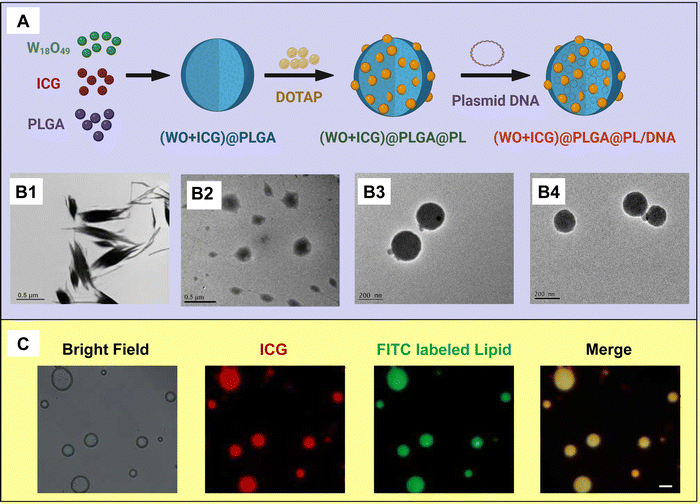
(WO + ICG)@PLGA@lipid/plasmid DNA nanocomplexes as core–shell vectors for synergistic genetic/photothermal therapy†
Yang
Bai‡
ab,
Guoqing
Feng‡
a,
Qingbin
Yang‡
a,
Tingting
Hua
a,
Bowen
Li
a,
Hao-Lin
Guo
c,
Yuan
Liu
d,
Qing
Yuan
e,
Niansong
Qian
*f and
Bin
Zheng
 *a
*a
aAcademy of Medical Engineering and Translational Medicine, Tianjin University, Tianjin, 300072, China. E-mail: binzheng@tju.edu.cn
bDepartment of Stomatology, Tianjin Medical University General Hospital, Heping District, Tianjin 300052, China
cSchool of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, 102401, China
dTianjin Anding Hospital, Tianjin, 300222, China
eDepartment of Urology, The Third Medical Center, Chinese People's Liberation Army (PLA) General Hospital, Beijing 100853, China
fDepartment of Respiratory, the Eighth Medical Center of Chinese PLA General Hospital, Beijing 100853, China. E-mail: qianniansong1@163.com
First published on 18th September 2024
Abstract
The synergistic therapeutic strategy of combining gene delivery and photothermal effects as an efficient cancer treatment method has garnered significant attention. Here, we developed a core–shell theragnostic platform ((WO + ICG)@PLGA@PL) capable of simultaneously delivering a fluorescent imaging agent, a photothermal agent, and genes. The self-assembled platform comprises four components: indocyanine green (ICG) for in vivo localization tracking, W18O49 (WO) nanoparticles for photothermal therapy, PLGA as a core for encapsulating ICG and WO, and positive liposomes for DNA interaction and particle stabilization. The results showed that (WO + ICG)@PLGA@PL could not only achieve a synergistic therapy effect of gene delivery and photothermal effect, but also effectively inhibit tumor growth in vivo. Additionally, the (WO + ICG)@PLGA@PL nanocomplex could be a promising tool for next-generation combined gene and photothermal therapy.
1. Introduction
Nanodiagnostics, a newly emerging strategy, can integrate diagnosis with therapeutics to explore novel treatment options using nanoparticles.1,2 The real-time, noninvasive monitoring in nanodiagnostics is highly significant for cancer therapy, enabling timely adjustments to the treatment plan.3 A critical aspect of nanodiagnostics is the ability to combine both diagnostic and therapeutic functions within a single integrated nanoparticle. Despite the tremendous development of theragnostic nanoparticles in recent years, significant challenges remain in developing effective nanodiagnostics agents that can provide fast and accurate imaging while simultaneously delivering therapy.4 Fluorescence-mediated imaging offers distinct advantages, including real-time monitoring, convenience, and accuracy, establishing it as a leading imaging technique in theragnostic applications.5 Indocyanine green (ICG) is particularly effective as a fluorescent imaging agent for theragnostic nanoparticles, as it strongly absorbs light around 800 nm, minimizing scattering and fluorescence interference by biomolecules at this wavelength.6,7 All these characteristics make ICG an ideal choice for fluorescent imaging in nanodiagnostics applications.To realize non-toxic, efficient treatment with nanodiagnostics in vivo, various clinical treatment modalities are employed. The commonly used treatment modalities include chemotherapy, radiotherapy and so on.8–10 Although chemotherapy and radiotherapy are effective for tumor treatment in clinical settings, they often cause significant side effects to the human body.11 To mitigate these side effects and address the issue of multidrug resistance (MDR), several new strategies have been developed. Among these new strategies, photothermal therapy (PTT) as a minimally invasive, non-toxic treatment methodology has attracted widespread interest.12,13 PTT employs photo-absorbing agents to generate heat from optical energy, leading to the ‘burning’ of cancer cells.14 Tungsten oxide (W18O49, WO) photothermal nanoparticles, known for their absorbance in the near-infrared (NIR) region (700–950 nm), high photothermal conversion efficiency and excellent photo-stability, are considered among the most valuable photothermal nanoparticles for clinical applications.15 Nevertheless, cancer is a complex disease, and the effectiveness of PTT and retention of therapeutic agents do not always yield optimal outcomes across all tumor types.16 To overcome these limitations of PTT, several new strategies have been developed. Gene therapy is one of the most promising strategies to treat cancer as an adjuvant therapy to PTT.17
Recently, a theragnostic strategy has been conceived on the basis of combining fluorescent imaging agent, a photothermal agent and gene therapy.18,19 In this article, the WO nanoparticles and ICG co-loaded poly(D,L-lactide-co-glycolide) (PLGA)@positive liposome core–shell theragnostic platform ((WO + ICG)@PLGA@PL) has been developed. The core–shell theragnostic platform is particularly appealing because it has the potential to simultaneously deliver a fluorescent imaging agent, a photothermal agent and gene therapy. The self-assembled (WO + ICG)@PLGA@PL is formed from four key components: (1) ICG, which exhibits strong absorption in the NIR region, functioning as a tracker for locating the whole system in vivo; (2) WO nanoparticles, which serve as an efficient photothermal agent for photodynamic therapy under 808 nm light irradiation; (3) PLGA, chosen as the core domain for encapsulating the ICG and WO nanoparticles; and (4) the positive liposome, which acts as a lipid shell that interacts electrostatically with negatively charged DNA, while also providing electrostatic repulsion to prevent particle aggregation (Fig. 1).
 | ||
| Fig. 1 The construction process and synergistic therapy process of the (WO + ICG)@PLGA@PL/DNA delivery system. | ||
To verify the theragnostic effectiveness of this platform in practical applications, we conducted a comprehensive evaluation of the (WO + ICG)@PLGA@PL nanoparticles, assessing various physical and chemical properties. These properties include structural integrity, morphology, size distribution, DNA-binding ability, gene transfection efficiency, bioimaging performance, photothermal conversion efficiency and in vivo therapeutic efficacy.
2. Materials and methods
2.1. Materials
Indocyanine green (ICG), tungsten(VI) chloride (WCl6, ≥99.9%), 1-propyl alcohol (anhydrous, 99.7%), ethanol (ACS reagent, ≥99.5%), polyvinyl alcohol (PVA), 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), poly(lactic-co-glycolic) acid (PLGA) and fluorescein isothiocyanate (FITC) were all obtained from Sigma-Aldrich (USA).2.2. Synthesis of the W18O49 nanostructures
The W18O49 (WO) nanoparticles were synthesized according to a previously reported method, with some improvements.20 Typically, WCl6 (0.5 g) was dissolved in 1-propyl alcohol under magnetic stirring. Then the above mixing solution was transferred into the reaction to obtain the WO nanorod-bundled nanostructures after maintaining at 200 °C for 10 h. The WO precipitate was ground, sonicated, and collected by centrifugation to obtain the WO prills. The final precipitate was kept in dichloromethane after washing.2.3. Synthesis of (WO + ICG)@PLGA nanoparticles
WO and PLGA co-assembling WO@PLGA and (WO + ICG)@PLGA nanoparticles were synthesized according to a previously reported method.20 Briefly, PLGA was dissolved in dichloromethane and then added to an aqueous stabilizer mixture containing PVA, ICG and WO in water under high-power ultrasound. Then the mixed emulsion solution was added to a larger amount of PVA water solution under high-power ultrasound. The mixed solution was magnetically stirred at room temperature to remove the organic solvent. Finally, after purification by centrifugation to remove PVA, the (WO + ICG)@PLGA nanoparticles samples were kept in water.2.4. Synthesis of (WO + ICG)@PLGA@PL nanoparticles
(WO + ICG)@PLGA@PL nanoparticles were prepared by the gentle hydration method reported in our lab. First, 3 mg of lipid DOTAP and 1 mg of cholesterol were dissolved in 4 mL of chloroform at room temperature. Then, the chloroform was evaporated using a vacuum rotary evaporator, forming a thin cationic lipid film on the wall of a 50 mL round-bottomed flask. Next, the cationic lipid film was dispersed in 5 mL of (WO + ICG)@PLGA nanoparticles solution (5 mg mL−1) and sonicated in a bath sonicator for 10 min. Finally, after purification by centrifugation to remove the uncoated cationic lipid, the (WO + ICG)@PLGA@PL nanoparticles samples were kept in water.2.5. Fluorescence imaging in vitro and in vivo
In vitro fluorescence imaging was operated by putting the samples into 96-well plates. The ICG fluorescence signal was monitored after excitation with 710 nm light. For the in vivo imaging, (WO + ICG)@PLGA-loaded macrophages were injected into mice locally or via the caudal vein, with 100 μL of phosphate buffer saline (PBS) used for the injection when the tumor size reached 300–350 mm3. Fluorescence imaging of mice was detected using an in vivo imaging system. The photothermal efficiency was tested as follows: samples were added into cuvette and irradiated for 10 min simultaneously with 1.5 W cm−2 laser (MDL-N-808 (Cnilaser, China)). Temperature variation was monitored using an infrared thermal imaging camera (Fluke, USA). To explore the photothermal treatment efficiency, the medium was replaced by new medium containing different treatments. The cells were incubated within the medium containing different samples for 24 h and then the medium was discarded and washed by PBS. Fresh medium was added and the plates were kept at 37 °C before and during laser irradiation. Cell viability was evaluated using the 3-((4,5)-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The cytotoxicity of the photothermal treatment was evaluated using the calcein-AM living cells staining assay according to the manufacturer's instructions (Invitrogen).2.6. Animal experiments
Healthy female C57BL/6 mice weighing 20–25 g body were purchased from HFK Technology Co., Ltd (Beijing). Animal experiments were performed in accordance with the statutory requirements of the People's Republic of China (GB14925-2010). To develop a melanoma tumor model, 5 × 104 B16-F10 melanoma cells were injected subcutaneously (s.c.). Once the tumor volume reached 300–350 mm3, subsequent experiments were conducted. The health status of the mice and the experimental parameters were strictly monitored and recorded throughout the experiment.2.7. In vivo antitumor assessment
The successfully modeled mice were randomly divided into four experimental groups, each receiving a different treatment regimen: mice injected with PBS (Group 1); mice receiving 808 nm laser irradiation only (Group 2); mice injected with ICG solution (Group 3); and mice injected with the (WO + ICG)@PLGA@PL/DNA complex and receiving 808 nm laser irradiation (Group 4).Subsequently, tumor size and body weight of each mouse were recorded daily. On day 15, a subset of the mice was sacrificed, and their tumors were collected for further analysis. Then photos of the tumors were taken with a digital camera (Nikon, Japan). After that, the tumors were washed with saline three times and fixed in 10% neutral-buffered formalin. For the hematoxylin and eosin (H&E), paraffin tumor sections were stained and observed using a fluorescence inversion microscope system (Olympus, Japan).
2.8. H&E staining
First, visceral organs collected from mice were fixed in glutaraldehyde and then dehydrated. After dehydration, the samples were cleared with xylene and embedded in paraffin. The embedded samples were sectioned into 10-micron thick slices using a rotary microtome. The sections were gradually dehydrated through a series of ethanol concentrations, including 50%, 70%, 80%, 95%, and 100%, each step lasted 3 min. The sections were then cleared with xylene or its substitute for 3 min. Subsequently, the sections were stained with hematoxylin solution for 10 min and quickly rinsed with 95% ethanol to remove excess hematoxylin. The sections were then stained with eosin solution for 2 min. The stained sections were dipped in 1% hydrochloric acid alcohol for 5 s to remove non-specific staining. Next, the sections were rinsed in Scott's tap water for 5 s to enhance the blue color of the nuclei. The sections were then dehydrated again through 95% and 100% ethanol and cleared with xylene. Finally, the sections were mounted with dimethyl ether hydroxymethyl benzoate or a similar sealing agent. The sections were examined under a microscope, and the results were documented.2.9. Graphical illustrations
Graphical illustrations were created with BioRender.com.2.10. Statistical analyses
Data were expressed as mean ± standard deviation (SD) of the experiments, and each experimental group contained 5 repeated samples. Data analysis was performed using Graphpad Prism 8.0.2 and Microsoft Excel.3. Results and discussion
3.1. Synthesis and characterization of (WO + ICG)@PLGA@PL/gene complexes
The synthesis process of (WO + ICG)@PLGA@PL is generally divided into three parts (Fig. 2(A)). Firstly, WO nanoparticles were produced by a hydrothermal method (Group 1). Subsequently, (WO + ICG)@PLGA was synthesized by the emulsion method (Group 2). The final stage involved coating the nanoparticles with the cationic lipid DOTAP using a film-dispersion technique (Group 3), followed by gene condensation through electrostatic adsorption onto the (WO + ICG)@PLGA surface (Group 4) (Fig. S1, ESI†). The size and morphology of the resulting nanoparticles were analyzed using transmission electron microscopy (TEM) (Fig. 2(B1)–(B4)). WO possesses uniformly nanostructures in the form of bundled nanorods. These WO nanorods were usually relatively larger, and formed agglomerates fast in solution, which cannot be used for PTT directly (Fig. S2, ESI†). Compared to the PLGA nanospheres in Group 2, the contrast of the nanospheres increased significantly, indicating successful encapsulation of WO and ICG within the PLGA matrix under sonication. In Group 3 and Group 4, there is no significant difference in the particle size or morphology compared with that in Group 2.To investigate the core–shell structure of the (WO + ICG)@PLGA@PL under an optical resolution fluorescence microscope, a small fraction of large (WO + ICG)@PLGA@PL particles was isolated through low-speed centrifugation after synthesis. In this experiment, red fluorescence corresponds to emission from ICG, while green fluorescence indicates the FITC-labeled lipid molecules (DOTAP). Upon excitation at 561 nm and 488 nm, the (WO + ICG)@PLGA@PL exhibited both red and green fluorescence, confirming that both ICG and the lipid shell were successfully co-localized on the same nanosphere (Fig. 2(C)). This finding substantiates the successful coating of the lipid shell on the (WO + ICG)@PLGA surface.
3.2. Cytotoxicity and cell uptake of (WO + ICG)@PLGA@PL
To investigate the cytotoxicity of nanoparticles, we employed the MTT assay to assess the relative viability of the B16-F10 cells (derived from rat macrophages). Various concentrations of WO@PLGA (Group 1), (WO + ICG)@PLGA (Group 2), and (WO + ICG)@PLGA@PL (Group 3) were incubated with B16-F10 cells at 37 °C for 24 h. Quantitative analysis revealed that as the concentration increased, the cell survival rate in all the samples began to decline. However, even at the highest concentration used (1 mg mL−1), cell viability remained above 80% (Fig. 3(A)). All the cell survival rates are above 90% when the concentration is below 0.2 mg mL−1. At the concentration of 0.1 mg mL−1, the cells incubated with these three groups were stained with calcein-AM, a kind of living cell fluorescence probe. In all these groups, most of the cells show strong green fluorescence which indicated that at the concentration of 0.1 mg mL−1, the nanoparticles incubated with the cells have no significant effect on cell activity (Fig. 3(B)).Besides, the uptake of the nanoparticles by cells was visualized using fluorescence microscopy (Fig. 3(C)). As exhibited in the fluorescence images, the blue fluorescence showed the Hoechst 33342 labeled nucleus, and the red fluorescence showed the ICG in cells. There are two groups including the cells incubated with ICG (Group 1) and the cells incubated with (WO + ICG)@PLGA@PL (Group 2). The red fluorescence strength in Group 2 is as high as that in Group 1, which suggests that the nanoparticle packing process and endocytosis process has no significant effect on the fluorescence of ICG.
3.3. Fluorescence imaging and photothermal performance of (WO + ICG)@PLGA@PL in vitro and in vivo
The fluorescence imaging system and thermal imaging instrument have been used for testing whether the (WO + ICG)@PLGA@PL still has both fluorescence and photothermal performance in vitro and vivo. Different concentrations of PLGA (Group 1), (WO + ICG)@PLGA (Group 2), and (WO + ICG)@PLGA@PL (Group 3) aqueous solutions were added to a 96-well plate. The fluorescence intensity of each sample was monitored using a fluorescence imaging system. As exhibited in Fig. 4(A), there is no red fluorescence in Group 1, which means that PLGA is unable to emit fluorescence. At the same concentration, the red fluorescence intensity in Group 3 is nearly the same as that in Group 2 in vitro and in vivo, which suggested that there was little effect on the fluorescence of ICG after the (WO + ICG)@PLGA was coated by lipid molecules. Subsequently, we performed in vivo imaging on mice after subcutaneous injection of these nanoparticles dissolved in PBS (Fig. 4(C)). The imaging results demonstrated that (WO + ICG)@PLGA (Group 2) and (WO + ICG)@PLGA@PL (Group 3) exhibited strong fluorescence at the injection sites, whereas the PLGA (Group 1) injection site showed no fluorescence. The combined in vivo and in vitro fluorescence imaging results indicated that the (WO + ICG)@PLGA@PL possesses excellent imaging capabilities.The photothermal performance in vitro and in vivo was tested by a thermal imaging instrument. The nanoparticles were added to different quartz cuvettes and irradiated with an 808 nm NIR laser at a power density of 1.5 W cm−2 for 10 min. The temperature changes for each group were monitored using a thermometer and an infrared thermal imager (Fig. 4(B) and Fig. S3, ESI†). Compared to Group 1, Group 2 and Group 3 exhibited significant differences in the temperature increase, indicating that (WO + ICG)@PLGA and (WO + ICG)@PLGA@PL demonstrated enhanced photothermal stability under 808 nm NIR laser irradiation. There was no significant difference in the temperature rise between Group 2 and Group 3, with temperatures rapidly rising to approximately 50 °C within 10 min, suggesting that the ICG loading did not affect the photothermal effect. To verify the in vivo photothermal conversion effect of (WO + ICG)@PLGA@PL, thermal imaging of mice under NIR laser irradiation showed that the local temperature in the injection areas of Group 2 and Group 3 increased rapidly, whereas no significant temperature increase was observed in the PLGA (Group 1) injection area (Fig. 4(D) and Fig. S4, ESI†). Consistent with the aforementioned in vitro thermal effects, after NIR laser irradiation for 600 s, the heat production efficiency in Group 3 is nearly the same as that in Group 2 which suggested that there was little effect on the photothermal conversion of the nanoparticles after the (WO + ICG)@PLGA coated by lipid molecules. (WO + ICG)@PLGA@PL effectively induced photothermal conversion both in vitro and in vivo, making it a promising photothermal agent for tumor therapy.
3.4. Gene therapy test in vitro
In order to test whether the plasmid DNA with (WO + ICG)@PLGA@PL can form complexes well, the Zeta PALS and 1% agarose gel electrophoresis experiments were conducted (Fig. 5(A) and (B)). We first conducted Zeta potential measurements for PLGA (Group 1), (WO + ICG)@PLGA (Group 2), and (WO + ICG)@PLGA@PL (Group 3). The Zeta potentials in both Group 1 and Group 2 are negative. The Zeta potential in Group 3 was nearly 40 mV, which is much higher than those in Group 1 and Group 2, which proved that the positive lipid molecules DOTAP had been successfully synthesized. As shown in Fig. 5(A), the free plasmid DNA was incubated with different amounts of nanocarrier. When the amount of plasmid DNA was kept constant at 100 ng, with the increase of the (WO + ICG)@PLGA@PL concentration, the mobility of the plasmid DNA toward the positive terminal was delayed. When the (WO + ICG)@PLGA@PL nanocarrier/plasmid DNA (N/P) ratio reached 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, most of the DNA was detained in a sample well, which indicated that the (WO + ICG)@PLGA@PL can effectively condense the plasmid DNA at this ratio. To ensure the maximization of gene delivery efficacy, the N/P ratio was fixed at 30
1, most of the DNA was detained in a sample well, which indicated that the (WO + ICG)@PLGA@PL can effectively condense the plasmid DNA at this ratio. To ensure the maximization of gene delivery efficacy, the N/P ratio was fixed at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in subsequent experiments.
1 in subsequent experiments.
Additionally, the gene-transfection test and gene therapy test in vitro were tested. As seen in Fig. 5(C), green fluorescence of green fluorescent protein (GFP) tags can be detected in cells, which suggested that the plasmid DNA was expressed successfully in cells. Besides, red fluorescence of ICG and green fluorescence of GFP tags are detected in the same cells (Fig. 5(D)). These results proved that ICG and plasmid DNA could efficiently co-deliver into the same cell by the (WO + ICG)@PLGA@PL/DNA complex. To demonstrate that (WO + ICG)@PLGA@PL nanoparticles can facilitate the transfection of plasmid DNA into B16-F10 cells and induce apoptosis, B16-F10 cells were transfected at an N/P ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After 48 h of Bax gene transfection, B16-F10 cells were lysed, and western blotting was performed (Fig. 5(E)). The band of caspase 3, which plays a central role in the execution-phase of cell apoptosis, was significantly decreased in Group 2 (Bax gene transfection), whereas the untransfected control group (Group 1) showed almost no change. As we know, after apoptosis is activated, the caspase 3 precursor protein would be degraded. Subsequently, flow cytometry was used to observe cells stained with calcein-AM and propidium iodide (PI), where calcein-AM penetrates live cells and fluoresces after hydrolysis by intracellular esterases, while PI fluoresces only in dead cells (Fig. 5(F)). These results indicated that (WO + ICG)@PLGA@PL/DNA nanoparticles could help the Bax gene transfect B16-F10 cells and induce cancer cell apoptosis.
1. After 48 h of Bax gene transfection, B16-F10 cells were lysed, and western blotting was performed (Fig. 5(E)). The band of caspase 3, which plays a central role in the execution-phase of cell apoptosis, was significantly decreased in Group 2 (Bax gene transfection), whereas the untransfected control group (Group 1) showed almost no change. As we know, after apoptosis is activated, the caspase 3 precursor protein would be degraded. Subsequently, flow cytometry was used to observe cells stained with calcein-AM and propidium iodide (PI), where calcein-AM penetrates live cells and fluoresces after hydrolysis by intracellular esterases, while PI fluoresces only in dead cells (Fig. 5(F)). These results indicated that (WO + ICG)@PLGA@PL/DNA nanoparticles could help the Bax gene transfect B16-F10 cells and induce cancer cell apoptosis.
3.5. Photothermal therapy effect in vitro and vivo
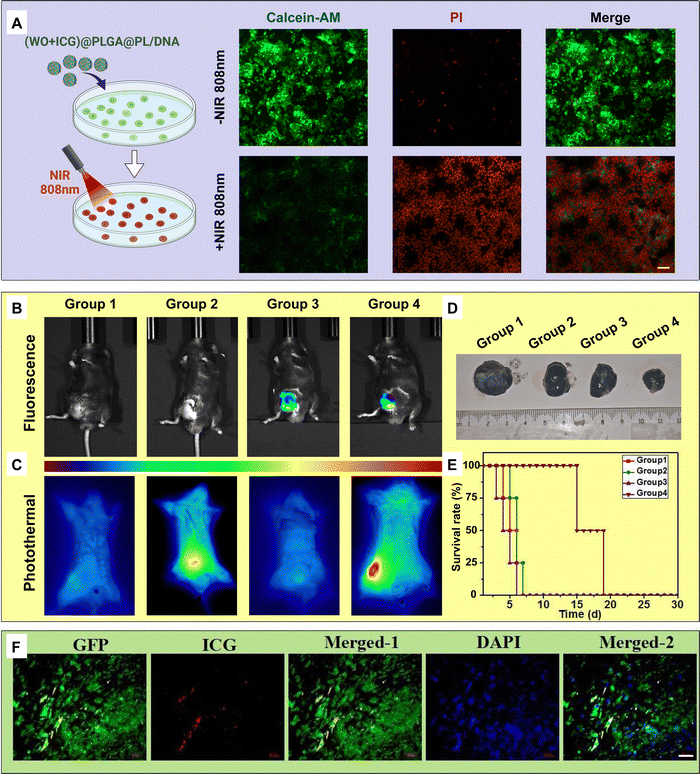
To evaluate the in vitro photothermal therapeutic efficacy of the nanoparticles, we investigated the synergistic therapeutic capability of Bax gene transfection combined with PTT to further kill tumor cells. B16-F10 cells transfected with the Bax gene via (WO + ICG)@PLGA@PL were stained with calcein-AM and PI (Fig. 6(A)). The results showed intense green fluorescence in cells not exposed to NIR irradiation, indicating that the (WO + ICG)@PLGA@PL/DNA complex did not affect cell viability. In contrast, cells exposed to NIR irradiation exhibited almost no green fluorescence, indicating cell death. These findings demonstrate that the (WO + ICG)@PLGA@PL/DNA complex can effectively kill tumor cells through a combination of gene therapy and PTT.To test the photothermal and genetic therapy efficacy in vivo, we used healthy C57BL/6 mice to establish a B16-F10 subcutaneous tumor model. The successfully modeled mice were randomly divided into four experimental groups, each receiving a different treatment regimen: the mice injected with PBS (Group 1), the mice exposed to 808 nm laser irradiation only (Group 2), the mice injected with ICG solution (Group 3) and the mice injected with (WO + ICG)@PLGA@PL/DNA complex and exposed to 808 nm laser irradiation (Group 4). There is a strong fluorescence signal an hour after the intratumoral injection of the nanoparticles in Group 3 and Group 4 (Fig. 6(B)). This indicates that the nanoparticles successfully accumulated at the tumor site. Subsequently, a thermal imaging camera was used to evaluate the in vivo photothermal conversion efficiency. The thermal imaging results showed a rapid temperature increase at the tumor sites of the mice following 808 nm NIR irradiation (Fig. 6(C)). Digital photographs of the excised tumors from each group revealed no significant antitumor effect in Groups 1–3, suggesting that NIR laser and pure ICG could not induce obvious tumor damage. In contrast, the mice in Group 4 showed much lower tumor growth (Fig. 6(D)). This result indicates that the (WO + ICG)@PLGA@PL/DNA complex effectively inhibited tumor growth under 808 nm laser irradiation. Additionally, the survival times of the mice were observed, with the experimental groups showing significantly prolonged survival periods (Fig. 6(E)). To determine whether the plasmid DNA was expressed in tumor cells, the tumor tissue section was observed after the mouse was injected with (WO + ICG)@PLGA@PL/DNA complex for 5 days. As shown in Fig. 6(F), positive green fluorescence was detected in the tumor tissue section, which suggested that the DNA was expressed in tumor cells. Besides, the red fluorescence of ICG was also detected in cells.
3.6. Analysis of histopathological sections
To comprehensively assess the biocompatibility of (WO + ICG)@PLGA@PL, we performed H&E staining on tumor and organ sections from the different experimental groups (Fig. 7). The staining results showed that the tissue structures of the heart, liver, spleen, lungs, and kidneys were normal, with no inflammatory infiltration or significant pathological damage, indicating that the nanoparticles did not exert notable toxic effects on the major organs of the mice. In tumor sections, the tumor cells in Group 4 exhibited significant tumor necrosis with severe structural damage and pyknosis, which indicated that the (WO + ICG)@PLGA@PL/DNA complex provides selective tumor cell killing.4. Conclusions
In summary, the (WO + ICG)@PLGA@PL/DNA complex was successfully developed for gene therapy and photothermal therapy simultaneously. It has been demonstrated that the (WO + ICG)@PLGA@PL has a core–shell structure with good DNA-binding ability. This system seamlessly integrates fluorescent imaging with photothermal therapeutic efficacy. ICG, as a photosensitizer, generates reactive oxygen species (ROS), including singlet oxygen and superoxide anions, upon exposure to the NIR laser. These ROS induce oxidative stress within cells, damaging intracellular lipids, proteins, and DNA, ultimately leading to tumor cell death. Additionally, ROS can enhance the sensitivity of tumor cells to other therapeutic modalities, such as chemotherapy or radiotherapy, by undermining the tumor's defense mechanisms. Upon incubation of the nanocomplexes with cells, bright red and green fluorescence were both observed in the cytoplasm around the nucleus, which indicated that the ICG and genes carried by the nanocomplexes were co-delivered into the tumor cells. In the in vivo experiment, the (WO + ICG)@PLGA@PL/DNA complex showed good inhibitory effects on the tumor. Hence, this (WO + ICG)@PLGA@PL delivery system has potential applications as a phosphorescent drug carrier for various tumor treatments. The possibility that ICG-mediated ROS generation could enhance the efficacy of combination therapies presents a valuable avenue for future research. For instance, when ICG is used in conjunction with gene therapy, the ROS produced may facilitate the release and activation of therapeutic genes, thereby improving overall treatment outcomes.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFC2600503), the National Natural Science Foundation of China (32271400), Key Project of Tianjin Natural Science Foundation (21JCZDJC00690), and Elderly Health & Happiness Major Program of China Ageing Development Foundation (EHH20211002 and EHH20211001).References
- C. Fang and M. Zhang, Nanoparticle-based theragnostics: Integrating diagnostic and therapeutic potentials in nanomedicine, J. Controlled Release, 2010, 146, 2–5 CrossRef CAS PubMed.
- J. H. Ryu, H. Koo, I. C. Sun, S. H. Yuk, K. Choi, K. Kim and I. C. Kwon, Tumor-targeting multi-functional nanoparticles for theragnosis: new paradigm for cancer therapy, Adv. Drug Delivery Rev., 2012, 64, 1447–1458 CrossRef CAS PubMed.
- D. Majumdar, X. H. Peng and D. M. Shin, The medicinal chemistry of theragnostics, multimodality imaging and applications of nanotechnology in cancer, Curr. Top. Med. Chem., 2010, 10, 1211–1226 CrossRef CAS PubMed.
- P. T. Hammond, Shooting for the Moon: Nanoscale Approaches to Cancer, ACS Nano, 2016, 10, 1711–1713 CrossRef CAS PubMed.
- A. Refaat, M. L. Yap, G. Pietersz, A. P. G. Walsh, J. Zeller, B. D. Rosal, X. Wang and K. Peter, In vivo fluorescence imaging: success in preclinical imaging paves the way for clinical applications, J. Nanobiotechnol., 2022, 20, 450 CrossRef PubMed.
- S. E. Boddington, T. D. Henning, P. Jha, C. R. Schlieve, L. Mandrussow, D. DeNardo, H. S. Bernstein, C. Ritner, D. Golovko, Y. Lu, S. Zhao and H. E. Daldrup-Link, Labeling human embryonic stem cell-derived cardiomyocytes with indocyanine green for noninvasive tracking with optical imaging: an FDA-compatible alternative to firefly luciferase, Cell Transplant, 2010, 19, 55–65 Search PubMed.
- T. H. Kim, Y. Chen, C. W. Mount, W. R. Gombotz, X. Li and S. H. Pun, Evaluation of temperature-sensitive, indocyanine green-encapsulating micelles for noninvasive near-infrared tumor imaging, Pharm. Res., 2010, 27, 1900–1913 CrossRef CAS.
- O. C. Farokhzad, J. Cheng, B. A. Teply, I. Sherifi, S. Jon, P. W. Kantoff, J. P. Richie and R. Langer, Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 6315–6320 CrossRef CAS.
- M. E. Werner, J. A. Copp, S. Karve, N. D. Cummings, R. Sukumar, C. Li, M. E. Napier, R. C. Chen, A. D. Cox and A. Z. Wang, Folate-targeted polymeric nanoparticle formulation of docetaxel is an effective molecularly targeted radiosensitizer with efficacy dependent on the timing of radiotherapy, ACS Nano, 2011, 5, 8990–8998 CrossRef CAS PubMed.
- Z. Liu, T. Lammers, J. Ehling, S. Fokong, J. Bornemann, F. Kiessling and J. Gätjens, Iron oxide nanoparticle-containing microbubble composites as contrast agents for MR and ultrasound dual-modality imaging, Biomaterials, 2011, 32, 6155–6163 CrossRef CAS PubMed.
- P. Cheng, S. Ming, W. Cao, J. Wu, Q. Tian, J. Zhu and W. Wei, Recent advances in sonodynamic therapy strategies for pancreatic cancer, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2024, 16, e1945 CAS.
- J. T. Robinson, S. M. Tabakman, Y. Liang, H. Wang, H. S. Casalongue, D. Vinh and H. Dai, Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy, J. Am. Chem. Soc., 2011, 133, 6825–6831 CrossRef CAS.
- S. Bhana, G. Lin, L. Wang, H. Starring, S. R. Mishra, G. Liu and X. Huang, Near-infrared-absorbing gold nanopopcorns with iron oxide cluster core for magnetically amplified photothermal and photodynamic cancer therapy, ACS Appl. Mater. Interfaces, 2015, 7, 11637–11647 CrossRef CAS.
- S. Shen, J. Qiu, D. Huo and Y. Xia, Nanomaterial-Enabled Photothermal Heating and Its Use for Cancer Therapy via Localized Hyperthermia, Small, 2024, 20, e2305426 CrossRef PubMed.
- A. W. Powell, A. Stavrinadis, S. Christodoulou, R. Quidant and G. Konstantatos, On-Demand Activation of Photochromic Nanoheaters for High Color Purity 3D Printing, Nano Lett., 2020, 20, 3485–3491 CrossRef CAS PubMed.
- Z. Wang, X. Zhen, P. K. Upputuri, Y. Jiang, J. Lau, M. Pramanik, K. Pu and B. Xing, Redox-Activatable and Acid-Enhanced Nanotheranostics for Second Near-Infrared Photoacoustic Tomography and Combined Photothermal Tumor Therapy, ACS Nano, 2019, 13, 5816–5825 CrossRef CAS PubMed.
- L. Zou, H. Wang, B. He, L. Zeng, T. Tan, H. Cao, X. He, Z. Zhang, S. Guo and Y. Li, Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics, Theranostics, 2016, 6, 762–772 CrossRef CAS PubMed.
- B. Zheng, H. B. Chen, P. Q. Zhao, H. Z. Pan, X. L. Wu, X. Q. Gong, H. J. Wang and J. Chang, Persistent Luminescent Nanocarrier as an Accurate Tracker in Vivo for Near Infrared-Remote Selectively Triggered Photothermal Therapy, ACS Appl. Mater. Interfaces, 2016, 8, 21603–21611 CrossRef CAS PubMed.
- H. Wang, W. Su, S. Wang, X. Wang, Z. Liao, C. Kang, L. Han, J. Chang, G. Wang and P. Pu, Smart multifunctional core-shell nanospheres with drug and gene co-loaded for enhancing the therapeutic effect in a rat intracranial tumor model, Nanoscale, 2012, 4, 6501–6508 RSC.
- B. Zheng, J. Wang, H. Pan, H. Chen, W. Ji, Z. Liao, X. Gong, H. Wang and J. Chang, A visual guide to gene/optothermal synergy therapy nanosystem using tungsten oxide, J. Colloid Interface Sci., 2017, 506, 460–470 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm00330f |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2024 |