Ultrafast and high-resolution X-ray imaging based on zero-dimensional organic silver halides†
Yongqiang
Zhou
a,
Zixian
Wang
a,
linfeng
Guo
a,
Lei
Huang
a,
Yichen
Liu
a,
Mengyue
Wu
a,
Qian
Zhang
a,
Kang
An
b,
Peng
He
b,
Fei
Wang
d,
Juan
Du
ef,
Zhengzheng
Liu
e,
Zhiping
Hu
f,
Yuxin
Leng
e,
Yayun
Pu
*a,
Jun’an
Lai
*b and
Xiaosheng
Tang
 *abc
*abc
aCollege of Optoelectronic Engineering, Chongqing University of Posts and Telecommunications, Chongqing, 400065, China. E-mail: puyy@cqupt.edu.cn
bKey Laboratory of Optoelectronic Technology & Systems (Ministry of Education), College of Optoelectronic Engineering, Chongqing University, Chongqing 400044, China. E-mail: ja.lai@cqu.edu.cn
cSchool of Materials Science and Engineering, Zhengzhou University, Zhengzhou 450001, China. E-mail: xstang@cqu.edu.cn
dCollege of Materials Science and Engineering, Sichuan University, Chengdu 610065, China
eState Key Laboratory of High Field Laser Physics and CAS Center for Excellence in Ultra-intense Laser Science, Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, ., 201800 Shanghai, China
fSchool of Physics and Optoelectronic Engineering, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 310024 Hangzhou, China
First published on 10th July 2024
Abstract
Supersensitive and fast X-ray imaging is of great importance in medical diagnosis, industrial flaw detection, security and safety inspection, and frontier science exploration. As the core of detection devices, new generation scintillators require small self-absorption capacity, short fluorescence lifetime, simple film-making protocol, excellent stability and non-toxicity. Herein, a new type of lead-free organic silver halide TPPAgX2 (TPP = C24H20P and X = I, Br, and Cl) is rationally developed with a large Stokes shift (176 nm) and ultralow photoluminescence decay (3.8 ns lifetime). It achieves an ultrafast fluorescent response that is the best among all the Pb-free perovskite scintillators. Temperature-dependent PL and DFT calculations together confirm that TPPAgCl2 follows an emission mechanism in which a triplet exciton can be rapidly upconverted via thermal activation. A series of TPPAgX2-based flexible scintillator films were fabricated and tested. A detection limit of 0.447 μGyair s−1 was obtained for TPPAgCl2, which is one order of magnitude lower than the medical X-ray diagnosis requirement. In addition, it exhibits a superior X-ray imaging resolution of 11.87 lp mm−1. The excellent performance and simple preparation methodology are expected to be potentially applicable for large-scale manufacturing.
1. Introduction
X-rays are highly energetic electromagnetic waves characterized by extremely short wavelengths and potent penetrating capabilities. They find extensive applications in medical diagnostics, industrial flaw detection, security and safety inspections, and non-destructive testing.1–7 In these applications, scintillators play an important role in converting high-energy X-rays into low-energy ultraviolet or visible light.8,9 Short life, high scintillation yield, low cost, scalable manufacturing, stability and non-toxicity are the main considerations for scintillators. Among these factors, a short life span is crucial for X-ray imaging.10–12 The response time of a scintillator to incident radiation particles directly determines the time resolution and response speed of X-ray imaging.13 For example, dynamic high-speed X-ray imaging requires a frame rate of 2 ns (GHz), with the scintillator's reaction time reaching the nanosecond level to achieve high-resolution images.14 In medicine-related applications, the impact of X-ray on the human body is mainly determined on the basis of irradiation dose and time. Only a short fluorescent life can reduce the damage caused by X-ray imaging.Recently, Wang et al. successfully fabricated the zero-dimensional organocopper halide composite scintillator (18-crown-6)2Na2(H2O)3Cu4I6(CNCI) with an ultrahigh optical yield of 109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV, but its fluorescence lifetime is as long as 1.98 μs.15 For MAPbI3 and MAPbBr3 hybrid lead halides, which exhibit a decay time of few nanoseconds,16,17 the luminous yield is less than 10
000 photons per MeV, but its fluorescence lifetime is as long as 1.98 μs.15 For MAPbI3 and MAPbBr3 hybrid lead halides, which exhibit a decay time of few nanoseconds,16,17 the luminous yield is less than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV at room temperature and is toxic. To reduce the lead content while preserving the perovskite structure, Pb2+ ions are often replaced by non-toxic and stable metal ions. In previous studies, Mn2+ ions were introduced into the lattice to replace the original Pb2+ ions, which reduced the content of toxic composition and improved the stability of the material.18 Inspired by this, manganese-based metal halides have been thoroughly synthesized and studied, with a focus on their optical properties.19 However, their long luminescence lifetime remains an unsolved problem.
000 photons per MeV at room temperature and is toxic. To reduce the lead content while preserving the perovskite structure, Pb2+ ions are often replaced by non-toxic and stable metal ions. In previous studies, Mn2+ ions were introduced into the lattice to replace the original Pb2+ ions, which reduced the content of toxic composition and improved the stability of the material.18 Inspired by this, manganese-based metal halides have been thoroughly synthesized and studied, with a focus on their optical properties.19 However, their long luminescence lifetime remains an unsolved problem.
According to Fermi's golden rule, excitonic radioluminescence can fundamentally provide fast and intense scintillation. For example, Shen et al. reported the successful synthesis of centimeter-scale BDAPbI4 (BDA = NH3C4H8NH3) single crystals. Their charge transport under X-ray was examined, and the shortest fluorescence lifetime to date (2.09 ns) was obtained.20 However, the presence of Pb2+ in this material greatly limits its practical application.
Therefore, it is particularly important to find scintillators with short decay lifetimes and no toxicity. Because the ability to decay X-rays is mainly determined by the effective atomic number of the material, lead-free silver halide with a higher atomic number and shorter decay time can be utilized as an excellent substitute for a short carrier decay time.21 In recent years, several polycrystalline silver halide powders and single crystals have been synthesized using expensive techniques,22,23 but their phase purity is low. Therefore, the development of high-quality silver halide scintillators with excellent performance is urgent and attractive.
In this paper, a series of nontoxic silver-based halide single crystals, TPPAgX2 (X = I, Br, Cl), with homogeneous size and morphology are systematically prepared using a simple antisolvent method. As-synthesized TPPAgI2 shows a Stokes shift of 84 nm and a short decay time of 105.8 ns, which can be further engineered and improved via the C-site regulation. Fluorescence lifetimes of 45.5 ns and 3.8 ns were therefore achieved on the TPPAgBr2 and TPPAgCl2, respectively. They also displayed Stokes shifts of 176 nm and 44 nm, respectively, without any self-absorption. Thermodynamically stable TPPAgCl2 exhibits thermally activated undelayed fluorescence emission, which is mainly derived from the triplet state of STEs under X-rays, ensuring a large Stokes shift and excellent X-ray imaging capability. In addition, homogeneous films with a lateral size of 16 cm2 and sub-millimeter in thickness were prepared by the mixture of TPPAgX2 particles, polydimethylsiloxane (PDMS) and coagulant. High-resolution static X-ray imaging resolution (11.87 lp mm−1) and detection limit (0.412 μGyair s−1) were consequently achieved on TPPAgCl2-based film. The newly developed X-ray imaging materials with extremely short fluorescence lifetime, non-toxicity, and large Stokes shift are expected to be promising alternatives for future commercial applications.
2. Experimental section
2.1 Chemical
Tetraphenylphosphonium iodide (C24H20PI), tetraphenylphosphonium bromide (C24H20PBr), tetraphenylphosphonium chloride (C24H20PCl), AgI, AgBr, and AgCl were all purchased from Aladdin Industries. Dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), tri-N-octylphosphine, polydimethylsiloxane (PDMS), and ether were all used as received without further purification.2.2 Synthesis of TPPAgX2 (X = I, Br, Cl) single crystals
The primary coordination model for Ag halides is the tetrahedral model. In the tetrahedral model, the halide ions are located around the silver ions, forming an orthotetrahedral structure. For charge balance in the compound system, the material ratio of AgI/Br/Cl to TPPI/Br/Cl should be controlled between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 because TPPI/Br/Cl, which is protonated, is only positively charged. To satisfy the coordination determination of metal Ag as much as possible, we finalized the ratio of AgI/Br/Cl to TPPI/Br/Cl to be 1
2 because TPPI/Br/Cl, which is protonated, is only positively charged. To satisfy the coordination determination of metal Ag as much as possible, we finalized the ratio of AgI/Br/Cl to TPPI/Br/Cl to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Specifically, the antisolvent method was used to synthesize TPPAgX2 single crystals. 1 mmol TPPI (466.29 mg) and 1 mmol AgI (234.77 mg) were added in a clean bottle, with 2 ml of DMF and 0.5 ml of DMSO. The sealed bottle was set on a heating table at 60 °C, stirring until the chemicals were completely dissolved and a transparent liquid was obtained (about 30 minutes). After filtering by applying a syringe, the solution was put into another clean bottle and set inside a closed jar with an ether counter-solvent. Single crystals were obtained while standing for at least 48 h.
1. Specifically, the antisolvent method was used to synthesize TPPAgX2 single crystals. 1 mmol TPPI (466.29 mg) and 1 mmol AgI (234.77 mg) were added in a clean bottle, with 2 ml of DMF and 0.5 ml of DMSO. The sealed bottle was set on a heating table at 60 °C, stirring until the chemicals were completely dissolved and a transparent liquid was obtained (about 30 minutes). After filtering by applying a syringe, the solution was put into another clean bottle and set inside a closed jar with an ether counter-solvent. Single crystals were obtained while standing for at least 48 h.
2.3 Synthesis of TPPAgX2 (X = I, Br, Cl) – PDMS films
As-synthesized TPPAgX2 (X = I, Br, Cl) crystals were ground by agate mortar to reduce their grain size and obtain a uniform size distribution. PDMS was prepared by mixing the prepolymer and curing agent in a volume ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. To synthesize TPPAgX2 (X = I, Br, Cl) – PDMS thin films, approximately 454 mg of TPPAgX2 (X = I, Br, Cl) single crystal powder was slowly mixed with 4 ml of PDMS (1.058 g ml−1) under ultrasound and then stirred for 5 minutes to obtain a uniformly mixed solution. The mixed solution was centrifuged at a speed of 800/min for 3 minutes. TPPAgX2 (X = I, Br, Cl) – PDMS thin films were prepared by spin coating and cured at 60–80 °C for 1 hour. The speed of spin coating can control the thickness of the film in the range of 50–500 μm.
1. To synthesize TPPAgX2 (X = I, Br, Cl) – PDMS thin films, approximately 454 mg of TPPAgX2 (X = I, Br, Cl) single crystal powder was slowly mixed with 4 ml of PDMS (1.058 g ml−1) under ultrasound and then stirred for 5 minutes to obtain a uniformly mixed solution. The mixed solution was centrifuged at a speed of 800/min for 3 minutes. TPPAgX2 (X = I, Br, Cl) – PDMS thin films were prepared by spin coating and cured at 60–80 °C for 1 hour. The speed of spin coating can control the thickness of the film in the range of 50–500 μm.
2.4 Physical characterization
Powder X-ray diffraction (PXRD) measurements were performed using a PANalytical X’Pert Powder diffractometer. During the measurement, Bragg's diffraction angle (2°) range was set to 10°–80° and the scanning time was 20 minutes. Scanning electron microscopy (SEM) images were taken using Quattro S. Cold FE-SEM, Hitachi High-Technologies with an acceleration voltage of 10 KV, and EDX were used to characterize the elements of the doping samples. Pure TPPAgX2 (X = I, Br, Cl) was scanned by applying a ZEISS GeminiSEM 300 field emission scanning electron microscope, and all elements were characterized by energy dispersive spectroscopy (EDS). The UV-Vis transmission spectra and absorption spectra were recorded using a UV-3600 spectrophotometer (Shimadzu, Japan) in the wavelength range of 200–800 nm. The decay curves were recorded by applying a time-resolved fluorescence spectrophotometer (FLS1000, Edinburgh Instrument Ltd., Edinburgh, UK). The photoluminescence (PL) spectra were measured using a 450 W Xe lamp for ozone removal as the excitation source and an FLS1000 spectrophotometer (Edinburgh Instrument Ltd., Edinburgh, UK). The thermogravimetric analysis of crystals was performed by TGA/DSC1/1600LF (Mettler Toledo, Switzerland) at a heating rate of 20 °C min−1 for up to 800 °C. The DFT calculation was performed using the Cambridge sequential total energy package (CASTEP) module.24 The generalized gradient approximation with the Burke-Ernzerhof potential was used for periodic solids. The cutoff energy was set as 400 eV, and the Brillouin zone used a 3 × 3 × 3 Monkhorst–Pack grid. Spin-polarized calculations within the DFT+U framework have been applied for Ag ions to correct the well-known DFT self-interaction errors.25,26 For the structural optimization, the maximum force limit, maximum energy variation tolerance and maximum displacement were set as 0.03 eV Å−1, 10−5 eV per atom and 0.002 Å, respectively. The self-consistent field (SCF) tolerance was set as 2 × 10−6 eV per atom. Excitation energy diagrams were performed using the B3LYP hybrid function implemented in the Gaussian-16 suite of program.27 The time-dependent density functional theory (TDDFT) calculations were performed with PBE0-D3(BJ)/def2-SVP level of theory on the geometries obtained from XRD experiments.28 An effective core potential associated with the def2-SVP basis set was used on the Ag atoms. This functional was shown to perform well for excited states29 and spin–orbit coupling (SOC) calculations.30 The above calculations were carried out using Gaussian 16 programs. The SOC matrix elements were calculated by applying ORCA 5.0.3 software based on the Douglas–Kroll–Hess 2nd order (DKH2) scalar relativistic method. The PBE0 functional and SARC/J auxiliary basis were used. Relativistically recontracted basis set DKH-def2-SVP was used for C, H and P atoms.31 For metal atoms, segmented all-electron relativistically contracted (SARC) DKH-TZVP was used for Ag.32 The radioluminescence (RL) spectrum was characterized using a Maya2000 Pro spectrometer. X-ray radiation for detection and imaging was produced by applying an X-ray source (HAMAMATSU, L10321). The type of charge-coupled device (CCD) chip used for detection and imaging was KAF-16803.3. Results and discussion
The TPPAgX2 (X = I, Br, Cl) single crystals (Fig. 1a) were synthesized by applying an anti-solvent method.33–35 All the structures were analyzed and confirmed by powder X-ray diffraction (PXRD) measurements (Fig. 1b) and single crystal X-ray diffraction (Fig. 1c–e). It is clear that the results are in perfect agreement, confirming the successful synthesis of TPPAgX2 single crystals. Detailed information is summarized in Tables S2–S4 (ESI†).Scanning electron microscope (SEM) graphs show that TPPAgI2 is a blocky-like solid, while TPPAgBr2/Cl2 is in the form of a small rod-like crystal (Fig. 1f–h and Fig. S1, ESI†). The dimensions of TPPAgX2 (X = I, Br, Cl) are confirmed as 1.25 mm, 0.75 mm, and 0.05 mm, respectively. Energy dispersive spectroscopy (EDS) mapping (Fig. S1 and Table S1, ESI†) shows that all the elements C, P, Ag, and I/Br/Cl are uniformly distributed. The detected elemental contents of C, P, Ag, and I in TPPAgX2 approached the stoichiometric ratios well. X-ray photoelectron spectroscopy (XPS) was used to further determine the composition and valence. By analyzing the integral area of the high-resolution spectra of C 1s, P 2p, Ag 3d, I 3d, Br 3d, and Cl 2p, the compositions were verified as TPP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag
Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X = 1
X = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. S2a–S4a, ESI†). Two distinct peaks at 368 and 374 eV were found on the Ag 3d spectra, proving the presence of Ag+ (Fig. S2bc–S4bc, ESI†).
2 (Fig. S2a–S4a, ESI†). Two distinct peaks at 368 and 374 eV were found on the Ag 3d spectra, proving the presence of Ag+ (Fig. S2bc–S4bc, ESI†).
The thermal stability of TPPAgX2 crystals was investigated by TG-DSC measurements (Fig. S5, ESI†). These materials show fairly excellent stability from room temperature to 200 °C. The first heat absorption occurs at 200 °C without significant loss of weight until the temperature rises up to 450 °C. The sharp weight loss occurs at 450–550 °C, concurrently accompanied by significant heat absorption. Higher than this temperature, the crystal is considered to be decomposed.
According to previous reports, various kinds of organic molecules can emit light when excited by ultraviolet light.36 Light emission largely originates from the electron transitions, after which an inherent loss channel is formed during the luminescence.10,37 Specifically, excited high-energy electrons and holes at the Lowest Unoccupied Molecular Orbital (LUMO) orbitals are expected to be in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for singlet and triplet states.38–41 Triplet excitons with 75% proportion follow the rule of phosphorescence at room temperature. To verify whether our materials exhibit phosphorescence behavior, PL, PLE and fluorescence lifetimes were tested for TPPI, TPPBr, TPPCl feedstock and TPPAgX2 crystals, respectively (Fig. 2a–d and Fig. S6, ESI†). The PL and PLE test results of TPPAgBr2 and TPPAgCl2 are similar.42
3 for singlet and triplet states.38–41 Triplet excitons with 75% proportion follow the rule of phosphorescence at room temperature. To verify whether our materials exhibit phosphorescence behavior, PL, PLE and fluorescence lifetimes were tested for TPPI, TPPBr, TPPCl feedstock and TPPAgX2 crystals, respectively (Fig. 2a–d and Fig. S6, ESI†). The PL and PLE test results of TPPAgBr2 and TPPAgCl2 are similar.42
From Fig. 2a and Fig. S6a (ESI†), the PL and PLE waveforms of pure organic TPPI/Br/Cl and TPPAgX2 are very similar with certain differences. Compared to the original organic emission, the hybrid Ag-halogen units with TPP molecules cause enhanced luminescence intensity of TPPAgBr/Cl without any shift, indicating that the luminescence behavior changes from pure molecules to the cooperation of AgX2 and molecules. As the size of the halogen atom increases, the luminescence center of TPPAgI2 changes from 429 nm to 462 nm, suggesting that the luminescence manner depends on halogen element regulation.
The fluorescence lifetimes of TPPI/Br/Cl and TPPAgX2 were compared in Fig. 2d (Fig. S6b, d, and f, ESI†). The fluorescence lifetime of TPPI reaches a microsecond level (0.62 μs) due to the emission inhibition of triplet excitons, which is far from being used for quick detection. The addition of Ag provides a new avenue for TPPI/Br/Cl to convert the triplet excitons efficiently. The triplet excitations can up-convert to singlet states through thermally activated reverse intersystem crossing (RISC) and ultimately take advantage of the fluorescence decay channel to rapidly emit photons.43 Fig. S7 (ESI†) shows the PLQY that the light yield of TPPI/Br/Cl is slightly higher than that of TPPAgX2. The addition of Ag improves the luminescence of organic molecules and greatly shortens the fluorescence lifetime.
From their PL and PLE, the Stokes shift can be calculated as 84 nm, 176 nm, and 44 nm for TPPAgI2/Br2/Cl2 cases. Minimized self-absorption is generally associated with a large Stokes shift. TPPAgBr2 thus possesses the largest Stokes shift with the corresponding smallest self-absorption. Because zero self-absorption is highly desirable for scintillators, especially for thick films, our materials exhibit considerable potential for scintillator device fabrication.
According to PL decay and the single exponential function line (Fig. 2d), the good fitting of the curves gives an accurate calculation of fluorescence time. The formulas for double exponential fitting and average life calculation are as follows:
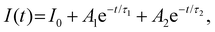 | (1) |
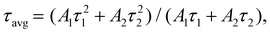 | (2) |
The fluorescence lifetime can be calculated as 105.8 ns for TPPAgI2, 45.5 ns for TPPAgBr2 and 3.8 ns for TPPAgCl2. The prominent value of 3.8 ns outperforms those of well-known materials, such as Gd2O2S:Tb (600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ns),44 BGO (300 ns),45 CsI:Tl (1000 ns),46 and CsPbBr3QDs (45 ns).47 To further compare the lifespan of our results with that of the literature, a series of data are presented in Table 1.
000 ns),44 BGO (300 ns),45 CsI:Tl (1000 ns),46 and CsPbBr3QDs (45 ns).47 To further compare the lifespan of our results with that of the literature, a series of data are presented in Table 1.
| Materials | PL decay | Ref. |
|---|---|---|
| TPPAgI 2 | 105.8 ns | This Work |
| TPPAgBr 2 | 45.5 ns | This Work |
| TPPAgCl 2 | 3.8 ns | This Work |
| Cs2Ag0.6Na0.4In0.85Bi0.15Cl6 | 16.0 μs | 48 |
| (DBA)4Cu4I4 | 20.3 μs | 49 |
| Cs2AgI3:Cu (5%) | 192.8 ns | 50 |
| Cs2AgBiBr6 | 1.5 μs | 51 |
| Rb2AgBr3 | 5.3 ns | 52 |
| (ETP)2MnBr4 | 295.0 μs | 53 |
| Cs2AgBiCl6 | 4.4 ns | 54 |
| Cs2AgInCl6:Mn (0.5%) | 0.6 ms | 55 |
| Cs2AgSbCl6:4%OA | 7.9 ns | 56 |
| SC-Ag (Ag6S6L6) | 15.5 μs | 57 |
| (DIET)3Cu3Br3 | 0.6 μs | 58 |
| Cu2AgBiI6 | 20.0 ns | 59 |
The UV-Vis absorption spectra were tested in the range of 250–800 nm (Fig. 2e), and TPPAgX2 started to absorb light in the region approaching the ultraviolet light, with bands at 410 nm, 320 nm, and 330 nm. According to Formula S1 in the ESI,†n = 1/2 is used to estimate the direct band gap of TPPAgX2. Fig. 2f shows that the band gap estimations of TPPAgX2 from the Tauc plots are 2.85 eV, 3.36 eV, and 3.61 eV, respectively. To enhance our understanding of the photoluminescent mechanism of TPPAgX2, the density of states (DOS), and band structures were calculated using periodic density functional theory (DFT).60,61 DFT calculations indicate (Fig. 2g–i) that the band gaps of TPPAgX2 (X = I, Br, Cl) are 2.457 eV, 3.017 eV, and 3.248 eV, respectively. Because the DFT generally underestimates the band gap of semiconductors, the calculated trends are basically consistent with the experimental results. The projected density of states (PDOS) of TPPAgX2 was also calculated, as shown in Fig. S8 (ESI†). The valence band maximum (VBM) is mainly contributed from I/Br/Cl p orbitals, while the conduction band minimum (CBM) is mainly associated with the orbitals of carbon. This suggests that the excitation is from AgX2 unit to TPP molecules, following a manner of inorganic–organic hybrid radioluminescence, which is in agreement with the PL results.
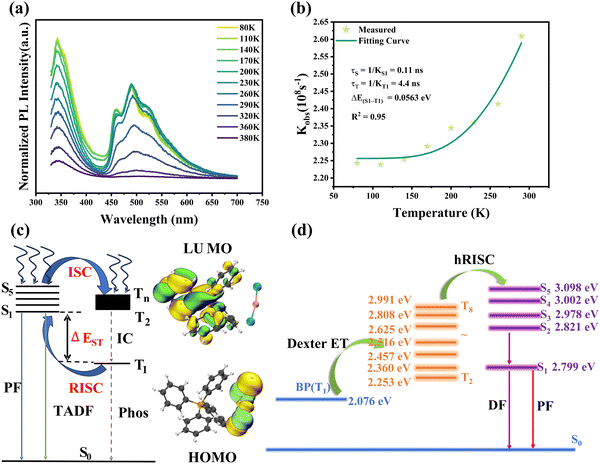
To better understand the relationship between excitation and emission of TPPAgX2 crystals, temperature-dependent PL spectra were measured in the range of 80–290(380) K (with an interval of 30 K). As shown in Fig. 3a, the most prominent broad emission band does not split into multiple peaks along the temperature drop, implying that it follows a single radiation mechanism rather than a multiple radiation mechanism. The major PL emission shows a monotonically decreasing behavior associated with the temperature-dependent thermal expansion interaction and electron–phonon interaction (Fig. 3d). The intensity of PL emission gradually increases from 80 K to 170 K and then slowly decreases until the temperature reaches 320 K. Higher PL emission intensity at lower temperatures is opposite to the normal thermal burst PL behavior.62 Similar phenomena can also be found in the literature of (C9NH20)2SnBr463 and (H2O)(C6H8N3)2Pb2Br10.64 To investigate the luminescence mechanism, TPPAgI2 was excited under the 305–445 nm irradiation (Fig. S9a, ESI†). Luminescence spectra centered at 462 nm come from the same excited state relaxation. Only one emission peak corresponded to the luminescence mechanism proposed in Fig. S10a (ESI†).
The temperature-dependent PL decay of 462 nm emission between 290 K and 80 K was monitored to study the radiation kinetics. Throughout the entire temperature range, the PL decay curve exhibits the same single exponential decay kinetics with almost no changes (Fig. S12a, ESI†).
Under UV excitation, photoinduced carriers relax simultaneously to self-trapped exciton (STE) via intersystem crossing (ISC) and are located in shallow trap states. At higher temperatures, thermal activation induces the carriers in the shallow trap state to return to the self-trapped state, which increases the carrier concentration and improves the emission efficiency. However, at lower temperatures, as the thermal energy is not sufficient to trap the carriers in the self-captured state, the carrier concentration of STE decreases. Consequently, we observe a decrease in the emission intensity. The capture process between shallow capture and excited states only affects the carrier concentration on the STE states rather than changing (Fig. S6, ESI†).65
E a gained by the Arrhenius formula (Formula S2, ESI†) is 462 MeV (Fig. S11a, ESI†), which is much higher than the room-temperature thermal ionization energy (∼26 MeV),66 (DADPA)In0.83Sb0.17Cl6H2O (44.42 MeV),67 indicating that the structural phase transition of TPPAgI2 crystal MCs is difficult to occur. To evaluate the deformability of the 0D structure and electron–phonon coupling strength, we calculate the Huang–Rhys factor (S) based on Formula S3 (ESI†). The calculated ħωphonon is 49.39 MeV. The extracted S factor of 11.34 is larger than those of traditional semiconducting materials, such as ZnSe (0.3)68 and CdSe (1.0).69
From Fig. 3b and c, the main emission signal of TPPAgBr2 and TPPAgCl2 did not split into multiple peaks, but the band width was sharply shrunk compared to TPPAgI2. When the temperature decreases from 380 K to 230 K (Fig. 3e and f), the PL emission intensity gradually increases. Excitation light with 270–370 nm for TPPAgBr2, and 265–375 nm for TPPAgCl2 were used to obtain the emission spectra of (290–700) nm and (285–700) nm, respectively. Fig. S9b and c (ESI†) shows the emission spectra centralized in a single peak and maintained the same shape. This indicates that the emission at 492 nm (TPPAgBr2) and 356/495 nm (TPPAgCl2) comes from excited state relaxation. The emission peaks around 466 nm and 492 nm (Fig. 2b) were observed for TPPAgBr2 and 356/467/495 nm (Fig. 2c) for TPPAgCl2 at 80–380 K, which correspondingly yielded the luminescence pathway (Fig. S10b and c). The temperature-dependent PL decay at 470 nm for TPPAgBr2 and TPPAgCl2 was further monitored between 290 K and 80 K to observe the radiation kinetics (Fig. S12b and c, ESI†). The curves exhibit the same single-exponential decay kinetics throughout the entire temperature range. Using formula (4–5), the activation energies of TPPAgBr2 and TPPAgCl2 can be determined as 244 MeV and 401 MeV, respectively (Fig. S11b and c, ESI†). S factors for TPPAgBr2 and TPPAgCl2 are 10.38 and 8.9, respectively. The phonons obtained through the phonon broadening model for TPPAgBr2 and TPPAgCl2 are 85.73 MeV and 64.65 MeV, respectively (Fig. 3e and f, ESI†). All these data are summarized in Table 2.
| Materials | E a (MeV) | ℏωphonon (MeV) | S |
|---|---|---|---|
| TPPAgI2 | 462 | 61.86 | 11.34 |
| TPPAgBr2 | 244 | 61.86 | 10.38 |
| TPPAgCl2 | 401 | 61.86 | 8.90 |
In addition, abnormal temperature-dependent PL luminescence was found on TPPAgCl2. The luminous intensity shows a positive relation with the temperature, while a slight blue-shift occurs along the temperature decreases (Fig. 4a). When the temperature is higher than 230 K, the PL intensity quickly decreases due to the influence of hot quenching. To understand this emission behavior in-depth, the relationship between lifetime and temperature was drawn (Fig. 4b). The lifetime of TPPAgCl2 exhibits a reverse relation with temperature variation. This is consistent with the delayed fluorescence mechanism proposed by Yersin.70,71 This phenomenon is mainly due to the reverse intersystem crossing (RISC) caused by the thermal activation transition from the long-lived lowest excited triplet (T1) to the short-lived lowest singlet (S1), as demonstrated in Fig. 4c. Because TPPAgCl2 freezes primarily in a triplet at low temperatures, for example, 40 K, the emission state can be assigned as a pure triplet. As the temperature increases, the singlet is filled with a continuous RISC process from the triplet, which makes the emission of the high-energy singlet possible, resulting in an overall reduction in the PL decay time. In addition, because the energy of the S1 state is higher than the T1 state, the emission spectrum shows a blue shift at 515–491 nm. The above results show that the emission of TPP comes from thermal activation delayed fluorescence (TADF), and the emission model can well fit the functional relationship between PL lifetime and temperature:72,73
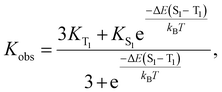 | (3) |
To verify the heat exciton trapping mechanism, the geometric and electronic structures of TPPAgCl2 were calculated (Fig. 4d). The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) cover almost the entire molecular skeleton. The calculated HOMO frontier orbitals of TPPAgCl2 are mainly distributed over the AgCl2 core, while the LUMO orbitals mainly originated from the TPP component, which is perfectly consistent with the DOS calculation (Fig. 4c and Fig. S8c, ESI†). The energy of the singlet and triplet states is calculated by applying the TD-DFT method based on the tam-dancoff approximation. The singlet states are listed as S1 (2.799 eV), S2 (2.821 eV), S3 (2.978 eV), S4 (3.002 eV) and S5 (3.098 eV), respectively. The triplet levels include T1 (2.076 eV), T2 (2.253 eV), T3 (2.360 eV), T4 (2.457 eV), T5 (2.516 eV), T6 (2.625 eV), T7 (2.808 eV) and T8 (2.991 eV).
The energy gap between T8 and T1 is as large as 0.915 eV, while it is much smaller between S5 and (T8–T5) because the lowest one is merely 0.107 eV. Therefore, TPPAgCl2 has a large Tn–T1 and a small Tn–Sm energy gap, which is necessary to inhibit the internal transformation of Tn to T1 and accelerate the RISC of Tn to Sm.74–76 It is worth noting that the experimental ΔEST fitting value (0.056 eV) (Fig. 4b) of TPPAgCl2 is significantly different compared with the theoretical calculated value (0.722 eV) (Fig. 4d), which may be related to the large spectral blue shift caused by the contraction of the distance between molecules with increasing temperature.57 The fitting curve (Fig. 4b) of TPPAgCl2 research to a platform below 170 K temperature indicates that excitons are mainly frozen in the T1 state. As temperature increases, the upper S1 populations participate in emission through the RISC pathway with a shorter life span. The nature of the excited state of luminescent materials under high-energy X-ray radiation is fundamentally different from those under low-energy photoexcitation, as shown in Fig. S13 (ESI†).36 The LUMO and LUMO of TPPCl were calculated for comparison. Fig. S14 (ESI†) shows that the difference between the HOMO and LUMO of TPPAgCl2 is smaller than that of TPPCl. It is generally known that the exchange interaction between HOMO and LUMO is directly proportional to the ΔEST.43 Smaller ΔEST means much easier electron excitation from HOMO to LUMO, indicating that the introduction of Ag guides all the thermal excitons towards the fast singlet state.
The ability to convert X-rays into visible light is key to scintillator materials in X-ray imaging.77–79 High-quality images with high repetition rates and no hysteresis are essentially important.80 Using the method described in the experimental section, a flexible film of TPPAgX2 and PDMS was prepared, with a thickness of 0.55 mm and an area of 16 cm2. The flexibility and fluorescence were tested under UV irradiation (Fig. S15 and S16, ESI†). Three TPPAgX2 films further examined the imaging capability under 70 KV/500 μA X-ray irradiation. The radioluminescence spectra exhibit almost the same profile as the PL spectra, indicating the same radiative complexation manner (Fig. 5b). To evaluate the light yield, commercial scintillator BGO with a light yield of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV was used as a standard reference. According to the relative method, the light yield can be determined by the following equation:81
000 photons per MeV was used as a standard reference. According to the relative method, the light yield can be determined by the following equation:81
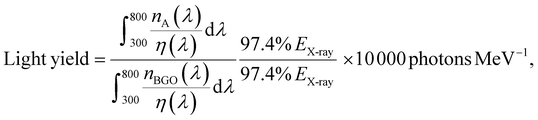 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons per MeV is the light yield of BGO (A represents TPPAgX2). The light yields are estimated to be 25
000 photons per MeV is the light yield of BGO (A represents TPPAgX2). The light yields are estimated to be 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 photons per MeV for TPPAgI2, 17
600 photons per MeV for TPPAgI2, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 photons per MeV for TPPAgBr2, and 21
420 photons per MeV for TPPAgBr2, and 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 photons per MeV for TPPAgCl2.
700 photons per MeV for TPPAgCl2.
As a proof of concept, a dedicated system consisting of an X-ray tube, imaging object, reflector, and a commercial digital camera was set up for the X-ray imaging study (Fig. 5a). The USB header and 74LS161 counter chip were selected as objects to examine the imaging ability of the TPPAgX2 scintillator. Under X-ray irradiation, four wires inside the USB header and the wiring configuration inside the chip could be clearly identified (Fig. 5f). Under natural light, it is impossible to distinguish an internal structure. Due to the different X-ray transmittances of metal and plastic, X-rays can finely reveal internal structures. When the signal-to-noise ratio is 3, the detection limit can be derived from the slope of the fitted line (Fig. 5c). We calculated the detection limits of 0.447 μGyair s−1 for TPPAgI2, 0.491 μGyair s−1 for TPPAgBr2, and 0.412 μGyair s−1 for TPPAgCl2, which are much lower than the dose rate required for standard medical X-ray diagnosis (5.500 μGyair s−1).82 To evaluate the spatial resolution of X-ray imaging of the best-performed TPPAgCl2, the resolution was evaluated using a standard card (Fig. S17, ESI†). It ended up with a resolution of 11.87 lp mm−1 (Fig. 5d). It is superior to other metal halide scintillators such as CsCu2I3 (7.5 lp mm−1), BA2PbBr4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10%Mn (10.7 lp mm−1), Cs2Ag0.6Na0.4In0.85Bi0.15Cl6 (4.3 lp mm−1), and (C8H20N)2MnBr4 (4.9 lp mm−1) (Fig. 5e).48,81,83–85 In summary, these solid experiment results support the hypothesis and potential of TPPAgX2 (X = I, Br, Cl) for X-ray imaging application.
10%Mn (10.7 lp mm−1), Cs2Ag0.6Na0.4In0.85Bi0.15Cl6 (4.3 lp mm−1), and (C8H20N)2MnBr4 (4.9 lp mm−1) (Fig. 5e).48,81,83–85 In summary, these solid experiment results support the hypothesis and potential of TPPAgX2 (X = I, Br, Cl) for X-ray imaging application.
4. Conclusion
In summary, non-toxicity TPPAgX2 (X = I, Br, Cl) scintillators with extremely low fluorescence lifetime, zero self-absorption, excellent optical yield and thermal stability are rationally designed and fabricated. The fluorescence lifetime of TPPAgCl2 is only 3.8 ns, which is the best among all the Pb-free perovskite scintillators. Together with temperature-related PL and DFT calculations, it was confirmed that TPPAgCl2 follows the emission mechanism of ultrafast up-conversion of triplet exciton upon thermal activation. Flexible TPPAgX2-PDMS films were carefully prepared and tested, and the best TPPAgCl2 exhibited considerably high resolution (11.87 lp mm−1), low detection limit (0.412 μGyair s−1) and good radiation stability to the X-ray imaging. Using the low-cost and rapid synthesis method, these flexible films can be extended to the large-scale mass production of scintillators. This work is expected to provide alternative materials for next-generation X-ray detection and imaging.Data availability
Research data are not shared.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (62375032), Natural Science Foundation of Chongqing (No. CSTB2023TIAD-KPX0017, CSTB2022NSCQ-MSX0360), China Postdoctoral Science Foundation (Grant No. BX20230355). Sponsored by Natural Science Foundation of Chongqing, China, cstc2019jcyj-msxmX0737; National Natural Science Foundation of China under Grant 52302059.References
- R. Zhuang, X. Wang, W. Ma, Y. Wu, X. Chen, L. Tang, H. Zhu, J. Liu, L. Wu, W. Zhou, X. Liu and Y. Yang, Highly sensitive X-ray detector made of layered perovskite-like (NH4)3Bi2I9 single crystal with anisotropic response, Nat. Photonics, 2019, 13, 602–608 CrossRef CAS.
- Y. C. Kim, K. H. Kim, D.-Y. Son, D.-N. Jeong, J.-Y. Seo, Y. S. Choi, I. T. Han, S. Y. Lee and N.-G. Park, Printable organometallic perovskite enables large-area, low-dose X-ray imaging, Nature, 2017, 550, 87–91 CrossRef CAS.
- B. Q. Wang, X. Yang, S. Chen, S. R. Lu, S. Y. Zhao, Q. K. Qian, W. S. Cai, S. H. Wang and Z. G. Zang, Flexible perovskite scintillators and detectors for X-ray detection, iScience, 2022, 25, 26 Search PubMed.
- J. X. Wang, X. J. Wang, J. Yin, L. Gutierrez-Arzaluz, T. Y. He, C. L. Chen, Y. Han, Y. H. Zhang, O. M. Bakr, M. Eddaoudi and O. F. Mohammed, Perovskite-Nanosheet Sensitizer for Highly Efficient Organic X-ray Imaging Scintillator, ACS Energy Lett., 2022, 7, 10–16 CrossRef CAS.
- L. Clinckemalie, D. Valli, M. B. J. Roeffaers, J. Hofkens, B. Pradhan and E. Debroye, Challenges and Opportunities for CsPbBr3 Perovskites in Low- and High-Energy Radiation Detection, ACS Energy Lett., 2021, 6, 1290–1314 CrossRef CAS.
- F. Cao, D. Yu, W. Ma, X. Xu, B. Cai, Y. M. Yang, S. Liu, L. He, Y. Ke, S. Lan, K.-L. Choy and H. Zeng, Shining Emitter in a Stable Host: Design of Halide Perovskite Scintillators for X-ray Imaging from Commercial Concept, ACS Nano, 2020, 14, 5183–5193 CrossRef CAS PubMed.
- J. H. Heo, J. K. Park, Y. Yang, D. S. Lee and S. H. Im, Self-powered flexible all-perovskite X-ray detectors with high sensitivity and fast response, iScience, 2021, 24, 102927 CrossRef CAS PubMed.
- C. Greskovich and S. Duclos, Ceramic scintillators, Annu. Rev. Mater. Sci., 1997, 27, 69–88 CrossRef CAS.
- P. Buechele, M. Richter, S. F. Tedde, G. J. Matt, G. N. Ankah, R. Fischer, M. Biele, W. Metzger, S. Lilliu, O. Bikondoa, J. E. Macdonald, C. J. Brabec, T. Kraus, U. Lemmer and O. Schmidt, X-ray imaging with scintillator-sensitized hybrid organic photodetectors, Nat. Photonics, 2015, 9, 843–848 CrossRef CAS.
- X. Wang, H. F. Shi, H. L. Ma, W. P. Ye, L. L. Song, J. Zan, X. K. Yao, X. Y. Ou, G. H. Yang, Z. Zhao, M. Singh, C. Y. Lin, H. Wang, W. Y. Jia, Q. Wang, J. H. Zhi, C. M. Dong, X. Y. Jiang, Y. G. Tang, X. J. Xie, Y. Yang, J. P. Wang, Q. S. Chen, Y. Wang, H. H. Yang, G. Q. Zhang, Z. F. An, X. G. Liu and W. Huang, Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence, Nat. Photonics, 2021, 15, 187–192 CrossRef CAS.
- X. Meng, S. J. Ji, Q. J. Wang, X. C. Wang, T. X. Bai, R. L. Zhang, B. Yang, Y. M. Li, Z. P. Shao, J. K. Jiang, K. L. Han and F. Liu, Organic-Inorganic Hybrid Cuprous-Based Metal Halides for Warm White Light-Emitting Diodes, Adv. Sci., 2022, 9, 10 Search PubMed.
- J. Perego, I. Villa, A. Pedrini, E. C. Padovani, R. Crapanzano, A. Vedda, C. Dujardin, C. X. Bezuidenhout, S. Bracco, P. E. Sozzani, A. Comotti, L. Gironi, M. Beretta, M. Salomoni, N. Kratochwil, S. Gundacker, E. Auffray, F. Meinardi and A. Monguzzi, Composite fast scintillators based on high-Z fluorescent metal–organic framework nanocrystals, Nat. Photonics, 2021, 15, 393–400 CrossRef CAS.
- H. Jiang, Q. He, X. Li, X. Su, Y. Zhang, S. Chen, S. Zhang, G. Zhang, J. Jiang, Y. Luo, P. M. Ajayan and L. Song, Tracking Structural Self-Reconstruction and Identifying True Active Sites toward Cobalt Oxychloride Precatalyst of Oxygen Evolution Reaction, Adv. Mater., 2019, 31, 1805127 CrossRef.
- C. Hu, L. Y. Zhang, R. Y. Zhu, M. Demarteau, R. Wagner, L. Xia, J. Q. Xie, X. Li, Z. H. Wang, Y. H. Shih and T. Smith, Ultrafast inorganic scintillator-based front imager for Gigahertz Hard X-ray imaging, Nucl. Instrum. Methods Phys. Res. Sect. A-Accel. Spectrom. Dect. Assoc. Equip., 2019, 940, 223–229 CrossRef CAS.
- H. Wang, J.-X. Wang, X. Song, T. He, Y. Zhou, O. Shekhah, L. Gutiérrez-Arzaluz, M. Bayindir, M. Eddaoudi, O. M. Bakr and O. F. Mohammed, Copper Organometallic Iodide Arrays for Efficient X-ray Imaging Scintillators, ACS Cent. Sci., 2023, 9, 668–674 CrossRef CAS PubMed.
- Y. Liu, Y. Zhang, X. Zhu, J. Feng, I. Spanopoulos, W. Ke, Y. He, X. Ren, Z. Yang, F. Xiao, K. Zhao, M. Kanatzidis and S. Liu, Triple-Cation and Mixed-Halide Perovskite Single Crystal for High-Performance X-ray Imaging, Adv. Mater., 2021, 33, 2006010 CrossRef CAS.
- J.-H. Wei, X.-D. Wang, J.-F. Liao and D.-B. Kuang, High Photoluminescence Quantum Yield (> 95%) of MAPbBr3 Nanocrystals via Reprecipitation from Methylamine-MAPbBr3 Liquid, ACS Appl. Electron. Mater., 2020, 2, 2707–2715 CrossRef CAS.
- S. H. Zou, Y. S. Liu, J. H. Li, C. P. Liu, R. Feng, F. L. Jiang, Y. X. Li, J. Z. Song, H. B. Zeng, M. C. Hong and X. Y. Chen, Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes, J. Am. Chem. Soc., 2017, 139, 11443–11450 CrossRef CAS PubMed.
- L. Q. Guan, S. Shi, X. W. Niu, S. C. Guo, J. Zhao, T. M. Ji, H. Dong, F. Y. Jia, J. W. Xiao, L. D. Sun and C. H. Yan, All-Inorganic Manganese-Based CsMnCl3 Nanocrystals for X-Ray Imaging, Adv. Sci., 2022, 9, 2201354 CrossRef CAS PubMed.
- L.-Z. Qiu, S.-Y. Wei, H.-S. Xu, Z.-X. Zhang, Z.-Y. Guo, X.-G. Chen, S.-Y. Liu, D. Wu and L.-B. Luo, Ultrathin Polymer Nanofibrils for Solar-Blind Deep Ultraviolet Light Photodetectors Application, Nano Lett., 2020, 20, 644–651 CrossRef CAS PubMed.
- Z. Zhang, R. Zhao, S. Teng, K. Huang, L. Zhang, D. Wang, W. Yang, R. Xie and N. Pradhan, Color Tunable Self-Trapped Emissions from Lead-Free All Inorganic IA-IB Bimetallic Halides Cs-Ag-X (X = Cl, Br, I), Small, 2020, 16, 2004272 CrossRef CAS.
- J.-L. Yao, Z.-X. Zhang, X.-Q. Sun, T. Chang, J.-F. Guo, K.-K. Huang, H.-B. Zeng, D.-Y. Wang, W.-S. Yang, R.-S. Zeng, X.-M. Li and R.-G. Xie, Doped Emitting Cesium Silver Halides as X-Ray Scintillator with Fast Response Time, High Absorption Coefficient, and Light Yield, Adv. Photonics Res., 2021, 2, 2100066 CrossRef CAS.
- P. Kumar, T. D. Creason, H. Fattal, M. Sharma, M. H. Du and B. Saparov, Composition-Dependent Photoluminescence Properties and Anti-Counterfeiting Applications of A2AgX3(A = Rb, Cs; X = Cl, Br, I), Adv. Funct. Mater., 2021, 31, 9 Search PubMed.
- J. P. Perdew, K. Burke and Y. Wang, Generalized gradient approximation for the exchange-correlation hole of a many-electron system (vol 54, pg 16 533, 1996), Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 14999 CrossRef CAS.
- V. I. Anisimov, F. Aryasetiawan and A. I. Lichtenstein, First-principles calculations of the electronic structure and spectra of strongly correlated systems: The LDA + U method, J. Phys.-Condes. Matter, 1997, 9, 767–808 CrossRef CAS.
- P. Luches, F. Pagliuca, S. Valeri, F. Illas, G. Preda and G. Pacchioni, Nature of Ag Islands and Nanoparticles on the CeO2(111) Surface, J. Phys. Chem. C, 2012, 116, 1122–1132 CrossRef CAS.
- N. Sieffert and G. Wipff, Uranyl extraction by N,N-dialkylamide ligands studied using static and dynamic DFT simulations, Dalton Trans., 2015, 44, 2623–2638 RSC.
- C. Adamo and V. Barone, Toward reliable density functional methods without adjustable parameters: The PBE0 model, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- D. Jacquemin, V. Wathelet, E. A. Perpète and C. Adamo, Extensive TD-DFT Benchmark: Singlet-Excited States of Organic Molecules, J. Chem. Theory Comput., 2009, 5, 2420–2435 CrossRef CAS PubMed.
- S. Kotaru, P. Pokhilko and A. I. Krylov, Spin-orbit couplings within spin-conserving and spin-flipping time-dependent density functional theory: Implementation and benchmark calculations, J. Chem. Phys., 2022, 157, 12 CrossRef PubMed.
- D. A. Pantazis and F. Neese, All-Electron Scalar Relativistic Basis Sets for the Actinides, J. Chem. Theory Comput., 2011, 7, 677–684 CrossRef CAS.
- J. D. Rolfes, F. Neese and D. A. Pantazis, All-electron scalar relativistic basis sets for the elements Rb-Xe, J. Comput. Chem., 2020, 41, 1842–1849 CrossRef CAS PubMed.
- C. Xing, B. Zhou, D. Yan and W.-H. Fang, Dynamic Photoresponsive Ultralong Phosphorescence from One-Dimensional Halide Microrods Toward Multilevel Information Storage, CCS Chem., 2023, 5, 2866–2876 CrossRef.
- C. Xing, Z. Qi, B. Zhou, D. Yan and W.-H. Fang, Solid-State Photochemical Cascade Process Boosting Smart Ultralong Room-Temperature Phosphorescence in Bismuth Halides, Angew. Chem., Int. Ed., 2024, 63, e202402634 CrossRef CAS PubMed.
- B. Zhou and D. Yan, Glassy inorganic-organic hybrid materials for photonic applications, Matter, 2024, 7, 1950–1976 CrossRef.
- W. B. Ma, Y. R. Su, Q. S. Zhang, C. Deng, L. Pasquali, W. J. Zhu, Y. Tian, P. Ran, Z. Chen, G. Y. Yang, G. J. Liang, T. Y. Liu, H. M. Zhu, P. Huang, H. Z. Zhong, K. W. Wang, S. Q. Peng, J. L. Xia, H. F. Liu, X. Liu and Y. M. Yang, Thermally activated delayed fluorescence (TADF) organic molecules for efficient X-ray scintillation and imaging (vol 21, p. 210, 2022), Nat. Mater., 2022, 21, 836 CrossRef CAS.
- Q. S. Chen, J. Wu, X. Y. Ou, B. L. Huang, J. Almutlaq, A. A. Zhumekenov, X. W. Guan, S. Y. Han, L. L. Liang, Z. G. Yi, J. Li, X. J. Xie, Y. Wang, Y. Li, D. Y. Fan, D. B. L. Teh, A. H. All, O. F. Mohammed, O. M. Bakr, T. Wu, M. Bettinelli, H. H. Yang, W. Huang and X. G. Liu, All-inorganic perovskite nanocrystal scintillators, Nature, 2018, 561, 88–93 CrossRef CAS PubMed.
- C. Dujardin, E. Auffray, E. Bourret-Courchesne, P. Dorenbos, P. Lecoq, M. Nikl, A. N. Vasil'ev, A. Yoshikawa and R. Y. Zhu, Needs, Trends, and Advances in Inorganic Scintillators, IEEE Trans. Nucl. Sci., 2018, 65, 1977–1997 CAS.
- C. W. E. Vaneijk, Fast scintillators and their applications, Radiat. Eff. Defects Solids, 1991, 119, 9–14 CrossRef.
- P. A. Rodnyi, P. Dorenbos and C. W. E. Vaneijk, Energy-Loss In Inorganic Scintillators, Phys. Status Solidi B-Basic Res, 1995, 187, 15–29 CrossRef CAS.
- P. Dorenbos, Scintillation mechanisms in Ce3+ doped halide scintillators, Phys. Status Solidi A-Appl. Res, 2005, 202, 195–200 CrossRef CAS.
- D. A. Popy, T. D. Creason, Z. Zhang, D. J. Singh and B. Saparov, Electronic structures and optical properties of (Ph4P)MX2 (M = Cu, Ag; X = Cl, Br), J. Solid State Chem., 2022, 316, 123626 CrossRef CAS.
- W. Ma, Y. Su, Q. Zhang, C. Deng, L. Pasquali, W. Zhu, Y. Tian, P. Ran, Z. Chen, G. Yang, G. Liang, T. Liu, H. Zhu, P. Huang, H. Zhong, K. Wang, S. Peng, J. Xia, H. Liu, X. Liu and Y. M. Yang, Thermally activated delayed fluorescence (TADF) organic molecules for efficient X-ray scintillation and imaging, Nat. Mater., 2022, 21, 210–216 CrossRef CAS PubMed.
- T. Y. He, Y. Zhou, P. Yuan, J. Yin, L. Gutierrez-Arzaluz, S. L. Chen, J. X. Wang, S. Thomas, H. N. Alshareef, O. M. Bakr and O. F. Mohammed, Copper Iodide Inks for High-Resolution X-ray Imaging Screens, ACS Energy Lett., 2023, 8, 1362–1370 CrossRef CAS.
- P. Lecoq, Development of new scintillators for medical applications, Nucl. Instrum. Methods Phys. Res., Sect. A, 2016, 809, 130–139 CrossRef CAS.
- P. Lecoq, Development of new scintillators for medical applications, Nucl. Instrum. Methods Phys. Res., Sect. A, 2016, 809, 130–139 CrossRef CAS.
- C. Wu, B. Du, W. Luo, Y. Liu, T. Li, D. Wang, X. Guo, H. Ting, Z. Fang, S. Wang, Z. Chen, Y. Chen and L. Xiao, Highly Efficient and Stable Self-Powered Ultraviolet and Deep-Blue Photodetector Based on Cs2AgBiBr6/SnO2 Heterojunction, Adv. Opt. Mater., 2018, 6, 1800811 CrossRef.
- W. J. Zhu, W. B. Ma, Y. R. Su, Z. Chen, X. Y. Chen, Y. G. Ma, L. Z. Bai, W. G. Xiao, T. Y. Liu, H. M. Zhu, X. F. Liu, H. F. Liu, X. Liu and Y. Yang, Low-dose real-time X-ray imaging with nontoxic double perovskite scintillators, Light Sci. Appl., 2020, 9, 10 CrossRef.
- Q. Hu, C. Zhang, X. Wu, G. Liang, L. Wang, X. Niu, Z. Wang, W. D. Si, Y. Han, R. Huang, J. Xiao and D. Sun, Highly Effective Hybrid Copper(I) Iodide Cluster Emitter with Negative Thermal Quenched Phosphorescence for X-Ray Imaging, Angew. Chem. Int. Ed., 2023, 62, e202217784 CrossRef CAS PubMed.
- T. Y. He, Y. Zhou, X. J. Wang, J. Yin, L. Gutiérrez-Arzaluz, J. X. Wang, Y. H. Zhang, O. M. Bakr and O. F. Mohammed, High-Performance Copper-Doped Perovskite-Related Silver Halide X-ray Imaging Scintillator, ACS Energy Lett., 2022, 7, 2753–2760 CrossRef CAS.
- J. A. Steele, P. Weicheng, C. Martin, M. Keshavarz, E. Debroye, Y. Haifeng, S. Banerjee, E. Fron, D. Jonckheere, K. Cheol Woong, W. Baekelant, N. Guangda, T. Jiang, J. Vanacken, M. Van der Auweraer, J. Hofkens and M. B. J. Roeffaers, Perovskite-Based Devices: Photophysical Pathways in Highly Sensitive Cs2AgBiBr6Double-Perovskite Single-Crystal X-Ray Detectors (Adv. Mater. 46/2018), Adv. Mater., 2018, 30, 1870353 CrossRef.
- M. Y. Zhang, X. M. Wang, B. Yang, J. S. Zhu, G. D. Niu, H. D. Wu, L. X. Yin, X. Y. Du, M. Niu, Y. S. Ge, Q. G. Xie, Y. F. Yan and J. Tang, Metal Halide Scintillators with Fast and Self-Absorption-Free Defect-Bound Excitonic Radioluminescence for Dynamic X-Ray Imaging, Adv. Funct. Mater., 2021, 31, 9 Search PubMed.
- B. H. Li, Y. Xu, X. L. Zhang, K. Han, J. C. Jin and Z. G. Xia, Zero-Dimensional Luminescent Metal Halide Hybrids Enabling Bulk Transparent Medium as Large-Area X-Ray Scintillators, Adv. Opt. Mater., 2022, 10, 7 Search PubMed.
- D. F. Wu, X. S. Zhao, Y. Y. Huang, J. N. Lai, H. Y. Li, J. Y. Yang, C. Q. Tian, P. He, Q. Huang and X. S. Tang, Lead-Free Perovskite Cs2AgBiX6Nanocrystals with a Band Gap Funnel Structure for Photocatalytic CO2 Reduction under Visible Light, Chem. Mat., 2021, 33, 4971–4976 CrossRef CAS.
- F. Locardi, M. Cirignano, D. Baranov, Z. Y. Dang, M. Prato, F. Drago, M. Ferretti, V. Pinchetti, M. Fanciulli, S. Brovelli, L. De Trizio and L. Manna, Colloidal Synthesis of Double Perovskite Cs2AgInCl6 and Mn-Doped Cs2AgInCl6 Nanocrystals, J. Am. Chem. Soc., 2018, 140, 12989–12995 CrossRef CAS.
- K. Lv, S. Qi, G. Liu, Y. Lou, J. Chen and Y. Zhao, Lead-free silver-antimony halide double perovskite quantum dots with superior blue photoluminescence, Chem. Commun., 2019, 55, 14741–14744 RSC.
- W. F. Wang, M. J. Xie, P. K. Wang, J. Lu, B. Y. Li, M. S. Wang, S. H. Wang, F. K. Zheng and G. C. Guo, Thermally Activated Delayed Fluorescence (TADF)-active Coinage-metal Sulfide Clusters for High-resolution X-ray Imaging, Angew. Chem., Int. Ed., 2024, 63, e202318026 CrossRef CAS.
- K. Han, J. C. Jin, B. B. Su, J. W. Qiao and Z. G. Xia, Promoting Single Channel Photon Emission in Copper(I) Halide Clusters for X-Ray Detection, Adv. Opt. Mater., 2022, 10, 8 Search PubMed.
- L. R. V. Buizza, A. D. Wright, G. Longo, H. C. Sansom, C. Q. Xia, M. J. Rosseinsky, M. B. Johnston, H. J. Snaith and L. M. Herz, Charge-Carrier Mobility and Localization in Semiconducting Cu2AgBiI6 for Photovoltaic Applications, ACS Energy Lett., 2021, 6, 1729–1739 CrossRef CAS PubMed.
- Y. Lin, S. Liu and D. Yan, Flexible Crystal Heterojunctions of Low-Dimensional Organic Metal Halides Enabling Color-Tunable Space-Resolved Optical Waveguides, Research, 2023, 6, 0259 CrossRef CAS PubMed.
- C. Xing, B. Zhou, D. Yan and W.-H. Fang, Integrating Full-Color 2D Optical Waveguide and Heterojunction Engineering in Halide Microsheets for Multichannel Photonic Logical Gates, Adv. Sci., 2024, 11, 2310262 CrossRef CAS.
- N. Mondal, A. De and A. Samanta, Achieving Near-Unity Photoluminescence Efficiency for Blue-Violet-Emitting Perovskite Nanocrystals, ACS Energy Lett., 2019, 4, 32–39 CrossRef CAS.
- B. B. Zhang, J. K. Chen, J. P. Ma, X. F. Jia, Q. Zhao, S. Q. Guo, Y. M. Chen, Q. Liu, Y. Kuroiwa, C. Moriyoshi, J. Y. Zhang and H. T. Sun, Antithermal Quenching of Luminescence in Zero-Dimensional Hybrid Metal Halide Solids, J. Phys. Chem. Lett., 2020, 11, 2902–2909 CrossRef CAS PubMed.
- A. Biswas, R. Bakthavatsalam, S. R. Shaikh, A. Shinde, A. Lohar, S. Jena, R. G. Gonnade and J. Kundu, Efficient Broad-Band Emission from Contorted Purely Corner-Shared One Dimensional (1D) Organic Lead Halide Perovskite, Chem. Mat., 2019, 31, 2253–2257 CrossRef CAS.
- J.-Q. Zhao, H.-S. Shi, L.-R. Zeng, H. Ge, Y.-H. Hou, X.-M. Wu, C.-Y. Yue and X.-W. Lei, Highly emissive zero-dimensional antimony halide for anti-counterfeiting and confidential information encryption-decryption, Chem. Eng. J., 2022, 431, 134336 CrossRef CAS.
- B. Moon, S. J. Kim, S. Lee, A. Lee, H. Lee, D. S. Lee, T. W. Kim, S. K. Lee, S. Bae and S. H. Lee, Rare-Earth-Element-Ytterbium-Substituted Lead-Free Inorganic Perovskite Nanocrystals for Optoelectronic Applications, Adv. Mater., 2019, 31, 7 Search PubMed.
- D. Y. Li, Y. B. Shang, Q. Liu, H. W. Zhang, X. Y. Zhang, C. Y. Yue and X. W. Lei, 0D hybrid indium halide as a highly efficient X-ray scintillation and ultra-sensitive fluorescent probe, Mater. Horiz., 2023, 10, 5004–5015 RSC.
- Q. Zhang, H. Q. Li, Y. Ma and T. Y. Zhai, ZnSe nanostructures: Synthesis, properties and applications, Prog. Mater. Sci., 2016, 83, 472–535 CrossRef CAS.
- A. M. Kelley, Electron-Phonon Coupling in CdSe Nanocrystals from an Atomistic Phonon Model, ACS Nano, 2011, 5, 5254–5262 CrossRef CAS PubMed.
- M. J. Leitl, V. A. Krylova, P. I. Djurovich, M. E. Thompson and H. Yersin, Phosphorescence versus Thermally Activated Delayed Fluorescence. Controlling Singlet–Triplet Splitting in Brightly Emitting and Sublimable Cu(I) Compounds, J. Am. Chem. Soc., 2014, 136, 16032–16038 CrossRef CAS.
- G. Xiao, Y.-J. Ma, Z. Qi, X. Fang, T. Chen and D. Yan, A flexible ligand and halogen engineering enable one phosphor-based full-color persistent luminescence in hybrid perovskitoids, Chem. Sci., 2024, 15, 3625–3632 RSC.
- Y. Zhang, T. S. Lee, J. M. Favale, D. C. Leary, J. L. Petersen, G. D. Scholes, F. N. Castellano and C. Milsmann, Delayed fluorescence from a zirconium(iv) photosensitizer with ligand-to-metal charge-transfer excited states, Nat. Chem., 2020, 12, 345–352 CrossRef CAS PubMed.
- A. Niwa, T. Kobayashi, T. Nagase and H. Naito, O. P. U. The Research Institute for Molecular Electronic Devices, Sakai 599-, K. Goushi, C. Adachi and K. U. International Institute for Carbon Neutral Energy Research, 744 Motooka, Nishi, Fukuoka 819-, Temperature dependence of photoluminescence properties in a thermally activated delayed fluorescence emitter, Appl. Phys. Lett., 2014, 104, 213301 CrossRef.
- Y. Luo, K. Zhang, Z. Ding, P. Chen, X. Peng, Y. Zhao, K. Chen, C. Li, X. Zheng, Y. Huang, X. Pu, Y. Liu, S.-J. Su, X. Hou and Z. Lu, Ultra-fast triplet-triplet-annihilation-mediated high-lying reverse intersystem crossing triggered by participation of nπ*-featured excited states, Nat. Commun., 2022, 13, 6892 CrossRef CAS PubMed.
- Y. Xu, X. Liang, X. Zhou, P. Yuan, J. Zhou, C. Wang, B. Li, D. Hu, X. Qiao, X. Jiang, L. Liu, S.-J. Su, D. Ma and Y. Ma, Highly Efficient Blue Fluorescent OLEDs Based on Upper Level Triplet–Singlet Intersystem Crossing, Adv. Mater., 2019, 31, 1807388 CrossRef.
- S. W. Park, D. Kim and Y. M. Rhee, Overcoming the Limitation of Spin Statistics in Organic Light Emitting Diodes (OLEDs): Hot Exciton Mechanism and Its Characterization, Int. J. Mol. Sci., 2023, 24, 12362 CrossRef CAS PubMed.
- L. Lu, M. Z. Sun, Q. Y. Lu, T. Wu and B. L. Huang, High energy X-ray radiation sensitive scintillating materials for medical imaging, cancer diagnosis and therapy, Nano Energy, 2021, 79, 21 Search PubMed.
- H. Zhang, Z. Yang, M. Zhou, L. Zhao, T. M. Jiang, H. Y. Yang, X. Yu, J. B. Qiu, Y. Yang and X. H. Xu, Reproducible X-ray Imaging with a Perovskite Nanocrystal Scintillator Embedded in a Transparent Amorphous Network Structure, Adv. Mater., 2021, 33, 7 Search PubMed.
- X. Zhao, T. Jin, W. R. Gao, G. D. Niu, J. S. Zhu, B. X. Song, J. J. Luo, W. C. Pan, H. D. Wu, M. Y. Zhang, X. He, L. C. Fu, Z. G. Li, H. T. Zhao and J. Tang, Embedding Cs3Cu2I5 Scintillators into Anodic Aluminum Oxide Matrix for High-Resolution X-Ray Imaging, Adv. Opt. Mater., 2021, 9, 8 Search PubMed.
- L. J. Xu, X. S. Lin, Q. Q. He, M. Worku and B. W. Ma, Highly efficient eco-friendly X-ray scintillators based on an organic manganese halide, Nat. Commun., 2020, 11, 7 CrossRef.
- T. M. Jiang, W. B. Ma, H. Zhang, Y. Tian, G. Lin, W. G. Xiao, X. Yu, J. B. Qiu, X. H. Xu, Y. Yang and D. X. Ju, Highly Efficient and Tunable Emission of Lead-Free Manganese Halides toward White Light-Emitting Diode and X-Ray Scintillation Applications, Adv. Funct. Mater., 2021, 31, 9 Search PubMed.
- H. Wei, Y. Fang, P. Mulligan, W. Chuirazzi, H.-H. Fang, C. Wang, B. R. Ecker, Y. Gao, M. A. Loi, L. Cao and J. Huang, Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals, Nat. Photonics, 2016, 10, 333–339 CrossRef CAS.
- Y. Zhang, Y. Ma, Y. Wang, X. Zhang, C. Zuo, L. Shen and L. Ding, Lead-Free Perovskite Photodetectors: Progress, Challenges, and Opportunities, Adv. Mater., 2021, 33, 2006691 CrossRef CAS.
- W. Y. Shao, X. Wang, Z. Z. Zhang, J. Huang, Z. Y. Han, S. Pi, Q. Xu, X. D. Zhang, X. C. Xia and H. W. Liang, Highly Efficient and Flexible Scintillation Screen Based on Manganese (II) Activated 2D Perovskite for Planar and Nonplanar High-Resolution X-Ray Imaging, Adv. Opt. Mater., 2022, 10, 9 Search PubMed.
- M. Y. Zhang, J. S. Zhu, B. Yang, G. D. Niu, H. D. Wu, X. Zhao, L. X. Yin, T. Jin, X. Y. Liang and J. Tang, Oriented-Structured CsCu2I3 Film by Close-Space Sublimation and Nanoscale Seed Screening for High-Resolution X-ray Imaging, Nano Lett., 2021, 21, 1392–1399 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2321717–2321719. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qm00362d |
| This journal is © the Partner Organisations 2024 |