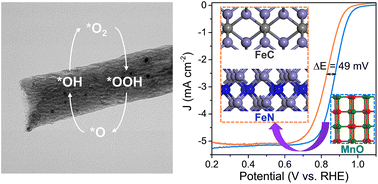
Incorporating metal oxides to optimize the charge distribution of iron species is an effective strategy, which develops efficient electrocatalysts for oxygen reduction. In this work, we fabricated a carbon-based catalyst (Fe4Mn4-NC-800) through the process of electrostatic spinning and high-temperature calcining, which involved co-doping with MnO, FeC, and FeN in carbon nanofibers. This one-step synthesis approach effectively increased the exposure of active sites, leading to improved catalytic reaction kinetics. The Fe4Mn4-NC-800 catalyst displays exceptional oxygen reduction reaction (ORR) performance (Eonset = 1.06 V and E1/2 = 0.86 V) and robust long-term stability in 0.1 M KOH. Acid washing experiments revealed the enhancement mechanism of MnO on the catalytic process of FeC and FeN. Furthermore, the zinc–air battery with Fe4Mn4-NC-800 assembly displays superior open circuit voltage (∼1.51 V), specific capacity (∼793 mA h gZn−1), and power density (∼170.8 mW cm−2) compared to Pt/C, indicating its feasibility in practical applications. Therefore, this study has proposed an efficient and promising method for the preparation of ORR catalysts, which could be a breakthrough in material design.