Integrin αvβ3-targeted engineered carbon dots for efficacious sonodynamic therapy and fluorescence navigation surgery against gliomas†
Xueli
Ren‡
ab,
Yu
Shi‡
ab,
Yanxi
Yang
ab and
Zhe
Liu
 *ab
*ab
aAcademy of Medical Engineering and Translational Medicine, Tianjin University, Tianjin, 300072, China. E-mail: zheliu@tju.edu.cn
bTianjin Key Laboratory of Brain Science and Neural Engineering, Tianjin University, Tianjin, 300072, China
First published on 24th May 2024
Abstract
Residual tumor margin is one of the major causes of cancer recurrence after surgical resection. Image-guided surgery is conducive to eradication of tumor lesions contributing to the avoidance of local recurrence, potential metastasis, and a poor survival rate, which is of significance to malignant tumor, especially invasive gliomas with high incidence and short survival time. Additionally, sonodynamic therapy (SDT) is a favorable method of tumor treatment with deep tissue penetration, non-invasiveness and reliable efficacy. Herein, engineered carbon dots of RB-CDs@RGD were fabricated for both SDT and fluorescence navigation surgery against gliomas. This engineered RB-CDs@RGD with αvβ3 integrin-targeting ability could generate sufficient reactive oxygen species (ROS) to induce glioma cell (rat glioma C6 cell) apoptosis. In vivo studies confirmed superior anti-tumor performance of RB-CDs@RGD with expected biocompatibility. Furthermore, RB-CDs@RGD as the tumor-targeted contrast agent was applied for fluorescence-guided surgery of gliomas given its outstanding fluorescence properties. Therefore, RB-CDs@RGD with distinctive fluorescence properties, SDT efficiency, and biosafety exhibited potential for application in distinguished fluorescence imaging-navigated surgery combined with SDT, and they could be used as a drug delivery nanoplatform for imaging-guided operative treatment with a promising prospect in clinic usage.
1. Introduction
Surgical resection remains the preferred strategy for many cancers in clinic. However, due to the inability of current clinical techniques to accurately locate tumor boundaries, incomplete tumor resection is the major cause of poor prognosis, local recurrence and low patient survival.1 Accurate visualization of tumor tissue is essential for surgery to reduce cancer recurrence and damage to normal tissue.2 The fluorescence of materials at tumor sites can be used to clearly highlight tumour tissues and show the boundary with normal tissues, which is conducive to carry out accurate tumor resection. Hence, fluorescence navigation surgery is a good choice to improve the effect of traditional cancer therapy with the advantages of real-time, high resolution and high specificity.3 Glioma originated from glial cells is a common primary brain tumor that poses a threat to human health.4 Although some strategies based on chemical or biological drug formulation has been accessible, the existence of the blood–brain barrier (BBB) hinders the transportation of these pharmaceutic agents to the lesions.5,6 Therefore, nanoparticle-based drug delivery systems have been developed to cross the BBB, especially functionalized with specific targeting ligands for targeted delivery to the brain and enhanced BBB transcytosis. All the same, the initial step in glioma treatment generally involves surgical intervention, and nanoparticle-based drug delivery systems combined with fluorescence navigation surgery would be a suitable therapeutic approach.Another method for improving the cancer curative effect is sonodynamic therapy (SDT). SDT is an emerging cancer therapy that uses low-intensity ultrasound irradiation to activate a sonosensitizer to generate reactive oxygen species (ROS), thereby promoting the apoptosis of tumor cells, and it overcomes the shortcomings of photodynamic therapy (PDT) in tissue penetration.7–9 Compared with conventional cancer therapy and PDT, SDT has the advantages of less invasiveness, reliable efficacy, less damage to normal tissues, stronger tissue penetration ability and controllable treatment time and space.10,11 Sonosensitizers are critical for SDT, and generate ROS when activated by ultrasound.12 However, the low chemical stability, potential phototoxicity and lack of tumor targeting ability of most sonosensitizers limit their further applications, so it is desirable to develop suitable methods to load sonosensitizers and apply them to SDT.13,14
Carbon dots (CDs) are a class of spherical fluorescent nanoparticles with diameters less than 10 nm and they have a synthetic history of 20 years since they were first accidentally discovered in 2004.15 The surface of CDs has –OH, –NH2, –COOH and other groups, which make them convenient for further modification and reaction.16 Because of their high stability, abundant and cheap raw materials, good biosafety, and many unique physicochemical and photochemical properties, they have great application prospects as functional materials and in biological imaging, cancer therapy, and catalysis.17–19 CDs have been shown to be promising materials for the treatment of cancer. They can be stimulated by ultrasound just like sonosensitizers and the electron–hole pairs in them can generate ROS, which enhances the killing effect of tumor cells.20 Furthermore, on account of their excellent fluorescence characteristics, water solubility, optical stability and biocompatibility, CDs are the ideal materials as contrast agents for fluorescence navigation surgery. Rose bengal (RB), a commercially available photoactive dye, has generally been exploited as a precursor for the synthesis of CDs given its merits such as low toxicity, good stability, and extraordinary hydrophilicity, as well as excellent optical properties with high fluorescence quantum yields and excitation-independent fluorescence emission.21,22 In addition, RB also serves as one of the sonosensitizers with the potential for application in SDT and can generate toxic singlet oxygen to eradicate tumorous cells and bacteria under ultrasound irradiation in company with mechanical disruption of cellular membranes.23 Nevertheless, its broader applications are limited by its rapid clearance and poor accumulation in tumors. Multifunctional peptides show promise in specific cell recognition, and particular research attention has been paid to the ability of the RGD sequence to target overexpressed αvβ3 receptors on tumor cell surfaces.24 It is noteworthy that cyclized peptides might provide enhanced stability and thus a cyclic RGD residue gives rise to a higher affinity for αvβ3 integrin compared with linear ones.25 Molecular probes conjugated with RGD motifs have been developed for clinical molecular imaging including PET/CT imaging of cancers.26
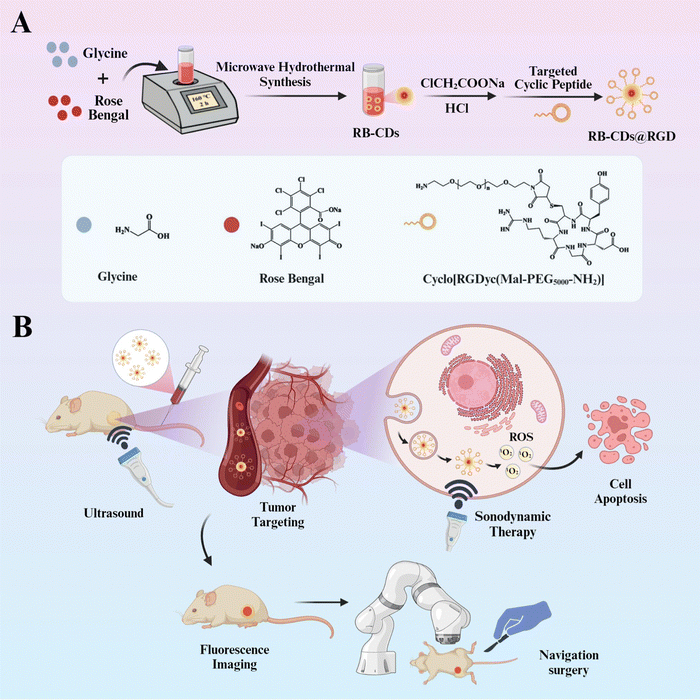
Based on these, we report a therapeutic and diagnostic platform with functions of fluorescence navigation surgery and targeted SDT (Fig. 1). Primarily, non-targeted CDs were prepared by microwave synthesis using glycine and sonosensitizer rose bengal (RB) as raw materials and they were denoted as RB-CDs. The surface groups of RB-CDs were then carboxylated to form RB-CDs–COOH. After activation by the EDC/NHS method, the targeted peptide cyclo[RGDyc(Mal-PEG5000–NH2)] was conjugated to the surface of RB-CDs to form targeted RB-CDs@RGD. The prepared RB-CDs@RGD has photoluminescence properties and can be used as imaging tracers in cells and in vivo. Meanwhile, due to the RGD groups on the surface of RB-CDs@RGD, it can specifically target the αvβ3 receptors which are overexpressed in many solid tumor cells such as gliomas, so it can be used as a molecular probe to detect glioma biomarkers for diagnosis.27 In addition, RB-CDs@RGD can generate ROS under ultrasound irradiation due to the characteristics of RB or CDs to promote cell apoptosis, and has excellent SDT therapeutic performance for gliomas. In addition, the tumour-targeting function and fluorescence properties of RB-CDs@RGD enable its use as a tumour-targeting contrast agent in fluorescence navigation surgery. This integrated diagnostic and therapeutic biomaterial (RB-CDs@RGD) used for SDT and navigation surgery is a promising oncology treatment strategy in oncology.
2. Materials and methods
2.1 Materials
Rose bengal (RB) was purchased from Sigma-Aldrich (USA). Glycine, L-alanine, citric acid, sodium hydroxide (NaOH), anhydrous ethanol, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) and N-hydroxysulfosuccinimide sodium (NHS) were obtained from Aladdin (Shanghai, China). Hydrochloric acid (HCl) was acquired from Jiangtian Chemical (Tianjin, China). Sodium chloroacetate (ClCH2COONa) was bought from MREDA (Beijing, China). The RGD peptide (cyclo[RGDyc(Mal-PEG5000–NH2)]) was synthesized by QYAOBIO ChinaPeptides Co. Ltd (Shanghai, China). BCA protein assay kit was obtained from Beyotime (Shanghai, China). 1,3-Diphenylisobenzofuran (DPBF), 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) and acetonitrile were obtained from MACKLIN (Shanghai, China). Dulbecco's Modified Eagle Medium (DMEM), Roswell Park Memorial Institute-1640 (RPMI-1640), fetal bovine serum (FBS) and trypsin were acquired from GIBCO (USA). Cell counting kit-8 (CCK-8), penicillin–streptomycin solution and 4′,6-diamidino-2′-phenylindole (DAPI) were purchased from Solarbio (Beijing, China). The live/dead double staining kit (calcein AM/PI) and the annexin V-FITC/PI apoptosis assay kit were bought from BIOSCIENCE (Shanghai, China). The HE staining kit was purchased from ZSGB-BIO (Beijing, China), and the caspase-3 antibody was bought from Cell Signaling Technology, Inc. (Shanghai, China). The TUNEL apoptosis assay kit was acquired from Servicebio (Wuhan, China) and the Ki67 antibody was purchased from Abcam (Shanghai, China). All chemicals were of analytical grade and were used without further purification.2.2 Preparation of RGD-tagged carbon dots
The non-tagged carbon dots (RB-CDs) were prepared via atypical microwave hydrothermal synthesis by reacting glycine and RB. Briefly, glycine (1.5 g, 1.3 M) and RB (50.0 mg, 3.3 mM) were, respectively, dissolved in ultrapure water. After mixing them together in a vial, the resulting solution was put into a microwave synthesizer (Monowave 400, Anton Paar, Austria) and maintained at 160 °C for 2 h with a programmed gradient heating process. After cooling down to room temperature, the raw product was dialyzed overnight (MW cut-off: 1000 Da) to obtain non-tagged RB-CDs, which were lyophilized at −50 °C and stored at 4 °C.In order to fabricate RGD-tagged CDs, the RB-CDs (1.0 g) were re-dispersed in ultrapure water containing NaOH (1.0 g, 500.0 mM) and ClCH2COONa (1.0 g, 171.7 mM). Followed by ultrasound treatment and neutralization with HCl, the carboxylated CDs (RB-CDs–COOH) were obtained. After overnight dialysis, RB-CDs–COOH was mixed with EDC·HCl (50 mg, 26.1 mM) and NHS (40 mg, 18.4 mM) to activate carboxylic groups. Then the peptide cyclo[RGDyc(Mal-PEG5000–NH2)] (50 mg) was added, and the bio-conjugation was achieved within 12 hours in darkness to obtain RGD-tagged CDs (RB-CDs@RGD). Excess EDC and NHS were removed by dialysis and RB-CDs@RGD was lyophilized at −50 °C before storage at 4 °C.
2.3 Characterization
The morphologies of various CDs were visualized on a transmission electronic microscope (TEM, Talos F200X, Thermofisher, USA) and a scanning electronic microscope (SEM, Apreo S, FEI, Czech Republic) and were used for the calculation of particle size distribution. The zeta potentials were measured using a dynamic light scattering instrument (Litesizer 500, Anton Paar, Austria) and triplicate measurements were carried out to give a standardized report of mean ± SD. Their optical performances were analyzed using a FT-IR spectrometer (TENSOR II, Bruker, Germany), a fluorescence spectrometer (Spectrofluorometer FS5, Edinburgh Instruments, Britain) and a UV-Vis spectrometer (Evolution 220, Thermo, USA), respectively. X-ray photoelectron spectra (XPS) were obtained using an XPS spectrometer (ESCALAB-Xi, Thermo Fisher, USA). The quantum yield (ϕ) was calculated using formula (1) where M indicates the slope of the curve, and η refers to the refractive index of solvent.28,29 | (1) |
2.4 Stability, ROS generation and RGD modification density
The stability of CDs was evaluated by measuring their zeta potentials and fluorescence intensity at regular intervals within 7 days. In addition, their optical performances were assessed by changing the pH values (from 1 to 12) of solutions, and their optical persistence was investigated by analyzing the fluorescence intensity at an excitation wavelength (λex) of 500 nm for varied durations of 1500 seconds.The sono-induced ROS generation of RB-CDs@RGD was assessed by using 1,3-diphenylisobenzofuran (DPBF) as an indicator.30,31 Briefly, RB-CDs@RGD was dissolved in acetonitrile solution containing DPBF (2 mg mL−1) and the acetonitrile solution without any CDs was used as the control group. Followed by ultrasound irradiation (frequency: 1.0 MHz, duty cycle: 50%, and power density: 1.0 W cm−2), the abosrption spectra and absorbance at 461 nm were measured at regular intervals and the ROS generation capacity of RB-CDs@RGD could be calculated based on the degradation of DPBF in solution.
The RGD modification density was determined using a BCA protein assay kit. According to the instruction, bovine serum albumin (BSA) and RB-CDs@RGD (1.0 mg mL−1) were mixed with the BCA reagent. After incubation for 30 min at 60 °C, the absorption at 562 nm was measured using a microplate reader (Synergy H1 Multimode Reader, Biotek, USA).
2.5 Cell culture and cytotoxicity assay
The rat glioma cells (C6) and human breast cancer cell lines (MCF-7) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). C6 cells were cultured with Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% FBS and 100 U mL−1 penicillin. MCF-7 cells were cultured in Roswell Park Memorial Institute-1640 (RPMI-1640) supplemented with 10% FBS and 100 U mL−1 penicillin. All of the cells were cultured at 37 °C with 5% CO2 in a humidified incubator.The cytotoxicity of RB-CDs and RB-CDs@RGD to C6 cells was determined using the CCK-8 method. C6 cells were seeded in 96-well plates at a density of 1 × 104 cells per well and incubated at 37 °C for 24 h. After washing with PBS, RB-CDs and RB-CDs@RGD were added at concentrations of 0, 25, 50, 100, 150 and 200 μg mL−1, and they were co-incubated for another 24 h. Then the DMEM medium containing 10% CCK-8 was added and the cell viability was measured at 450 nm using a microplate reader (Synergy H1 Multimode Reader, Biotek, USA). The cytotoxicity of RB-CDs and RB-CDs@RGD was measured under additional ultrasound irradiation (frequency: 1.0 MHz, duty cycle: 50%, power density: 1.0 W cm−2, and duration: 3 min).
2.6 In vitro cell uptake and targeting
C6 cells and MCF-7 cells were seeded in 24-well plates (5 × 104 cells per well) and incubated overnight. The medium was then replaced with fresh medium containing RB-CDs and RB-CDs@RGD (CCDs = 20 μg mL−1). Followed by further co-incubation for 4 h, the cells were washed twice with PBS and stained with DAPI (1 μg mL−1) for 15 min in the dark. After washing with PBS, the cellular uptake was analyzed by inverted fluorescence microscopy (Ti 2, Nikon, Japan). To quantify the cellular uptake, C6 cells and MCF-7 cells were inoculated on 6-well plates (3 × 105 cells per well) and incubated for 24 h. After washing with PBS, the fresh medium containing RB-CDs and RB-CDs@RGD (CCDs = 50 μg mL−1) was added. Followed by incubation for 4 h, the cells were washed twice and the specific targeting of CDs was quantitatively detected using a flow cytometry instrument (CytoFLEX S, BECKMAN COULTER, USA).2.7 Sono-induced intracellular ROS production and cell apoptosis assay
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was used as a redox fluorescence probe to detect the production of intracellular ROS.32,33 C6 cells were seeded in 24-well plates at a density of 5 × 104 cells per well and incubated for 24 h. After washing with PBS, the medium was replaced with fresh medium containing RB-CDs and RB-CDs@RGD (CCDs = 40 μg mL−1). Followed by incubation for 4 h, the cells were washed and the medium containing 10 μM DCFH-DA was added. After further incubation for 30 min, ultrasound groups were exposed to ultrasound irradiation (frequency: 1.0 MHz, duty cycle: 50%, power density: 1.0 W cm−2, duration: 3 min) and the ROS production of each group was observed using an inverted fluorescence microscope.C6 cells were seeded in 24-well plates (5 × 104 cells per well) and incubated for 24 h to perform the live/dead staining assay. After washing with PBS, the medium containing RB-CDs and RB-CDs@RGD (CCDs = 40 μg mL−1) was added, respectively. Followed by incubation for 18 h, the medium was replaced and the cells were treated with ultrasound (frequency: 1.0 MHz, duty cycle: 50%, power density: 1.0 W cm−2, duration: 3 min). After further incubation for 6 h, the cells were stained with the calcein-AM/PI kit and observed using an inverted fluorescence microscope. As for the cell apoptosis assay, C6 cells were seeded in 6-well plates (3 × 105 cells per well) and co-incubated with RB-CDs and RB-CDs@RGD (CCDs = 40 μg mL−1). After ultrasound irradiation and further incubation, the cells were treated with the annexin V-FITC/PI apoptosis kit and quantitatively analyzed using a flow cytometry instrument.
2.8 Hemolysis assay, in vivo imaging and sonodynamic therapy with RB-CDs@RGD
As for the hemolysis assay, fresh blood was collected from healthy mice and centrifuged (1000 rpm, 5 min) to obtain the red blood cells (RBCs). After washing with PBS, the RBCs were diluted 10 times and 0.3 mL suspension was incubated with 1.2 mL RB and various CDs (RB-CDs, RB-CDs–COOH and RB-CDs@RGD) at 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 2
°C for 2 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h, followed by centrifugation. The optical density (OD) of the supernatant was measured at 541 nm using a microplate reader (Synergy H1 Multimode Reader, Biotek, USA). Triton X-100 was used as a positive control and PBS as a negative control to calculate the hemolysis ratio.
h, followed by centrifugation. The optical density (OD) of the supernatant was measured at 541 nm using a microplate reader (Synergy H1 Multimode Reader, Biotek, USA). Triton X-100 was used as a positive control and PBS as a negative control to calculate the hemolysis ratio.
The Balb/c nude mice (male, 5 weeks old, 16–18 g) were obtained from Beijing Huafukang Biotechnology Co. Ltd (Beijing, China). PBS suspension containing 1.0 × 106 C6 cells was injected subcutaneously into the right hind leg of each nude mouse to establish mice glioma models. Animal experiments were performed when the tumor volume reached 100 mm3. All animal experiments were approved by the Animal Ethics Committee of Tianjin University Experimental Animal Center (approval no. TJUE-2024-212) according to the Guidelines for the Care and Use of Experimental Animals in Tianjin University. In order to validate the tumor targeting ability of RB-CDs@RGD in mice, the in vivo imaging experiments were performed. A series of RB-CDs@RGD solutions with various concentrations (0, 0.5, 1, 2, 5 and 10 mM) were imaged before in vivo imaging to optimize the injection dose of RB-CDs@RGD. Subsequently, 200 μL of RB-CDs@RGD solution (CCDs = 10 mM) was intravenously administered into mice through the tail vein and the control group was injected with 200 μL normal saline. In vivo imaging was performed at predetermined time points before and after injection using an in vivo imaging system (IVIS Spectrum, PerkinElmer, USA).
The C6 tumor bearing nude mice were randomly divided into 3 groups (n = 3): the control, RB-CDs@RGD and RB-CDs@RGD + US. 200 μL of RB-CDs@RGD (CCDs = 10 mM) was intravenously administered into the nude mice of the RB-CDs@RGD group and the RB-CDs@RGD + US group via the tail vein and the control group was injected with normal saline. After 1 h, the mice of the RB-CDs@RGD + US group were treated with ultrasound irradiation (frequency: 1.0 MHz, duty cycle: 50%, power density: 1.0 W cm−2, and duration: 5 min). This treatment was repeated once every two days and continued for 14 days. During this period, the tumor images, size and mice body weight were recorded, and the tumor volumes were calculated from the following formula: tumor volume = (length × width2)/2. The mice were sacrificed after the treatment cycle and the tumor tissues and major organs (heart, liver, spleen, lung, and kidneys) were collected. The harvested major organs of mice were used for HE staining and the isolated tumor tissues were weighed, photographed and fixed for H&E, Ki67, caspase-3 and TUNEL staining.
2.9 Fluorescence navigation surgery
Navigation surgery was performed when the tumor volume reached around 300 mm3. In short, the mice were injected intravenously with 200 μL RB-CDs@RGD solution (CCDs = 10 mM) through the tail vein and after half an hour, the mice were injected intraperitoneally with tribromoethanol (20 μL g−1) to be anaesthetized and immobilized on the operating table. Then the fluorescence at tumor sites was observed under a surgical microscope (OPMI PENTERO 800, Carl Zeiss Meditec AG, Germany) at 560 nm and the tumor tissue was surgically resected under the guidance of fluorescence. After operation, the skin of the mice was sutured and the postoperative recovery was observed.2.10 Statistical analysis
All data were presented in the form of mean ± SD. The statistical analysis was performed using SPSS Statistics 23.0 and GraphPad Prism 8.0 with a Student's t-test (*p < 0.05, **p < 0.01 and ***p < 0.001). A probability value of p < 0.05 was considered to be statistically significant.3. Results and discussion
3.1 Preparation and characterization
The RB-CDs were synthesized by a green and efficient one-pot synthesis method using glycine and RB in a microwave synthesis instrument (Fig. 1(A)). Subsequently, the surface of RB-CDs was carboxylated to form RB-CDs–COOH and then activated by the EDC/NHS method, followed by the conjugation of the RGD-targeted cyclic peptide (cyclo[RGDyc(Mal-PEG5000–NH2)]) to form the targeted RB-CDs@RGD. In order to optimize the reaction conditions, the fluorescence intensity of RB-CDs was investigated at various reaction times and temperatures, and the optimal reaction condition was found to be 160 °C for 2 h (Fig. 2(A) and (B)). The particle size and zeta potential of RB-CDs and RB-CDs@RGD were 2.7 ± 0.6 nm/−27.5 ± 0.7 mV and 3.6 ± 0.4 nm/−14.3 ± 0.4 mV, respectively (Fig. 2(C)). The morphology of RB-CDs and RB-CDs@RGD was spherical with good dispersion and uniform size distribution in aqueous solution (Fig. 2(D)–(F)). As shown in Fig. 2(G), the fluorescence intensity of RB-CDs and fluorescein had an obvious linear relationship with absorbance and the quantum yield of RB-CDs was calculated to be 5.22%. The elemental composition of RB-CDs was investigated by XPS. As shown in Fig. 2(H), the XPS spectra had five peaks at the binding energies of 201.5 eV, 284.8 eV, 402.2 eV, 533.0 eV and 620.5 eV, which are Cl 2p, C 1s, N 1s, O 1s and I 3d peaks, respectively. The presence of elements Cl and I in RB-CDs confirmed the successful reaction between RB and glycine, as these two elements can only be derived from RB molecules.22The FT-IR spectra of glycine, RB and RB-CDs are shown in Fig. 2(I). The N–H absorption peaks at 1517 cm−1 and 3176 cm−1, the C–N absorption peak at 1334 cm−1 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption peak at 1662 cm−1 of RB-CDs were the characteristic peaks of the amide bond, indicating that the reaction of glycine and RB was mediated by the amino groups of glycine and the carboxyl groups of RB. The reactant RB had stretching vibration peaks of C–I and C–Cl at 613 cm−1 and 660 cm−1, respectively, and these halogen peaks also appeared for RB-CDs which further proved the the successful preparation of RB-CDs.34,35 The structure and functional groups of RB-CDs@RGD were further analyzed by FT-IR spectroscopy (Fig. 2(J)). The N–H absorption peak at 3176 cm−1 of RB-CDs–COOH decreased sharply compared to that of RB-CDs, indicating that the amino groups might be hidden in the core, proving the conversion of the amino group to carboxyl group. The C–O–C stretching vibration peak at 1102 cm−1 and the C–H stretching vibration peak at 2881 cm−1 of RB-CDs@RGD were the characteristic peaks of the targeted cyclic peptide cyclo[RGDyc(Mal-PEG5000–NH2)]. The above results indicate the successful conjugation of RB-CDs and the targeted cyclic peptide to form targeted RB-CDs@RGD.36Fig. 2(K) shows the UV-Vis spectra of RB, RB-CDs and RB-CDs@RGD. Both RB-CDs and RB-CDs@RGD had the characteristic absorption peak of RB at 530 nm, indicating the successful reaction between RB and glycine. As shown in Fig. 2(L), compared with raw rose bengal, the fluorescence excitation peak of RB-CDs@RGD has a blue shift of 28 nm (from 545 nm (RB) to 517 nm (RB-CDs@RGD)) with broader absorption, while the fluorescence emission peak of RB-CDs@RGD has a red shift of 10 nm (from 570 nm (RB) to 580 nm (RB-CDs@RGD)). The blue shift of RB-CDs@RGD is a common phenomenon, and it is more often attributed to its superior fluorescence properties due to the presence of carbon dots.37,38
O absorption peak at 1662 cm−1 of RB-CDs were the characteristic peaks of the amide bond, indicating that the reaction of glycine and RB was mediated by the amino groups of glycine and the carboxyl groups of RB. The reactant RB had stretching vibration peaks of C–I and C–Cl at 613 cm−1 and 660 cm−1, respectively, and these halogen peaks also appeared for RB-CDs which further proved the the successful preparation of RB-CDs.34,35 The structure and functional groups of RB-CDs@RGD were further analyzed by FT-IR spectroscopy (Fig. 2(J)). The N–H absorption peak at 3176 cm−1 of RB-CDs–COOH decreased sharply compared to that of RB-CDs, indicating that the amino groups might be hidden in the core, proving the conversion of the amino group to carboxyl group. The C–O–C stretching vibration peak at 1102 cm−1 and the C–H stretching vibration peak at 2881 cm−1 of RB-CDs@RGD were the characteristic peaks of the targeted cyclic peptide cyclo[RGDyc(Mal-PEG5000–NH2)]. The above results indicate the successful conjugation of RB-CDs and the targeted cyclic peptide to form targeted RB-CDs@RGD.36Fig. 2(K) shows the UV-Vis spectra of RB, RB-CDs and RB-CDs@RGD. Both RB-CDs and RB-CDs@RGD had the characteristic absorption peak of RB at 530 nm, indicating the successful reaction between RB and glycine. As shown in Fig. 2(L), compared with raw rose bengal, the fluorescence excitation peak of RB-CDs@RGD has a blue shift of 28 nm (from 545 nm (RB) to 517 nm (RB-CDs@RGD)) with broader absorption, while the fluorescence emission peak of RB-CDs@RGD has a red shift of 10 nm (from 570 nm (RB) to 580 nm (RB-CDs@RGD)). The blue shift of RB-CDs@RGD is a common phenomenon, and it is more often attributed to its superior fluorescence properties due to the presence of carbon dots.37,38
3.2 Stability, ROS production and RGD modification density
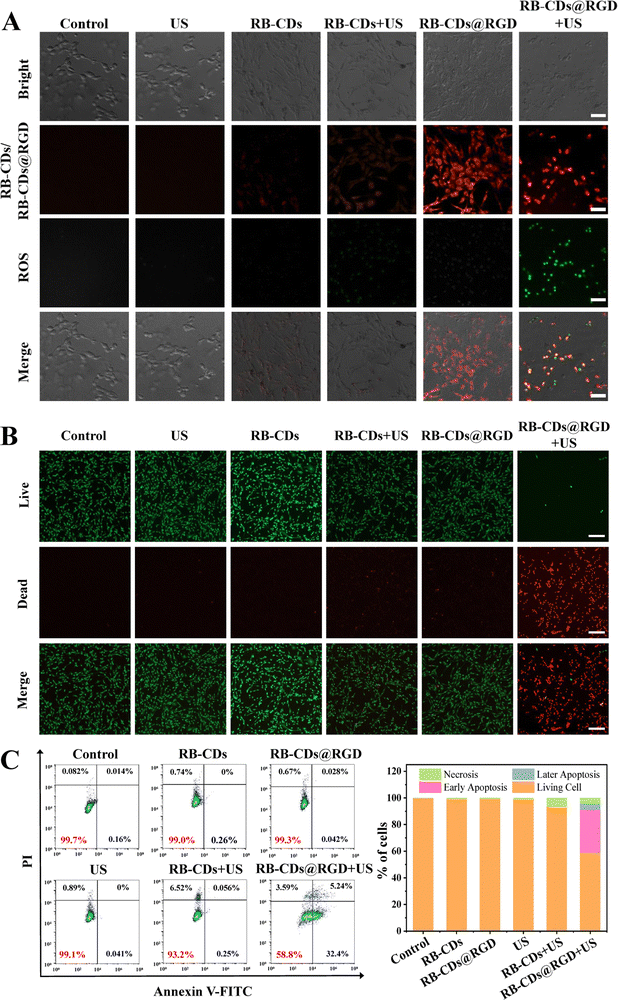
The zeta potentials and fluorescence intensity of RB-CDs and RB-CDs@RGD did not change significantly within a week, indicating that they had good stability (Fig. 3(A) and (B)). Under the irradiation of a 150 W xenon lamp for 30 min, the change of fluorescence intensity of RB-CDs at 580 nm was no more than 3%, and the fluorescence intensity of RB-CDs@RGD was still 97% of the initial intensity, which indicate that RB-CDs and RB-CDs@RGD have excellent photobleaching resistance and have the potential to be used as fluorescence imaging tracers (Fig. 3(C)). In order to investigate the influence of pH values on fluorescence characteristics, the fluorescence intensity of RB-CDs and RB-CDs@RGD solutions with various pH values was measured (Fig. 3(D)). It could be seen that the fluorescence intensity of RB-CDs and RB-CDs@RGD solutions was the strongest at pH 7 and decreased sharply at low pH values of 1–3 or high pH values of 9–11. The fluorescent colors of solutions changed from pale yellow to bright yellow and then to pale orange as the pH values changed from 1 to 12. These results show that RB-CDs and RB-CDs@RGD have excellent fluorescence properties in wide pH ranges (pH 4–10), which is conducive to their stability in an acidic tumor microenvironment and the applications in fluorescence imaging. Moreover, different from most of the CDs, the fluorescence emission of RB-CDs@RGD was independent of the excitation of the fluorescence spectra of RB-CDs@RGD, which enables the avoidance of fluorescence overlap with other fluorescent dyes, contributing to its potential applications in various fields (Fig. 3(E)).34,381,3-Diphenylisobenzofuran (DPBF) was used as an indicator to measure the extracellular ROS generation of RB-CDs@RGD under ultrasound irradiation and it could be oxidized by ROS, resulting in reduced absorbance.39 The absorbance at 461 nm of RB-CDs@RGD solution with DPBF added was measured after ultrasound irradiation for various durations to investigate the ROS generation capacity of RB-CDs@RGD (Fig. 3(F)). The results show that the absorbance of RB-CDs@RGD decreased significantly with the increase of the ultrasound time. DPBF in solution was continuously degraded by the generated ROS, and the DPBF content of RB-CDs@RGD solution was only 66% after 300 seconds. These results indicate that RB-CDs@RGD has excellent ROS generation performance and the potential for application in sonodynamic therapy.
Considering the targeting function of RGD in RB-CDs@RGD, the RGD modification density was measured using a BCA protein assay kit and found that the peptide bonds react with the BCA reagent to form a chelate complex absorbing at 562 nm.40 According to a calibration curve, the RGD modification density of RB-CDs@RGD was measured to be 0.463 ± 0.031 mg mg−1.
3.3 Targeted uptake and cytotoxicity assay
To validate the specific targeting and uptake of RB-CDs@RGD, MCF-7 cells (αvβ3−) and C6 cells (αvβ3+) were co-incubated with RB-CDs and RB-CDs@RGD, respectively.41 As shown in Fig. 3(G), the control groups for MCF-7 and C6 cell lines presented negligible fluorescence, and trace red fluorescence of RB-CDs after co-incubation with MCF-7 and C6 cells became extremely weak due to the non-targeting profile. Similar faint fluorescence was observed when RB-CDs@RGD was incubated with MCF-7 cells, while sparkling fluorescence was found for the group of RB-CDs@RGD + C6 cells, which indicates a strong and specific targeting between the RGD residues and C6 cells to afford enhanced cellular uptake.42 The flow cytometry further revealed this result with a significantly higher fluorescence (up to 97.3%) compared to those (less than 1%) of other groups (Fig. 3(H)), and the mean fluorescence intensity also verified the RGD-mediated targeting capability between MCF-7 and C6 cell lines (inset in Fig. 3(H)).For further studying their potential for biomedical applications, the cytotoxicity of RB-CDs and RB-CDs@RGD to C6 cells was measured by the CCK-8 method. As shown in Fig. 3(I), RB-CDs and RB-CDs@RGD had almost no toxicity to C6 cells without ultrasound irradiation, and the cell viability was above 80%, indicating the safety and biocompatibility of RB-CDs and RB-CDs@RGD. When additional ultrasound irradiation was applied, the cytotoxicity of RB-CDs and RB-CDs@RGD increased with increasing concentration, and the cell viability of the RB-CDs@RGD + US group was only 24.3% at 200 μg mL−1 (Fig. 3(J)). In addition, the toxicity of RB-CDs@RGD to C6 cells was significantly higher than that of RB-CDs, which was due to the targeting of RGD groups and enhanced cellular uptake. These results confirmed the excellent cytotoxicity of RB-CDs@RGD on glioma cells with ultrasound irradiation and its potential for application in SDT against gliomas.
3.4 Intracellular ROS production, live/dead staining and apoptosis of glioma cells
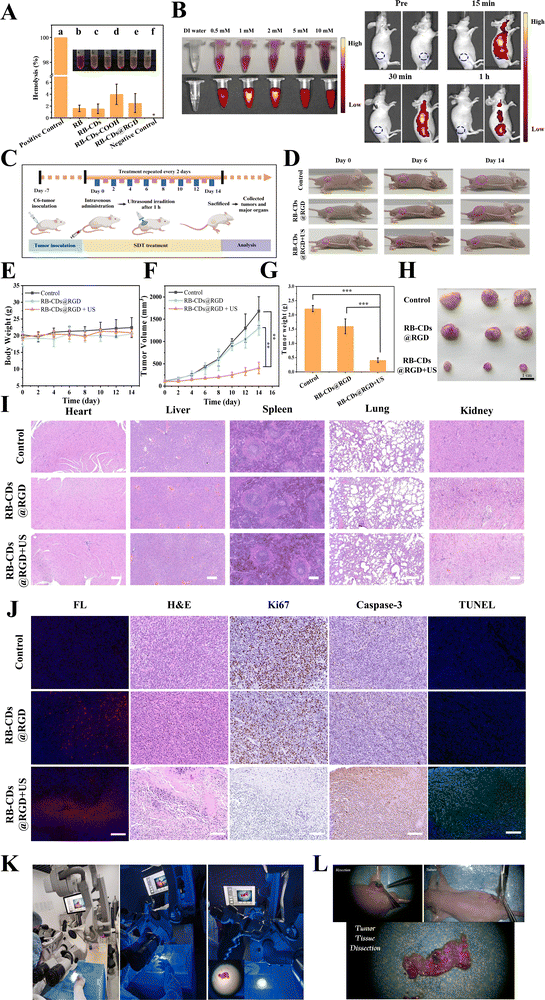
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) was used as an indicator for the detection of intracellular ROS. It could cross the cell membrane freely due to its liposolubility and was then hydrolyzed to DCFH by intracellular esterase and subsequently oxidized by ROS to produce DCF with green fluorescence.43 As shown in Fig. 4(A), the RB-CDs@RGD + US group showed obvious green fluorescence of ROS, while the fluorescence of other groups was very weak, indicating that RB-CDs@RGD had excellent intracellular ROS production ability under ultrasound irradiation. Fig. 4(B) shows the live/dead staining of C6 cells after treatment with RB-CDs and RB-CDs@RGD. It could be seen that only a small number of cells were dead in the RB-CDs + US group and the RB-CDs@RGD group, due to the lack of targeting ability and ultrasound irradiation, respectively. While a large number of cells were dead in the RB-CDs@RGD + US group, demonstrating the excellent SDT performance of RB-CDs@RGD. The apoptosis experiment further confirmed the ability of RB-CDs@RGD to promote apoptosis (Fig. 4(C)). Compared with other groups, the apoptosis of C6 cells in the RB-CDs@RGD + US group was significant and reached 37.64%.3.5 In vivo imaging and sonodynamic therapy
Hemolysis assay is an important method to investigate the damage of biomaterials due to erythrocytes and the hemolysis ratio more than 5% indicates a hemolytic effect.44 As shown in Fig. 5(A), the hemolysis ratios of RB, RB-CDs, RB-CDs–COOH and RB-CDs@RGD were all below 5%, indicating that they had good blood compatibility and could be applied to living organisms.After confirming the specific uptake of RB-CDs@RGD by C6 cells, it is vital to validate the tumor targeting and accumulation of RB-CDs@RGD in mice. Before in vivo imaging, the fluorescence of various concentrations of RB-CDs@RGD was initially imaged to optimize the injection dose of RB-CDs@RGD (Fig. 5(B)). Then 200 μL of RB-CDs@RGD (10 mM) was intravenously administered through the tail vein, and the fluorescence distribution and tumor targeting were visualized by in vivo imaging at predetermined time points. As shown in Fig. 5(B), the injected RB-CDs@RGD gradually accumulated at the tumor site and significant fluorescence could be observed at 0.5–1 h after injection, confirming the tumor targeting and accumulation ability of RB-CDs@RGD in mice due to the RGD groups on the surface.
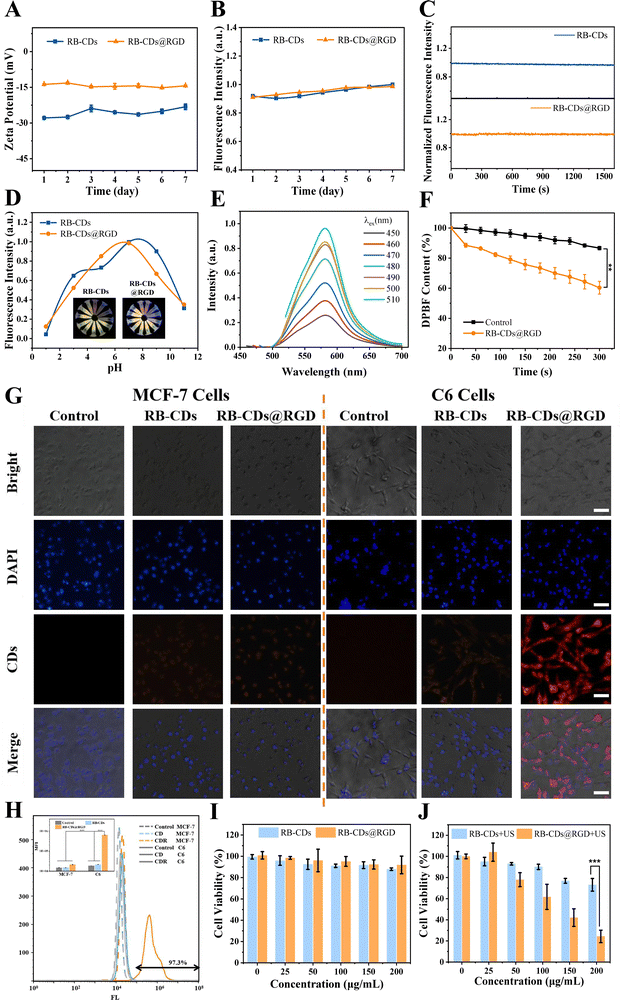
Encouraged by the tumor accumulation and cytotoxicity of RB-CDs@RGD, the therapeutic performance of RB-CDs@RGD on C6 tumor-bearing nude mice was evaluated. Fig. 5(C) shows the periodic treatment schedule used for performing ultrasound treatment on the tumor site of mice 1 h after RB-CDs@RGD injection and repeated every 2 days. The mice were sacrificed after 14 days and the tumor tissues and main organs were collected for further analysis. There was no significant difference in the body weight between the experimental and control groups during the treatment period, indicating that RB-CDs@RGD and RB-CDs@RGD + US did not cause serious systemic toxicity (Fig. 5(E)). Compared with the control group and the RB-CDs@RGD group, tumour growth was significantly inhibited in the RB-CDs@RGD + US group (Fig. 5(D) and (F)–(H)), demonstrating the excellent anti-tumor efficiency of RB-CDs@RGD.
To further investigate the damage to normal organs and SDT efficiency of RB-CDs@RGD, fluorescence imaging, H&E, Ki67, caspase-3 and TUNEL staining were performed on the sections of tumor tissues and major organs (heart, liver, spleen, lung and kidneys) after treatment. Fig. 5(I) shows the H&E staining of heart, liver, spleen, lung and kidneys of mice. Compared with the control group, the major organs of mice in RB-CDs@RGD and RB-CDs@RGD + US groups had no obvious cell damage and morphological changes, confirming the biosafety and great potential for clinical application of RB-CDs@RGD. Fig. 5(J) shows the fluorescence imaging, H&E, Ki67, caspase-3, and TUNEL staining of tumor tissues. Significant red fluorescence of RB-CDs@RGD could be observed in the RB-CDs@RGD group and RB-CDs@RGD + US group, which further demonstrates the accumulation ability of RB-CDs@RGD at tumor sites. The H&E stained tumor cells in the control group and the RB-CDs@RGD group were tightly arranged, while the cells in the RB-CDs@RGD + US group were arranged loosely. In addition, obvious tissue destruction could be seen in the RB-CDs@RGD + US group, which further reflects the excellent therapeutic effect of RB-CDs@RGD on mice glioma with ultrasound. Ki67 was a nuclear protein closely related to the cell cycle and proliferation.45,46 Compared with the control group and the RB-CDs@RGD group, the expression of Ki67 in the RB-CDs@RGD + US group was the lowest, indicating that the malignancy and proliferation rate of tumors in the RB-CDs@RGD + US group were much lower than those in the other two groups. Caspase-3 was an important terminal shear enzyme in the process of apoptosis.47,48 The level of caspase-3 in tumor tissues of the RB-CDs@RGD + US group was significantly higher than that of the other groups, indicating a higher apoptosis rate, and this was confirmed by TUNEL staining.
3.6 Fluorescence navigation surgery
Due to the excellent fluorescence properties, water solubility and tumor targeting characteristics, RB-CDs@RGD could be used as a tumor-targeted contrast agent for surgical resection of tumors under the guidance of imaging, so as to better label the tumors and realize fluorescence navigation surgery.49Fig. 5(K) shows the photographs taken during the navigation surgery process under bright field and fluorescence field, and Fig. 5(L) shows a video of three main steps in the surgery: tumor resection, skin suture and tumor tissue dissection (Supporting Video in the ESI†). These results show that RB-CDs@RGD could quickly target the tumor sites of mice after injection and exhibit strong fluorescence under excitation light, which could be used to highlight the tumor sites of mice for fluorescence navigation surgery. In addition, the mice recovered well after operation without death and obvious side effects, indicating the safety of RB-CDs@RGD for fluorescence-guided surgical resection of tumors. This reveals the excellent application potential and broad prospects of RB-CDs@RGD as a tumor targeting contrast agent and in navigation surgery.4. Conclusions
In this study, engineered carbon dots of RB-CDs@RGD with integrin αvβ3 receptor targeting function were successfully fabricated by a green and efficient method, and were applied to fluorescence imaging, navigation surgery and SDT against gliomas. The synthesized RB-CDs@RGD had small size, excellent fluorescence properties, optical stability and photobleaching resistance and have the potential to be applied in fluorescence imaging. In addition, it had excellent ROS production performance under ultrasound irradiation and could efficiently kill tumor cells. Cell-targeted uptake and in vivo imaging experiments confirmed that RB-CDs@RGD could target the αvβ3 receptors of C6 tumor cells driven by the targeted RGD groups on the surface, thereby enhancing the therapeutic effect of SDT. In comparison, RB-CDs@RGD exhibits an ultra-small size of less than 10 nm, which facilitates a convenient evacuation out of the circulation and therefore an active targeting accumulation in tumor tissues. In addition, the matrix of CDs renders desirable stability, stable photo-activity and resistance to photobleaching of RB-CDs@RGD which make it an excellent candidate for biomedical imaging. Moreover, hemolysis and H&E staining of major organs demonstrated the biosafety of RB-CDs@RGD, showing a great prospect for clinical applications. In vivo studies showed that RB-CDs@RGD could significantly inhibit the growth of tumors. Thanks to its excellent fluorescence properties, tumor targeting ability and water solubility, RB-CDs@RGD has a broad application prospect as a targeted contrast agent for the resection of tumors under imaging guidance and the feasibility was confirmed by fluorescence navigation surgery. In summary, this engineered carbon dots provided an advanced strategy for the fluorescence navigation surgery and sonodynamic therapy against gliomas. Furthermore, some studies have reported that ultrasound can help open the blood–brain barrier, and that RB also has an effect in enhancing immunity, so the carbon dots are expected to be further investigated for the SDT of in situ gliomas and synergistic SDT and immunotherapy to enhance efficacy.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (grant no. 82072057 and 82311540023). We are also appreciated for Ms. Chenxi Liu's technical assistance.References
- J. Zhu, C. Chu, D. Li, Y. Zhang, Y. Cheng, H. Lin, X. Wang, J. Liu, X. Pang, J. Cheng and G. Liu, Superior Fluorescent Nanoemulsion Illuminates Hepatocellular Carcinoma for Surgical Navigation, Front. Bioeng. Biotechnol., 2022, 10, 890668 CrossRef PubMed.
- K. Hasegawa and N. Kokudo, Surgical treatment of hepatocellular carcinoma, Surg. Today, 2009, 39, 833–843 CrossRef PubMed.
- W. Shang, X. Xia, N. Lu, P. Gao, L. Peng, Y. Liu, H. Deng, J. Jiang, Z. Li and J. Liu, Colourful fluorescence-based carbon dots for tumour imaging-guided nanosurgery, Nanomedicine, 2022, 45, 102583 CrossRef CAS PubMed.
- K. Yang, Z. Wu, H. Zhang, N. Zhang, W. Wu, Z. Wang, Z. Dai, X. Zhang, L. Zhang, Y. Peng, W. Ye, W. Zeng, Z. Liu and Q. Cheng, Glioma targeted therapy: insight into future of molecular approaches, Mol. Cancer, 2022, 21, 39 CrossRef CAS PubMed.
- Y. Zhang, W. Xiao, S. He, X. Xia, W. Yang, Z. Yang, H. Hu, Y. Wang, X. Wang, H. Li, Y. Huang and H. Gao, Lipid-mediated protein corona regulation with increased apolipoprotein A–I recruitment for glioma targeting, J. Controlled Release, 2024, 368, 42–51 CrossRef CAS PubMed.
- S. Ruan, Y. Zhou, X. Jiang and H. Gao, Rethinking CRITID Procedure of Brain Targeting Drug Delivery: Circulation, Blood Brain Barrier Recognition, Intracellular Transport, Diseased Cell Targeting, Internalization, and Drug Release, Adv. Sci., 2021, 8, 2004025 CrossRef CAS PubMed.
- Y. He, S. H. Liu, J. Yin and J. Yoon, Sonodynamic and chemodynamic therapy based on organic/organometallic sensitizers, Coord. Chem. Rev., 2021, 429, 213610 CrossRef CAS.
- H. Hu, W. Feng, X. Qian, L. Yu, Y. Chen and Y. Li, Emerging Nanomedicine-Enabled/Enhanced Nanodynamic Therapies beyond Traditional Photodynamics, Adv. Mater., 2021, 33, e2005062 CrossRef PubMed.
- Y. Zhou, M. Wang and Z. Dai, The molecular design of and challenges relating to sensitizers for cancer sonodynamic therapy, Mater. Chem. Front., 2020, 4, 2223–2234 RSC.
- L. Wang, Y. Tian, K. Lai, Y. Liu, Y. Liu, J. Mou, S. Yang and H. Wu, An Ultrasound-Triggered Nanoplatform for Synergistic Sonodynamic-Nitric Oxide Therapy, ACS Biomater. Sci. Eng., 2023, 9, 797–808 CrossRef CAS PubMed.
- Y. Zhu, Y. Liu, K. Khan, G. Arkin, A. K. Tareen, Z. Xie, T. He, L. Su, F. Guo, X. Lai, J. Xu and J. Zhang, Ultrasound combined with nanomaterials for cancer therapy, Mater. Today Adv., 2023, 17, 100330 CrossRef CAS.
- P. Liu, T. Yang, Y. Li, J. Guo, S. Li, H. Chen and Y. Liu, Nanomaterial-based sonosensitizers: from exemplary design towards purposeful improvement, Mater. Chem. Front., 2023, 7, 985–1003 RSC.
- B. Geng, S. Xu, L. Shen, F. Fang, W. Shi and D. Pan, Multifunctional carbon dot/MXene heterojunctions for alleviation of tumor hypoxia and enhanced sonodynamic therapy, Carbon, 2021, 179, 493–504 CrossRef CAS.
- X. Xing, S. Zhao, T. Xu, L. Huang, Y. Zhang, M. Lan, C. Lin, X. Zheng and P. Wang, Advances and perspectives in organic sonosensitizers for sonodynamic therapy, Coord. Chem. Rev., 2021, 445, 214087 CrossRef CAS.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- G. Nocito, G. Calabrese, S. Forte, S. Petralia, C. Puglisi, M. Campolo, E. Esposito and S. Conoci, Carbon Dots as Promising Tools for Cancer Diagnosis and Therapy, Cancers, 2021, 13, 1991 CrossRef CAS PubMed.
- Z. Kang and S.-T. Lee, Carbon dots: advances in nanocarbon applications, Nanoscale, 2019, 11, 19214–19224 RSC.
- H. Wang, J. Bi, B.-W. Zhu and M. Tan, Multicolorful Carbon Dots for Tumor Theranostics, Curr. Med. Chem., 2018, 25, 2894–2909 CrossRef CAS PubMed.
- N. Panwar, A. M. Soehartono, K. K. Chan, S. Zeng, G. Xu, J. Qu, P. Coquet, K.-T. Yong and X. Chen, Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery, Chem. Rev., 2019, 119, 9559–9656 CrossRef CAS PubMed.
- J. Xu, J. Ning, Y. Wang, M. Xu, C. Yi and F. Yan, Carbon dots as a promising therapeutic approach for combating cancer, Bioorg. Med. Chem., 2022, 72, 116987 CrossRef CAS PubMed.
- C.-Y. Li, W.-J. Zhang, W.-Z. Bi, Q.-J. Ma, S.-X. Feng, X.-L. Chen and L.-B. Qu, Rose Bengal-Derived Carbon Quantum Dots as a Fluorescence Probe for the Highly Sensitive Detection of Fe3+ Ions, ChemistrySelect, 2023, 8, e202302661 CrossRef CAS.
- X.-W. Yu, X. Liu, Y.-W. Jiang, Y.-H. Li, G. Gao, Y.-X. Zhu, F. Lin and F.-G. Wu, Rose Bengal-Derived Ultrabright Sulfur-Doped Carbon Dots for Fast Discrimination between Live and Dead Cells, Anal. Chem., 2022, 94, 4243–4251 CrossRef CAS PubMed.
- Y. Gao, Z. Li, C. Wang, J. You, B. Jin, F. Mo, J. Chen, Y. Zheng and H. Chen, Self-assembled chitosan/rose bengal derivative nanoparticles for targeted sonodynamic therapy: preparation and tumor accumulation, RSC Adv., 2015, 5, 17915–17923 RSC.
- J. Cossu, F. Thoreau and D. Boturyn, Multimeric RGD-Based Strategies for Selective Drug Delivery to Tumor Tissues, Pharmaceutics, 2023, 15, 525 CrossRef CAS PubMed.
- M. A. Dechantsreiter, E. Planker, B. Mathä, E. Lohof, G. Hölzemann, A. Jonczyk, S. L. Goodman and H. Kessler, N-Methylated Cyclic RGD Peptides as Highly Active and Selective αVβ3 Integrin Antagonists, J. Med. Chem., 1999, 42, 3033–3040 CrossRef CAS PubMed.
- L. Li, X. Chen, J. Yu and S. Yuan, Preliminary Clinical Application of RGD-Containing Peptides as PET Radiotracers for Imaging Tumors, Front. Oncol., 2022, 12, 837952 CrossRef CAS PubMed.
- N. Li, S. Qiu, Y. Fang, J. Wu and Q. Li, Comparison of Linear vs. Cyclic RGD Pentapeptide Interactions with Integrin αvβ3 by Molecular Dynamics Simulations, Biology, 2021, 10, 688 CrossRef CAS PubMed.
- S. Arab, S. Masoum and E. Seyed Hosseini, Engineering the Synthesis of Luminescent Carbon Dots With Ultra High-Quantum Yield Using Experimental Design Approach in Order to Develop Sensing Applications, IEEE Sens. J., 2020, 20, 1705–1711 CAS.
- H. Lin, J. Huang and L. Ding, Preparation of Carbon Dots with High-Fluorescence Quantum Yield and Their Application in Dopamine Fluorescence Probe and Cellular Imaging, J. Nanomater., 2019, 2019, 1–9 Search PubMed.
- K. Żamojć, M. Zdrowowicz, P. B. Rudnicki-Velasquez, K. Krzymiński, B. Zaborowski, P. Niedziałkowski, D. Jacewicz and L. Chmurzyński, The development of 1,3-diphenylisobenzofuran as a highly selective probe for the detection and quantitative determination of hydrogen peroxide, Free Radical Res., 2016, 51, 38–46 CrossRef PubMed.
- X.-H. Wang, X.-F. Wei, J.-H. Liu, W. Yang, Y.-A. Liu, K. Cheng, X.-Y. He, X.-L. Fu, Y. Zhang and H.-X. Zhang, Chlorin e6-1,3-diphenylisobenzofuran polymer hybrid nanoparticles for singlet oxygen-detection photodynamic abaltion, Methods Appl. Fluoresc., 2021, 9, 025003 CrossRef CAS PubMed.
- D. Yu, Y. Zha, Z. Zhong, Y. Ruan, Z. Li, L. Sun and S. Hou, Improved detection of reactive oxygen species by DCFH-DA: New insight into self-amplification of fluorescence signal by light irradiation, Sens. Actuators, B, 2021, 339, 129878 CrossRef CAS.
- Z. Liu, J. Li, Y. Jiang and D. Wang, Multifunctional nanocapsules on a seesaw balancing sonodynamic and photodynamic therapies against superficial malignant tumors by effective immune-enhancement, Biomaterials, 2019, 218, 119251 CrossRef CAS PubMed.
- L. Tong, X. Wang, Z. Chen, Y. Liang, Y. Yang, W. Gao, Z. Liu and B. Tang, One-Step Fabrication of Functional Carbon Dots with 90% Fluorescence Quantum Yield for Long-Term Lysosome Imaging, Anal. Chem., 2020, 92, 6430–6436 CrossRef CAS PubMed.
- C. Zhang, M. Liu, T. Li, S. Liu, Q. Chen, J. Zhang and K. Zhang, One-pot hydrothermal synthesis of dual-emission fluorescent carbon dots for hypochlorous acid detection, Dyes Pigm., 2020, 180, 108507 CrossRef CAS.
- Y. Zhao, L. Shi, J. Fang and X. Feng, Bio-nanoplatforms based on carbon dots conjugating with F-substituted nano-hydroxyapatite for cellular imaging, Nanoscale, 2015, 7, 20033–20041 RSC.
- M. Cheng, P. Wang, L. Cao, W.-F. Dong, L. Li and R. Yan, Novel visible-light-excited afterglow rose-bengal-derived carbon dots and their applications, J. Lumin., 2022, 252, 119370 CrossRef CAS.
- Y. Guo, Z. Wang, Y. Chen, F. Chao, Y. Xu, L.-L. Qu, F.-G. Wu and X. Dong, Ultrabright Green-Emissive Nanodots for Precise Biological Visualization, Nano Lett., 2024, 24, 2264–2272 CrossRef CAS PubMed.
- H. Sun, S. Li, W. Qi, R. Xing, Q. Zou and X. Yan, Stimuli-responsive nanoparticles based on co-assembly of naturally-occurring biomacromolecules for in vitro photodynamic therapy, Colloids Surf., A, 2018, 538, 795–801 CrossRef CAS.
- F. Causa, E. Battista, R. Della Moglie, D. Guarnieri, M. Iannone and P. A. Netti, Surface Investigation on Biomimetic Materials to Control Cell Adhesion: The Case of RGD Conjugation on PCL, Langmuir, 2010, 26, 9875–9884 CrossRef CAS PubMed.
- Z. Liu, J. Li, W. Chen, L. Liu and F. Yu, Light and sound to trigger the Pandora’s box against breast cancer: A combination strategy of sonodynamic, photodynamic and photothermal therapies, Biomaterials, 2020, 232, 119685 CrossRef CAS PubMed.
- X. Lv, C. Zhang, Q. Shuaizhen, R. Yu and Y. Zheng, Design of integrin αvβ3 targeting self-assembled protein nanoparticles with RGD peptide, Biomed. Pharmacother., 2020, 128, 110236 CrossRef CAS PubMed.
- S. Benedetti, S. Catalani, S. De Stefani, M. Primiterra, A. Fraternale, F. Palma and S. Palini, A microplate-based DCFH-DA assay for the evaluation of oxidative stress in whole semen, Heliyon, 2022, 8, e10642 CrossRef CAS PubMed.
- B. Zhang, J. Jiang, P. Wu, J. Zou, J. Le, J. Lin, C. Li, B. Luo, Y. Zhang, R. Huang and J. Shao, A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy, Acta Pharm. Sin. B, 2021, 11, 246–257 CrossRef CAS PubMed.
- Q. He, Y. Liu, F. Pan, H. Duan, J. Guan, Z. Liang, H. Zhong, X. Wang, Y. He, W. Huang and T. Guan, Unsupervised domain adaptive tumor region recognition for Ki67 automated assisted quantification, Int. J. Comput. Assist. Radiol. Surg., 2022, 18, 629–640 CrossRef PubMed.
- Q. Liu, D. Ran, L. Wang, J. Feng, W. Deng, D. Mei, Y. Peng and C. Du, Association between Ki67 expression and therapeutic outcome in colon cancer, Oncol. Lett., 2023, 25, 272 CrossRef CAS PubMed.
- J. Liu, F. Wu, M. Wang, M. Tao, Z. Liu and Z. Hai, Caspase-3-Responsive Fluorescent/Photoacoustic Imaging of Tumor Apoptosis, Anal. Chem., 2023, 95, 9404–9408 CrossRef CAS PubMed.
- X. Pu, S. J. Storr, Y. Zhang, E. A. Rakha, A. R. Green, I. O. Ellis and S. G. Martin, Caspase-3 and caspase-8 expression in breast cancer: caspase-3 is associated with survival, Apoptosis, 2016, 22, 357–368 CrossRef PubMed.
- Y. Wu, Z. Chen, D. Shen, Z. He, J. Lv, H. Li, M. Yang, J. Tan, J. Yuan, J. Gao and Z. Yuan, A Lysosome-Targeted Near-Infrared Fluorescent Probe with Excellent Water Solubility for Surgery Navigation in Breast Cancer, ACS Omega, 2023, 8, 12481–12488 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d0qm00311j |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2024 |