Chalcogen modification: one-step strategy for tuning the photophysical properties and NIR phototherapy of iodinated BODIPY†
Hongyi
Liu
a,
Hui
Li
a,
Wen
Li
a,
Jinjin
Zhang
a,
Jingtao
Ye
a,
Shenglong
Liao
 b,
Yang
Li
*a and
Shouchun
Yin
b,
Yang
Li
*a and
Shouchun
Yin
 *a
*a
aKey Laboratory of Organosilicon Chemistry and Materials Technology of Ministry of Education, College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou 311121, P. R. China. E-mail: liyang@hznu.edu.cn; yinsc@hznu.edu.cn
bSchool of Engineering, Hangzhou Normal University, Hangzhou 311121, P. R. China
First published on 31st July 2024
Abstract
Near-infrared (NIR) photosensitizers have immense potential for in vivo phototherapy due to minimal scattering of NIR light in biological tissues. Among various types of photosensitizers, BODIPY dyes are potential candidates for phototherapy owing to their high molar extinction coefficient and tunable photophysical properties. However, most NIR BODIPY photosensitizers have relatively complicated structures and lengthy synthesis approaches, restricting their practical application. In this work, a simple strategy of chalcogen modification was applied to tune the photophysical properties of iodinated BODIPY for enhanced NIR phototherapy. As the atomic radius of chalcogen atoms increases, the BODIPY-X (X = O, S, Se, and Te) dyes exhibit a red-shifted absorption from 558 nm, 610 nm, and 618 nm to 660 nm, a faster singlet oxygen generation rate, and higher photothermal conversion efficiency due to the heavy atom effect. This modification facilitates intramolecular charge transfer (ICT) and enhances intersystem crossing (ISC), critical for effective PDT and PTT. To improve hydrophilicity and delivery efficiency, we encapsulated BODIPY-X using the amphiphilic copolymer Pluronic F127, creating F127/BODIPY-X nanoparticles (NPs). These NPs exhibited enhanced solubility and bioavailability, crucial for therapeutic efficacy. Moreover, the F127-encapsulated BODIPY-Te nanoparticles exhibit the best anti-tumor efficiency on U87-bearing mice, which is consistent with their outstanding photothermal conversion and photodynamic performance. Hence, a chalcogen modification strategy with a simple synthesis approach paves a new way for tuning the photophysical properties of NIR photosensitizers and could stimulate the rapid development of NIR phototheranostic agents.
Introduction
Near-infrared (NIR) phototherapy, as a cutting-edge non-invasive medical treatment, harnesses the power of NIR light to treat various health conditions and has gained increasing attention in recent years.1 Unlike visible light, NIR light experiences minimal scattering and absorption when traversing biological tissues, thus it can penetrate deep into tissues and provide a range of therapeutic benefits.2 In general, phototherapy can be divided into two categories, photodynamic therapy (PDT) and photothermal therapy (PTT), both of which have shown promising results in the treatment of solid tumors and may become the next revolutionary technique in clinical surgery.3 The key factors of PDT and PTT are based on photosensitive agents that can generate cytotoxic reactive oxygen species (ROS) such as singlet oxygen (1O2),4 or hyperthermia under light irradiation,5 and then destroy tumor cells. Therefore, tuning the photophysical performance of photosensitive agents is critical for NIR phototherapy.6 Firstly, photosensitive agents must have spectral activity within the phototherapeutic window (>600 nm) to ensure sufficient tissue penetration depth, while avoiding light absorption by endogenous biomolecules.7 Secondly, they must have a high rate of intersystem crossing (ISC) to generate triplet excitons, promote electron transfer or energy transfer between triplet excitons and the ground state oxygen (triplet oxygen, 3O2), and finally generate ROS.8In recent years, NIR photosensitizers have emerged as a promising frontier in the realm of in vivo phototherapy.9 Among the myriad of NIR photosensitizers, 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) dyes have attracted considerable attention since their discovery in 1968 due to their exceptional photophysical properties.10 These unique properties include, but are not limited to, a high molar extinction coefficient,11 high fluorescence quantum yield,12 good photostability,13 low cytotoxicity,14 and good biocompatibility,15 which make them particularly attractive for NIR phototherapy. In particular, BODIPY has a stable and planar core structure with multiple reactive sites, which could be easily modified to tailor the photophysical properties according to specific requirements.16,17 In order to meet the above requirements for NIR phototherapy, various chemical modification approaches have been developed over the past decades.18 For example, to elongate the absorption wavelength of BODIPY, nitrogen atoms have been employed to substitute the methine meso-spacer of dipyrromethene19 and strong conjugated groups have been inserted via the Knoevenagel condensation,20 Heck reaction,21 or Suzuki coupling.22 However, the majority of reported BODIPY derivatives with absorption in the NIR region often suffer from complex synthetic procedures, difficult separation and purification, and relatively low yields, which have hindered their widespread utilization in clinical settings.23,24 Therefore, exploring a BODIPY-derived photosensitizer that keeps a balance between simple synthesis routes and high NIR phototherapy efficiency is an essential requirement for the development of NIR phototherapy.25
To address this issue, our research endeavors to simplify the synthesis process of BODIPY-based NIR photosensitizers without sacrificing the NIR phototherapy efficiency. In this manuscript, we designed and synthesized four types of photosensitizers, BODIPY-X (X = O, S, Se, and Te), via a one-step nucleophilic substitution approach based on iodinated BODIPY (Scheme 1). Specifically, heavy atom modification (e.g. bromine and iodine) as one of the most classic strategies has been widely applied to elongate the absorbance and enhance the photodynamic efficiency,26,27 while the absorption peak of BODIPY could hardly exceed 600 nm via iodine modification. To further elongate the absorption wavelength and enhance the phototherapy efficiency, chalcogen atoms (O, S, Se, and Te) are subsequently applied to modify the iodinated BODIPY. On one hand, the electron-donating ability of the substituent group increases with the increasing atomic number, which facilitates intramolecular charge transfer (ICT). The enhanced ICT could effectively extend the absorption wavelength and promote non-radiative decay and photothermal conversion.28 On the other hand, as heavy atoms, iodine, selenium, and tellurium can promote the ISC from the singlet to the triplet state and thus enhance the efficacy of photodynamic therapy.29 To conclusively assess the viability of BODIPY-X in tumor phototherapy, we leveraged the amphiphilic copolymer Pluronic F127 for encapsulation. This step was crucial for enhancing the hydrophilicity of BODIPY-X, thereby improving its delivery efficiency. The resulting F127/BODIPY-X nanoparticles (F127/BODIPY-X NPs) demonstrated enhanced solubility and bioavailability, which are pivotal for effective therapeutic intervention. Subsequently, we applied these optimized nanoparticles in a study involving tumor-bearing mice, aiming to evaluate their potential in applications. The results suggest that the BODIPY-Te NPs displayed the best phototherapeutic effect on malignant tumors, validating that the chalcogen modification strategy based on iodinated BODIPY could effectively enhance the efficiency of NIR phototherapy and stimulate the rapid development of NIR phototherapeutic agents.
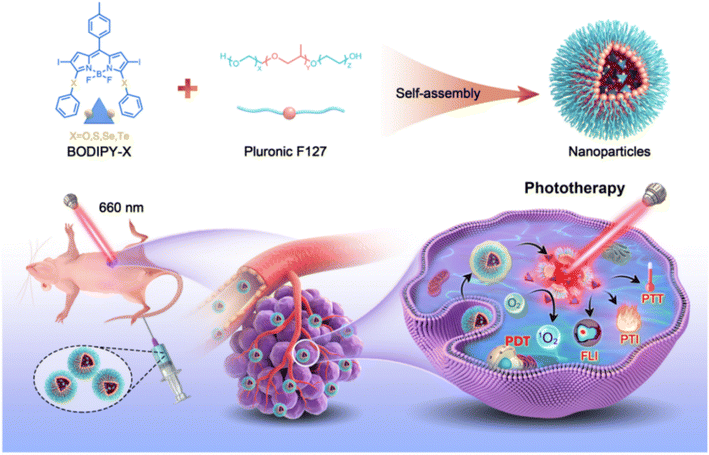 | ||
| Scheme 1 Schematic illustration of the preparation procedure of F127/BODIPY-X (X = O, S, Se, and Te) nanoparticles and phototherapy against tumor. | ||
Results and discussion
Synthesis and characterization of BODIPY-X and F127/BODIPY-X nanoparticles
Here, a simple nucleophilic aromatic substitution reaction between 3,5-dichloro-4,4-difluoro-2,6-diiodo-4-bora-3a,4a-diaza-s-indacene and phenol, diphenyl disulfide, diphenyl diselenide, or diphenyl ditelluride was utilized to synthesize four types of heavy-atom-functionalized BODIPY photosensitizers, BODIPY-X (X = O, S, Se, and Te). The synthetic procedures of BODIPY-X are clearly described in the ESI.† According to Scheme S1 and Fig. S1–S13 (ESI†), all the compounds were prepared with moderate yields and the structures of the four types of BODIPY-X compounds were confirmed by nuclear magnetic resonance (1H NMR and 13C NMR) and mass spectroscopy (MALDI-TOF). Specifically, the yields of BODIPY-O, BODIPY-S, and BODIPY-Se are 50.4%, 41.3%, and 42.0% respectively and their single crystal are successfully achieved and verified via single crystal X-ray diffraction (Tables S1–S3, ESI†). The yield of BODIPY-Te is 31.7%, which is lower than those of the others owing to the relatively poor nucleophilicity of the phenyl telluride group.30Subsequently, a series of photophysical characterization studies on BODIPY-X and F127/BODIPY-X NPs were conducted. As depicted in Fig. 1a, BODIPY-O, BODIPY-S, BODIPY-Se, and BODIPY-Te in THF exhibited a single-peak absorption with maximum absorption wavelengths of 548 nm, 600 nm, 605 nm, and 639 nm, respectively. Their fluorescence emission also displayed single peaks at 572 nm, 628 nm, 636 nm, and 694 nm with the corresponding fluorescence quantum yields of 8.9%, 2.1%, 1.2%, and 0.01%, respectively (Fig. 1b). In comparison with BODIPY-O, both the maximum absorption and emission of BODIPY-S and BODIPY-Se displayed similar redshift values. With the further increase in the atomic number, the BODIPY-Te displays a much larger redshift in the maximum absorption and emission in comparison with BODIPY-O, which are 89 nm and 122 nm, respectively. According to the spectral variation, it could be concluded that the increase of the atomic radius of chalcogen atoms results in the redshift of maximum absorption and emission, which could be attributed to the enhanced intramolecular charge transfer (ICT) as well as the electron-donating ability of chalcogen substituents.31 Besides, due to the heavy atom-accelerated ISC and the faster internal conversion at longer wavelengths, the fluorescence quantum yields of BODIPY-X also exhibited a declining trend as the radius increase of chalcogen atoms.32 Notably, although the fluorescence quantum yield decreased in the order of BODIPY-O, BODIPY-S, BODIPY-Se, and BODIPY-Te, the redshift of maximum absorption and emission often means better light penetration as well as deeper photo imaging and therapy depth, suggesting that such chalcogen modification strategy for iodinated BODIPY might play an important role in tuning the efficiency of phototheranostics in deep tissue.33
In order to enhance the hydrophilicity and delivery efficiency into tumors, hydrophobic BODIPY-X were directly encapsulated with an amphiphilic block copolymer (F127) through a nanoprecipitation process to obtain composite nanoparticles F127/BODIPY-X. From the UV-Vis absorption spectrum, it is proved that BODIPY-X is successfully encapsulated by F127 and the drug loading efficiency could be calculated by standard curves, which were 3.31%, 1.70%, 7.40%, and 1.24%, respectively (Fig. S14, ESI†). Then, the size and morphology of F127/BODIPY-X were characterized via dynamic light scattering (DLS) and transmission electron microscopy (TEM). As shown in Fig. S15 and S16 (ESI†), four types of F127/BODIPY-X NPs display a spherical shape with relatively narrow-dispersed sizes and good stability in water. The average hydrodynamic diameters of F127/BODIPY-O, F127/BODIPY-S, F127/BODIPY-Se, and F127/BODIPY-Te are about 238.2, 196.3, 160.4, and 100.8 nm, respectively, which are well consistent with the TEM images. Subsequently, the absorption and fluorescence emission spectra of F127/BODIPY-X NPs were evaluated (Fig. 1c and d). Similar to BODIPY-X in THF, the F127/BODIPY-X NPs displayed an obvious redshift in both absorption and emission spectra as the atomic radius of chalcogen atoms increased. Notably, in comparison with the solution state, the maximum absorption of F127/BODIPY-X NPs also exhibits a slight redshift, which are 10 nm, 10 nm, 13 nm, and 21 nm in the order of O, S, Se, and Te. The spectral variation between the solution state and the solid state suggests the effective J-aggregation of the conjugated molecules in nanoparticles,34 which could also benefit NIR phototherapy owing to the elongated absorption wavelength.
To investigate the mechanism behind the different optical absorption and emission properties of BODIPY-X, the electron energy levels and corresponding energy bandgaps are evaluated via density functional theory (DFT, Fig. 2a) calculations and electrochemical characterization (Fig. 2b). The HOMO/LUMO energy levels (EHOMO/ELUMO) and optical bandgaps (Eg) of BODIPY-X were obtained by cyclic voltammetry (CV). The results show that the EHOMO/ELUMO of BODIPY-O, BODIPY-S, BODIPY-Se, and BODIPY-Te are −5.48/−3.56, −5.58/−3.83, −5.47/−3.77, and −5.38/−3.77 eV, with optical bandgaps of 1.92, 1.75, 1.70, and 1.61 eV, respectively. A similar trend was also discovered through DFT calculations. As depicted in Fig. 2c, the Eg from DFT calculation fell steadily from BODIPY-O (2.77 eV) to BODIPY-S (2.56 eV), BODIPY-Se (2.54 eV), and BODIPY-Te (2.38 eV). Generally, the redshift value of the maximum absorption and emission increases as the bandgap narrows.35 Hence, the decrease in optical bandgaps of BODIPY-X in the order of O, S, Se, and Te is fully consistent with the UV-Vis absorption and fluorescence emission spectra shown in Fig. 1. Moreover, the Eg of BODIPY-S and BODIPY-Se obtained from either CV measurements or DFT calculation are very close, which reflects their similar redshift values of maximum absorption and fluorescence emission in comparison with BODIPY-O. BODIPY-Te has the lowest Eg value, accounting for its highest redshift of absorption and fluorescence emission. These findings suggest that increasing the radius of the chalcogen atom in the substituents can result in the decrease of Eg, thus benefiting the NIR absorption and phototherapy of BODIPY-X.
Photodynamic and photothermal properties of BODIPY-X and F127/BODIPY-X nanoparticles
As the potential candidates for NIR phototherapy, BODIPY-X and F127/BODIPY-X were used to conduct a series of in vitro validation experiments, including 1O2 production capability and photothermal conversion efficiency. Firstly, to determine whether BODIPY-X and F127/BODIPY-X can generate ROS under light irradiation, 1,3-diphenylisobenzofuran (DPBF) was used as an indicator. As exemplified in Fig. 3a, when F127/BODIPY-Te NPs were irradiated with a 660 nm laser beam, the distinctive absorption peak of DPBF at 414 nm dropped quickly, demonstrating the successful generation of ROS that can oxidize DPBF to a colorless state. Accordingly, the absorbance dropping kinetics of DPBF with F127/BODIPY-X NPs or BODIPY-X after 660 nm laser irradiation are presented in Fig. 3b and Fig. S17 (ESI†). Obviously, the absorbance drop kinetics for DPBF with F127/BODIPY-O NPs is almost the same as for the blank DPBF group, referring to the poor ROS generation capability of F127/BODIPY-O NPs under laser irradiation. In contrast, the other three groups displayed significant ROS generation. Notably, F127/BODIPY-Te NPs displayed a faster ROS generation rate than F127/BODIPY-S and F127/BODIPY-Se NPs (Fig. 3b), which arose from the stronger heavy-atom effect of the tellurium atom that increases the spin–orbit coupling constant and promotes ISC as well as the production of ROS.36 A similar trend was also observed for the DBPF solution with dissolved BODIPY-X. In order to support the above explanation, the singlet–triplet energy gaps (ΔEST) of BODIPY-X were calculated via DFT calculations. BODIPY-Te (ΔEST = 0.27 eV) exhibits a smaller ΔEST in comparison to BODIPY-O (ΔEST = 1.01 eV), BODIPY-S (ΔEST = 0.58 eV), and BODIPY-Se (ΔEST = 0.48 eV), which favors the occurrence of ISC37 (Table S4, ESI†). Subsequently, the type of ROS was further investigated by electron spin resonance (ESR) spectroscopy. Using 2,2,6,6-tetramethyl-4-piperidinone (TEMP) as a spin-trapping reagent for 1O2, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 characteristic peak of 1O2 was observed,38 demonstrating that the type of ROS generated is singlet oxygen (Fig. S18, ESI†) that could be potentially applied for photodynamic therapy of tumors.
1 characteristic peak of 1O2 was observed,38 demonstrating that the type of ROS generated is singlet oxygen (Fig. S18, ESI†) that could be potentially applied for photodynamic therapy of tumors.
In addition to the generation of singlet oxygen, the photothermal conversion of F127/BODIPY-X NPs was further investigated to evaluate their potential in photothermal therapy. According to the temperature elevation tests during laser irradiation, it is found that F127/BODIPY-O, F127/BODIPY-S, and F127/BODIPY-Se NPs displayed quite weak photothermal conversion and only F127/BODIPY-Te NPs exhibited significant photothermal conversion ability. As depicted in Fig. 3c and d, the temperature increases along with the irradiation time and the temperature elevation presents a strong dependence on the concentration and laser power density. This trend could also be observed from the infrared thermal images (Fig. 3e). Particularly, after 10 min of laser irradiation (660 nm, 1 W cm−2), aqueous solution with dispersed 10 μM F127/BODIPY-Te NPs can reach a high temperature over 50 °C, which meets the hyperthermia condition that is sufficient to destroy tumor cells.39 Furthermore, based on the linear time data versus −ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ (Fig. S19a, ESI†), the photothermal conversion efficiency of F127/BODIPY-Te NPs was calculated to be 36.4%. Moreover, F127/BODIPY-Te NPs displayed good photothermal stability across five on–off irradiation cycles, which could meet the demand for multiple treatments in photothermal therapy (Fig. S19b, ESI†). According to the aforementioned research, F127/BODIPY-Te NPs exhibit the fastest 1O2 generating capabilities and the highest photothermal conversion efficiency, which is in line with our initial goal of molecular design.
θ (Fig. S19a, ESI†), the photothermal conversion efficiency of F127/BODIPY-Te NPs was calculated to be 36.4%. Moreover, F127/BODIPY-Te NPs displayed good photothermal stability across five on–off irradiation cycles, which could meet the demand for multiple treatments in photothermal therapy (Fig. S19b, ESI†). According to the aforementioned research, F127/BODIPY-Te NPs exhibit the fastest 1O2 generating capabilities and the highest photothermal conversion efficiency, which is in line with our initial goal of molecular design.
In vitro cytotoxicity and cell imaging of BODIPY-X and F127/BODIPY-X nanoparticles
High phototoxicity upon light irradiation as well as low dark toxicity is highly essential for phototherapy to minimize side effects and enhance therapeutic efficiency.40 Thus, MTT experiments were firstly conducted to evaluate the cytotoxicity of BODIPY-X and F127/BODIPY-X under dark and light conditions. As demonstrated in Fig. 4a and Fig. S20 (ESI†), human glioblastoma cells (U87) and human embryonic kidney cells (HEK293) displayed excellent cell viability of about 100% after 24 hours of cultivation with BODIPY-X or F127/BODIPY-X at a concentration of 200–2000 ng mL−1 without irradiation, suggesting that both BODIPY-X and F127/BODIPY-X have excellent biological safety with very low dark toxicity. Under laser irradiation (660 nm, 1 W cm−2), both BODIPY-X and F127/BODIPY-X exhibited significant cytotoxicity against U87 cells and HEK 293 cells, while F127/BODIPY-Te NPs exhibited the best phototoxicity with a half maximal inhibitory concentration (IC50) of about 2000 ng mL−1 (Fig. 4c and Fig. S20, ESI†). Additionally, phototoxicity also exhibits strong dependence on the light power density, especially for F127/BODIPY-Te NPs (Fig. 4b). To investigate the basic principle of phototoxicity of F127/BODIPY-Te NPs, cells co-incubated with F127/BODIPY-Te NPs were divided into five groups, including “control”, “no laser”, “laser + Vc”, “laser + ice”, and “laser”, in which vitamin C (Vc) and ice were applied as the scavenger of singlet oxygen and heat, respectively. As depicted in Fig. 4d, cell viability in “control” and “no laser” groups are both about 100%, while it drops to 38.5% after treatment with laser irradiation (“laser” group). In the group of “laser + Vc”, cell viability drops to 45.3% after laser irradiation, which is higher than the “laser” group, suggesting that Vc as the scavenger of singlet oxygen would weaken the phototoxicity of F127/BODIPY-Te NPs. Similarly, in the “laser + ice” group, cell viability drops to 49.4% after laser irradiation, which is also higher than the “laser” group, indicating that inhibiting the suppression of the photothermal effect with ice would also weaken the phototoxicity of F127/BODIPY-Te NPs. Therefore, it could be concluded that the high phototoxicity of F127/BODIPY-Te NPs is the synergistic effect of photothermal conversion and photodynamic process, which is well consistent with the previous characterization by photothermal and photodynamic tests.Except for MTT experiments, live/dead cell staining was then performed to visualize the phototoxicity of F127/BODIPY-Te using a confocal laser scanning microscope (CLSM). As seen in Fig. 4e, the majority of the U87 cells treated with F127/BODIPY-Te and laser irradiation perished, while no substantial cytotoxicity was seen in U87 cells treated with F27/BODIPY-Te without laser irradiation, demonstrating that F127/BODIPY-Te exhibits high phototoxicity and little dark toxicity, which is fully consistent with the MTT results shown in Fig. 4a and c. Besides, no red fluorescence signal was observed for cells that were exposed only to laser irradiation, suggesting the ignorable adverse effect of laser irradiation. Furthermore, to validate the photodynamic effect of F127/BODIPY-X NPs at the cellular level, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was selected as the probe for singlet oxygen detection in cells. As depicted in Fig. S21 (ESI†), very weak green fluorescence can be detected when U87 cells are not irradiated, implying that F127/BODIPY-X NPs alone can’t generate ROS without light irradiation. However, bright green fluorescence can be observed after being irradiated with a 660 nm laser, indicating that F127/BODIPY-X NPs are able to generate strong singlet oxygen in cells under irradiation (Fig. 5a). Notably, flow cytometry result in Fig. 5b shows that the fluorescence intensity of cells treated with F127/BODIPY-Te NPs and laser irradiation is stronger than those treated with F127/BODIPY-O, F127/BODIPY-S, and F127/BODIPY-Se NPs, suggesting the stronger ROS generation capability of F127/BODIPY-Te NPs in cells.
Except for photothermal and photodynamic effects, BODIPY-X as the derivatives of BODIPY also displays significant fluorescence emission according to the emission spectra in Fig. 1, which might be valuable in cell imaging or even tumor imaging.41 Hence, the cellular uptake of F127/BODIPY-X NPs was investigated via CLSM together with DAPI staining. As shown in Fig. 5c, it is revealed that F127/BODIPY-X NPs could be rapidly taken up by the tumor cells, and the red fluorescence surrounding the cell nucleus can be observed, indicating F127/BODIPY-X NPs can be used for cell imaging in vitro. The fluorescence intensities of F127/BODIPY-Te groups are also significantly lower than those of the other groups due to the heavy-atom effect of Te,42 which is consistent with their fluorescence quantum yields. However, the sacrifice of fluorescence emission is acceptable in this regard, as it represents that non-radiative transitions play the dominant role and can improve the phototherapy efficiency and imaging depth.43 All the above results indicate that F127/BODIPY-Te NPs are superb photosensitizers with long absorption wavelength, negligible dark toxicity, high phototoxicity, and have potential for fluorescence imaging.
In vivo fluorescence and photothermal imaging of F127/BODIPY-Te NPs
After being validated in vitro, the in vivo NIR fluorescence imaging and photothermal imaging capabilities of F127/BODIPY-Te were investigated based on the U87 tumor-bearing mice model. As shown in Fig. 6a and b, after the tail vein injection of F127/BODIPY-Te NPs, the NIR fluorescence intensity at the tumor site steadily rose over time and reached maximum values at around 8 hours post-injection. Owing to the enhanced permeation and retention effect in the tumor, F127/BODIPY-Te NPs clearly depict the contour of the tumor on the dorsal side.44 The fluorescence signal began to fade after 8 h, while it remained strong enough for imaging within 24 h. In addition, the U87 tumor-bearing mice were irradiated with a 660 nm laser 8 h after F127/BODIPY-Te injection. The PBS group shows a slight temperature increase, while the F127/BODIPY-Te group shows a significant temperature increase (Fig. 6c and d). After 10 min of laser irradiation, the temperature at tumor sites in the F127/BODIPY-Te group reached 50.1 °C. With the temperature variation, infrared thermal images can also clearly depict the tumor position and outline. In a short conclusion, it is proven that the tumor-targeted retention ability of F127/BODIPY-Te could be visualized by NIR fluorescence and photothermal imaging, providing accurate guidance for subsequent photo-therapeutic therapy.In vivo photodynamic and photothermal effect of F127/BODIPY-X NPs
To evaluate the actual therapeutic effect of F127/BODIPY-X in vivo, U87 tumor-bearing mice were stochastically divided into nine groups for different treatments as follows: “PBS”, “F127/BODIPY-O”, “F127/BODIPY-O + laser”, “F127/BODIPY-S”, “F127/BODIPY-S + laser”, “F127/BODIPY-Se”, “F127/BODIPY-Se + laser”, “F127/BODIPY-Te”, and “F127/BODIPY-Te + laser”. All mice bearing U87 tumor were first injected with the corresponding substance via the tail vein. Four hours later, the tumor sites of the mice were exposed to a 660 nm laser with 0.6 W cm−2 for 5 min. During the whole treatment for 24 days, tumor volume and mice body weight were carefully monitored every 3 days to qualitatively assess the therapeutic effect of each group (Fig. 6e and f). For the groups of “F127/BODIPY-X + laser”, tumor growth was gradually greatly suppressed during 24 days, although the tumor size could not be immediately diminished in the early stage (5–6 days). In contrast, F127/BODIPY-X without laser irradiation exhibited poor tumor inhibition, suggesting the great efficiency of NIR phototherapy. Among all the groups, “F127/BODIPY-Te + laser” presented the best tumor inhibition effect, which is consistent with previous phototoxicity results shown in Fig. 4c. Notably, all of the mice survived during the whole procedure and no significant body weight loss or variation was found for all groups (Fig. 6f), suggesting the ignorable influence on mice growth during the in vivo phototherapy based on F127/BODIPY-X NPs.Subsequently, biosafety and other side effects during the in vivo NIR phototherapy were examined. The major organs of mice including the heart, liver, spleen, lungs, and kidneys were harvested and analyzed by hematoxylin and eosin (H&E) staining after phototherapy. As shown in Fig. 7a, no distinct lesions and side effects were observed in these major organs, indicating little harm caused by F127/BODIPY-X NPs to normal tissues. Besides, nine blood biochemical indices of the liver and kidney function including total protein (TP), albumin (ALB), white cell ratio (A/G), alanine aminotransferase (ALT), aspartate aminotransferase (AST), aspartate/alanine ratio (AST/ALT), urea nitrogen (BUN), and uric acid (UA) were detected after treatment. As shown in Fig. S22 (ESI†), these nine blood biochemical indicators are all within the normal reference range, indicating that the above treatment approach shows no influence on the normal body function.
To further investigate the in vivo therapeutic mechanism, cell proliferation and apoptosis in the tumors were analyzed by hematoxylin and eosin (H&E) staining, Ki67, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay after treatment (Fig. 7b). The H&E-stained sections of tumor tissues in the PBS group displayed the most hypercellular structure and presented obvious nuclear polymorphism. Among all groups, the tumor tissues from the treatment of F127/BODIPY-Te NPs presented the fewest tumor cells and the highest level of tumor necrosis. The Ki67 assay indicated that F127/BODIPY-Te NPs with laser irradiation effectively reduced the percentage of proliferating tumor cells and showed a particularly significant decrease in Ki67-positive tumor cells. As expected, TUNEL assay confirmed that treatment with F127/BODIPY-Te NPs and laser irradiation resulted in significantly reduced proliferation and increased apoptosis compared to that of the other groups, which is in full agreement with the results obtained from H&E and Ki67 analysis.
Conclusions
In summary, with the aim of exploring a photosensitizer with a simple synthesis route, significant NIR absorption, and highly efficient phototherapy of the tumor, four types of iodinated BODIPY modified with different chalcogen atoms have been designed and synthesized via a one-step nucleophilic aromatic substitution reaction. The photophysical properties of these photosensitizers are clearly investigated. As the atomic radius of chalcogen elements increases, the electron-donating impact of substituents also increases, resulting in a significant improvement of ICT and thus narrowing the ΔEg and ΔEST, which creates a redshift in the absorption and emission peaks of the prepared BODIPY-X.45 Moreover, the enhancement of the heavy-atom effect increases the spin-orbital coupling constant, which promotes the non-radiative transition and ISC,46 thus improving the efficiency of photothermal and photodynamic processes. In comparison with BODIPY-O, BODIPY-S, and BODIPY-Se, BODIPY-Te exhibits longer maximum absorption and emission wavelength as well as better photothermal and photodynamic effects. Subsequently, F127/BODIPY-X NPs were prepared and applied for in vitro and in vivo phototherapy. The results of in vitro and in vivo experiments indicate that F127/BODIPY-X NPs have negligible dark toxicity and high phototoxicity that can effectively inhibit tumor growth under the guidance of multimodal imaging, demonstrating the superiority of PTT and PDT combinational therapy. Meanwhile, F127/BODIPY-Te NPs exhibit the best phototherapy effect, which agrees with its high photothermal conversion and photodynamic efficiency. In light of these findings, this line of research proposes a simple strategy of chalcogen atom modification for constructing NIR photosensitizers with one-step synthesis and dual phototherapy effects. By further integrating advanced tumor-targeting methods, such as the attachment of specific tumor-targeting ligands or the utilization of cell membrane cloaking techniques, the selectivity and accumulation of these nanoparticles in tumor tissues can be significantly enhanced. In the following research, the integration of chalcogen modification with refined tumor-targeting approaches would present a promising avenue for the advancement of NIR phototherapy, holding potential to transform cancer treatment through more precise and effective therapeutic interventions.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (LZ23B040001, LY23E030003, and LY24B030005), the National Natural Science Foundation of China (22105222), the Interdisciplinary Research Project of Hangzhou Normal University (2024JCXK05) and the Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Soochow University.Notes and references
- C. Yan, Y. Zhang and Z. Guo, Recent progress on molecularly near-infrared fluorescent probes for chemotherapy and phototherapy, Coord. Chem. Rev., 2021, 427, 213556 CrossRef CAS.
- J. Li and K. Pu, Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation, Chem. Soc. Rev., 2019, 48, 38–71 RSC.
- J. Li, J. Rao and K. Pu, Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy, Biomaterials, 2018, 155, 217–235 CrossRef CAS PubMed.
- H. Bian, D. Ma, X. Zhang, K. Xin, Y. Yang, X. Peng and Y. Xiao, Tailored engineering of novel xanthonium polymethine dyes for synergetic PDT and PTT triggered by 1064 nm laser toward deep-seated tumors, Small, 2021, 17, 2100398 CrossRef CAS PubMed.
- L. Feng, C. Li, L. Liu, Z. Wang, Z. Chen, J. Yu, W. Ji, G. Jiang, P. Zhang, J. Wang and B. Z. Tang, Acceptor planarization and donor rotation: A facile strategy for realizing synergistic cancer phototherapy via type I PDT and PTT, ACS Nano, 2022, 16, 4162–4174 CrossRef CAS PubMed.
- M. Lan, S. Zhao, W. Liu, C. S. Lee, W. Zhang and P. Wang, Photosensitizers for photodynamic therapy, Adv. Healthcare. Mater., 2019, 8, 1900132 CrossRef PubMed.
- B. Li, A. A. Ansari, A. K. Parchur and R. Lv, Exploring the influence of polymeric and non-polymeric materials in synthesis and functionalization of luminescent lanthanide nanomaterials, Coord. Chem. Rev., 2024, 514, 215922 CrossRef CAS.
- R. Qu, X. Zhen and X. Jiang, Emerging designs of aggregation-induced emission agents for enhanced phototherapy applications, CCS Chem., 2021, 4, 401–419 CrossRef.
- P. Chinna Ayya Swamy, G. Sivaraman, R. N. Priyanka, S. O. Raja, K. Ponnuvel, J. Shanmugpriya and A. Gulyani, Near infrared (NIR) absorbing dyes as promising photosensitizer for photo dynamic therapy, Coord. Chem. Rev., 2020, 411, 213233 CrossRef CAS.
- A. Treibs and F.-H. Kreuzer, Difluorboryl-komplexe von di- and tripyrrylmethenen, Justus Liebigs Ann. Chem., 1968, 718, 208–223 CrossRef CAS.
- A. C. Benniston and G. Copley, Lighting the way ahead with boron dipyrromethene (Bodipy) dyes, Phys. Chem. Chem. Phys., 2009, 11, 4124–4131 RSC.
- G. Ulrich, R. Ziessel and A. Harriman, The chemistry of fluorescent bodipy dyes: versatility unsurpassed, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- Q. Zheng, G. Xu and P. N. Prasad, Conformationally restricted dipyrromethene boron difluoride (BODIPY) dyes: highly fluorescent, multicolored probes for cellular imaging, Chem. – Eur. J., 2008, 14, 5812–5819 CrossRef CAS PubMed.
- T. Zhang, C. Ma, T. Sun and Z. Xie, Unadulterated BODIPY nanoparticles for biomedical applications, Coord. Chem. Rev., 2019, 390, 76–85 CrossRef CAS.
- D. Kand, P. Liu, M. X. Navarro, L. J. Fischer, L. Rousso-Noori, D. Friedmann-Morvinski, A. H. Winter, E. W. Miller and R. Weinstain, Water-soluble BODIPY photocages with tunable cellular localization, J. Am. Chem. Soc., 2020, 142, 4970–4974 CrossRef CAS PubMed.
- H. Lu, J. Mack, Y. Yang and Z. Shen, Structural modification strategies for the rational design of red/NIR region BODIPYs, Chem. Soc. Rev., 2014, 43, 4778–4823 RSC.
- X. Lin, F. Chen, X. Yu, H. Wang, H. Qiu, Y. Li, S. Yin and P. J. Stang, Phenylthiol-BODIPY-based supramolecular metallacycles for synergistic tumor chemo-photodynamic therapy, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2203994119 CrossRef CAS PubMed.
- A. Turksoy, D. Yildiz and E. U. Akkaya, Photosensitization and controlled photosensitization with BODIPY dyes, Coord. Chem. Rev., 2019, 379, 47–64 CrossRef CAS.
- J. Wang, C. Yu, E. Hao and L. Jiao, Conformationally restricted and ring-fused aza-BODIPYs as promising near infrared absorbing and emitting dyes, Coord. Chem. Rev., 2022, 470, 214709 CrossRef CAS.
- D. Xi, N. Xu, X. Xia, C. Shi, X. Li, D. Wang, S. Long, J. Fan, W. Sun and X. Peng, Strong pi–pi stacking stabilized nanophotosensitizers: improving tumor retention for enhanced therapy for large tumors in mice, Adv. Mater., 2022, 34, e2106797 CrossRef PubMed.
- P. Li, Z. Liu, X. Huo and W. Zhang, Stereodivergent construction of 1,5/1,7-nonadjacent tetrasubstituted stereocenters enabled by Pd/Cu-cocatalyzed asymmetric Heck cascade reaction, Angew. Chem., Int. Ed., 2024, e202407498, DOI:10.1002/anie.202407498.
- N. A. Bumagina, E. V. Antina, A. A. Ksenofontov, L. A. Antina, A. A. Kalyagin and M. B. Berezin, Basic structural modifications for improving the practical properties of BODIPY, Coord. Chem. Rev., 2022, 469, 214684 CrossRef CAS.
- H.-B. Cheng, X. Cao, S. Zhang, K. Zhang, Y. Cheng, J. Wang, J. Zhao, L. Zhou, X.-J. Liang and J. Yoon, BODIPY as a multifunctional theranostic reagent in biomedicine: self-assembly, properties, and applications, Adv. Mater., 2023, 35, 2207546 CrossRef CAS PubMed.
- N. Boens, B. Verbelen, M. J. Ortiz, L. Jiao and W. Dehaen, Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core, Coord. Chem. Rev., 2019, 399, 213024 CrossRef CAS.
- Y. Li, M. Jiang, M. Yan, J. Ye, Y. Li, W. Dehaen and S. Yin, Near-infrared boron–dipyrrin (BODIPY) nanomaterials: Molecular design and anti-tumor therapeutics, Coord. Chem. Rev., 2024, 506, 215718 CrossRef CAS.
- A. Gorman, J. Killoran, C. O’Shea, T. Kenna, W. M. Gallagher and D. F. O’Shea, In vitro demonstration of the heavy-atom effect for photodynamic therapy, J. Am. Chem. Soc., 2004, 126, 10619–10631 CrossRef CAS PubMed.
- M. Jiang, J. Zhang, Y. Li, T. Shi, T. Ma, Y. Sun, H. Qiu, Y. Li and S. Yin, Rational design of amphiphilic BODIPY-based photosensitizers for multimodal imaging-guided phototherapy, Mater. Chem. Front., 2023, 7, 3668–3679 RSC.
- Y. Lyu, J. Zeng, Y. Jiang, X. Zhen, T. Wang, S. Qiu, X. Lou, M. Gao and K. Pu, Enhancing both biodegradability and efficacy of semiconducting polymer nanoparticles for photoacoustic imaging and photothermal therapy, ACS Nano, 2018, 12, 1801–1810 CrossRef CAS PubMed.
- Y. Zhu, C. Chen, G. Yang, Q. Wu, J. Tian, E. Hao, H. Cao, Y. Gao and W. Zhang, Inhibiting radiative transition-mediated multifunctional polymeric nanoplatforms for highly efficient tumor phototherapeutics, ACS Appl. Mater. Interfaces, 2020, 12, 44523–44533 CrossRef CAS PubMed.
- E. Fron, E. Coutiño-Gonzalez, L. Pandey, M. Sliwa, M. Van der Auweraer, F. C. De Schryver, J. Thomas, Z. Dong, V. Leen, M. Smet, W. Dehaen and T. Vosch, Synthesis and photophysical characterization of chalcogen substituted BODIPY dyes, New J. Chem., 2009, 33, 1490–1496 RSC.
- K. Wen, H. Tan, Q. Peng, H. Chen, H. Ma, L. Wang, A. Peng, Q. Shi, X. Cai and H. Huang, Achieving efficient NIR-II type-I photosensitizers for photodynamic/photothermal therapy upon regulating chalcogen elements, Adv. Mater., 2022, 34, e2108146 CrossRef PubMed.
- V.-N. Nguyen, Y. Yim, S. Kim, B. Ryu, K. M. K. Swamy, G. Kim, N. Kwon, C. Y. Kim, S. Park and J. Yoon, Molecular design of highly efficient heavy-atom-free triplet BODIPY derivatives for photodynamic therapy and bioimaging, Angew. Chem., Int. Ed., 2020, 59, 8957–8962 CrossRef CAS PubMed.
- L.-L. Wu, X. Meng, Q. Zhang, X. Han, F. Yang, Q. Wang, H.-Y. Hu and N. Xing, Heavy-atom engineered hypoxia-responsive probes for precisive photoacoustic imaging and cancer therapy, Chin. Chem. Lett., 2024, 35, 108663 CrossRef CAS.
- W. Sun, X. Zhao, J. Fan, J. Du and X. Peng, Boron dipyrromethene nano-photosensitizers for anticancer phototherapies, Small, 2019, 15, 1804927 CrossRef PubMed.
- H. Ma, Q. Peng, Z. An, W. Huang and Z. Shuai, Efficient and long-lived room-temperature organic phosphorescence: theoretical descriptors for molecular designs, J. Am. Chem. Soc., 2019, 141, 1010–1015 CrossRef CAS PubMed.
- L. Birnoschi, M. S. Oakley, E. J. L. McInnes and N. F. Chilton, Relativistic quantum chemical investigation of actinide covalency measured by electron paramagnetic resonance spectroscopy, J. Am. Chem. Soc., 2024, 146, 14660–14671 CrossRef CAS PubMed.
- S. Yao, Y. Chen, W. Ding, F. Xu, Z. Liu, Y. Li, Y. Wu, S. Li, W. He and Z. Guo, Single-atom engineering of hemicyanine and its amphiphilic derivative for optimized near infrared phototheranostics, Chem. Sci., 2023, 14, 1234–1243 RSC.
- L. Hang, T. Zhang, H. Wen, L. Liang, W. Li, X. Ma and G. Jiang, Controllable photodynamic performance via an acidic microenvironment based on two-dimensional metal-organic frameworks for photodynamic therapy, Nano Res., 2021, 14, 660–666 CrossRef CAS.
- H. Wang, J. Chang, M. Shi, W. Pan, N. Li and B. Tang, A dual-targeted organic photothermal agent for enhanced photothermal therapy, Angew. Chem., Int. Ed., 2019, 58, 1057–1061 CrossRef CAS PubMed.
- Q. Wu, G. Chen, K. Gong, J. Wang, X. Ge, X. Liu, S. Guo and F. Wang, MnO2-Laden black phosphorus for MRI-guided synergistic PDT, PTT, and Chemotherapy, Matter, 2019, 1, 496–512 CrossRef.
- X. Liu, H. Su, W. Shi, Y. Liu, Y. Sun and D. Ge, Functionalized poly(pyrrole-3-carboxylic acid) nanoneedles for dual-imaging guided PDT/PTT combination therapy, Biomaterials, 2018, 167, 177–190 CrossRef CAS PubMed.
- V.-N. Nguyen, Y. Yan, J. Zhao and J. Yoon, Heavy-atom-free photosensitizers: from molecular design to applications in the photodynamic therapy of cancer, Acc. Chem. Res., 2021, 54, 207–220 CrossRef CAS PubMed.
- W. Zhang, W. Deng, H. Zhang, X. Sun, T. Huang, W. Wang, P. Sun, Q. Fan and W. Huang, Bioorthogonal-targeted 1064 nm excitation theranostic nanoplatform for precise NIR-IIa fluorescence imaging guided efficient NIR-II photothermal therapy, Biomaterials, 2020, 243, 119934 CrossRef CAS PubMed.
- L. He, Y. Huang, Y. Chang, Y. You, H. Hu, K. W. Leong and T. Chen, A highly selective dual-therapeutic nanosystem for simultaneous anticancer and antiangiogenesis therapy, J. Mater. Chem. B, 2017, 5, 8228–8237 RSC.
- D. Xu, Y. Li, S. Yin and F. Huang, Strategies to address key challenges of metallacycle/metallacage-based supramolecular coordination complexes in biomedical applications, Chem. Soc. Rev., 2024, 53, 3167–3204 RSC.
- Y. P. Rey, D. G. Abradelo, N. Santschi, C. A. Strassert and R. Gilmour, Quantitative profiling of the heavy-atom effect in BODIPY dyes: correlating initial rates, atomic numbers, and 1O2 quantum yields, Eur. J. Org. Chem., 2017, 2170–2178 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2202487–2202489. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qm00508b |
| This journal is © the Partner Organisations 2024 |







