resulted in highly smooth films, whereas +ve grid biasing led to columnar growth. Interestingly, −ve grid biasing significantly reduced the carbon and hydrocarbon content of GaN films. Similarly, by placing metal-covered Si half-rings around an Ir/YSZ/Si(001) substrate, Yoshikawa et al.489 demonstrated wafer-scale bias-enhanced nucleation and heteroepitaxial growth of single-crystalline diamond by PECVD, with the half-rings and their thickness influencing the bias-enhanced nucleation density and the plasma density distribution. Some studies have reported innovative ways to execute the EF-matter interaction in CVD. For example, Reiprich et al.490 reported an EF-guided localized and programmable CVD method to deposit 3D materials. The electrically charged precursor molecules were guided by the arrays of electrodynamic funnels to nanosized (≥250 nm) deposition locations, where nearest neighbor coupling led to the deposition of 3D nanostructures. Shi et al.491 reported double bias induced post-deposition nanostructuring of a boron-doped diamond film via a reactive ion etching process, introduced by negative substrate bias (250–300 V) and positive bias (0–60 V) of a strategically placed grid in the HFCVD system. The double biasing greatly improved the etching efficiency, forming nanocone structures with enhanced electrochemically active surface area.
Some studies simulated the physical processes on the scale of the apparatus in EF-assisted PECVD to present mathematical models relating the electrical bias with the deposition rate.492,493 For example, to explain the diamond growth from the CH4/H2 gas mixture in EFCVD, Lifang et al.494,495 performed Monte Carlo simulation correlating the energy carried by electron and precursor fragments as a function of experimental parameters. For metal-catalyzed CVD of CNTs, Saeidi and coworkers496,497 presented a theoretical model relating the external EF with the phonon oscillations of metal catalysts. They demonstrated that a catalyst-specific optimum electric field was required, depending upon the metal–carbon van der Waals interactions, atomic mass, and free charge carrier density of metal atoms.497 Wang et al.438 proposed a two-dimensional axisymmetric model to understand the influence of positive bias and deposition pressure on the plasma flow properties in the deposition chamber during the bias-enhanced PECVD process, predicting a bias voltage threshold phenomenon with a narrow range of suitable voltage.
4.3 Perspective on electric field-assisted chemical vapor deposition
The imposition of external EF around the assembly of precursors, carrier gas, and substrate has provided a non-contaminating way to manipulate the CVD process. However, except for bias-enhanced nucleation in diamond growth and aligned growth of nanostructures, the potentials of EF-assisted modulations seem to be underutilized in CVD. Previous studies have focused mainly on the realization of EF-assistance on isolated, lab-scale deposition processes, which should be tested at the industrial scale, given the importance of CVD in modern manufacturing. In this regard, recent reports of employing EF-assisted modulations in tuning the ion energies in plasma-assisted atomic layer deposition,498,499 an offshoot technique of PECVD, warrant renewed attention in EFCVD.Electric field-driven, potentially non-thermal mechanisms have proven their significance in materials synthesis and processing,500 manifesting far-from-equilibrium effects at a range of length scales – from the atomic level to microstructural level – and therefore should not remain ignored in CVD. The reports of using an external electric field to influence defect creation and defect migration during materials processing432,433,435,501,502 should be extended to the EFCVD of diverse functional materials.
Most reports on EFCVD remain confined to lab-scale demonstrations, primarily due to economic constraints and challenges of process integration. In addition, limited understanding of EF-matter interaction in CVD parametric space also poses a significant barrier to broader the adoption of EFCVD. As nucleation and film growth are mediated by charged nanoclusters generated in the gas phase during CVD,466,473,503–505 there is a growing need for studies investigating the influence of external EF effects on electrostatic energy and drag force acting on these nanoparticles, particularly in relation to floating, grounded, or biased substrates. Such investigations accompanied by appropriate modeling efforts, specifically focusing on the influence of external EF on the reactivity, mass and heat transfer, and reaction kinetics would provide valuable insights to optimize EFCVD processes and scale them for practical applications.
5 Magnetic field-assisted chemical vapor process
5.1 Magnetic field-matter interaction in chemical vapor deposition
Depending upon the time-space properties of the field, an MF-matter interaction has manifested into any or a combination of phenomena – Zeeman effect, magneto-thermodynamic effect, magnetic torque, Lorentz force, Faraday force, eddy current, and energy injection.506 Quantitatively, an external magnetic field of 1 T induces a ∼0.05 J mol−1 change in Gibbs free energy of a chemical reaction at room temperature, influencing the rate constant by a seemingly insignificant factor of ∼10−5.507 However, these low-energy magnetic interactions have displayed a noticeable influence on chemical kinetics and the outcome of the chemical reactions.508 For example, when a magnetic field is applied in the course of precursor flow in CVD, polar molecules and charged particles move in a cyclical way under the influence of the MF-induced Lorenz force, making their movement trajectories overlap more frequently. This increased the probability of collision between reacting species and carrier gas molecules.26 An external MF influenced the nucleation process and, depending upon the difference in magnetization between the vapor phase and the substrate, provided an extra driving force in the form of Zeeman energy, thus lowering the energy barrier for nucleation on the substrate surface.509 A sufficiently strong and favorably oriented MF influenced the mobility and diffusion of precursor molecules onto the substrate surface, altering the crystallization temperature, phase separation, and morphology in the resulting film.510 The coupling of MF with other field effects-electric field,27,28,31 plasma,29,30,511 or both26,27,31,512,513 has resulted in a synergistic effect, leading to the deposition of films with unique nanostructures.5.2 Overview of magnetic field-assisted chemical vapor deposition
The initial reports on externally controlled MF assistance in the CVD process can be traced back to the 1980s when it was used to influence the plasma properties during PECVD, displaying variable effects of MF on the plasma and resulting deposition, depending upon the MF strength, precursor characteristics, and plasma and thermodynamic parameters.514–516 For example, MF was used to confine the plasma density over the substrate, enhancing the deposition rate and film quality of DLC films.517–519 The confinement of a low RF power plasma by an MF parallel to the substrate surface effectively enhanced the plasma density without increasing the ion energy, resulting in a high deposition rate at low DC self-bias (sheath voltage). In the absence of MF, only a high RF power input could ensure a high growth rate. However, a high RF power resulted in a high sheath voltage and ion energy, causing a potential deterioration in film quality. The external MF influenced the plasma sheath parameters by affecting the ion flux and ion energy.516 A streamlined magnetic field has been used to stabilize the RF plasma over long-range propagation, enabling high plasma density with a decay-free, uniformly distributed ion energy over an extended space, thus offering an energy-efficient means for scalable fabrication of films of 1D/2D materials.511,520–522 Table 4 presents selected examples of MF assistance employed in CVD of functional coatings.| Material/substrate | Process parameters (precursor, MF, substrate temperature) | Key features | Application | Ref. |
|---|---|---|---|---|
| a a-C:H:SiOx-SiOx doped amorphous hydrogenated carbon; J = photocurrent density; L = (N,N-(4,4,4-trifluorobut-1-en-3-on)-dimethyl propylene diamine; LDHs – FeMgAl layered double hydroxides. | ||||
| Diamond/Si (111) | (H2 + CH4; 98/2 sccm); dynamic magnetic field (DMF, ω = 83–600 π rad s−1) | Enhanced nucleation density due to an induced EF excited by the DMF and electron-stimulated H desorption | — | 523 |
| Diamond/rod-shaped tungsten carbide | (H2 + CH4; 2000/10 sccm); ring magnet 400 mT; 800 °C | Simulation studies on MF distribution, ion movement, and deposition | — | 524 |
| Diamond/Si (100) | (H2 + CH4; 99/1 vol.); periodic MF (ω = 0–1 kHz); ∼700 °C | (110) or (100) orientation favored with lower or higher ω | — | 525 |
| Nanostructured carbon films/Si | (H2 + CH4) ∼8 mT; ∼700 °C | MF assistance in PECVD resulted in finer nanostructures with higher crystallinity | Electron field emission; MFCVD sample gave turn-on field 6.5 V μm−1 (vs. ∼15.5 for zero-field) | 442 |
| Diamond film/Si (100) | (H2 + CH4); ∼8 mT; −ve substrate bias (0–40 mA current); ∼700 °C | Combined EF- and MF-assisted control; film thickness varied with field | Electron field emission; MFCVD sample gave turn-on field 6.1 V μm−1 (vs. 11.2 for zero-field) | 31 |
| Diamond film/Si | (H2 + CH4); ∼4 mT; ∼808–872 °C | Use of a rotating MF; film thickness 7.41 μm (without MF 6.88 μm) | — | 526 |
| Branched or Fe-encapsulated carbon nanotubes/Si | Iron(II) phthalocyanine + H2; ∼0.2 T (gradient 50 mT cm−1); 550 °C | MF promoted the coalescence or division of catalyst particles, forming branched or encapsulated CNTs | — | 527 |
| DLC on YG8 carbide substrate | Cathode arc discharge (120 A DC), (N2, Ar, C2H2), 0.9 Pa, 450 °C | Role of MF and bias controlled on structure and properties | Cutting tool protection film; coefficient of friction = 0.112 | 91 |
| Carbon nanofibers (CNFs)/Ni-deposited Cu | C2H2; 0–0.5 T; 700 °C | CNFs changed to homogenized, narrower, bamboo-like CNTs with increasing MF | — | 528 |
| Single-walled carbon nanotubes | 10 T | MF-assisted preferential growth of metallic single-walled CNTs (1 nm diameter) | — | 529 |
| a-C:H:SiOx film/stainless steel | (Polyphenylmethylsiloxane + Ar); (0–1.7 mT) | Use of MF for enhancing plasma confinement in PECVD; elastic modulus increased from 90 to 117–125 GPa | Tribological application; hardness increased from 8.7 to 11.7–12.5 GPa | 29 |
| Vertically standing graphene | (5–50 mT) | Use of MF for plasma confinement and vertical growth | Na+ battery anode; capacity retention of 86% after 2000 cycles at 1 A g−1 | 511 |
| Carbon nanotubes on LDH-catalyst | (N2/H2/C3H6 70/40/40 ml min−1); (∼0.02 T); ∼700 °C | MF affects catalyst arrangement, inducing higher yield, increased length and graphitic nature | — | 521 |
| Iron oxide/Si (100) | [Fe(OtBu)3]2; 0–0.5 T perpendicular to the substrate; 500 °C | Magnetite with increased particulate size in MFCVD, whereas (hematite + amorphous iron(III)oxide) in CVD | — | 530 |
| Iron on FTO or Si (100) | Fe(CO)5; 0–1 T; 300 °C | Anisotropic columnar growth in MFCVD, isotropic grain growth in CVD | Water-splitting; J values were 0.027 and 0.050 mA cm−2 for no field and MF film | 531 |
| α-Fe2O3/FTO | Fe(CO)5 + 20 sccm O2; 0–120 mT | MF assistance in PECVD | Water-splitting; J values were 0.484 and 0.659 mA cm−2 for no field and MF film | 532 |
| UO2/Si | U(OtBu)6; 0–1 T; 400–1000 °C | Preferred 〈111〉 growth in CVD, polycrystalline growth in MFCVD | — | 533 |
| TiO2/Si | TiCl2(OiPr)(pyridine)3; 0–0.5 T with (perpendicular/parallel) field orientation; 300 °C | Well-defined anatase-type TiO6 subunits in MFCVD, whereas rutile-type distorted TiO6 units in CVD | — | 534 |
| MgFe2O4 films | MgFe2(OtBu)8; 0–1 T; 300 °C | MF induces higher grain growth and densification, influencing the magnetic domains and inversion of the crystal lattice | — | 535 |
| ReN films/Si | [fac-Re(I)(CO)3(L)]; 0–1 T; 600 °C | Preferred growth along the 〈100〉 direction in MFCVD | — | 536 |
Researchers also employed an external MF to influence the normal CVD processes for the deposition of numerous functional materials. For example, metal catalysts used for the growth of CNTs are influenced by the application of magnetic field, thus altering the growth mechanism. During MFCVD of Fe-encapsulated CNTs, Wei et al.527 observed that an MF (0.2 T with a gradient of ∼50 mT cm−1) transverse to the growth direction resulted in the growth of branched CNTs having Fe particles at their base, whereas an MF parallel to the growth direction led to less-branched Fe-encapsulated CNTs. Similarly, the CVD of carbon nanofibers over a Ni catalyst led to random nanofibers with entangled orientation, but an external MF assistance led to an aligned growth perpendicular to the substrate.528 Interestingly, with increasing field strength (up to 0.5 T) the morphology changed from disordered solid-cored nanofibers to bamboo-like CNTs. A growth mechanism based on the particle size of the catalyst, which decreased with increasing magnetic field and the diamagnetic nature of carbon atoms, was proposed to explain the morphological variations in CNFs with the field strength. A similar growth mechanism, induced by an ordered arrangement of magnetic FeMgAl layered double hydroxide catalyst flakes in an MF-assisted fluidized bed CVD, resulted in a higher yield of bundles of longer, narrower, and highly graphitic CNTs.521
Magnetic field assistance has played a key role in enabling the growth of diamond at relatively low temperatures and low pressures in catalytic HFCVD.537–539 Based on the atomic scale dynamics and electronic spin effects for carbon rehybridization and fixation, Little and Goddard537 demonstrated the influence of a static MF on the nucleation and growth mechanism of diamond. A strong MF (∼19.3 T) was capable of initiating the nucleation, thus eliminating the requirement of chemical or abraded nucleation centers.537 The constriction effect induced by Lorentz force of the static MF increased the effective concentration of electrons and polarized hydrocarbon species in the reactive gas mixture by minimizing their collisions with the reactor walls.539 In a periodic MF (with magnetic field strength B = B0 sin ωft, where B0 is maximum field strength and ωf is angular frequency, Fig. 8(a)), the concentration of active carbon particles increased with increasing ωf, resulting in enhanced nucleation density and diminished crystal size. Due to the rotation of magnetic lines in a periodic field, the electrons and polarized hydrocarbon species prolong their moving path, leading to a higher probability of collision with reaction gas molecules to produce more carbon precursors.539 However, based on the intricate relationship between B0 and vibrational frequency of spiral movement, it is proposed that the positive effect of increasing ωf on prolonging the moving path of particles becomes negative beyond a ωf value (Fig. 8(b)).525,539
 | ||
| Fig. 8 (a) Schematic diagram of a periodic MF-assisted HFCVD system and (b) the measured relationship between B0 and angular frequencies, reused with permission from Elsevier.525 (c) A schematic displaying the distribution of MF strength in magnetic and electric field coupled HFCVD. The numerical values in figures are taken from the work of Wang et al.31 and represent the Gaussian value of the MF strength. | ||
As diamond films are known for their orientation-dependent functional properties, Wang et al.525 demonstrated that depending upon lower or higher ωf values the MF-assisted HFCVD grown diamond film preferred a (110) or (100) orientation, respectively. Using a methane and hydrogen (1/99 vol%) gas mixture, the molar ratio of C2H2 and CH3 fragments (C[C2H2]/C[CH3]) in a reactive gas mixture influenced the orientation of the diamond film. The C–H bond dissociation energy in CH3 (462 kJ mol−1) is lower than that in C2H2 (556 kJ mol−1), indicating an easier dehydrogenation of CH3 than C2H2. The rising collision possibility with increasing ωf raised the C[C2H2] more rapidly compared to the C[CH3], resulting in a higher C[C2H2]/C[CH3] ratio that favored the (100) orientation over (110) orientation. Also, higher ωf values resulted in continuous nano-diamond films with fine grains, displaying an improved field emission performance.539
In a related study, Wang et al.31 investigated the effect of the coupled magnetic and electric field on the growth of a diamond film, presenting a qualitative simulation of the moving paths of particles in the coupled fields with different electric field intensities, direction of initial velocity, and charge-to-mass ratio (Fig. 8(c)). The experimental results indicated that the coupled fields enhanced the graphitization and refinement of diamond crystals. The simulation study pointed out that MF suppressed the (100) orientation by changing the precursor distribution, influencing the ratio of two precursor fragments −C2H2 and CH3, a key factor deciding the preferred orientation of the as-prepared film.
Liu et al.540 investigated the effect of a dynamic MF (where the induced EF points alternately in the ±z directions, orthogonal to the substrate surface523) with different angular frequencies on the growth rate, diamond quality, growth orientation, and deposition uniformity. At higher angular frequencies, the electrons move with a higher velocity and a larger radius, leading to an increased range of electron motion. With this, the electron-molecule collisional excitations are intensified, enabling more activated molecules to participate in growth and enhance the growth rate.540 Additionally, similar to the EF-assisted bias-enhanced nucleation,90,419,420 a dynamic MF drives electrons and ions to enhance the diamond nucleation. However, it does not cause a persistent bombardment of charged particles to the diamond growth front, which otherwise causes graphitization. Thus, a dynamic MF suppressed the sp2 carbon generation and improved the diamond quality.540 Presenting a detailed investigation of the effect of dynamic MF on diamond growth, Liu et al.523 demonstrated that the dynamic MF-induced EF alternately pointed in the ±z directions, driving the electrons to continuously oscillate during motion and leading to non-persistent bombarding at the substrate surface.523 As a result, the dynamic MF-induced diamond nucleation has an entirely different surface chemistry compared to the EF bias-assisted nucleation. As the thermal energy of the hot filament was well below the energy required to generate atomic H and CH3 components via gas phase electron–molecule collisions, the gas phase electron–molecule collision excitation could not support the enhancement of diamond nucleation in CVD. The nucleation density (calculated from SEM images) displayed a dependence on ω (Fig. 9(a)) and the Fourier-transformed infrared spectroscopy (FTIR) of the MF-assisted HFCVD film displayed strong peaks at 1750 and 2240–2400 cm−1 (Fig. 9(b)), indicating a highly activated film surface. Therefore, the authors proposed that dynamic MF mainly influenced the surface chemistry rather than the gas chemistry. The enhancement was attributed to a dynamic MF-induced EF, leading to the electron-stimulated desorption of hydrogen and formation of carbon dangling bonds on the growth front, which subsequently morphed into mono-radical and bi-radical sites for enhanced diamond nucleation (Fig. 9(c)).
 | ||
| Fig. 9 (a) Dynamic MF intensity and diamond nucleation density as a function of angular frequencies; (b) square root of diamond nucleation density fitted (red curve) as a function of the induced electric field; and (c) schematic diagram of the diamond growth front and hydrogen desorption, adopted with permission from Elsevier.523 | ||
Magnetic field-assisted CVD has been also employed for the deposition of numerous non-carbonaceous materials, such as metalloids and metal oxides. For example, in PECVD of hydrogenated microcrystalline silicon (μc-Si:H) thin films, Kim et al.541 observed that an MF assist using a geometrically aligned magnetic mirror resulted in a 7-fold increase in deposition rate and enhanced crystallinity. This was attributed to the increased formation of Si and SiH radicals from the SiH4 precursor under MF-induced enhanced plasma confinement, as observed in the analysis of plasma properties using optical emission spectroscopy.
For magnetically active materials, the application of MF during CVD influences not only the deposition and growth process but also the orientation of grains and other field effects.542 Mathur and coworkers have extensively used MF-assisted manipulation in the CVD of magnetically active materials.530–536,543 For example, PECVD of hematite film using an iron pentacarbonyl precursor displayed excellent MF-assisted control on phase and surface properties in static MF generated by different arrangements (parallel/perpendicular; par/perp) of rod-type or disk-type magnets (RTMs or DTMs) along the substrate (Fig. 10(a)–(f)).532 In crystallographic analysis, the zero field and RTM-perp samples experienced preferred growth along the (110) crystallographic plane, whereas the RTM-par sample, along with the DTM-par and DTM-perp samples, experienced preferred growth along the (104) plane (Fig. 10(g) and (h)). Compared to zero field deposition, field-assisted depositions resulted in a more homogeneous particle shape and size, forming a denser microstructure (Fig. 10(i)–(n)). The films deposited under the DTM-perp arrangement creating an overall attractive field interaction exhibited larger aggregates with fewer grain boundaries (Fig. 10(j)). The films deposited under the DTM-par arrangement creating an overall repulsive interaction exhibited more aggregates of densified crystallites (Fig. 10(k)). However, the films deposited under DTM-perp and DTM-par arrangements displayed no difference in crystallite sizes. More importantly, the hematite film prepared under MF-assistance displayed significant enhancement in photoelectrochemical properties in water splitting, which was attributed to the presence of smooth particle surfaces and fewer grain boundaries in field-assisted growth, favoring charge transportation and reduced charge recombination.
 | ||
| Fig. 10 Photographs of plasma under (a) parallel and (b) perpendicular field geometry and corresponding qualitative magnetic field profiles (d) and (e) for RTMs; photographs of plasma (c) and corresponding qualitative magnetic field profile (f) for arrangement of DTMs; XRD patterns of the hematite films deposited under different MFs. Asterisks (*) represent SnO2 from FTO substrates (g) and (h); surface morphologies of deposition using RTM under (i) zero field, (j) parallel field, and (k) perpendicular field; surface morphologies of deposition using DTM under (l) zero field, (m) parallel field, and (n) perpendicular field, adopted with permission from Wiley.532 | ||
The MFCVD of hematite on F-doped SnO2 and Si(100) substrates displayed substrate-independent anisotropic growth of α-Fe2O3 columns, as against an isotropic grain growth in zero field deposition.533 Moreover, a parallel field resulted in larger crystallites with cuboidal morphology, whereas a 45° inclination led to a tilted orientation of anisotropic grains. The TEM analysis displayed a gradual increase in average crystallite size with increasing field strength, supporting the observation that even under low MF strength, the interactions between the crystalline nuclei and MF can greatly influence the nucleation and grain growth on the substrate.31,530,536
Mathur and coworkers further broadened the horizon of MFCVD by using a paramagnetic metalorganic Ti(III) precursor [TiCl2(OiPr)(pyridine)3] for the deposition of TiO2 films, demonstrating a remarkable shape anisotropy in deposited films.534 The MF-assisted (B = 0.5 T) deposition resulted in the growth of well-defined anatase-type TiO6 subunits, whereas zero-field deposition led to distorted rutile-type TiO6 subunits. The zero-field deposition displayed preferential growth along the (200) plane, whereas the MFCVD resulted in preferential growth along the (101) and (200) planes in perpendicular-field and parallel-field orientations, respectively. Elaborating on the MF-matter interaction, similar experiments performed using a diamagnetic precursor Ti(OiPr)4 indicated no such field-dependent microstructural variations, highlighting the importance of the d1 electronic configuration in triggering the MF-matter interaction. However, noticeably, irrespective of the field strength and orientation, the MFCVD with paramagnetic precursors resulted in diamagnetic TiO2 films.534
MFCVD displayed similar variations in shape, morphology, and crystallographic orientation during <1.0 T MF-assisted CVD of rhenium nitride,536 UO2,531 and MgFe2O4.535 Interestingly, although MF-assist has invariably increased the nucleation and growth and has a homogenization effect on shape, particle size distribution, and composition, it has exerted a varying influence on particle size evolution, depending upon the MF orientations and magnetic behavior of participating and resulting species. More precisely, except for UO2 (ref. 531) and YBa2Cu3O7 films,509 the MF assistance led to increased particle size in CVD of magnetite,530 hematite,532 TiO2,534 rhenium nitride,536 and MgFe2O4.535 This was explained based on the induced internal magnetic field inside the growing diamagnetic UO2 and YBa2Cu3O7 being antiparallel to the applied external MF, creating an energetically unfavorable situation for grain growth.509 Notwithstanding, in case of YBa2Cu3O7 a low MF (≤2.0 T) caused a decrease in grain size compared to the zero field, but at higher MF (≥4.0 T) the grain size increased, due to the change in growth mode—from being 2D at low MF to 3D at higher MF,509 and most likely due to, in our opinion, a high MF field overcoming the internal diamagnetic field effect.
5.3 Perspective on magnetic field-assisted CVD
Over the years, numerous results have strengthened the utility of external MF in inducing a profound influence on the nucleation, grain growth, phase composition, and crystallographic orientation during CVD, thus altering the morphology, chemical topography, and functional properties of the resulting films. Therefore, it is expected to increasingly become a preferred pathway, compared to conventional experimental controls-temperature and pressure or precursor chemistry, for in situ manipulation of the material properties of the CVD-grown films. Magnetic fields with spatial and temporal variations such as periodic, dynamic, or rotating configurations have shown significant potential in influencing nucleation and growth mechanisms during CVD. However, such approaches have mostly been limited to the deposition of diamond materials and their application to other material systems remains underexplored. Most reports on magnetic field-assisted CVD are limited to lab-scale set-ups with small-area deposition. A key challenge lies in generating strong and uniform magnetic fields over larger deposition areas. This limitation is reflected in the fact that most MFCVD studies have employed magnetic field strengths of ≤1 T. New CVD setup designs capable of accommodating even stronger magnetic fields (≥5 T) carry the potential to broaden the scope of MFCVD enabling field-assisted control over growth kinetics and materials properties. Nevertheless, the integration of strong magnets brings added instrumentation complexity, cost, and technical expertise requirements, which must be carefully considered for practical scalability.Most MF-matter interaction studies in CVD so far have focused mainly on the precursor-MF interactions, and the interaction of MF with a carrier gas and substrate533,544,545 is less explored. Moreover, the interaction of MF with temperature, an important process control parameter, has been less precisely monitored and understood, especially in the case of a magnetically active precursor and deposited materials. The influence of external MF in those CVD processes where paramagnetic oxygen is used as a reactant/carrier gas546 is less exploited. Most importantly, the field of MFCVD lacks sufficient mathematical models and simulation studies relating the effects of external magnetic fields with the CVD growth parameters.
6 Concluding remarks
Field-assisted chemical vapor deposition (CVD) represents a promising future direction for controlling thin-film growth, with precise control over film properties and microstructure. This technique involves applying external fields, such as magnetic or electric fields, during the CVD process to influence the deposition dynamics, leading to novel material characteristics and improved process efficiency. The various field-assisted modulations in CVD have emerged as a powerful additional control method, often operating independently of precursor chemistry influence and thermodynamic parameters. These modulations have enabled significant advancements in growth control, microstructure tuning, and material properties for targeted applications.This review underscores the importance of non-conventional process parameters in CVD and highlights their impact on material synthesis. Additionally, it addresses key limitations, particularly the lack of theoretical studies that could provide deeper insights into the role of these modulators in CVD nucleation and growth. Understanding their effects on microstructure, composition, and functional properties remains a crucial challenge. In particular, the emerging field of magnetic-field-assisted CVD would benefit from further research, enabling reliable control over the process and material characteristics.
According to current estimates, the global CVD market has reached approximately 24 billion USD and is projected to grow to nearly 48 billion USD by 2032.547 Field-assisted manipulations are expected to play a key role in capitalizing on this trend; however, the existing challenges, as schematically highlighted in Fig. 11, need to be effectively addressed to unfold the potential of field-enhanced CVD techniques. Key advancements will likely involve optimizing field modulations in terms of intensity and configuration and innovations in reactor design to accommodate such modulations in high-throughput systems. Additionally, advancing our understanding of field-matter interactions, coupled with extensive computational analysis using 3D reactor-scale models, will be crucial for overcoming current limitations. Finally, the integration of operando and in situ techniques into conventional CVD systems and processes to develop a robust framework for depositing materials with enhanced functional properties can bring a paradigm shift in thin film engineering.
 | ||
| Fig. 11 Schematic of existing challenges in field-assisted CVD and the future directions to overcome them. | ||
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
All authors contributed equally.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Authors gratefully thank the University of Cologne for the infrastructural and financial support. SM acknowledges the financial support by the German Science Foundation (DFG) in the frame of the priority program SPP 1959 “Manipulation of matter controlled by electric and magnetic field: towards novel synthesis and processing routes of inorganic materials”.Notes and references
- B. Sun, J. Pang, Q. Cheng, S. Zhang, Y. Li, C. Zhang, D. Sun, B. Ibarlucea, Y. Li, D. Chen, H. Fan, Q. Han, M. Chao, H. Liu, J. Wang, G. Cuniberti, L. Han and W. Zhou, Adv. Mater. Technol., 2021, 6, 2000744 CrossRef CAS.
- L. Sun, G. Yuan, L. Gao, J. Yang, M. Chhowalla, M. H. Gharahcheshmeh, K. K. Gleason, Y. S. Choi, B. H. Hong and Z. Liu, Nat. Rev. Methods Primers, 2021, 1, 1–20 CrossRef.
- M. Nakaya, A. Uedono and A. Hotta, Coatings, 2015, 5, 987–1001 CrossRef CAS.
- M. Bahri, S. H. Gebre, M. A. Elaguech, F. T. Dajan, M. G. Sendeku, C. Tlili and D. Wang, Coord. Chem. Rev., 2023, 475, 214910 CrossRef CAS.
- L. Wang, G. Sun and S. Yuan, Adv. Mater. Technol., 2024, 9, 2301973 CrossRef CAS.
- J. Zhang, J. Wang, G. Zhang, Z. Huo, Z. Huang and L. Wu, Mater. Des., 2024, 237, 112577 CrossRef CAS.
- M. Yaseen, M. A. K. Khattak, A. Khan, S. Bibi, M. Bououdina, M. Usman, N. A. Khan, A. A. A. Pirzado, R. A. Abumousa and M. Humayun, Nanocomposites, 2024, 10, 1–40 CrossRef.
- L. Tang, J. Tan, H. Nong, B. Liu and H.-M. Cheng, Acc. Mater. Res., 2021, 2, 36–47 CrossRef CAS.
- M. D. Straub, J. Leduc, M. Frank, A. Raauf, T. D. Lohrey, S. G. Minasian, S. Mathur and J. Arnold, Angew. Chem., Int. Ed., 2019, 58, 5749–5753 CrossRef CAS PubMed.
- U. Atamtürk, E. Jung, T. Fischer and S. Mathur, Chem. Mater., 2022, 34, 7344–7356 CrossRef.
- C. K. Amadi, T. Karimpour, M. Jafari, Z. Peng, D. V. Gerven, V. Brune, F. Hartl, M. Siaj and S. Mathur, Dalton Trans., 2024, 53, 9874–9886 RSC.
- A. Sutorius, R. Weißing, C. R. Pèrez, T. Fischer, F. Hartl, N. Basu, H. Suk Shin and S. Mathur, Nanoscale, 2024, 16, 15782–15792 RSC.
- V. Brune, C. Hegemann and S. Mathur, Inorg. Chem., 2019, 58, 9922–9934 CrossRef CAS PubMed.
- B. Qin, H. Ma, M. Hossain, M. Zhong, Q. Xia, B. Li and X. Duan, Chem. Mater., 2020, 32, 10321–10347 CrossRef CAS.
- Y. Hamedani, P. Macha, T. J. Bunning, R. R. Naik, M. C. Vasudev, Y. Hamedani, P. Macha, T. J. Bunning, R. R. Naik and M. C. Vasudev, in Chemical Vapor Deposition – Recent Advances and Applications in Optical, Solar Cells and Solid State Devices, IntechOpen, 2016 Search PubMed.
- F. Loyer, S. Bulou, P. Choquet and N. D. Boscher, Plasma Processes Polym., 2018, 15, 1800121 CrossRef.
- G. Franz, Processes, 2021, 9, 980 CrossRef CAS.
- H. Kun, J. Li, K. Li, N. Yan, C. bian, Y. Guan, Y. Yang and H. Li, Polym. Degrad. Stab., 2022, 196, 109816 CrossRef.
- R. Panickar, C. B. Sobhan and S. Chakravorti, Vacuum, 2020, 172, 109108 CrossRef CAS.
- M. Bi, J. Zhu, Y. Luo, H. Cai, X. Li, X. Wang, Y. Wei, X. Wang, C. Hu, J. Hu, G. Zhang, X. Wang and X. Zhang, Coatings, 2022, 12, 1731 CrossRef CAS.
- K. Alberi and M. A. Scarpulla, J. Phys. D: Appl. Phys., 2017, 51, 023001 CrossRef.
- X. Zhao, C. Wei, Z. Gai, S. Yu and X. Ren, Chem. Pap., 2020, 74, 767–778 CrossRef CAS.
- M. E. A. Warwick, A. J. Roberts, R. C. T. Slade and R. Binions, J. Mater. Chem. A, 2014, 2, 6115–6120 RSC.
- A. Sawabe and T. Inuzuka, Appl. Phys. Lett., 1985, 46, 146–147 CrossRef CAS.
- S. Yugo, T. Kimura and T. Muto, Vacuum, 1990, 41, 1364–1367 CrossRef CAS.
- J. Li, Y. Wang, Q. Wei, Y. Xie, H. Long, Z. Deng, L. Ma, Z. Yu, Y. Jiang, N. Hu and K. Zhou, Surf. Coat. Technol., 2017, 324, 413–418 CrossRef CAS.
- Y. Wang, Q. Wei, Z. Yu, H. Long, Z. Deng, Y. Xie, J. Li, C.-T. Lin, L. Ma and K. Zhou, Appl. Surf. Sci., 2017, 423, 788–792 CrossRef CAS.
- W. In-Hwang, X. Chen, T. Kuzuya, K. Kawabe and S. Motojima, Carbon, 2000, 38, 565–571 CrossRef CAS.
- A. S. Grenadyorov, А. А. Solovyev, K. V. Oskomov and V. O. Oskirko, J. Vac. Sci. Technol., A, 2019, 37, 061512 CrossRef.
- T. Tabuchi, M. Takashiri and K. Ishida, Surf. Coat. Technol., 2007, 202, 114–120 CrossRef CAS.
- Y. Wang, J. Li, N. Hu, Y. Jiang, Q. Wei, Z. Yu, H. Long, H. Zhu, Y. Xie, L. Ma, C.-T. Lin and W. Su, Mater. Res. Express, 2018, 5, 035009 CrossRef.
- X. Lu, P. J. Bruggeman, S. Reuter, G. Naidis, A. Bogaerts, M. Laroussi, M. Keidar, E. Robert, J.-M. Pouvesle, D. Liu and K. Ostrikov, Front. Phys., 2022, 10, 1–12 CAS.
- Plasma Chemistry, ed. A. Fridman, Cambridge University Press, Cambridge, 2008, pp. 12–91 Search PubMed.
- I. B. Denysenko, S. V. Ivko and N. A. Azarenkov, in 2018 IEEE 8th International Conference Nanomaterials: Application & Properties (NAP), 2018, pp. 1–5 Search PubMed.
- L. Martinu, O. Zabeida and J. E. Klemberg-Sapieha, in Handbook of Deposition Technologies for Films and Coatings, ed. P. M. Martin, William Andrew Publishing, 3rd edn, Boston, 2010, pp. 392–465 Search PubMed.
- S. Sovizi, S. Angizi, S. A. Ahmad Alem, R. Goodarzi, M. R. R. Taji Boyuk, H. Ghanbari, R. Szoszkiewicz, A. Simchi and P. Kruse, Chem. Rev., 2023, 123, 13869–13951 CrossRef CAS PubMed.
- C. Vallée, M. Bonvalot, S. Belahcen, T. Yeghoyan, M. Jaffal, R. Vallat, A. Chaker, G. Lefèvre, S. David, A. Bsiesy, N. Possémé, R. Gassilloud and A. Granier, J. Vac. Sci. Technol., A, 2020, 38, 033007 CrossRef.
- J. Chen, Z. Bo and G. Lu, in Vertically-Oriented Graphene: PECVD Synthesis and Applications, ed. J. Chen, Z. Bo and G. Lu, Springer International Publishing, Cham, 2015, pp. 19–34 Search PubMed.
- J. Zhou, J. Liao, J. Huang, T. Chen, B. Lv and Y. Peng, Vacuum, 2022, 195, 110678 CrossRef CAS.
- M. Yamamoto, H. Murata, N. Miyata, H. Takashima, M. Nagao, H. Mimura, Y. Neo and K. Murakami, ACS Omega, 2023, 8, 5497–5505 CrossRef CAS PubMed.
- J. Chen, P. Ji, Y. Yang, C. Jin, L. Zhuge and X. Wu, Diamond Relat. Mater., 2021, 112, 108243 CrossRef CAS.
- J. Chen, P. Ji, Y. Yang, C. Jin, L. Zhuge and X. Wu, Thin Solid Films, 2020, 709, 138167 CrossRef CAS.
- J. Wang, P. Bulkin, I. Florea, J.-L. Maurice and E. Johnson, J. Phys. D: Appl. Phys., 2016, 49, 285203 CrossRef.
- S. Lin, D. Xie, Y. Tang, Y. Wang, F. Jing, N. Huang and Y. Leng, Vacuum, 2021, 189, 110223 CrossRef CAS.
- P. Mandracci and P. Rivolo, Coatings, 2023, 13, 1075 CrossRef CAS.
- N. M. Santhosh, G. Filipič, E. Tatarova, O. Baranov, H. Kondo, M. Sekine, M. Hori, K. Ken Ostrikov and U. Cvelbar, Micromachines, 2018, 9, 565 CrossRef PubMed.
- K. Ollegott, P. Wirth, C. Oberste-Beulmann, P. Awakowicz and M. Muhler, Chem. Ing. Tech., 2020, 92, 1542–1558 CrossRef CAS.
- R. Snyders, D. Hegemann, D. Thiry, O. Zabeida, J. Klemberg-Sapieha and L. Martinu, Plasma Sources Sci. Technol., 2023, 32, 074001 CrossRef CAS.
- Y. Hua, J. Song, Z. Hao, G. Zhang and C. Ren, Plasma Res. Express, 2018, 1, 015008 CrossRef.
- K. Yi, D. Liu, X. Chen, J. Yang, D. Wei, Y. Liu and D. Wei, Acc. Chem. Res., 2021, 54, 1011–1022 CrossRef CAS PubMed.
- V. Cech and M. Branecky, Plasma Processes Polym., 2023, 20, 2300019 CrossRef CAS.
- G. S. Oehrlein and S. Hamaguchi, Plasma Sources Sci. Technol., 2018, 27, 023001 CrossRef.
- D. W. Hess, Annu. Rev. Mater. Sci., 1986, 16, 163–183 Search PubMed.
- S. Mathur and T. Ruegamer, Int. J. Appl. Ceram. Technol., 2011, 8, 1050–1058 CrossRef CAS.
- L. Czympiel, M. Frank, A. Mettenbörger, S.-M. Hühne and S. Mathur, C. R. Chim., 2018, 21, 943–951 CrossRef CAS.
- A. S. M. de Freitas, C. C. Maciel, J. S. Rodrigues, R. P. Ribeiro, A. O. Delgado-Silva and E. C. Rangel, Vacuum, 2021, 194, 110556 CrossRef CAS.
- M. Li, D. Liu, D. Wei, X. Song, D. Wei and A. T. S. Wee, Adv. Sci., 2016, 3, 1600003 CrossRef PubMed.
- N.-C. Yeh, C.-C. Hsu, J. Bagley and W.-S. Tseng, Nanotechnology, 2019, 30, 162001 CrossRef CAS PubMed.
- E. Bertran-Serra, S. Rodriguez-Miguel, Z. Li, Y. Ma, G. Farid, S. Chaitoglou, R. Amade, R. Ospina and J.-L. Andújar, Nanomaterials, 2023, 13, 2533 CrossRef CAS PubMed.
- G. L. Kabongo, B. M. Mothudi and M. S. Dhlamini, Front. Mater., 2021, 8, 1–11 Search PubMed.
- H. Nagasawa and T. Tsuru, in Advanced Materials for Membrane Fabrication and Modification, CRC Press, 2018 Search PubMed.
- D. Patrun, S. Zhao, Z. Aytuna, T. Fischer, M. Miess, Z. Hong and S. Mathur, Nano Energy, 2024, 128, 109836 CrossRef CAS.
- M. Pyeon, T.-P. Ruoko, J. Leduc, Y. Gönüllü, M. Deo, N. V. Tkachenko and S. Mathur, J. Mater. Res., 2018, 33, 455–466 CrossRef CAS.
- L. Jürgensen, M. Frank, M. Pyeon, L. Czympiel and S. Mathur, Organometallics, 2017, 36, 2331–2337 CrossRef.
- R. Garg, N. Rajagopalan, M. Pyeon, Y. Gönüllü, T. Fischer, A. S. Khanna and S. Mathur, Surf. Coat. Technol., 2018, 356, 49–55 CrossRef CAS.
- L. Jürgensen, D. Höll, M. Frank, T. Ludwig, D. Graf, A. Katrin Schmidt-Verma, A. Raauf, I. Gessner and S. Mathur, Dalton Trans., 2020, 49, 13317–13325 RSC.
- F. Zhou, J. Shan, L. Cui, Y. Qi, J. Hu, Y. Zhang and Z. Liu, Adv. Funct. Mater., 2022, 32, 2202026 CrossRef CAS.
- F. Sanchette, M. El Garah, S. Achache, F. Schuster, C. Chouquet, C. Ducros and A. Billard, Coatings, 2021, 11, 1225 CrossRef CAS.
- J. Li and H. Chae, Korean J. Chem. Eng., 2023, 40, 1268–1276 CrossRef CAS.
- Y. Liu, J. He, N. Zhang, W. Zhang, Y. Zhou and K. Huang, J. Mater. Sci., 2021, 56, 12559–12583 CrossRef CAS.
- S. V. Baryshev and M. Muehle, IEEE Trans. Plasma Sci., 2024, 52, 1082–1103 CAS.
- N. Chen, D. H. Kim, P. Kovacik, H. Sojoudi, M. Wang and K. K. Gleason, Annu. Rev. Chem. Biomol. Eng., 2016, 7, 373–393 CrossRef CAS PubMed.
- Z. Yang, Y. Liu, Z. Guo, J. Wei, J. Liu, L. Chen and C. Li, Diamond Relat. Mater., 2024, 142, 110767 CrossRef CAS.
- C.-W. Chuang and F. C.-N. Hong, Cryst. Growth Des., 2023, 23, 37–48 CrossRef CAS.
- F. Movassagh-Alanagh, A. Abdollah-Zadeh, M. A. Zolbin, N. Nemati and R. Aghababaei, Tribol. Int., 2023, 179, 108137 CrossRef CAS.
- O.-G. Simionescu, O. Brîncoveanu, C. Romaniţan, S. Vulpe and A. Avram, Coatings, 2022, 12, 943 CrossRef CAS.
- G. Moradkhani, J. Profili, M. Robert, G. Laroche, S. Elkoun and F. Mighri, Polymers, 2024, 16, 360 CrossRef CAS PubMed.
- A. D. Nguyen, T. K. Nguyen, C. T. Le, S. Kim, F. Ullah, Y. Lee, S. Lee, K. Kim, D. Lee, S. Park, J.-S. Bae, J. I. Jang and Y. S. Kim, ACS Omega, 2019, 4, 21509–21515 CrossRef CAS PubMed.
- J. Zheng, A. Xu, A. Wu and X. Li, ACS Appl. Mater. Interfaces, 2021, 13, 21231–21240 CrossRef CAS PubMed.
- S. P. Flynn, M. McKenna, R. Monaghan, S. M. Kelleher, S. Daniels and A. MacCormac, J. Chem. Educ., 2017, 94, 221–225 CrossRef CAS.
- Y. Huang, L. Cao and P. Zeng, Russ. J. Phys. Chem., 2023, 97, 3399–3404 CrossRef CAS.
- M. Zhao, D. Chen, Y. Guo, Q. Wang, T. Zhao, J. Lu and Y. Zhou, Thin Solid Films, 2023, 784, 140064 CrossRef CAS.
- J.-H. Oh, T. K. Lee, R. Y. Kim, J.-H. An, S.-I. Mo, J.-E. Hong, S.-W. Kim, M. J. Keum, H. Song and K.-H. Kim, Small Struct., 2024, 5, 2300218 CrossRef CAS.
- J. Cho, H. Seok, I. Lee, J. Lee, E. Kim, D. Sung, I.-K. Baek, C.-H. Lee and T. Kim, Sci. Rep., 2022, 12, 10335 CrossRef CAS PubMed.
- L. Himics, D. Gál, P. Csíkvári, R. Holomb, M. Koós, A. Sulyok, B. Pécz and M. Veres, Vacuum, 2023, 216, 112493 CrossRef CAS.
- V. A. Terekhov, E. I. Terukov, Y. K. Undalov, K. A. Barkov, N. A. Kurilo, S. A. Ivkov, D. N. Nesterov, P. V. Seredin, D. L. Goloshchapov, D. A. Minakov, E. V. Popova, A. N. Lukin and I. N. Trapeznikova, Symmetry, 2023, 15, 1800 CrossRef CAS.
- C. Wu, D. Y. Guo, L. Y. Zhang, P. G. Li, F. B. Zhang, C. K. Tan, S. L. Wang, A. P. Liu, F. M. Wu and W. H. Tang, Appl. Phys. Lett., 2020, 116, 072102 CrossRef CAS.
- V. K. Shukla, J. Lekshmi, B. S. Yadav, M. Kumari, S. Dalal, A. Goyal and P. Rai, Int. J. Refract. Met. Hard Mater., 2024, 119, 106559 CrossRef CAS.
- H.-U. Kim, H. Seok, W. S. Kang and T. Kim, Nanoscale Adv., 2022, 4, 2962–2972 RSC.
- V. S. Jayaseelan and R. N. Singh, J. Mater. Res., 2024, 39, 825–835 CrossRef CAS.
- H. Tian, S. Jie, P. Zhang, J. Li and S. An, Diamond Relat. Mater., 2024, 141, 110671 CrossRef CAS.
- A. M. Mumlyakov, E. A. Pershina, Ju. V. Bondareva, P. A. Nekludova, A. A. Shibalova, M. V. Shibalov, Yu. V. Anufriev, A. M. Tagachenkov and M. A. Tarkhov, Carbon, 2023, 214, 118332 CrossRef CAS.
- Y. Ma, J. Han, D. Yue, Z. Tong, M. Wang, L. Xiao, S. Jia and X. Chen, Inorg. Chem., 2023, 62, 13505–13511 CrossRef CAS PubMed.
- R. Muñoz, E. López-Elvira, C. Munuera, F. Carrascoso, Y. Xie, O. Çakıroğlu, T. Pucher, S. Puebla, A. Castellanos-Gomez and M. García-Hernández, npj 2D Mater. Appl., 2023, 7, 1–11 CrossRef.
- T.-T. Zhang, B.-H. Lv, C.-C. Fan, B.-Y. Shi, Q.-J. Cao, W. Wang, F.-F. Tao and W.-D. Dou, ACS Omega, 2023, 8, 36245–36252 CrossRef CAS PubMed.
- W. J. Lee, T.-Y. Cho, J.-H. Eom, J.-S. Park, J. Ryu and S.-K. Cho, Plasma Processes Polym., 2022, 19, 2200001 CrossRef CAS.
- A. M. Wrobel and P. Uznanski, Plasma Processes Polym., 2023, 20, 2200190 CrossRef CAS.
- G. Akiki, D. Suchet, D. Daineka, S. Filonovich, P. Bulkin and E. V. Johnson, Appl. Surf. Sci., 2020, 531, 147305 CrossRef CAS.
- B. Jugdersuren, X. Liu, J. C. Culbertson, N. Mahadik, O. Thomas and Y. Shu, J. Appl. Phys., 2023, 134, 214303 CrossRef CAS.
- C. Yu, K. Gao, C.-W. Peng, C. He, S. Wang, W. Shi, V. Allen, J. Zhang, D. Wang, G. Tian, Y. Zhang, W. Jia, Y. Song, Y. Hu, J. Colwell, C. Xing, Q. Ma, H. Wu, L. Guo, G. Dong, H. Jiang, H. Wu, X. Wang, D. Xu, K. Li, J. Peng, W. Liu, D. Chen, A. Lennon, X. Cao, S. De Wolf, J. Zhou, X. Yang and X. Zhang, Nat. Energy, 2023, 8, 1375–1385 CrossRef CAS.
- D. Liu, X. Chen, Y. Yan, Z. Zhang, Z. Jin, K. Yi, C. Zhang, Y. Zheng, Y. Wang, J. Yang, X. Xu, J. Chen, Y. Lu, D. Wei, A. T. S. Wee and D. Wei, Nat. Commun., 2019, 10, 1188 CrossRef PubMed.
- K. Chakrabarty, I. Arnold and S. A. Catledge, J. Vac. Sci. Technol., A, 2019, 37, 061507 CrossRef.
- K. Yi, Z. Jin, S. Bu, D. Wang, D. Liu, Y. Huang, Y. Dong, Q. Yuan, Y. Liu, A. T. S. Wee and D. Wei, ACS Appl. Mater. Interfaces, 2020, 12, 33113–33120 CrossRef CAS PubMed.
- B. Schurink, W. T. E. van den Beld, R. M. Tiggelaar, R. W. E. van de Kruijs and F. Bijkerk, Coatings, 2022, 12, 685 CrossRef CAS.
- H. Nadhom, D. Lundin, P. Rouf and H. Pedersen, J. Vac. Sci. Technol., A, 2020, 38, 033402 CrossRef CAS.
- D. E. Gomersall and A. J. Flewitt, J. Appl. Phys., 2022, 131, 215301 CrossRef CAS.
- P. M. Campbell, C. J. Perini, J. Chiu, A. Gupta, H. S. Ray, H. Chen, K. Wenzel, E. Snyder, B. K. Wagner, J. Ready and E. M. Vogel, 2D Mater., 2017, 5, 015005 CrossRef.
- H. He, C. Wu, H. Hu, S. Wang, F. Zhang, D. Guo and F. Wu, J. Phys. Chem. Lett., 2023, 14, 6444–6450 CrossRef CAS PubMed.
- C. A. Beaudette, J. T. Held, K. A. Mkhoyan and U. R. Kortshagen, ACS Omega, 2020, 5, 21853–21861 CrossRef CAS PubMed.
- K. M. M. D. K. Kimbulapitiya, B. Rehman, S. S. Wani, C.-T. Chen, R.-H. Cyu, A. Manikandan, M. K. Date, Y.-R. Peng, R. J. G. L. R. Kumara, F.-C. Chuang and Y.-L. Chueh, Nano Energy, 2024, 122, 109235 CrossRef CAS.
- E. Z. Frątczak, J. Balcerzak and M. Rogala, Materials, 2024, 17, 314 CrossRef PubMed.
- Y. Xia, Z. Xu, J. Peng, Q. Shen and C. Wang, Surf. Coat. Technol., 2022, 441, 128522 CrossRef CAS.
- M. A. Kudryashov, L. A. Mochalov, I. O. Prokhorov, M. A. Vshivtsev, Y. P. Kudryashova, V. M. Malyshev and E. A. Slapovskaya, High Energy Chem., 2023, 57, 532–536 CrossRef CAS.
- T.-H. Yang, Z.-Z. Lin, S.-C. Tsai, J.-Z. Dai, S.-M. Chen, M.-W. Lin and S. Chen, Mater. Sci. Semicond. Process., 2023, 162, 107515 CrossRef CAS.
- C. Yadav, M. Kumar, K. Lodhi and S. Kumar, Mater. Today Commun., 2023, 36, 106736 CrossRef CAS.
- M. Gürsoy, Coatings, 2024, 14, 347 CrossRef.
- O. Richard, H. Mziouek, R. Arès, V. Aimez and A. Jaouad, Surf. Interfaces, 2023, 40, 103104 CrossRef CAS.
- K. D. Lee, K.-S. Ji, S. Bae, S. Kim, H. Kim, J. E. Kim, Y. C. Nam, S. Choi, M. S. Jeong, M. G. Kang, H.-E. Song, Y. Kang, H.-S. Lee and D. Kim, J. Nanosci. Nanotechnol., 2017, 17, 4687–4693 CrossRef CAS.
- S. Ahn, S. J. Hong, H. S. Yang and S. M. Cho, Mater. Sci. Semicond. Process., 2022, 143, 106538 CrossRef CAS.
- H.-L. Chen, Y.-C. Tu, C.-C. Hsieh, D.-L. Lin and K.-C. Leou, J. Appl. Phys., 2014, 116, 103307 CrossRef.
- B. B. Sahu and J. G. Han, Phys. Plasmas, 2016, 23, 123504 CrossRef.
- G. Das, S. Mandal, S. Dhar, S. Bose, J. R. Sharma, S. Mukhopadhyay, C. Banerjee and A. K. Barua, J. Mater. Sci.: Mater. Electron., 2017, 28, 10382–10390 CrossRef CAS.
- X. Li, R. Jin, L. Li, J. Lu, Y. Gu, F. Ren and J. Huang, Optik, 2019, 180, 104–112 CrossRef CAS.
- M. A. Yusuf, A. Rosikhin, J. D. Malago, F. A. Noor and T. Winata, Mater. Sci. Forum, 2019, 966, 100–106 Search PubMed.
- S. Juneja and S. Kumar, Silicon, 2021, 13, 3927–3940 CrossRef CAS.
- X. Zhou, X. Tan, Y. Lv, Y. Wang, J. Li, S. Liang, Z.-H. Zhang, Z. Feng and S. Cai, Opt. Express, 2020, 28, 29245–29252 CrossRef CAS PubMed.
- P. R. Y. Gangavarapu, A. M. R. Sharma and A. K. Naik, Semicond. Sci. Technol., 2019, 34, 065018 CrossRef CAS.
- B. B. Sahu, Y. Yin and J. G. Han, Phys. Plasmas, 2016, 23, 033512 CrossRef.
- C. Chen, W. Fu, C. Zhang, D. Lu, M. Han and Y. Yan, Appl. Sci., 2021, 11, 9873 CrossRef CAS.
- M. Cantoro, S. Hofmann, C. Mattevi, S. Pisana, A. Parvez, A. Fasoli, C. Ducati, V. Scardaci, A. C. Ferrari and J. Robertson, J. Appl. Phys., 2009, 105, 064304 CrossRef.
- T. Labbaye, E. Kovacevic, T. Lecas, M.-R. Ammar, A. Canizarès, N. Raimboux, T. Strunskus, C. Jaeger, P. Simon and C. Boulmer-Leborgne, Appl. Surf. Sci., 2018, 453, 436–441 CrossRef CAS.
- U. Sharma and S. C. Sharma, IEEE Trans. Plasma Sci., 2022, 50, 888–898 CAS.
- U. Khalilov, A. Bogaerts, S. Hussain, E. Kovacevic, P. Brault, C. Boulmer-Leborgne and E. C. Neyts, J. Phys. D: Appl. Phys., 2017, 50, 184001 CrossRef.
- Z. Jiang, B. Zhang, Q. Shen and Z.-J. Jiang, J. Alloys Compd., 2019, 806, 864–873 CrossRef CAS.
- W. Wang, É. Ngo, P. Bulkin, Z. Zhang, M. Foldyna, P. Roca i Cabarrocas, E. V. Johnson and J.-L. Maurice, Nanomaterials, 2023, 13, 2061 CrossRef CAS PubMed.
- S. Djoumi, F. Kail, P. R. i Cabarrocas and L. Chahed, Thin Solid Films, 2022, 758, 139447 CrossRef CAS.
- J. Tang, J.-L. Maurice, W. Chen, S. Misra, M. Foldyna, E. V. Johnson and P. Roca i Cabarrocas, Nanoscale Res. Lett., 2016, 11, 455 CrossRef CAS PubMed.
- M. Hývl, M. Müller, T.-H. Stuchlíková, J. Stuchlík, M. Šilhavík, J. Kočka, A. Fejfar and J. Červenka, Nanotechnology, 2020, 31, 225601 CrossRef PubMed.
- A. O. Zamchiy, Е. А. Baranov, S. Ya. Khmel, E. A. Maximovskiy, D. V. Gulyaev and K. S. Zhuravlev, Thin Solid Films, 2018, 654, 61–68 CrossRef CAS.
- E. Azrak, W. Chen, S. Moldovan, S. Gao, S. Duguay, P. Pareige and P. Roca i Cabarrocas, J. Phys. Chem. C, 2018, 122, 26236–26242 CrossRef CAS.
- L. Zheng, E. Azrak, R. Gong, C. Castro, S. Duguay, P. Pareige, P. Roca i Cabarrocas and W. Chen, J. Alloys Compd., 2022, 899, 163273 CrossRef CAS.
- L. Dai, I. Maurin, M. Foldyna, J. Alvarez, W. Wang, H. Mohsin, W. Chen, J.-P. Kleider, J.-L. Maurice, T. Gacoin and P. R. i Cabarrocas, Nanotechnology, 2018, 29, 435301 CrossRef PubMed.
- W. Yang, X. Zhang, F. Huang, Z. Zhang, J. Muhammad, X. Li, Z. Rong, X. Guo, Y. Jung and X. Dong, Appl. Surf. Sci., 2021, 557, 149848 CrossRef CAS.
- E. Azrak, Z. Xue, S. Liu, W. Chen, C. Castro, S. Duguay, P. Pareige, L. Yu and P. Roca i Cabarrocas, Appl. Surf. Sci., 2023, 618, 156637 CrossRef CAS.
- J. W. Miller, Z. Khatami, J. Wojcik, J. D. B. Bradley and P. Mascher, Surf. Coat. Technol., 2018, 336, 99–105 CrossRef CAS.
- F. Azmi, Y. Gao, Z. Khatami and P. Mascher, J. Vac. Sci. Technol., A, 2022, 40, 043402 CrossRef CAS.
- Z. Khatami, L. Wolz, J. Wojcik and P. Mascher, J. Mater. Res., 2024, 39, 150–164 CrossRef CAS PubMed.
- F. Azmi, Y. Gao, Z. Khatami and P. Mascher, Meet. Abstr., 2020, MA2020-01, p. 2934 Search PubMed.
- R. B. Namin, P. Mascher, F. Chibante and Z. Khatami, ECS J. Solid State Sci. Technol., 2023, 12, 106002 CrossRef CAS.
- J. Kulczyk-Malecka, D. Donaghy, B. Delfour-Peyrethon, M. Werner, P. R. Chalker, J. W. Bradley and P. J. Kelly, J. Vac. Sci. Technol., A, 2020, 38, 033410 CrossRef CAS.
- N. R. Lee, Y. S. Jun, K. I. Moon and C. S. Lee, Jpn. J. Appl. Phys., 2017, 56, 035506 CrossRef.
- K. H. Kim, K. S. Kim, Y. J. Ji, J. E. Kang and G. Y. Yeom, Appl. Surf. Sci., 2021, 541, 148313 CrossRef CAS.
- Z. Huang, Z. Chen, W. Lang and X. Wang, Materials, 2021, 14, 2954 CrossRef CAS PubMed.
- R. Song, S. Chen, Z. Liu, C. Huo and Q. Chen, Diamond Relat. Mater., 2023, 132, 109687 CrossRef CAS.
- S.-C. Lin, C.-C. Wang, C.-L. Tien, F.-C. Tung, H.-F. Wang and S.-H. Lai, Micromachines, 2023, 14, 279 CrossRef PubMed.
- N. Q. Minh, N. Van Nong, M. S. D. C. Dela Vega, O. Oda and M. Hori, Vacuum, 2023, 213, 112118 CrossRef CAS.
- A. Dias, N. Bundaleska, E. Felizardo, D. Tsyganov, A. Almeida, A. M. Ferraria, A. M. Botelho do Rego, M. Abrashev, T. Strunskus, N. M. Santhosh, U. Cvelbar, J. Zavašnik, M. F. Montemor, M. M. Almeida, P. A. Carvalho, J. Kissovski, L. L. Alves and E. Tatarova, Chem. Eng. J., 2022, 430, 133153 CrossRef CAS.
- M. Su, H. Yang, Z. Liu, E. Wu, X. Chen, Z. Bo, L. Dai and K. Ken Ostrikov, Carbon, 2022, 197, 301–310 CrossRef CAS.
- B. Dey, S. Bulou, W. Ravisy, N. Gautier, M. Richard-Plouet, A. Granier and P. Choquet, Surf. Coat. Technol., 2022, 436, 128256 CrossRef CAS.
- C. T. Tran, M. Raco, L. M. Casey and D. R. McKenzie, Plasma Processes Polym., 2022, 19, 2200019 CrossRef CAS.
- A. Capote, G. Capote, E. J. Corat and V. J. Trava-Airoldi, Surf. Coat. Technol., 2022, 445, 128716 CrossRef CAS.
- K. Yamasaki, T. Matsutani, N. Imaeda and T. Kawasaki, Vacuum, 2015, 122, 332–336 CrossRef CAS.
- M. Murano, T. Matsutani and T. Kawasaki, Vacuum, 2018, 158, 60–64 CrossRef CAS.
- D. Batryshev, A. Utegenov, R. Zhumadilov, N. Akhanova, S. Orazbayev, S. Ussenkhan, J. Lin, K. Takahashi, N. Bastykova, S. Kodanova, M. Gabdullin and T. Ramazanov, Contrib. Plasma Phys., 2022, 62, e202100238 CrossRef CAS.
- Z. Guo, L. Sang, Z. Wang, Q. Chen, L. Yang and Z. Liu, Surf. Coat. Technol., 2016, 307, 1059–1064 CrossRef CAS.
- Y. Hu, X. Tian, Q. Fan, Z. Wang, B. Liu, L. Yang and Z. Liu, Plasma Sci. Technol., 2019, 21, 105502 CrossRef CAS.
- S. Bulou, E. Lecoq, F. Loyer, G. Frache, T. Fouquet, M. Gueye, T. Belmonte and P. Choquet, Plasma Processes Polym., 2019, 16, 1800177 CrossRef.
- C. Ruhmlieb, Y. J. Lee, C. Strelow, T. Kipp and A. Mews, J. Mater. Chem. C, 2019, 7, 10098–10110 RSC.
- M. Taplick, C. Ruhmlieb, T. Kipp and A. Mews, Nano Lett., 2023, 23, 1313–1319 CrossRef CAS PubMed.
- M. A. A. Mamun, H. Furuta and A. Hatta, Jpn. J. Appl. Phys., 2018, 57, 06JF02 CrossRef.
- R. Hatada, S. Flege, M. N. Ashraf, A. Timmermann, C. Schmid and W. Ensinger, Coatings, 2020, 10, 360 CrossRef CAS.
- R. Hatada, K. Baba, S. Flege and W. Ensinger, Surf. Coat. Technol., 2016, 305, 93–98 CrossRef CAS.
- S. de F. M. Mariano, M. Ueda, R. M. Oliveira, E. J. de D. M. Pillaca and N. M. dos Santos, Surf. Coat. Technol., 2017, 312, 47–54 CrossRef CAS.
- S. F. M. Mariano and M. Ueda, Appl. Surf. Sci., 2019, 465, 824–832 CrossRef CAS.
- Z. Wu, E. Wang, G. Zhang, Y. Shen and G. Shao, Small, 2024, 11, 2307923 CrossRef PubMed.
- H. Wang, Y. Han, P. Luo, Y. Zhou, Q. Chen, H. Zhu, Y. Yang, B. Zhang and K. Huang, ChemistrySelect, 2022, 7, e202200103 CrossRef CAS.
- S. Sahoo, G. Sahoo, S. M. Jeong and C. S. Rout, J. Energy Storage, 2022, 53, 105212 CrossRef.
- M. A. Zafar and M. V. Jacob, Rev. Mod. Plasma Phys., 2022, 6, 37 CrossRef.
- S. Zheng, Q. Wang, K. Guo, J. Bai, Z. Yang, H. Yu, H. Liu, H. Wei, J. Zhu and Q. Hu, J. Cryst. Growth, 2024, 627, 127538 CrossRef CAS.
- O. Baranov, I. Levchenko, S. Xu, J. W. M. Lim, U. Cvelbar and K. Bazaka, 2D Mater., 2018, 5, 044002 CrossRef CAS.
- W. Zheng, X. Zhao and W. Fu, ACS Appl. Mater. Interfaces, 2021, 13, 9561–9579 CrossRef CAS PubMed.
- A. Khan, J. Cong, R. R. Kumar, S. Ahmed, D. Yang and X. Yu, ACS Appl. Nano Mater., 2022, 5, 17544–17555 CrossRef CAS.
- S. Ghosh, S. R. Polaki, M. Kamruddin, S. M. Jeong and K. Ken Ostrikov, J. Phys. D: Appl. Phys., 2018, 51, 145303 CrossRef.
- M. R. Maschmann, P. B. Amama, A. Goyal, Z. Iqbal and T. S. Fisher, Carbon, 2006, 44, 2758–2763 CrossRef CAS.
- A. Thapa, S. Neupane, R. Guo, K. L. Jungjohann, D. Pete and W. Li, Diamond Relat. Mater., 2018, 90, 144–153 CrossRef CAS.
- J. Sun, T. Rattanasawatesun, P. Tang, Z. Bi, S. Pandit, L. Lam, C. Wasén, M. Erlandsson, M. Bokarewa, J. Dong, F. Ding, F. Xiong and I. Mijakovic, ACS Appl. Mater. Interfaces, 2022, 14, 7152–7160 CrossRef CAS PubMed.
- A. Tewari, S. Ghosh and P. Srivastava, Plasma Res. Express, 2021, 3, 035003 CrossRef CAS.
- S. Xu, S. Wang, Z. Chen, Y. Sun, Z. Gao, H. Zhang and J. Zhang, Adv. Funct. Mater., 2020, 30, 2003302 CrossRef CAS.
- C. Shen, S. Xu, Z. Chen, N. Ji, J. Yang and J. Zhang, Small, 2023, 19, 2207745 CrossRef CAS PubMed.
- S. Xu, T. Cheng, Q. Yan, C. Shen, Y. Yu, C.-T. Lin, F. Ding and J. Zhang, Adv. Sci., 2022, 9, 2200737 CrossRef CAS PubMed.
- S. Xu, Y. Wen, Z. Chen, N. Ji, Z. Zou, M. Wu, L. Qu and J. Zhang, Angew. Chem., Int. Ed., 2021, 60, 24505–24509 CrossRef CAS PubMed.
- S.-F. Wang, D. Xue, J. Liang, L.-Y. Chen, Y. Xie and J.-M. Zhang, Diamond Relat. Mater., 2023, 140, 110426 CrossRef CAS.
- D. H. Seo, S. Yick, Z. J. Han, J. H. Fang and K. Ken Ostrikov, ChemSusChem, 2014, 7, 2317–2324 CrossRef CAS PubMed.
- L.-A. Gautier, V. Le Borgne and M. A. El Khakani, Carbon, 2016, 98, 259–266 CrossRef CAS.
- W.-H. Chiang, T.-C. Lin, Y.-S. Li, Y.-J. Yang and Z. Pei, RSC Adv., 2016, 6, 2270–2278 RSC.
- X. Peng, Z. Wang, Z. Wang, J. Gong and H. Hao, Catal. Today, 2019, 337, 63–68 CrossRef CAS.
- E. P. Neustroev, A. R. Prokopyev, S. O. Semenov, V. I. Popov, F. F. Protopopov, A. S. Andreev, N. A. Savvinova and E. S. Lukin, IOP Conf. Ser.:Mater. Sci. Eng., 2021, 1079, 042086 CAS.
- A. Dias, E. Felizardo, N. Bundaleska, M. Abrashev, J. Kissovski, A. M. Ferraria, A. M. Rego, T. Strunskus, P. A. Carvalho, A. Almeida, J. Zavašnik, E. Kovacevic, J. Berndt, N. Bundaleski, M.-R. Ammar, O. M. N. D. Teodoro, U. Cvelbar, L. L. Alves, B. Gonçalves and E. Tatarova, Appl. Mater. Today, 2024, 36, 102056 CrossRef.
- J. Zheng, R. Yang, Y. Lou, W. Li and X. Li, Thin Solid Films, 2012, 521, 137–140 CrossRef CAS.
- H. Seok, Y. T. Megra, C. K. Kanade, J. Cho, V. K. Kanade, M. Kim, I. Lee, P. J. Yoo, H.-U. Kim, J. W. Suk and T. Kim, ACS Nano, 2021, 15, 707–718 CrossRef CAS PubMed.
- Y. Kim, S. Kwon, E.-J. Seo, J. H. Nam, H. Y. Jang, S.-H. Kwon, J.-D. Kwon, D.-W. Kim and B. Cho, ACS Appl. Mater. Interfaces, 2018, 10, 36136–36143 CrossRef CAS PubMed.
- R. K. Joshi, M. Yoshimura, K. Tanaka, K. Ueda, A. Kumar and N. Ramgir, J. Phys. Chem. C, 2008, 112, 13901–13904 CrossRef CAS.
- S. A. Ahmad Kamal, R. Ritikos and S. Abdul Rahman, Appl. Surf. Sci., 2015, 328, 146–153 CrossRef CAS.
- H. Hamidinezhad, A. Akbar Ashkarran and Z. Abdul-Malek, Mater. Sci. Semicond. Process., 2014, 27, 26–32 CrossRef CAS.
- M. Macias-Montero, A. Borras, Z. Saghi, J. P. Espinos, A. Barranco, J. Cotrino and A. R. Gonzalez-Elipe, Nanotechnology, 2012, 23, 255303 CrossRef PubMed.
- N. Filippin, J. Castillo-Seoane, M. C. López-Santos, C. T. Rojas, K. Ostrikov, A. Barranco, J. R. Sánchez-Valencia and A. Borrás, ACS Appl. Mater. Interfaces, 2020, 12, 50721–50733 CrossRef CAS PubMed.
- M. C. Vasudev, H. Koerner, K. M. Singh, B. P. Partlow, D. L. Kaplan, E. Gazit, T. J. Bunning and R. R. Naik, Biomacromolecules, 2014, 15, 533–540 CrossRef CAS PubMed.
- Z. He, C. S. Lee, J.-L. Maurice, D. Pribat, P. Haghi-Ashtiani and C. S. Cojocaru, Carbon, 2011, 49, 4710–4718 CrossRef CAS.
- J. Jiang, N. M. Chetuya, E. I. Meletis, J. H. Ngai, G. J. Grzybowski and B. Claflin, J. Vac. Sci. Technol. B, 2024, 42, 034001 CrossRef CAS.
- H.-U. Kim, M. Kim, H. Seok, K.-Y. Park, J.-Y. Moon, J. Park, B.-S. An, H. J. Jung, V. P. Dravid, D. Whang, J.-H. Lee and T. Kim, ChemSusChem, 2021, 14, 1344–1350 CrossRef CAS PubMed.
- Y. Xing, S. Dong, X. Zhang, Y. Zhang, J. Han, X. Zhang, L. Zhang, B. Zhang and Z. Zeng, Cryst. Growth Des., 2023, 23, 2257–2263 CrossRef CAS.
- V. Brune, M. Grosch, R. Weißing, F. Hartl, M. Frank, S. Mishra and S. Mathur, Dalton Trans., 2021, 50, 12365–12385 RSC.
- P. A. L. Sino, T.-C. Lin, S. Wani, L. Lee, C.-T. Chen, M.-J. Liu, Y.-Z. Kuo, B. Rehman, K. Tuyen Le, J.-M. Wu, F.-C. Chuang and Y.-L. Chueh, Mater. Today, 2023, 69, 97–106 CrossRef.
- M. Chaudhary, T.-Y. Yang, C.-T. Chen, P.-C. Lai, Y.-C. Hsu, Y.-R. Peng, A. Kumar, C.-H. Lee and Y.-L. Chueh, Adv. Funct. Mater., 2023, 33, 2303697 CrossRef CAS.
- J. Park, I. Cho, H. Jeon, Y. Lee, J. Zhang, D. Lee, M. K. Cho, D. J. Preston, B. Shong, I. S. Kim and W.-K. Lee, Adv. Mater., 2024, 36, 2314031 CrossRef CAS PubMed.
- H.-U. Kim, V. Kanade, M. Kim, K. S. Kim, B.-S. An, H. Seok, H. Yoo, L. E. Chaney, S.-I. Kim, C.-W. Yang, G. Y. Yeom, D. Whang, J.-H. Lee and T. Kim, Small, 2020, 16, 1905000 CrossRef CAS PubMed.
- R. Zhang, Q. Zhang, X. Jia, S. Wen, H. Wu, Y. Gong, Y. Yin, C. Lan and C. Li, Nanotechnology, 2023, 34, 345704 CrossRef CAS PubMed.
- K. Aydin, C. Kanade, V. Kaluram Kanade, G. Bahit, C. Ahn and T. Kim, Nanoscale, 2023, 15, 17326–17334 RSC.
- C. Ahn, J. Lee, H.-U. Kim, H. Bark, M. Jeon, G. H. Ryu, Z. Lee, G. Y. Yeom, K. Kim, J. Jung, Y. Kim, C. Lee and T. Kim, Adv. Mater., 2015, 27, 5223–5229 CrossRef CAS PubMed.
- H. Seok, M. Kim, J. Cho, E. Kim, S. Son, K.-W. Kim, J. K. Kim, P. J. Yoo, M. Kim, H.-U. Kim and T. Kim, ACS Sustainable Chem. Eng., 2023, 11, 568–577 CrossRef CAS.
- J. H. Kim, S. J. Yun, H. S. Lee, J. Zhao, H. Bouzid and Y. H. Lee, Sci. Rep., 2018, 8, 10284 CrossRef PubMed.
- Y. Li, J. Duan, Y. Berencén, R. Hübner, H.-S. Tsai, C.-N. Kuo, C. Shan Lue, M. Helm, S. Zhou and S. Prucnal, Nanoscale Adv., 2023, 5, 443–449 RSC.
- J. Cho, H. Seok and T. Kim, Meet. Abstr., 2023, MA2023-02, p. 1157 Search PubMed.
- M. Chaudhary, Y.-C. Shih, S.-Y. Tang, T.-Y. Yang, T.-W. Kuo, C.-C. Chung, Y.-C. Shen, A. kumar Anbalagan, C.-H. Lee, T.-H. Hou, J.-H. He and Y.-L. Chueh, ACS Appl. Mater. Interfaces, 2023, 15, 33858–33867 CrossRef CAS PubMed.
- W. Fan, H. Shen, B. Liu, L. Zhao, X. Zhang and H. Pan, Energies, 2023, 16, 6963 CrossRef CAS.
- B. Grübel, H. Nagel, B. Steinhauser, F. Feldmann, S. Kluska and M. Hermle, Phys. Status Solidi A, 2021, 218, 2100156 CrossRef.
- J.-S. Song, Y. S. Park and N.-H. Kim, Appl. Sci., 2021, 11, 358 CrossRef CAS.
- Z. Zhong, X. Luo, L. Zhou, S. Hu, L. Dai, S. Wang and S. Yang, Opt. Quantum Electron., 2023, 55, 264 CrossRef CAS.
- Y.-R. Zhang, Y.-T. Hu and Y.-N. Wang, Plasma Sources Sci. Technol., 2020, 29, 084003 CrossRef CAS.
- H. J. Kim, K. Lee and H. Park, Plasma Sources Sci. Technol., 2024, 33, 015008 CrossRef CAS.
- H. J. Kim, K. Lee and H. Park, Plasma Sources Sci. Technol., 2023, 32, 115008 CrossRef CAS.
- D. Li, X. Feng, Z. Wen, Z. Shang and Y. She, Optoelectron. Lett., 2016, 12, 285–289 CrossRef.
- H. Lim, Y. Park, N. Baek, S.-Y. Jun, S. Lee, J. Yang, D. Jung and S. Yu, J. Nanosci. Nanotechnol., 2021, 21, 4477–4483 CrossRef CAS PubMed.
- E. Bertran-Serra, A. Musheghyan-Avetisyan, S. Chaitoglou, R. Amade-Rovira, I. Alshaikh, F. Pantoja-Suárez, J.-L. Andújar-Bella, T. Jawhari, A. Perez-del-Pino and E. Gyorgy, Appl. Surf. Sci., 2023, 610, 155530 CrossRef CAS.
- G. Sato, T. Kato, W. Oohara and R. Hatakeyama, Thin Solid Films, 2006, 506–507, 550–554 CrossRef CAS.
- C. R. Yang, C. H. Yeh, L. C. Hu, T. C. Wei, C. C. Lee, J. Y. Chang and T. T. Li, Plasma Chem. Plasma Process., 2018, 38, 247–259 CrossRef CAS.
- J. Miller, A. Ceballos, L. B. Bayu Aji, A. Moore, C. Wasz, S. O. Kucheyev, S. Elhadj and S. Falabella, Thin Solid Films, 2020, 714, 138394 CrossRef CAS.
- L. C. Hu, G. M. Ruan, T. C. Wei, C. J. Wang, Y. W. Lin, C. C. Lee, Y. Kawai and T. T. Li, Thin Solid Films, 2014, 570, 574–579 CrossRef CAS.
- P. Ji, J. Chen, T. Huang, L. Zhuge and X. Wu, Diamond Relat. Mater., 2020, 109, 108067 CrossRef CAS.
- Y. Xia, X. Yang, L. Chang, H. Zhou, J.-H. Zhang, D. Jing, Q. Xu, G.-J. Niu, H.-S. Zhou and G.-N. Luo, Rev. Sci. Instrum., 2023, 94, 125110 CrossRef CAS PubMed.
- Y. Yang, T. Huang, M. Li, Y. Yu, J. Huang, B. Yu, X. Wu and P. Ji, Plasma Sci. Technol., 2022, 24, 105502 CrossRef CAS.
- J. Chen, P. Ji, M. Li, T. Huang, L. Zhuge and X. Wu, Plasma Sci. Technol., 2022, 24, 054001 CrossRef CAS.
- P. Ji, J. Chen, T. Huang, C. Jin, L. Zhuge and X. Wu, Diamond Relat. Mater., 2020, 108, 107958 CrossRef CAS.
- P. Ji, J. Chen, T. Huang, C. Jin, L. Zhuge and X. Wu, Appl. Phys. A, 2020, 126, 247 CrossRef CAS.
- X. Ma, D. Xu, P. Ji, C. Jin, J. Lin, Y. Ding and C. Xu, Vacuum, 2019, 164, 355–360 CrossRef CAS.
- D. Li, S. Dai, A. Goullet and A. Granier, Nano, 2018, 13, 1850124 CrossRef CAS.
- P. Ji, J. Yu, T. Huang, C. Jin, Y. Yang, L. Zhuge and X. Wu, Plasma Sci. Technol., 2018, 20, 025505 CrossRef.
- J. Chen, P. Ji, C. JIN, L. Zhuge and X. Wu, Plasma Sci. Technol., 2018, 21, 025502 CrossRef.
- J. Qian, P. Ji, C. Jin, L. Zhuge and X. Wu, IEEE Trans. Plasma Sci., 2020, 48, 2431–2436 CAS.
- T. Goto, K.-I. Sato, Y. Yabuta, S. Sugawa and S. Hara, IEEE J. Electron Devices Soc., 2018, 6, 512–517 CAS.
- H. Bae, I. Hamaguchi, K. Sasai, H. Suzuki and H. Toyoda, Jpn. J. Appl. Phys., 2021, 60, 126002 CrossRef CAS.
- S. Patil, S. Sharma, S. Sengupta, A. Sen and I. Kaganovich, Phys. Rev. Res., 2022, 4, 013059 CrossRef CAS.
- M. Magarotto, D. Melazzi and D. Pavarin, J. Plasma Phys., 2019, 85, 905850404 CrossRef CAS.
- Q.-Z. Zhang, J.-Y. Sun, W.-Q. Lu, J. Schulze, Y.-Q. Guo and Y.-N. Wang, Phys. Rev. E, 2021, 104, 045209 CrossRef CAS PubMed.
- S. Sharma, I. D. Kaganovich, A. V. Khrabrov, P. Kaw and A. Sen, Phys. Plasmas, 2018, 25, 080704 CrossRef.
- T. Zhang, R. Cui, R. Han, F. He, W. Zhu, Z. Xia, Y. Cui and J. Ouyang, Plasma Sources Sci. Technol., 2022, 31, 105008 CrossRef CAS.
- A. O. Zamchiy, E. A. Baranov and S. Y. Khmel, Vacuum, 2018, 147, 99–106 CrossRef CAS.
- A. O. Zamchiy, E. A. Baranov, I. E. Merkulova, V. A. Volodin, M. R. Sharafutdinov and S. Y. Khmel, Vacuum, 2018, 152, 319–326 CrossRef CAS.
- E. A. Baranov, A. O. Zamchiy, N. A. Lunev, I. E. Merkulova, V. A. Volodin, M. R. Sharafutdinov and A. A. Shapovalova, J. Appl. Mech. Tech. Phys., 2022, 63, 757–764 CrossRef CAS.
- I. E. Merkulova, J. Phys.: Conf. Ser., 2019, 1382, 012160 CrossRef CAS.
- T. Tabuchi, Y. Toyoshima, S. Fujimoto and M. Takashiri, Thin Solid Films, 2020, 694, 137714 CrossRef CAS.
- T. Tabuchi, Y. Toyoshima, S. Fujimoto and M. Takashiri, AIP Adv., 2019, 9, 055125 CrossRef.
- H. Sugiura, H. Kondo, T. Tsutsumi, K. Ishikawa and M. Hori, C, 2019, 5, 8 CAS.
- L. Jia, H. Sugiura, H. Kondo, K. Takeda, K. Ishikawa, O. Oda, M. Sekine, M. Hiramatsu and M. Hori, Plasma Processes Polym., 2016, 13, 730–736 CrossRef CAS.
- H. Sugiura, L. Jia, Y. Ohashi, H. Kondo, K. Ishikawa, T. Tsutsumi, T. Hayashi, K. Takeda, M. Sekine and M. Hori, Jpn. J. Appl. Phys., 2019, 58, 030912 CrossRef CAS.
- T. Ichikawa, N. Shimizu, K. Ishikawa, M. Hiramatsu and M. Hori, Carbon, 2020, 161, 403–412 CrossRef CAS.
- X.-F. Wang, W.-Z. Jia, Y.-H. Song, Y.-Y. Zhang, Z.-L. Dai and Y.-N. Wang, Phys. Plasmas, 2017, 24, 113503 CrossRef.
- D. Lundin, J. Jensen and H. Pedersen, J. Vac. Sci. Technol., A, 2014, 32, 030602 CrossRef.
- M. A. A. Mamun, H. Furuta and A. Hatta, IEEE Trans. Plasma Sci., 2019, 47, 22–31 Search PubMed.
- H. Kakiuchi, H. Ohmi and K. Yasutake, J. Phys. D: Appl. Phys., 2020, 53, 415201 CrossRef CAS.
- Z. Gong, J. Shi, W. Ma, B. Zhang and J. Zhang, RSC Adv., 2016, 6, 115092–115100 RSC.
- Ö. Çelikel and H. Kavak, Diamond Relat. Mater., 2021, 120, 108610 CrossRef.
- M. Y. Yoon, J.-R. Jeong, H.-C. Lee and J.-H. Kim, Appl. Surf. Sci., 2023, 636, 157814 CrossRef CAS.
- G. Kalita and M. Umeno, AppliedChem, 2022, 2, 160–184 CrossRef CAS.
- A. M. Coclite and K. K. Gleason, Plasma Processes Polym., 2012, 9, 425–434 CrossRef CAS.
- M. Gürsoy, Plasma Chem. Plasma Process., 2020, 40, 1063–1079 CrossRef.
- M. Gürsoy, J. Appl. Polym. Sci., 2021, 138, 49722 CrossRef.
- F. Loyer, G. Bengasi, G. Frache, P. Choquet and N. D. Boscher, Plasma Processes Polym., 2018, 15, 1800027 CrossRef.
- D. D. Burkey, in CVD Polymers, John Wiley & Sons, Ltd, 2015, pp. 65–85 Search PubMed.
- M. Wang, N. D. Boscher, K. Heinze and K. K. Gleason, Adv. Funct. Mater., 2017, 27, 1606652 CrossRef.
- A. Kumar, D. S. Grant, K. Bazaka and M. V. Jacob, J. Appl. Polym. Sci., 2018, 135, 45771 CrossRef.
- H. Akther, M. M. Rahman, A. H. Bhuiyan, H. Kabir, S. A.-A. Zumahi, J. A. Syed and R. Nasrin, Mater. Today Commun., 2022, 31, 103377 CrossRef CAS.
- A. Loesch-Zhang, A. Geissler and M. Biesalski, Plasma Processes Polym., 2023, 20, e2300016 CrossRef CAS.
- T. Dufour, Polymers, 2023, 15, 3607 CrossRef CAS PubMed.
- S. Matsui, in Encyclopedia of Nanotechnology, ed. B. Bhushan, Springer Netherlands, Dordrecht, 2012, pp. 866–876 Search PubMed.
- W. J. MoberlyChan, D. P. Adams, M. J. Aziz, G. Hobler and T. Schenkel, MRS Bull., 2007, 32, 424–432 CrossRef CAS.
- M. Manoccio, M. Esposito, A. Passaseo, M. Cuscunà and V. Tasco, Micromachines, 2021, 12, 6 CrossRef PubMed.
- R. Winkler, J. D. Fowlkes, P. D. Rack and H. Plank, J. Appl. Phys., 2019, 125, 210901 CrossRef.
- K. Höflich, G. Hobler, F. I. Allen, T. Wirtz, G. Rius, L. McElwee-White, A. V. Krasheninnikov, M. Schmidt, I. Utke, N. Klingner, M. Osenberg, R. Córdoba, F. Djurabekova, I. Manke, P. Moll, M. Manoccio, J. M. De Teresa, L. Bischoff, J. Michler, O. De Castro, A. Delobbe, P. Dunne, O. V. Dobrovolskiy, N. Frese, A. Gölzhäuser, P. Mazarov, D. Koelle, W. Möller, F. Pérez-Murano, P. Philipp, F. Vollnhals and G. Hlawacek, Appl. Phys. Rev., 2023, 10, 041311 Search PubMed.
- R. Winkler, B. B. Lewis, J. D. Fowlkes, P. D. Rack and H. Plank, ACS Appl. Nano Mater., 2018, 1, 1014–1027 CrossRef CAS.
- P. Niiranen, H. Nadhom, M. Zanáška, R. Boyd, M. Sortica, D. Primetzhofer, D. Lundin and H. Pedersen, Rev. Sci. Instrum., 2023, 94, 023902 CrossRef CAS PubMed.
- P. Niiranen, A. Kapran, H. Nadhom, M. Čada, Z. Hubička, H. Pedersen and D. Lundin, J. Vac. Sci. Technol., A, 2024, 42, 023006 CrossRef CAS.
- H. Nadhom, Y. Yuan, P. Rouf, N. Solin and H. Pedersen, J. Vac. Sci. Technol., A, 2021, 39, 043411 CrossRef CAS.
- G. Pozina, C.-W. Hsu, N. Abrikossova and C. Hemmingsson, Crystals, 2023, 13, 373 CrossRef CAS.
- M. Xu, T. Bearda, M. Hasan, H. S. Radhakrishnan, I. Gordon, J. Szlufcik and J. Poortmans, in 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion (WCPEC) (A Joint Conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC), 2018, pp. 3148–3151 Search PubMed.
- H. Nadhom, R. Boyd, P. Rouf, D. Lundin and H. Pedersen, J. Phys. Chem. Lett., 2021, 12, 4130–4133 CrossRef CAS PubMed.
- G. Akiki, M. Frégnaux, I. Florea, P. Bulkin, D. Daineka, S. Filonovich, M. Bouttemy and E. V. Johnson, J. Vac. Sci. Technol., A, 2020, 39, 013201 CrossRef.
- I. V. Otto IV, C. Vallée, S. Kal and P. Biolsi, J. Vac. Sci. Technol. B, 2023, 41, 032202 CrossRef.
- D. N. Polyakov, V. V. Shumova and L. M. Vasilyak, Plasma Sources Sci. Technol., 2021, 30, 07LT01 CrossRef CAS.
- T. Faraz, K. Arts, S. Karwal, H. C. M. Knoops and W. M. M. Kessels, Plasma Sources Sci. Technol., 2019, 28, 024002 CrossRef CAS.
- S. J. C. Irvine and D. Lamb, in Chemical Vapour Deposition: Precursors, Processes and Applications, 2008, pp. 477–493 Search PubMed.
- C. P. Grigoropoulos, Int. J. Extreme Manuf., 2019, 1, 012002 CrossRef CAS.
- J. Niu, S. Meng, J. Li, H. Jin, F. Yi and Y. Qin, Ceram. Int., 2020, 46, 2086–2092 CrossRef CAS.
- S. Miyata, K. Matsuse, A. Ibi, T. Izumi, Y. Shiohara and T. Goto, Supercond. Sci. Technol., 2013, 26, 045020 CrossRef CAS.
- W. Arpavate, K. Roongraung and S. Chuangchote, in Green Sustainable Process for Chemical and Environmental Engineering and Science, ed. Inamuddin, R. Boddula, A. M. Asiri and M. M. Rahman, Elsevier, 2021, pp. 189–203 Search PubMed.
- D. J. Hwang, S.-G. Ryu and C. P. Grigoropoulos, Nanotechnology, 2011, 22, 385303 CrossRef PubMed.
- Y. van de Burgt, J. Laser Appl., 2014, 26, 032001 CrossRef.
- R. Tu, Z. Liu, Q. Xu, S. Zhang, Q. Li, X. Zhang, M. L. Kosinova and T. Goto, J. Eur. Ceram. Soc., 2023, 43, 5214–5222 CrossRef CAS.
- K.-H. Dahmen, in Encyclopedia of Physical Science and Technology ed. R. A. Meyers, Academic Press, New York, 3rd edn, 2003, pp. 787–808 Search PubMed.
- M. R. Baklanov, V. Jousseaume, T. V. Rakhimova, D. V. Lopaev, Yu. A. Mankelevich, V. V. Afanas'ev, J. L. Shohet, S. W. King and E. T. Ryan, Appl. Phys. Rev., 2019, 6, 011301 Search PubMed.
- Y. Zhang, Z. Chen, K. Zhang, Z. Feng and H. Zhao, Phys. Status Solidi RRL, 2021, 15, 2100202 CrossRef CAS.
- S. J. C. Irvine, in Chemical Physics of Thin Film Deposition Processes for Micro- and Nano-Technologies, ed. Y. Pauleau, Springer Netherlands, Dordrecht, 2002, pp. 199–222 Search PubMed.
- H. Cheng, R. Tu, S. Zhang, M. Han, T. Goto and L. Zhang, J. Eur. Ceram. Soc., 2017, 37, 509–515 CrossRef CAS.
- Y. Fujita, J. Cryst. Growth, 2000, 221, 382–387 CrossRef CAS.
- D. Farhanian, G. De Crescenzo and J. R. Tavares, Langmuir, 2017, 33, 1780–1791 CrossRef CAS PubMed.
- C. P. Christensen and K. M. Lakin, Appl. Phys. Lett., 2008, 32, 254–256 CrossRef.
- G. Leyendecker, D. Bäuerle, P. Geittner and H. Lydtin, Appl. Phys. Lett., 1981, 39, 921–923 CrossRef CAS.
- B. Liu, R. F. Hicks and J. J. Zinck, J. Cryst. Growth, 1993, 129, 111–118 CrossRef CAS.
- B. Liu, R. F. Hicks and J. J. Zinck, J. Cryst. Growth, 1992, 123, 500–518 CrossRef CAS.
- A. Ruzin and Y. Nemirovsky, J. Electron. Mater., 1993, 22, 281–288 CrossRef CAS.
- S. J. C. Irvine, A. Stafford, M. U. Ahmed, A. Brown and H. Kheyrandish, J. Electron. Mater., 1997, 26, 723–727 CrossRef CAS.
- M. J. Santos, A. J. Silvestre and O. Conde, Surf. Coat. Technol., 2002, 151–152, 160–164 CrossRef CAS.
- J. Mazumder and A. Kar, Theory and Application of Laser Chemical Vapor Deposition, Springer US, Boston, MA, 1995 Search PubMed.
- C. Duty, D. Jean and W. J. Lackey, Int. Mater. Rev., 2001, 46, 271–287 CrossRef CAS.
- H. Sankur, Thin Solid Films, 1992, 218, 161–169 CrossRef CAS.
- E. Hwang, J. Choi and S. Hong, Nanoscale, 2022, 14, 16065–16076 RSC.
- M. Yang, S. Bai, Q. Xu, J. Li, T. Shimada, Q. Li, T. Goto, R. Tu and S. Zhang, Diamond Relat. Mater., 2020, 109, 108094 CrossRef CAS.
- L. Constantin, L. Fan, C. Azina, K. Keramatnejad, J.-F. Silvain and Y. F. Lu, Cryst. Growth Des., 2018, 18, 2458–2466 CrossRef CAS.
- Z. Wu, W. Sun, A. Mao, Q. Zhu, X. Chen, X. Zhang, L. Trinh, N. Li, X. Huang, N. Kraiem, J.-F. Silvain, B. Cui and Y. Lu, Diamond Relat. Mater., 2024, 142, 110744 CrossRef CAS.
- L. S. Fan, Y. S. Zhou, M. X. Wang, Y. Gao, L. Liu, J. F. Silvain and Y. F. Lu, Laser Phys. Lett., 2014, 11, 076002 CrossRef CAS.
- A. Odusanya, I. Rahaman, P. K. Sarkar, A. Zkria, K. Ghosh and A. Haque, C, 2022, 8, 24 CAS.
- S. Yu, R. Tu and T. Goto, J. Eur. Ceram. Soc., 2016, 36, 403–409 CrossRef CAS.
- Z. Liu, Q. Xu, Q. Sun, J. Li, R. Tu, S. Zhang, M. Yang, Q. Li, Z. Deng, L. Zhang, T. Goto, H. Ohmori and M. Kosinova, Thin Solid Films, 2019, 678, 8–15 CrossRef CAS.
- H. Katsui, K. Shimoda and M. Hotta, Ceram. Int., 2023, 49, 38813–38823 CrossRef CAS.
- K. An, H.-N. Lee, K.-H. Cho, Y. J. Han and K.-T. Kang, Org. Electron., 2021, 91, 106078 CrossRef CAS.
- H. Katsui, K. Harada, Z. Liu, N. Kondo and M. Hotta, Ceram. Int., 2022, 48, 31016–31022 CrossRef CAS.
- B. G. Salazar, C. R. Brewer, L. McElwee-White and A. V. Walker, J. Vac. Sci. Technol., A, 2022, 40, 023404 CrossRef CAS.
- B. Sharma and A. Sharma, Appl. Surf. Sci., 2021, 567, 150724 CrossRef CAS.
- Y. D. Zhao, X. Dong, Z. Z. Ma, Y. T. Zhang, B. Wu, S. W. Zhuang, B. L. Zhang, W. C. Li and G. T. Du, J. Cryst. Growth, 2016, 454, 30–34 CrossRef CAS.
- T. Wang, R. Tu, C. Zhang, S. Zhang, K. Wang, T. Goto and L. Zhang, J. Asian Ceram. Soc., 2021, 9, 197–207 CrossRef.
- Y. Hashimoto and A. Ito, Mater. Lett., 2024, 366, 136558 CrossRef CAS.
- R. Tu, Z. Liu, C. Wang, P. Lu, B. Guo, Q. Xu, B.-W. Li and S. Zhang, RSC Adv., 2022, 12, 15555–15563 RSC.
- T.-H. Feng and X.-C. Xia, Opt. Mater. Express, 2017, 7, 1281–1288 CrossRef CAS.
- Y. Mitsuhashi, S. Matsumoto and A. Ito, Mater. Trans., 2023, 64, 1107–1111 CrossRef CAS.
- Y. Mitsuhashi, S. Matsumoto and A. Ito, J. Am. Ceram. Soc., 2023, 106, 5140–5146 CrossRef CAS.
- N. Yamaguchi and A. Ito, Mater. Lett., 2024, 369, 136721 CrossRef CAS.
- W. Li, Y. Zhang, Z. Chen, H. Zhao, S. A. Ringel and A. R. Arehart, Appl. Phys. Lett., 2023, 123, 112101 CrossRef CAS.
- E. Güney, J. Karimzadeh Khoei, B. S. Sengul, F. Can and G. Ozaydin Ince, J. Appl. Polym. Sci., 2024, 141, e55210 CrossRef.
- A. Aufoujal, U. Legrand, J.-L. Meunier and J. R. Tavares, Catalysts, 2020, 10, 534 CrossRef CAS.
- D. Kim, A. Park, Y. Choi, H. Seong, S. J. Shin, Y. H. Cho and Y. Yoo, ACS Appl. Polym. Mater., 2023, 5, 8474–8482 CrossRef CAS.
- K. R. Johnson, P. Arevalo Rodriguez, C. R. Brewer, J. A. Brannaka, Z. Shi, J. Yang, B. Salazar, L. McElwee-White and A. V. Walker, J. Chem. Phys., 2016, 146, 052816 CrossRef PubMed.
- T. Tsuchiya, J. Ceram. Soc. Jpn., 2018, 126, 889–899 CrossRef CAS.
- K. K. Gleason, Adv. Mater., 2024, 36, 2306665 CrossRef CAS PubMed.
- H. Akazawa, J. Vac. Sci. Technol., A, 2018, 36, 041505 CrossRef.
- H. Akazawa, J. Vac. Sci. Technol. B, 2022, 40, 062204 CrossRef CAS.
- H. Nasri Lari, J. Chaouki and J. R. Tavares, Chem. Eng. J., 2020, 390, 124526 CrossRef CAS.
- E. Kasparek, J. R. Tavares, M. R. Wertheimer and P.-L. Girard-Lauriault, Langmuir, 2018, 34, 12234–12243 CrossRef CAS PubMed.
- H. Wang, Y. Zhao, J. Li, Y. Zhou, Z. Shi, C. Yin, S. Zhuang and F. Yang, Mater. Res. Express, 2019, 6, 095914 CrossRef CAS.
- C.-S. Xu and P.-W. Lv, Chin. J. Struct. Chem., 2021, 40, 1223–1230 CAS.
- H. Liu, C. R. Brewer, A. V. Walker and L. McElwee-White, Organometallics, 2020, 39, 4565–4574 CrossRef CAS.
- C. R. Brewer, N. C. Sheehan, J. Herrera, A. V. Walker and L. McElwee-White, Organometallics, 2022, 41, 761–775 CrossRef CAS.
- C. R. Brewer, O. M. Hawkins, N. C. Sheehan, J. D. Bullock, V. D. Kleiman, A. V. Walker and L. McElwee-White, Organometallics, 2019, 38, 4363–4370 CrossRef CAS.
- S. P. Murzin, Appl. Sci., 2022, 12, 12133 CrossRef CAS.
- K. Jeong, J. Lee, I. Byun, M. Seong, J. Park, H. Nam and J. Lee, Thin Solid Films, 2017, 626, 145–153 CrossRef CAS.
- L.-S. Fan, L. Constantin, D. Li, L. Liu, K. Keramatnejad, C. Azina, X. Huang, H. R. Golgir, Y. Lu, Z. Ahmadi, F. Wang, J. Shield, B. Cui, J.-F. Silvain and Y.-F. Lu, Light:Sci. Appl., 2018, 7, 17177 CrossRef CAS PubMed.
- S. Kuk, H. K. Nam, Z. Wang and D. J. Hwang, J. Nanosci. Nanotechnol., 2018, 18, 7085–7089 CrossRef CAS PubMed.
- Q. Xu, P. Zhu, Q. Sun, R. Tu, S. Zhang, M. Yang, Q. Li, J. Shi, H. Li, L. Zhang, T. Goto, M. Han, J. Yan, S. Li and H. Ohmori, J. Am. Ceram. Soc., 2018, 101, 1471–1478 CrossRef CAS.
- H. R. Golgir, Y. S. Zhou, D. Li, K. Keramatnejad, W. Xiong, M. Wang, L. J. Jiang, X. Huang, L. Jiang, J. F. Silvain and Y. F. Lu, J. Appl. Phys., 2016, 120, 105303 CrossRef.
- L. Fan, L. Constantin, Z. P. Wu, K. A. McElveen, X. G. Chen, T. He, F. Wang, C. Debiemme-Chouvy, B. Cui, R. Y. Lai, X. Li, J. F. Silvain and Y. F. Lu, Sci. Adv., 2021, 7, eabc7547 CrossRef CAS PubMed.
- Y. S. Zhou, L. S. Fan, Z. Q. Xie, L. Jiang, J.-F. Silvain and Y. F. Lu, Curr. Opin. Solid State Mater. Sci., 2015, 19, 107–114 CrossRef CAS.
- J. Lasseter, P. D. Rack and S. J. Randolph, Nanomaterials, 2023, 13, 757 CrossRef CAS PubMed.
- W. Zhang, X.-y. Chen, Y.-s. Ma, W.-x. Fu, L. Wang, H. Zhou, Z.-j. Xu, B. Wang and Y.-s. Ruan, Chin. J. Liq. Cryst. Disp., 2019, 34, 755–763 CrossRef CAS.
- K. Jeong, J. Lee, I. Byun, M. Seong, J. Park, H. W. Kim, M. J. Kim, J.-H. Kim and J. Lee, Mater. Sci. Semicond. Process., 2017, 68, 245–251 CrossRef CAS.
- K. An, H.-N. Lee, K. H. Cho, S.-W. Lee, D. J. Hwang and K.-T. Kang, Micromachines, 2020, 11, 88 CrossRef PubMed.
- N. Khosla, J. Narayan and R. Narayan, J. Mater. Res., 2024, 39, 716–725 CrossRef CAS.
- S. J. Panchu, S. Dhani, A. Chuturgoon and M. K. Moodley, J. Photochem. Photobiol., B, 2018, 187, 10–17 CrossRef CAS PubMed.
- S. J. Panchu, M. A. Adebisi, E. Manikandan and M. K. Moodley, J. Electron. Mater., 2020, 49, 1957–1968 CrossRef CAS.
- S. J. Panchu, K. Raju, H. C. Swart, B. Chokkalingam, M. Maaza, M. Henini and M. K. Moodley, ACS Omega, 2021, 6, 4542–4550 CrossRef CAS PubMed.
- J. W. Um, S.-Y. Kim, B. H. Lee, J. B. Park and S. Jeong, Carbon, 2020, 169, 163–171 CrossRef CAS.
- Z. Liu, Y. Cai, R. Tu, Q. Xu, M. Hu, C. Wang, Q. Sun, B.-W. Li, S. Zhang, C. Wang, T. Goto and L. Zhang, Carbon, 2021, 175, 377–386 CrossRef CAS.
- J. Lasseter, P. D. Rack and S. J. Randolph, ACS Appl. Nano Mater., 2022, 5, 10890–10899 CrossRef CAS.
- F. Lisha, L. Fan, W. Guolong, V. S. Kovalenko and Y. Jianhua, Opto-Electron. Eng., 2022, 49, 210333 Search PubMed.
- C. Zhang, J. Zhang, K. Lin and Y. Huang, Rev. Sci. Instrum., 2017, 88, 053907 CrossRef PubMed.
- B. Fotovvati, A. Dehghanghadikolaei and N. Namdari, Part. Sci. Technol., 2021, 39, 738–747 CrossRef CAS.
- T. Goto, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2016, 31, 1–5 CrossRef CAS.
- Y. Zhang, Y. Jiao, C. Li, C. Chen, J. Li, Y. Hu, D. Wu and J. Chu, Int. J. Extrem. Manuf., 2020, 2, 032002 CrossRef CAS.
- A. Ito, J. Ceram. Soc. Jpn., 2021, 129, 646–653 CrossRef CAS.
- S. Bai, L. Ruan, H. Chen, Y. Du, H. Deng, N. Dai and Y. Tang, Chem. Eng. J., 2024, 493, 152805 CrossRef CAS.
- Z. Wu, A. Mao, L. Wadle, X. Huang, N. Kraiem, J.-F. Silvain, B. Cui and Y. Lu, Diamond Relat. Mater., 2024, 149, 111549 CrossRef CAS.
- Z. Q. Xie, J. Bai, Y. S. Zhou, Y. Gao, J. Park, T. Guillemet, L. Jiang, X. C. Zeng and Y. F. Lu, Sci. Rep., 2014, 4, 4581 CrossRef PubMed.
- Q. Xu, Z. Deng, Q. Sun, R. Tu, S. Zhang, M. Yang, Q. Li, L. Zhang, T. Goto and H. Ohmori, Carbon, 2018, 139, 76–84 CrossRef CAS.
- Q. Sun, R. Tu, Q. Xu, C. Zhang, J. Li, H. Ohmori, M. Kosinova, B. Basu, J. Yan, S. Li, T. Goto, L. Zhang and S. Zhang, J. Power Sources, 2019, 444, 227308 CrossRef CAS.
- R. Tu, Z. Hu, Q. Xu, L. Li, M. Yang, Q. Li, J. Shi, H. Li, S. Zhang, L. Zhang, T. Goto, H. Ohmori, M. Kosinova and B. Basu, J. Asian Ceram. Soc., 2019, 7, 312–320 CrossRef.
- J. S. Ten, M. Sparkes and W. O'Neill, in Laser Applications in Microelectronic and Optoelectronic Manufacturing (LAMOM) XXII, SPIE, 2017, vol. 10091, pp. 52–58 Search PubMed.
- M. Trippel, J. Bläsing, M. Wieneke, A. Dadgar, G. Schmidt, F. Bertram, J. Christen and A. Strittmatter, Rev. Sci. Instrum., 2022, 93, 113904 CrossRef CAS PubMed.
- H. Rabiee Golgir, D. W. Li, K. Keramatnejad, Q. M. Zou, J. Xiao, F. Wang, L. Jiang, J.-F. Silvain and Y. F. Lu, ACS Appl. Mater. Interfaces, 2017, 9, 21539–21547 CrossRef CAS PubMed.
- Y. Zhang, V. G. Thirupakuzi Vangipuram, K. Zhang and H. Zhao, Appl. Phys. Lett., 2023, 122, 162101 CrossRef CAS.
- C. S. Torres-Castillo and J. R. Tavares, Can. J. Chem. Eng., 2023, 101, 1410–1420 CrossRef CAS.
- A. Khlyustova, Y. Cheng and R. Yang, J. Mater. Chem. B, 2020, 8, 6588–6609 RSC.
- R. Fan and T. L. Andrew, J. Electrochem. Soc., 2021, 168, 077518 CrossRef CAS.
- C. A. Dorval Dion and J. R. Tavares, Powder Technol., 2013, 239, 484–491 CrossRef CAS.
- S. Hosseininasab, N. Faucheux, G. Soucy and J. R. Tavares, J. Nanopart. Res., 2019, 21, 114 CrossRef.
- S. Yousefi, M. A. Makarem, E. Rahimpour and M. R. Rahimpour, in Advances in Synthesis Gas : Methods, Technologies and Applications, ed. M. R. Rahimpour, M. A. Makarem and M. Meshksar, Elsevier, 2023, vol. 3, pp. 395–410 Search PubMed.
- H. Nasri Lari, D. Farhanian, D. C. Boffito, G. S. Patience, G. De Crescenzo, J. Chaouki and J. R. Tavares, Catal. Commun., 2017, 100, 19–23 CrossRef CAS.
- V. Labonté, A. Marion, N. Virgilio and J. R. Tavares, Ind. Eng. Chem. Res., 2016, 55, 7362–7372 CrossRef.
- D. Farhanian, G. De Crescenzo and J. R. Tavares, Sci. Rep., 2018, 8, 12223 CrossRef PubMed.
- S. Hosseininasab, N. Faucheux, G. Soucy and J. R. Tavares, Chem. Eng. J., 2017, 325, 101–113 CrossRef CAS.
- A. Bérard, G. S. Patience, G. Chouinard and J. R. Tavares, Sci. Rep., 2016, 6, 31574 CrossRef PubMed.
- P. R. Riley, P. Joshi, N. Khosla, R. J. Narayan and J. Narayan, Carbon, 2022, 196, 972–978 CrossRef CAS.
- M. J. Jeon, S.-K. Hyeong, H. Y. Jang, J. Mun, T.-W. Kim, S. Bae and S.-K. Lee, Nanomaterials, 2023, 13, 2937 CrossRef CAS PubMed.
- D. A. Boyd, L. Greengard, M. Brongersma, M. Y. El-Naggar and D. G. Goodwin, Nano Lett., 2006, 6, 2592–2597 CrossRef CAS PubMed.
- J. Svensson, N. M. Bulgakova, O. A. Nerushev and E. E. B. Campbell, Phys. Rev. B:Condens. Matter Mater. Phys., 2006, 73, 205413 CrossRef.
- D. Dey and A. K. Tiwari, ACS Omega, 2020, 5, 17857–17867 CrossRef CAS PubMed.
- I. Angelov, L. Zaharieva and L. Antonov, Molecules, 2023, 28, 695 CrossRef CAS PubMed.
- N. Wang and L. Weatherley, Curr. Opin. Chem. Eng., 2023, 39, 100895 CrossRef.
- S. Xu, R. Eisenberg, Z. Song and H. Huang, Phys. Rev. E, 2023, 108, 064413 CrossRef CAS PubMed.
- F. Che, J. T. Gray, S. Ha, N. Kruse, S. L. Scott and J.-S. McEwen, ACS Catal., 2018, 8, 5153–5174 CrossRef CAS.
- I. Lyubinetsky, S. Mezhenny, W. J. Choyke and J. T. Yates Jr, J. Vac. Sci. Technol., A, 1999, 17, 1445–1450 CrossRef CAS.
- S. Yugo, T. Kanai, T. Kimura and T. Muto, Appl. Phys. Lett., 1991, 58, 1036–1038 CrossRef CAS.
- V. S. Jayaseelan and R. N. Singh, J. Appl. Phys., 2023, 133, 155302 CrossRef CAS.
- A. Saravanan, B.-R. Huang, K. J. Sankaran, S. Kunuku, C.-L. Dong, K.-C. Leou, N.-H. Tai and I.-N. Lin, ACS Appl. Mater. Interfaces, 2014, 6, 10566–10575 CrossRef CAS PubMed.
- Y. Zhang, A. Chang, J. Cao, Q. Wang, W. Kim, Y. Li, N. Morris, E. Yenilmez, J. Kong and H. Dai, Appl. Phys. Lett., 2001, 79, 3155–3157 CrossRef CAS.
- Appendix B - Bias Enhanced Nucleation, https://www.chm.bris.ac.uk/pt/diamond/stuthesis/appendb.htm#_ednref15, accessed February 12, 2024 Search PubMed.
- N. M. Hwang, Non-Classical Crystallization of Thin Films and Nanostructures in CVD and PVD Processes, Springer Netherlands, Dordrecht, 2016, vol. 60 Search PubMed.
- J. J. Alcantar-Peña, E. de Obaldia, J. Montes-Gutierrez, K. Kang, M. J. Arellano-Jimenez, J. E. Ortega Aguilar, G. P. Suchy, D. Berman-Mendoza, R. Garcia, M. J. Yacaman and O. Auciello, Diamond Relat. Mater., 2017, 78, 1–11 CrossRef.
- L. Osman, A. Zkria, A. M. Ali, S. Nagano, H. Naragino and T. Yoshitake, Appl. Phys. Express, 2023, 16, 075501 CrossRef CAS.
- W. Wang, S. Yang, B. Liu, X. Hao, J. Han, B. Dai and J. Zhu, Carbon Lett., 2023, 33, 517–530 CrossRef CAS.
- A. Saravanan, B. R. Huang, K. J. Sankaran, G. Keiser, J. Kurian, N. H. Tai and I. N. Lin, J. Appl. Phys., 2015, 117, 215307 CrossRef.
- W.-K. Youn, C.-S. Kim, J.-Y. Lee, S.-S. Lee and N.-M. Hwang, J. Phys. Chem. C, 2012, 116, 25157–25163 CrossRef CAS.
- D.-S. Kim and N.-M. Hwang, J. Phys. D: Appl. Phys., 2018, 51, 463002 CrossRef.
- J. Wang, J.-H. Park, A.-Y. Lu and J. Kong, J. Am. Chem. Soc., 2022, 144, 22925–22932 CrossRef CAS PubMed.
- M.-S. Chae, T. H. Lee, K. R. Son, Y. W. Kim, K. S. Hwang and T. G. Kim, Nanoscale Horiz., 2019, 4, 610–618 RSC.
- Q.-J. Feng, H.-W. Liang, Y.-Y. Mei, J.-Y. Liu, C. C. Ling, P.-C. Tao, D.-Z. Pan and Y.-Q. Yang, J. Mater. Chem. C, 2015, 3, 4678–4682 RSC.
- H. Bae, K. Sasai, H. Suzuki and H. Toyoda, Vacuum, 2021, 192, 110429 CrossRef CAS.
- J. Langer, V. Cimalla, V. Lebedev, L. Kirste, M. Prescher, T. Luo, J. Jeske and O. Ambacher, Phys. Status Solidi A, 2022, 219, 2100756 CrossRef CAS.
- K. An, S. Zhang, S. Shao, J. Liu, J. Wei, L. Chen, Y. Zheng, Q. Liu and C. Li, Plasma Sci. Technol., 2022, 24, 045502 CrossRef CAS.
- V. Beladiya, M. Becker, T. Faraz, W. M. M. Erwin Kessels, P. Schenk, F. Otto, T. Fritz, M. Gruenewald, C. Helbing, K. D. Jandt, A. Tünnermann, M. Sierka and A. Szeghalmi, Nanoscale, 2020, 12, 2089–2102 RSC.
- B. Wang, D. Yang, X. Zhu, Y. Zhao, S. Wang, J. Zhu and M. Zhai, Processes, 2022, 10, 2665 CrossRef CAS.
- T. Yoshimura, S. Tatsuura and W. Sotoyama, Thin Solid Films, 1992, 207, 9–11 CrossRef CAS.
- S. Tatsuura, W. Sotoyama and T. Yoshimura, Appl. Phys. Lett., 1992, 60, 1661–1663 CrossRef.
- S. Tatsuura, W. Sotoyama, K. Motoyoshi, A. Matsuura, T. Hayano and T. Yoshimura, Appl. Phys. Lett., 1993, 62, 2182–2184 CrossRef CAS.
- N. Hu, Y. Wang, J. Li, Q. Wei, Y. Jiang, L. Ma, Z. Yu, K. Zhou and H. Long, Surf. Coat. Technol., 2019, 359, 459–467 CrossRef CAS.
- B. Peng, S. Jiang, Y. Zhang and J. Zhang, Carbon, 2011, 49, 2555–2560 CrossRef CAS.
- N. Panjawi, A. Naik, M. E. A. Warwick, G. Hyett and R. Binions, Chem. Vap. Deposition, 2012, 18, 102–106 CrossRef CAS.
- A. J. T. Naik, M. E. A. Warwick, S. J. A. Moniz, C. S. Blackman, I. P. Parkin and R. Binions, J. Mater. Chem. A, 2013, 1, 1827–1833 RSC.
- M. E. A. Warwick, I. Ridley and R. Binions, Surf. Coat. Technol., 2013, 230, 163–167 CrossRef CAS.
- L. Romero and R. Binions, Surf. Coat. Technol., 2013, 230, 196–201 CrossRef CAS.
- A. J. T. Naik, C. Bowman, N. Panjwani, M. E. A. Warwick and R. Binions, Thin Solid Films, 2013, 544, 452–456 CrossRef CAS.
- L. Romero, A. B. Jorge, P. F. McMillan and R. Binions, ECS J. Solid State Sci. Technol., 2014, 3, N107 CrossRef CAS.
- F. Qiu-Ju, L. Fang, L. Tong-Tong, L. Yun-Zheng, S. Bo, L. Meng-Ke and L. Hong-Wei, Acta Phys. Sin., 2018, 67, 218101 CrossRef.
- I. Top, R. Binions, M. E. A. Warwick, C. W. Dunnill, M. Holdynski and I. Abrahams, J. Mater. Chem. C, 2018, 6, 4485–4493 RSC.
- M. E. A. Warwick, I. Ridley and R. Binions, Sol. Energy Mater. Sol. Cells, 2016, 157, 686–694 CrossRef CAS.
- L. Romero, A.-B. Jorge-Sobrido, P. F. McMillan and R. Binions, Thin Solid Films, 2015, 584, 320–325 CrossRef CAS.
- L. Romero, C. Piccirillo, P. M. L. Castro, C. Bowman, M. E. A. Warwick and R. Binions, Chem. Vap. Deposition, 2015, 21, 63–70 CrossRef CAS.
- W.-J. Lee, S.-K. Rha, S.-Y. Lee and C.-O. Park, J. Electrochem. Soc., 1997, 144, 683 CrossRef CAS.
- E. Pawlas-Foryst, W. Przybyło, M. Kopyto and K. Fitzner, Mater. Res. Bull., 2001, 36, 915–923 CrossRef CAS.
- Y.-T. Jang, J.-H. Ahn, B.-K. Ju and Y.-H. Lee, Solid State Commun., 2003, 126, 305–308 CrossRef CAS.
- G. Shaw, I. P. Parkin, K. F. E. Pratt and D. E. Williams, J. Mater. Chem., 2005, 15, 149–154 RSC.
- M. E. A. Warwick, I. Ridley and R. Binions, J. Nanosci. Nanotechnol., 2011, 11, 8158–8162 CrossRef CAS PubMed.
- J. Crane, M. Warwick, R. Smith, N. Furlan and R. Binions, J. Electrochem. Soc., 2010, 158, D62 CrossRef.
- R. Naeem, S. Ahmed, K. M. Lo, W. J. Basirun, R. Yahya, M. Misran, T. A. N. Peiris, J. S. Sagu, K. G. U. Wijayantha, A. K. Thapa, G. U. Sumanasekera and M. Mazhar, Chem. Vap. Deposition, 2015, 21, 360–368 CrossRef CAS.
- J.-Y. Lee and J.-H. Yoon, Solid State Commun., 2004, 132, 627–630 CrossRef CAS.
- R. Naeem, R. Yahya, A. Pandikumar, H. N. Ming and M. Mazhar, J. Mater. Sci.: Mater. Electron., 2017, 28, 868–877 CrossRef CAS.
- L. Issman, P. A. Kloza, J. Terrones Portas, B. Collins, A. Pendashteh, M. Pick, J. J. Vilatela, J. A. Elliott and A. Boies, ACS Nano, 2022, 16, 9583–9597 CrossRef CAS PubMed.
- S. Zhao, Q. Feng, C. Gao, D. Wang, Y. Xing, J. Xie, Z. Dong, M. Li and H. Liang, Mater. Sci. Semicond. Process., 2020, 116, 105142 CrossRef CAS.
- S.-H. Park, J.-W. Park, S.-M. Yang, K.-H. Kim and N.-M. Hwang, J. Phys. Chem. C, 2015, 119, 25047–25052 CrossRef CAS.
- S. Park, J. Jung, K. Kim, K. Kim and N. Hwang, Cryst. Growth Des., 2018, 18, 5816–5823 CrossRef CAS.
- D. Kim, D.-Y. Kim, J.-H. Kwon and N.-M. Hwang, Coatings, 2020, 10, 726 CrossRef CAS.
- A. Gamboa, L. M. Marques and E. C. Fernandes, Diamond Relat. Mater., 2021, 113, 108274 CrossRef CAS.
- C. Luo, G. Liu and M. Zhang, Front. Mater. Sci., 2019, 13, 270–276 CrossRef.
- C. Liao, Y. Zhang and C. Pan, J. Appl. Phys., 2012, 112, 114310 CrossRef.
- H. Tani, R. Lu, S. Koganezawa and N. Tagawa, IEEE Trans. Magn., 2017, 53, 1–6 Search PubMed.
- N. M. Hwang, in Non-Classical Crystallization of Thin Films and Nanostructures in CVD and PVD Processes, ed. N. M. Hwang, Springer Netherlands, Dordrecht, 2016, pp. 261–289 Search PubMed.
- J.-W. Park, K.-H. Kim and N.-M. Hwang, Adv. Mater. Lett., 2018, 9, 638–642 CrossRef CAS.
- S.-W. Yoo, N.-M. Hwang, S.-J. You, J.-H. Kim and D.-J. Seong, J. Nanopart. Res., 2017, 19, 374 CrossRef.
- T. Sharda, M. Umeno, T. Soga and T. Jimbo, Appl. Phys. Lett., 2000, 77, 4304–4306 CrossRef CAS.
- W. Chen, C. Xiao, Q. Yang, A. Moewes and A. Hirose, Can. J. Phys., 2005, 83, 753–759 CrossRef CAS.
- J.-I. Lee and N.-M. Hwang, Carbon, 2008, 46, 1588–1592 CrossRef CAS.
- E. Plaza, H. Briceño-Fuenmayor, J. Arévalo, R. Atencio and L. Corredor, J. Nanopart. Res., 2015, 17, 246 CrossRef.
- H. Kanayama, K. Ikesugi, K. Shimanaka and H. Sato, Diamond Relat. Mater., 2012, 24, 83–87 CrossRef CAS.
- J. Zhang and C. Pan, J. Alloys Compd., 2010, 495, 93–96 CrossRef CAS.
- S. A. Linnik, A. V. Gaydaychuk, A. S. Mitulinsky and S. P. Zenkin, Mater. Lett., 2022, 324, 132670 CrossRef CAS.
- K. Ishikawa, Adv. Eng. Mater., 2024, 26, 2400679 CrossRef CAS.
- A. Roy and D. Das, Diamond Relat. Mater., 2018, 88, 204–214 CrossRef CAS.
- D. Das and A. Roy, Appl. Surf. Sci., 2020, 515, 146043 CrossRef CAS.
- M. G. Cuxart, I. Šics, A. R. Goñi, E. Pach, G. Sauthier, M. Paradinas, M. Foerster, L. Aballe, H. M. Fernandez, V. Carlino and E. Pellegrin, Carbon, 2017, 117, 331–342 CrossRef CAS.
- Y. Qi, B. Deng, X. Guo, S. Chen, J. Gao, T. Li, Z. Dou, H. Ci, J. Sun, Z. Chen, R. Wang, L. Cui, X. Chen, K. Chen, H. Wang, S. Wang, P. Gao, M. H. Rummeli, H. Peng, Y. Zhang and Z. Liu, Adv. Mater., 2018, 30, 1704839 CrossRef PubMed.
- K. S. A. Butcher, V. Georgiev, D. Georgieva, R. Gergova, P. Terziyska and P. W. Binsted, Coatings, 2022, 12, 1581 CrossRef CAS.
- T. Yoshikawa, D. Herrling, F. Meyer, F. Burmeister, C. E. Nebel, O. Ambacher and V. Lebedev, J. Vac. Sci. Technol. B, 2019, 37, 021207 CrossRef.
- J. Reiprich, N. A. Isaac, L. Schlag, T. Kups, M. Hopfeld, G. Ecke, T. Stauden, J. Pezoldt and H. O. Jacobs, ACS Nano, 2020, 14, 12885–12894 CrossRef CAS PubMed.
- L. Shi, F. Xu, J. Gao, M. Yuen, S. Sun, J. Xu, K. Jia and D. Zuo, Diamond Relat. Mater., 2020, 109, 108098 CrossRef CAS.
- J. Geiser and M. Arab, Spec. Top. Rev. Porous Media, 2010, 1, 215–229 CrossRef.
- L. Rudniak, Comput. Chem. Eng., 1998, 22, S755–S758 CrossRef CAS.
- D. Li-Fang, C. Jun-Ying, D. Guo-Yi and S. Yong, Chinese Phys., 2002, 11, 419 CrossRef.
- D. Lifang, M. Boqin, S. Yong and W. Zhijun, Plasma Sci. Technol., 2005, 7, 2845 CrossRef.
- M. Saeidi, M. Vaezzadeh and F. Badakhshan, Phys. B, 2011, 406, 1038–1040 CrossRef CAS.
- M. Saeidi, Phys. E, 2015, 70, 225–230 CrossRef CAS.
- M. Yan, T. Zhang, B. Wang, J. Liu, X. Liang, Y. Xu and F. Yi, J. Appl. Phys., 2023, 134, 155303 CrossRef CAS.
- V. Beladiya, T. Faraz, P. Schmitt, A.-S. Munser, S. Schröder, S. Riese, C. Mühlig, D. Schachtler, F. Steger, R. Botha, F. Otto, T. Fritz, C. van Helvoirt, W. M. M. Kessels, H. Gargouri and A. Szeghalmi, ACS Appl. Mater. Interfaces, 2022, 14, 14677–14692 CrossRef CAS PubMed.
- B. Reeja-Jayan and J. Luo, MRS Bull., 2021, 46, 26–35 CrossRef CAS.
- J. W. Strand, J. Cottom, L. Larcher and A. L. Shluger, Phys. Rev. B, 2020, 102, 014106 CrossRef CAS.
- A. Ansh, U. Patbhaje, J. Kumar, A. Meersha and M. Shrivastava, Commun. Mater., 2023, 4, 1–11 CrossRef.
- W.-K. Youn, S.-S. Lee, J.-Y. Lee, C.-S. Kim, N.-M. Hwang and S. Iijima, J. Phys. Chem. C, 2014, 118, 11946–11953 CrossRef CAS.
- M. G. Byun, J. W. Yang, J. H. Park, N. M. Hwang, J. Park and B. D. Yu, Cryst. Growth Des., 2022, 22, 2490–2498 CrossRef CAS.
- M. G. Byun, J. H. Park, J. W. Yang, N. M. Hwang, J. Park and B. D. Yu, Electron. Mater. Lett., 2023, 19, 218–228 CrossRef CAS.
- Magneto-Science: Magnetic Field Effects on Materials: Fundamentals and Applications, ed. M. Yamaguchi and Y. Tanimoto, Springer, Berlin, Heidelberg, 2006, pp. 1–40 Search PubMed.
- U. E. Steiner and T. Ulrich, Chem. Rev., 1989, 89, 51–147 CrossRef CAS.
- A. A. J. Kipriyanov and P. A. Purtov, Bull. Korean Chem. Soc., 2012, 33, 1009–1014 CrossRef CAS.
- Y. Ma, K. Watanabe, S. Awaji and M. Motokawa, J. Cryst. Growth, 2001, 233, 483–489 CrossRef CAS.
- Y. Lu, C. Yang, H. Wang, L. Ma, M. Xu and L. Xi, Vacuum, 2023, 211, 111912 CrossRef CAS.
- Z. Wu, Y. Zhang, Y. Shen, W. Zhang and G. Shao, Adv. Mater. Interfaces, 2020, 7, 2000854 CrossRef CAS.
- M. Baghgar, Y. Abdi and E. Arzi, Commun. Mater., 2009, 48, 20603 Search PubMed.
- H. Yasuda, Magneto Luminous Chemical Vapor Deposition, CRC Press, Boca Raton, 2012 Search PubMed.
- M. Taniguchi, M. Hirose, T. Hamasaki and Y. Osaka, Appl. Phys. Lett., 2008, 37, 787–788 CrossRef.
- T. Hamasaki, H. Kurata, M. Hirose and Y. Osaka, Appl. Phys. Lett., 2008, 37, 1084–1086 CrossRef.
- T. H. Yuzuriha, W. E. Mlynko and D. W. Hess, J. Vac. Sci. Technol., A, 1985, 3, 2135–2140 CrossRef CAS.
- Z. Sun, X. Shi and E. Liu, Thin Solid Films, 1999, 355–356, 146–150 CrossRef CAS.
- S. R. P. Silva, K. J. Clay, S. P. Speakman and G. A. J. Amaratunga, Diamond Relat. Mater., 1995, 4, 977–983 CrossRef CAS.
- J. Schwan, S. Ulrich, K. Jung, H. Ehrhardt, R. Samlenski and R. Brenn, Diamond Relat. Mater., 1995, 4, 304–308 CrossRef CAS.
- C. Luo, D. Wan, J. Jia, D. Li, C. Pan and L. Liao, Nanoscale, 2016, 8, 13017–13024 RSC.
- G.-L. Tian, J.-Q. Huang, J. Li, Q. Zhang and F. Wei, Carbon, 2016, 108, 404–411 CrossRef CAS.
- C. B. Lugod and J. Auresenia, MATEC Web Conf., 2019, 268, 05004 CrossRef CAS.
- X. Liu, K. Wen, X. Duan, C. Wang and H. Long, Vacuum, 2023, 218, 112661 CrossRef CAS.
- F. Kwok, X. Du, Z. Sun, M. Ng, Q. Liu, L. L. Fan, W. Yip, D. Kwok and S. To, Surf. Coat. Technol., 2024, 483, 130802 CrossRef CAS.
- Y. Wang, J. Li, H. Long, H. Luo, B. Zhou, Y. Xie, S. Li, Q. Wei and Z. Yu, Surf. Coat. Technol., 2016, 292, 49–53 CrossRef CAS.
- F. M. Kwok, X. Du, Z. Sun, M. C. Ng, W. S. Yip, K. Y. D. Kwok and S. To, Surf. Coat. Technol., 2025, 495, 131588 CrossRef CAS.
- D. Wei, Y. Liu, L. Cao, L. Fu, X. Li, Y. Wang and G. Yu, J. Am. Chem. Soc., 2007, 129, 7364–7368 CrossRef CAS PubMed.
- C. Luo, Q. Fu and C. Pan, Sci. Rep., 2015, 5, 9062 CrossRef CAS PubMed.
- A. Hamasaki, A. Furuse, J. Uchimura, Y. Takashima and S. Ozeki, Int. J. Appl. Electromagn. Mech., 2023, 71, S393–S401 CrossRef.
- D. Stadler, D. N. Mueller, T. Brede, T. Duchoň, T. Fischer, A. Sarkar, M. Giesen, C. M. Schneider, C. A. Volkert and S. Mathur, J. Phys. Chem. Lett., 2019, 10, 6253–6259 CrossRef CAS PubMed.
- A. Raauf, J. Leduc, M. Frank, D. Stadler, D. Graf, M. Wilhelm, M. Grosch and S. Mathur, Inorg. Chem., 2021, 60, 1915–1921 CrossRef CAS PubMed.
- M. Pyeon, V. Rauch, D. Stadler, M. Gürsoy, M. Deo, Y. Gönüllü, T. Fischer, T. Hwang and S. Mathur, Adv. Eng. Mater., 2019, 21, 1900195 CrossRef.
- D. Stadler, T. Brede, D. Schwarzbach, F. Maccari, T. Fischer, O. Gutfleisch, C. A. Volkert and S. Mathur, Nanoscale Adv., 2019, 1, 4290–4295 RSC.
- P. Tutacz, D. Stadler, T. Karimpour, T. Duchoň, S. Cramm, C. M. Schneider, T. Fischer, D. N. Mueller and S. Mathur, Chem. Mater., 2023, 35, 8050–8056 CrossRef CAS.
- H. Lee, Z. T. Aytuna, A. Bhardwaj, M. Wilhelm, K. Lê, B. May, D. N. Mueller and S. Mathur, Adv. Eng. Mater., 2023, 25, 2300021 CrossRef CAS.
- M. Frank, L. Jürgensen, J. Leduc, D. Stadler, D. Graf, I. Gessner, F. Zajusch, T. Fischer, M.-A. Rose, D. N. Mueller and S. Mathur, Inorg. Chem., 2019, 58, 10408–10416 CrossRef CAS PubMed.
- R. B. Little and R. Goddard, J. Appl. Phys., 2004, 95, 2702–2712 CrossRef CAS.
- X. You, Z. Yu, L. Shi and L. Wang, J. Cryst. Growth, 2009, 311, 4675–4678 CrossRef CAS.
- H. Long, S. Li, H. Luo, Y. Wang, Q. P. Wei and Z. M. Yu, Appl. Surf. Sci., 2015, 353, 548–552 CrossRef CAS.
- X. Liu, K. Wen, X. Duan, C. Wang and H. Long, Coatings, 2023, 13, 441 CrossRef CAS.
- Y. J. Kim, Y. S. Choi, K. S. Shin, S. H. Cho, I. S. Choi and J. G. Han, Curr. Appl. Phys., 2010, 10, S354–S356 CrossRef.
- D.-H. Kim, H.-S. Jang, H.-R. Lee, C.-D. Kim and H.-D. Kang, Appl. Phys. Lett., 2004, 85, 109–111 CrossRef CAS.
- A. Mettenbörger, T. Singh, A. P. Singh, T. T. Järvi, M. Moseler, M. Valldor and S. Mathur, Int. J. Hydrogen Energy, 2014, 39, 4828–4835 CrossRef.
- S. Awaji, K. Watanabe, Y. Ma and M. Motokawa, Phys. B, 2001, 294–295, 482–485 CrossRef.
- M. Tahashi, K. Sassa and S. Asai, Mater. Trans., 2002, 43, 2813–2817 CrossRef CAS.
- Y. Wu, H. Tao, S. Su, H. Yue, H. Li, Z. Zhang, Z. Ni and X. Chen, Sci. Rep., 2017, 7, 46583 CrossRef CAS PubMed.
- PMR, Chemical Vapor Deposition Market Size, Growth Report, 2032, https://www.polarismarketresearch.com Search PubMed; https://www.polarismarketresearch.com/industry-analysis/chemical-vapor-deposition-cvd-market, accessed December 3, 2024.
| This journal is © The Royal Society of Chemistry 2025 |




