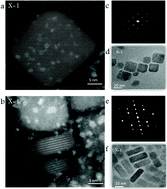
In this work we synthesized vacancy-ordered lead-free layered double perovskite (LDP) nanoparticles. This structure consists of two layers of trivalent metal halide octahedra [B(III)X6]3− separated by a layer of divalent metal [B(II)X6]4− (B is a divalent or trivalent metal). The chemical formula of this structure is based on A4B(II)B(III)2X12 where A is Cs, B(III) is Bi, X is Cl and B(II) is a different ratio between Mn2+ and Cd2+. Well-defined colloidal nanoplates of Cs4CdxMn1−xBi2Cl12 were successfully synthesized. These nanoplates show photoluminescence (PL) in the orange to red region that can be tuned by changing the Cd/Mn ratio. High resolution scanning transmission electron microscopy (HR-STEM) and atomic resolution elemental analysis were performed on these lead free LDP nanoplates revealing two different particle compositions that can be controlled by the Cd/Mn ratio. Ultraviolet Photoelectron Spectroscopy (UPS) and scanning tunneling spectroscopy (STS) reveal the band gap structure of these LDP nanoplates. Density functional theory (DFT) calculations show the existence of [MnCl6]4− in-gap states. While the absorption occurs from the valence band maximum (VBM) to the conduction band minimum (CBM), the emission may occur from the CBM to an in-gap band maximum (IGM), which could explain the PL in the orange to red region of these nanoplates. This work provides a detailed picture of the chemical and electronic properties of LDP nanoparticles.